Abstract
Rottlerin is a polyphenolic compound derived from Mallotus philipinensis. In the present study, we show that rottlerin decreased tumor size and stimulated apoptosis in an orthotopic model of pancreatic cancer with no effect on normal tissues in vivo. Rottlerin also induced apoptosis in pancreatic cancer (PaCa) cell lines by interacting with mitochondria and stimulating cytochrome c release. Immunoprecipitation results indicated that rottlerin disrupts complexes of prosurvival Bcl-xL with Bim and Puma. Furthermore, siRNA knockdown showed that Bim and Puma are necessary for rottlerin to stimulate apoptosis. We also showed that rottlerin and Bcl-2 and Bcl-xL inhibitor BH3I-2′ stimulate apoptosis through a common mechanism. They both directly interact with mitochondria, causing increased cytochrome c release and mitochondrial depolarization, and both decrease sequestration of BH3-only proteins by Bcl-xL. However, the effects of rottlerin and BH3I-2′ on the complex formation between Bcl-xL and BH3-only proteins are different. BH3I-2′ disrupts complexes of Bcl-xL with Bad but not with Bim or Puma, whereas rottlerin had no effect on the Bcl-xL interaction with Bad. Also BH3I-2′, but not rottlerin, required Bad to stimulate apoptosis. In conclusion, our results demonstrate that rottlerin has a potent proapoptotic and antitumor activity in pancreatic cancer, which is mediated by disrupting the interaction between prosurvival Bcl-2 proteins and proapoptotic BH3-only proteins. Thus rottlerin represents a promising novel agent for pancreatic cancer treatment.
Keywords: phytochemical, polyphone, mitochondria, proapoptotic protein
pancreatic adenocarcinoma is an aggressive malignancy and is resistant to chemo- and radiotherapy (7, 38). One mechanism mediating pancreatic cancer aggressiveness and unresponsiveness to treatment is its resistance to apoptosis. Finding approaches to stimulate the death-signaling mechanisms is critical for the development of effective therapeutic strategies in pancreatic cancer.
Rottlerin is a polyphenolic compound derived from Mallotus philipinensis (Euphorbiaceae) (15). Rottlerin is widely used as an inhibitor of the δ-isoform of protein kinase C (15). Another cellular target of rottlerin is mitochondria. Rottlerin was shown to cause uncoupling of mitochondrial respiration from oxidative phosphorylation and a collapse of mitochondrial membrane potential (ΔΨm) in several cell types (8). Recently, rottlerin was shown to stimulate apoptosis in various cancer cells, including colon (40) and lung (6) cancer cells, chronic leukemia, and multiple myeloma cells (31, 34). The mechanisms whereby rottlerin stimulates apoptosis have not yet been elucidated.
The key signaling event in apoptosis pathway is the release of mitochondrial cytochrome c into the cytosol, which is usually accompanied by a decrease in ΔΨm. Once in the cytosol, cytochrome c activates caspases, leading to apoptosis. Proteins of the Bcl-2 family are major regulators of cytochrome c release. On the basis of their function and structure, Bcl-2 proteins can be divided into three groups, namely proapoptotic proteins containing three homologous BH domains (BH1-BH3), proapoptotic proteins containing BH3 domain only, and prosurvival proteins containing four BH domains (BH1-BH4). Proapoptotic multidomain Bax and Bak form channels in the outer mitochondrial membrane through which mitochondrial cytochrome c is released into the cytosol (25, 29). BH3-only proteins, such as Bad, Bim, and Puma, activate Bax/Bak channels (1, 24, 46). Oppositely, prosurvival Bcl-2 proteins, such as Bcl-xL and Bcl-2, bind to and sequester Bad, Bim, and Puma, preventing them from activating Bax/Bak channels (1, 24, 28, 46).
During the past several years, small-molecule pharmacological inhibitors of prosurvival Bcl-2/Bcl-xL have been developed and shown to stimulate apoptosis in cancer cells, demonstrating prosurvival Bcl-2 proteins as promising therapeutic targets in cancer treatment. At the same time, there is a growing interest in testing the therapeutic potential and studying the mechanisms of action of natural phytochemical compounds as anticancer drugs (3, 21, 27).
The present study shows that rottlerin inhibits tumor growth in orthotopic models of pancreatic cancer and stimulates apoptosis both in vivo in the pancreatic tumor and in vitro in pancreatic cancer (PaCa) cells. BH3-only proteins Bim and Puma are required for rottlerin to stimulate apoptosis. Rottlerin prevents sequestration of Bim and Puma by Bcl-xL, resulting in cytochrome c release, loss of ΔΨm, and PaCa cell death. Rottlerin and the Bcl-2/Bcl-xL inhibitor, BH3I-2′, activate a common signaling pathway in PaCa cells by preventing sequestration of BH3-only proteins by Bcl-xL, thus stimulating apoptosis.
MATERIALS AND METHODS
Materials.
