Abstract
The blood-testis barrier (BTB) is one of the tightest blood-tissue barriers in mammals. As such, it poses a challenge to deliver any drugs to the seminiferous epithelium of the testis, such as a nonhormonal male contraceptive. To circumvent this problem, a genetically engineered follicle-stimulating hormone (FSH) mutant protein was produced in Spodoptera furgiperda (Sf)-9 insect cells to serve as a testis-specific carrier. Subsequently, a 22-amino acid peptide corresponding to the second extracellular loop of occludin, which was known to disrupt BTB integrity in vivo, was inserted to the FSH mutant by polymerase chain reaction (PCR), as well as chemical cross-linking. This molecule was found to have negligible hormonal activity but was still capable of binding to FSH receptors, which are restricted to Sertoli cells in mammals. When this FSH mutant-occludin peptide conjugate was administered to adult rats at 40 µg/adult rat (~300 gm b.w.) via intraperitoneally (i.p.) injection, it induced transient and reversible disruption of the BTB, while at 150 µg/rat, it induced partial germ cell loss from the testis, particularly elongating/elongate spermatids. Most importantly, this effect was limited to the BTB without compromising the TJ-barrier integrity or cell adhesion in epithelia of other organs, such as kidney, liver, and small intestine. In summary, the use of an FSH mutant-occludin peptide conjugate is a feasible nanodevice to transiently compromise the BTB.—Wong, C.-H., Mruk, D. D., Lee, W. M., Cheng, C. Y. Targeted and reversible disruption of the blood-testis barrier by an FSH mutant-occludin peptide conjugate.
Keywords: spermatogenesis, tight junction, adherens junction, ectoplasmic specialization, serfoli-germ cell interactions, male contraception
The blood-testis barrier (BTB) in mammals is a functional definition that refers to the specialized junction structures found between Sertoli cells in the seminiferous epithelium, located closely to the basement membrane (1–3). Unlike blood-brain and blood-retina barriers which are composed of TJ between the capillary endothelial cells, the BTB is constituted by coexisting TJ, actin-based adherens junctions (AJ, e.g., basal ectoplasmic specialization) and intermediate-filament-based desmosome-like junctions (for reviews, see 3, 4). The BTB also divides the seminiferous epithelium into the basal and the apical compartment and is crucial for spermatogenesis by limiting the access of hormones, electrolytes, nutrients and other biological substances to the developing germ cells from the systemic circulation (for reviews, see 1, 2, 5). It also forms an immunological barrier to segregate postmeiotic germ cell antigens from the host immune system, creating a unique environment for germ cell development (for reviews, see 3, 6, 7). However, this barrier also creates a natural obstacle for delivering substances across the BTB, including contraceptives. If a delivery vehicle can be developed to reversibly and transiently disrupt the BTB, it will become a valuable tool to target contraceptives behind the BTB to disrupt spermatogenesis.
A previous study has demonstrated that a 44-amino acid synthetic peptide corresponding to the second extracellular loop of chicken occludin was capable of perturbing the TJ-permeability barrier and reducing endogenous occludin production when added to kidney cells cultured in vitro (8). This observation was reproduced in primary cultures of Sertoli cells. A 22-amino acid peptide (NH2-GSQIYTICSQFYTPGGTG-LYVD-COOH) (occludin peptide), which corresponded to residues 209–230 in the second extracellular loop of rat occludin, indeed, reversibly perturbed the Sertoli cell TJ-barrier (9). Most importantly, when administered directly to testes of adult rats via intrates-ticular injection, this peptide reversibly disrupted the BTB in vivo (9). On the basis of these observations, it is plausible that if this peptide can be conjugated to a delivery vehicle targeted to the testis, it is an excellent candidate to transiently open the BTB for drug delivery without compromising other epithelial barriers.
Herein, we report the use of a deglycosylated mutant of FSH as a testis-specific vehicle for this 22-amino acid occludin peptide. FSH receptors are limited to Sertoli cells in mammals (10–12). It was also reported that deglycosylated FSH possessed relatively little hormonal activity is still but capable of binding onto its receptors (for a review, see 13). Theoretically, this FSH mutant (ΔFSH) can serve as a specific carrier for the occludin peptide via the blood circulation to the testis, where FSH can bind onto its receptor and brings the peptide to close proximity to the BTB. As such, the occludin peptide can induce a short-term BTB disruption and provide a window for drug delivery. To test this hypothesis, the occludin peptide was conjugated to the FSH mutant by genetic engineering and/or chemical cross-linking techniques, and its effects on the BTB and other TJ barriers were examined.
MATERIALS AND METHODS
Animals
Sprague-Dawley rats (outbreds) were purchased from Charles River Laboratories (Kingston, NY, USA). The use of animals reported herein was approved by The Rockefeller University Animal Care and Use Committee with Protocol Numbers 97113, 00111, 03017, and 06018.
Preparation of recombinant FSH mutant-occludin peptide (ΔFSH-occludin) conjugate
The ΔFSH-occludin conjugate was prepared by PCR as detailed in Supplementary Method 1. Additional occludin peptides were chemically conjugated to the N terminus of each of the two subunits of the ΔFSH-conjugate, which was performed at SoluLink (San Diego, CA, USA). In brief, ΔFSH (at a concentration of ~2 mg/ml) was modified with succinimidyl 4-formylbenzoate (SFB) at pH 7.2 with 10 equivalents of SFB to ΔFSH to yield aldehyde group at the N-terminus, the sample was desalted and the aldehydes were quantified (step 1, see Fig. 1). The 22-amino acid synthetic occludin peptide was reacted with SANH [acetone 5-(succinimidyloxycarbonyl)-pyridine-2-ylhydrazone] (Merck Biosciences, Darmstadt, Germany) to generate hydrazine group at its N terminus, and the sample was desalted, and hydrazines were quantified (step 2). The SFB-modified ΔFSH was then reacted with the activated occludin peptide in a Conjugation Buffer (0.1 M sodium phosphate, 0.15M NaCl, pH 6 at 22°C) at room temperature overnight (see Fig. 1). Unreacted hydrazine or aldehyde groups were then capped by 2-sulfobenzal-dehyde, and the conjugate was isolated by gel filtration chromatography. It is noted that SFB is a heterobifunctional cross-linker in which its N-hydroxysuccinimide ester (NHS-ester) can react with the amine-containing Lys residues on ΔFSH-peptide conjugate besides the N-terminal amino-groups, to yield additional free aldehydes. The aldehyde can subsequently react with the hydrazine at the occludin peptide to form the stable hydrazone conjugate. Thus, the mass of occludin peptide in the ΔFSH conjugate as shown in Table 1 may be an underestimate.
