Abstract
Objective. To assess the efficacy of rituximab (RTX) in SSc.
Methods. Fourteen patients with SSc were evaluated. Eight patients were randomized to receive two cycles of RTX at baseline and 24 weeks [each cycle consisted of four weekly RTX infusions (375 mg/m2)] in addition to standard treatment, whereas six patients (control group) received standard treatment alone. Lung involvement was assessed by pulmonary function tests (PFTs) and chest high-resolution CT (HRCT). Skin involvement was assessed both clinically and histologically.
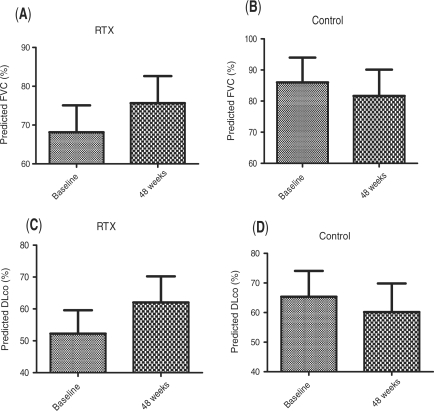
Results. There was a significant increase of forced vital capacity (FVC) in the RTX group compared with baseline (mean ± s.d.: 68.13 ± 19.69 vs 75.63 ± 19.73, at baseline vs 1-year, respectively, P = 0.0018). The median percentage of improvement of FVC in the RTX group was 10.25%, whereas that of deterioration in the controls was 5.04% (P = 0.002). Similarly, diffusing capacity of carbon monoxide (DLCO) increased significantly in the RTX group compared with baseline (mean ± s.d.: 52.25 ± 20.71 vs 62 ± 23.21, at baseline vs 1-year respectively, P = 0.017). The median percentage of improvement of DLCO in the RTX group was 19.46%, whereas that of deterioration in the control group was 7.5% (P = 0.023). Skin thickening, assessed with the Modified Rodnan Skin Score (MRSS), improved significantly in the RTX group compared with the baseline score (mean ± s.d.: 13.5 ± 6.84 vs 8.37 ± 6.45 at baseline vs 1-year, respectively, P < 0.001).
Conclusion. Our results indicate that RTX may improve lung function in patients with SSc. To confirm our encouraging results we propose that larger scale, multicentre studies with longer evaluation periods are needed.
Keywords: Scleroderma, Systemic sclerosis, Rituximab, Interstitial lung disease, Fibrosis, B cells
Introduction
SSc is a chronic systemic autoimmune disease characterized by vasculopathy and progressive fibrosis. SSc—interstitial lung disease (ILD)—is not uncommon in patients with the diffuse form of the disease and represents the clinical manifestation that dictates prognosis; this manifestation responds poorly to treatment. Therapeutic options for treating SSc-associated ILD are limited and most of the drugs tested so far have shown poor or modest results. Cyclophosphamide (CYC) has shown statistically significant but clinically questionable efficacy in the treatment of SSc-associated ILD, but is associated with immunosuppression underscoring the necessity for novel, more effective and less toxic therapies [1–4]. We and others [5–7] have employed mycophenolate mofetil (MMF) in the treatment of a limited number of patients with SSc-related ILD with encouraging, yet preliminary, results.
It has been suggested that targeting B cells could be a candidate therapy for SSc, since several lines of evidence point in the direction of B cells having a possible pathogenic role in this debilitating disease [8,9]. B cells from tight skin mice—an animal model of SSc—exhibit chronic hyperactivity and exaggerated calcium responses after B-cell receptor (BCR) cross-linking. B cells from this animal model show augmented CD19 (an important positive BCR response regulator) signalling caused by impaired function of CD22, a negative BCR response regulator [10]. Likewise, B cells from patients with SSc overexpress CD19, compared with B cells from healthy subjects and disease control patients, and are chronically activated [11]. Furthermore, studies revealed that B-cell genes were specifically transcribed in SSc skin [12] and that B-cell infiltration was a prominent feature of SSc-associated ILD [13].
Rituximab (RTX) is a chimeric mAb against human CD20 that depletes peripheral B cells. It has been successfully introduced in the treatment of systemic rheumatic diseases and exhibits an acceptable safety profile. In animal models of SSc, administration of anti-CD20 mAb to newborn tight skin mice led to significant suppression of skin fibrosis [14]. On the other hand, there are encouraging data from the literature regarding the use of RTX in chronic graft vs host disease (GVHD) [15–18]. GVHD is a late complication of heterologous haematopoietic stem-cell transplantation and exhibits several similarities to SSc, such as scleroderma-like skin manifestations and circulating autoantibodies. Furthermore, chronic GVHD has been considered by some as a systemic autoimmune disease [19–22]. The observed microchimerism in a significant percentage of patients with SSc may further suggest pathogenetic similarities between the two entities, justifying similar therapeutic trials [23,24]. Recently, two uncontrolled studies have explored the potential clinical efficacy of RTX in SSc. In the first one, skin fibrosis as assessed clinically and histologically improved significantly in the RTX-treated patients [25]. In the second one, even though no overt clinical benefit was observed, skin biopsies from RTX-treated patients exhibited a significant reduction in the myofibroblast score and the patients remained clinically stable throughout the study period [26]. There are also two additional reports (in abstract form) showing improvement of skin fibrosis (27, 28) and a case report of improvement of SSc-associated ILD (29). The preliminary encouraging results from the use of RTX in animal models of SSc and in humans with chronic GVHD and SSc has led us to investigate more thoroughly the potential efficacy of RTX in patients with SSc in an open-label, proof-of-principle, randomized, controlled study. We report herein that RTX treatment of patients with SSc and SSc-associated ILD led to improvement of lung function and was well tolerated.