Antibodies against caspase-3 caspase-9, PKC-δ, and Bcl-2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); anti-Bcl-xL, Puma, Bim, and Bad, total Akt, and phospho473S-Akt antibodies were from Cell Signaling Technology (Denver, MA); anti-cytochrome c antibody was from BD Pharmingen (San Diego, CA); anti-cytochrome c oxidase subunit IV antibody was from Molecular Probes (Carlsbad, CA); rottlerin was from Sigma (St. Louis, MO); BH3I-2′ and PKC-δ peptide substrate were from Calbiochem (San Diego, CA). PKC assay kit was purchased from Upstate Biotechnology (Lake Placid, NY). All other reagents were purchased from Sigma. PKC inhibitors GF-109203X and Ro-32-0432 were from Calbiochem. A specific PKC-δ translocation inhibitor (δV1-1: S-F-N-S-Y-E-L-G-S-L) was synthesized as we described previously (35).
Pancreatic cancer animal models.
Animal studies were approved by the Chancellor's Animal Research Committee of the University of California, Los Angeles in accordance with the NIH Guide for the Care and Use of Laboratory Animals. One model used was the subcutaneous model. Pancreatic cancer cells (MIA PaCa-2, 2 × 106) were subcutaneously injected into the flanks of nude mice. After the tumor reached a size of ∼2 × 2 mm, animals were randomly allocated to receive either control vehicle (n = 6) or rottlerin (0.5 mg/kg, n = 6). Drugs were prepared fresh each day and injected intraperitoneally in a total volume of 50 μl for 2 wk. The other model used was the orthotopic model. Pancreatic tumors grown subcutaneously were harvested, and tumor pieces (1 mm3) were transplanted into the tail of the pancreas of recipient nude mice (20). Animals were randomized and treated with either daily intraperitoneal injections of rottlerin (0.5 mg/kg, n = 8) or control vehicle (n = 8) for 4 wk. At euthanasia, pancreatic tumors, liver, spleen, lungs, and kidney were harvested. Tumor volume was assessed as described earlier (12). For further analyses, the tumor was either fixed in formalin and embedded in paraffin or frozen in 2-methyl-butane/dry ice and embedded in optimal cutting temperature compound.
In situ TUNEL assay.
The percentage of apoptotic cells in tissue sections was determined as described previously (16). Briefly, cryostat sections were fixed in 4% paraformaldehyde, and in situ terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay was done as described by the manufacturer (Roche Molecular Biochemicals, Manheim, Germany). The number of apoptotic cells was determined in relation to the total number of cells.
Cell culture.
Human pancreatic adenocarcinoma cell lines, the poorly differentiated MIA PaCa-2, and moderately differentiated PANC-1 were obtained from the American Type Culture Collection (Manassas, VA). MIA PaCa-2 and PANC-1 cells were grown in 1:1 DMEM-F-12 medium from GIBCO Invitrogen (Grand Island, NY) supplemented with 15% FBS, 4 mM l-glutamine, and antibiotic/antimycotic solution from Omega Scientific (Tarzana, CA). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 and were used between passages 4 and 12. For analyses, cells were plated at a density of 5 × 105/ml in either 100-mm culture dishes or 150 cm2, and cells were cultured for up to 48 h.
Transfection.
Puma, Bim, Bad, and negative control siRNAs were obtained from Dharmacon (Lafayette, CO). PaCa cells were transiently transfected using Nucleofector system from Amaxa Biosystems (Cologne, Germany). The 2 × 106 cells were resuspended in corresponding Nucleofector solution. siRNA and cells were mixed in the electroporation Amaxa cuvette, and transfection was performed with the electrical setting T20. After nucleofection the cells were immediately transferred into prewarmed medium and cultured at 37°C, 5% CO2.
Measurement of apoptosis in cells.
Internucleosomal DNA fragmentation was measured by using Cell Death Detection ELISAPlus kit (Roche Molecular Biochemicals) according to the manufacturer's instructions. Phosphatidylserine externalization was analyzed with the annexin-V (AnV)-FLUOS Staining Kit from Roche Biochemicals (Indianapolis, IN) as we described before (9, 41, 42). Caspase-3, -9, and -8 activities were measured as we described previously (9, 41, 42) using a fluorogenic assay with the substrates specific for caspase-3 [Ac-DEVD amino-4-methylcoumarin (AMC)], caspase-9 (Ac-LEHD-AMC), and caspase-8 (Ac-IETD-AMC). The data were expressed as moles of AMC/mg of protein/min and normalized to the control.
Cell fractionation for the measurement of cytochrome c release.
Cells were resuspended in a lysis buffer (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1 mM Na-EGTA, 1 mM Na-EDTA, 2 mM MgCl2, pH 7.0); disrupted by 80 strokes in a Dounce homogenizer, and centrifuged at 1,000 g to pellet nuclei and cell debris. Supernatants were further centrifuged at 13,000 g for 30 min, and the cytosolic fractions (supernatants) and the pellets (membrane fractions enriched with mitochondria) were collected. To validate the quality of cytosolic and mitochondrial separation, both fractions were assessed by immunoblotting for the mitochondrial marker cytochrome c oxidase subunit IV.
Total cell lysates.
Cells were resuspended in RIPA phosphorylation buffer (50 mM NaCl, 50 mM Tris·HCl pH 7.2, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, 10 mM Na2HPO4 + NaH2PO4 pH 7.0, 100 mM NaF, 2 mM Na3VO4, 20% glycerol, 80 μM glycerophosphate, 1 mM PMSF, 5 μg/ml of pepstatin, leupeptin, chymostatin, antipain, and aprotinin), sonicated, and centrifuged for 15 min at 15,000 g at 4°C.
