Abstract
Excessive adiposity has long been associated with increased incidence of breast cancer in postmenopausal women, as well as with increased mortality of breast cancer, regardless of menopausal status. While adipose tissue-derived estrogen contributes to obesity-associated risk for estrogen receptor (ER)-positive breast cancer, the estrogen-independent impact of adipose tissue on tumor invasion and progression remains to be elucidated. Here we show that adipose stromal cells (ASCs) significantly stimulate migration and invasion of ER-negative breast cancer cells in vitro and tumor invasion in a co-transplant xenograft mouse model. Our study also identifies cofilin-1, a known regulator of actin dynamics, as a determinant for the tumor-promoting activity of ASCs. The cofilin-1-dependent pathway affects the production of interleukin 6 (IL-6) in ASCs. Depletion of IL-6 from ASC-conditioned medium abrogated the stimulatory effect of ASCs on the migration and invasion of breast tumor cells. Thus, our work uncovers a link between cytoskeleton-based pathway in ASCs and the stromal impact on breast cancer cells.
Keywords: adipose stromal cells, cofilin-1, IL-6, breast tumor cells, migration, invasion
INTRODUCTION
Obesity has reached epidemic proportions throughout the western world in recent years and has become a major health risk factor for a variety of human diseases including cardiovascular diseases, diabetes, and cancer (Hjartaeker et al., 2008; Zalesin et al., 2008). In particular, a wealth of epidemiological evidence has established a strong association between obesity and higher incidence of breast cancer in postmenopausal women (Carmichael, 2006a; Li et al., 2005). In fact, it is estimated that approximately 20% of all postmenopausal breast cancer cases can be attributed to overweight and obesity (La Vecchia et al., 1997). In addition, a high body mass index (BMI) at disease onset has a well-recognized predictive value for poor prognosis in both pre- and post-menopausal breast cancer patients (Carmichael, 2006b; Whiteman et al., 2005). In a recent clinical study (Dawood et al., 2008), obese or overweight patients with locally advanced breast cancer (LABC) had significantly worse survival outcomes and a higher incidence of recurrence compared to patients with lower BMI (<25). Thus, it is of paramount importance to understand the molecular basis for the association of obesity with breast cancer risk and mortality.
In addition to its function as an energy depot, adipose tissue also serves as a major endocrine organ (Ailhaud, 2006). Numerous studies have demonstrated that an abundance of growth factors and cytokines is released from adipose tissue and can exert a substantial impact on the progression and outcome of many human diseases, including breast cancer (Schaeffler et al., 2007). For instance, breast adipose tissue is an important site for estrogen production among post-menopausal women (Cooke and Naaz, 2004). Elevated estrogen biosynthesis from intratumoral adipose tissue is thought to contribute to estrogen receptor (ER)-positive, postmenopausal breast cancer (Bulun et al., 2005; Kamat et al., 2002). However, a possible estrogen-independent effect of adipose tissue on breast cancer development remains to be elucidated.
Mammary epithelia are surrounded by multiple types of stromal cells including preadipocytes, adipocytes, fibroblasts, vasculature, pericytes, and macrophages (Hennighausen and Robinson, 2005). These stromal cells, in concert with the extracellular matrix (ECM), create a microenvironment that tightly controls proliferation and differentiation of epithelial cells (Bissell et al., 2002). At the onset and during the progression of breast cancer, the tissue microenvironment is reorganized by the tumor cells in order to support tumor cell proliferation and invasion into the surrounding tissue (Pupa et al., 2002). Numerous studies have shown that tumors recruit stromal fibroblasts in a process referred to as the desmoplasmic reaction (Schaeffler et al., 2007). These carcinoma-associated-fibroblasts (CAF) are then reprogrammed to produce growth factors, cytokines, and ECM-remodeling proteins (Orimo et al., 2005). While adipose tissue-derived cells have been linked with cancer development (Celis et al., 2005; Tessitore et al., 2004), much less is known about the molecular basis of the impact of adipose tissue on the behavior of breast tumor cells.
Recent advances in regenerative medicine have led to the discovery of a new adipose cell type termed adipose stromal cells (ASCs) with great developmental plasticity (Gimble et al., 2007; Tholpady et al., 2006). These cells share characteristics of mesenchymal stem cells (MSCs) isolated from other tissues including the capability to differentiate into multiple cell lineages (Lin et al., 2006). Given that bone-marrow-derived MSCs recruited by breast carcinomas promote breast cancer invasion and metastasis (Karnoub et al., 2007), we hypothesized that ASCs might possess a similar tumor-promoting capability. Using in vitro co-culture assays and a xenograft model, we studied the effect of ASCs on the migratory and invasive behaviors of tumor cells.
RESULTS
ASCs stimulate migration and invasion of breast tumor cells in vitro
ASCs were isolated from adipose tissue obtained by abdominal liposuction or reduction mammoplasty from cancer-free donors using a previously published protocol (Katz et al., 2005). These cells were tested in a Boyden-chamber based transmigration assay for their ability to induce migration of ER-positive (MCF-7) and ER-negative breast cancer cells (MDA-MB-231). As a comparison, we included skin fibroblasts that were previously shown to stimulate tumor growth in a xenograft model (Liu & Hornsby, 2007a). The various stromal cells, together with medium alone as the negative control, were seeded in the lower chamber of a Boyden-chamber apparatus 48 hours prior to the assay. Breast cancer cells were subsequently placed into the top chamber and allowed to migrate across the porous membrane. ASCs of both breast and abdominal adipose tissue origins substantially stimulated migration of breast cancer cells, as evidenced by the large number of tumor cells migrating through the membrane in the presence of ASCs (Fig. 1A, top panel). In contrast, skin fibroblasts only gave rise to modest increases in the number of migrated breast cancer cells. ASCs stimulated migration of ER-positive and ER-negative cells to a comparable extent (Fig. 1A), suggesting that the effect of ASCs was estrogen independent. Matrigel-based cell invasion assay demonstrated a similar stimulatory effect of ASCs on tumor cell invasion (Fig. 1B).
Figure 1. ASCs promote migration and invasion of breast cancer cells.
