Abstract
In the last few years, there has been a greater understanding of the spectrum and biology of Hodgkin lymphoma. In standard texts, Hodgkin lymphoma is classified as two distinct entities, namely nodular lymphocyte predominant Hodgkin lymphoma and classical Hodgkin lymphoma. However, recent evidence suggests that classical Hodgkin lymphoma is not a single disease. While the mixed cellularity and lymphocyte depleted subtypes may be part of a biologic continuum, the nodular sclerosis subtype has a distinct epidemiology, clinical presentation and histology. Nodular sclerosis Hodgkin lymphoma may also be related to primary mediastinal B-cell lymphoma and mediastinal grey zone lymphomas. We present an update on the pathobiology of Hodgkin lymphoma and discuss these biologic and clinical differences in this review.
Keywords: Classical Hodgkin lymphoma, Nodular lymphocyte predominant Hodgkin lymphoma, Primary mediastinal large B-cell lymphoma, Grey zone lymphomas, Epstein Barr virus, Epidemiology, Biology, Immunophenotyping
The eponym ‘Hodgkin’s disease’ was conferred by Samuel Wilks in 1856 1, almost 25 years after the first description of ‘morbid appearances of the absorbent glands and spleen’ by Thomas Hodgkin. Interestingly, of the seven original cases described by Dr. Hodgkin at the Guy’s hospital, only three were later shown to be truly Hodgkin’s disease in 1926, by the diagnostic criteria in use at that time 2. Also of interest is the fact that Dr. Hodgkin’s contributions in other fields of medicine and social sciences were far greater and had much more impact in his lifetime. Hodgkin lymphoma, as it is now termed, has an incidence rate of 2.7 per 100,000 population and is estimated to account for 11.7% of all lymphomas diagnosed in 2006 3. The last decade has seen tremendous advances in the understanding of its biology and we present an update on the pathobiology of Hodgkin lymphoma [HL] along with newer insights into its classification.
HODGKIN LYMPHOMA – HOW MANY DISEASE ENTITIES?
Presently, HL is classified into two largely distinct entities, namely nodular lymphocyte predominance HL (NLPHL) and classical HL (CHL), the latter being further subtyped as nodular sclerosis (NSCHL), lymphocyte rich (LRCHL), mixed cellularity (MCCHL), and lymphocyte depletion (LDCHL) subtypes 4. Salient clinical and histopathologic features of each subtype are shown in tables 1 and 2. While CHL tends to be regarded in the clinical and experimental literature as a single disease, consideration of all epidemiological, biological and clinical data suggests that CHL probably consists of more than one entity.
Table 1.
Salient Clinical Features of Hodgkin lymphoma
| Subtype | Incidence/Epidemiology | Sites | Prognosis |
|---|---|---|---|
| NLPHL | M:F (3:1), unimodal peak in 4th decade, also occurs in children | Peripheral LN; single node rather than group of nodes | Good response to Rx, slow progression, frequent relapses, may ‘progress’ to THRBCL or DLBCL |
| LRCHL | M:F (2:1) | Peripheral LN | Usually low stage, good prognosis, infrequent relapses |
| MCCHL | M:F (2:1), Developing countries, young children and older adults, HIV, B- symptoms | Peripheral LN, spleen, advanced stage | Prognosis intermediate between LRHL and LDHL |
| LDCHL | M:F (4:1), Rare, HIV, Developing countries, B- symptoms | Retroperitoneal and abdominal Advanced stage | Aggressive course |
| NSCHL | M:F (1:1), Most common subtype in West, adolescents and young adults, B-symptoms | Cervical,, axillary and mediastinal | Prognosis intermediate between LRHL and LDHL |
Table 2.
Salient Histopathologic Features of Hodgkin lymphoma
| Subtype | Salient histopathologic features |
|---|---|
| NLPHL | LP cells Background of small lymphocytes in a nodular or nodular and diffuse pattern (nodular pattern must be present at least focally). Background small B-cells with mantle cell phenotype (IgD+, IgM−). Expanded meshworks of FDCs, as seen by CD21 immunostain Epithelioid histiocytes are present in varying numbers |
| LRCHL | Classical HRS cells few in number. Most often nodular. HRS cells found in marginal/mantle zone around regressed GCs with dense FDC networks). May show aggregates of epithelioid histiocytes, but eosinophils and neutrophils are rare. |
| MCCHL | Diffuse lymph node effacement with numerous HRS cells; mixed inflammatory background with lymphocytes, eosinophils, plasma cells, histiocytes. Granulomas may be present. |
| LDCHL | Diffuse fibrosis variant - Hypocellular background and abundant disordered non-birefringent fibrosis, abundant histiocytes but fewer lymphocytes. Reticular variant - Numerous HRS cells with bizarre cytological features. |
| NSCHL | Capsular fibrosis and broad collagen bands, often perivascular Lacunar cells and mummified cells Grades I and II differ in number of HRS cells and presence of necrosis in latter. |
Nodular Sclerosis CHL
NSCHL stands apart from other forms of CHL and NLPHL. Young adults are more often affected than the elderly and this subtype is more frequently seen in developed countries, accounting for the early peak in Western populations 5. It differs from all other forms of classical Hodgkin lymphoma as being more common in females than males, and less frequently associated with EBV. The epidemiologic risk patterns of NSCHL are distinct from those of MCCHL, suggesting that the two may not share a common etiology 6. Over the last few decades, the incidence of NSCHL has continued to rise 7. The incidence of NSCHL in HIV-positive individuals decreases with decreasing CD4 counts, suggesting that it needs an intact immune system for its development 8.
Clinically, mediastinal involvement is more common in NSCHL than in other types of CHL. Recent studies have indicated a close relationship to primary mediastinal large B-cell lymphoma (PMLBL), and a possible origin from a thymic B-cell 9–11. Both NSCHL and PMLBL lack immunoglobulin expression and functional expression of HLA class I antigens, and share cytogenetic abnormalities. MAL, a gene involved in T-cell activation that is expressed in PMLBL, is only expressed by NSCHL and no other HL subtypes 12. CD23, which is expressed on normal thymic B-cells and PMLBL, is also expressed in some cases of NSCHL. The existence of mediastinal grey zone lymphomas (MGZL) with transitional morphology and phenotype between NSCHL and PMLBL, further supports a relationship between these two entities 11.
Studies employing gene expression profiling as well as immunohistochemistry have provided evidence for underlying biological and clinical differences between NSCHL and other forms of CHL, in particular MCCHL 13–15. A histological hallmark of NSCHL is the presence of broad bands of birefringent collagen (Fig. 1), suggesting differences in cytokine networks as compared to other subtypes, with increased production of IL-13 16. Residual B cells are often relatively abundant in NSCHL, with preservation of follicular structures. The lacunar and other HRS-cell variants may be associated with lymphoid follicles, and sometimes intimately associated with the follicular dendritic cell meshworks 17. This preservation of the nodal structures contrasts with MCCHL and LDHL, which have a high ratio of T:B lymphocytes (with an increase of the CD4+ subset) 18.
Figure 1.
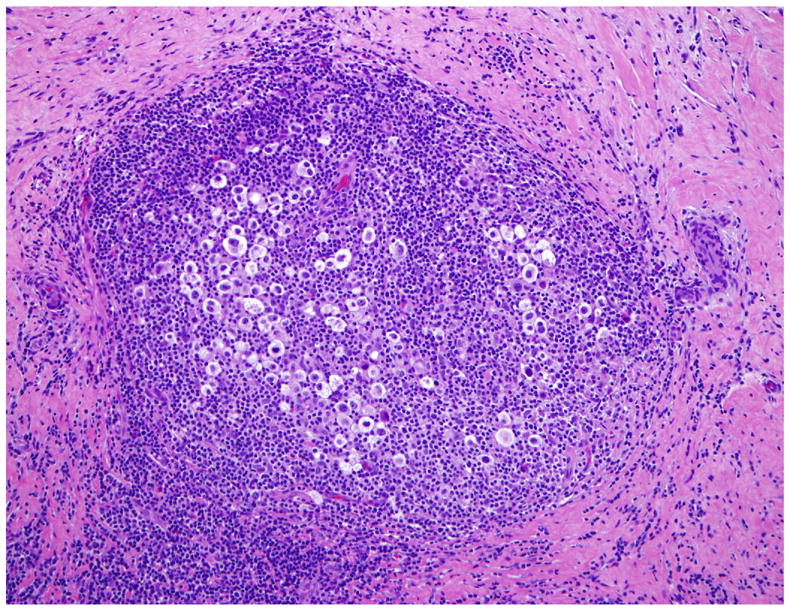
Nodular sclerosis Hodgkin lymphoma is characterized by broad bands of birefringent collagen. Lacunar variants of HRS cells often cluster within the nodules (H&E).
In addition to the abundant lymphocytes in the background, there are variable numbers of neutropils and eosinophils, sometimes forming microabscesses within the nodules. Some cases may have a high proportion of HRS cells, resulting in a “lymphocyte-depleted” appearance 19. A high content of HRS cells, extensive necrosis, and a prominent fibrohistiocytic stroma all are features associated with Grade 2 NSCHL 20. While grading is optional in the WHO classification, in some studies Grade 2 NSCHL has been associated with a higher relapse rate and poorer response to therapy, particularly in patients with advanced stage disease.
Mixed Cellularity and Lymphocyte Depleted CHL
In contrast to NSCHL, MCCHL and LDCHL are associated with lower socio-economic status, greater prevalence in males, frequent EBV infection of the neoplastic cells, and a different pattern of spread within the immune system – typically sparing the mediastinum and thymus gland. MCCHL and LDCHL have overlapping clinical, epidemiological and biological features, differing largely in the extent of depletion of normal background lymphocytes and the degree of immunosuppression in the host. Just as NSCHL can be graded according the proportion of tumor cells, MCCHL and LDCHL can be viewed as two grades of a single disease entity. Both occur often in the setting of HIV-infection.
Although histologic subtype has historically been known to impact prognosis 21, 22, current multimodality treatment protocols achieve cure in over 90% of patients with early-stage disease and have blunted the effect of histology on outcomes. Presently, disease stage, presence of B symptoms and risk factors such as coincidental AIDS are considered to be the most important prognostic factors 23–25. However, even in the modern treatment era, morphologic groups continue to have prognostic significance, with LDCHL (Fig. 2) and MCCHL subtypes conferring a significantly worse prognosis 26.
Figure 2.
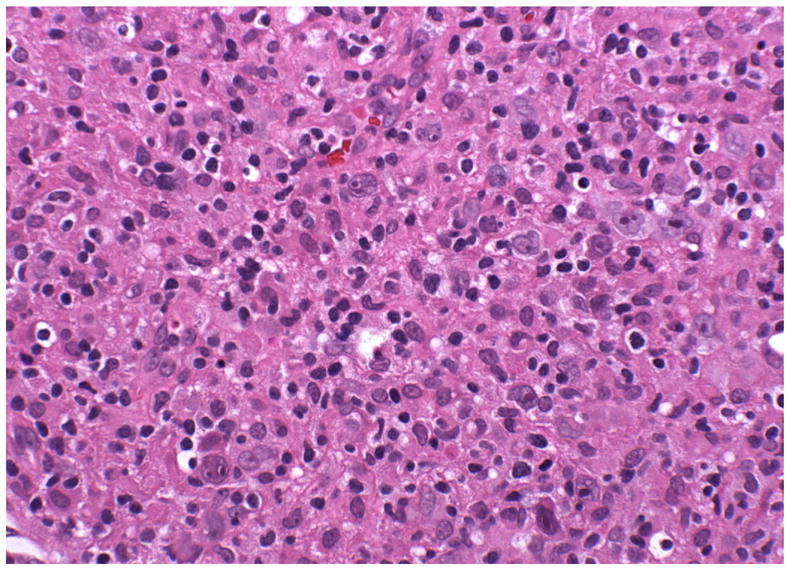
Lymphocyte depleted CHL with abundant HRS-cells in a background rich in histiocytes, but with few lymphocytes (H&E).
MCCHL exhibits a bimodal age-incidence curve and represents most cases of CHL in the pediatric age group. It is relatively uncommon in young adults, but increases in incidence after the age of 50. These incidence patterns mirror that of EBV infection, which are seen in the very young and the elderly 27. The epidemiological observations with respect to EBV and HL have provided support for a three disease hypothesis, first offered by MacMahon, prior to the modern classification of Hodgkin lymphoma 28.