Figure 1.
A schematic illustration for the conjugation of additional 22-amino acid occludin peptide to the N terminus of ΔFSH. ΔFSH was initially modified with SFB (succinimidyl 4-formylbenzoate) to incorporate benzaldehyde moieties to its N-terminus, and likely to the free amino group-containing Lys residues. Then, N-terminal-hydrazido-terephthlate-modified occludin peptides were added to the activated ΔFSH, forming stable ΔFSH-occludin conjugates via the hydrazone linkage.
TABLE 1.
Effects of different doses of ΔFSH-occludin conjugate on the BTB integrity and germ cell loss from the seminiferous epithelium in adult Sprague-Dawley rats
| Treatment | ΔFSH-occludin conjugate* (protein/rat) |
FSH (Mr 40 kDa) (Mr ~2200) |
Occludin Peptide** | Effects on BTB*** |
Effects on germ cell loss*** |
|---|---|---|---|---|---|
| Regimen 1 | 40 µg | 1 nmol (40 µg) | 3 nmol (~6.6 µg) | + | ns |
| Regimen 2 | 150 µg | 3.5 nmol (140 µg) | 10.5 nmol (~20 µg) | + | + |
The protein concentration was estimated by Coomassie blue dye-binding assay using BSA as a standard and was administered to each rat (~300 gm b.w.) via i.p. (n=3 rats).
The mass of occludin containing in the FSH mutant-occludin peptide conjugate was estimated based on the stoichiometric ratio of FSH mutant:occludin peptide in the conjugate at ~1:3 (see Fig. 1). It is noted that the mass of occludin in the conjugate may be an underestimate since succinimidyl 4-formylbenzoate (SFB) also reacted with other primary amines found in Lys in addition to the N-terminus of the α and β subunit, yielding additional aldehyde groups that reacted with the hydrazine functional groups in the 22-amino acid peptide, forming the stable hydrazone conjugate (see Fig. 1).
This scoring is based on results of electron microscopy and fluorescent microscopy studies to assess BTB integrity, and/or histological analysis (to assess effects on germ cell loss) on rats sacrificed by week 9 after treatment. In Regimen 1, <10% of the seminiferous tubules were affected having tubules with signs of germ cell loss from the epithelium; in Regimen 2, ~30% of the tubules were affected having tubules with visible germ cell loss (e.g., elongating and round spermatids, and spermatocytes found in tubule lumen (see Fig. 5). About 600 tubules were examined and scored from three rats. ns, not statistically significant; +, detectable germ cell loss.
Measurements of intracellular levels of cAMP
The intrinsic biological activity of the ΔFSH-occludin conjugate was assessed by its ability to induce the production of cAMP in cultured rat Sertoli cells vs. native FSH. In short, Sertoli cells were isolated from 20-day-old rat testes and cultured in F12/Dulbecco’s modified Eagle medium as described (14). Cells were plated on Matrigel (BD Biosciences)-coated 24-well plates at a cell density of 0.5 × 106 cells/cm2. About 1-day after cell isolation and plating on dishes, ΔFSH-occludin conjugate and native rat FSH were added to Sertoli cells at a concentration of 100 ng or 500 ng/ml culture medium. Cells were terminated and lysed in 0.1M HCl after a 10-h incubation. The levels of cAMP in these lysates were quantified by using an ELISA kit (BIOMOL, Plymouth Meeting, PA, USA), according to the manufacturer’s protocol. Data were analyzed using an ALL-FIT computer program (GRAFIT, Version 3.0). The inter- and intra-assay coefficients of variation (CVs) of the cAMP enzyme immunoassay (EIA) were estimated to be 8% and 12%, respectively.
FSH receptor binding of the ΔFSH-conjugate vs. native FSH
Sertoli cells isolated from 20-day-old rat testes were plated on 100-mm dishes for 4 days in F12/Dulbecco’s modified Eagle medium and incubated at 35°C in a humidified atmosphere of 95% air/5% CO2, as described above. Cells were scrapped on day 5. Rat FSH (1 µg) obtained from the National Hormone and Peptide Program (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD) was iodinated using Iodogen (Pierce) with [125I]-Na (Amersham Biosciences, Piscataway, NJ) (15). About 1 × 106 cpm [125I]-rat FSH was then incubated with ~4 × 106 Sertoli cells suspended in F12/Dulbecco’s modified Eagle medium with and without native rat FSH or ΔFSH-occludin conjugate (1 µg/sample) in a final sample vol of 500 µl (in triplicates) at 35°C for 1.5 h. Thereafter, cells were pelleted (800 g for 10 min), supernatant was aspirated, and the radioactivity in cells were determined using a gamma counter.
Administration of ΔFSH-occludin conjugate and sample preparation
ΔFSH-occludin conjugate was suspended in 200 µl of sterile saline, which was administrated to adult male rats (~300 gm b.w., n=3) by i.p. injection using 27-gauge needles. Rats without any treatment or injected with 40 µg native rat FSH for each animal via i.p. served as negative controls. In selected experiments, another set of controls in which rats received the FSH mutant protein without the peptide conjugate were included (n=2). Rats were sacrificed at specified time points by CO2 asphyxiation. Testes, liver, kidney, and small intestines were removed, frozen immediately in liquid N2 and stored at −80°C until use. In selected experiments, organs were also fixed in Bouin’s fixative and processed for paraffin-embedded sections.
Immunoblotting
Immunoblot analyses were performed as described (16, 17) using testis lysates prepared in a modified radio-immunoprecipitation assay (RIPA) buffer [50 mM Tris-HCl, pH 7.4 at 22°C containing 150 mM NaCl, 1 mM EGTA, 1% Nonidet P-40 (v/v), 0.25% Na-deoxycholate (v/v), 1 mM PMSF, 1 mM sodium orthovanadate, leupeptin (1 µg/ml) and aprotinin (1 µg/ml)]. An equal amount of proteins from lysates (~100 µg) was resolved by SDS-PAGE under reducing or nonreducing conditions. Proteins were electroblotted onto nitrocellulose membranes and probed with corresponding antibodies (Supplemental Material 1).
Immunofluorescence and electron microscopy
Frozen sections of rat testes, liver, kidney, and small intestine (~8 µm thickness) were fixed in ice-cold methanol and nonspecific binding sites were blocked with 10% normal goat serum. Sections were then incubated with a rabbit anti-occludin antibody (Ab) (1:100) following by a goat anti-rabbit-IgG-Cy-3. Testis sections were also incubated with a mouse anti-ZO-1 IgG-FITC (1:100). Sections were then washed, mounted in Vectashield Hardset with 4′,6′-diamidino-2-phenylidole (DAPI) (Vector Lab, Burlingame, CA), and examined by a fluorescent microscope. Electron microscopy (EM) was performed essentially as earlier described (16–18).