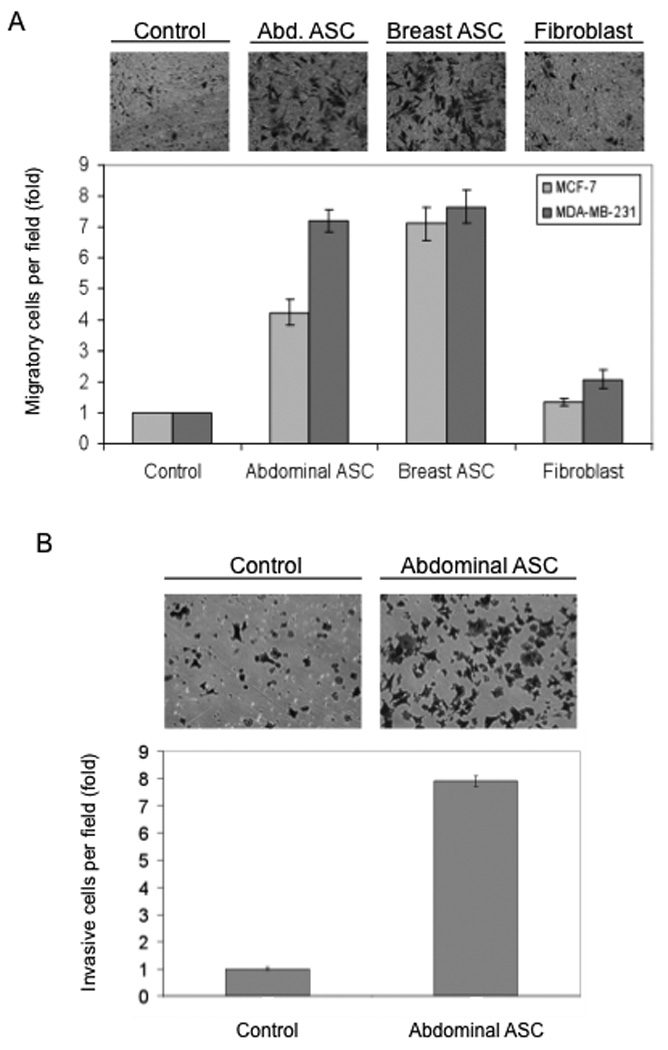
(A) Boyden-chamber migration assay. ASCs isolated from breast and abdominal adipose tissue and a skin fibroblast cell line were plated in 24-well plates for 48h. Normal growth medium without cells was used as control. MDA-MB-231 cells or MCF-7 cells were loaded in the upper chambers. MDA-MB-231 cells were assayed after 7 h and MCF-7 cells after 24 h. The top panels illustrate the migrated MDA-MB-231 cells. (B) Matrigel-based invasion assay. Abdominal ASCs were plated as described above in normal growth medium. MDA-MB-231 cells were loaded on the top chambers coated with Matrigel. The breast tumor cells were allowed to invade for 7 h. Columns are the mean of a representative experiment assayed in triplicate and normalized to the control column. In this and following figures, error bars = standard deviation (SD). All experiments were repeated at least 3 times.
Because there was no direct contact between ASCs and breast cancer cells under these assay conditions, an ASC-secreted factor(s) was most likely responsible for the stimulation of tumor cell migration and invasion. In support of this notion, ASC-conditioned medium was sufficient to stimulate tumor cell migration in the Boyden-chamber assay (data not shown, but see below). To complement the Boyden-chamber assay, the ability of ASC-conditioned medium to stimulate tumor cell migration was tested in a “wound-healing” assay, where a “wound” was created in a confluent culture of MDA-MB-231 cells and movement of the tumor cell front into the open space was monitored by live-cell imaging. Tumor cells that were exposed to ASC-conditioned medium displayed a 20% higher velocity than those exposed to control medium (p=0.0001; Suppl. Fig. 1A), thus further supporting the existence of an ASC-secreted factor(s) that influences the migratory behavior of breast cancer cells. It is also worth noting that, upon exposure to ASC-conditioned medium, a small number of the tumor cells migrated at a much faster pace than the rest of the cell population (Suppl. Fig. 1B). This type of “outlier” cells was not observed in MDA-MB-231 cells treated with control medium.
ASCs stimulate invasion of breast tumor cells in a xenograft model
To validate the in vitro effect of ASCs on tumor cell behaviors, we used a renal capsule-based xenograft mouse model (Liu & Hornsby, 2007a) to investigate the impact of ASCs on tumor invasion in vivo. MDA-MB-231 cells were transplanted alone or with ASCs under the renal kidney capsule of immunodeficient mice (n=4 per treatment). Two weeks after transplantation, tumor development was evaluated by immunohistochemistry of the green fluorescent protein (GFP)-positive tumor cells in the xenograft mouse kidney tissue. As shown in panels a and b in Fig. 2A, MDA-MB-231 cells without ASCs formed a quite even front at the boundary between tumor cells and the mouse kidney tissue. In contrast, MDA-MB-231 cells that were co-transplanted with ASCs did not form a distinct boundary between the xenograft and kidney tissue (panels c and d in Fig. 2A). Rather, tumor cells in this case adopted a quite aggressive phenotype, as evidenced by invasion of finger-like protrusions plus individual tumor cells into the kidney parenchyma. To quantitate the ASC effect, the depth of the invading cell front was taken as an indicator for tumor invasiveness. There was a striking increase in the depth of invasion when ASCs were co-transplanted with the breast tumor cells (Fig. 2B). Taken together, both in vitro and in vivo findings clearly demonstrate a stimulatory effect of ASCs on the migratory behavior and invasive capability of breast cancer cells.
Figure 2. ASCs promote MDA-MB-231 cell invasion in a xenograft mouse model.