Patients and methods
Patients
We enrolled 14 patients with a diagnosis of SSc, fulfilling the preliminary ACR criteria for the classification of the disease (30). Baseline demographic and clinical characteristics of the patients are presented in Table 1. All patients underwent a complete physical examination and a detailed review of their medical records prior to study enrolment. Other variables were also evaluated (full blood count, biochemistry profile, autoantibody profiles, urinalysis, ECG and cardiac ultrasound). Inclusion criteria were: (i) the detection of anti-Scl-70 autoantibodies in their sera; (ii) the presence of SSc-associated ILD as indicated by findings in either high-resolution CT (HRCT) of the chest or pulmonary function tests (PFTs) or both; and (iii) the absence of any changes in medications and/or dosage of treatment administered during the last 12 months before enrolment. All patients belonged to the diffuse variety of the disease as documented by the clinical presentation of skin involvement at the time of the study and/or its course over time since diagnosis. Moreover, all patients were anti-Scl-70 positive and had significant ILD, a feature of diffuse SSc. No changes in medication were allowed during the study.
Table 1.
Baseline characteristics of RTX and control group
| RTX | Control | P-value | |
|---|---|---|---|
| Age, median (range), years | 53 (41–66.5) | 56 (47.7–68.5) | NS |
| Disease duration, mean ± s.d., years | 6.87 ± 4.88 | 8.33 ± 5.6 | NS |
| HAQ-DI, median (range) | 0.687 (0.28–1.25) | 0.312 (0.09–0.90) | NS |
| MRSS, mean ± s.d. | 13.5 ± 6.84 | 11.5 ± 2.16 | NS |
| FVC, mean ± s.d. | 68.13 ± 19.69 | 86 ± 19.57 | NS |
| DLCO, mean ± s.d. | 52.25 ± 20.71 | 65.33 ± 21.43 | NS |
| HRCT score, mean ± s.d. | 13.1 ± 4.5 | 16.4 ± 6.4 | NS |
FVC and DLCO are expressed as a percentage of normal predicted values based on age, sex and height. HAQ-D1: HAQ-disability index; MRSS: Modified Rodnan Skin Score; NS: non-significant.
A local (Patras University Hospital, Patras, Greece) ethics committee approved the study (which fulfilled the Declaration of Helsinki requirements) and a written informed consent was obtained from all participating individuals.
Randomization and treatment
Patients born on an even-numbered date (n = 8) were assigned to the RTX group and those born on an odd-numbered date (n = 6) to the control group. Patients in the RTX group received four weekly pulses of RTX (375 mg/m2) at baseline and at 6 months on top of the already administered treatment. Patients in the control group continued their previously administered treatment unchanged (details in Table 2). Four patients in the RTX group (Patients 2, 4, 5 and 6) and two in the control group (Patients 11 and 14) were on MMF therapy during study enrolment and remained so throughout the study. They have been on that therapy for at least 4 years prior to study enrolment (apart from Patient 14 who was on MMF therapy for 2 years prior to enrolment). Three patients in the RTX group (Patients 2, 3 and 4) and one in the control group (Patient 10) had received CYC in the past but were off that therapy for at least 3 years prior to study enrolment. There were no significant differences between the two patient groups as shown in Table 2.
Table 2.
Demographics, clinical and laboratory parameters of the cohort
| Patient no./sex/age in years | Disease duration, years | FVC at baseline | FVC at 1 year | DLCO at baseline | DLCO at 1 year | MRSS at baseline | MRSS at 1 year | Histological improvement | Skin B-cell depletion | Concurrent medications | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RTX | 1/F/47 | 6 | 85 | 88 | 67 | 78 | 14 | 10 | No | No | Pred, Bos |
| 2/M/72 | 5 | 55 | 62 | 40 | 49 | 8 | 2 | Yes | Yes | Pred, MMF | |
| 3/F/56 | 6 | 70 | 75 | 33 | 38 | 10 | 4 | Yes | No | Pred, Bos | |
| 4/M/39 | 13 | 30 | 35 | 14 | 27 | 29 | 21 | Yes | Yes | Pred, Bos, MMF | |
| 5/F/33 | 15 | 85 | 90 | 71 | 96 | 16 | 14 | No | No | Pred, MMF | |
| 6/F/56 | 7 | 90 | 97 | 67 | 67 | 12 | 4 | Yes | Yes | MMF | |
| 7/F/70 | 1 | 68 | 84 | 64 | 81 | 11 | 8 | NA | NA | – | |
| 8/F/50 | 2 | 62 | 74 | 62 | 60 | 8 | 4 | NA | NA | Pred | |
| Control | 9/F/60 | 9 | 72 | 75 | 61 | 59 | 13 | 14 | No | No | Pred, CYC |
| 10/F/73 | 15 | 57 | 52 | 26 | 16 | 12 | 10 | No | No | Pred | |
| 11/F/48 | 5 | 114 | 110 | 84 | 79 | 14 | 9 | NA | NA | Pred, Bos, MMF | |
| 12/F/47 | 4 | 91 | 85 | 66 | 60 | 12 | 9 | No | No | Bos, CYC | |
| 13/F/67 | 5 | 88 | 70 | 84 | 65 | 8 | 4 | NA | NA | Pred | |
| 14/F/52 | 2 | 94 | 98 | 71 | 82 | 10 | 12 | NA | NA | Pred, MMF |
Pred: low-dose prednisone; Bos: Bosentan; NA: not applicable.