Lymphocyte Rich CHL
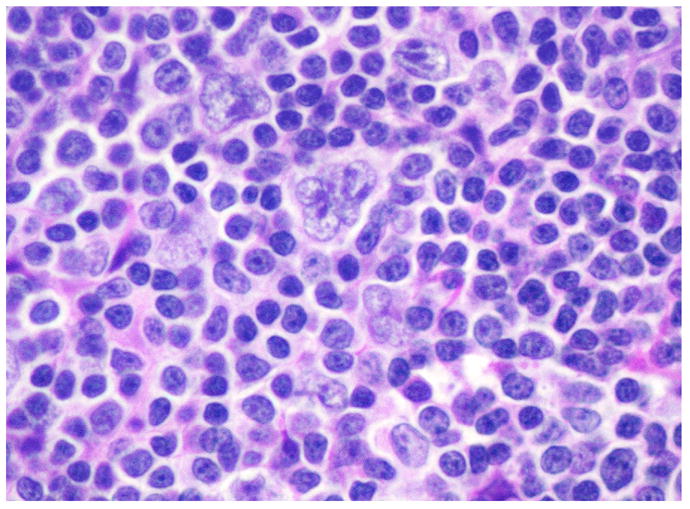
The most recently identified subtype of HL is lymphocyte-rich CHL (LRCHL), a subtype that was often mistaken for NLPHL in older studies. First recognized as follicular Hodgkin lymphoma by Ashton-Key et al. 29, this form of CHL usually presents with Stage I or II disease. As the name implies, there is a rich background of normal lymphocytes, with the malignant cells found in a B-cell rich milieu in the mantle and marginal zones of reactive follicles 30. The neoplastic cells have the phenotype of classical HRS cells, but are smaller and, in H&E stained sections, may resemble LP cells. Thus, immunohistochemical studies are critical for correct diagnosis (Fig. 3).
Figure 3.
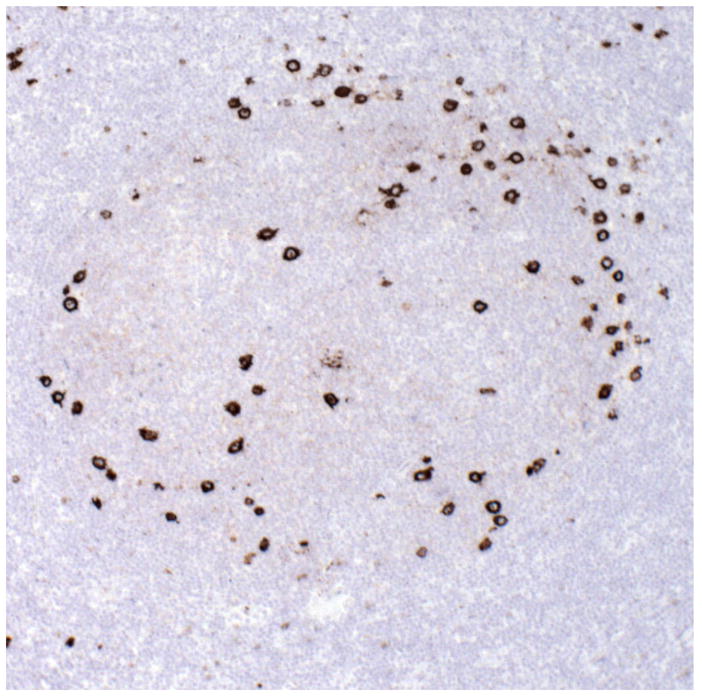
Lymphocyte rich CHL with HRS cells within expanded follicles, mainly at the periphery in the mantle and marginal zone (CD15 immunostain).
It has been questioned whether LRCHL is just an early form of NSCHL, but in patients with sequential biopsies, the histological pattern is usually constant. Clinically, the cases differ from NSCHL in having infrequent mediastinal involvement, and an older age at presentation 31. The prognosis is excellent, with event-free and overall survival of 97% at 30 months.
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma, and classical Hodgkin lymphoma
A new category was created in the WHO classification of 2008 to accommodate those cases in which the distinction between CHL and diffuse large B-cell lymphoma (DLBCL) is not possible 32 Cases with these features have been referred to in the literature as “grey zone lymphomas 11, 33. The majority of such patients present with mediastinal masses, and there is an increased male:female ratio, in contrast to both NSCHL and PMLBCL, which are more common in females 11, 34 In the past many of these lymphomas were diagnosed as Hodgkin’s-related anaplastic large cell lymphoma 35. However, a biological relationship to anaplastic large cell lymphoma is lacking 36. The optimal therapy has not been determined 37, 38. However, use of both chemotherapy and radiation therapy appears required for prolonged relapse-free survival 39.
There are other instances in which the distinction between CHL and DLBCL is problemmatic. For exmaple, EBV-positive DLBCL of the elderly may display Hodgkin-like features with neoplastic cells resembling HRS cells 40. In any given case the diagnosis is based on a combination of clinical, histological, immunophenotypic, and genotypic features. However, the borderline or grey zone cases usually demonstrate a discordance between the morphology and the expected immunophenotype. In most such cases the therapy is based on DLBCL-type regimens, and as CD20 is usually strongly expressed, rituximab is included in the treatment protocol.
Nodular lymphocyte predominant HL
NLPHL was the first subtype to be recognized as a distinct biologic entity and to be distinguished from CHL 41. It typically presents in peripheral lymph nodes, and is the only HL subtype to involve mesenteric lymph nodes; the mediastinum is nearly always spared. Other common sites of involvement are periparotid and inguinal lymph nodes. While the peak incidence is in the fourth decade, NLPHL is also seen in children, much more commonly in males. Patients lack B-symptoms and have a good prognosis, sometimes even without therapy 26, 42, 43.
At the cellular level, NLPHL lacks HRS cells. The neoplastic cell of NLPHL was originally termed the L&H cell, after the original description of this form of HL by Lukes and Butler as “lymphocytic and histiocytic predominance” 44. These cells have also been referred to as “popcorn” cells, but the WHO classification of 2008 recommended the use of the term “LP cell” 32. LP cells arise in the germinal center or follicular environment (Fig. 4), and NLPHL is sometimes seen in association with progressive transformation of germinal centers (PTGC) 45. Overall, PTGC has a low incidence of progression to NLHPL, when diagnosed independently 46.
Figure 4.
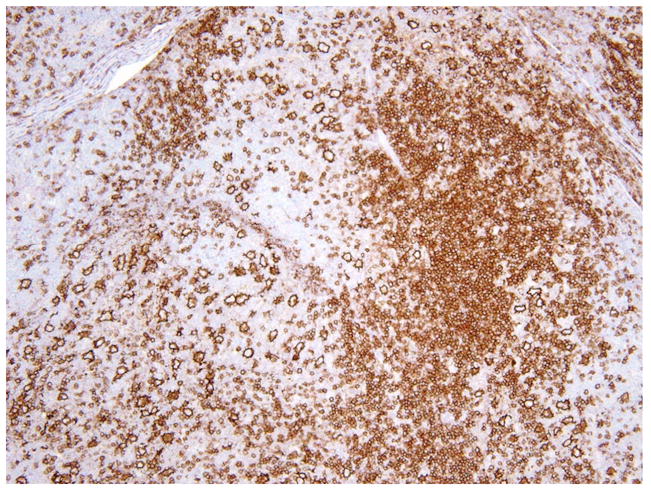
In nodular lymphocyte predominant HL, LP cells are strongly positive for CD20 and are seen in the context of lymphoid follicles (CD20 immunostain).
While NLPHL arises in a B-cell rich follicular environment, T-cells are recruited to the lesion and eventually become predominant over time 41, 47. With time, there is also a loss of the nodular growth pattern, such that NLPHL may progress to a process that is almost indistinguishable from T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) 48, 49. Features that are helpful in distinguishing NLPHL from THRLBCL include evidence of nodular pattern as manifested by CD21+ follicular dendritic cells and admixed small IgD+ positive B-cells. In addition, the nature of the T-cell component differs with CD8+ T-cells predominating in THRLBCL, as contrasted with CD4+ CD57+ T-cells in NLPHL 49. A recent study found that de novo THRLBCL lacks follicular T-helper cells (TFH) identified by PD-1, yet PD-1-positive cells are abundant in NLPHL, as well as in cases showing borderline features between THRLBCL and NLPHL 50. The clinical significance of a T-cell rich pattern, or diffuse areas is controversial. One study found that NLPHL patients with T-cell rich nodules often presented at high stage and with B symptoms, but still had a good prognosis, similar to NLPHL 49. Fan et al. found that NLPHL patients with diffuse areas or T-cell rich nodules were more likely to have recurrent disease 47. However, whether recurrence leads to long term survival differences is not clear.
NLPHL is generally associated with a good prognosis, but patients presenting with advanced stage disease do not respond well to CHL treatment regimens 42. Based on the biology of NLPHL, which is closer to that of other B-cell lymphomas than CHL, alternative treatment regimens, including the use of rituximab, have been employed more recently 51. NLPHL progresses in approximately 5% of patients to diffuse large B-cell lymphoma (DLBCL), which may be composite with the NLPHL in the same anatomic site 52, 53. As a rule NLPHL is not associated with EBV, but a recent report described three cases of EBV-positive NLPHL in Vietnamese children, suggesting that as B-cells, LP cells are at risk to become EBV-transformed 54.
The Neoplastic Cells in Hodgkin Lymphoma
Hodgkin lymphoma is unique in that the neoplastic cells constitute a minority population (less than 1%) in the affected lymph nodes. The classic binucleate Reed-Stenberg (RS) cell was independently described by Carl Sternberg in 1898 [Sternberg, 1898 #543] and Dorothy Reed in 1902 55 (Fig. 5A). The binucleation is an artifact seen as a result of deep indentations and folds in the nuclear membrane. Classical RS cells are seen relatively infrequently; cells exhibiting a similar phenotype but varied morphology are more common. These are collectively referred to as Hodgkin/RS (HRS) cells. Well-recognized variants include lacunar cells and mummified cells, but in actuality the morphological spectrum is very broad. The LP cells of NLPHL exhibit a similar broad range in morphological features, but, in general, lack prominent nucleoli (Fig. 5B). The immunophenotype of HRS and LP cells is summarized in Table 3.
Figure 5.
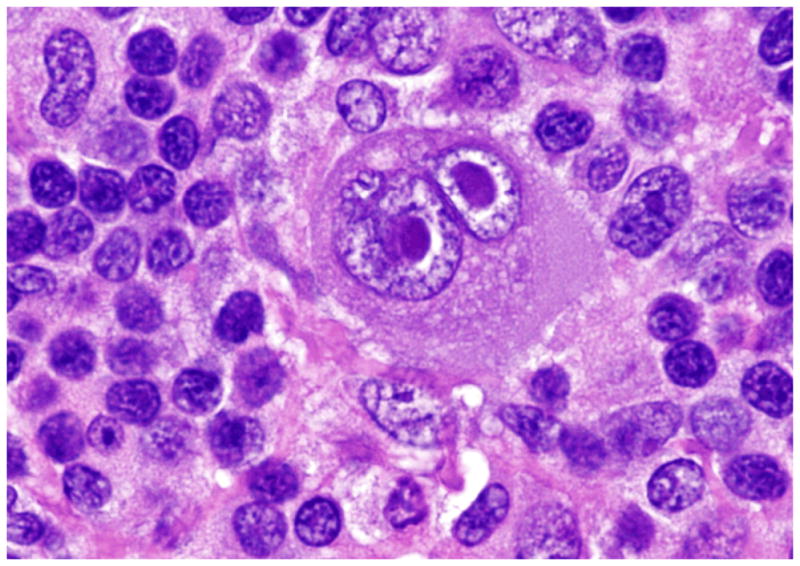
A. The classical RS cell is binucleate, each nucleus contains a prominent eosinophilic nucleolus with perinucleolar halos giving the cell an “owl-eye” appearance (H&E). B. The LP cell, the neoplastic cell in NLPHL, has a multilobate/folded nucleus with smaller basophilic nucleoli giving the cell a “popcorn” appearance (H&E).