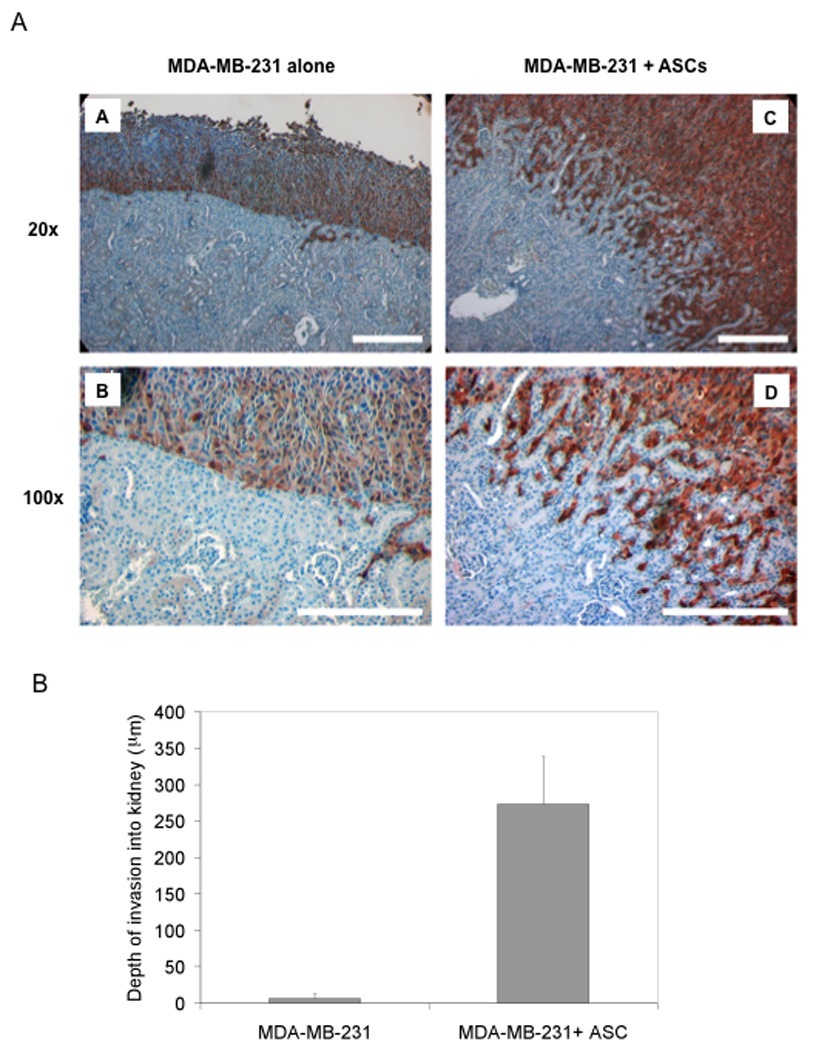
MDA-MB-231-GFP cells either alone or with ASCs were transplanted under the renal capsule of immunodeficient mice (n=4 per treatment). The mice were sacrificed 2 weeks after transplantation. Sections were stained with a GFP-specific antibody for visualizing the GFP-expressing breast tumor cells. MDA-MB-231-GFP cells appear brown and mouse renal cells blue as a result of hematoxylin counter-staining. (A) Panels a and b show MDA-MB-231 transplanted alone, magnification x20 and x100, respectively. Panels c and d show MDA-MB-231 cells co-transplanted with ASCs. The white bars stand for 200 µm. (B) The depth of breast tumor cells invading into the mouse kidney tissue was used as a measure of invasiveness.
The stimulatory effect of ASCs involves a cofilin-1-dependent pathway in the stromal cells
To identify the potential molecular players that mediate the effect of ASCs on tumor cell migration, we used an siRNA mini-library that was used in a separate study of ASCs (Ghosh et al., manuscript submitted). The siRNA-transfected ASCs were seeded in the lower chamber of the Boyden-chamber system to assess their effects on the migration of MDA-MB-231 cells. Of all the genes targeted in the siRNA screen, cofilin-1 knockdown resulted in the greatest reduction in the ASC-mediated stimulation of tumor cell migration (Suppl. Fig, 2). To ascertain the specificity of the siRNA pool, two independent cofilin-1 siRNA oligos were tested for their effects on ASC-mediated stimulation of tumor cell migration. As shown in Fig. 3, both the siRNA pool and individual oligos were effective in reducing the cofilin-1 expression and the migration-promoting activity of ASCs. Notably, the protein levels of cofilin-1 in the knockdown cell populations correlate well with their ability to stimulate migration of MDA-MB-231 cells.
Figure 3. siRNA knock-down of Cofilin-1 in ASCs reduces their migration-promoting activity.
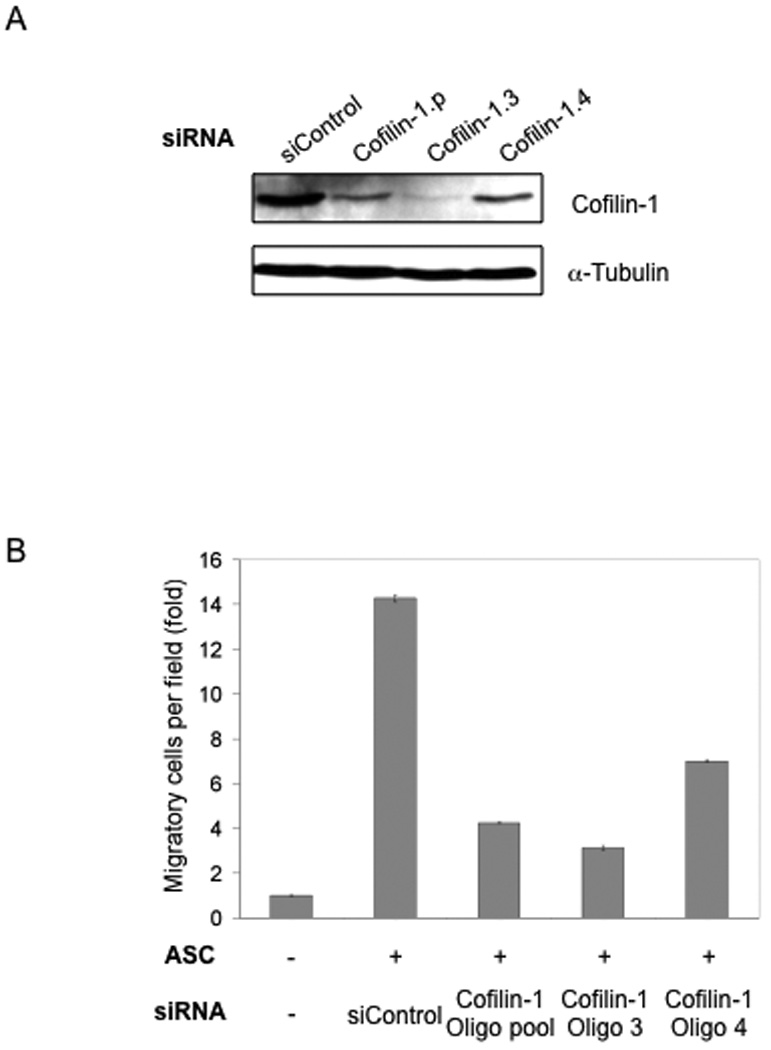
(A) Cofilin-1 knocked down in ASCs by siRNA using an oligo pool and individual oligos. α-Tubulin is used as loading control. (B) Cofilin-1-knockdown ASCs were tested in the Boyden-chamber migration assay using MDA-MB-231 cells.