PFT
Standard PFTs were performed, at baseline, at 24 weeks and at 1 year in all patients, including assessments of forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), total lung capacity and diffusing capacity of carbon monoxide (DLCO) corrected for haemoglobin concentration. PFT parameters are expressed as a percentage of normal predicted values based on age, sex and height. All tests were performed at the same laboratory, at our institution (Patras University Hospital).
Chest HRCT
All patients had an HRCT performed at baseline and at 24 weeks using a 16 multi-detector GE CT Scan (General Electric, Waukesha WI, USA) with slice thickness of 0.625 mm. Imaging findings were interpreted separately by two experienced radiologists (C.K. and A.K.) in a blinded fashion. Acquisition parameters of tube voltage, tube current and slice thickness were 140 kilovolt potential (kVp), 300 mA and 0.625 mm, respectively. In order to obtain contiguous images of lung abnormalities, a second low-dose scanning (120 kV, 200 mA) was performed with slice thickness of 1.25 mm, covering the whole thorax. This protocol permitted data reconstruction and evaluation of images in coronal and saggital levels using multiplanar reformatting algorithm. The severity and extent of lung involvement was assessed according to the scoring system proposed by Warrick et al. [31] as follows: one point was assigned to ground glass appearance, two points to irregular pleural margins, three to septal/subpleural lines, four to honeycombing and five to subpleural cysts. The sum of points results in the severity score (0–15 points). Extent score was calculated giving 1 point when involvement of one to three lung segments was present, 2 points for four to nine segments and 3 points to more than 10 segments (0–15 points). Total score was obtained by adding the two above scores (0–30 points).
Clinical assessment of skin thickening
The MRSS was used for clinical assessment of skin thickening at baseline and at 1 year, by an experienced assessor in a blinded fashion [32,33].
Skin histology
Histological assessment of skin fibrosis was made by skin biopsies performed at baseline and at 24 weeks of evaluation (a 5-mm punch biopsy of lesional skin). Skin biopsies were performed in six patients of the RTX group and three patients of the control group (including those receiving CYC) and were performed prior to RTX administration. Biopsies were taken from lesional skin in the forearm; the second biopsy was taken from lesional skin adjacent (always < 2 cm) to the site of the baseline biopsy (supplementary Fig. 1, available as supplementary data at Rheumatology Online).
Pathological evaluation of skin biopsies
All skin biopsies were fixed in 10% neutral buffered formalin and embedded in paraffin. Four micrometre-thick paraffin sections were obtained and stained with haematoxylin and eosin, and Masson's trichrome (fibrosis evaluation). All trichrome stains of biopsies were performed at the same time, using the same staining set (04-011802-Masson trichrome, Goldner-Bioptica, Milan, Italy) in order to have comparable results.
Fibrosis quantification
To quantify collagen accumulation in the dermis, we employed the Image J software (freely downloadable and developed by Wayne Rasband at the Research Services Branch of the NIH) according to the method described previously [34]. A similarly and simultaneously stained skin biopsy from skin keloid was analysed as well, representing a positive control for collagen deposition. Collagen deposition was examined separately for the papillary and the reticular dermis.
Evaluation of immunostains for the presence of B and T cells
All biopsies were examined immunohistochemically for the presence of T and B cells as described previously using the BenchMark® XT automated slide stainer (Ventana, Tucson AZ, USA) and a standard streptavidin biotin method [i view DAB-DAB MapTM Detection kit (streptavidin horseradish peroxidase detection kit), Ventana]. Antibodies used included: anti-CD4 (Neomarkers, Fremont CA, USA), anti-CD8 (Novocastra, Newcastle, UK) and anti-CD20 (Dako, Carpinteria CA, USA). As has been previously reported [35], 10 cell counts were performed manually at ×400 magnification using a 10 × 10 microscope grid. Numbers of immunochemically stained cells were determined by visual inspection of three different fields per section. The average scores then were calculated. All biopsies were evaluated simultaneously in a blinded (to treatment and date) fashion.
Overall functional impairment
We used the 20-item HAQ-DI [36,37]. Clinically significant improvement in functional status was defined as a 0.2 decrease in HAQ-DI score, as previously described [38].
Levels of circulating inter-cellular adhesion molecule-1, E-selectin, vascular cell adhesion molecule and ET-1
Serum samples were obtained from all patients at baseline and at 24 weeks and were stored at −20°C. Serum levels of the three soluble endothelium activation/injury markers and of ET-1 were measured employing ELISA methodology, using commercially available kits, according to the manufacturer's instructions (R&D Systems, Minneapolis MN, USA).
Efficacy end points
Primary end points included evaluations of (i) changes in pulmonary function as assessed by PFT and (ii) clinical assessment of skin involvement by the MRSS. Secondary outcome measures included changes in (i) skin histology including collagen deposition and lymphocytic infiltration, (ii) HRCT scores, (iii) serum levels of soluble markers and (iv) overall functional impairment.