Table 3.
Comparison of LP and HRS cells
| LP cell | HRS cells | |
|---|---|---|
| Nucleus | Polylobated with finely dispersed chromatin | Single to multilobated with peripherally condensed chromatin |
| Nucleoli | Small, usually basophilic | Large, often eosinophilic |
| Cytoplasm | Relatively sparse | Abundant |
| CD15 | Negative | Membrane and/or Golgi positivity |
| CD30 | Negative; rare weak positive cells | Membrane and/or Golgi positivity |
| CD20 | Positive | Variable (30–40% cases +) |
| CD79a | Positive | Negative to variably weak |
| CD45 | Positive | Negative |
| J-chain | Positive | Negative |
| OCT 2 | Positive | Often negative |
| BOB.1 | Positive | Often negative |
| PU.1 | Positive | Negative |
| PAX5 | Positive | Positive (weak) |
| EBV | Negative | Variably positive |
HRS cells can be characterized by lineage specific markers, as well as those representing activation markers and transcription factors. In approximately 85% of cases, the HRS cells are positive for CD15 (the Lewis × blood group carbohydrate, 3-fucosyl N-acetyl lactosamine), whereas CD30 is expressed nearly universally. Most B-lineage associated markers are absent, but CD20 is expressed weakly in up to 50% of cases of CHL.
Analysis of B-cell-specific transcription factors is useful in understanding the differentiation-linked phenotype in HL. Oct-2, BOB.1, Pax-5, and PU.1 belong to a group of transcription factors whose expression correlates with cell lineage and/or the stage of B-cell differentiation 56. Oct-2 and BOB.1 are required for germinal center formation and immunoglobulin production 57. PU.1 plays an essential role in the development of both lymphoid and myeloid lineages, by regulating the cytokine-dependent proliferation and differentiation of precursor cells. Pax-5, also known as B-cell specific activator protein (BSAP), is essential for B-cell commitment in early B cells and also the maintenance of B-cell identity in mature cells 58, 59, and is nearly always positive, albeit weak, in CHL 60. HRS cells are universally negative for B-cell transcription factor PU.1; transcription factors Oct-2 and BOB.1 are either negative or inconsistently expressed 61–63. On the other hand, transcription factors Pax-5, Oct-1, Oct-2, BOB.1, and PU.1 are universally expressed in all cases of NLPHL 62, 64, 65..
Approximately 10% of cases of CHL have been reported to express surface T-cell markers on HRS cells 66, 67. Lineage-inappropriate markers of dendritic cells, monocytes and plasma cells such as CD2, CD3, CD4, CD5, CD8, granzyme B, fascin, CD138 and MUM1 have also been detected on HRS cells 68–72. Although most cases that show a T-cell immunophenotype are also of B-cell origin on molecular analysis 73, a T-cell origin was suggested in three reported cases, based on the presence of T cell receptor gene rearrangements 74, 75. However, conclusive evidence for a T-cell form of CHL is lacking. For one, at the time the two reports were published, it was not appreciated that peripheral T-cell lymphomas could express both CD30 and CD15, and mimic CHL at both the phenotypic and morphological levels 76. Additionally, cases of pleomorphic T-cell lymphomas following primary cutaneous anaplastic large cell lymphoma, mycosis fungoides and lymphomatoid papulosis may closely simulate CHL 77. Much of the biology that we understand regarding CHL is related to its derivation from rescued germinal center B-cells 78. Conceptually, suggesting that the same disease entity may be of T-cell derivation runs counter to the view that lineage is a primary factor in defining disease entities 79.
Histogenesis of the neoplastic cells of Hodgkin lymphoma
As suggested by immunophenotypic studies, both HRS cells and LP cells are derived from B-cells at the germinal center or post-germinal center stage of differentiation 80, 81. Immunoglobulin gene rearrangement studies have established the clonal nature of HRS cells 82. A high load of somatic mutations in the rearranged immunoglobulin genes supports a germinal center derivation, specifically preapoptotic germinal center B-cells 81, 83–85. HRS cells show evidence for a partial deletion of the IGH constant region by interphase cytogenetics, suggesting the presence of class switch recombination 86. Further, chromosomal breakpoints affecting the immunoglobulin loci are recurrent in CHL. In a recent study of CHL with respect to B-cell differentiation stage based on phenotypic markes (bcl6/CD10/MUM1/CD138), most cases of CHL were at a late germinal center or post-germinal center stage 87.
In spite of their B-cell origin, HRS cells have a global loss of B-cell gene expression 88 in that they neither produce immunoglobulin nor have a functional B-cell antigen receptor 89, 90. Although HRS cells do harbor somatic mutations, these may be ‘crippling mutations’, meaning that these mutations lead to lack of IGH@ gene function 91. It also was speculated that HRS cells are unable to transcribe immunoglobulin genes due to a lack of key B-cell gene transcriptional regulators Oct-2, BOB.1 and PU.1 61, 62, 65, 84 and/or aberrant expression of suppressors of B-cell genes 92. However, in at least a proportion of cases the transcription apparatus is intact. An alternative hypothesis is epigenetic silencing of immunoglobulin gene transcription by promoter hypermethylation 93. Genomic imbalances or rearrangements are not a cause of PU.1, BOB1, and OCT2 deficiency in CHL and argue for another mechanism underlying this phenomenon 94 Ordinarily, crippling mutations in a germinal center B-cell will arrest further differentiation and cause apoptosis of the cells. The fact that HRS cells continue to proliferate in spite of having these deleterious mutations suggests that they somehow acquire the capacity to escape apoptosis, survive and continue proliferating. Escape from apoptosis probably represents the major oncogenic event in CHL lymphomagenesis. Apoptosis appears to be inhibited by several means in HRS cells 78, 95–97:
Constitutive activation of the transcription factor NFkB either autonomously, by rosetting T-cells, by EBV or by inactivation of its inhibitors such as IkB 98–103.
Inhibition of executors of apoptosis by expressing X-linked inhibitor of apoptosis (XIAP) 106, 107.
Protection from Fas-induced cell death by expression of FLICE-like inhibitory protein molecule, a potent inhibitor of Fas-induced death 111.
The transforming events that lead to the development of HL are largely unknown. Mutations in tumor suppressor genes p53 and RB have only been inconsistently reported 112, 113. While most HRS cells express proliferation associated molecules such as Ki-67 and PCNA 99, a considerable fraction of these undergo abortive mitoses with arrested metaphases, multinucleation and single cell death 114. In addition to multinucleation, which is not due to cellular fusion 115, HRS cells are near tetraploid, and almost all show rather random numerical chromosomal aberrations 116. Comparative genomic hybridization (CGH) studies have identified a set of recurrent chromosomal abnormalities in CHL, with gain of 17q being the most frequent in one series 117. These features suggest that HRS cells undergo nuclear division but may have defects in cell division and cytokinesis. Indeed, profound deregulation of cell cycle checkpoints with cyclin E persistency have been reported in HRS cells 118. Recently, COX-2 expression has been associated with cell proliferation and angiogenesis in HL 119. HRS cells also elaborate cytokines that act as paracrine or autocrine factors that assist cell survival and proliferation 120, 121, and produce the characteristic inflammatory background of CHL.
LP cells are also presumably derived from selected germinal center B-cells. However, unlike HRS cells that carry crippling immunoglobulin gene rearrangements, LP cells often show ongoing somatic hypermutation. Activation-induced cytidine deaminase (AID), a factor indispensable for class switch recombination and somatic hypermutation of immunoglobulin genes, is consistently expressed in LP cells but only infrequently in CHL 122. Frequent occurrence of BCL6 gene rearrangements also support the hypothesis of a germinal center B cell origin of LP cells and indicate a significant role of BCL6 in the pathogenesis of NLPHL 123.
The role of cytokines and infiltrating cells
Interactions between HRS cells and background inflammatory cells are important in the pathogenesis and progression of Hodgkin lymphoma 124. A variety of cytokines, chemokines, growth factors and their receptors including interleukins (IL1 to IL10), interferon, TNF-a, TGF-b, G-CSF, GM-CSF and others play a role in creating this microenvironment 120, 121. The clinical manifestations (such as B symptoms and immunosuppression) and pathologic features reflect imbalances in chemokines, cytokines and their receptors elaborated both by the neoplastic cells and surrounding tissues 18, 99, 125, 126. The T-cell infiltrate in CHL predominantly comprises Th2 and T-regulatory cells, and generally lacks Th1 cells, CD8 cytotoxic T cells and NK cells, thereby preventing cytotoxic anti-tumor immune responses. Cytokine production is driven by expression of T-cell transcription factors 127. HRS cells secrete Th2 type chemokines and cytokines (such as TARC, MDC, MIG, and IP-10), and cytokines that inhibit Th1 responses (such as IL-10 and TGF-beta) which induce apoptosis of activated Th1 and CD8 T cells 93, 128, 129. The expression of Th2 cytokines and chemokines leads to the reactive infiltrate of eosinophils, Th2 cells, and fibroblasts characteristic of CHL. Cytokines such as IL1, IL6 and TNF have been shown to proffer an unfavorable prognosis including advanced stage, the presence of ‘B’ symptoms, decreased response to therapy and reduced survival 130, 131. The production and induction of various other cytokines may also explain the influx of eosinophils (IL-5, eotaxin, IL-9), mast cells (IL-9) and plasma cells (IL-6) 132, 133. Nodal fibrosis is mediated by cytokines such as IL-13, TGF-beta, matrix metalloproteases and their inhibitors (TIMP-1 and TIMP-2)16, 134, 135. Certain cytokines also function as autocrine growth factors (e.g. IL-13/IL-13R, IL-3/IL-3R, TIMP-1), perpetuating the proliferation of neoplastic cells 16, 126, 134. 136, 137. Cytokine signaling is probably mediated through aberrant activation of transcription factors of the signal transducer and activator of transcription (STAT) family 138–140. HRS cells also express a number of molecules that are important for T-cell and B-cell interactions (CD40, MHC class II, CD80, CD86). The presence of T-rosettes around HRS cells suggests that T-cells play an important role for HRS cell survival 78. CD30 and CD40 ligands found on HRS cells have pleiotropic biologic activities and their activation might be a critical element in the deregulated cytokine network and cell contact-dependent activation cascade typical for CHL 141.
The cytokine milieu in CHL may lead to the generation of regulatory T (Treg) cells, positive for CD4, CD25, and CCR4, which may be associated with immune escape 142, 143. Many of the cells resetting the HRS cells in CHL have the phenotype of Treg cells. Low numbers of infiltrating CD8, CD 56, CD 57+ cells and high numbers of granzyme B and TIA-1+ cells have been associated with an unfavorable clinical course (presence of leukocytosis, B symptoms, advanced clinical stage (III/IV) and non-response to therapy) 144, 145. Imbalances in T-cell subsets are not confined to the involved lymph nodes, but are also found in the peripheral blood 146, 147. A lack of MHC class I antigen expression on HRS cells also has been postulated to decrease the population of CD8+ T-cells and contribute to the altered CD4:CD8 ratio 130, 148. Patients with CHL demonstrate impairment of cellular immunity, including a diminished delayed-type hypersensitivity reaction, reduced antigen-dependent proliferation of T and B lymphocytes and a decreased CD4:CD8 ratio 130, 132. The background of NLPHL differs from that of CHL. LP cells are closely associated with TFH cells, and these cells lead to a distinct cytokine mRNA profile 149, 151. Patients with NLPHL do not demonstrate impaired immunity.
ETIOLOGIC CONSIDERATIONS
Genetic predisposition
Familial HL represents 4.5% of all newly diagnosed cases 151. Anticipation (i.e. earlier onset and/or increasing severity in successive generations) occurs in families that exhibit both HL and NHL and it has been suggested that both neoplasms may have a common genetic basis 152. The relative risk of the development of HL increases approximately 100-fold in monozygous twins 153, 154 and seven-fold in siblings of patients less than 45 years of age 155. However, the cumulative lifetime risks are very small for the development of HL de novo or in first-degree relatives of affected patients 154. HLA associations have been described in familial HL including HLA A1, B5, B18, DPB1, DRB1, DQA1 and DQB1 156, 157. HLA phenotypes may determine the immune response to EBV and maybe implicated in the pathogenesis of HL 158. Risk of HL is reported to be lower among young adults with multiple older siblings, at least to some extent explained by the fact that having older siblings is associated with earlier exposure to common childhood pathogens 159. Obviously, the interrelations between CHL and infectious agents are complex and are dependent, at least to some degree, on genetics 153, 154. Familial cases of NLPHL have also been reported but the genetics are less well studied 160. Interestingly, both NLPHL and CHL have been reported with increased frequency in patients with the autoimmune lymphoproliferative syndrome (ALPS), which is associated with defective lymphocyte apoptosis 161.
