Abstract
Mice lacking both Prx1 and Prx2 display severe abnormalities in the mandible. Our analysis showed that complete loss of Prx gene products leads to growth abnormalities in the mandibular processes evident as early as E10.5 associated with changes in the survival of the mesenchyme in the medial region. Changes in the gene expression in the medial and lateral regions were related to gradual loss of a subpopulation of mesenchyme in the medial region expressing eHand. Our analysis also showed that Prx gene products are required for the initiation and maintenance of chondrogenesis and terminal differentiation of the chondrocytes in the caudal and rostral ends of Meckel’s cartilage. The fusion of the mandibular processes in the Prx1/Prx2 double mutants is caused by accelerated ossification. These observations together show that during mandibular morphogenesis Prx gene products play multiple roles including the cell survival, the region-specific terminal differentiation of Meckelian chondrocytes and osteogenesis.
Keywords: Prx1, Prx2, Mandibular processes, Meckel’s cartilage, Osteogenesis
Introduction
The development of the lower arch is a multi-step process that starts with the formation of mandibular processes from the first branchial arch. After its formation, the first branchial arch is subdivided into the maxillary and mandibular processes that give rise to the upper and lower jaws respectively. Initially, the mandibular processes consist of a mesenchymal core encased by epithelium. The epithelium covering the mandibular processes is derived from embryonic ectoderm and endoderm. The mesenchyme of the mandibular processes is derived from mesoderm and cranial neural crest cells (CNCC) (reviewed by Knight and Schilling, 2006; Noden and Schneider, 2006). CNCC that populate the mandibular processes arise from the posterior mesencephalon and rhombomeres 1 and 2 and eventually differentiate into bone, cartilage, cranial ganglia, and connective tissue (reviewed by Knight and Schilling, 2006; Noden and Schneider, 2006). These cells do not express Hox genes and carry some species-specific patterning information (reviewed by Brugmann et al., 2006a; Brugmann et al., 2006b; Knight and Schilling, 2006; Noden and Schneider, 2006). However, the fate and differentiation of CNCC into skeletal structures within the mandibular arch are determined by signaling interactions between CNCC and the surrounding tissues including the endoderm of the foregut and the mandibular epithelium (reviewed by Knight and Schilling, 2006; Noden and Schneider, 2006).
Several studies provided evidence that morphogenesis of mandibular processes is regulated by two independent functional regions: two large lateral regions, and a small medial region (reviewed by Mina, 2001b; Mina, 2001a). These regions are characterized by exclusive patterns of expression of signaling molecules including Bmp4 and Fgf8 in the epithelium and several transcription factors in the mandibular mesenchyme (Mina, 2001b; Mina, 2001a).
Lateral regions, where chondrogenesis and osteogenesis are initiated in vivo, give rise to the portion of the mandibular arch containing molar teeth, to most of the rod of Meckel’s cartilage and its associated bones, and, in mammals, to the middle ear structures. It has been shown that Fgf8 expressed by the epithelium of the lateral region together with several genes expressed in the underlying mesenchyme, including Lhx6, Barx1, Gsc, Dlx, and Pitx1, regulates cell proliferation, apoptosis and morphogenesis of the two lateral regions (Trumpp et al., 1999; Abu-Issa et al., 2002; Frank et al., 2002; Macatee et al., 2003). The restricted expression of the Fgf8 is the epithelium of the lateral regions is regulated by SHH expressed by the endoderm (Haworth et al., 2007) and BMP4 expressed by epithelium of the medial region (Liu et al., 2005).
The small region, where the two mandibular processes merge, gives rise to the medial region of the mandibular arch containing incisor teeth, and the most medially located skeletal elements including the symphysial portion of Meckel’s cartilage and its associated bones. The medial region contains highly proliferative mesenchyme and makes a significant contribution to the overall growth of the developing mandible (McGonnell et al., 1998; Mina et al., 2002). Several studies have shown that morphogenesis of the medial region is independent of FGF8 (Trumpp et al., 1999) and dependent on complex interactions of multiple signaling pathways including Bone morphogenetic proteins (BMP) (reviewed by Nie et al., 2006; Liu et al., 2005; Dudas et al., 2004; Ko et al., 2007), Endothelin1 (ET1) (reviewed by Clouthier and Schilling, 2004; Thomas et al., 1998; Clouthier et al., 2000; Fukuhara et al., 2004 Sato et al., 2008b Fukuhara et al., 2004; Ozeki et al., 2004; Sato et al., 2008a), and Hedgehog (HH)- mediated signaling (Jeong et al., 2004; Yamagishi et al., 2006). Candidate transcription factors involved in morphogenesis of the medial region are many including dHAND (Hand2), eHAND (Hand1), Fox genes, Dlx5, Dlx6, Msx1 and Msx2 (Satokata and Maas, 1994; Srivastava et al., 1995; Thomas et al., 1998; Sato et al., 2008b; Fukuhara et al., 2004; Ozeki et al., 2004; Sato et al., 2008a; Jeong et al., 2004).
Prx1 and Prx2 are closely related members of the paired-related family of homeobox genes co-expressed in variety of sites including craniofacial mesenchyme (reviewed by Meijlink et al., 1999). In the mandibular processes, Prx genes are co-expressed at high levels in the mesenchyme of the medial region (Meijlink et al., 1999; Doufexi and Mina, 2008). Prx2 null mice show no obvious craniofacial and skeletal abnormalities (ten Berge et al., 1998), whereas Prx1 mutant mice show defects in skeletal elements derived from the maxillary processes and the caudal part of the mandibular processes, including hypoplastic coronoid, condylar and angular processes and malformed malleus (Martin et al., 1995). In contrast to single mutants, Prx1/Prx2 double mutants die a few hours after birth, exhibit many novel abnormalities, including the highly penetrant and noticeable abnormalities in the developing mandible (ten Berge et al., 1998; Lu et al., 1999a; ten Berge et al., 2001). The mandibular processes of the neonate Prx1/Prx2 double mutants are shortened, fused at the midline, lack the midline symphysis, and have only a single or no incisor. These studies also showed that abnormalities in the mandibular processes of Prx1/Prx2 double mutants were associated with changes in the expression of Fgf8, Bmp4 and Shh in the medial mandibular epithelium, reduced proliferation in the mesenchyme underneath the oral epithelium, and small changes in the domains of Alx3 and Dlx2 expression in the mandibular mesenchyme (ten Berge et al., 1998; ten Berge et al., 2001). Furthermore, it was shown the formation of a single mandibular incisor was associated with the downregulation of Pax9 and Patched underneath the medial domain of Fgf8 expression (ten Berge et al., 1998; Lu et al., 1999a; ten Berge et al., 2001).
The phenotypic abnormalities in the various Prx mutants indicated that in addition to their involvement in mandibular outgrowth, Prx might play roles in chondrogenesis in the mandibular processes. Meckel’s cartilage in Prx1 mutants displayed abnormal sigmoidal morphology (Martin et al., 1995) and for the most part was absent in the Prx1/Prx2 double mutants (ten Berge et al., 1998; Lu et al., 1999a; Meijlink et al., 1999).
Abnormalities in the mandibular processes and Meckel’s cartilage in Prx1/Prx2 double mutants provided direct evidence for the essential roles of Prx gene products in the morphogenesis of the medial region and mandibular chondrogenesis. However, the underlying mechanisms leading to these abnormalities and the genetic pathway(s) in the medial region, which include Prx and gene products regulated by or dependent on Prx gene products, are still not well identified.
In this study, to gain a better understanding of the underlying mechanisms of abnormalities in growth and morphogenesis of the mandibular processes, we examined the patterns of expression of additional transcription factors with essential roles in mandibular morphogenesis including Msx1, Msx2, dHand, eHand, Barx1, Dlx5 and Pitx1 in the Prx1/Prx2 double mutants. We also re-examined the effects of the absence of Prx gene products on cell proliferation and apoptosis in mandibular mesenchyme. Our analysis showed that complete loss of Prx gene products leads to growth abnormalities in the mandibular processes evident as early as E10.5 and is associated with changes in cell proliferation, apoptosis, and expression of regulatory genes in the medial (where Prx genes are expressed) and lateral (where Prx genes are not expressed) regions.
Furthermore, we also examined the underlying mechanisms for abnormlities in Meckel’s cartilage in Prx1/Prx2 double mutants. Our analysis showed roles for Prx gene products in terminal differentiation of chondrocytes in the caudal and rostral ends as well as osteogenic differentiation by mandibular mesenchyme.
RESULTS
Skeletal preparations at E19 showed that the Prx1/Prx2 double mutants embryos developed much smaller and malformed upper and lower jaws compared with the Prx1+/-;Prx2-/- and Prx1+/+;Prx2-/- (supplemental Figure 1). The mandibular processes in the Prx1 +/+; Prx2 -/- and Prx1 +/-; Prx2 -/- contained bones, two molar tooth germs, two incisor tooth germs and the symphysial cartilage between the mandibular processes (supplemental Figure 1B & 1D, data not shown). In contrast, Prx1/Prx2 double mutant embryos had significantly smaller mandibles that were fused at the midline and lacked symphysial cartilage (supplemental Figure 1F). The Prx1/Prx2 double mutant mandibles contained two molar tooth germs and mostly no incisors and occasionally one incisor (data not shown). Prx1/Prx2 double mutants also displayed wider mandibular processes and an increase in the angle between the right and left mandibular processes compared with Prx1 +/-; Prx2 -/- and Prx1 +/+; Prx2 -/- (supplemental Figure 1B, 1D, 1F). The abnormalities in the Prx1/Prx2 double mutants at E19 were similar to those reported for newborns (ten Berge et al., 1998; Lu et al., 1999a; Lu et al., 1999b). The lack of noticeable abnormalities in Prx1+/+; Prx2-/- as compared to wild type, is consistent with the lack of abnormalities in Prx2-/- mutants (ten Berge et al., 1998). In the experiments described in this paper Prx1+/+; Prx2-/- embryos were used as the control.
The formation of significantly smaller mandibles in the Prx1/Prx2 double mutants at E19 (supplemental Figure 1) suggests that Prx gene products are involved in proper outgrowth of the mandibular processes. To determine the onset of growth abnormalities in the mandibular processes, the size of the mandibular processes were compared between the Prx1/Prx2 double mutants and controls (Prx1+/+;Prx2-/-) at early stages of development. These studies showed that at E10.5 (soon after the fusion of the mandibular processes) and at E13.5 mandibular processes in the Prx1/Prx2 double mutants were 56% and 65% smaller than controls respectively (Table 1).
Table 1. Quantitative analysis of the effects of loss of Prx on the size, proliferation, apoptosis and osteogenesis of the mandibular processes.