Statistical analysis
Statistical analysis was performed using the SPSS software (SPSS Inc., Chicago, IL, USA), version 14. Data are presented as mean ± s.d., median (upper and lower quartile values) or percentages, as appropriate. The paired Student's t-test, Wilcoxon matched pairs test, Mann–Whitney test and Fisher's exact test were used where indicated. Values of P < 0.05 were considered as statistically significant.
Results
Effects of RTX treatment on SSc-associated ILD
PFTs and HRCT were used to assess the potential effect of RTX administration on SSc-associated ILD.
PFTs improve following RTX treatment
At the 1-year evaluation, there was a significant increase of FVC in the RTX group compared with baseline (mean ± s.d.: 68.13 ± 19.69 vs 75.63 ± 19.73, at baseline vs 1 year, respectively, P = 0.0018), whereas no change was noticed in the control group (mean ± s.d.: 86 ± 19.57 vs 81.67 ± 20.69, at baseline vs 1 year, respectively, P = 0.23), as shown in Fig. 1A and B. The median (upper and lower quartile values) percentage of improvement of FVC in the RTX group was 10.25% (6.19–18.65), whereas in the control group FVC deteriorated [median percentage of deterioration (upper and lower quartile values) 5.04% (4.11–11.6)]. Direct comparison of FVC changes recorded at 1 year revealed that the RTX-treated group improved significantly (P = 0.002) compared with the standard treatment (control) group.
Fig. 1.
Effects of RTX treatment on PFTs. B-cell depletion treatment mediates a significant improvement of FVC (P = 0.002) and DLCO (P = 0.023) at 1 year (A and C, respectively) compared with the values of the control group (B and D). FVC and DLCO values are presented as percentages of predicted values.
There was a significant increase of DLCO in the RTX group compared with baseline (mean ± s.d.: 52.25 ± 20.71 vs 62 ± 23.21, at baseline vs 1 year, respectively, P = 0.017), whereas no changes were noticed in the control group (mean ± s.d.: 65.33 ± 21.43 vs 60.17 ± 23.69, at baseline vs 1 year, respectively, P = 0.25), as shown in Fig. 1C and D. The median (upper and lower quartile values) percentage of improvement of DLCO in the RTX group was 19.46% (3.7–30.8), whereas in the control group the median percentage of deterioration was 7.5% (1.4–26.57) (P = 0.023).
The improvement of lung function tests in the RTX-treated patients was already evident in the 24-week evaluation (mean ± s.d.: 71.5 ± 21.3 and 55.2 ± 25.1 for FVC and DLCO, respectively).
Effects of RTX treatment on HRCT
HRCT scores were identical at baseline and at 24 weeks in all patients in the RTX group (mean ± s.d.: 13.1 ± 4.5). In the control group, there was a modest increase in the HRCT score that was not statistically significant (mean ± s.d.: 16.4 ± 6.4 vs 16.8 ± 6.5, at baseline vs 24 weeks, respectively, P = 0.170).
Effects of RTX treatment on skin disease in patients with SSc
To evaluate any potential effect of RTX on skin involvement we performed standard clinical assessment and skin biopsy analysis.
Effects of RTX treatment on skin thickening, as clinically assessed
Skin thickening, assessed with the MRSS, was similar in the two treatment groups at baseline (Table 1, P = 0.50). However, at the 1-year evaluation, there was a significant decrease of MRSS in the RTX group compared with the baseline score (mean ± s.d.: 13.5 ± 6.84 vs 8.37 ± 6.45 at baseline vs 1 year, respectively, P = 0.0003). On the contrary, no significant change in skin scores was noticed in the control group at 1 year when compared with the baseline MRSS (mean ± s.d., 11.50 ± 2.16 vs 9.66 ± 3.38 at baseline vs 1 year, respectively, P = 0.16). The median (upper and lower quartile values) percentage of improvement in the RTX-treated group was 39.25% (27.33–64.95) compared with 20.80% (10.78–39.28) in the control group. Statistical analysis revealed that despite differences, the values were not statistically significant (P = 0.06).
Effects of RTX treatment on collagen deposition
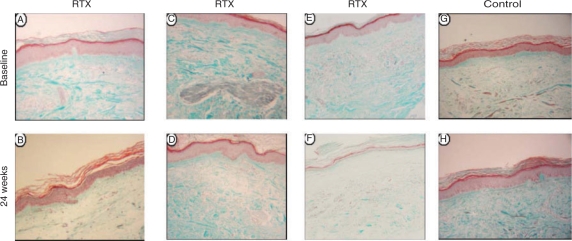
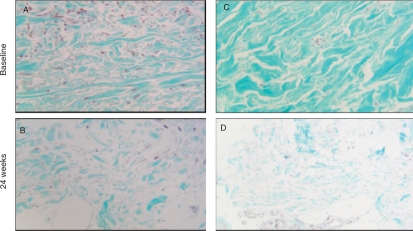
In the RTX-treated group, there was a significant reduction of collagen deposition in the papillary dermis at 24 weeks compared with baseline (mean ± s.d.: 51.75 ± 19.78 vs 31.68 ± 14.02 at Week 0 vs Week 24, respectively, P = 0.030). The control group showed no change in collagen deposition in the papillary dermis at 24 weeks compared with baseline values (mean ± s.d.: 46.53 ± 22.43 vs 46.27 ± 10.49 at baseline vs Week 24, respectively, P = 0.980). The median (upper and lower quartile values) percentage of improvement of skin fibrosis in the RTX group was 38.33% (6.86–59.9), whereas in the control group skin fibrosis worsened (median percentage of worsening of skin fibrosis was 5.23%). Histological improvement was evident in four patients (Patients 2, 3, 4 and 6) of the RTX group and coincided with clinical improvement in these patients (Table 2). Differences were not statistically significant (P = 0.09). Representative skin histology is shown in Figs 2 and 3.