EBV and Classical HL
20–100% of HL appear to be associated with EBV infection, the association varying with age (more frequent in children and older adults), gender(more frequent in males), geography (higher in Asia than in the US) and histology (more likely in MCCHL and LDCHL than in other subtypes) 54, 162–166. EBV infection increases the risk of CHL by 3–4-fold 167. Importantly, EBV infection is localized to HRS cells and is clonal, suggesting a possible causal role 168. HRS cells positive for EBV express a limited number of latency genes, exhibiting a type II latency phenotype 165. LMP-1 expression is not a constitutive characteristic and may be induced by extracellular signals 169. LMP-1 functions as a viral oncogene in that it can immortalize B cells 104, 113, 137, 170 and can constitutively activate TNF receptor/CD40 signaling pathways and induce NF- k B. It is also interesting to note that EBV-associated CHL occurs in patients without clinically manifest deficiencies in anti-viral immunity. In spite of expressing viral proteins, tumors are apparently able to escape EBV-specific immunity in vivo 171. The expression of galectin-1 may play a role in blocking the T-cell cytotoxic response 172.
Nevertheless, most adults that carry EBV never develop CHL. A variety of mechanisms are proposed to determine lymphomagenesis in affected individuals including promotion of genetic instability and alteration of normal processes of apoptosis 173. Loss of function of one or more tumor suppressor proteins (p16, p53, Rb) may be involved in defective cell regulation of H/RS cells. EBV may have a role in inhibiting P16(INK4A) expression, thus resulting in a perturbed p16(INK4A)-Rb cell cycle checkpoint 113. Whether or not EBV-positivity has prognostic significance remains controversial, with data on both sides of the question 162, 174–178. In addition to an epidemiologic association and potential role in pathogenesis, viral antigens may pose theoretical targets for anti-cancer therapies, including vaccination 173, 179. {Ambinder, 1996 #3920; Meyer, 2004 #3914}.
HIV infection and Hodgkin lymphoma
HIV-infected individuals (especially those with AIDS) have up to 10-fold increase in incidence of CHL 180–183. HIV-associated CHL (HIV-CHL) is usually MCCHL or LDCHL, is of advanced stage at diagnosis, and has a near-universal association with EBV infection. HIV-HL patients are infected by multiple EBV variants and, with progression, LMP-1 deletion mutants may preferentially accumulate within neoplastic tissues. In HIV-HL, there is intratumoral loss of CD4+ T cells and a decrease in intratumoral activated cytotoxic T lymphocytes leading to a striking inversion in the CD4/CD8 ratio 184. The risk of Hodgkin lymphoma in persons with HIV/AIDS (PWHA) increased substantially over the 1990–2002 period at a time when highly active antiretroviral therapy (HAART) was introduced, and associated with HAART-related improvements in CD4 counts 8, 185. Interestingly, this increase was coincident with a decrease in immunoblastic lymphomas, usually seen in late stage AIDS associated with profound loss of T-cell function.
Hodgkin lymphoma and other immune disorders
An increased incidence of CHL is also reported in other immunodeficiencies including ataxia telangiectasia, Wiskott-Aldrich syndrome, Bloom’s syndrome, autoimmune lymphoproliferative (Canale-Smith) syndrome 161 and following transplantation, as a post-transplantation lymphoproliferative disorder 186. An increased risk of CHL has also been reported in patients with a personal or family history of sarcoidosis 187 {Landgren, 2006 #3553} and multiple sclerosis 188. EBV-positive CHL is increased in incidence in patients receiving immunosuppression for a variety of immune conditions, including rheumatoid arthritis 189, 190. Immunodeficiency-related Hodgkin lymphoma has been recently reviewed 191.
Association with non-Hodgkin lymphoma
Synchronous and/or metachronous occurrence of Hodgkin lymphoma and B-cell non-Hodgkin lymphoma is not unexpected given the B-cell origin of the neoplastic cells in NLPHL and CHL 192–196. In selected cases subjected to sequence analysis of the immunoglobulin genes in both lesions, a clonal relationship has been shown in most instances 11, 197. CHL is also increased in patients with chronic lymphocytic leukemia (CLL). In patients with CLL, the secondary CHL are nearly always EBV-positive, and immunosuppressive therapy, in particular fludarabine, appears to increase this risk 198, 199. A further discussion of the relationship between CHL and B-cell lymphoma is beyond the scope of this review.
Geographic and socioeconomic considerations
In the West, HL is most prevalent among whites, followed by blacks and Hispanics, with the lowest incidence in Asians. The age-incidence patterns of the subtypes vary, with MCCHL seen in the very young, followed by NSCHL in young adults, and MCCHL and LDCHL in the elderly. In contrast, in developing countries, , there is an expanded early peak in young children associated with MCCHL, probably related to the age of first EBV infection 27, 200, 201. Incidence rates are low in Asian subgroups, but approximately double in US Asians as in native Asians. The consistently low rates of HL in Asians may be due to genetic resistance to disease development or environmental influences in its etiology 202.
The risk of CHL is inversely associated with socioeconomic status lending support to the hypothesis that CHL in young adults may occur as a result of aberrant host responses to a delay in first infection by common infections 203, 204. Early exposure to other children at nursery school and day care seems to decrease the risk of Hodgkin lymphoma in young adults, most likely by facilitating childhood exposure to common infections and promoting maturation of cellular immunity 159.
SUMMARY
Hodgkin lymphoma is a biologically heterogeneous group of neoplasms brought together by morphologic and phenotypic similarities. Although NLPHL is clearly identified as a separate entity, there is also a greater appreciation today for the differences between NSCHL and the other subtypes, mainly MCCHL/LDCHL and LRCHL. NSCHL affects young adults, is associated with mediastinal involvement and requires an intact immune system for its development. In addition to histological differences, its cytokine milieu and background lymphocyte population differ from other subtypes of CHL. The presence of an overlap with PMLBL suggests a thymic origin for mediastinal NSCHL. MCCHL and LDHL represent a spectrum, sharing many features related to incidence, pattern of spread, and association with immunodeficiency.
Acknowledgments
This work was prepared with the support of the Center for Cancer Research, NCI.
References
- 1.Wilks S. Unpublished papers of Thomas Hodgkin. Bull N Y Acad Med. 1970;46(1):67–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Kass EH, Bartlett AH, Thomas Hodgkin MD. (1798–1866): an annotated bibliography. Bull Hist Med. 1969;43(2):138–75. [PubMed] [Google Scholar]
- 3.Stat bite: U.S. incidence of childhood Hodgkin lymphoma by age group, 1975–2002. J Natl Cancer Inst. 2006;98(1):7. doi: 10.1093/jnci/djj024. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe ES, Harris NL, Stein H, Vardiman J. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 5.Engert A, Ballova V, Haverkamp H, et al. Hodgkin’s lymphoma in elderly patients: a comprehensive retrospective analysis from the German Hodgkin’s Study Group. J Clin Oncol. 2005;23(22):5052–60. doi: 10.1200/JCO.2005.11.080. [DOI] [PubMed] [Google Scholar]
- 6.Cozen W, Katz J, Mack TM. Risk patterns of Hodgkin’s disease in Los Angeles vary by cell type. Cancer Epidemiol Biomarkers Prev. 1992;1(4):261–8. [PubMed] [Google Scholar]
- 7.Clavel J, Steliarova-Foucher E, Berger C, Danon S, Valerianova Z. Hodgkin’s disease incidence and survival in European children and adolescents (1978–1997): report from the Automated Cancer Information System project. Eur J Cancer. 2006;42(13):2037–49. doi: 10.1016/j.ejca.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108(12):3786–91. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–62. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102(12):3871–9. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 11.Traverse-Glehen A, Pittaluga S, Gaulard P, et al. Mediastinal Gray Zone Lymphoma: The Missing Link Between Classic Hodgkin’s Lymphoma and Mediastinal Large B-Cell Lymphoma. Am J Surg Pathol. 2005;29(11):1411–21. doi: 10.1097/01.pas.0000180856.74572.73. [DOI] [PubMed] [Google Scholar]
- 12.Hsi ED, Sup SJ, Alemany C, et al. MAL is expressed in a subset of Hodgkin lymphoma and identifies a population of patients with poor prognosis. Am J Clin Pathol. 2006;125(5):776–82. doi: 10.1309/98KL-HRDA-M5CM-DHE2. [DOI] [PubMed] [Google Scholar]
- 13.Devilard E, Bertucci F, Trempat P, et al. Gene expression profiling defines molecular subtypes of classical Hodgkin’s disease. Oncogene. 2002;21(19):3095–102. doi: 10.1038/sj.onc.1205418. [DOI] [PubMed] [Google Scholar]
- 14.Levy A, Armon Y, Gopas J, et al. Is classical Hodgkin’s disease indeed a single entity? Leuk Lymphoma. 2002;43(9):1813–8. doi: 10.1080/1042819021000006286. [DOI] [PubMed] [Google Scholar]
- 15.Levy A, Diomin V, Gopas J, Ariad S, Sacks M, Benharroch D. Hodgkin’s lymphoma in the Bedouin of southern Israel: epidemiological and clinical features. Isr Med Assoc J. 2000;2(7):501–3. [PubMed] [Google Scholar]
- 16.Ohshima K, Akaiwa M, Umeshita R, Suzumiya J, Izuhara K, Kikuchi M. Interleukin-13 and interleukin-13 receptor in Hodgkin’s disease: possible autocrine mechanism and involvement in fibrosis. Histopathology. 2001;38(4):368–75. doi: 10.1046/j.1365-2559.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 17.Cossman J, Schnitzer B, Deegan MJ. Immunologic surface markers in non-Hodgkin’s lymphomas. Am J Pathol. 1977;87(1):19–32. [PMC free article] [PubMed] [Google Scholar]
- 18.Pituch-Noworolska A, Drabik G, Kacinska E, Klekawka T. Lymphocyte populations in lymph nodes in different histological types of Hodgkin’s disease in children. Acta Haematol. 2004;112(3):129–35. doi: 10.1159/000079723. [DOI] [PubMed] [Google Scholar]
- 19.DeVita VT, Jr, Simon RM, Hubbard SM, et al. Curability of advanced Hodgkin’s disease with chemotherapy. Long-term follow-up of MOPP-treated patients at the National Cancer Institute. Ann Intern Med. 1980;92:587–595. doi: 10.7326/0003-4819-92-5-587. [DOI] [PubMed] [Google Scholar]