Immunoprecipitation.
Cells were collected, washed twice in a buffer containing 20 mM Tris (pH 7.5) and 10 mM DTT, then resuspended in a lysis buffer (50 mM Tris·HCl, 150 mM NaCl, 2 mM EGTA, 10 μg/ml each leupeptin and aprotinin, 1 mM PMSF, 1% NP-40), and sonicated for 30 s. Lysates were clarified by centrifugation, and 500 μg of protein was subjected to overnight immunoprecipitation with either Bcl-xL or Bcl-2 antibody at 4°C using Catch and Release Reversible Immunoprecipitation System from Millipore (Billerica, MA).
Western blot analysis.
Proteins from total cell lysates or immunoprecipitates were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. Nonspecific binding was blocked with 5% bovine serum albumin or nonfat dry milk in Tris-buffered saline (4 mM Tris base, 100 mM NaCl, pH 7.5) containing 0.05% Tween 20 for 1 h. Membranes were incubated with primary antibody for overnight at 4°C and then for 1 h with peroxidase-conjugated secondary antibody. The blots were developed with Supersignal Chemiluminescent Substrate from Pierce (Rockford, IL).
Measurements of ΔΨm in intact PaCa cells.
Changes in ΔΨm were detected with the potential-sensitive probe MitoTracker Red (CMX-Ros) from Invitrogen (Carlsbad, CA) as we described previously (41). During the last 30 min of the incubation period, cells were loaded with 10 nM CMX-Ros for 30 min at 37°C in the dark, washed twice with phosphate-buffered saline, and analyzed by flow cytometry using the FACScan with FL-3 detector. To completely dissipate ΔΨm, cells were treated with the uncoupling agent CCCP (50 μM) for 1 h before CMX-Ros staining.
Measurements of ΔΨm and cytochrome c release in PaCa cells permeabilized with digitonin.
For permeabilization, cells were resuspended in the medium that mimics ionic composition of cytosol. It contained 250 mM sucrose, 22 mM KCl, 11 mM triethanolamine, 3 mM MgCl2, 5 mM KH2PO4 (pH 7.0), 0.5 mM EGTA, and 10 mM succinate incubated for 3 min with 1.25 × 10−3% digitonin as described (24, 26). Efficiency of permeabilization was confirmed by positive Trypan blue staining in greater than 90% of the cells. Changes in ΔΨm were measured with a ΔΨm-sensitive tetraphenyl phosphonium (TPP+)-selective electrode (23). An increase in ΔΨm causes TPP+ uptake by mitochondria and, correspondingly, a decrease in external TPP+ measured by the electrode. Cytochrome c levels in soluble and membrane fractions were measured with Western blot. Cytochrome c release and ΔΨm were measured in the same preparations of permeabilized cells.
Fluorescence polarization assay.
Complex formation between a fluorescein-labeled peptide [NLWAAQRYGRELRRMSDK(fluorescein)FVD] of BH3 domain of Bad (synthesized by CURE Research center Peptide CORE) and the recombinant Bcl-xL fragment lacking the flexible loop and the carboxyterminal hydrophobic region (Calbiochem) was performed as described in Ref. 48. Briefly, Bcl-xL was mixed with 15 nM fluorescently labeled Bad in a 96-well plate with and without rottlerin, BH3I-2′, or vehicle in a final volume of 50 μl, and fluorescence polarization was measured using Analyst 96-well plate reader (LJL; Molecular Devices, Sunnyvale, CA).
Measurements of PKC-δ activity.
Measurements of PKC-δ activity were performed as we described in Ref. 35. Briefly, cells were homogenized in ice-cold homogenization buffer containing (in mM) 130 NaCl, 50 Tris·HCl (pH 7.5), 5 EGTA, 5 EDTA, 1.5 MgCl2, 10 NaF, 1 Na3VO4, 10 Na4P2O7, 1 PMSF, and 10% (vol/vol) glycerol plus 5 μg/ml each of pepstatin, leupeptin, chymostatin, antipain, and aprotinin, sonicated five times for 10 s on ice, and incubated for 45 min at 4°C. PKC-δ was immunoprecipitated using specific antibody against PKC-δ isoform (1:100 dilution). The beads were washed and resuspended in the kinase buffer [(in mM) 20 MOPS (pH 7.2), 25 β-glycerophosphate, 5 EGTA, 1 Na3VO4, and 1 DTT]. The kinase assay was performed by using the PKC assay kit (Upstate Biotechnology).
Statistical analysis.
For statistical analysis, the results were from at least three independent experiments. Differences between two groups were analyzed using Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Rottlerin inhibited tumor growth and stimulated apoptosis in pancreatic cancer animal models.