Assessing BTB integrity by a functional in vivo assay
The BTB integrity in rats treated with ΔFSH-occludin conjugate using Regimen 1 (see Table 1) at 40 µg protein/rat via i.p. vs. controls was monitored by a functional assay. In short, it assessed the ability of the BTB to block the diffusion of FITC-inulin (Mr 4,600) (Sigma) from the systemic circulation administered via the jugular vein in adult rats to the adluminal compartment of the seminiferous epithelium. Experimental groups of rats (n=2 per time point in each treatment group) (~270–300 g b.w.) include animals receiving ΔFSH-occludin conjugate treatment for 3 and 9 wk; control groups include normal rats (negative control), rats treated with ΔFSH without the occludin peptide at 3-week posttreatment (negative control), and rats treated with CdCl2 (3 mg/kg b.w.) at 5-day posttreatment, which is known to disrupt the BTB (16) (positive control). Rats were placed under anesthesia by ketamine HCl (60 mg/kg b.w., intramuscularly (i.m.)) with xylazine (3 mg/kg b.w., i.m.) that served as an analgesic. Hair was shaved at the surgical site over the jugular vein, cleaned with 70% ethanol, and an incision of ~0.8-cm was made to expose the jugular vein. FITC-inulin dissolved in PBS (1 mg/200 µl) was administered gently via the jugular vein using an insulin syringe with a 28-gauge 0.1-inch needle. After the fluorescent marker was administered, the wound was closed with a wound clip, and rats were allowed to recover. About 90 min later, rats were sacrificed by CO2 asphyxiation, and their testes were removed and frozen immediately in liquid nitrogen. Sections (~10 µm) were cut in a cryostat at −20°C, and all samples within an experimental group were placed on poly-L-lysine coated slide, air-dried, and examined under an Olympus BX40 microscope equipped with UPlanF1 fluorescent optics and an Olympus DP70 Digital Camera. Fluorescent micrographs were acquired using QImaging QCapture Software Suite (Version 2.65) (Quantitative Imaging Corp., Burnaby, BC, Canada) and printed using a Canon i9900. The distance traveled by the fluorescence probe vs. the radius in each tubule was measured and calculated. For tubules from oblique sections, the mean of the longest and shortest axis served as the radii. A total of 100 tubules were scored from each testis, and two testes from both rats were scored (a total of 200 tubules).
Assessment of antisperm Ab titer in serum samples by ELISA
The antisperm Ab titer in rat serum samples were estimated by ELISA. Positive sera were obtained from female rats (~250 gm b.w., n=2) immunized via subcutaneously (s.c.) injection with germ cell lysates. Total germ cells were isolated from adult male rats as described previously (19), and lysates were obtained by sonication using 20 × 106 total germ cells/ml PBS containing 0.1% Triton X-100 and 2 mM EDTA. ELISA was performed essentially as earlier described (20). Following color development using p-Npp, an alkaline phosphatase (AP)-specific substrate, reaction was stopped by adding 1M NaOH, and absorbance was read at 405 nm. The inter- and intra-assay CVs of this assay were estimated to be 9% and 14%, respectively.
Statistical analysis
Statistical analyses were performed by ANOVA with Tukey’s honestly significant difference (HSD) tests or Student’s t tests using the GB-STAT Statistical Analysis Software Package (Version 7.0; Dynamic Microsystems, Silver Spring, MD).
RESULTS
Production of the ΔFSH-occludin conjugate
To produce a FSH mutant without hormonal activity (ΔFSH), mutagenic PCR was performed to introduce mutations and deletions at the corresponding glycosylation sites on the α and β subunits of FSH, respectively (see Supplemental Table 1). The signal peptide on each subunit was also deleted so that the recombinant protein would not be secreted by the transfected Sf-9 cells. This also retained the glutathione S-transferase (GST)-tag that fused to the N terminus of the recombinant protein to permit its subsequent purification by affinity chromatography. The GST-tag was then removed by thrombin cleavage. To produce the ΔFSH-occludin peptide conjugate, two approaches were applied. First, a single occludin peptide was genetically engineered to the 3′ end of the ΔFSH α-subunit construct by PCR (see Suppl. Table 2), interspaced by a linker of KAKAK (see Suppl. Table 2) to minimize steric hindrance. Second, another occludin peptide was covalently conjugated to both ΔFSH subunits via a hydrazone linkage (see Fig. 1). As such, each ΔFSH molecule carried at least three occludin peptides. In selected experiments, only a single occludin peptide was used without the chemical conjugation, which yielded similar effects as of the conjugate having about three peptides per ΔFSH mutant protein.
The mutation and deletion introduced to ΔFSH and the presence of occludin peptide in the construct were confirmed by DNA sequencing. The authenticity of the ΔFSH-occludin conjugate produced by Sf-9 cells was also examined by immunoblot analysis. A polyclonal antibody (pAb) that recognized native rat FSH obtained from the National Hormone and Peptide Program (NIDDK, NIH) was used to identify the α- and β-subunit of the ΔFSH (Fig. 2A). However, the affinity of this Ab to ΔFSH apparently was weaker than the native FSH when the same amount of protein (~50 ng) was used for analysis (see Fig. 2A), possibly as a result of the deglycosylation. The mutant also displayed a smaller electrophoretic mobility vs. native FSH plausibly for the same reason (Fig. 2A). After purifying the FSH mutant from Sf-9 cell lysates by affinity chromatography using Glutathione-Sepharose, the purity of the mutant protein was verified by silver staining on an SDS-polyacrylamide gel under reducing conditions (Fig. 2B). Two prominent bands, corresponding to the α and β subunits of FSH, were identified (Fig. 2B). This study also illustrated the purity of the recombinant mutant protein, which was used for all subsequent experiments. A total of five different batches were used for all the experiments described in this report, including some preliminary dosing experiments during the past four years.
Figure 2.
A study to assess the immunoreactivity and identity of the FSH mutant protein to be used for preparing ΔFSH-occludin conjugate. A) After purification and thrombin cleavage, the recombinant ΔFSH protein was resolved onto 8% T SDS-polyacrylamide gels under nonreducing conditions and processed for immunoblotting using an Ab against native rat FSH obtained from the National Hormone and Peptide Program (NIDDK, NIH). Equal amount of native and recombinant rat FSH mutant protein (~50 ng) was loaded onto the same gel as a positive control. Note that the mutant protein appears to have a smaller MW than the native protein, which is a result of deglycosylation. B) The purity of the ΔFSH mutant protein was visualized by silver staining of proteins in a 12.5% T SDS-polyacrylamide gel under reducing conditions. D, dye-front.