Fig. 2.
RTX-induced changes of skin histology at 24 weeks. Improvement of fibrosis in the papillary dermis of Patients 6 and 4 before and following RTX treatment (A, B and C, D, respectively). Improvement of fibrosis in full thickness dermis (E and F) in Patient 2. Worsening of fibrosis at 24 week in full thickness dermis is seen in the control-treated Patient 8 (H) compared with baseline (G).
Fig. 3.
RTX-induced reduction of collagen deposition in the dermis (Masson's trichrome X40) in Patients 4 (B) and 2 (D) compared with baseline (A and C, respectively).
When collagen deposition in the reticular dermis was evaluated comparatively at baseline and at Week 24, there were no differences either in the RTX (mean ± s.d.: 76.57 ± 16.04 vs 73.07 ± 9.86 at Week 0 vs Week 24, respectively, P = 0.758) or in the control group (mean ± s.d.: 50.97 ± 28.88 vs 57.03 ± 22.63 at Week 0 vs Week 24, respectively, P = 0.498) of patients with SSc. Exceptionally, a striking improvement was observed in Patient 2 (RTX group), who displayed a significant reduction of skin fibrosis not only in the papillary but in the reticular dermis as well, and had clinically an almost complete resolution of sclerodermatous skin lesions (Fig. 2C and D).
Effects of RTX on skin infiltrating B cells
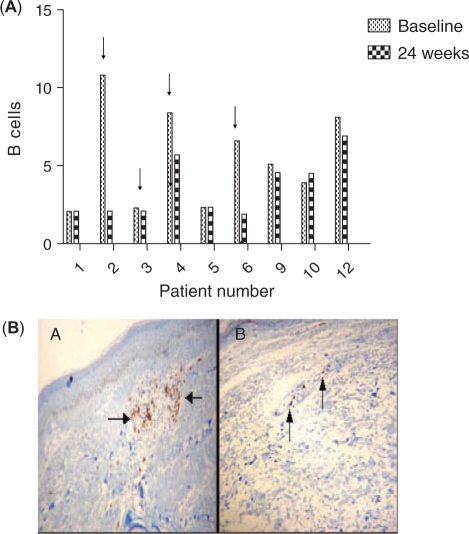
The presence of T and B cells was assessed by immunohistochemistry in all biopsies. Representation of T cells was substantially limited in all patients; these were predominantly CD8+ T cells and no differences were observed at 24 weeks, compared with baseline (data not shown). The number of B cells was relatively low as well, but they were more abundant than T cells. RTX administration significantly reduced the number of B cells in three patients (Patients 2, 4 and 6) but had no effect on the remaining three patients of the RTX group (Patients 1, 3 and 5). Patients exhibiting B-cell depletion in the skin following RTX administration were the ones with the higher numbers of B cells at baseline. All three patients with skin B-cell depletion improved histologically. However, among the three non-B-cell depletors, there was a single patient who improved histologically (Patient 3). In the control group no difference was recorded in B cell numbers at 24 weeks compared with baseline. All data and representative skin immunohistochemistry are shown in Fig. 4.
Fig. 4.
(A) Effects of RTX on skin infiltrating B cells. Numbers of infiltrating B cells in the dermis (y-axis) at baseline and at 24 weeks. The mean ± s.d. of infiltrating B cells was 5.41 ± 3.73 vs 2.70 ± 1.47 at week 0 vs week 24, respectively, P = 0.110 for the RTX group and 5.69 ± 2.16 vs 5.32 ± 1.36 at week 0 vs week 24, respectively, P = 0.54 for the control group. The arrows indicate patients with histological evidence of improvement of skin fibrosis. Apart from Patient 3, in all other patients improvement of skin histology is associated with RTX-induced decreases in the numbers of skin-infiltrating B cells. (B) Elimination of skin-infiltrating B cells of Patient 2 before (A) and following RTX (B) at 24 weeks.
Overall functional impairment
There was a significant improvement in HAQ scores at 1 year compared with baseline in the RTX group [median (lower and upper quartile values), 0.687 (0.28–1.25) vs 0.312 (0.125–0.687) at baseline vs 1 year, respectively, P = 0.03]. No change was noticed in the control group [median (lower and upper quartile values), 0.312 (0.09–0.90) vs 0.125 (0.09–0.40) at baseline vs 1 year, respectively, P = 0.130]. In the RTX group, six patients exhibited a clinically significant improvement of functional status (as defined by a decrease of HAQ score by 0.2) and two patients remained unchanged. By contrast, in the control group one patient worsened, two remained unchanged and three patients improved.