- 20.MacLennan K, Bennett M, Tu A, et al. Relationship of histopathologic features to survival and relapse in nodular sclerosing Hodgkin’s disease. Cancer. 1989;64:1686–1693. doi: 10.1002/1097-0142(19891015)64:8<1686::aid-cncr2820640822>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Lukes R, Butler J, Hicks E. Natural history of Hodgkin’s disease as related to its pathological picture. Cancer. 1966;19:317–44. [Google Scholar]
- 22.Wijlhuizen TJ, Vrints LW, Jairam R, et al. Grades of nodular sclerosis (NSI-NSII) in Hodgkin’s disease. Are they of independent prognostic value? Cancer. 1989;63(6):1150–3. doi: 10.1002/1097-0142(19890315)63:6<1150::aid-cncr2820630618>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Hasenclever D. The disappearance of prognostic factors in Hodgkin’s disease. Ann Oncol. 2002;13 (Suppl 1):75–8. doi: 10.1093/annonc/13.s1.75. [DOI] [PubMed] [Google Scholar]
- 24.Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol. 2002;20(1):221–30. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- 25.Feltl D, Vitek P, Zamecnik J. Hodgkin’s lymphoma in the elderly: the results of 10 years of follow-up. Leuk Lymphoma. 2006;47(8):1518–22. doi: 10.1080/10428190500518602. [DOI] [PubMed] [Google Scholar]
- 26.Allemani C, Sant M, De Angelis R, Marcos-Gragera R, Coebergh JW. Hodgkin disease survival in Europe and the U.S: prognostic significance of morphologic groups. Cancer. 2006;107(2):352–60. doi: 10.1002/cncr.21995. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong AA, Alexander FE, Cartwright R, et al. Epstein-Barr virus and Hodgkin’s disease: further evidence for the three disease hypothesis. Leukemia. 1998;12(8):1272–6. doi: 10.1038/sj.leu.2401097. [DOI] [PubMed] [Google Scholar]
- 28.MacMahon B. Epidemiology of Hodgkin’s disease. Cancer Res. 1966;26(6):1189–201. [PubMed] [Google Scholar]
- 29.Ashton-Key M, Thorpe PA, Allen JP, Isaacson PG. Follicular Hodgkin’s disease. Am J Surg Pathol. 1995;19(11):1294–9. [PubMed] [Google Scholar]
- 30.Anagnostopoulos I, Hansmann ML, Franssila K, et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96(5):1889–99. [PubMed] [Google Scholar]
- 31.Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-rich classical Hodgkin’s lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin’s Study Group trials. J Clin Oncol. 2005;23(24):5739–45. doi: 10.1200/JCO.2005.17.970. [DOI] [PubMed] [Google Scholar]
- 32.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 33.Rudiger T, Jaffe ES, Delsol G, et al. Workshop report on Hodgkin’s disease and related diseases (‘grey zone’ lymphoma) Ann Oncol. 1998;9 (Suppl 5):S31–8. doi: 10.1093/annonc/9.suppl_5.s31. [DOI] [PubMed] [Google Scholar]
- 34.Garcia JF, Mollejo M, Fraga M, et al. Large B-cell lymphoma with Hodgkin’s features. Histopathology. 2005;47(1):101–10. doi: 10.1111/j.1365-2559.2005.02175.x. [DOI] [PubMed] [Google Scholar]
- 35.Pileri S, Bocchia M, Baroni CD, et al. Anaplastic large cell lymphoma (CD30 +/Ki-1+): results of a prospective clinico-pathological study of 69 cases. Br J Haematol. 1994;86(3):513–23. doi: 10.1111/j.1365-2141.1994.tb04781.x. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe ES. Anaplastic large cell lymphoma: the shifting sands of diagnostic hematopathology. Mod Pathol. 2001;14(3):219–28. doi: 10.1038/modpathol.3880289. [DOI] [PubMed] [Google Scholar]
- 37.Zinzani PL, Martelli M, Magagnoli M, et al. Anaplastic large cell lymphoma Hodgkin’s-like: a randomized trial of ABVD versus MACOP-B with and without radiation therapy. Blood. 1998;92(3):790–4. [PubMed] [Google Scholar]
- 38.Cazals-Hatem D, Andre M, Mounier N, et al. Pathologic and clinical features of 77 Hodgkin’s lymphoma patients treated in a lymphoma protocol (LNH87): a GELA study. Am J Surg Pathol. 2001;25(3):297–306. doi: 10.1097/00000478-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Dunleavy D, Pittaluga S, Grant N, et al. Gray zone lymphomas: clinical and histological characteristics and treatment with dose-adjusted EPOCH-R. Blood. 2008;112(11):1228. [Google Scholar]
- 40.Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B-Cell Lymphoproliferative Disorders: A Clinicopathologic Study of 22 Patients. Am J Surg Pathol. 2003;27(1):16–26. doi: 10.1097/00000478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Mason D, Banks P, Chan J, et al. Nodular lymphocyte predominance Hodgkin’s disease: a distinct clinico-pathological entity. Am J Surg Pathol. 1994;18:528–30. doi: 10.1097/00000478-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte- predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol. 1999;17(3):776–83. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 43.Nogova L, Reineke T, Josting A, et al. Lymphocyte-predominant and classical Hodgkin’s lymphoma--comparison of outcomes. Eur J Haematol Suppl. 2005;66:106–10. doi: 10.1111/j.1600-0609.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 44.Lukes RJ, Butler JJ. The pathology and nomenclature of Hodgkin’s disease. Cancer Res. 1966;26(6):1063–83. [PubMed] [Google Scholar]
- 45.Poppema S, Kaiserling E, Lennert K. Nodular paragranuloma and progressively transformed germinal centers: ultrastructural and immunohistochemical findings. Virchows Arch [B] 1979;31:211–25. doi: 10.1007/BF02889938. [DOI] [PubMed] [Google Scholar]
- 46.Ferry JA, Zukerberg LR, Harris NL. Florid progressive transformation of germinal centers. A syndrome affecting young men, without early progression to nodular lymphocyte predominance Hodgkin’s disease. Am J Surg Pathol. 1992;16(3):252–8. [PubMed] [Google Scholar]
- 47.Fan Z, Natkunam Y, Bair E, Tibshirani R, Warnke RA. Characterization of variant patterns of nodular lymphocyte predominant hodgkin lymphoma with immunohistologic and clinical correlation. Am J Surg Pathol. 2003;27(10):1346–56. doi: 10.1097/00000478-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Rudiger T, Gascoyne RD, Jaffe ES, et al. Workshop on the relationship between nodular lymphocyte predominant Hodgkin’s lymphoma and T cell/histiocyte-rich B cell lymphoma. Ann Oncol. 2002;13(Suppl 1):44–51. doi: 10.1093/annonc/13.s1.44. [DOI] [PubMed] [Google Scholar]
- 49.Boudova L, Torlakovic E, Delabie J, et al. Nodular lymphocyte-predominant Hodgkin lymphoma with nodules resembling T-cell/histiocyte-rich B-cell lymphoma: differential diagnosis between nodular lymphocyte-predominant Hodgkin lymphoma and T-cell/histiocyte-rich B-cell lymphoma. Blood. 2003;102(10):3753–8. doi: 10.1182/blood-2003-02-0626. [DOI] [PubMed] [Google Scholar]
- 50.Nam-Cha SH, Roncador G, Sanchez-Verde L, et al. PD-1, a follicular T-cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. Am J Surg Pathol. 2008;32(8):1252–7. doi: 10.1097/PAS.0b013e318165b0d6. [DOI] [PubMed] [Google Scholar]
- 51.Ekstrand BC, Lucas JB, Horwitz SM, et al. Rituximab in lymphocyte-predominant Hodgkin disease: results of a phase 2 trial. Blood. 2003;101(11):4285–9. doi: 10.1182/blood-2002-08-2644. [DOI] [PubMed] [Google Scholar]
- 52.Sundeen JT, Cossman J, Jaffe ES. Lymphocyte predominant Hodgkin’s disease, nodular subtype with coexistent “large cell lymphoma.” Histological progression or composite malignancy? Am J Surg Pathol. 1988;12:599–606. [PubMed] [Google Scholar]
- 53.Huang JZ, Weisenburger DD, Vose JM, et al. Diffuse large B-cell lymphoma arising in nodular lymphocyte predominant Hodgkin lymphoma: A report of 21 cases from the Nebraska Lymphoma Study Group. Leukemia & Lymphoma. 2004;45(8):1551–7. doi: 10.1080/1042819031000149421. [DOI] [PubMed] [Google Scholar]
- 54.Chang K-C, Khen NT, Jones D, Su I-J. Epstein-Barr virus is associated with all histological subtypes of Hodgkin lymphoma in Vietnamese children with special emphasis on the entity of lymphocyte predominance subtype. Human Pathology. 2005;36(7):747–55. doi: 10.1016/j.humpath.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Reed DM. On the pathological changes in Hodgkin’s disease, with especial reference to its relation to tuberculosis. Johns Hopkins Hosp Rep. 1902;1902(10):133–96. [Google Scholar]
- 56.Schebesta M, Heavey B, Busslinger M. Transcriptional control of B-cell development. Curr Opin Immunol. 2002;14(2):216–23. doi: 10.1016/s0952-7915(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 57.Schubart DB, Rolink A, Kosco-Vilbois MH, Botteri F, Matthias P. B-cell-specific coactivator OBF-1/OCA-B/Bob1 required for immune response and germinal centre formation. Nature. 1996;383(6600):538–42. doi: 10.1038/383538a0. [DOI] [PubMed] [Google Scholar]
- 58.Krenacs L, Himmelmann AW, Quintanilla-Martinez L, et al. Transcription factor B-cell-specific activator protein (BSAP) is differentially expressed in B cells and in subsets of B-cell lymphomas. Blood. 1998;92(4):1308–16. [PubMed] [Google Scholar]
- 59.Schebesta M, Pfeffer PL, Busslinger M. Control of pre-BCR signaling by Pax5-dependent activation of the BLNK gene. Immunity. 2002;17(4):473–85. doi: 10.1016/s1074-7613(02)00418-1. [DOI] [PubMed] [Google Scholar]
- 60.Foss HD, Reusch R, Demel G, et al. Frequent expression of the B-cell-specific activator protein in Reed-Sternberg cells of classical Hodgkin’s disease provides further evidence for its B-cell origin. Blood. 1999;94(9):3108–13. [PubMed] [Google Scholar]
- 61.Stein H, Marafioti T, Foss HD, et al. Down-regulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood. 2001;97(2):496–501. doi: 10.1182/blood.v97.2.496. [DOI] [PubMed] [Google Scholar]
- 62.Torlakovic E, Tierens A, Dang HD, Delabie J. The transcription factor PU.1, necessary for B-cell development is expressed in lymphocyte predominance, but not classical Hodgkin’s disease. Am J Pathol. 2001;159(5):1807–14. doi: 10.1016/S0002-9440(10)63027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCune RC, Syrbu SI, Vasef MA. Expression profiling of transcription factors Pax-5, Oct-1, Oct-2, BOB.1, and PU.1 in Hodgkin’s and non-Hodgkin’s lymphomas: a comparative study using high throughput tissue microarrays. Mod Pathol. 2006;19(7):1010–8. doi: 10.1038/modpathol.3800622. [DOI] [PubMed] [Google Scholar]
- 64.Browne P, Petrosyan K, Hernandez A, Chan JA. The B-cell transcription factors BSAP, Oct-2, and BOB.1 and the pan-B-cell markers CD20, CD22, and CD79a are useful in the differential diagnosis of classic Hodgkin lymphoma. Am J Clin Pathol. 2003;120(5):767–77. doi: 10.1309/YCH8-DWUF-FQBK-GPVB. [DOI] [PubMed] [Google Scholar]
- 65.Re D, Muschen M, Ahmadi T, et al. Oct-2 and Bob-1 deficiency in Hodgkin and Reed Sternberg cells. Cancer Res. 2001;61(5):2080–4. [PubMed] [Google Scholar]
- 66.Tzankov A, Bourgau C, Kaiser A, et al. Rare expression of T-cell markers in classical Hodgkin’s lymphoma. Mod Pathol. 2005;18(12):1542–9. doi: 10.1038/modpathol.3800473. [DOI] [PubMed] [Google Scholar]