Hormonal and receptor binding activity of the ΔFSH-occludin conjugate vs. native rat FSH
Hormonal activity of the ΔFSH protein vs. native FSH was estimated by an in vitro assay, based on the ability of FSH to induce cAMP production in cultured Sertoli cells by stimulating adenylate cyclase (for a review, see 21). It was shown that native FSH, but not the ΔFSH-occludin conjugate, significantly stimulated the production of cAMP from Sertoli cells dose dependently, consistent with its intrinsic hormonal activity (Fig. 3A). However, the ΔFSH-occludin conjugate competed equally well with native rat FSH to bind onto FSH receptors residing in Sertoli cells (Fig. 3B) as FSH receptors are restricted to Sertoli cells (10, 11). This also illustrates that the ΔFSH-occludin conjugate did not possess any hormonal activity of native FSH.
Figure 3.
A study to assess the hormonal and receptor activity of the ΔFSH-occludin conjugate vs. native FSH in vitro. A) Different concentrations of ΔFSH-occludin conjugate (FSH-ocln conjugate) and native recombinant rat FSH were added to cultured Sertoli cells, and their abilities to induce the production of cAMP in the cells were measured by immunoassays as described in Materials and Methods. Cultures without any addition of FSH served as controls. B) Competition experiments showing native rat FSH and FSH-occludin conjugate competed equally well to the binding of [125I]-FSH to Sertoli cells obtained from 20-day-old rat testes. Each bar is the mean ± sd of 3 determinations from one experiment. Two additional experiments using different batches of cells yielded similar results. Student’s t test was performed to compare results between treatment groups and controls. ns, not significantly different; *Significantly different, P < 0.05; **significantly different, P < 0.01.
Effects of the ΔFSH-occludin conjugate on the BTB integrity
Previous studies have shown an acute and local administration of occludin peptide to rat testes at 2–10 mg/testis, a transient disruption of the BTB was induced (9). To assess whether the ΔFSH-occludin conjugate (at 40 µg/rat i.p.) (Table 1) was indeed delivered to the testis after i.p. administration, several BTB-associated structural proteins were examined by immunoblottings and immunofluorescent microscopy (Fig. 4, Fig 5). Immunoblot analyses showed that after rats were administered with the ΔFSH-occludin conjugate, a significant reduction in occludin protein level was detected by 3 wk after treatment (Fig. 4A, B). By 6 wk and thereafter, the occludin protein level was restored to its basal level (Fig. 4A, B). However, other structural components of the BTB, such as JAM-A, ZO-1 (Fig. 4A, B), N-cadherin and β-catenin (Fig. 4A, C) (3–5) were largely unaffected, illustrating occludin was the only target of the ΔFSH-occludin conjugate at the BTB (Fig. 4A, B, C). These results are consistent with the earlier in vitro and in vivo observations via direct administration of the occludin peptide to Sertoli cell cultures or testes (9). Also, these data have illustrated that ΔFSH is a functional carrier that targets this occludin peptide specifically to the testis. This conclusion was further supported by the fluorescent microscopy study colocalizing occludin and ZO-1 to the seminiferous epithelium of treated rats (Fig. 5A–L) using the regimens shown in Table 1. In normal rat testes, immunoreactive occludin and ZO-1 coexisted at the basal compartment of the epithelium, consistent with their localization at the BTB (Fig. 5A–D). However, 3-wk after the ΔFSH-occludin conjugate administration, the intensity of occludin, but not ZO-1, staining was greatly reduced (Fig. 5E–H). By 9 wk, the staining pattern and intensity of occludin in the epithelium bounced back to the level indistinguishable from normal rats, indicating that a recovery had occurred (Fig. 5I–L). These results were further validated by a study using EM that demonstrated dose-dependent ultrastructural changes at the BTB following treatments of rats with low (40 µg/rat) and high (150 µg/rat) doses (Supplemental Fig. 1).
Figure 4.
Effects of the administration of ΔFSH-occludin conjugate (40 µg/rat i.p.) on TJ-and AJ-associated proteins in the rat testis. Immunoblot analyses were performed using specific antibodies that recognized junction-associated structural proteins (A), which are known to be involved in junction-restructuring events in the testis. Testes from rats without any treatment (control, Ctrl) or rats received recombinant rat FSH mutant without the peptide conjugate (FSH) (terminated at 3 wk) served as controls. The protein level of actin was also quantified to serve as a protein loading control. (B–C) These are the corresponding densitometrically scanned results using immunoblots, such as those shown in (A) for TJ-(B) and AJ-(C) associated proteins. The level of each protein in testes of rats without any treatment (Ctrl) was arbitrarily set at 1, against which ANOVA was performed. Each bar in (B–C) is the mean ± sd of 3 determinations from 3 rats. ns, not significantly different. *Significantly different, P < 0.05.
Figure 5.
Immunolocalization of occludin and ZO-1 in testes and the status of spermatogenesis in testes from rats administered with ΔFSH-occludin conjugate via at 40 µg/rat i.p. (A–P) or 150 µg/rat (Q–T). A–L) These are immunofluorescent micrographs of cross sections of normal rat testes (Ctrl, n=3, A–D), testes from rats receiving ΔFSH-occludin conjugate at 3 wk (E–H) and 9 wk (I–L) after the treatment. In another group of controls (n=2), rats received the same amount of ΔFSH without the occludin peptide and terminated on 3 and 9 wk, as this group of animals did not display any changes in the status of spermatogenesis, data were not shown herein. Occludin and ZO-1 appeared as red (Cy-3) and green fluorescence (FITC), respectively, near the basement membrane consistent with their localization at the BTB. Merged images in (C), (G), and (K) correspond to micrographs in which occludin and ZO-1 were colocalized as orange fluorescence. (D), (H) and (L) are the DAPI staining of the corresponding cross sections that indicate the relative location of the basement membrane. Scale Bar in (A) = 60 µm, which applies to (B–L). M–P) Hematoxylin/eosin-stained paraffin sections of testes from control (Ctrl) rats (M), rats terminated at 3 wk after treatment (3 wk) (N and O) and rats terminated at 9 wk after receiving treatment (9 wk) (P). The boxed area in (N) was magnified and shown in (O). Scale Bar in (M) = 50 µm, which applies to (N) and (P), (O) = 20 µm. The boxed area in N, (asterisk denotes seminiferous tubule with spermatocytes and spermatids found in the tubule lumen) was enlarged and shown in (O), illustrating in some tubules (~10%), germ cell loss from the epithelium was detected (see O). However, in rats received a higher dose of the ΔFSH-occludin conjugate at 150 µg/rat (see Table 1), virtually all the tubules by 3-wk were devoid of elongating/elongate spermatids (see R–T vs. Q, Q is a control normal testis) and that immature germ cells such as spermatocytes and round spermatids were found in the tubule lumen (see solid arrowheads in S and T; S is the magnified view of the boxed area shown in R). Scale Bar in (Q) = 25 µm, (R) = 80 µm, (S) = 12 µm, which applies to (T).