Markers of endothelium activation/injury and ET-1
To examine any potential effects of RTX administration on endothelium, which is a key player in SSc pathogenesis, we measured serum levels of three markers of endothelium activation/injury [E-selectin, vascular cell adhesion molecule (VCAM) and inter-cellular adhesion molecule-1 (ICAM-1)] and ET-1. There was a trend towards a decrease in all three endothelium activation/injury markers and ET-1 serum levels in the RTX group at 24 weeks, compared with baseline, which did not reach statistical significance. It was worth noticing though that the patients with histological improvement in skin biopsy (Patients 2, 3, 4 and 6) were the ones who displayed a decrease in serum levels of at least three of the four markers studied. In the control group, even though no statistically significant changes were observed, VCAM and ET-1 levels showed an upward trend at 24 weeks compared with baseline (data not shown).
Adverse events
Patient 4 (RTX group) suffered a respiratory tract infection 3 months after the second cycle of RTX. The patient was hospitalized for 3 days, treated with four antibiotics and oxygen supplementation and recovered fully in a few days.
Discussion
This is the first randomized controlled study in the literature to evaluate the efficacy of RTX in patients with SSc. In this study, we report an improvement in lung function with an increase in FVC and DLCO at 1 year, following two cycles (composed of four weekly doses each) of RTX in patients with SSc compared with the control group. Perhaps more importantly, none of the RTX-treated patients exhibited worsening of either FVC or DLCO, whereas five out of six patients had declining FVC and DLCO values at 1 year in the control group. These results indicate that RTX may favourably affect lung function parameters in patients with SSc. We should note, however, that patients in the control group tended to have more early disease and better lung function parameters (although not statistically different from the RTX group) making them more likely to deteriorate over the time of the study. Radiological assessment of ILD by HRCT revealed no changes in the RTX group, whereas there was a minor deterioration of two patients in the control group. It is of importance to note that none of the patients in the RTX group displayed worsening of lung fibrosis assessed by HRCT. Therefore, our HRCT studies support the idea that RTX treatment may stabilize pulmonary lesions apparent on imaging in diffuse SSc. The contrast between the RTX-induced improvement of lung function and the lack of improvement in the imaging studies may stem from using the Warrick scoring system, which is a rather crude method of assessment, or because the time interval of evaluation (24 weeks) was too short. Alternatively, most of the lesions apparent on HRCT may represent established fibrosis and not active lesions even though the effects of RTX are incompletely understood on both these parameters.
Although improvement in skin thickening, assessed by MRSS, has been reported in some studies evaluating the efficacy of different therapeutic agents in SSc, histological confirmation of improvement has only been documented following stem-cell transplantation [39]. Improvement of skin fibrosis also occurs in the long term during the physical course of the disease. In this study, improvement of skin thickening, as assessed clinically, was evident in the isolated analysis in the RTX group but head-to-head comparisons with the control group revealed that differences strongly tended to reach statistical significance, but did not reach it. This may be due to the small number of participants and the short time interval (24 weeks) between biopsies. Nevertheless, the improvement depicted in our figures from skin histology may suggest a potentially modifying role of RTX in the pathological process of skin fibrosis in SSc.
Two recent uncontrolled studies have explored the potential clinical efficacy of a single cycle of RTX in SSc. In the study by Smith et al. [25], clinical and histological improvement of skin fibrosis was observed at 24 weeks following RTX treatment and in the study by Lafyatis et al. [26], a significant reduction of the myofibroblast score in skin biopsies of RTX-treated patients was reported. These data are in agreement with ours and suggest that RTX may favourably affect skin fibrosis in SSc. Lung function tests at 24 weeks were stable in both the previously mentioned studies. We should note, however, that only 7 out of 15 patients in the study by Lafyatis et al. and 5 out of 8 patients in the study by Smith et al. had evidence of mild ILD (since patients with significant ILD were excluded), whereas in our study the presence of ILD was one of the inclusion criteria (and most patients had significant ILD). Furthermore, our study is the first in the literature to report lung function parameters at 1 year following RTX administration and the first to report the effects of two consecutive cycles of RTX on lung function and skin thickening in SSc.
Although RTX has been successfully introduced lately in the treatment of various autoimmune diseases, its exact mechanism of action is not completely understood. Pathogenesis of SSc is largely unknown but the results of the present study indicate that B cells may play a role and RTX may have a favourable effect on the disease process. RTX targets B cells that are present in skin biopsies of patients with scleroderma [40] and are the source of autoantibodies, some of which may contribute to pathogenesis [41]. In addition, it has become clear that apart from peripheral B-cell depletion, RTX indirectly affects other immune cells such as T cells [42,43], which have been implicated in SSc pathogenesis as well [44,45]. In our study, improvement of skin fibrosis was more commonly encountered in patients with evidence of B-cell depletion in the skin, indirectly supporting a potential role of B cells in SSc. Although our data are preliminary, we may propose that the number of infiltrating B cells in the dermis, as assessed by skin biopsy, could serve as a marker of response to RTX therapy in patients with SSc.