Cofilin-1 is a key regulator of actin dynamics. It acts by severing actin filaments (F-actin) and thereby creating barbed ends for polymerization and extension of actin filaments as well as increasing available monomeric G-protein (Marcoux and Vuori, 2005), (Song et al., 2006). The activity of cofilin-1 is governed through the coordinated action of several kinases, including RhoA and Rock1 (Wang et al., 2007) (Fig. 4A). To ascertain the role of the cofilin-1-dependent pathway in ASC-stimulated tumor cell migration, we knocked down several additional players in the same pathway: N-Wasp, RhoA, Rock1, and Rock2, another member of the Rock family (Suppl. Fig. 3). Knockdown of N-Wasp, RhoA or Rock1 consistently reduced the stimulatory activity of ASCs on breast tumor cells (Fig. 4B). In contrast, siRNA knockdown of Rock2, which is not involved in regulation of cofilin-1 activity, did not have as much of an effect on the migration of breast cancer cells (Fig. 4B). In yet another approach to determine the involvement of Rock1 in the ASC-mediated stimulation of breast cancer cell migration, ASCs were pre-treated with a ROCK-specific inhibitor (Y27632). At concentrations that do not affect cell viability, the drug treatment reduced ASC-stimulated migration of breast cancer cells in a dose-dependent manner (Fig. 4C). Taken together, these data indicate that the cofilin-1-dependent pathway in ASCs plays an important role in conferring stimulation of tumor cell migration.
Figure 4. The migration-promoting activity of ASCs is controlled by the Cofilin-1 signaling pathway.
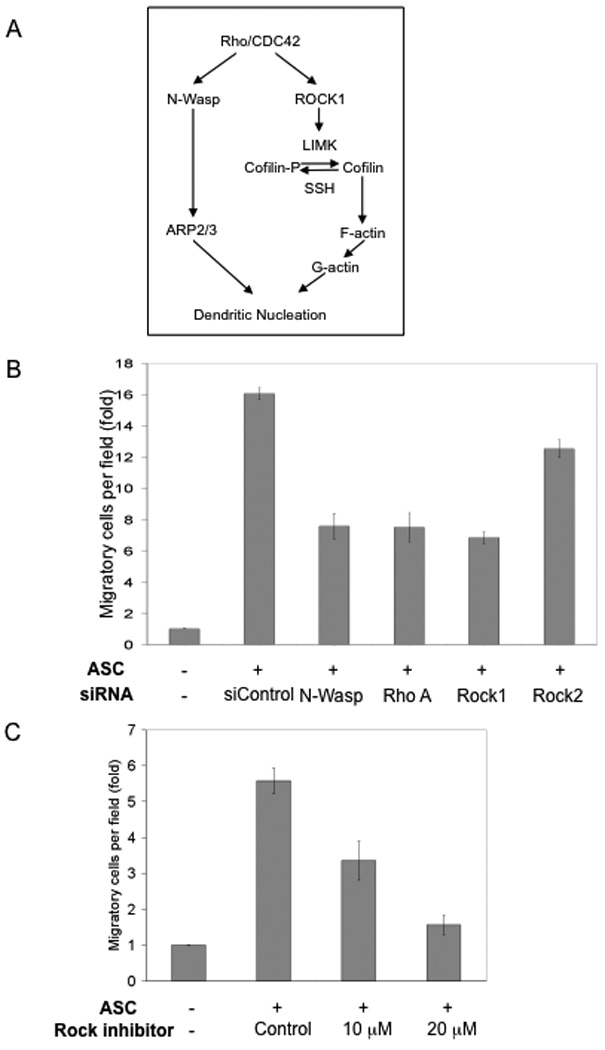
(A) A diagram illustrating the Cofilin-1-dependent pathway, adapted from Wang et al. (Wang et al., 2007). (B) siRNA knockdown of several players of the Cofilin-1 pathway reduced the migration-promoting activity of ASCs. (C) Pre-treatment of ASCs with the specific Rock inhibitor Y27632 reduced the migration-promoting activity of ASC in a dose-dependent manner. All experiments were carried out in triplicate and repeated at least 3 times.
ASC-secreted Interleukin-6 (IL-6) is responsible for the ASC-dependent stimulation of breast cancer cell migration and invasion
Our data from the in vitro migration and invasion assays strongly suggest the presence of a diffusible factor(s) in ASC-conditioned medium that stimulates tumor cell migration. Furthermore, medium that was conditioned with cofilin-knockdown ASCs exhibited a significantly lower stimulatory activity in the Boyden-chamber assay in comparison with medium from the control knockdown cells (Fig. 5A), suggesting that the cofilin-1 knockdown reduces the production of the putative migration-promoting factor(s).
Figure 5. siRNA knock down of Cofilin-1 in ASCs results in reduced production of IL-6.
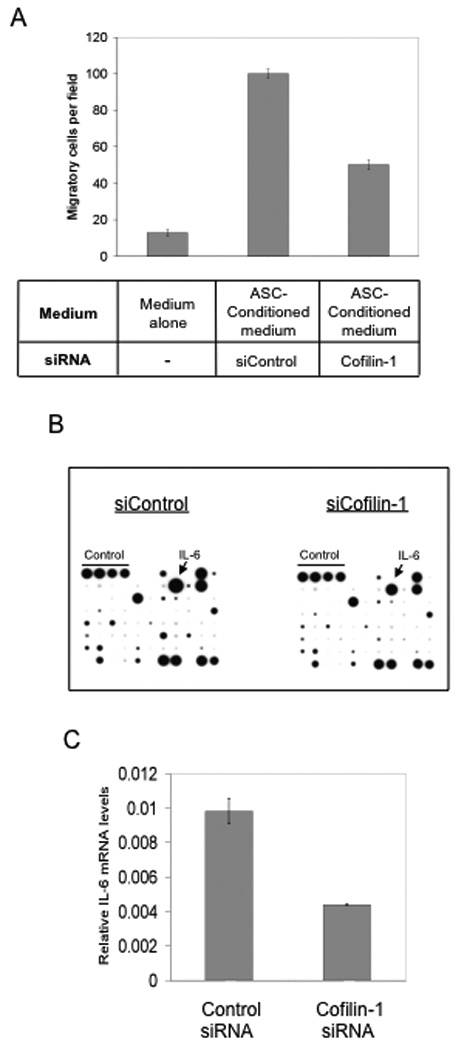
(A) MDA-MB-231 migration assay using growth medium alone or ASC-conditioned medium, with or without Cofilin-1 knockdown. (B) Antibody-based cytokine array (RayBio®) using conditioned medium from control and Cofilin-1 knockdown ASCs. Experiment was repeated twice. (C) RT-PCR of IL-6 in ASCs transfected either with control or Cofilin-1 siRNA. All experiments were carried out in triplicate.