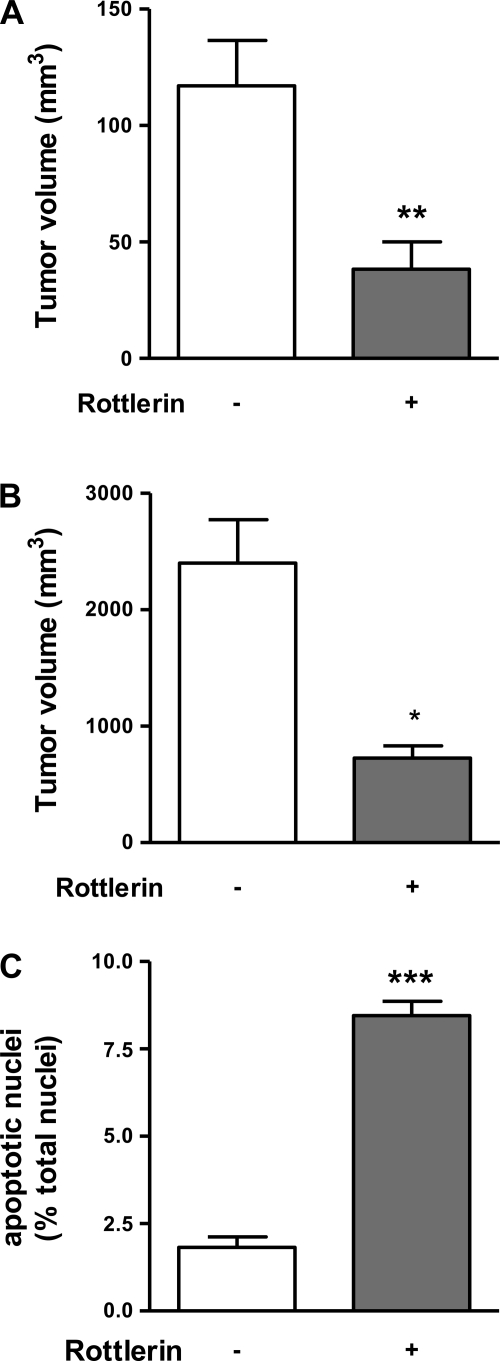
To determine the effects of rottlerin on the growth of xenografted pancreatic cancers we used a subcutaneous and orthotopic pancreatic cancer model in nude mice as described before (11, 12, 20). Mice received either 0.5 mg/kg of rottlerin or vehicle (DMSO) daily for 2 (subcutaneous model) or 4 wk (orthotopic model). There was no difference in body weight throughout the study period between both experimental groups (not shown). In both models, rottlerin significantly inhibited tumor growth by 60–70% (Fig. 1, A and B). The decrease in tumor growth was associated with an increase in apoptotic cell death in PaCa cells. In the orthotopic model, treatment of mice with rottlerin increased apoptosis in PaCa cells fourfold compared with control animals (Fig. 1C), suggesting that rottlerin inhibits pancreatic cancer growth by induction of apoptosis. Importantly, normal extratumoral tissues, e.g., liver and kidney, were histologically normal; there was no increase in apoptosis or necrosis in these organs (not shown).
Fig. 1.
Rottlerin inhibited pancreatic cancer growth in vivo. The effects of rottlerin on pancreatic cancer growth were measured in a subcutaneous (A) and orthotopic (B) xenograft model in nude mice. Animals received either rottlerin (0.5 mg/kg) or vehicle by daily intraperitoneal injections. The tumor volumes of rottlerin- and vehicle- treated animals were determined after 2 wk in the subcutaneous (A) and after 4 wk in the orthotopic model (B). C: percentage of apoptotic cells in tissues sections was determined in cryostat sections fixed in 4% paraformaldehyde using terminal deoxynucleotidyl transferase-mediated nick end labeling assay. The number of apoptotic cells was determined in relation to the total number of cells. The values in A–C represent means ± SE (at least 5 animals in each group). *P < 0.05, **P < 0.02, ***P < 0.0005 vs. the same parameter in tumors from mice treated without rottlerin.
Rottlerin stimulated PaCa cell death.
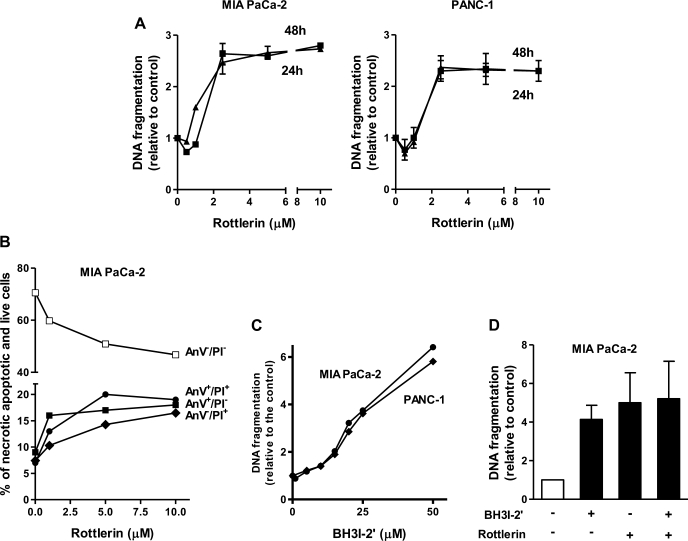
Rottlerin at micromolar concentrations dose dependently stimulated apoptosis in PaCa cells (Fig. 2). Increase in DNA fragmentation in both MIA PaCa-2 and PANC-1 cells was already significant at 1.3 μM and reached maximum at 2.5 μM rottlerin (Fig. 2A). Of note, application of 2.5 μM rottlerin in vitro is approximately equivalent to the in vivo application of 0.5 mg/kg. Stimulation of apoptosis by rottlerin increased with the time and reached plateau at 24-h culturing with rottlerin.