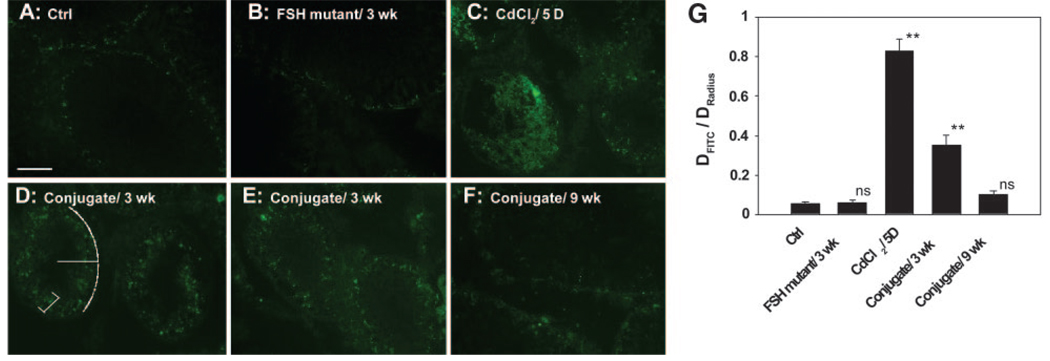
These results are consistent with data obtained from another study using a functional in vivo assay that monitored BTB integrity by assessing the ability of the BTB to block the movement of a fluorescent molecular probe (FITC-inulin, Mr 4,600) across the BTB from the systemic circulation (Fig. 6). For instance, it was shown that in normal rats and rats treated with ΔFSH only, the fluorescence was blocked from entering into the adluminal compartment and restricted to the BTB site near the basement membrane in virtually all tubules scored (Fig. 6A, B, G). However, in rats treated with CdCl2 at 3 mg/kg b.w. i.p., which is known to irreversibly disrupt the BTB integrity (16), fluorescence traveled to the lumen in most tubules (Fig. 6C vs. 6A, B). In rats treated with the ΔFSH-occludin conjugate at 40 µg protein/rat (see Regimen 1, Table 1), the BTB also became “leaky” since some fluorescence was found in the seminiferous epithelium beyond the BTB (see white bracket in Fig. 6D), moving away from the BTB site near the basement membrane (see white broken line) (Fig. 6D, E vs. 6A, B). When the BTB recovered by 9-wk, it was capable of blocking the fluorescence probe again limiting the fluorescence behind the BTB (Fig. 6F vs. 6A, B, D, E, G). In short, this functional assay further confirmed that the ΔFSH-occludin conjugate induced reversible damage to the BTB (Fig. 6G).
Figure 6.
A functional assay to assess BTB integrity following ΔFSH-occludin conjugate treatment. Rats were treated with ΔFSH-occludin conjugate at 40 µg/rat i.p. (see Regimen 1 in Table 1 and Materials and Methods) and used for this assay by 3 (D, E) and 9 (F) wk vs. control (Ctrl, normal rats) (A), rats treated with FSH mutant only and used by 3 wk (B), and rats treated with CdCl2 (2 mg/kg b.w.) and used by day 5 (positive control) (C). The BTB integrity was monitored by measuring the distance traveled by the fluorescent tracer (FITC-inulin, Mr 4,600) from the BTB (see white broken line in D) to the adluminal compartment (see white bracket in D) vs. the radius of the tubule (see white line in D). The ratios obtained from each sample group are shown in (G). Student’s t test was performed to compare results from each group and the ctrl group. ns, not significantly different; **Significantly different, P < 0.01.
Effects of the ΔFSH-occludin conjugate on germ cell adhesion in the epithelium
Because an acute administration of the 22-amino acid occludin peptide to the testis via intratesticular injection at 2–10 mg/testis was shown to trigger massive and reversible germ cell loss (9), testes obtained from rats treated with the regimen shown in Table 1 were also examined for this phenomenon (Fig. 5M–P). While direct and acute administration of the peptide caused germ cell detachment from the epithelium by ~4-wk (9), no significant germ cell loss was detected by 3- or 9-wk after conjugate administration using the low-dose regimen shown in Table 1, Regimen 1 (Fig. 5N–P vs. 5M) except ~10% of tubules examined by 3 wk (see Fig. 5N, O). This indicated that although the ΔFSH-occludin conjugate induced damages at the BTB, it did not trigger a secondary loss of germ cells from the epithelium, provided that only ~6.6 µg peptide (from 40 µg of the conjugate) (see Table 1) was administered to each rat. However, virtually no elongating/elongate spermatids were found in the seminiferous epithelium by 3 wk at a high dose (Regimen 2 at 150 µg/rat, i.e., ~20 µg occludin peptide/rat), and spermatocytes and round spermatids were also found in many of the tubules examined (Fig. 5R–T vs. 5Q). These results also illustrate the dose-dependent effects of this ΔFSH-occludin conjugate in the testis. It is important to note that while the ΔFSH-occludin conjugate is effective in perturbing the BTB and Sertoli-germ cell adhesion in the epithelium as shown in Fig. 5, it did not interfere with the TJ barrier or cell adhesion in other organs, such as kidney, liver, and small intestine using both Regimens (see Supplemental Fig. 2), illustrating the FSH mutant indeed specifically carried the occludin peptide to the testis to exert its disruptive effects.