To identify the putative migration-promoting factor(s), we used conditioned medium from control and cofilin-1-knockdown ASCs to probe an antibody-based cytokine array that is capable of detecting 79 different cytokines and growth factors. To reduce the high background of growth factors and cytokines present in normal fetal bovine serum, the FBS concentration in the ASC-culturing medium was reduced to 1% (v/v). The reduced serum concentration was still sufficient to sustain the ASC-mediated stimulatory effect on tumor cell migration (Suppl. Fig. 4). As shown in Fig. 5B, the control ASC-conditioned medium contained a number of cytokines and growth factors at detectable levels. These include ENA-78, ILβ1, IL-6, IL-8, MCP-1, RANTES, VEGF, FGF-4, HGF, TIMP-1, and TIMP-2 (Fig. 5B, left panel). Among all the detected factors, the protein level of IL-6 was most significantly reduced in the cofilin-1-knockdown conditioned medium (arrows in Fig. 5B). Consistent with the result from the cytokine array, the mRNA level of IL-6 was also reduced in the cofilin-1-knockdown ASCs (Fig. 5C).
To determine whether IL-6 contributes to ASC-mediated stimulation of breast tumor cell migration, IL-6 was depleted from the ASC-conditioned medium by an IL-6-specific antibody and the resulting medium was tested for its ability to induce migration of breast cancer cells in the Boyden-chamber assay. As shown in Fig. 6A, IL-6 depletion abrogated the migration-promoting activity of ASCs. As a control, non-conditioned medium that was treated with the IL-6 antibody did not affect breast cancer cell migration, suggesting that the neutralizing effect of the IL-6 antibody was specific to the ASC-conditioned medium. In a similar manner, IL-6 depletion substantially reduced the capability of ASC cells to stimulate invasion of breast cancer cells in the Matrigel-based invasion assay (Fig. 6B). Therefore, our work identifies IL-6 as an important ASC-secreted factor that is responsible for stimulating the migratory and invasive behavior of breast tumor cells.
Figure 6. IL-6 depletion reduces the migration and invasion-promoting effect of ASCs.
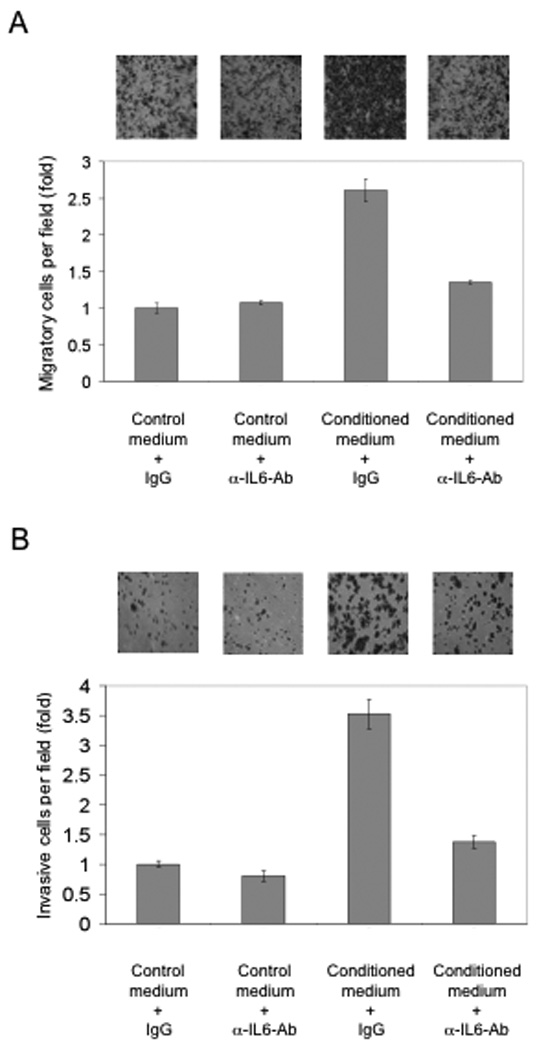
(A) IL-6 was removed from ASC-conditioned medium by incubation with a mouse monoclonal IL-6 specific antibody. An isomeric IgG was used as control antibody. The Boyden-chamber migration assay was conducted as shown in the previous figures. (B) Matrigel-based invasion assay of MDA-MB-231 cells using control or IL-6-depleted ASC-conditioned medium. Experiments were carried out in triplicate and repeated three times. Columns were normalized to Control Medium + IgG.
DISCUSSION
Excessive adiposity is a well-documented risk factor for breast cancer. Adipose tissue exerts both paracrine and endocrine effects on breast tumor development. Our study shows that ASCs, a resident cell population in adipose tissue with multipotent potential, provide a potent stimulus for tumor cell migration and invasion in vitro and in vivo. Because ASCs used in our study were derived from cancer-free individuals and had never been exposed to any tumor milieu, our work suggests that ASCs have an innate ability to promote tumor migration and invasion. Furthermore, we demonstrate that the cofilin-dependent signal transduction pathway has a previously unappreciated function in controlling the production of adipose cell-secreted tumor-promoting factors. Lastly, we provide evidence that ASC-secreted IL-6 is an important factor in promoting tumor cell migration and invasion.
It has been well documented that tumor cells have the ability to recruit stromal cells to the vicinity of the tumor and re-program the latter to support tumor growth (Schaeffler et al., 2007). In addition, accumulating evidence suggests that chemokines produced by bone marrow-derived mesenchymal stem cells (BM-MSC) play a key role in promoting tumor growth and progression (Raman et al., 2007). For example, it has been shown recently that BM-MSCs can be programmed for promotion of tumor invasion when exposed to breast cancer cell-conditioned medium (Lin et al., 2008). Work by the Weinberg group also demonstrates that BM-MSCs in tumor stroma are induced to secrete the chemokine CCL5 (RANTES), which in turn enhances the migration, invasion, and metastatic capacity of the tumor (Karnoub et al., 2007). In addition, a recently published study indicates that IL-6 secreted by bone marrow stromal cells creates a bone marrow microenvironment supporting growth and metastasis of neuroblastoma cells (Ara et al., 2009). In this regard, it is noteworthy that cytokine profiles produced from adipose-derived stem cells are similar to those displayed by bone marrow-derived stem cells, with IL-6 and IL-8 being the most abundantly expressed chemokines amongst a variety of others (Kilroy et al., 2007).