Our study has several potential limitations. The first one is the small number of patients recruited, which does not provide the study with sufficient statistical power to prove efficacy. Indeed, this is a proof-of-principle study that was performed in order to obtain preliminary data regarding the effect of RTX on a limited number of patients with SSc. We designed the study as an open label randomized controlled one, so that we can compare the results of the RTX-treated patients with a similarly affected control group of patients receiving standard treatment and care. An additional limitation is that most patients had long-standing disease and had received various forms of immunosuppressive treatment in the past. The patients enrolled in our study are heterogeneous in terms of disease duration, severity and previous treatments. Ideally, one should have recruited patients with early disease and with no previous exposure to other forms of immunosuppressive treatment, but this approach might be hampered by disease rarity. Yet another limitation is that patients in both the RTX and the control group received several concurrent immune-based therapies and even though they were on the same treatment for a significant amount of time prior to enrolment, one cannot rule out the possibility that these therapies contributed to the outcomes reported in this study. Furthermore, improvement of skin fibrosis may associate with the natural course of the disease; nevertheless, improvement of skin fibrosis was seen only in our RTX-treated group and not in the control group.
We report herein the results of a controlled study evaluating the efficacy of RTX in patients with SSc and SSc-associated ILD. Our data, although preliminary and on a small cohort, indicate a possible disease-modifying role of RTX in SSc, particularly in SSc-associated lung disease. However, in SSc treatment several therapeutic agents have shown some efficacy in small-scale studies but failed to do so in larger scale studies. Taking into consideration the limitations of the present study, definite conclusions should not be drawn. Nevertheless, our data could serve as a good starting point for the design of larger scale, multicentre studies with longer evaluation periods and especially in earlier stages of the disease.

Supplementary data
Supplementary data are available at Rheumatology Online.
Supplementary Material
Acknowledgements
D.D., S.N.C.L and A.P.A had full access to all data and were responsible for data analysis and interpretation of the results. Study design and manuscript preparation was by D.D., S.N.C.L and A.P.A. Acquisition of data was by D.D., S.N.C.L, M.K. and G.Y. Assessment of HRCT was by C.K. and A.K. Pathological evaluation of skin biopsies was by A.C.T. Immunohistochemistry was by C.S. Statistical analysis was by D.D.
Funding: This work was supported by the Hellenic Rheumatology Society (a non-profitable organization that did not interfere in any stage of this study). Funding to pay the Open Access publication charges for this article was provided by Roche Hellas.
Disclosure statement: The authors have declared no conflicts of interest.
Footnotes
See page 201 for the editorial comment on this article (doi:10.1093/rheumatology/kep421)
References
- 1.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 2.Tashkin DP, Elashoff R, Clements PJ, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 4.Yiannopoulos G, Pastromas V, Antonopoulos I, et al. Combination of intravenous pulses of cyclophosphamide and methylprednizolone in patients with systemic sclerosis and interstitial lung disease. Rheumatol Int. 2007;27:357–61. doi: 10.1007/s00296-006-0217-1. [DOI] [PubMed] [Google Scholar]
- 5.Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology. 2006;45:1005–8. doi: 10.1093/rheumatology/kei211. [DOI] [PubMed] [Google Scholar]
- 6.Nihtyanova SI, Brough GM, Black CM, Denton CP. Mycophenolate mofetil in diffuse cutaneous systemic sclerosis—a retrospective analysis. Rheumatology. 2007;46:442–5. doi: 10.1093/rheumatology/kel244. [DOI] [PubMed] [Google Scholar]
- 7.Plastiras SC, Vlachoyiannopoulos PG, Tzelepis GE. Mycophenolate mofetil for interstitial lung disease in scleroderma. Rheumatology. 2006;45:1572. doi: 10.1093/rheumatology/kel335. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto M, Sato S. B lymphocytes and systemic sclerosis. Curr Opin Rheumatol. 2005;17:746–51. doi: 10.1097/01.bor.0000179945.73518.28. [DOI] [PubMed] [Google Scholar]
- 9.Sato S, Fujimoto M, Hasegawa M, Takehara K, Tedder TF. Altered B lymphocyte function induces systemic autoimmunity in systemic sclerosis. Mol Immunol. 2004;41:1123–33. doi: 10.1016/j.molimm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Asano N, Fujimoto M, Yazawa N, et al. B Lymphocyte signaling established by the CD19/CD22 loop regulates autoimmunity in the tight-skin mouse. Am J Pathol. 2004;165:641–50. doi: 10.1016/S0002-9440(10)63328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato S, Fujimoto M, Hasegawa M, Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum. 2004;50:1918–27. doi: 10.1002/art.20274. [DOI] [PubMed] [Google Scholar]
- 12.Whitfield ML, Finlay DR, Murray JI, et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci USA. 2003;100:12319–24. doi: 10.1073/pnas.1635114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafyatis R, O’Hara C, Feghali-Bostwick CA, Matteson E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 2007;56:3167–8. doi: 10.1002/art.22847. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa M, Hamaguchi Y, Yanaba K, et al. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol. 2006;169:954–66. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canninga-van Dijk MR, van der Straaten HM, Fijnheer R, Sanders CJ, van den Tweel JG, Verdonck LF. Anti-CD20 monoclonal antibody treatment in 6 patients with therapy-refractory chronic graft-versus-host disease. Blood. 2004;104:2603–6. doi: 10.1182/blood-2004-05-1855. [DOI] [PubMed] [Google Scholar]