Determination of the size, quantification of H3-p positive cells and TUNEL positive cells, as well as the analysis of osteogenesis were performed as described in Materials and Methods. The areas for quantitative analysis included the entire area of the mandibular mesenchyme and the mesenchyme of the medial region. All parameters were measured using ImageJ and values represent means ± S.E. Asterisk indicates statistically significant difference (p< 0.05).
| Stage of development | Prx1+/+; Prx2-/- (control) | Prx1-/-; Prx2-/- | |
|---|---|---|---|
| Size (mm2) | E10.5 n=4 | 0.75 ± 0.01 | 0.44 ± 0.02* |
| E13.5 n=3 | 2.62 ± 0.04 | 1.71 ± 0.09* | |
| TUNEL positive cells (cells/mm2) | E10.5 n=4 | 59 ± 21 | 290 ± 145* |
| E11.5 n=4 | 30 ± 18 | 76 ± 32* | |
| H3-P positive cells (cells/mm2) | E10.5 (whole mandible) n=4 | 444 ± 105 | 206 ± 85* |
| E10.5 (medial region) n=4 | 297 ± 97 | 101 ± 62* | |
| Osteogenesis (mm2) | Runx2 expression n=3 | 0.36 ± 0.01 | 0.47 ± 0.02* |
| Mason Tricrome n=3 | 0.06 ± 0.004 | 0.12 ± 0.01* |
There are multiple possible mechanisms that could cause defects in the outgrowth of the mandibular processes of Prx1/Prx2 double mutants. One possibility is that the defects are due to lack of migration of the CNCC into the mandibular processes. However, Prx genes are not expressed in premigratory neural crest and cells expressing Prx1 and Prx2 are initially normally distributed in the mandibular processes of the mutants but disappear later (Lu et al., 1999; ten Berge et al., 2001). This suggests that the abnormalities in the outgrowth in Prx1/Prx2 double mutants may be due to disturbances in the signaling pathways regulating mandibular morphogenesis and/or negative effects of Prx in maintenance and survival of the of mandibular mesenchyme.
Loss of Prx genes affected the patterns of expression of genes in the medial (rostral) region of the mandibular processes
Next we examined the effects of absence of Prx gene products on the expression of the components of the signaling pathways implicated in proper growth and morphogenesis of the mandibular processes by in situ hybridization using E10.5 and E11.5 embryos. Since Prx1 and Prx2 are expressed in mesenchyme of the medial region, we first focused on components of other signaling pathways expressed in the medial region of the mandibular processes including Bmp4, Msx1, Msx2, dHand, and eHand (Figures 1-3).
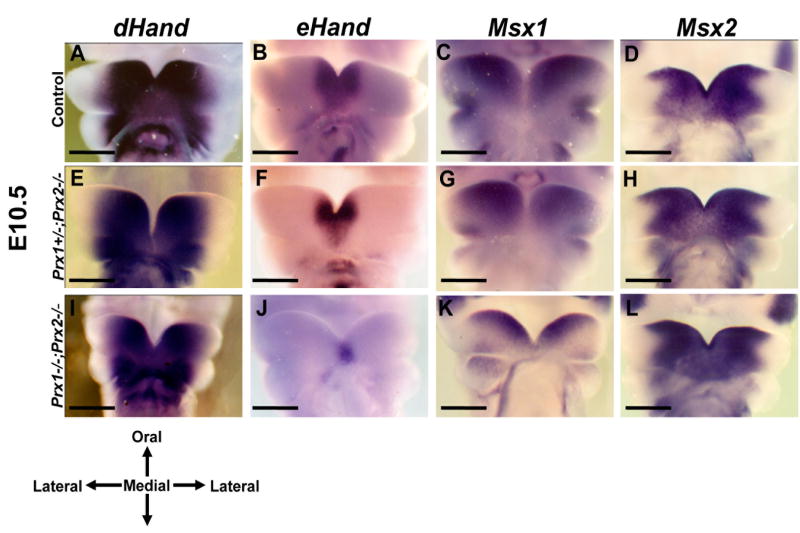
Figure 1. Effects of absence of Prx on the expression of regulatory factors in the medial region of the developing mandibles at E10.5.
Frontal views on whole mount in situ hybridization analysis of control (A-D), Prx1+/-;Prx2-/- (E-H) and Prx1;Prx2 double mutant (I-L) embryos at E10.5. A, E, I show that dHand is expressed in a similar domain in the medial region of the mandibular processes of all three genotypes. The expression of eHand in the medial region of the mandibular processes in control (B) is wider than those in the Prx1+/-;Prx2-/- (F) and Prx1;Prx2 double mutant (J). Expression of the Msx1 in the medial region of the mandibular processes of the Prx1;Prx2 double mutant (K) is more restricted to the oral side as compared to those in Prx1+/-;Prx2-/- (G) and controls (C). D, H, L show that Msx2 is expressed in a similar domain in all three genotypes. Scale bar in all pictures=1mm.
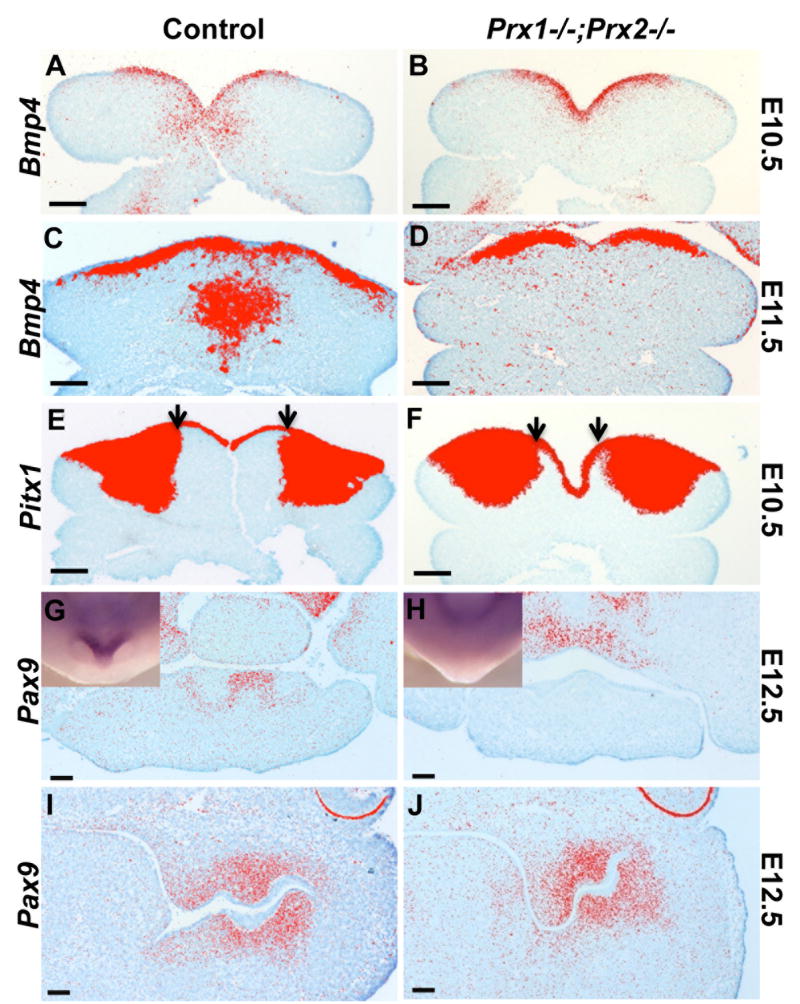
Figure 3. Effects of absence of Prx on the expression of Bmp4, Pitx1 and Pax9.
A-D are pseudo colored images of in situ hybridization analysis with Bmp4 on transverse sections of mandibular processes of control (A, C) and Prx1-/-;Prx2-/- (B, D) embryos at E10.5 (A, B) and E11.5 (C, D). The expression of the Bmp4 in the mesenchyme of the medial region is reduced in the Prx1/Prx2 double mutants as compared to control. There are no significant changes in the expression of Bmp4 in the mandibular epithelium between the two genotypes.
E, F are pseudo colored images of in situ hybridization analysis with Pitx1 on transverse sections of mandibular processes of control (E) and Prx1-/-;Prx2-/- (F) embryos at E10.5. The expression of Pitx1 in the mandibular epithelium is similar between the control and Prx1/Prx2 double mutants. The expression of the Pitx1 in the mandibular mesenchyme in extended into the medial region in the Prx1/Prx2 double mutants as compared to control.
G-J are pseudo colored images of in situ hybridization analysis of Pax9 expression in the dental papillae of the mandibular incisors (G, H) and molars (I, J) in transverse sections of control (G, I) and Prx1/Prx2 double mutant (H, J) mandibles at E12.5. Note Pax9 expressed in the dental papillae of the mandibular incisors in the control (G), but not in the Prx1/Prx2 double mutants (H). Whole-mount in situ hybridization analysis with Pax9 is shown in the insets. Note that Pax9 is expressed in the rostral region of the control mandible but not in Prx1;Prx2 double mutants. Also note Pax9 is expressed in similar domain and intensity in the dental papillae of the mandibular molars in the control (I) and the Prx1/Prx2 double mutants (J). Scale bars =100 um.
eHand (Hand 1) and dHand (Hand 2) are members of the class II bHLH proteins expressed in partially overlapping domains in the neural crest derived mesenchyme in the branchial arches (reviewed by Firulli, 2003). eHand−/− embryos die at E8.5 due to abnormalities in the yolk sac (Firulli et al., 1998) and mice with deletion of eHand in neural crest derived mesenchyme did not display abnormalities in the mandibular processes nor in embryonic development (Barbosa et al., 2007). dHand−/− embryos die at E10.5 due to heart abnormalities and display hypoplastic mandibular processes secondary to programmed cell death (reviewed by Firulli et al., 1998). The compound Hand mutants with neural crest specific deletion of the eHand combined with various mutant alleles of dHand displayed hypoplastic mandibular processes due to severe defect in the medial region of the mandibular processes including defects in the development of lower incisors and the symphysis of Meckel’s cartilage (Barbosa et al., 2007).
The shared subset of abnormalities in the medial region of Prx and Hand compound mutants provide evidence for their essential roles in morphogenesis of the medial region of mandibular processes and suggest that products of these genes may directly or indirectly interact and function in the same pathway. To test this possibility we examined expression of eHand and dHand in Prx1/Prx2 double mutant embryos. At E10.5 and E11.5, in control embryos, dHand and eHand were expressed in overlapping domains in the mesenchyme in the medial region of the mandibular process (Figures 1A, 1B, 2A, 2B). In the medial region, eHand expression was within the domain of dHand expression and more restricted to the midline (Figures 1A, 1B, 2A, 2B). In the Prx1/Prx2 double mutants there were no significant changes in the domain of expression of dHand in the medial region of the mandibular processes (Figures 1I & 2I) as compared to controls (Figures 1A & 2A) and Prx1+/-; Prx2-/- (Figures 1E & 2E). On the other hand, the domain of expression of eHand in the Prx1/Prx2 double mutants was reduced at E10.5 (Figure 1J) and absent at E11.5 (Figure 2J). At both stages of development, the domain of eHand in Prx1+/-; Prx2-/- was intermediate between the Prx1/Prx2 double mutants and control (Figures 1F & 2F). These observations suggest that in the medial region of the mandibular processes, expression of eHand (but not dHand) is regulated by Prx gene products.