- 67.Tzankov A, Zimpfer A, Pehrs AC, et al. Expression of B-cell markers in classical hodgkin lymphoma: a tissue microarray analysis of 330 cases. Mod Pathol. 2003;16(11):1141–7. doi: 10.1097/01.MP.0000093627.51090.3F. [DOI] [PubMed] [Google Scholar]
- 68.Pinkus GS, Pinkus JL, Langhoff E, et al. Fascin, a sensitive new marker for Reed-Sternberg cells of hodgkin’s disease. Evidence for a dendritic or B cell derivation? Am J Pathol. 1997;150(2):543–62. [PMC free article] [PubMed] [Google Scholar]
- 69.Sorg UR, Morse TM, Patton WN, et al. Hodgkin’s cells express CD83, a dendritic cell lineage associated antigen. Pathology. 1997;29(3):294–9. doi: 10.1080/00313029700169125. [DOI] [PubMed] [Google Scholar]
- 70.Oudejans JJ, Jiwa NM, Kummer JA, et al. Analysis of major histocompatibility complex class I expression on Reed-Sternberg cells in relation to the cytotoxic T-cell response in Epstein-Barr virus-positive and -negative Hodgkin’s disease. Blood. 1996;87(9):3844–51. [PubMed] [Google Scholar]
- 71.Kuppers R, Schwering I, Brauninger A, Rajewsky K, Hansmann ML. Biology of Hodgkin’s lymphoma. Ann Oncol. 2002;13 (Suppl 1):11–8. doi: 10.1093/annonc/13.s1.11. [DOI] [PubMed] [Google Scholar]
- 72.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin’s lymphoma. Am J Pathol. 1999;154(6):1685–91. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuppers R, Brauninger A. Reprogramming of the tumour B-cell phenotype in Hodgkin lymphoma. Trends In Immunology. 2006;27(5):203–5. doi: 10.1016/j.it.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Muschen M, Rajewsky K, Brauninger A, et al. Rare occurrence of classical Hodgkin’s disease as a T cell lymphoma. J Exp Med. 2000;191(2):387–94. doi: 10.1084/jem.191.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seitz V, Hummel M, Marafioti T, Anagnostopoulos I, Assaf C, Stein H. Detection of clonal T-cell receptor gamma-chain gene rearrangements in Reed-Sternberg cells of classic Hodgkin disease. Blood. 2000;95(10):3020–4. [PubMed] [Google Scholar]
- 76.Barry TS, Jaffe ES, Sorbara L, Raffeld M, Pittaluga S. Peripheral T-cell lymphomas expressing CD30 and CD15. Am J Surg Pathol. 2003;27(12):1513–22. doi: 10.1097/00000478-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Davis T, Morton C, Miller-Cassman R, Balk S, Kadin M. Hodgkin’s disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med. 1992;326:1115–22. doi: 10.1056/NEJM199204233261704. [DOI] [PubMed] [Google Scholar]
- 78.Kuppers R, Hansmann ML. The Hodgkin and Reed/Sternberg cell. Int J Biochem Cell Biol. 2005;37(3):511–7. doi: 10.1016/j.biocel.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 79.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361–92. [PubMed] [Google Scholar]
- 80.Theil J, Laumen H, Marafioti T, et al. Defective octamer-dependent transcription is responsible for silenced immunoglobulin transcription in Reed-Sternberg cells. Blood. 2001;97(10):3191–6. doi: 10.1182/blood.v97.10.3191. [DOI] [PubMed] [Google Scholar]
- 81.Cossman J, Annunziata CM, Barash S, et al. Reed-Sternberg cell genome expression supports a B-cell lineage. Blood. 1999;94(2):411–6. [PubMed] [Google Scholar]
- 82.Kuppers R, Yahalom J, Josting A. Advances in biology, diagnostics, and treatment of Hodgkin’s disease. Biol Blood Marrow Transplant. 2006;12(1 Suppl 1):66–76. doi: 10.1016/j.bbmt.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 83.Kanzler H, Kuppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med. 1996;184(4):1495–505. doi: 10.1084/jem.184.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marafioti T, Hummel M, Foss HD, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95(4):1443–50. [PubMed] [Google Scholar]
- 85.Rajewsky K, Kanzler H, Hansmann ML, Kuppers R. Normal and malignant B-cell development with special reference to Hodgkin’s disease. Ann Oncol. 1997;8 (Suppl 2):79–81. [PubMed] [Google Scholar]
- 86.Martin-Subero JI, Klapper W, Sotnikova A, et al. Chromosomal breakpoints affecting immunoglobulin loci are recurrent in Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma. Cancer Res. 2006;66(21):10332–8. doi: 10.1158/0008-5472.CAN-06-1992. [DOI] [PubMed] [Google Scholar]
- 87.Bai M, Panoulas V, Papoudou-Bai A, et al. B-cell differentiation immunophenotypes in classical Hodgkin lymphomas. Leuk Lymphoma. 2006;47(3):495–501. doi: 10.1080/10428190500306784. [DOI] [PubMed] [Google Scholar]
- 88.Schwering I, Brauninger A, Klein U, et al. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;101(4):1505–12. doi: 10.1182/blood-2002-03-0839. [DOI] [PubMed] [Google Scholar]
- 89.Hertel CB, Zhou XG, Hamilton-Dutoit SJ, Junker S. Loss of B cell identity correlates with loss of B cell-specific transcription factors in Hodgkin/Reed-Sternberg cells of classical Hodgkin lymphoma. Oncogene. 2002;21(32):4908–20. doi: 10.1038/sj.onc.1205629. [DOI] [PubMed] [Google Scholar]
- 90.Jox A, Zander T, Kuppers R, et al. Somatic mutations within the untranslated regions of rearranged Ig genes in a case of classical Hodgkin’s disease as a potential cause for the absence of Ig in the lymphoma cells. Blood. 1999;93(11):3964–72. [PubMed] [Google Scholar]
- 91.Kuppers R, Schmitz R, Distler V, Renne C, Brauninger A, Hansmann ML. Pathogenesis of Hodgkin’s lymphoma. Eur J Haematol Suppl. 2005;66:26–33. doi: 10.1111/j.1600-0609.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 92.Renne C, Willenbrock K, Kuppers R, Hansmann ML, Brauninger A. Autocrine-and paracrine-activated receptor tyrosine kinases in classic Hodgkin lymphoma. Blood. 2005;105(10):4051–9. doi: 10.1182/blood-2004-10-4008. [DOI] [PubMed] [Google Scholar]
- 93.Ushmorov A, Leithauser F, Sakk O, et al. Epigenetic processes play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood. 2006;107(6):2493–500. doi: 10.1182/blood-2005-09-3765. [DOI] [PubMed] [Google Scholar]
- 94.Cavazzini F, De Wolf-Peeters C, Wlodarska I. Alterations of loci encoding PU.1, BOB1, and OCT2 transcription regulators do not correlate with their suppressed expression in Hodgkin lymphoma. Cancer Genet Cytogenet. 2005;158(2):167–71. doi: 10.1016/j.cancergencyto.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Taylor CR. Apoptosis and cell cycle-related genes and proteins in classical Hodgkin lymphoma: application of tissue microarray technique. Appl Immunohistochem Mol Morphol. 2003;11(3):206–13. doi: 10.1097/00129039-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Felberbaum RS. The molecular mechanisms of classic Hodgkin’s lymphoma. Yale J Biol Med. 2005;78(4):203–10. [PMC free article] [PubMed] [Google Scholar]
- 97.Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109(7):2700–7. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 98.Hinz M, Loser P, Mathas S, Krappmann D, Dorken B, Scheidereit C. Constitutive NF-kappaB maintains high expression of a characteristic gene network, including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed-Sternberg cells. Blood. 2001;97(9):2798–807. doi: 10.1182/blood.v97.9.2798. [DOI] [PubMed] [Google Scholar]
- 99.Tzankov A, Dirnhofer S. Pathobiology of classical Hodgkin lymphoma. Pathobiology. 2006;73(3):107–25. doi: 10.1159/000095558. [DOI] [PubMed] [Google Scholar]
- 100.Flavell KJ, Murray PG. Hodgkin’s disease and the Epstein-Barr virus. Mol Pathol. 2000;53(5):262–9. doi: 10.1136/mp.53.5.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horie R, Watanabe T, Morishita Y, et al. Ligand-independent signaling by overexpressed CD30 drives NF-kappaB activation in Hodgkin-Reed-Sternberg cells. Oncogene. 2002;21(16):2493–503. doi: 10.1038/sj.onc.1205337. [DOI] [PubMed] [Google Scholar]
- 102.Jungnickel B, Staratschek-Jox A, Brauninger A, et al. Clonal deleterious mutations in the IkappaBalpha gene in the malignant cells in Hodgkin’s lymphoma. J Exp Med. 2000;191(2):395–402. doi: 10.1084/jem.191.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Emmerich F, Theurich S, Hummel M, et al. Inactivating I kappa B epsilon mutations in Hodgkin/Reed-Sternberg cells. J Pathol. 2003;201(3):413–20. doi: 10.1002/path.1454. [DOI] [PubMed] [Google Scholar]
- 104.Kuppers R. Molecular biology of Hodgkin’s lymphoma. Adv Cancer Res. 2002;84:277–312. doi: 10.1016/s0065-230x(02)84009-x. [DOI] [PubMed] [Google Scholar]
- 105.Mathas S, Lietz A, Anagnostopoulos I, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med. 2004;199(8):1041–52. doi: 10.1084/jem.20031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kashkar H, Seeger JM, Hombach A, et al. XIAP targeting sensitizes Hodgkin lymphoma cells for cytolytic T-cell attack. Blood. 2006;108(10):3434–40. doi: 10.1182/blood-2006-05-021675. [DOI] [PubMed] [Google Scholar]
- 107.Akyurek N, Ren Y, Rassidakis GZ, Schlette EJ, Medeiros LJ. Expression of inhibitor of apoptosis proteins in B-cell non-Hodgkin and Hodgkin lymphomas. Cancer. 2006;107(8):1844–51. doi: 10.1002/cncr.22219. [DOI] [PubMed] [Google Scholar]
- 108.Chabay P, Pesce P, De Matteo E, Lombardi MG, Rey G, Preciado MV. No Influence of bcl-2, p53, and p21waf1 protein expression on the outcome of pediatric Hodgkin lymphomas. J Pediatr Hematol Oncol. 2006;28(9):552–8. doi: 10.1097/01.mph.0000212955.43350.bb. [DOI] [PubMed] [Google Scholar]
- 109.Bai M, Papoudou-Bai A, Horianopoulos N, Grepi C, Agnantis NJ, Kanavaros P. Expression of bcl2 family proteins and active caspase 3 in classical Hodgkin’s lymphomas. Hum Pathol. 2007;38(1):103–13. doi: 10.1016/j.humpath.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 110.Stetler-Stevenson M, Crush-Stanton S, Cossman J. Involvement of the bcl-2 gene in Hodgkin’s disease. J Natl Cancer Inst. 1990;82:855–8. doi: 10.1093/jnci/82.10.855. [DOI] [PubMed] [Google Scholar]
- 111.Dutton A, Young LS, Murray PG. The role of cellular FLICE inhibitory protein (c-FLIP) in the pathogenesis and treatment of cancer. Expert Opin Ther Targets. 2006;10(1):27–35. doi: 10.1517/14728222.10.1.27. [DOI] [PubMed] [Google Scholar]
- 112.Feuerborn A, Moritz C, Von Bonin F, et al. Dysfunctional p53 deletion mutants in cell lines derived from Hodgkin’s lymphoma. Leuk Lymphoma. 2006;47(9):1932–40. doi: 10.1080/10428190600667721. [DOI] [PubMed] [Google Scholar]