Our in vitro study clearly implicates the role of ASC-secreted IL-6 in promoting tumor cell migration of ER-positive and ER-negative breast cancer cells. This finding is in line with previously published data showing the growth promoting effect of IL-6 (Paduch and Kandefer-Szerszen, 2005; Sasser et al., 2007). IL-6 secretion increases with body mass and obese patients have greatly elevated IL-6 serum levels (Hoene and Weigert, 2008). Furthermore, high serum level of IL-6 is a viable marker for poor prognosis in breast cancer patients (Hong et al., 2007). It is conceivable that high IL-6 serum levels provide an environment that is conducive to tumor growth and progression (Gao et al., 2007; Sansone et al., 2007), which offers a reasonable explanation for the poor survival rates of obese breast cancer patients (Knuepfer and Preiss, 2007).
Depletion of IL-6 from the ASC-conditioned medium did not totally abolish the ASC-mediated stimulatory effect on migration and invasion of the breast cancer cells. This could be due to incomplete depletion of IL-6 from the conditioned medium. Alternatively, additional factor(s) in the ASC conditioned medium may also contribute to the tumor-promoting effect of ASCs. Consistent with this possibility, the cytokine blotting used in this work indicates differential levels of additional factors secreted by the control and Cofilin-knockdown ASCs. It will be of importance to determine whether IL-6 could act synergistically with another putative ASC-secreted factor to promote tumor invasion.
Our finding does not exclude an additional effect of ASCs in guiding tumor cell invasion through physical contact with the tumor cells in vivo. It was recently reported that fibroblasts are capable of generating tracks and leading the movement of carcinoma cells when the two cell types were in physical contact (Gaggioli et al., 2007). Given the highly migratory nature of the ASC itself (M.W. and R.L., unpublished data), it is conceivable that the cytokine-producing and track-generating capabilities of ASC may both contribute to its observed tumor invasion-promoting effect in vivo.
The function of Cofilin-1 has been extensively characterized in epithelial cells. By severing actin filaments (F-actin), Cofilin-1 can increase the number of free barbed ends for polymerization and at the same time replenish the pool of monomeric G-actin in the cells (Pollard and Borisy, 2003; Wang et al., 2007). Deregulation of the Cofilin-1-mediated pathways in breast tumor cells is a critical determinant for the invasive and metastatic phenotype of tumor cells (Wang et al., 2007). However, function of the same actin-regulatory pathways in the stromal compartment of the tumor microenvironment is not well understood. Our study strongly implicates a Cofilin-1-dependent pathway in controlling the gene expression level of IL-6, which is distinct from its well-recognized role in supporting cytoskeleton dynamics. While the exact mechanism by which Cofilin-1 regulates IL-6 production remains to be elucidated, we envision the following two possible scenarios. First, Cofilin-1-mediated cytoskeleton dynamics may lead to changes in the activity of a number of kinases known to be associated with the cytoskeleton network. Alternatively, Cofilin-1 may influence the location and activity of nuclear actin, which has been shown to more directly regulate transcription via its interactions with various transcription factors, chromatin remodeling complexes, and even RNA polymerase II itself (Pederson, 2008). Regardless of the underlying mechanism, we speculate that excessive adiposity may exert significant stress on cellular structure and maintenance of the stress fibers in ASCs. Constant activation of the Cofilin-1 pathway may therefore result in increased expression and secretion of cytokines like IL-6. Weight loss via pharmacological intervention or physical activity in obese breast cancer patients may greatly improve their survival by reducing the adipose production of inflammatory factors. Furthermore, given the known function of Cofilin-1 pathway in breast tumor cells, simultaneous blockage of the Cofilin-1 pathway in tumor and the surrounding stromal cells may provide an effective way to blunt tumor invasion.
Adiposity is often equated with the abundance of the fully differentiated, fat-laden mature adipocytes. However, adipose tissue contains a variety of other resident cell types including preadipocytes. While it was reported that mature adipocytes rather than preadipocytes are promoters of breast carcinoma growth (Manabe et al., 2003), more recent work demonstrated the involvement of both adipocytes and preadipocytes in promoting the proliferation of colon cancer cells (Amemori et al., 2007). The complex composition of adipose tissue is reflected by the presence of various precursors of adipocytes at earlier stages of adipogenesis, as evidenced by the isolation and characterization of ASCs for their regenerative potentials (Gimble et al., 2007; Tholpady et al., 2006). Similar to multipotent adult stem cells from other tissues, ASCs display extensive developmental plasticity, as evidenced by their ability to differentiate into multiple lineages including adipocytes, osteoblasts, chondrocytes, and myocytes, etc. In our study, ASCs isolated from either abdominal or breast adipose tissue greatly increased migration of both ER-positive and ER-negative breast tumor cells, suggesting that the induction of tumor cell migration and invasion is independent of the source of fat depot and estrogenic function of ASCs. The recent identification of white fat progenitor cells in the mouse adipose vasculature provides more insight into the molecular identity and locations of these adipocyte pregenitor cell population (Tang et al., 2008). Therefore, it will be of importance to determine in future work whether human ASCs and mouse fat progenitor cells share the same property in stimulating tumor cell migration and invasion.
While most of the published work has focused on the tumor-promoting activity of stromal cells in response to the initial activating cue from tumor cells, the current work reveals an innate capability of ASCs from cancer-free individuals to promote tumor cell migration and invasion. Adipose tissue is not only a fat depository, but also an important endocrine organ that releases a wide variety of chemokines or adipokines (Ailhaud, 2006). Obesity is characterized by a vast increase in fat cell size and number (Spalding et al., 2008). Moreover, obesity is associated with an altered cytokine profile, characterized by a reduction in the release of anti-inflammtory cytokines such as adiponectin and a concurrent increase in secretion of pro-inflammatory cytokines (Ferrante, 2007). Consequently, obesity is considered to be an important source of chronic inflammation. Prolonged exposure to high levels of pro-inflammatory cytokines secreted by ASCs in adipose tissue prior to tumor formation may provide an environment conducive to the initiation and subsequent development of breast cancer. It will be of interest to determine in future work whether the tumor migration-promoting activity of ASCs varies in the general population and if so, whether it serves as a predisposing factor for breast cancer development.
METHODS AND MATERIALS
Cell culture and reagents
The human breast cancer cell line MCF-7 was obtained from the American Type Culture Collection (ATCC, Rockville, MD). MDA-MB-231-GFP and the primary human fibroblast cells (CRL2703) were previously reported (Liu & Hornsby, 2007b). Human adipose stromal cells (ASCs) were isolated from abdominal and breast adipose tissue from cancer-free donors undergoing liposuction and reduction mammoplasty, respectively, using previously published protocols (Ghosh et al., 2007). For all experiments, ASCs between passages 4 and 10 were used.