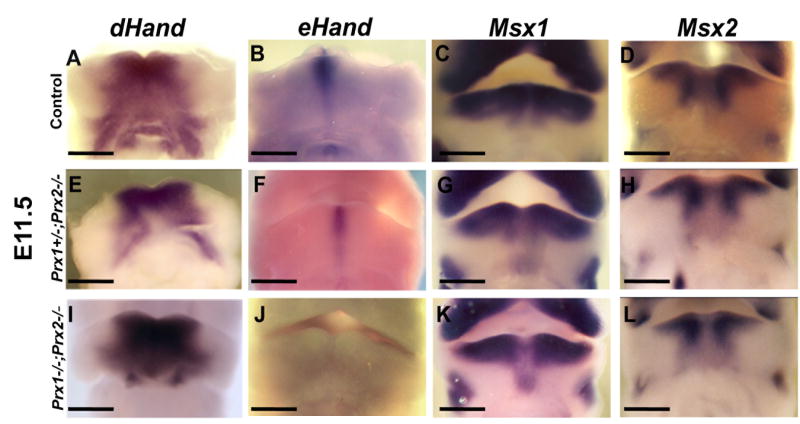
Figure 2. Effects of absence of Prx on the expression of regulatory factors in the medial region of the developing mandibles at E11.5.
Frontal views on whole mount in situ hybridization analysis of control (A-D), Prx1+/-;Prx2-/- (E-H) and Prx1;Prx2 double mutant (I-L) embryos at E11.5. A, E, I show that the expression of dHand in the medial region of the mandibular processes is similar in all three genotypes. eHand is expressed in a restricted domain in the midline of the mandibular processes in the control (B). Note the reduced domain of expression of eHand in Prx1+/-;Prx2-/- (F) and the absence of expression of eHand in the Prx1;Prx2 double mutant (J). C, G, K show that the expression of Msx1 in the mandibular processes of the Prx1+/-;Prx2-/- (G) and Prx1;Prx2 double mutant (K) is extended into the aboral side in the midline region as compared to control (C). D, H, L show that the expression of Msx2 in the mandibular processes is similar in all three genotypes. Scale bars =1 mm.
Msx1 and Msx2 are two transcription factors with restricted expression in the mesenchyme of the medial region and are well-known targets of BMP signaling. The abnormalities in the medial region of the Msx1, Msx2 and Msx1;Msx2 (Satokata and Maas, 1994; Satokata et al., 2000) mutants as well as in mice with targeted deletion of Bmp4 in the mandibular process (Liu et al., 2005), in Alk2/Wnt1-Cre mutants (Dudas et al., 2004) and in Wnt1-Cre;Smad4fl/fl mutants (Ko et al., 2007) indicated their essential functions in morphogenesis of the medial region. Further analysis of various mutants indicated that ET-1-dHAND-Msx1 constitutes one of the genetic pathways regulating morphogenesis of the mandibular process and that the expression of Msx1 in the medial mandibular mesenchyme is dependent on dHand (Thomas et al., 1998.) As the next step we examined the effects of loss of Prx on the expression of Bmp4, Msx1 and Msx2.
In the control embryos, Bmp4 was expressed in the epithelium of the medial region and the underlying mesenchyme at E10.5 and E11.5 (Figures 3A, 3C). In situ hybridization to whole mounts and tissue sections showed little or no changes in the Bmp4 expression in the epithelium of the medial region in Prx1/Prx2 double mutants (Figures 3B, 3D). However, at both stages, expression of the Bmp4 in the mesenchyme of the medial region was downregulated (Figures 3B, 3D).
At E10.5, in the control embryos, Msx1 and Msx2 were expressed in partially overlapping domains in the mesenchyme of the medial region of the mandibular processes (Figures 1C, 1D). At E11.5, in the controls, Msx1 expression was restricted to the oral side and the expression of Msx2 in the medial region extended from oral to aboral side (Figure 2C, 2D). Compared to controls, in the Prx1/Prx2 double mutants the expression of Msx1 was more restricted to the oral side of the mandibular processes at E10.5 (Figure 1K) and extended into the aboral side in the midline region at E11.5 (Figure 2K). The pattern of expression of Msx1 in Prx1+/-; Prx2-/- was intermediate between the Prx1/Prx2 double mutants and control (Figures 1G & 2G). As compared to controls, in Prx1/Prx2 double mutants Msx2 expression appeared unaffected at E10.5 (Figures 1D, 1H, 1L) and at E11.5 (Figure 2D, 2H, 2L). These observations suggest that in the medial region of the mandibular processes, expression of Bmp4 and Msx1 in the mesenchyme may be directly or indirectly regulated by Prx gene products.
The unchanged pattern of the expression of Bmp4 in the mandibular epithelium of the Prx1/Prx2 double mutants in our study is different from the absence of Bmp4 in the medial mandibular epithelium reported by ten Berge et al, (1998) but consistent with the expression of Msx genes in the mandibular mesenchyme shown to be regulated by BMP signaling. Taken together, these observation suggest that Prx gene products may function upstream of eHand, Msx1 and Bmp4 in the medial mandibular mesenchyme.
Loss of Prx genes affected the patterns of expression of genes in the lateral (Caudal) regions of the mandibular processes
We next examined the patterns of expression of selected factors that are expressed complementarily to Prx1 and Prx2 and primarily in the lateral regions of the mandible including Fgf8, Dlx5, Barx1 and Pitx1 (Figures 3E, 3F & 4).
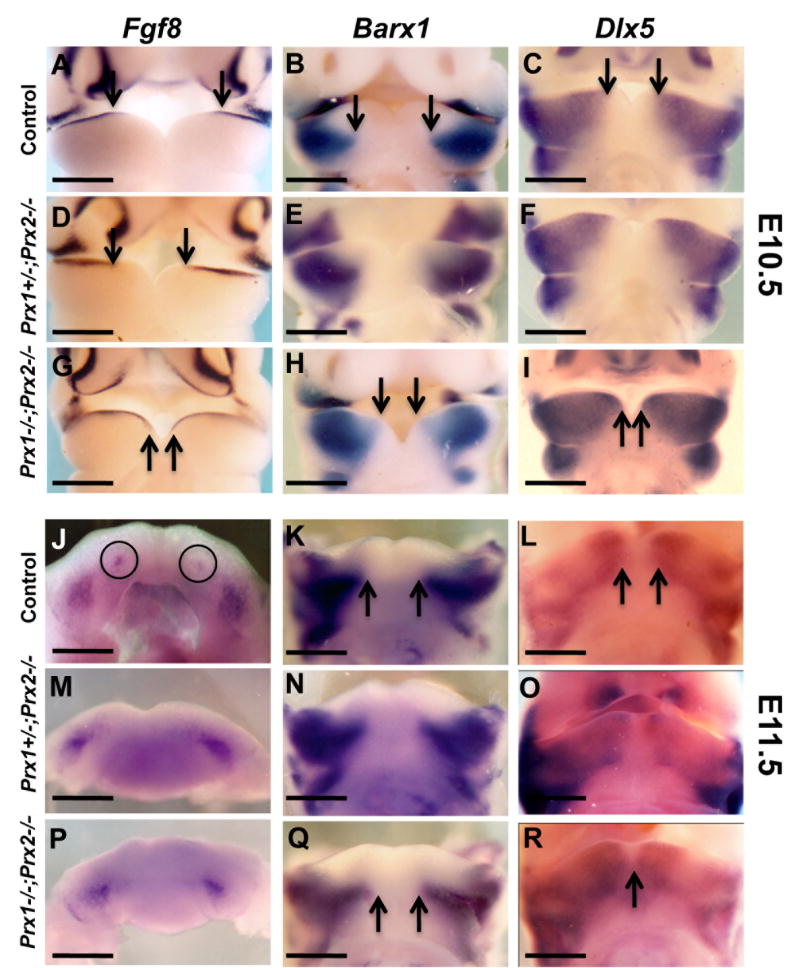
Figure 4. Effects of absence of Prx gene products on the expression of regulatory factors in the lateral region of the developing mandibles.
Whole mount in situ hybridization analysis with Fgf8 (A, D, G, J, M, P), Barx1 (B, E, H, K, N, Q) and Dlx5 (C, F, I, L, O, R) probes on E10.5 (A-I) and E11.5 (J-R) of control (A-C and J-L), Prx1+/-;Prx2-/- (D-F and M-O) and Prx1;Prx2 double mutant (G-I and P-R) heads.
A is a frontal view of E10.5 head showing expression of Fgf8 in the mandibular epithelium of the lateral region (A). The medial boundary of the expression of Fgf8 in the mandibular epithelium (indicated by arrows) in the Prx1/Prx2 double mutants (G) is extended into the medial region as compared to control (A). The medial boundary of the expression of Fgf8 in the mandibular epithelium of Prx1+/-;Prx2-/- (D) is intermediate between those in the control and Prx1/Prx2 double mutants.
Expression of Barx1 and Dlx5 in the mandibular processes is restricted to the lateral mesenchyme in the controls (B, C) and extended more into the mesenchyme of the medial region in the Prx1+/-;Prx2-/- (E, F) and Prx1/Prx2 double mutants (H, I).
J, M and P are top views of mandibular processes showing Fgf8 expression in E11.5 embryos. Note that in control embryos Fgf8 is expressed in presumptive incisors (circles) and molars (J). M and P are views of mandibular processes from Prx1+/-;Prx2-/- and Prx1;Prx2 double mutant showing that the expression of Fgf8 is detected in the molars but not in the incisors. Note that the expressions of Barx1 and Dlx5 in the Prx1;Prx2 double mutant (Q, R) are extended into the medial region as compared to controls (K, L). Scale bars =1 mm.
At E10.5, Pitx1 was expressed in the mandibular epithelium of the medial and lateral region and the mesenchyme of the lateral regions (Figure 3E). In the control embryos at E10.5, Fgf8 was expressed in the epithelium of the lateral region and Barx1 and Dlx5 in the mesenchyme of lateral regions (Figures 4A-C). Compared to their patterns of expression in the control, in the Prx1/Prx2 double mutants, the expression of Fgf8 in the mandibular epithelium, as well as expression of Dlx5, Barx1 and Pitx1 in the mandibular mesenchyme extended into the medial region (Figures 3F, 4G-I). The pattern of expression of Fgf8 in the Prx1/Prx2 double mutants at E10.5 in our study is consistent with the results reported by ten Berge et al., (2001).