Antisperm Ab level in rat serum after ΔFSH-occludin conjugate treatment
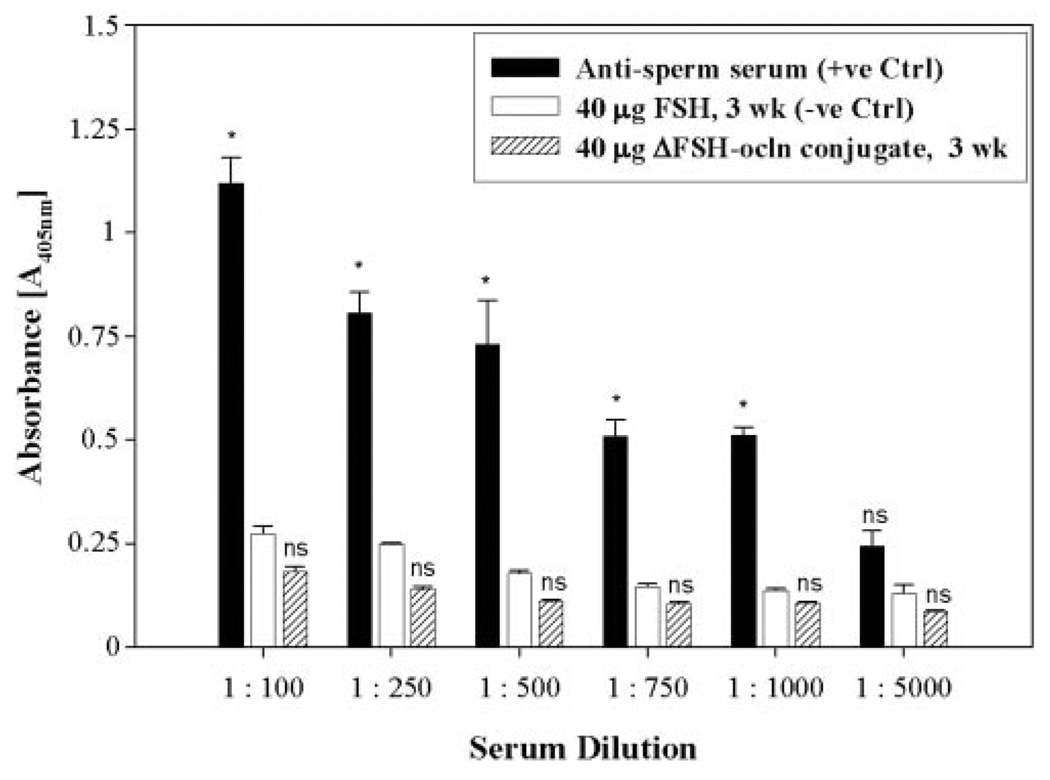
Transient damages to the BTB caused by the ΔFSH-occludin conjugate may trigger undesirable immune response against postmeiotic germ cells within the seminiferous epithelium. To examine this possibility, the level of antisperm Ab in rat serum after treatments was estimated by ELISA (Fig. 7). Sera from rats that were administered with the ΔFSH-occludin conjugate were similar to negative controls (normal rat sera) (Fig. 7). This illustrates that although the ΔFSH-occludin conjugate induced damages to the BTB, the transient effects did not result in any immune response against postmeiotic germ cells in the seminiferous epithelium behind the BTB.
Figure 7.
A study to estimate the antisperm Ab titer in serum samples after treatment of rats with the ΔFSH-occludin conjugate. Antisperm Ab titer was estimated in serum samples from treated rats by ELISA vs. controls (see Materials and Methods). Sera from two female rats that were previously immunized by male rat germ cell extracts served as positive controls, while sera from male rats administrated with native rat FSH served as negative controls. The assay was performed three times using different sets of samples, and results were shown herein as mean ± sd. Samples from the same experimental group were assayed simultaneously in a single session to eliminate interassay variations. Student’s t test was performed to compare results from treatment groups and negative controls. ns, not significantly different; * significantly different, P < 0.05.
DISCUSSION
The aim of this study was to examine whether ΔFSH is a feasible carrier to deliver a 22-amino acid peptide, which was designed based on the sequence of the second extracellular loop of occludin, to the testis and induce transient BTB disruption. Occludin is a transmembrane protein found in most TJ barriers in mammalian organs, including the testis (for reviews, see refs. 22 and 23). The two extracellular loops of occludin, which homotypically interact with those present at adjacent epithelial cells (24), are responsible for sealing the TJ. It was shown that peptides derived from the first loop disrupted adhesion in cell lines without TJ (25) but did not affect TJ assembly in a TJ-bearing cell line (8). In contrast, a peptide that contained a sequence from the second loop perturbed TJ integrity and reduced endogenous occludin production (8). These observations plausibly resulted from the interaction between the peptide and the extracellular domain of the endogenous occludin, which, in turn, compromised the interlocking structure that was necessary to maintain TJ integrity. The ability of this occludin peptide to suppress endogenous occludin production by Sertoli cells in the testis as reported herein is consistent with results of an earlier report using MDCK epithelial cells (8), even though the underlying physiological mechanism(s) is not immediately known. It may be that a surge of the occludin peptides in the microenvironment at the BTB that interact with intact occludins between adjacent Sertoli cells somehow either “shut-down” the de novo synthesis of occludin, or “enhances” endocytosis and intracellular degradation of occludin, or both. This thus contributes to a significant decline in the steady-state protein level of occludin. It is obvious that further studies should include an ultrastructural localization of occludin and/or FSH in testes from rats treated with the FSH mutant-occludin peptide conjugate. Additionally, overexpression of this occludin peptide using seminiferous epithelium cultured in vitro should be investigated since this will potentially yield similar findings as those shown herein.
Since occludin is abundantly found at the BTB in rats (24) and acute administration of this 22-amino acid peptide to rat testes by intratesticular injection indeed reversibly disrupted the BTB (9), we sought to target this peptide to the testis to disrupt the BTB as a mean to transport contraceptives specifically to the seminiferous epithelium. This is the rationale of the present study. In fact, there are studies in the literature based on studies of other epithelial barriers indicating that the TJ barrier function can be manipulated in vivo by interfering with junction proteins. For example, the permeability of rat intestine TJ barrier was increased by a short peptide derived from Clostridium perfringens enterotoxin via its ability to bind and inhibit claudin, another TJ-transmembrane molecule (26). In another study, an Ab that targets the endothelial barrier antigen, a blood-brain barrier (BBB)-associated membrane protein, also induced a transient opening of the BBB when administered to rats (27). However, because occludin is found in most TJ barriers in mammalian epithelia and/or endothelia, it is crucial to ensure that the occludin peptide is specifically targeted to the BTB. Therefore, an FSH mutant, which serves as a specific carrier for the occludin peptide, was developed as reported herein.