Fig. 2.
Rottlerin stimulated apoptosis in pancreatic cancer (PaCa) cells. MIA PaCa-2 and PANC-1 cells were cultured for indicated times (A) or for 48 h (B, C, and D) with indicated concentrations of rottlerin (A and B), BH3I-2′ (C), combination of 2.5 μM rottlerin, and 25 μM BH3I-2′ (D), or vehicle. Apoptosis was determined by measuring DNA fragmentation (A, C, and D) and annexin-V/propidium iodide (AnV/PI) staining (B). A, C, and D: DNA fragmentation was measured by Cell Death ELISA kit. The values were normalized to those in cells cultured without rottlerin. B: cells were stained with AnV and PI, analyzed by flow cytometry, and the percentages of cells in groups AnV+/PI− (early apoptosis), AnV−/PI+ (early necrosis), AnV+/PI+ (mixture of apoptosis associated with secondary necrosis and late necrosis), and AnV−/PI− (live cells) were measured. The values in represent means ± SE, n = 3.
To assess the differential effects of rottlerin on apoptosis and necrosis, we stained MIA PaCa-2 cells with AnV and propidium iodide (PI). As we showed before (41), AnV+/PI− group represents cells with early apoptosis; cells stained with PI only (AnV−/PI+) are early necrotic ones, whereas AnV+/PI+ group includes cells with primary necrosis and also cells with apoptosis associated with secondary necrosis (28a, 41). Rottlerin increased the number of cells in each group up to twofold and as a result decreased the number of live (AnV−/PI−) cells (Fig. 2B). These data indicate that rottlerin stimulated not only apoptotic but also necrotic death of PaCa cells.
We compared the proapoptotic effects of rottlerin with those induced by BH3I-2′, an inhibitor of Bcl-2 and Bcl-xL. BH3I-2′ is a mimetic of BH3 domain; it binds to the hydrophobic pocket of Bcl-2 and Bcl-xl, preventing their interaction with BH3-only proteins (13, 18). BH3I-2' stimulated apoptosis in both MIA PaCa-2 and PANC-1 cells (Fig. 2C). The effects of 25 μM of BH3I-2′ and 2.5 μM of rottlerin on apoptosis were nonadditive, suggesting a common underlying mechanism (Fig. 2D).
Rottlerin stimulated mitochondrial pathway of apoptosis in PaCa cells.
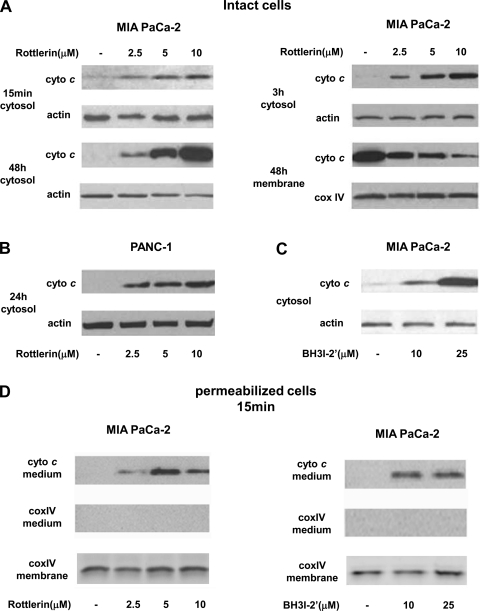
Rottlerin dose and time dependently stimulated cytochrome c release from mitochondria into cytosol in both MIA PaCa-2 and PANC-1 cells (Fig. 3, A and B). Cytochrome c release increased with the concentration of rottlerin in the range of 2.5 to 10 μM. In MIA PaCa-2 cells, the induction of cytochrome c release was evident as early as at 15 min and continued to increase for up to 48 h (Fig. 3, A and B). BH3I-2′ also dose dependently stimulated cytochrome c release in MIA PaCa cells (Fig. 3C).
Fig. 3.
Rottlerin stimulated cytochrome c (cyto c) release in PaCa cells. MIA PaCa-2 cells (A and C) and PANC-1 cells (B) were cultured for indicated times with indicated concentrations of rottlerin (A and B), BH3I-2′ (C), or vehicle. A–C: cytochrome c levels in cytosolic and membrane fractions were measured with Western blot analysis. The blots were reprobed for actin and Cox IV to confirm equal protein loading in cytosolic (actin) and membrane fractions (CoxIV). D: MIA PaCa-2 cells were permeabilized for 3 min with 1.25 × 10−3% digitonin and then incubated with indicated concentrations of rottlerin (left) or BH3I-2′ (right) for 15 min. Cytochrome c levels in the medium were measured using Western blot analysis. The blots were reprobed for Cox IV to confirm no contamination of the medium with broken mitochondria and to confirm equal amount of mitochondria in all conditions. Immunoblots are representative of 3 independent experiments, which all gave the same results.