At E11.5, in control embryos, the expression of Fgf8 was restricted to presumptive molar and incisor forming regions (Figure 4J). The mandibular processes of the Prx1/Prx2 double mutants contained the two domains of Fgf8 in the lateral regions (presumptive molars) but not the domains of Fgf8 in the medial region (presumptive incisors) (Figure 4P). The absence of Fgf8 in the mandibular epithelium of the medial region in Prx1/Prx2 double mutants at E11.5 in our study is different from the results reported by ten Berge et al., (2001). We speculated that the difference was related to abnormalities in the developing mandibular incisors (presence of only a single or no incisor) in the Prx1/Prx2 double mutants (ten Berge et al., 1998; Lu et al., 1999a; ten Berge et al., 2001). To test this possibility, we analyzed the expression of Pax9, a transcription factor with essential roles in tooth formation and morphogenesis (Neubuser et al., 1997) by whole mount and radioactive in situ hybridization in sections from E12.5 embryos (Figure 3). In the control embryos, Pax9 expression was detected in the molars and incisors dental mesenchyme (Figures 3G, 3I). In the Prx1/Prx2 double mutants Pax9 expression was detected in the dental mesenchyme of the developing molars (Figure 3 J) but was not detected in the incisors regions (Figure 3H). Thus, the lack of expression of Fgf8 and Pax9 in our study is related to the absence of the mandibular incisors in Prx1/Prx2 double mutants.
Similar to their patterns of expression at E10.5, at E11.5 the domains of expression of Barx1, Dlx5 and Pitx1 in the Prx1/Prx2 double mutants were extended into the medial region as compared to controls (Figures 4Q, 4R and data not shown). The non-overlapping domains of expression of Prx1 and Prx2 in the medial mandibular mesenchyme with the regulatory factors expressed in the lateral regions of the mandibular processes does not support the direct interactions between the Prx gene products and these regulatory factors. These observations suggest that Prx gene products may be necessary for the establishment of the boundaries of the expression domains of regulatory genes in the lateral regions of the mandibular processes.
Loss of Prx genes affected the cell proliferation and apoptosis of the mesenchyme of the medial region
We then examined the effects of absence of Prx gene products on proliferation and survival of mandibular mesenchyme. These studies were limited to the Prx1/Prx2 double mutants and controls. Cell proliferation was examined by immunohistochemistry using H3-p antibody that marks cells in M phase in tissue sections of E10.5 embryo mandibular processes. Apoptosis was examined by TUNEL assay. We found significant differences in the numbers of apoptotic and proliferative cells in the mandibular processes in the Prx1/Prx2 double mutants compared to controls (Table 1 and data not shown). At E10.5, there was approximately 46% reduction in the number of proliferative cells and a 5-fold increase in the number of apoptotic cells in the mandibular mesenchyme of the Prx1/Prx2 double mutants compared to controls (Table 1 and supplemental Figure 2A and 2B and data not shown). In the Prx1/Prx2 double mutants, apoptotic cells were detected primarily in the mesenchyme of the medial (rostral) and most caudal regions of the mandibular processes (data not shown). The number of proliferative cells in mesenchyme of the medial region in the Prx1/Prx2 double mutants was approximately one third of that in the controls (Table 1 and supplemental Figure 2A and 2B).
The changes in the cell proliferation and apoptosis in the mandibular mesenchyme and the mesenchyme of the medial region observed in the Prx1/Prx2 double mutants in our studies are different from results reported by ten Berge et al., 2001) and most likely related to the sensitivity of the different assays (immunohistochemistry vs. immunofluorescence) used in these studies. This previous study did not detect significant differences between the number of apoptotic cells in the mandibular processes in the Prx1/Prx2 double mutants and wild type at E10.5 and E11.5 (ten Berge et al., 2001). In vivo BrdU labeling showed reduction in the number of labeled cells only in the mesenchyme underneath the oral epithelium of Prx1-/-Prx2-/- mutants as compared to Wild-type (ten Berge et al., 2001).
The significant changes in the number of proliferative and apoptotic cells in the mesenchyme in the medial region of the mandibular processes in the Prx1/Prx2 double mutants compared to controls suggest a role for the Prx gene products in the proliferation and survival of specific population of the CNC mesenchyme in the medial region of the developing mandible.
Effects of absence of Prx gene products on the Meckel’s cartilage
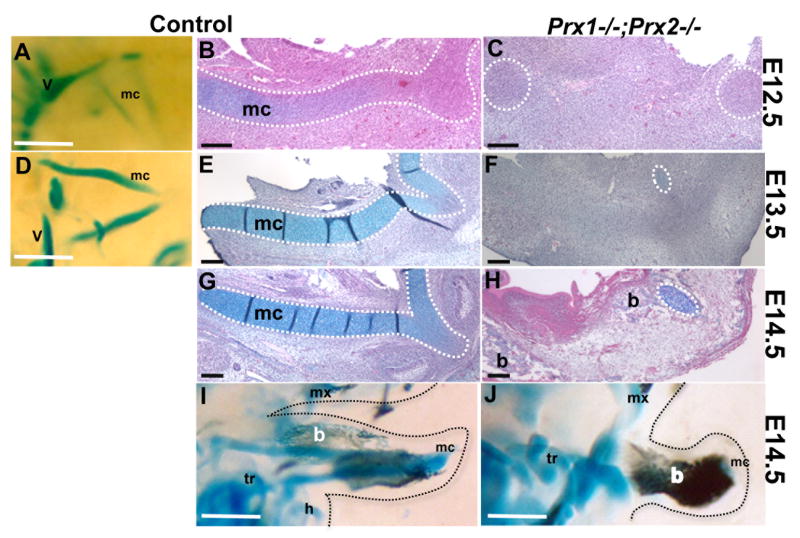
Previous studies showed that one of the most noticeable abnormalities in the Prx1/Prx2 double mutants is reduction in the size of Meckel’s cartilage (MC) (ten Berge et al., 1998; Lu et al., 1999b). Skeletal staining of embryos at E14.5 showed that, in contrast to the rod-shaped cartilage in the wild-type, most of MC was absent in the Prx1/Prx2 double mutants (ten Berge et al., 1998; Lu et al., 1999b). To gain insight into the underlying mechanism leading to abnormalities in MC, we compared the development of MC in the mandibular processes in the Prx1/Prx2 double mutants and control embryos using bone and cartilage staining, histological evaluations and in situ hybridization analysis between E11.5 and E14.5.
The formation of MC in the mouse mandibular processes begins at E11.5 with the formation of chondrogenic condensations. One day later, at E12.5 chondrogenic differentiation is initiated and the primordium of MC becomes apparent as rods within each mandibular processes and a short rostral process (Ishizeki et al., 2003; Ramaesh and Bard, 2003). As development proceeds, the two rods lengthens by appositional growth at both ends and fuse with the small rostral process at E13.5. At E14.5 mandibular processes contain the fully differentiated Meckel’s cartilage that becomes surrounded by mandibular bones. During later stages of embryonic development, chondrocytes located in the most rostral (symphysial portion of MC) and caudal ends of MC (giving rise to the incus and the malleus of the middle ear) undergo endochondral ossification, whereas chondrocytes in the main body of MC are either resorbed or transformed into fibroblasts that give rise to the sphenomandibular ligament (Richman and Diewert, 1988; Ishizeki et al., 2003; Ramaesh and Bard, 2003).
Skeletal staining and histological evaluation of control embryos at E12.5, showed the presence of rods of precartilage primordia (identified by light staining with Alcian Blue) within each mandibular process (Figures 5A & 5B) and the condensations for the rostral processes in (Figure 5B). During the next two days (E13.5 and E14.5) the rods of MC elongated, thickened, stained more intensely with Alcian Blue, and fused with the rostral processes (Figure 5D, 5E, 5G, 5I). In contrast, in the Prx1/Prx2 double mutants the precartilage primordia for the MC rods and the rods of MC were absent (Figure 5C, 5F, 5H, 5J). However, despite the absence of the main rod of MC, Prx1/Prx2 double mutants contained a very small rostral processes and a small cartilaginous structure in the most caudal ends revealing the region-specific abnormalities in the MC in the Prx1/Prx2 double mutants (Figure 5F, 5H, data not shown).
Figure 5. Effect of absence of Prx genes on development of Meckel’s Cartilage.
Whole mount staining (A, D, I, J) and histological analysis (B, C, E, F, G, H) of longitudinal sections of mandibular processes from control (A, B, D, E, G, I) and Prx1;Prx2 double mutant (C, F, H, J) embryos at E12.5 (A-C), E13.5 (D-F) and E14.5 (G-J). In all images the symphyseal portion of the Meckel’s cartilage and the medial region of the developing mandible is on the right.
Side view (A) and longitudinal section (B) of the mandibular processes from control embryos, showing the presence of two rods of Meckel’s cartilage at E12.5. The dashed white line oulines the forming Meckel’s cartilage. C is a longitudinal section of a mandibular process from Prx1;Prx2 double mutant embryo at E12.5 showing the absence of rods of Meckel’s cartilage. The dashed white circles outlines the areas of condensation for the most rostral and caudal ends of Meckel’s cartilge.
Side view (D) and longitudinal section (E) of the mandibular processes from control embryos showing the presence of the two rods of Meckel’s cartilage (outlined by dashed white line) at E13.5. F is a longitudinal section of a mandibular process from Prx1;Prx2 double mutant embryo at E13.5 showing the absence of rods of Meckel’s cartilage. Note the small remnants of Meckel’s cartilage (indicated by dashed white circle) in the rostral region of the mandibular processes of Prx1;Prx2 double mutant at E13.5.
(G) Longitudinal section through the mandibular process isolated from E14.5 control embryo showing the fully formed rod of Meckel’s cartilage (outlined by dashed white line). (H) Longitudinal section through the mandibular process from Prx1;Prx2 double mutant embryo at E14.5 showing the absence of the fully formed rod of Meckel’s cartilage. Note the remnant of Meckel’s cartilage (indicated by dashed white circle) in the rostral region of the mutant mandibular process.
I and J are side views of stained head from control (I) and Prx1;Prx2 double mutant (J) embryos at E14.5 showing the formation of mandibular bones stained with Alizarin Red (indicated by b). Note the increased bones stained with Alizarin Red in the mandibular processes of Prx1;Prx2 double mutant. The soft tissue of the face is highlighted by dashed black lines. Abbreviations: b, mandibular bones; h, hyoid; mc, Meckel’s cartilage; mx, maxilla; tr, tympanic ring; v, vertebrate column.Scale bars =100 um.