- 113.Kim LH, Peh SC, Poppema S. Expression of retinoblastoma protein and P16 proteins in classic Hodgkin lymphoma: relationship with expression of p53 and presence of Epstein-Barr virus in the regulation of cell growth and death. Hum Pathol. 2006;37(1):92–100. doi: 10.1016/j.humpath.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 114.Spina D, Leoncini L, Close P, et al. Growth vs. DNA strand breaks in Hodgkin’s disease: impaired proliferative ability of Hodgkin and Reed-Sternberg cells. Int J Cancer. 1996;66(2):179–83. doi: 10.1002/(SICI)1097-0215(19960410)66:2<179::AID-IJC7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 115.Kuppers R, Sousa AB, Baur AS, Strickler JG, Rajewsky K, Hansmann ML. Common germinal-center B-cell origin of the malignant cells in two composite lymphomas, involving classical Hodgkin’s disease and either follicular lymphoma or B-CLL. Mol Med. 2001;7(5):285–92. [PMC free article] [PubMed] [Google Scholar]
- 116.Martin-Subero JI, Knippschild U, Harder L, et al. Segmental chromosomal aberrations and centrosome amplifications: pathogenetic mechanisms in Hodgkin and Reed-Sternberg cells of classical Hodgkin’s lymphoma? Leukemia. 2003;17(11):2214–9. doi: 10.1038/sj.leu.2403129. [DOI] [PubMed] [Google Scholar]
- 117.Chui DT, Hammond D, Baird M, Shield L, Jackson R, Jarrett RF. Classical Hodgkin lymphoma is associated with frequent gains of 17q. Genes Chromosomes Cancer. 2003;38(2):126–36. doi: 10.1002/gcc.10266. [DOI] [PubMed] [Google Scholar]
- 118.Tzankov A, Zimpfer A, Went P, et al. Aberrant expression of cell cycle regulators in Hodgkin and Reed-Sternberg cells of classical Hodgkin’s lymphoma. Mod Pathol. 2005;18(1):90–6. doi: 10.1038/modpathol.3800276. [DOI] [PubMed] [Google Scholar]
- 119.Ohsawa M, Fukushima H, Ikura Y, et al. Expression of cyclooxygenase-2 in Hodgkin’s lymphoma: its role in cell proliferation and angiogenesis. Leuk Lymphoma. 2006;47(9):1863–71. doi: 10.1080/10428190600685442. [DOI] [PubMed] [Google Scholar]
- 120.Cossman J, Messineo C, Bagg A. Reed-Sternberg cell: survival in a hostile sea. Lab Invest. 1998;78(3):229–35. [PubMed] [Google Scholar]
- 121.Poppema S, Potters M, Visser L, van den Berg AM. Immune escape mechanisms in Hodgkin’s disease. Ann Oncol. 1998;9 (Suppl 5):S21–4. doi: 10.1093/annonc/9.suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 122.Greiner A, Tobollik S, Buettner M, et al. Differential expression of activation-induced cytidine deaminase (AID) in nodular lymphocyte-predominant and classical Hodgkin lymphoma. J Pathol. 2005;205(5):541–7. doi: 10.1002/path.1746. [DOI] [PubMed] [Google Scholar]
- 123.Wlodarska I, Nooyen P, Maes B, et al. Frequent occurrence of BCL6 rearrangements in nodular lymphocyte predominance Hodgkin lymphoma but not in classical Hodgkin lymphoma. Blood. 2003;101(2):706–10. doi: 10.1182/blood-2002-05-1592. [DOI] [PubMed] [Google Scholar]
- 124.Nishikori M, Uchiyama T. Molecular pathogenesis of Hodgkin lymphoma. Int J Hematol. 2006;83(5):398–403. doi: 10.1532/IJH97.06049. [DOI] [PubMed] [Google Scholar]
- 125.Gorschluter M, Bohlen H, Hasenclever D, Diehl V, Tesch H. Serum cytokine levels correlate with clinical parameters in Hodgkin’s disease. Ann Oncol. 1995;6(5):477–82. doi: 10.1093/oxfordjournals.annonc.a059218. [DOI] [PubMed] [Google Scholar]
- 126.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99(12):4283–97. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 127.Atayar C, Poppema S, Blokzijl T, Harms G, Boot M, van den Berg A. Expression of the T-cell transcription factors, GATA-3 and T-bet, in the neoplastic cells of Hodgkin lymphomas. Am J Pathol. 2005;166(1):127–34. doi: 10.1016/S0002-9440(10)62238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohshima K, Karube K, Hamasaki M, et al. Imbalances of chemokines, chemokine receptors and cytokines in Hodgkin lymphoma: classical Hodgkin lymphoma vs. Hodgkin-like ATLL. Int J Cancer. 2003;106(5):706–12. doi: 10.1002/ijc.11301. [DOI] [PubMed] [Google Scholar]
- 129.Ohshima K, Tutiya T, Yamaguchi T, et al. Infiltration of Th1 and Th2 lymphocytes around Hodgkin and Reed-Sternberg (H&RS) cells in Hodgkin disease: Relation with expression of CXC and CC chemokines on H&RS cells. Int J Cancer. 2002;98(4):567–72. doi: 10.1002/ijc.10218. [DOI] [PubMed] [Google Scholar]
- 130.Gruss HJ, Pinto A, Duyster J, Poppema S, Herrmann F. Hodgkin’s disease: a tumor with disturbed immunological pathways. Immunol Today. 1997;18(4):156–63. doi: 10.1016/s0167-5699(97)84661-0. [DOI] [PubMed] [Google Scholar]
- 131.Chan WC. The Reed-Sternberg cell in classical Hodgkin’s disease. Hematol Oncol. 2001;19(1):1–17. doi: 10.1002/hon.659. [DOI] [PubMed] [Google Scholar]
- 132.Poppema S. Immunobiology and pathophysiology of hodgkin lymphomas. Hematology Am Soc Hematol Educ Program. 2005:231–8. doi: 10.1182/asheducation-2005.1.231. [DOI] [PubMed] [Google Scholar]
- 133.Glimelius I, Edstrom A, Fischer M, et al. Angiogenesis and mast cells in Hodgkin lymphoma. Leukemia. 2005;19(12):2360–2. doi: 10.1038/sj.leu.2403992. [DOI] [PubMed] [Google Scholar]
- 134.Oelmann E, Herbst H, Zuhlsdorf M, et al. Tissue inhibitor of metalloproteinases 1 is an autocrine and paracrine survival factor, with additional immune-regulatory functions, expressed by Hodgkin/Reed-Sternberg cells. Blood. 2002;99(1):258–67. doi: 10.1182/blood.v99.1.258. [DOI] [PubMed] [Google Scholar]
- 135.Pennanen H, Kuittinen O, Soini Y, Turpeenniemi-Hujanen T. Clinicopathological correlations of TIMP-1 and TIMP-2 in Hodgkin’s lymphoma. Eur J Haematol. 2004;72(1):1–9. doi: 10.1046/j.0902-4441.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 136.Aldinucci D, Poletto D, Gloghini A, et al. Expression of functional interleukin-3 receptors on Hodgkin and Reed-Sternberg cells. Am J Pathol. 2002;160(2):585–96. doi: 10.1016/S0002-9440(10)64878-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee IS, Kim SH, Song HG, Park SH. The molecular basis for the generation of Hodgkin and Reed-Sternberg cells in Hodgkin’s lymphoma. Int J Hematol. 2003;77(4):330–5. doi: 10.1007/BF02982639. [DOI] [PubMed] [Google Scholar]
- 138.Skinnider BF, Elia AJ, Gascoyne RD, et al. Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2002;99(2):618–26. doi: 10.1182/blood.v99.2.618. [DOI] [PubMed] [Google Scholar]
- 139.Kube D, Holtick U, Vockerodt M, et al. STAT3 is constitutively activated in Hodgkin cell lines. Blood. 2001;98(3):762–70. doi: 10.1182/blood.v98.3.762. [DOI] [PubMed] [Google Scholar]
- 140.Cochet O, Frelin C, Peyron JF, Imbert V. Constitutive activation of STAT proteins in the HDLM-2 and L540 Hodgkin lymphoma-derived cell lines supports cell survival. Cell Signal. 2006;18(4):449–55. doi: 10.1016/j.cellsig.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 141.Gruss HJ, Scheffrahn I, Hubinger G, Duyster J, Hermann F. The CD30 ligand and CD40 ligand regulate CD54 surface expression and release of its soluble form by cultured Hodgkin and Reed-Sternberg cells. Leukemia. 1996;10(5):829–35. [PubMed] [Google Scholar]
- 142.Ishida T, Ishii T, Inagaki A, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66(11):5716–22. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 143.Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103(5):1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 144.Alvaro-Naranjo T, Lejeune M, Salvado-Usach MT, et al. Tumor-infiltrating cells as a prognostic factor in Hodgkin’s lymphoma: a quantitative tissue microarray study in a large retrospective cohort of 267 patients. Leuk Lymphoma. 2005;46(11):1581–91. doi: 10.1080/10428190500220654. [DOI] [PubMed] [Google Scholar]
- 145.Asano N, Oshiro A, Matsuo K, et al. Prognostic significance of T-cell or cytotoxic molecules phenotype in classical Hodgkin’s lymphoma: a clinicopathologic study. J Clin Oncol. 2006;24(28):4626–33. doi: 10.1200/JCO.2006.06.5342. [DOI] [PubMed] [Google Scholar]
- 146.Mainou-Fowler T, Taylor PR, Miller S, Dickinson AM, Proctor SJ. Intracellular cytokine profiles by peripheral blood CD3+ T-cells in patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2003;44(8):1325–31. doi: 10.1080/1042819031000090246. [DOI] [PubMed] [Google Scholar]
- 147.Franzke A, Koenecke C, Geffers R, et al. Classical Hodgkin’s lymphoma: molecular evidence for specific alterations in circulating T lymphocytes. Tumour Biol. 2006;27(6):329–33. doi: 10.1159/000096151. [DOI] [PubMed] [Google Scholar]
- 148.Martin SE, Zhang HZ, Magyarosy E, Jaffe ES, Hsu SM, Chu EW. Immunologic methods in cytology: definitive diagnosis of non-Hodgkin’s lymphomas using immunologic markers for T- and B-cells. Am J Clin Pathol. 1984;82(6):666–73. doi: 10.1093/ajcp/82.6.666. [DOI] [PubMed] [Google Scholar]
- 149.Atayar C, Poppema S, Visser L, van den Berg A. Cytokine gene expression profile distinguishes CD4+/CD57+ T cells of the nodular lymphocyte predominance type of Hodgkin’s lymphoma from their tonsillar counterparts. J Pathol. 2006;208(3):423–30. doi: 10.1002/path.1894. [DOI] [PubMed] [Google Scholar]
- 150.Atayar C, van den Berg A, Blokzijl T, et al. Hodgkin’s lymphoma associated T-cells exhibit a transcription factor profile consistent with distinct lymphoid compartments. J Clin Pathol. 2007;60(10):1092–7. doi: 10.1136/jcp.2006.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kerzin-Storrar L, Faed MJ, MacGillivray JB, Smith PG. Incidence of familial Hodgkin’s disease. Br J Cancer. 1983;47(5):707–12. doi: 10.1038/bjc.1983.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alexandrescu DT, Garino A, Brown-Balem KA, Wiernik PH. Anticipation in families with Hodgkin’s and non-Hodgkin’s lymphoma in their pedigree. Leuk Lymphoma. 2006;47(10):2115–27. doi: 10.1080/10428190600724928. [DOI] [PubMed] [Google Scholar]
- 153.Mack TM, Cozen W, Shibata DK, et al. Concordance for Hodgkin’s disease in identical twins suggesting genetic susceptibility to the young-adult form of the disease. N Engl J Med. 1995;332(7):413–8. doi: 10.1056/NEJM199502163320701. [DOI] [PubMed] [Google Scholar]
- 154.Goldin LR, Pfeiffer RM, Gridley G, et al. Familial aggregation of Hodgkin lymphoma and related tumors. Cancer. 2004;100(9):1902–8. doi: 10.1002/cncr.20189. [DOI] [PubMed] [Google Scholar]
- 155.Grufferman S, Cole P, Smith PG, Lukes RJ. Hodgkin’s disease in siblings. N Engl J Med. 1977;296(5):248–50. doi: 10.1056/NEJM197702032960504. [DOI] [PubMed] [Google Scholar]
- 156.Hors J, Dausset J. HLA and susceptibility to Hodgkin’s disease. Immunol Rev. 1983;70:167–92. doi: 10.1111/j.1600-065x.1983.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 157.Harty LC, Lin AY, Goldstein AM, et al. HLA-DR, HLA-DQ, and TAP genes in familial Hodgkin disease. Blood. 2002;99(2):690–3. doi: 10.1182/blood.v99.2.690. [DOI] [PubMed] [Google Scholar]
- 158.Diepstra A, Niens M, Vellenga E, et al. Association with HLA class I in Epstein-Barr-virus-positive and with HLA class III in Epstein-Barr-virus-negative Hodgkin’s lymphoma. Lancet. 2005;365(9478):2216–24. doi: 10.1016/S0140-6736(05)66780-3. [DOI] [PubMed] [Google Scholar]