Boyden-chamber transmigration assay
Untreated or siRNA-treated ASCs were plated (4×104 cells/0.5 ml DMEM/F12 plus FBS) in a 24-well plate (cat.# 353504, BD Biosciences, Bedford, MA) and incubated for 48h at 37°C. MDA-MB-231-GFP (5×104 cells/0.2 ml) were placed in the upper compartment of the Boyden-chamber system, using Boyden-chamber inserts fitted with 3 micron pore membranes (BD Biocoat cat.# 354575). For experiments using MCF-7 cells, 2×105 cells/0.2 ml were loaded into the Boyden-chamber inserts fitted with 8 micron pore membranes (BD BioCoat cat.# 354578). MDA-MB-231-GFP cells were assayed for 7 h and MCF-7 cells for 24 h. At the end of the assay, inserts were removed and the cells were fixed in 4% paraformaldehyde and then stained with a 0.1% Crystal-Violet solution. Tumor cells on the upper membrane surface were removed with a paper towel. The air-dried membranes were viewed under 20x magnification and migrated cells were counted in 3 randomly chosen fields per membrane. Each cell line was assayed at least three times and assays were performed in triplicate. Error bars show standard deviation (SD). For invasion assays, Matrigel-coated inserts with 8 micron pore membranes (BD BioCoat cat.# 354480) were used. MDA-MB-231-GFP cells (5×104 cells/0.2 ml) were processed in the same manner as the migration assay.
Wound-healing migration assay
MDA-MB-231-GFP cells (4×104 cells/0.5 ml) were plated into silicone cell culture inserts (quadriPERM, cat.# Z376647, Sigma-Aldrich, St, Louis, MO) that had been placed into a 35 mm Petri-dish. The control medium was DMEM + 10% FBS while the conditioned medium consisted of DMEM + 10% FBS supplemented with ASC supernatant. The cells were incubated for 48 h at 37°C. The cell monolayer was scraped with a pipette tip to create a wound. Closure of the wound was monitored with a NIKON BioStation IM Time Lapse Imaging System at 5 randomly chosen fields per sample in 15-min intervals over a period of 24 h. The speed of wound-closure or velocity of the moving cell fronts was measured at 3 different points per field using NIS – Elements Advanced Research (V 3.0) software. Statistical analysis was performed by an unpaired Student’s t-test.
Xenograft in immunodeficient mice
Male and female immunodeficient mice (RAG2−/− γc−/−, Taconic, Germantown, NY) at the age of > 6 weeks and with an average weight of ∼ 25 g were used in this experiment. Procedures were executed in accordance with the National Institutes of Health (NIH) Guide for the Use and Care of Laboratory Animals. Cell transplantations were performed as described previously (Liu and Hornsby, 2007a). Either MDA-MB-231-GFP cells (2 × 106 cells) alone or mixed with an equal number of ASCs were injected under the renal capsule. Two weeks after cell transplantation, xenografts were fixed in 4% paraformaldehyde and processed by conventional methods as described in (Liu & Hornsby, 2007a). Penetration of the invading cell front into the host kidney was measured under 100x magnification. Statistical analysis was performed by an unpaired Student’s t-test.
RNA isolation and real-time RT-PCR
Total RNA was extracted using the TRIzol method according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). 1 µg of total RNA was then used to produce cDNA by reverse transcription using random primers of the ImPrompII Reverse Transcription System kit (Promega, Madison, WI). SYBR Green-based RT-PCR was carried out in a 7900 HT Real-Time PCR System (Applied Biosystems) according to the manufacturer’s instructions. The primer sets used in these experiments are listed in Suppl. Table 1. Values for each gene were normalized to the expression levels of 18S rRNA.
siRNA knockdown
Dharmacon siGENOME SMARTpool oligos were purchased from Thermo Fisher Scientific (Lafayette, CO). Catalog numbers for the specific siRNA oligo pools are listed in Suppl. Table 2. 1 × siRNA buffer (cat.# B-002000-UB-015) was used in mock and OTP (cat.# M-013549-00) in control transfections. siRNA knockdown experiments were carried out as described previously (Ghosh et al., 2007). In brief, ASCs at ∼ 60% confluency were transfected with Lipofectamine RNAiMax reagent (Invitrogen, Carlsbad, CA) in Opti-MEM (GIBCO) and siRNA oligos at a concentration of 20 nM overnight. Individual siRNA oligo sets were also tested at a final concentration of 10 nM (cat.# D-012707-03, −04).
Immunoblotting
After electrophoretic separation and immunoblotting of whole ASC lysates, Cofilin-1 was detected with a mouse monoclonal anti-Cofilin-1 antibody (Santa Cruz Biotechnology, CA) and α-Tubulin with a mouse monoclonal anti-α-Tubulin antibody (CalBiochem, La Jolla, CA).
Cytokine Antibody Array
An antibody-based cytokine array system was used to detect the levels of cytokines and growth factors in supernatants from ASCs that had been either treated with a control siRNA or Cofilin-1 siRNA. In order to minimize the effect of exogenous cytokines and growth factors in FBS, the FBS concentration in the ASC-culturing medium was decreased to 1% (v/v). The experiment was carried out using the RayBio Human Cytokine Array V kit (cat.# AAH-CYT-5) from RayBiotech (Norcross, GA) following the manufacturer’s instructions. siRNA-treated ASCs were incubated with culture medium for 2 days. The cell-free supernatant was used undiluted. All incubation steps were carried out overnight at 4°C.
IL-6 antibody depletion
Culture medium and ASC-conditioned culture medium were incubated with 2 µg/ml of a mouse monoclonal anti-hIL6-antibody or a mouse monoclonal IgG1 (R&D Systems, Minneapolis, MN) for 4 h on a rotary shaker at 4°C. Protein G-Agarose beads (Roche, Indianapolis, IN) were then added and incubation continued overnight at 4°C. The beads were removed by centrifugation. The supernatant was warmed at 37°C and subsequently used in a Boyden-chamber assay as described above.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Adam Katz for ASCs. The work was supported by grants to R.L. from the NCI (CA93506) and DOD (W81XWH-06-1-034), and by grants to P.J.H. from the NIA, the Owens Medical Research Foundation, and the Glenn Foundation for Medical Research.
Contributor Information
M Walter, Department of Molecular Medicine, Institute of Biotechnology, University of Texas Health Science Center at San Antonio, San Antonio, TX 78245.
S Liang, Department of Physiology, University of Texas Health Science Center at San Antonio, San Antonio, TX 78245.
S Ghosh, Department of Molecular Medicine, Institute of Biotechnology, University of Texas Health Science Center at San Antonio, San Antonio, TX 78245.