We next examined the direct effect of rottlerin and BH3I-2′ on mitochondria using MIA PaCa-2 cells permeabilized with digitonin (Fig. 3D). When added to permeabilized cells both rottlerin and BH3I-2′ dose-dependently stimulated cytochrome c release (Fig. 3D), suggesting that both these agents stimulate cytochrome c release by directly affecting mitochondria.
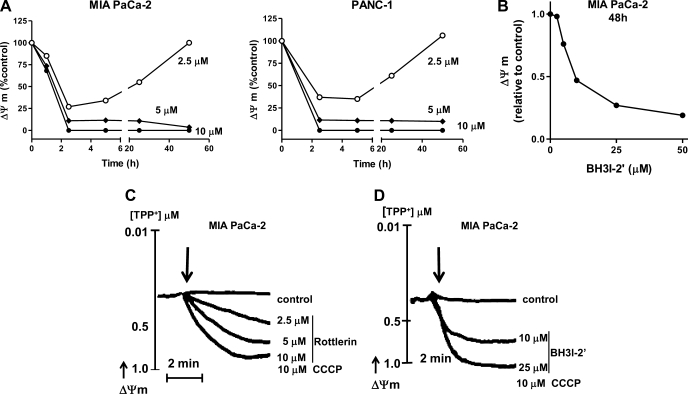
Cytochrome c release in apoptosis is usually associated with mitochondrial depolarization (14). Rottlerin dose dependently decreased ΔΨm in MIA PaCa-2 and PANC-1 cells (Fig. 4A). Depolarization was first observed at 15 min of rottlerin treatment. Importantly, low concentrations of rottlerin (2.5 μM) transiently depolarized mitochondria, whereas higher concentrations (5 and 10 μM) caused permanent loss of ΔΨm (Fig. 4A). The reason for transient depolarization remains to be determined. However, the differences in the time dependencies of rottlerin-induced cytochrome c release and depolarization suggest that cytochrome c release and depolarization are mediated through different mitochondrial permeability systems. BH3I-2′ also dose dependently stimulated mitochondrial depolarization of MIA PaCa cells (Fig. 4B). In contrast to rottlerin, the effect of BH3I-2′ on ΔΨm was not transient at concentrations tested.
Fig. 4.
Rottlerin stimulated loss of ΔΨm in PaCa-2 cells. MIA PaCa-2 (A and B) and PANC-1 (A) cells were cultured for indicated times (A) or 48 h (B) with indicated concentrations of rottlerin (A), BH3I-2′ (B), or vehicle. A and B: mitochondrial membrane potential was measured in cells labeled with ΔΨm-sensitive dye, MitoTracker Red (CMX-Ros) and analyzed with flow cytometry. The values in A and B were normalized to those at 0 time (A) or 48 h (B) in the absence of rottlerin or BH3I-2′. The values represent means ± SE, n = 3. C and D: MIA PaCa-2 cells were permeabilized for 3 min with 1.25 × 10−3% digitonin and then incubated with indicated concentrations of rottlerin (C), BH3I-2′(D), CCCP, or vehicle for indicated times. Changes in ΔΨm were measured using ΔΨm-sensitive tetraphenyl phosphonium (TPP+) electrode. Protonophore CCCP was applied to cancel membrane potential. The results are representative of 3 independent experiments, which all gave the same results.
Both rottlerin and BH3I-2′ stimulated loss of ΔΨm in digitonin-permeabilized MIA PaCa-2 cells (Fig. 4, C and D), indicating direct interaction of rottlerin and BH3I-2′ with mitochondria.
Once in cytosol, cytochrome c activates the initiator caspase-9, followed by activation of effector caspase-3, resulting in apoptosis (33). We found that rottlerin increased activity of caspase-9 (LEHDase) as well as caspase-9 processing manifest by a decrease in procaspase-9 (47 kDa) and increase in 35-kDa cleaved form (Fig. S1A; supplemental material for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website). Rottlerin dose and time dependently stimulated caspase-3 (DEVDase) activity and its processing, which also manifests by a decrease in 32-kDa band of procaspase-3 and by a concomitant increase in 17-kDa band corresponding to active (i.e., cleaved) caspase-3 (Fig. S1, A and C). Of note, apoptosis induced by rottlerin in MIA PaCa-2 cells was prevented by pan-caspase inhibitor z-VAD-fmk, indicating a necessary role of caspases (not shown). Importantly, rottlerin did not activate caspase-8 (Fig. S1B), which acts upstream of mitochondria (33). These confirm that it stimulates mitochondrial pathway of apoptosis.
The results of the Figs. 3, 4, and S1 suggest that rottlerin and BH3I-2′ stimulate apoptosis through a common mechanism; they both directly interact with mitochondria, stimulating cytochrome c release and loss of ΔΨm. This leads to caspase-9 and -3 activation and apoptosis.
BH3I-2′ but not rottlerin stimulated apoptosis through disrupting complexes of Bcl-xL with Bad.