FSH is a pituitary-secreted glycoprotein that is critical for regulating reproductive function (for reviews, see 28, 29, 30). In males, FSH targets testes exclusively, where its receptor is predominantly found in Sertoli cells with very low abundance in germ cells (31, 32). As such, FSH is a perfect candidate as a testis-specific delivery vehicle. FSH is composed of two subunits, α and β, which noncovalently bind to each other to form a heterodimer. Both subunits contain two glycosylation sites (see Supplemental Table 1) that are crucial to the bioactivity and metabolic clearance rate of the hormone (for reviews, see refs. 13, 29, 33). For instance, the mutation at the first glycosylation site of the α-subunit (Asn-52 in humans) that leads to its deglycosylation can greatly reduce the bioactivity of FSH in vitro (34–36), whereas mutations in both glycosylation sites (Asn-52 and Asn-78 in humans) of this subunit further stripped the hormonal activity (35–37). Although in vitro studies investigating the effect of double-deglycosylation in the β-subunit (Asn-7 and Asn-24 in humans) were not conclusive because the mutant showed contradictory results in different assay systems (35, 36), it did reduce the hormonal potency in vivo (38). More important, it was reported that the receptor binding ability and the bioactivity of FSH were dissociated from each other, conferring by different stretches of amino acid sequences in the two subunits (34). Furthermore, deglycosylation of FSH usually resulted in an enhancement of receptor binding ability (34–36). As such, a FSH mutant that has low or virtually no bioactivity can still bind to its receptor. This is crucial for establishing a reliable delivery system because this mutant FSH must be capable of binding onto its receptor on the Sertoli cell surface. Moreover, it is known that deglycosylated FSH molecules have significantly shorter plasma half-life than the wild-type (WT) hormone (33, 38). As such, the mutant would not stay in the host systemic circulation for an exceedingly long period to interfere with normal FSH function. We thus sought to prepare a completely deglycosylated rat FSH mutant, which was produced by mutating the Asn residues 56 and 82 to Asp in the α subunit and by deleting the glycosylation site at amino acid residue 6 and residues 22–24 in the β subunit (see Supplemental Table 1). The resulting ΔFSH molecule indeed failed to induce the production of cAMP when added to cultured rat Sertoli cells, indicating that this mutant is a candidate testis-specific drug-delivery vehicle. To produce the ΔFSH-occludin conjugate, additional occludin peptides were linked to the N terminus of ΔFSH α and β subunits, and another occludin peptide was genetically engineered to the C-terminus of ΔFSHα but not to the C-terminus of ΔFSHβ-subunit. This is because previous studies have shown that the C-terminal region of the β subunit is crucial for receptor binding (39, 40).
To clinically serve as a delivery system, it is also critical that the ΔFSH-occludin conjugate only affects BTB transiently and reversibly. Furthermore, such disruption should not elicit any adverse immune response in host animals. To assess whether the ΔFSH-occludin conjugate was successfully delivered to the testis and targeted the BTB, the protein level of occludin in the testis was used as a marker. In a previous in vitro study, the expression of occludin in Sertoli cells was suppressed after treatment with the 22-amino acid synthetic occludin peptide (9). Herein, we also reported a reduction in endogenous occludin level at the BTB in vivo, indicating that the conjugate indeed suppressed occludin production at the BTB. This loss of occludin was transient, since such a reduction was detected at 3-wk after the treatment but not at 6 and 9 wk. The effect was specific because other junction-associated proteins were not affected as illustrated by immunoblottings and fluorescent microscopy. The conjugate also did not immediately affect the signaling transducers that regulate TJ and AJ dynamics (e.g., MAPKs), although changes in these molecules were detected at 9 wk after the treatment (data not shown). This suggests that these signaling pathways may be involved in the recovery process. These findings may also explain the transient damaging effect of the ΔFSH-occludin conjugate to the BTB since it only targets one structural component of the TJ strands. This postulate is supported by the lack of antisperm antibodies in treated rats. As such, no adverse immunological response was triggered, making this conjugate a suitable therapeutic agent.
As mentioned previously, occludin can also be found in many TJ barriers other than the BTB, the potential effects of the conjugate in other organs have been examined. These include liver and kidney, two organs where FSH is usually cleared from the systemic circulation (for a review, see ref. 28); and small intestine, which may have immediate contact with the ΔFSH-occludin conjugate when it is administered i.p. Immunofluorescent microscopy staining for occludin to examine the tight junction barrier and histological analysis revealed that the epithelium of these organs was not affected throughout the treatment period (see Supplemental Fig. 2), illustrating the conjugate was targeted to the BTB since FSH receptors are restricted to Sertoli cells in the testis (10–12).
As reported herein, it is obvious that this ΔFSH-occludin peptide conjugate fulfills the criteria as a specific carrier of therapeutic agent(s) to the testis. First, it only transiently perturbs the BTB, as illustrated in a functional test to assess BTB integrity. At a low dose, it does not even interfere with germ cell attachment in the seminiferous epithelium, in contrast to the acute administration. Second, it does not provoke the host immune system to generate antibodies against postmeiotic germ cell antigens, at least during the experimental period reported herein. Third, its damaging effect to the BTB is dose-dependent, as illustrated in an electron microscopic study and an in vivo functional test to assess BTB integrity. Fourth, it does not interfere with TJ structures other than that at the BTB. Nonetheless, the possibility of using this conjugate as a nanodevice to target contraceptives to the seminiferous epithelium behind the BTB must be vigorously investigated in future studies. For instance, it is not known whether repeated administrations of the conjugate to mice (or rats, humans) would elicit secondary immune response, causing orchitis.
In summary, it was shown that the administration of a ΔFSH-occludin conjugate to adult rats via i.p. induced reversible BTB damage, while at high dose it also affected germ cell adhesion in the seminiferous epithelium. Besides its possible application as a male contraceptive device, this conjugate can likely be used as a novel in vivo model to study BTB dynamics with adequate characterization.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the CONRAD Program (CICCR CIG 01–72 to C.Y.C.), National Institutes of Health (NICHD, U01 HD045908; U54 HD029990 Project 3; to C.Y.C.), and Hong Kong Research Grant Council (HKU 7536/OSM to W.M.L.).