PJ Hornsby, Department of Physiology, University of Texas Health Science Center at San Antonio, San Antonio, TX 78245.
R Li, Department of Molecular Medicine, Institute of Biotechnology, University of Texas Health Science Center at San Antonio, San Antonio, TX 78245.
REFERENCES
- Ailhaud G. Adipose tissue as a secretory organ: from adipogenesis to the metabolic syndrome. Comptes Rendus Biologies. 2006;329:570–577. doi: 10.1016/j.crvi.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol. 2007;292:G923–G929. doi: 10.1152/ajpgi.00145.2006. [DOI] [PubMed] [Google Scholar]
- Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Research. 2009;68:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- Carmichael AR. Obesity and prognosis of breast cancer. Obesity Reviews. 2006a;7:333–340. doi: 10.1111/j.1467-789X.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Carmichael AR. Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG. 2006b;113:1160–1166. doi: 10.1111/j.1471-0528.2006.01021.x. [DOI] [PubMed] [Google Scholar]
- Celis JE, Moreira JMA, Cabezon T, Gromov P, Friis E, Rank F, Gromova I. Identification of extracellular and intracellular signaling components of the mammary adipose tissue and its interstitial fluid in high risk breast cancer patients. Mol Cell Prot. 2005;4:492–522. doi: 10.1074/mcp.M500030-MCP200. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Experimental Biology and Medicine. 2004;229:1127–1135. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- Dawood S, Broglio K, Gonzalez-Angulo AM, Kau SW, Islam R, Hortobagyi GN, et al. Prognostic Value of Body Mass Index in Locally Advanced Breast Cancer. Clin Cancer Res. 2008;14:1718–1725. doi: 10.1158/1078-0432.CCR-07-1479. [DOI] [PubMed] [Google Scholar]
- Ferrante AWJ. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Int Medicine. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung carcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Lu Y, Katz A, Hu Y, Li R. Tumor suppressor BRCA1 inhibits a breast cancer-associated promoter of the aromatase gene (CYP19) in human adipose stromal cells. Am J Physiol Endocrinol Metab. 2007;292:E246–E252. doi: 10.1152/ajpendo.00242.2006. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nature Reviews Molecular Cell Biology. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Hjartaeker A, Langseth H, Weiderpass E. Obesity and diabetes epidemics: cancer repercussions. Adv Exp Med Biol. 2008;630:72–93. doi: 10.1007/978-0-387-78818-0_6. [DOI] [PubMed] [Google Scholar]
- Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obesity Rev. 2008;9:20–29. doi: 10.1111/j.1467-789X.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- Kamat A, Hinshelwood MM, Murry BA, Mendelson CR. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endo. Metab. 2002;13:122–128. doi: 10.1016/s1043-2760(02)00567-2. [DOI] [PubMed] [Google Scholar]
- Karnoub A, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:707–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- Knuepfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer. Breast Cancer Research & Treatment. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Negri E, Franceschi S, Talamini R, Bruzzi P, Palli D, et al. Body mass index and post-menopausal breast cancer: an age-specific analysis. Br J Cancer. 1997;75:441–444. doi: 10.1038/bjc.1997.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Bowerman S, Heber D. Health ramifications of the obesity epidemic. Surg Clin North Am. 2005;85:681–701. doi: 10.1016/j.suc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Lin S, Yang J, Everett AD, Clevenger CV, Koneru M, Mishra PJ, et al. The isolation of novel mesenchymal stromal cell chemotactic factors from the conditioned medium of tumor cells. Exp Cell Res. 2008;314:3107–3117. doi: 10.1016/j.yexcr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Chen X, Yan Z, Liu L, Tang W, Zheng X, et al. Multilineage differentiation of adipose-derived stromal cells from GFP transgenic mice. Mol Cell Biochem. 2006;285:69–78. doi: 10.1007/s11010-005-9056-8. [DOI] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Fibroblast stimulation of blood vessel development and cancer cell invasion in a subrenal capsule xenograft model: stress-induced premature senescence does not increase effect. Neoplasia. 2007a;9:418–426. doi: 10.1593/neo.07205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007b;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix cuture through cancer-stromal interactions. J Path. 2003;201:221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- Marcoux N, Vuori K. EGF receptor activity is essential for adhesion-induced stress fiber formation and cofilin phosphorylation. Cellular Signalling. 2005;17:1449–1455. doi: 10.1016/j.cellsig.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCl12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Paduch R, Kandefer-Szerszen M. Vitamin D, tamoxifen, and β-estradiol modulate breast cancer cell growth and interleukin-6 and metalloproteinase-2 production in three-dimensional co-cultures of tumor cell spheroids with endothelium. Cell Biol Toxicol. 2005;21:247–256. doi: 10.1007/s10565-005-0002-z. [DOI] [PubMed] [Google Scholar]
- Pederson T. As functional actin comes into view, is it globular, filamentous, or both? J Cell Biol. 2008;180:1061–1064. doi: 10.1083/jcb.200709082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Pupa SM, Menard S, Forti S, Tagliabue E. New insights into the role of extracellular matrix during tumor onset and progression. J Cell Physiol. 2002;192:259–267. doi: 10.1002/jcp.10142. [DOI] [PubMed] [Google Scholar]
- Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Letters. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser KA, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-α-positive human breast cancer. FASEB Journal. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- Schaeffler A, Schoelmerich J, Buechler C. Mechanisms of disease: adipokines and breast cancer - endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nature Clinical Practice. 2007;3:345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- Song X, Chen X, Yamaguchi H, Mouneimme G, Condeelis JS, Eddy RJ. Initiation of cofilin activity in response to EGF is uncoupled from cofilin phosphorylation and dephosphorylation in carcinoma cells. J Cell Science. 2006;119:2871–2881. doi: 10.1242/jcs.03017. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore L, Vizio B, Pesola D, Cecchini F, Mussa A, Argiles JM, et al. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int J Oncol. 2004;24:1529–1535. [PubMed] [Google Scholar]
- Tholpady SS, Llull R, Ogle RC, Rubin JP, Futrell JW, Katz AJ. Adipose tissue: stem cells and beyond. Clin Plast Surg. 2006;33:55–62. doi: 10.1016/j.cps.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Wang W, Eddy RJ, Condeelis JS. The cofilin pathway in breast cancer invasion and metastasis. Nature Reviews Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2005;14:2009–2014. doi: 10.1158/1055-9965.EPI-05-0106. [DOI] [PubMed] [Google Scholar]
- Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinology Metabolism Clinics of North America. 2008;37:663–684. doi: 10.1016/j.ecl.2008.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