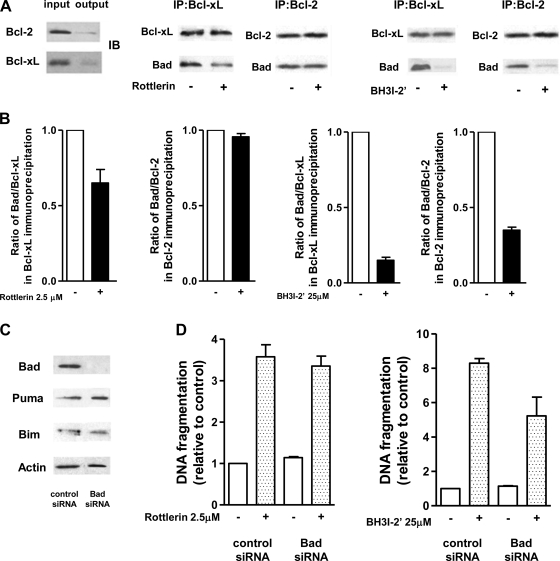
The pharmacological inhibitors of prosurvival of Bcl-xL and Bcl-2 induce apoptosis by preventing sequestration by prosurvival proteins of proapoptotic BH3-only proteins (18). Our immunoprecipitation experiments showed that, in MIA PaCa-2 cells, the proapoptotic BH3-only protein Bad is in complex with Bcl-xL and Bcl-2 (Fig. 5A). BH3I-2′ markedly decreased the amount of Bad coimmunoprecipitated with Bcl-xL and Bcl-2 (Fig. 5, A and B). Differently, rottlerin only slightly decreased the amount of Bad in complex with Bcl-xL (Fig. 5, A and B) and had no effect on the interaction of Bad with Bcl-2 (Fig. 5A).
Fig. 5.
BH3I-2′, but not rottlerin, decreased the amount of Bad sequestered by Bcl-xL. Bad is required for apoptosis induced by BH3I-2′ but not by rottlerin. A and B: MIA PaCa-2 cells were cultured for 1 h with 25 μM BH3I-2′, 2.5 μM of rottlerin, or vehicle. Bcl-xL and Bcl-2 were immunoprecipitated from total cell lysates. A: immunoprecipitates (IP) were immunoblotted (IB) with antibody against Bcl-xL, Bcl-2, and Bad as indicated. B: intensities of the bands were densitometrically quantified and expressed as the ratios of the intensities of Bad to that of Bcl-xL or Bcl-2. The ratios were normalized to those in cells cultured without BH3I-2′ and rottlerin. The results are means ± SE of 2 experiments. C and D: MIA PaCa-2 cells were transfected with Bad siRNA or negative-control siRNA. C: transfection efficiency and lack of the effect of Bad knockdown on the levels of Puma and Bim were confirmed by immunoblot. D: transfected cells were cultured for 24 h with 2.5 μM rottlerin (B), 25 μM BH3I-2′, or vehicle. DNA fragmentation was measured by Cell Death ELISA kit. The values were normalized to those in cells cultured without rottlerin or BH3I-2′. Values are means ± SE (n = 3); *P < 0.05 compared with cells transfected with control siRNA and incubated without BH3I-2′ and rottlerin.
We further showed that Bad knockdown with siRNA greatly decreased apoptosis induced by BH3I-2′ but had no effect on apoptosis induced by rottlerin (Fig. 5, C and D), indicating that proapoptotic effect of rottlerin does not involve Bad.
To compare the effects of rottlerin and BH3I-2′ on the interaction of Bcl-xL with Bad in cell-free system we examined the effect of rottlerin on the binding of fluorescein-labeled peptide of the BH3 domain of Bad protein to Bcl-xL using fluorescence polarization assay (48). Figure S2 shows that recombinant Bcl-xL forms complex with the BH3 domain of Bad manifested by an increase in the fluorescence polarization. BH3I-2′ dose dependently disrupted complex formation between Bcl-xL and BH3 domain of Bad, whereas rottlerin at concentrations up to 200 μM was without effect. Thus the results in MIA PaCa-2 cells, as well as the results in cell-free system, indicate that BH3I-2′ but not rottlerin disrupts the complex of Bad with Bcl-xL and Bcl-2.
Rottlerin stimulated apoptosis through disrupting complexes of Bcl-xL with Bim and Puma.
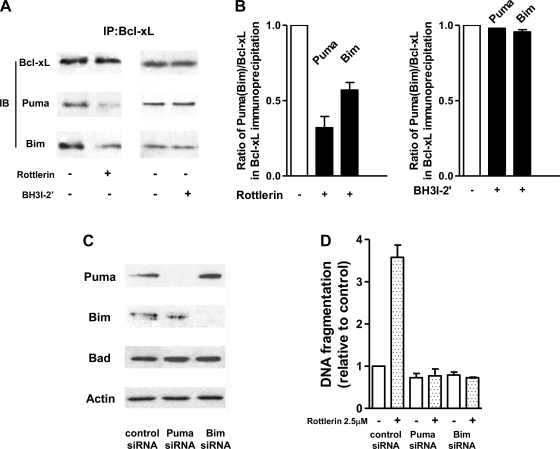
To determine whether the proapoptotic effect of rottlerin in PaCa cells is mediated through Puma and Bim we measured the effect of rottlerin on the sequestration of these BH3-only proteins by Bcl-xL (Fig. 6). Rottlerin greatly decreased the amount of Puma and Bim coimmunoprecipitated with Bcl-xL (Fig. 6, A and B). Differently, BH3I-2′ had no effect on the interaction of Bcl-xL with Puma (Fig. 6A, B). Of note, neither rottlerin nor BH3I-2′ affected the interaction of Bcl-2 with Puma or Bim (data not shown).