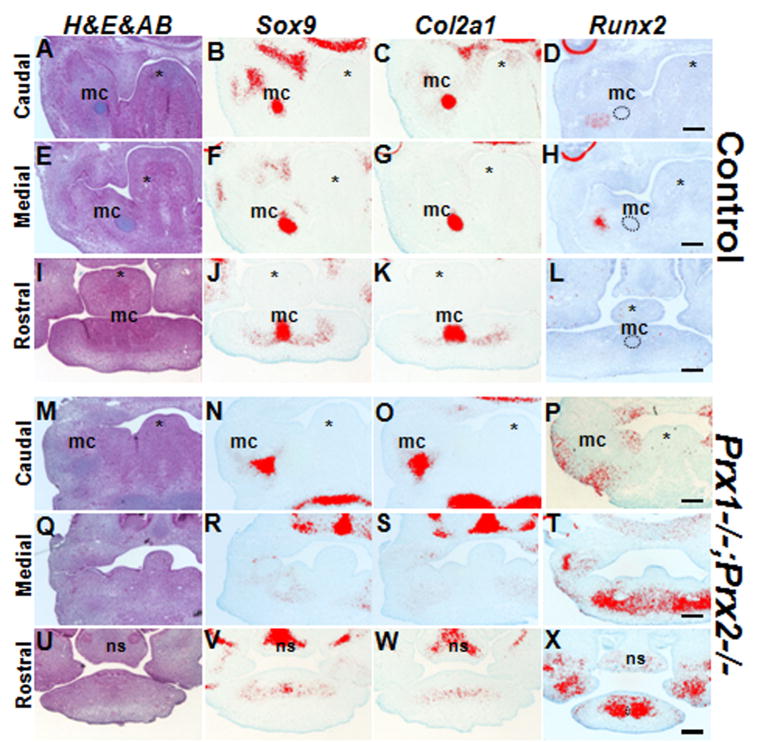
To determine the mechanisms leading to the region-specific abnormalities in MC in the Prx1/Prx2 double mutants, the expression of Sox9 and Col2a1, markers for chondrogenic condensation and immature and proliferating chondrocytes respectively, were examined by radioactive in situ hybridization to sections (supplemental Figure 2C and 2D and Figure 6). In situ hybridization on sections of the mandibular processes at E11.5 showed similar patterns of expression of Sox9 in the control and Prx1/Prx2 double mutants (supplemental Figure 2C and 2D) and is consistent with whole mount in situ hybridization reported by (Lu et al., 1999a) indicating that the initial specification of the chondrogenic mesenchyme in the mandibular processes in the Prx1/Prx2 double mutants was unaffected.
Figure 6. Effect of absence of Prx on the expression of Sox9, Col2a1 and Runx2 at E12.5.
Stained and pseudo colored images of in situ hybridization for Sox9, Col2a1, and Runx2 on transverse sections from the mandibular processes of control (Prx1+/-;Prx2-/-) (A-L) and Prx1;Prx2 double mutant (M-X) embryos at E12.5.
(A-D) are adjacent transverse sections through the caudal region of the mandibular processes from a control embryo showing that Sox9 and Col2a1 are expressed in the Meckel’s cartilage. Runx2 is not expressed in the Meckel’s cartilage (indicated by black circle) and is expressed in the mandibular bones.
(E-H) are adjacent transverse sections through the middle region of the mandibular processes from a control embryo showing that Sox9 and Col2a1 are expressed in the Meckel’s cartilage. Runx2 is not expressed in the Meckel’s cartilage but is expressed in the developing bone adjacent to Meckel’s’ cartilage (H).
(I-L) are adjacent transverse sections through the rostral region of the mandibular processes from a control embryo showing the Sox9 and Col2a1 are expressed in the Meckel’s cartilage in the rostral tip. Runx2 is not expressed in the rostral region.
(M-P) are adjacent transverse sections through the caudal region of the mandibular processes from a Prx1;Prx2 double mutant embryo showing the expression of Sox9 and Col2a1 in the Meckel’s cartilage. Runx2 is not expressed in the Meckel’s cartilage but is expressed at slightly higher levels and in a larger domain as compared to control (D).
(Q-T) are adjacent transverse sections through the middle region of the mandibular processes from a Prx1;Prx2 double mutant embryo and lack of expression Sox9 and Col2a1 in the medial region of the mandibular processes. Note the robust expression of Runx2 in mandibular processes of the Prx1;Prx2 double mutant embryo as compared to control (H).
(U-X) are adjacent cross sections through the rostral region of the mandibular processes from a Prx1;Prx2 double mutant embryo showing reduced domains and levels of expression of Sox9 and Col2a1 as compared to controls (J, K). Note the high levels of Runx2 in mandibular processes of the Prx1;Prx2 double mutant embryo as compared to control (L). Abbreviations: mc, Meckel’s cartilage; ns, nasal septum; asterisk, tongue. Scale bars =100 um.
At E12.5, in the control embryos, Sox9 and Col2a1 expression was detected in the precartilage primordium in the most caudal (Figures 6A-C), middle (Figures 6E-G) and rostral (Figures 6I-K) regions of the developing mandible. In the Prx1/Prx2 double mutants, expression of Sox9 and Col2a1, was detected in the most caudal (Figures 6M-O) and rostral (Figures 6U-W) ends but not in the middle (Figures 6Q-S) region that corresponds to the main body of MC. The expression of Sox9 and Col2a1 in the rostral ends in the Prx1/Prx2 double mutants (Figures 6U-W) was reduced as compared to the control (Figures 6I-K).
The absence of the expression of Sox9 in the main body of MC in the Prx1/Prx2 double mutants indicates that the loss of the major portion of MC in the Prx1/Prx2 double mutants may be related to defects in the maintenance of chondrogenic mesenchyme and/or appositional growth of chondrogenic mesenchyme.
Effects of absence of Prx gene products on the mandibular bones
Skeletal staining at E14.5 showed increased intense Alizarin Red staining in the hypoplastic mandibular processes of Prx1/Prx2 double mutant embryos compared to controls (Figures 5I, 5J).
The bones in the mandibular processes are formed by intramembranous ossification. During this process CNCC derived mesenchyme forms osteogenic condensations composed of osteoprogenitor cells, which proliferate and directly differentiate into pre-osteoblasts and then into mature osteoblasts secreting bone matrix. In the mandibular processes, the osteogenic condensations are formed first in the caudal region at around E13.5 and later in more rostral regions (Ramaesh and Bard, 2003; Mina et al., 2007).
To determine the onset of skeletal abnormalities and to gain further insight into underlying mechanisms of increased bone formation, we examined the expression of Runx2, a master regulator of osteoblast differentiation and a crucial early determinant of the osteoblast lineage (reviewed by Karsenty, 2008). In situ hybridization analysis at E12.5 showed that in control embryos, Runx2 was barely detectable in the caudal, medial and rostral regions of the mandibular processes (Figures 6D, 6H, 6L). By contrast, the Prx1/Prx2 double mutants contained large populations of Runx2 expressing cells in the middle and rostral regions (Figures 6T & 6X) indicating early/accelerated initiation of osteogenesis in the mandibular processes of the Prx1/Prx2 double mutants.
In situ hybridization and histomorphometric analysis at E13.5 showed expanded/larger domains of expression of Runx2 and Mason trichrome staining in the mandibular processes of the Prx1/Prx2 double mutants as compared to control (Table1 and Supplemental Figure 3). In the Prx1/Prx2 double mutants the domains of Runx2 expression in the rostral region of the mandibular processes were expanded and fused together in the midline (supplemental Figure 3H) and clusters of cells stained with Mason trichrome were detected in the rostral region (supplemental Figure 3D). By contrast, in the controls, domains of Runx2 expression in the rostral region were separated (supplemental Figure 3G) and the rostral region did mot contain many Mason trichrome stained cells (supplemental Figure 3C and Table 1). These observations suggest that fusion of the mandibular processes in the midline was related to a larger population of the osteogenic cells that undergo accelerated ossification.
Micro CT analysis of the mandible at P1 showed that the mineralized volumes of mandibles from Prx1/Prx2 double mutants (0.73 ± 0.08 mm3, n=3) were significantly decreased in comparison to controls (1.56 ± 0.08 mm3, n=3), attributable to the decreased size of the mandibles in the Prx1/Prx2 double mutants (supplemental Figure 4). However, despite the changes in the rate and timing of osteogenesis there were no significant differences in mineral density of the mandibles between the Prx1/Prx2 double mutants (496 ± 36 mg/cm3 HA) and controls (503 ± 6 mg/cm3 HA) indicating that the absence of the Prx gene did not affect the mineralization and matrix synthesis by osteoblasts. The architectural appearance and the porosity of the mandibular bones in the Prx1/Prx2 double mutants appeared to be similar to those in the control (supplemental Figure 4).
DISSCUSION
In the mandibular processes, Prx1 and Prx2 are expressed in overlapping domains with Prx2 domain entirely within the domain of Prx1. Deletion of Prx2 did not lead to any developmental defects in the mandibular processes suggesting that Prx1 compensates for loss of Prx2. This possibility was supported by severe abnormalities in the Prx1/Prx2 double mutants including abnormalities in the outgrowth of the mandibular processes, morphogenesis of the medial region, development of the incisors, formation of MC and, as reported in this paper, mandibular osteogenesis.
In the present paper we have examined the underlying mechanisms of abnormalities in growth and morphogenesis of the mandibular processes and Meckel’s cartilage in Prx1/Prx2 double mutants. Our analysis showed that growth abnormalities in the mandibular processes are evident as early as E10.5 and are associated with reduced proliferation and increased apoptosis in the mesenchyme of the medial region of the mandibular processes which in return results in changes in the patterns and domains of expression of the components of signaling pathways involved in proper morphogenesis of the mandibular processes.
We have also examined the underlying mechanisms that lead to the absence of the main body of Meckel’s cartilage in Prx1/Prx2 double mutants. Our analysis suggests roles for Prx gene products in the endochondral ossification at the two ends of MC and suggests that the absence of the main body of MC may be indirect and related to changes in the fate of precursors from chondrogenic to osteogenic mesenchyme. Our results show that fusion of the mandibular process is caused by accelerated osteogenesis.
Loss of Prx genes affected morphogenesis of the medial region of the developing mandible
Our observations showed changes in the domain and intensity of the expression of a number of regulatory factors in the medial mandibular mesenchyme of the Prx1/Prx2 double mutants compared to controls. The most striking change in the Prx1/Prx2 double mutant mandible was the reduction and subsequent elimination of eHand expression from the medial mandibular mesenchyme at E10.5 and E11.5 respectively suggesting that eHand expression may be directly or indirectly regulated by Prx gene products. This epistatic relationship predicts unchanged patterns of expression of Prx genes in the medial mesenchyme of the various Hand mutants. The early embryonic lethality in eHand null mutants complicated detailed analysis of the effects of loss of eHand in mandibular morphogenesis. The unchanged expression of Prx1 in E9.5 dHAND-/- (Thomas et al., 1998) and in E12.5 Hand compound mutants (Barbosa et al., 2007) provides evidence that at least Prx1 acts upstream of dHAND. However, the down-regulated expression of Prx2 in the compound Hand mutants (Barbosa et al., 2007) suggests that Hand genes act upstream of Prx2. The expression of Hand genes in the medial mandibular mesenchyme is regulated by ET1 signaling (reviewed by Firulli, 2003) whereas expression of Prx is dependent on members of the fibroblast growth factor and hedgehog families of signaling molecules and not on ET1 (Doufexi and Mina, 2008; Clouthier et al., 2000) indicating that Hand and Prx genes belong to different pathways.
Our study also showed changes in the expression of Msx1 and Bmp4 in the mesenchyme of the medial region in the Prx1/Prx2 double mutants as compared to controls suggesting direct or indirect interactions between the Prx and Msx genes which in turn, as suggested by studies in developing teeth, regulate the expression of Bmp4 in the mesenchyme (Chen et al., 1996). The expanded expression of a Msx2 transgene, containing a multimerized 52-bp core element shown to be Bmp4-responsive (Brugger et al., 2004) and capable of binding Prx1 (Liu et al., 2005) provides evidence for direct roles of at least Prx1 in regulation of Msx2 (but not Msx1) transcription in the mandibular mesenchyme (Liu et al., 2005).