REFERENCES
- 1.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol. Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 2.Setchell BP. The functional significance of the blood-testis barrier. J. Androl. 1980;1:3–10. [Google Scholar]
- 3.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 4.Wong CH, Cheng CY. The blood-testis barrier: Its biology, regulation and physiological role in spermatogenesis. Curr. Top. Dev. Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 6.Bart J, Groen HJM, van der Graaf WTA, Hollema H, Hendrikse NH, Vaalburg W, Sleijfer DT, de Vries EGE. An oncological view on the blood-testis barrier. Lancet. Oncol. 2002;3:357–363. doi: 10.1016/s1470-2045(02)00776-3. [DOI] [PubMed] [Google Scholar]
- 7.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin. Cell. Dev. Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 8.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol. 1997;136:399–408. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung NPY, Mruk D, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol. Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- 10.Walker W, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 11.Sprengel R, Braun T, Nikolics K, Segaloff D, Seeburg P. The testicular receptor for follicle stimulating hormone: Structure and functional expression of cloned cDNA. Mol. Endocrinol. 1990;4:525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- 12.Griswold M, Heckert L, Linder C. The molecular biology of the FSH receptor. J. Steroid. Biochem. Mol. Biol. 1995;53:215–218. doi: 10.1016/0960-0760(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 13.Ulloa-Aguirre A, Timossi C, Barrios-de-Tomasi J, Maldonado A, Nayudu P. Impact of carbohydrate heterogeneity in function of follicle-stimulating hormoneL studies derived from in vitro and in vivo models. Biol. Reprod. 2003;69:379–389. doi: 10.1095/biolreprod.103.016915. [DOI] [PubMed] [Google Scholar]
- 14.Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, α2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol. Cell. Endocrinol. 1992;89:127–140. doi: 10.1016/0303-7207(92)90219-v. [DOI] [PubMed] [Google Scholar]
- 15.Fraker P, Speck JJ. Protein and cell membrane iodination with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3α,6α-diphenylglycoluril. Biochem. Biophys. Res. Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 16.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of the blood-testis barrier dynamics in the testis: An in vivo study. J. Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 17.Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 18.Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 19.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J. Biol. Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 20.Silverstrini B, Guglielmotti A, Saso L, Milanese C, Melanitou E, Grima J, Cheng CY. Development of an enzyme-linked immunosorbent assay with a monoclonal antibody prepared against α1-antitrypsin for diagnostic screening of inflammatory disorders. Clin. Chem. 1990;36:277–282. [PubMed] [Google Scholar]
- 21.Means AR, Dedman JR, Tash JS, Tindall DJ, Sickle M, Welsh MJ. Regulation of the testis Sertoli cell by follicle stimulating hormone. Ann. Rev. Physiol. 1980;42:59–70. doi: 10.1146/annurev.ph.42.030180.000423. [DOI] [PubMed] [Google Scholar]
- 22.Balda MS, Matter K. Transmembrane proteins of tight junctions. Semin. Cell. Dev. Biol. 2000;11:281–289. doi: 10.1006/scdb.2000.0177. [DOI] [PubMed] [Google Scholar]
- 23.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 24.Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, Tsukita S. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am. J. Physiol. 1998;274:C1708–C1717. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- 25.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in firoblasts. J. Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- 26.Kondoh M, Masuyama A, Takahashi A, Asano N, Mizuguchi H, Koizumi N, Fujii M, Hayakawa T, Horiguchi Y, Watanbe Y. A novel strategy for the enhancement of drug absorption using a claudin modulator. Mol. Pharmacol. 2005;67:749–756. doi: 10.1124/mol.104.008375. [DOI] [PubMed] [Google Scholar]
- 27.Ghabriel MN, Lu JJ, Tadros R, Hermanis G. A narrow time-window for access to the brain by exogenous protein after immunological targeting of a blood-brain barrier antigen. J. Comp. Path. 2004;131:52–60. doi: 10.1016/j.jcpa.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Rose MP, Gaines Das RE, Balen AH. Definition and measurement of follicle stimulating hormone. Endocr. Rev. 2000;21:5–22. doi: 10.1210/edrv.21.1.0388. [DOI] [PubMed] [Google Scholar]
- 29.Dias JA, Lindau-Shepard B, Hauer C, Auger I. Human follicle-stimulating hormone structure-activity relationships. Biol. Reprod. 1998;58:1331–1336. doi: 10.1095/biolreprod58.6.1331. [DOI] [PubMed] [Google Scholar]
- 30.Sairam MR, Krishnamurthy H. The role of follicle-stimulating hormone in spermatogenesis: lessons from knockout animal models. Arch. Med. Res. 2001;32:601–608. doi: 10.1016/s0188-4409(01)00328-9. [DOI] [PubMed] [Google Scholar]
- 31.Baccetti B, Collodel G, Costantino-Ceccarini E, Eshkol A, Gambera L, Moretti E, Strazza M, Piomboni P. Localization of human follicle-stimulating hormone in the testis. FASEB J. 1998;12:1045–1054. doi: 10.1096/fasebj.12.11.1045. [DOI] [PubMed] [Google Scholar]
- 32.Wahlstrom T, Huhtaniemi I, Hoatta O, Seppala M. Localization of luteinizing hormone, follicle-stimulating hormone, prolactin, and their receptors in human and rat testis using immunohistochemistry and radioreceptor assay. J. Clin. Endocrinol. Metab. 1983;57:825–830. doi: 10.1210/jcem-57-4-825. [DOI] [PubMed] [Google Scholar]
- 33.Ulloa-Aguirre A, Timossi C, Damian-Matsumura P, Dias JA. Role of glycosylation in function of follicle-stimulating hormone. Endocrine. 1999;11:205–215. doi: 10.1385/ENDO:11:3:205. [DOI] [PubMed] [Google Scholar]
- 34.Valove FM, Finch C, Anasti JN, Froehlich J, Flack MR. Receptor binding and signal transduction are dissociable functions requiring different sites on follicle-stimulating hormone. Endocrinology. 1994;135:2657–2661. doi: 10.1210/endo.135.6.7988456. [DOI] [PubMed] [Google Scholar]
- 35.Flack MR, Froehlich J, Bennet AP, Anasti J, Nisula BC. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J. Biol. Chem. 1994;269:14015–14020. [PubMed] [Google Scholar]
- 36.Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol. Endocrinol. 1994;8:722–731. doi: 10.1210/mend.8.6.7935488. [DOI] [PubMed] [Google Scholar]
- 37.Keene JL, Matzuk MM, Otani T, Fauser BCJM, Galway AB, Hsueh AJW, Boime I. Expression of biologically active human follitropin in Chinese hamster ovary cells. J. Biol. Chem. 1989;264:4769–4775. [PubMed] [Google Scholar]
- 38.Bishop LA, Nguyen TV, Schofield PR. Both of the β-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and in vivo potency. Endocrinology. 1995;136:2635–2640. doi: 10.1210/endo.136.6.7750487. [DOI] [PubMed] [Google Scholar]
- 39.Santa Coloma TA, Reichert LE. Identification of a follicle-stimulating hormone receptor-binding region in hFSH-β-(81–95) using synthetic peptides. J. Biol. Chem. 1990;265:5037–5042. [PubMed] [Google Scholar]
- 40.Lindau-Shepard B, Roth KE, Dias JA. Identification of amino acids in the C-terminal region of human follicle-stimulating hormone (FSH) β-subunit involved in binding to human FSH receptor. Endocrinology. 1994;135:1235–1240. doi: 10.1210/endo.135.3.8070368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.