- 159.Chang ET, Montgomery SM, Richiardi L, Ehlin A, Ekbom A, Lambe M. Number of siblings and risk of Hodgkin’s lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1236–43. [PubMed] [Google Scholar]
- 160.Campbell GN, Lloyd J, Wotherspoon A, Coulter C, Bain BJ. Nodular lymphocyte predominant Hodgkin lymphoma in siblings. Leuk Lymphoma. 2004;45(3):609–11. doi: 10.1080/10428190310001602354. [DOI] [PubMed] [Google Scholar]
- 161.Straus SE, Jaffe ES, Puck JM, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98(1):194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 162.Keresztes K, Miltenyi Z, Bessenyei B, et al. Association between the Epstein-Barr virus and Hodgkin’s lymphoma in the North-Eastern part of Hungary: effects on therapy and survival. Acta Haematol. 2006;116(2):101–7. doi: 10.1159/000093639. [DOI] [PubMed] [Google Scholar]
- 163.Nakatsuka S, Aozasa K. Epidemiology and pathologic features of Hodgkin lymphoma. Int J Hematol. 2006;83(5):391–7. doi: 10.1532/IJH97.05184. [DOI] [PubMed] [Google Scholar]
- 164.Araujo I, Bittencourt AL, Barbosa HS, et al. The high frequency of EBV infection in pediatric Hodgkin lymphoma is related to the classical type in Bahia, Brazil. Virchows Arch. 2006;449(3):315–9. doi: 10.1007/s00428-006-0244-z. [DOI] [PubMed] [Google Scholar]
- 165.Andersson J. Epstein-Barr virus and Hodgkin’s lymphoma. Herpes. 2006;13(1):12–6. [PubMed] [Google Scholar]
- 166.Chang KC, Huang GC, Jones D, Tsao CJ, Lee JY, Su IJ. Distribution and prognosis of WHO lymphoma subtypes in Taiwan reveals a low incidence of germinal-center derived tumors. Leuk Lymphoma. 2004;45(7):1375–84. doi: 10.1080/10428194042000198849. [DOI] [PubMed] [Google Scholar]
- 167.Cartwright RA, Watkins G. Epidemiology of Hodgkin’s disease: a review. Hematol Oncol. 2004;22(1):11–26. doi: 10.1002/hon.723. [DOI] [PubMed] [Google Scholar]
- 168.Gulley ML, Eagan PA, Quintanilla-Martinez L, et al. Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin’s disease: association with mixed cellularity subtype and Hispanic American ethnicity. Blood. 1994;83(6):1595–602. [PubMed] [Google Scholar]
- 169.Kis LL, Takahara M, Nagy N, Klein G, Klein E. Cytokine mediated induction of the major Epstein-Barr virus (EBV)-encoded transforming protein, LMP-1. Immunol Lett. 2006;104(1–2):83–8. doi: 10.1016/j.imlet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 170.Jarrett RF. Viruses and lymphoma/leukaemia. J Pathol. 2006;208(2):176–86. doi: 10.1002/path.1905. [DOI] [PubMed] [Google Scholar]
- 171.Niedobitek G, Pazolt D, Teichmann M, Devergne O. Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol. 2002;198(3):310–6. doi: 10.1002/path.1217. [DOI] [PubMed] [Google Scholar]
- 172.Gandhi MK, Moll G, Smith C, et al. Galectin-1 mediated suppression of Epstein-Barr virus specific T-cell immunity in classic Hodgkin lymphoma. Blood. 2007;110(4):1326–9. doi: 10.1182/blood-2007-01-066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Meyer RM, Ambinder RF, Stroobants S. Hodgkin’s lymphoma: evolving concepts with implications for practice. Hematology Am Soc Hematol Educ Program. 2004:184–202. doi: 10.1182/asheducation-2004.1.184. [DOI] [PubMed] [Google Scholar]
- 174.Jarrett RF, Stark GL, White J, et al. Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: a population-based study. Blood. 2005;106(7):2444–51. doi: 10.1182/blood-2004-09-3759. [DOI] [PubMed] [Google Scholar]
- 175.Claviez A, Tiemann M, Luders H, et al. Impact of latent Epstein-Barr virus infection on outcome in children and adolescents with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(18):4048–56. doi: 10.1200/JCO.2005.01.701. [DOI] [PubMed] [Google Scholar]
- 176.Keegan TH, Glaser SL, Clarke CA, et al. Epstein-Barr virus as a marker of survival after Hodgkin’s lymphoma: a population-based study. J Clin Oncol. 2005;23(30):7604–13. doi: 10.1200/JCO.2005.02.6310. [DOI] [PubMed] [Google Scholar]
- 177.Herling M, Rassidakis GZ, Medeiros LJ, et al. Expression of Epstein-Barr virus latent membrane protein-1 in Hodgkin and Reed-Sternberg cells of classical Hodgkin’s lymphoma: associations with presenting features, serum interleukin 10 levels, and clinical outcome. Clin Cancer Res. 2003;9(6):2114–20. [PubMed] [Google Scholar]
- 178.Kwon JM, Park YH, Kang JH, et al. The effect of Epstein-Barr virus status on clinical outcome in Hodgkin’s lymphoma. Ann Hematol. 2006;85(7):463–8. doi: 10.1007/s00277-006-0081-9. [DOI] [PubMed] [Google Scholar]
- 179.Ambinder RF, Robertson KD, Moore SM, Yang J. Epstein-Barr virus as a therapeutic target in Hodgkin’s disease and nasopharyngeal carcinoma. Seminars in cancer biology. 1996;7(4):217–26. doi: 10.1006/scbi.1996.0029. [DOI] [PubMed] [Google Scholar]
- 180.Thompson LD, Fisher SI, Chu WS, Nelson A, Abbondanzo SL. HIV-associated Hodgkin lymphoma: a clinicopathologic and immunophenotypic study of 45 cases. Am J Clin Pathol. 2004;121(5):727–38. doi: 10.1309/PNVQ-0PQG-XHVY-6L7G. [DOI] [PubMed] [Google Scholar]
- 181.Spina M, Berretta M, Tirelli U. Hodgkin’s disease in HIV. Hematol Oncol Clin North Am. 2003;17(3):843–58. doi: 10.1016/s0889-8588(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 182.Pantanowitz L, Schlecht HP, Dezube BJ. The growing problem of non-AIDS-defining malignancies in HIV. Curr Opin Oncol. 2006;18(5):469–78. doi: 10.1097/01.cco.0000239886.13537.ed. [DOI] [PubMed] [Google Scholar]
- 183.Bellas C, Santon A, Manzanal A, et al. Pathological, immunological, and molecular features of Hodgkin’s disease associated with HIV infection. Comparison with ordinary hodgkin’s disease. Am J Surg Pathol. 1996;20(12):1520–4. doi: 10.1097/00000478-199612000-00012. [DOI] [PubMed] [Google Scholar]
- 184.Bosch Princep R, Lejeune M, Salvado Usach MT, Jaen Martinez J, Pons Ferre LE, Alvaro Naranjo T. Decreased number of granzyme B+ activated CD8+ cytotoxic T lymphocytes in the inflammatory background of HIV-associated Hodgkin’s lymphoma. Ann Hematol. 2005;84(10):661–6. doi: 10.1007/s00277-005-1051-3. [DOI] [PubMed] [Google Scholar]
- 185.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids. 2006;20(12):1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 186.Rowlings PA, Curtis RE, Passweg JR, et al. Increased incidence of Hodgkin’s disease after allogeneic bone marrow transplantation. J Clin Oncol. 1999;17(10):3122–7. doi: 10.1200/JCO.1999.17.10.3122. [DOI] [PubMed] [Google Scholar]
- 187.Landgren O, Engels EA, Pfeiffer RM, et al. Autoimmunity and susceptibility to Hodgkin lymphoma: a population-based case-control study in Scandinavia. J Natl Cancer Inst. 2006;98(18):1321–30. doi: 10.1093/jnci/djj361. [DOI] [PubMed] [Google Scholar]
- 188.Hjalgrim H, Rasmussen S, Rostgaard K, et al. Familial clustering of Hodgkin lymphoma and multiple sclerosis. J Natl Cancer Inst. 2004;96(10):780–4. doi: 10.1093/jnci/djh135. [DOI] [PubMed] [Google Scholar]
- 189.Kamel OW, Weiss LM, van de Rijn M, Colby TV, Kingma DW, Jaffe ES. Hodgkin’s disease and lymphoproliferations resembling Hodgkin’s disease in patients receiving long-term low-dose methotrexate therapy. Am J Surg Pathol. 1996;20(10):1279–87. doi: 10.1097/00000478-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 190.Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46(12):3151–8. doi: 10.1002/art.10679. [DOI] [PubMed] [Google Scholar]
- 191.Said JW. Immunodeficiency-related Hodgkin lymphoma and its mimics. Adv Anat Pathol. 2007;14(3):189–94. doi: 10.1097/PAP.0b013e31805048fc. [DOI] [PubMed] [Google Scholar]
- 192.Jaffe ES, Zarate OA, Medeiros LJ. The interrelationship of Hodgkin’s disease and non-Hodgkin’s lymphomas--lessons learned from composite and sequential malignancies. Semin Diagn Pathol. 1992;9(4):297–303. [PubMed] [Google Scholar]
- 193.Jaffe ES, Wilson WH. Gray zone, synchronous, and metachronous lymphomas: Diseases at the interface of non-Hodgkin’s lymphomas and Hodgkin’s Lymphoma. In: Mauch PM, Armitage JO, Coiffier B, Dalla-Favera R, Harris NL, editors. Non-Hodgkin’s Lymphoma. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2004. pp. 69–80. [Google Scholar]
- 194.Zarate-Osorno A, Medeiros LJ, Jaffe ES. Hodgkin’s disease coexistent with plasma cell dyscrasia. Arch Pathol Lab Med. 1992;116(9):969–72. [PubMed] [Google Scholar]
- 195.Zarate-Osorno A, Medeiros LJ, Kingma DW, Longo DL, Jaffe ES. Hodgkin’s disease following non-Hodgkin’s lymphoma. A clinicopathologic and immunophenotypic study of nine cases. Am J Surg Pathol. 1993;17(2):123–32. [PubMed] [Google Scholar]
- 196.Zarate-Osorno A, Medeiros LJ, Longo DL, Jaffe ES. Non-Hodgkin’s lymphomas arising in patients successfully treated for Hodgkin’s disease. A clinical, histologic, and immunophenotypic study of 14 cases. Am J Surg Pathol. 1992;16(9):885–95. doi: 10.1097/00000478-199209000-00007. [DOI] [PubMed] [Google Scholar]
- 197.Brauninger A, Hansmann ML, Strickler JG, et al. Identification of common germinal-center B-cell precursors in two patients with both Hodgkin’s disease and non-Hodgkin’s lymphoma. N Engl J Med. 1999;340(16):1239–47. doi: 10.1056/NEJM199904223401604. [DOI] [PubMed] [Google Scholar]
- 198.Momose H, Jaffe ES, Shin SS, Chen YY, Weiss LM. Chronic lymphocytic leukemia/small lymphocytic lymphoma with Reed-Sternberg-like cells and possible transformation to Hodgkin’s disease. Mediation by Epstein-Barr virus. Am J Surg Pathol. 1992;16(9):859–67. doi: 10.1097/00000478-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 199.Fong D, Kaiser A, Spizzo G, Gastl G, Tzankov A. Hodgkin’s disease variant of Richter’s syndrome in chronic lymphocytic leukaemia patients previously treated with fludarabine. Br J Haematol. 2005;129(2):199–205. doi: 10.1111/j.1365-2141.2005.05426.x. [DOI] [PubMed] [Google Scholar]
- 200.Correa P, O’Conor GT. Epidemiologic patterns of Hodgkin’s disease. Int J Cancer. 1971;8(2):192–201. doi: 10.1002/ijc.2910080203. [DOI] [PubMed] [Google Scholar]
- 201.Dinand V, Arya LS. Epidemiology of childhood Hodgkins disease: is it different in developing countries? Indian Pediatr. 2006;43(2):141–7. [PubMed] [Google Scholar]
- 202.Glaser SL, Hsu JL. Hodgkin’s disease in Asians: incidence patterns and risk factors in population-based data. Leuk Res. 2002;26(3):261–9. doi: 10.1016/s0145-2126(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 203.Gutensohn N, Cole P. Childhood social environment and Hodgkin’s disease. N Engl J Med. 1981;304(3):135–40. doi: 10.1056/NEJM198101153040302. [DOI] [PubMed] [Google Scholar]
- 204.Soares A, Biasoli I, Scheliga A, et al. Socioeconomic inequality and short-term outcome in Hodgkin’s lymphoma. Int J Cancer. 2007;120(4):875–9. doi: 10.1002/ijc.22417. [DOI] [PubMed] [Google Scholar]