Our results showed essential roles for Prx gene products in proliferation and survival of the mesenchymal cells in the medial region and suggest that changes in the expression of the genes expressed in the medial and lateral regions of the mandibular processes in the Prx1/Prx2 double mutants are indirect and related to changes in the shape of the mandible resulting from loss of a subpopulation of the mesenchyme in the medial region (See model in Figure 7). We suggest that the reduction and/or absence of expression of eHand and Bmp4 from the mesenchyme of the medial region of the Prx1/Prx2 double mutant mandible are caused by loss of a subpopulation of cells from the medial mandibular mesenchyme. Similarly, the reduced number and/or absence of the lower incisors in the Prx1/Prx2 double mutants are related to loss of cells and not to direct roles of Prx gene products in odontogenesis. This model also suggest that extension of expression of Fgf8, and its target genes (Barx1, Dlx5 and Pitx1) into the medial region in the Prx1/Prx2 double mutants is due to the loss of a subpopulation of cells from the medial mandibular mesenchyme resulting in closer proximity of their domains of expression.
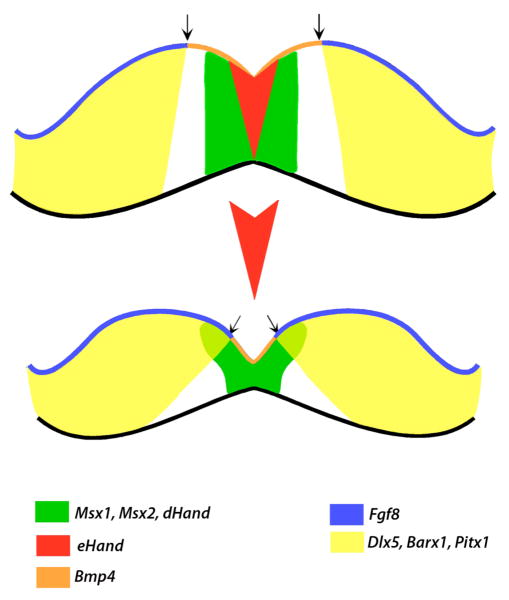
Figure 7. A model representing the role for Prx genes during the development of the medial region of the mandible.
Schematic diagram summarizing the changes in gene expression in the Prx1/Prx2 double mutants compared to control and a model for the role of the Prx gene products in morphogenesis of the mandibular processes. We propose that changes in the pattern of expression of eHand and Bmp4 in the mesenchyme of the medial region and extension of the expression of factors expressed in lateral regions (Fgf8, Barx1, Dlx5 and Pitx1) into the medial region in Prx1/Prx2 double mutants embryos are due to the apoptosis and the absence of the mesenchyme expressing eHand in the most medial region of the mandibular processes (represented by the red arrowhead shaped domain between the two mandibles).
This model shares both similarities and differences with the model proposed by ten Berge et al., 2001) who also suggested that changes in the patterns of expression of various regulatory factors in the Prx1/Prx2 double mutants are related to changes in the shape of the mandibular processes. Based on the changes in the patterns of expression of Fgf8, Bmp4 and Shh in the mandibular epithelium, and Alx3 and Dlx2 in the mandibular mesenchyme between the Prx1/Prx2 double mutants and wildtype, these authors suggested that reduced cell proliferation in the mesenchyme underneath the oral epithelium resulted in a rotation in the mandibular process in which the oral region develops in a more medial position and the aboral region in a more lateral position.
The mechanism by which Prx genes regulate the cell proliferation and cell death in the mandibular mesenchyme is not fully understood. However, previous studies have implicated Prx1 as an activator of c-Fos, a member of AP1 family of transcription factors involved in controlling cell proliferation and apoptosis through interactions with Cyclin D1 proteins (Grueneberg et al., 1992; Cserjesi et al., 1994; Martin et al., 1997; Shaulian and Karin, 2001). This activation is by binding of Prx1 to the serum responsive element (SRE), or CARG motif (Grueneberg et al., 1992; Martin et al., 1997). This implies that reduced cell proliferation and increased apoptosis in the mandibular mesenchyme of Prx1/Prx2 double mutants may be related to decreased levels of activated c-Fos.
It remains possible that the mechanisms by which Prx genes regulate the cell proliferation and cell death may be indirect. ten Berge et al., 2001 showed downregulation of Shh in the medial mandibular epithelium and suggested that Prx gene products regulate the high levels of expression of Shh in the overlying epithelium which in turn promotes cell proliferation in part of the underlying mesenchyme (ten Berge et al., 2001). Our previous observations showed that Hh signaling is a positive regulator of Prx expression in the medial mandibular mesenchyme (Doufexi and Mina, 2008). These observations suggest a reciprocal signaling interactions between Hh signaling and Prx in the mandibular process in which a member of Hh family is involved in the expression of Prx genes in the mandibular mesenchyme. Prx gene products in turn regulate a signal required for maintaining the high levels of expression of a member of Hh family (i.e., Shh) in mandibular epithelium.
Our data showed that, despite the increased cell death and reduced size of the mandibular processes in the Prx1/Prx2 double mutants, the domain of the expression of dHand, the oral domain of expression of Msx1 and Msx2 in the mesenchyme of the medial region as well as Bmp4 expression in the epithelium of the medial region were similar to those in the control embryos. These observations suggest that, in addition to their roles in cell survival and proliferation, Prx gene products have roles in restricting the expression of these regulatory genes to the medial region of the mandibular processes and in establishing the boundaries between Fgf8 and Bmp4 and, consequently, their downstream targets. This possibility is partially supported by previous studies that suggested that Prx gene products function in the mandibular processes as repressors of Msx2 transcription (Liu et al., 2005).
Role of Prx genes in development of Meckel’s cartilage and mandibular bones
Our analyses of the developing MC in the Prx1/Prx2 double mutants suggest that Prx gene products are not required for the initial specification of chondrogenic mesenchyme but may be required for the initiation and maintenance of condensation giving rise to MC. However, lack of expression of Prx1 and Prx2 in prechondrogenic condensation at many sites including the developing MC (Meijlink et al., 1999; Doufexi and Mina, 2008) does not support a direct role for the Prx gene products in the initiation of chondrogenesis. We propose that the absence of the main body of MC in the Prx1/Prx2 double mutant most likely is related to the indirect effects of Prx on other processes including cell survival, cell proliferation and the expression of CAMs (cell adhesion molecules) and Tenascin-C, shown to be regulated by Prx1 in a variety of cell types including limb mesenchyme (Norris and Kern, 2001; Ihida-Stansbury et al., 2004).
The remnant of the MC in the most rostral and caudal ends in the Prx1/Prx2 double mutants suggest roles of Prx gene products in endochondral ossification. The abnormalities in the chondrocytes in the rostral ends of mandibular processes in the Prx1/Prx2 double mutants are similar to those in the Hand compound mutants that also exhibited a fused mandible, absence of symphysial cartilage with a single incisor present (Barbosa et al., 2007). It was shown that, unlike in wild type, in the Hand compound mutants chondrocytes in the symphysis of Meckel’s cartilage did not express Ihh and collagen X (Barbosa et al., 2007). These similarities suggest cooperative roles of Prx and Hand transcription factors in terminal differentiation and hypertrophy of chondrocytes in the mandibular symphysis.
Interestingly our observations unraveled a previously uncharacterized phenotype involving accelerated and expanded osteoblast differentiation in the middle and rostral regions of the mandibular processes in the Prx1/Prx2 double mutants. The negative roles of Prx gene products in osteogenesis are further supported by studies that showed the negative effects of Prx1 on osteocalcin and collagen1α1 promoter-CAT constructs (Hu et al., 1995; Jiang and Stefanovic, 2008).
Thus, it appears the decreased cell proliferation and increased apoptosis in the mesenchyme of the medial region did not affect the proliferation and differentiation of the osteogenic mesenchyme. The accelerated osteogenesis in the Prx1/Prx2 double mutants results in similar abnormalities as those in the Hand compound mutants (Barbosa et al., 2007) and abnormalities in mice with branchial arch-specific deletion of dHand (Funato et al., 2009) which also exhibited accelerated osteoblasts differentiation. The mandibles in mice with branchial arch-specific deletion of dHand contained the symphysial portion of MC surrounded by porotic mandibular bones, which exhibited multiple holes due to insufficient mineralization (Funato et al., 2009). This study indicated that dHand acts as an inhibitor of the Runx2-DNA interaction and thereby regulates osteoblasts differentiation in branchial arch development (Funato et al., 2009).
However, we did not detect bony defects in Prx1/Prx2 double mutants at P1. Thus, the accelerated and expanded domain of Runx2 expression in the Prx1/Prx2 double mutants may be related to increased proliferation or/decreased apoptosis of osteogenic mesenchyme and/or recruitment of additional osteo-progenitor cells. It is possible that the increase in osteogenesis in the Prx1/Prx2 double mutants occurs as a compensation for the lack of MC or due to conversion of the chondrogenic mesenchyme to osteogenic mesenchyme. Alternatively, it is also possible that the expanded domain of expression of Runx2 may reflect fusion of a number of osteogenic condensations in a significantly smaller mandible.
EXPERIMENTAL PRODUCERS
Transgenic animals and genotyping
Prx1/Prx2 double mutants were established by crossing Prx1+/-;Prx2-/- mice on a 129 SV/J × C57Bl/6 background (a kind gift from Dr. JF. Martin). This cross resulted in generation of three genotypes. Genotyping was done by PCR on DNA isolated from tail tip of neonates and embryos. Four primer pairs were used to amplify the Prx1 wild-type allele (ccctcagtggatagtagtatatcgaacacaatata/gaccagttgaactctgaggagaagaagaagagaaa; ~500 bp product), Prx1 mutant allele (ctctcactatagatggcagtaaatc/gccactcccactgtcctttc; ~300 bp product), Prx2 wild-type allele (ccgttggcaccaaacgaaag/atctgggctcatcgtggtag; ~1.3kb product) and Prx2 mutant allele (tgactaggggaggagtagaa/atctgggctcatcgtggtag; ~1.0 kb product).
Tissue fixation, processing and staining
Mandibular processes from various embryonic stages were fixed in freshly prepared 4% paraformaldehyde in Phosphate Buffered Saline (PBS) at 4°C overnight. All fixed tissues were dehydrated and processed for paraffin embedding. Seven μm sections were mounted on Probe-On Plus slides and processed for various analyses. Sections were stained with hematoxylin/eosin (H&E), Mason’s trichrome (to detect collagenous matrix) and Alcian Blue (to detect sulfated proteoglycans in cartilage).