- 16.Carella AM, Biasco S, Nati S, Congiu A, Lerma E. Rituximab is effective for extensive steroid-refractory chronic graft-vs.-host-disease. Leuk Lymphoma. 2007;48:623–4. doi: 10.1080/10428190601094362. [DOI] [PubMed] [Google Scholar]
- 17.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–62. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto M, Okano A, Akamatsu S, et al. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006;20:172–3. doi: 10.1038/sj.leu.2403996. [DOI] [PubMed] [Google Scholar]
- 19.Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105:4885–91. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20:349–60. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Perruche S, Marandin A, Kleinclauss F, et al. Association of mixed hematopoietic chimerism with elevated circulating autoantibodies and chronic graft-versus-host disease occurrence. Transplantation. 2006;81:573–82. doi: 10.1097/01.tp.0000183878.53367.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Simon JA, Sanchez-Abarca I, Diez-Campelo M, Caballero D, San Miguel J. Chronic graft-versus-host disease: pathogenesis and clinical management. Drugs. 2006;66:1041–57. doi: 10.2165/00003495-200666080-00002. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JL, Furst DE, Maloney S, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559–62. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- 24.Murata H, Nakauchi H, Sumida T. Microchimerism in Japanese women patients with systemic sclerosis. Lancet. 1999;354:220. doi: 10.1016/S0140-6736(99)00164-6. [DOI] [PubMed] [Google Scholar]
- 25.Smith VP, Van Praet JT, Vandooren BR, et al. Rituximab in diffuse cutaneous systemic sclerosis: an open-label clinical and histopathological study. Ann Rheum Dis. doi: 10.1136/ard.2008.095463. Advance Access published December 22, 2008, doi: 0:ard.2008.095463v1. [DOI] [PubMed] [Google Scholar]
- 26.Lafyatis R, Kissin E, York M, et al. B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2009;60:578–83. doi: 10.1002/art.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosello S, De Santis M, Lama G, et al. Clinical improvement in systemic slerosis patients treated with anti-CD20. Arthritis Rheum Sup. 2007;56:494. [Google Scholar]
- 28.Lombardi S, Quartuccio L, Franzolini N, et al. Rituximab for the long term treatment of severe cutaneous involvement in systemic sclerosis. Arthritis Rheum Sup. 2008;58:S822–3. [Google Scholar]
- 29.McGonagle D, Tan AL, Madden J, et al. Successful treatment of resistant scleroderma-associated interstitial lung disease with rituximab. Rheumatology. 2008;47:552–3. doi: 10.1093/rheumatology/kem357. [DOI] [PubMed] [Google Scholar]
- 30.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 31.Warrick JH, Bhalla M, Schabel SI, Silver RM. High resolution computed tomography in early scleroderma lung disease. J Rheumatol. 1991;18:1520–8. [PubMed] [Google Scholar]
- 32.Valentini G, D’Angelo S, Della RA, Bencivelli W, Bombardieri S. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. IV. Assessment of skin thickening by modified Rodnan skin score. Ann Rheum Dis. 2003;62:904–5. doi: 10.1136/ard.62.9.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czirjak L, Nagy Z, Aringer M, Riemekasten G, Matucci-Cerinic M, Furst DE. The EUSTAR model for teaching and implementing the modified Rodnan skin score in systemic sclerosis. Ann Rheum Dis. 2007;66:966–9. doi: 10.1136/ard.2006.066530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangan GK, Tesch GH. Quantification of renal pathology by image analysis. Nephrology. 2007;12:553–8. doi: 10.1111/j.1440-1797.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 35.Scopa CD, Vagianos C, Kardamakis D, Kourelis TG, Kalofonos HP, Tsamandas AC. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with rectal cancer. Appl Immunohistochem Mol Morphol. 2001;9:329–34. doi: 10.1097/00129039-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Khanna D, Furst DE, Clements PJ, et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol. 2005;32:832–40. [PubMed] [Google Scholar]
- 37.Poole JL, Steen VD. The use of the Health Assessment Questionnaire (HAQ) to determine physical disability in systemic sclerosis. Arthritis Care Res. 1991;4:27–31. doi: 10.1002/art.1790040106. [DOI] [PubMed] [Google Scholar]
- 38.Redelmeier DA, Lorig K. Assessing the clinical importance of symptomatic improvements. An illustration in rheumatology. Arch Intern Med. 1993;153:1337–42. [PubMed] [Google Scholar]
- 39.Nash RA, McSweeney PA, Crofford LJ, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood. 2007;110:1388–96. doi: 10.1182/blood-2007-02-072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wollheim FA. Is rituximab a potential new therapy in systemic sclerosis?: New evidence indicates the presence of CD20-positive B-lymphocytes in scleroderma skin. J Clin Rheumatol. 2004;10:155. doi: 10.1097/01.rhu.0000129090.86550.1e. [DOI] [PubMed] [Google Scholar]
- 41.Baroni SS, Santillo M, Bevilacqua F, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–76. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 42.Sfikakis PP, Boletis JN, Lionaki S, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52:501–13. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 43.Sfikakis PP, Souliotis VL, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123:66–73. doi: 10.1016/j.clim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Sakkas LI, Chikanza IC, Platsoucas CD. Mechanisms of disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:679–85. doi: 10.1038/ncprheum0346. [DOI] [PubMed] [Google Scholar]
- 45.Sakkas LI, Platsoucas CD. Is systemic sclerosis an antigen-driven T cell disease? Arthritis Rheum. 2004;50:1721–33. doi: 10.1002/art.20315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.