In situ hybridization to whole-mounts and sections
Whole-mount hybridization using digoxigenin-labeled RNA probes and hybridization to tissue sections using 33P-labeled antisense RNA probes were performed as previously described (Mina et al., 2002). The patterns of expression of each probe were analyzed in at least four embryos per genotype. The cDNAs for this study included dHand and eHand (Srivastava et al., 1995), Msx1 (Mackenzie et al., 1991), Msx2 (MacKenzie et al., 1992), Bmp4 (Wozney et al., 1988), Barx1 (Tissier-Seta et al., 1995), Dlx5 (Qiu et al., 1995), Fgf8 (Trumpp et al., 1999), Pitx1 (Lu et al., 1999c), Sox9 (Wright et al., 1995), Runx2 (Ducy et al., 1997), Col2a1 (Metsaranta et al., 1991) and Pax9 (Neubuser et al., 1997). Individuals who kindly provided these cDNAs include Drs. Olson, Ferguson, Wozney, Neubuser, Rubenstein and Martin. Following whole-mount in situ hybridization, embryos were photographed using a Nikon stereomicroscope with an attached SPOT digital camera. The pseudo-coloring of the silver grains in the dark-field image on tissue section were done as previously described (Havens et al., 2008).
Skeletal whole-mount staining and analysis
Embryos were processed for staining with Alcian Blue and Alizarin Red as previously described, with some modifications (Wang et al., 1999). Embryos were fixed and stained with Alcian Blue (0.015% Alcian Blue in 95% Ethanol/Glacial Acetic Acid) overnight, then destained with 95% Ethanol for at least 2 days and treated with 1% KOH until most of the background was cleared. A subset of embryos was also stained overnight with 0.1% Alizarin Red in 1%KOH. All stained embryos were cleared through the graded glycerol/1% KOH series and stored in 100% glycerol. The patterns of cartilage and bone at each developmental stage were analyzed in at least four embryos per genotype.
Cell proliferation and apoptosis assays
Mandibular processes isolated from E10.5 were fixed and subjected to immunohistochemistry with anti-phosphorylated histone 3 (H3-p) antibody for analysis of cell proliferation as described before (Havens et al., 2008). An in situ Cell Death Detection Kit (Roche) using Fast Red as a substrate solution for Alkaline Phosphatase was used to detect apoptotic cells in sections from E10.5 and E11.5. Cell proliferation and apoptosis were analyzed in four embryos per genotype.
Quantitative analysis
For quantitative analysis of cell proliferation and apoptosis, equivalently defined areas were outlined and measured in controls and Prx1; Prx2 double mutant mandibles using ImageJ software. These areas included the entire area of the mandibular mesenchyme and medial region corresponding to the region expressing Msx2 (400 × 600 pixel2 area outlined in 10x images). In each defined area, H3-p positive and TUNEL-positive cells were individually counted in 8-13 sections. Values for cell proliferation and apoptosis represent the mean ± SE of positively stained cells/mm2 that were determined in 8-13 sections from four different embryos at each stage of development from each genotype.
Areas of osteogenic mesenchyme expressing Runx2, the newly formed bone stained with Mason trichrome, and entire area of the mandibular mesenchyme at E13.5 were quantified in adjacent section using ImageJ software in total of 6-9 sections from at least three different embryos from each genotype.
The size of the mandibular processes were quantified from images captured from freshly dissected mandibles using ImageJ software in total of 4-6 mandibles from each genotype.
Micro-Computed Tomography Imaging (MicroCT)
Mandibles were isolated from Prx1-/-, Prx2-/- and control newborns (P1). Following fixation in 70% ethanol, the mandibles were imaged at 6 μm resolution using cone beam micro-focus X-ray computed tomography (μCT40, Scanco Medical AG, Brüttisellen, Switzerland). Serial tomographic images were acquired at 45kV and 177μA, collecting 2000 projections per rotation at 300 msec integration time. Three-dimensional 16-bit grayscale images were reconstructed using standard convolution back-projection algorithms with Shepp and Logan filtering, and rendered within a 12.3 mm field of view at a discrete density of 4,629,630 voxels/mm3 (isometric 6-μm voxels). Mineral density (mg/cm3) was measured by calibrating X-ray attenuation to mineral content using a graded hydroxyapatite phantom and voltage-specific beam hardening correction. Segmentation of mineralized bone from marrow and soft tissue was performed in conjunction with a constrained Gaussian filter to reduce noise, applying a hydroxyapatite-equivalent density threshold of 160 mg/cm3. Total mineralized volume (mm3) and average mineral density (mg/cm3) were measured directly. Values represent the mean ± SE determined from at least three different embryos for each genotype.
Statistical analysis
Unpaired, two-tailed t-tests were performed to determine statistically significance differences and p < 0.05 was considered statistically significant.
Supplementary Material
Side views of the heads (A, C, E) and top views of the mandibles (B, D, F) from stained control (Prx1+/+;Prx2-/-)(A, B), Prx1+/-;Prx2-/- (C, D) and Prx1;Prx2 double mutant (E, F) embryos at E19. Note reduced size of the maxillary and mandibular processes in the Prx1;Prx2 double mutant (E) as compared to Prx1+/+;Prx2-/- (A) and Prx1+/-;Prx2-/- (C). Scale bar in all pictures=1mm.
A and B are transverse sections through mandibular processes of the control (A) and Prx1;Prx2 double mutant (B) processed for immunohistochemistry with an H3-p antibody. Note the decreased number of stained cells (brown) in the mandibular processes of the Prx1/Prx2 double mutants compared to controls.
C and D are pseudo-colored images of in situ hybridization analysis for Sox9 in longitudinal sections through mandibular processes of control (E) and Prx1;Prx2 double mutant (F) at E11.5. Scale bars =100 um.
Masson’s trichrome stained (A-D) and pseudo-colored images of in situ hybridization for Runx2, (E-H) on adjacent transverse sections through the middle (A, B, E, F) and rostral (C, D, G, H) regions of the mandibular processes of control (A, C, E, G) and Prx1;Prx2 double mutant (B, D, F, H) embryos at E13.5. Note the expanded area of Runx2 expression in the rostral region of the mandibular processes of the Prx1/Prx2 double mutants. Also note the significant increases in the number of cells stained with Masson’s trichrome (indicated by arrowheads) in mandibular processes of the Prx1/Prx2 double mutants as compared to control. Scale bars =100 um.
A and B are superior and inferior views of a control mandible. C and D are micro-CT images of a mandible from Prx1;Prx2 double mutant and E, F are images from a mandible from another Prx1-/-;Prx2-/-. Note the reduced size of mandibles and bones in the Prx1;Prx2 double mutant mutants as compared to control. Note the similarities in the architectures of the mandibular bones in the control and Prx1-/-;Prx2-/-. Scale bars =1 mm.
Acknowledgments
We thank all the individuals who provided reagents, including Drs. Jim Martin for providing the transgenic animals, Drs. Bruce Havens, Aikaterini-Elisavet Doufexi and Mrs. Barbara Rodgers for technical assistance in all aspects of this work, Dr. Stephen Clark for his help and assistance on mouse management and Dr. William B. Upholt for critical reading of the manuscript. This work was supported by NIH grant R01 DE08682 to MM.
Grant sponsor: National Institute of Health (NIDCR)
Grant numbers: DE08682
References
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- Barbosa AC, Funato N, Chapman S, McKee MD, Richardson JA, Olson EN, Yanagisawa H. Hand transcription factors cooperatively regulate development of the distal midline mesenchyme. Dev Biol. 2007;310:154–168. doi: 10.1016/j.ydbio.2007.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, Cho JY, Dobias SL, Yi SE, Lyons K, Bell JR, Arora K, Warrior R, Maxson R. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development. 2004;131:5153–5165. doi: 10.1242/dev.01390. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Kim J, Helms JA. Looking different: understanding diversity in facial form. Am J Med Genet A. 2006a;140:2521–2529. doi: 10.1002/ajmg.a.31361. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Tapadia MD, Helms JA. The molecular origins of species-specific facial pattern. Curr Top Dev Biol. 2006b;73:1–42. doi: 10.1016/S0070-2153(05)73001-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Schilling TF. Understanding endothelin-1 function during craniofacial development in the mouse and zebrafish. Birth Defects Res C Embryo Today. 2004;72:190–199. doi: 10.1002/bdrc.20007. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Williams SC, Yanagisawa H, Wieduwilt M, Richardson JA, Yanagisawa M. Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Dev Biol. 2000;217:10–24. doi: 10.1006/dbio.1999.9527. [DOI] [PubMed] [Google Scholar]
- Cserjesi P, Lilly B, Hinkley C, Perry M, Olson EN. Homeodomain protein MHox and MADS protein myocyte enhancer-binding factor-2 converge on a common element in the muscle creatine kinase enhancer. J Biol Chem. 1994;269:16740–16745. [PubMed] [Google Scholar]
- Doufexi AE, Mina M. Signaling pathways regulating the expression of Prx1 and Prx2 in the chick mandibular mesenchyme. Dev Dyn. 2008;237:3115–3127. doi: 10.1002/dvdy.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121:173–182. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Firulli AB. A HANDful of questions: the molecular biology of the heart and neural crest derivatives (HAND)-subclass of basic helix-loop-helix transcription factors. Gene. 2003;312:27–40. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–270. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Kurihara Y, Arima Y, Yamada N, Kurihara H. Temporal requirement of signaling cascade involving endothelin-1/endothelin receptor type A in branchial arch development. Mech Dev. 2004;121:1223–1233. doi: 10.1016/j.mod.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Funato N, Chapman SL, McKee MD, Funato H, Morris JA, Shelton JM, Richardson JA, Yanagisawa H. Hand2 controls osteoblast differentiation in the branchial arch by inhibiting DNA binding of Runx2. Development. 2009;136:615–625. doi: 10.1242/dev.029355. [DOI] [PubMed] [Google Scholar]
- Grueneberg DA, Natesan S, Alexandre C, Gilman MZ. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992;257:1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- Havens BA, Velonis D, Kronenberg MS, Lichtler AC, Oliver B, Mina M. Roles of FGFR3 during morphogenesis of Meckel’s cartilage and mandibular bones. Dev Biol. 2008;316:336–349. doi: 10.1016/j.ydbio.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth KE, Wilson JM, Grevellec A, Cobourne MT, Healy C, Helms JA, Sharpe PT, Tucker AS. Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of Fgf8 in head ectoderm. Dev Biol. 2007;303:244–258. doi: 10.1016/j.ydbio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Hu Y, Flanagan J, Brennan DP, Zhou H, Ng KW, Eisman JA, Morrison NA. rHox: a homeobox gene expressed in osteoblastic cells. J Cell Biochem. 1995;59:486–497. doi: 10.1002/jcb.240590409. [DOI] [PubMed] [Google Scholar]
- Ihida-Stansbury K, McKean DM, Gebb SA, Martin JF, Stevens T, Nemenoff R, Akeson A, Vaughn J, Jones PL. Paired-related homeobox gene Prx1 is required for pulmonary vascular development. Circ Res. 2004;94:1507–1514. doi: 10.1161/01.RES.0000130656.72424.20. [DOI] [PubMed] [Google Scholar]
- Ishizeki K, Shinagawa T, Nawa T. Origin-associated features of chondrocytes in mouse Meckel’s cartilage and costal cartilage: an in vitro study. Ann Anat. 2003;185:403–410. doi: 10.1016/S0940-9602(03)80097-3. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Stefanovic B. Homeobox gene Prx1 is expressed in activated hepatic stellate cells and transactivates collagen alpha1(I) promoter. Exp Biol Med (Maywood) 2008;233:286–296. doi: 10.3181/0707-RM-177. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- Knight RD, Schilling TF. Cranial neural crest and development of the head skeleton. Adv Exp Med Biol. 2006;589:120–133. doi: 10.1007/978-0-387-46954-6_7. [DOI] [PubMed] [Google Scholar]
- Ko SO, Chung IH, Xu X, Oka S, Zhao H, Cho ES, Deng C, Chai Y. Smad4 is required to regulate the fate of cranial neural crest cells. Dev Biol. 2007;312:435–447. doi: 10.1016/j.ydbio.2007.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005;283:282–293. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Lu MF, Cheng HT, Kern MJ, Potter SS, Tran B, Diekwisch TG, Martin JF. prx-1 functions cooperatively with another paired-related homeobox gene, prx-2, to maintain cell fates within the craniofacial mesenchyme. Development. 1999a;126:495–504. doi: 10.1242/dev.126.3.495. [DOI] [PubMed] [Google Scholar]
- Lu MF, Cheng HT, Lacy AR, Kern MJ, Argao EA, Potter SS, Olson EN, Martin JF. Paired-related homeobox genes cooperate in handplate and hindlimb zeugopod morphogenesis. Dev Biol. 1999b;205:145–157. doi: 10.1006/dbio.1998.9116. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999c;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm-and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130:6361–6374. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A, Ferguson MW, Sharpe PT. Expression patterns of the homeobox gene, Hox-8, in the mouse embryo suggest a role in specifying tooth initiation and shape. Development. 1992;115:403–420. doi: 10.1242/dev.115.2.403. [DOI] [PubMed] [Google Scholar]
- Mackenzie A, Leeming GL, Jowett AK, Ferguson MW, Sharpe PT. The homeobox gene Hox 7.1 has specific regional and temporal expression patterns during early murine craniofacial embryogenesis, especially tooth development in vivo and in vitro. Development. 1991;111:269–285. doi: 10.1242/dev.111.2.269. [DOI] [PubMed] [Google Scholar]
- Martin JF, Bradley A, Olson EN. The paired-like homeo box gene MHox is required for early events of skeletogenesis in multiple lineages. Genes Dev. 1995;9:1237–1249. doi: 10.1101/gad.9.10.1237. [DOI] [PubMed] [Google Scholar]
- Martin KA, Gualberto A, Kolman MF, Lowry J, Walsh K. A competitive mechanism of CArG element regulation by YY1 and SRF: implications for assessment of Phox1/MHox transcription factor interactions at CArG elements. DNA Cell Biol. 1997;16:653–661. doi: 10.1089/dna.1997.16.653. [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Clarke JD, Tickle C. Fate map of the developing chick face: analysis of expansion of facial primordia and establishment of the primary palate. Dev Dyn. 1998;212:102–118. doi: 10.1002/(SICI)1097-0177(199805)212:1<102::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Meijlink F, Beverdam A, Brouwer A, Oosterveen TC, Berge DT. Vertebrate aristaless-related genes. Int J Dev Biol. 1999;43:651–663. [PubMed] [Google Scholar]
- Metsaranta M, Toman D, De Crombrugghe B, Vuorio E. Specific hybridization probes for mouse type I, II, III and IX collagen mRNAs. Biochim Biophys Acta. 1991;1089:241–243. doi: 10.1016/0167-4781(91)90014-d. [DOI] [PubMed] [Google Scholar]
- Mina M. Morphogenesis of the medial region of the developing mandible is regulated by multiple signaling pathways. Cells Tissues Organs. 2001a;169:295–301. doi: 10.1159/000047894. [DOI] [PubMed] [Google Scholar]
- Mina M. Regulation of mandibular growth and morphogenesis. Crit Rev Oral Biol Med. 2001b;12:276–300. doi: 10.1177/10454411010120040101. [DOI] [PubMed] [Google Scholar]
- Mina M, Havens B, Velonis DA. FGF signaling in mandibular skeletogenesis. Orthod Craniofac Res. 2007;10:59–66. doi: 10.1111/j.1601-6343.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Mina M, Wang YH, Ivanisevic AM, Upholt WB, Rodgers B. Region- and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev Dyn. 2002;223:333–352. doi: 10.1002/dvdy.10056. [DOI] [PubMed] [Google Scholar]
- Neubuser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Nie X, Luukko K, Kettunen P. BMP signalling in craniofacial development. Int J Dev Biol. 2006;50:511–521. doi: 10.1387/ijdb.052101xn. [DOI] [PubMed] [Google Scholar]
- Noden DM, Schneider RA. Neural crest cells and the community of plan for craniofacial development: historical debates and current perspectives. Adv Exp Med Biol. 2006;589:1–23. doi: 10.1007/978-0-387-46954-6_1. [DOI] [PubMed] [Google Scholar]
- Norris RA, Kern MJ. Identification of domains mediating transcription activation, repression, and inhibition in the paired-related homeobox protein, Prx2 (S8) DNA Cell Biol. 2001;20:89–99. doi: 10.1089/104454901750070292. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Kurihara Y, Tonami K, Watatani S, Kurihara H. Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech Dev. 2004;121:387–395. doi: 10.1016/j.mod.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Qiu M, Bulfone A, Martinez S, Meneses JJ, Shimamura K, Pedersen RA, Rubenstein JL. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9:2523–2538. doi: 10.1101/gad.9.20.2523. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Bard JB. The growth and morphogenesis of the early mouse mandible: a quantitative analysis. J Anat. 2003;203:213–222. doi: 10.1046/j.1469-7580.2003.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JM, Diewert VM. The fate of Meckel’s cartilage chondrocytes in ocular culture. Dev Biol. 1988;129:48–60. doi: 10.1016/0012-1606(88)90160-1. [DOI] [PubMed] [Google Scholar]
- Sato T, Kawamura Y, Asai R, Amano T, Uchijima Y, Dettlaff-Swiercz DA, Offermanns S, Kurihara Y, Kurihara H. Recombinase-mediated cassette exchange reveals the selective use of Gq/G11-dependent and -independent endothelin 1/endothelin type A receptor signaling in pharyngeal arch development. Development. 2008a;135:755–765. doi: 10.1242/dev.012708. [DOI] [PubMed] [Google Scholar]
- Sato T, Kurihara Y, Asai R, Kawamura Y, Tonami K, Uchijima Y, Heude E, Ekker M, Levi G, Kurihara H. An endothelin-1 switch specifies maxillomandibular identity. Proc Natl Acad Sci U S A. 2008b;105:18806–18811. doi: 10.1073/pnas.0807345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6:348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–1999. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Brouwer A, Korving J, Martin JF, Meijlink F. Prx1 and Prx2 in skeletogenesis: roles in the craniofacial region, inner ear and limbs. Development. 1998;125:3831–3842. doi: 10.1242/dev.125.19.3831. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Brouwer A, Korving J, Reijnen MJ, van Raaij EJ, Verbeek F, Gaffield W, Meijlink F. Prx1 and Prx2 are upstream regulators of sonic hedgehog and control cell proliferation during mandibular arch morphogenesis. Development. 2001;128:2929–2938. doi: 10.1242/dev.128.15.2929. [DOI] [PubMed] [Google Scholar]
- Thomas T, Kurihara H, Yamagishi H, Kurihara Y, Yazaki Y, Olson EN, Srivastava D. A signaling cascade involving endothelin-1, dHAND and msx1 regulates development of neural-crest-derived branchial arch mesenchyme. Development. 1998;125:3005–3014. doi: 10.1242/dev.125.16.3005. [DOI] [PubMed] [Google Scholar]
- Tissier-Seta JP, Mucchielli ML, Mark M, Mattei MG, Goridis C, Brunet JF. Barx1, a new mouse homeodomain transcription factor expressed in cranio-facial ectomesenchyme and the stomach. Mech Dev. 1995;51:3–15. doi: 10.1016/0925-4773(94)00343-l. [DOI] [PubMed] [Google Scholar]
- Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999;13:3136–3148. doi: 10.1101/gad.13.23.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Rutherford B, Upholt WB, Mina M. Effects of BMP-7 on mouse tooth mesenchyme and chick mandibular mesenchyme. Dev Dyn. 1999;216:320–335. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<320::AID-DVDY2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- Yamagishi C, Yamagishi H, Maeda J, Tsuchihashi T, Ivey K, Hu T, Srivastava D. Sonic hedgehog is essential for first pharyngeal arch development. Pediatr Res. 2006;59:349–354. doi: 10.1203/01.pdr.0000199911.17287.3e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Side views of the heads (A, C, E) and top views of the mandibles (B, D, F) from stained control (Prx1+/+;Prx2-/-)(A, B), Prx1+/-;Prx2-/- (C, D) and Prx1;Prx2 double mutant (E, F) embryos at E19. Note reduced size of the maxillary and mandibular processes in the Prx1;Prx2 double mutant (E) as compared to Prx1+/+;Prx2-/- (A) and Prx1+/-;Prx2-/- (C). Scale bar in all pictures=1mm.
A and B are transverse sections through mandibular processes of the control (A) and Prx1;Prx2 double mutant (B) processed for immunohistochemistry with an H3-p antibody. Note the decreased number of stained cells (brown) in the mandibular processes of the Prx1/Prx2 double mutants compared to controls.
C and D are pseudo-colored images of in situ hybridization analysis for Sox9 in longitudinal sections through mandibular processes of control (E) and Prx1;Prx2 double mutant (F) at E11.5. Scale bars =100 um.
Masson’s trichrome stained (A-D) and pseudo-colored images of in situ hybridization for Runx2, (E-H) on adjacent transverse sections through the middle (A, B, E, F) and rostral (C, D, G, H) regions of the mandibular processes of control (A, C, E, G) and Prx1;Prx2 double mutant (B, D, F, H) embryos at E13.5. Note the expanded area of Runx2 expression in the rostral region of the mandibular processes of the Prx1/Prx2 double mutants. Also note the significant increases in the number of cells stained with Masson’s trichrome (indicated by arrowheads) in mandibular processes of the Prx1/Prx2 double mutants as compared to control. Scale bars =100 um.
A and B are superior and inferior views of a control mandible. C and D are micro-CT images of a mandible from Prx1;Prx2 double mutant and E, F are images from a mandible from another Prx1-/-;Prx2-/-. Note the reduced size of mandibles and bones in the Prx1;Prx2 double mutant mutants as compared to control. Note the similarities in the architectures of the mandibular bones in the control and Prx1-/-;Prx2-/-. Scale bars =1 mm.