Fig. 6.
Rottlerin decreased the amount of Puma and Bim sequestered by Bcl-xL. Puma and Bim are required for apoptosis induced by rottlerin. A and B: MIA PaCa-2 cells were cultured for 1 h with 2.5 μM of rottlerin, 25 μM BH3I-2′, or vehicle. Bcl-xL and Bcl-2 were immunoprecipitated from total cell lysates. A: immunoprecipitates were immunoblotted with antibody against Bcl-xL, Puma, Bim as indicated. B: intensities of the bands were densitometrically quantified and expressed as the ratios of the intensities of Puma or Bim to that of Bcl-xL. The ratios were normalized to those in cells cultured without rottlerin BH3I-2′ and rottlerin. The results are means ± range of 2 experiments. C and D: MIA PaCa-2 cells were transfected with Puma or Bim siRNA or negative-control siRNA. C: transfection efficiency and lack of the effects of Puma or Bim knockdown on the Bad levels were confirmed by immunoblot. D: transfected cells were cultured for 24 h with 2.5 μM rottlerin, 25 μM BH3I-2′, or vehicle. DNA fragmentation was measured by Cell Death ELISA kit. The values were normalized to those in cells cultured without rottlerin or BH3I-2′. Values are means ± SE (n = 3).
We further showed that Puma and Bim knockdown with siRNA prevented stimulation of apoptosis by rottlerin in MIA PaCa-2 cells, indicating a critical role for Puma and Bim in the proapoptotic effects of rottlerin (Fig. 6, C and D). Of note, Puma and Bim knockdown with siRNA also prevented stimulation of apoptosis by rottlerin in PANC-1 cells (not illustrated), suggesting that the mechanisms of proapoptotic effects of rottlerin are the same in both MIA PaCa-2 and PANC-1 cells.
Rottlerin-induced apoptosis was not mediated by the inhibition of PKC-δ or Akt.
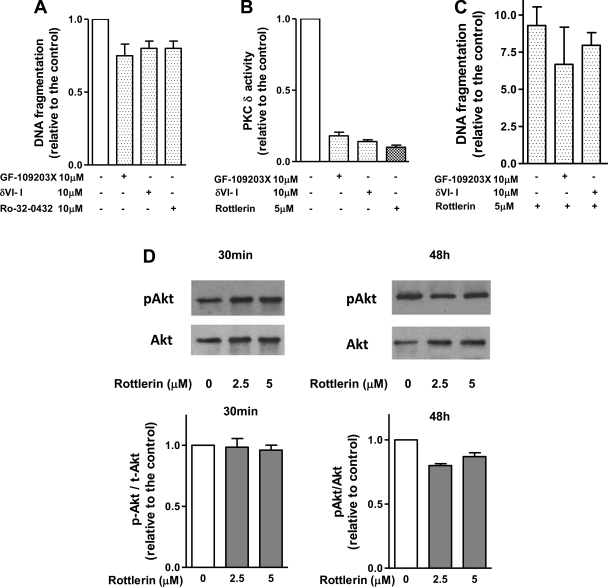
Apoptotic effects of rottlerin were often attributed to the inhibition of PKC-δ (32, 36). Therefore, we next examined the involvement of PKC-δ in the effects of rottlerin on apoptosis. We showed (Fig. 7A) that neither broad spectrum PKC inhibitor nor specific peptide inhibitor of PKC-δ (35) stimulated apoptosis in MIA PaCa-2 cells. We also showed that the inhibitors blocked PKC-δ activity in MIA PaCa-2 cells (Fig. 7B and Ref. 46a). Pretreatment with PKC inhibitors also did not prevent apoptosis induced by rottlerin (Fig. 7C). Furthermore, rottlerin similarly stimulated apoptosis in MIA PaCa-2 cells, which express PKC-δ, and in PANC-1 cells, which do not express this isoform of PKC. (17) As shown in several publications (33, 36), PKC-δ could inhibit apoptosis through activating prosurvival Akt kinase. However, rottlerin did not have any significant effect on Akt phosphorylation in PaCa cells (Fig. 7D). These data indicate that PKC-δ and Akt are not involved in proapoptotic effects of rottlerin.
Fig. 7.
Rottlerin-induced apoptosis is not mediated through PKC or Akt. MIA PaCa-2 cells were cultured for 48 h with indicated concentrations of specific PKC-δ translocation inhibitor, broad-spectrum PKC inhibitors GF-109203X and R032–0432, rottlerin, or vehicle. A and C: apoptosis was determined by measuring DNA fragmentation using Cell Death ELISA kit. The values were normalized to those in cells cultured without inhibitors. The values are means ± SE, n = 3. B: changes in PKC-δ kinase activities were measured in PKC-δ immunoprecipitates by kinase assay using PKC-δ-specific peptide substrate. Activities were normalized to the basal activity in the absence of the inhibitors. Values are means ± SE (n = 2). C: Akt phosphorylation, an indicator of Akt activation, was measured by immunoblots of Akt phosphorylated at 473S and total Akt. D: intensities of pAkt and total Akt were densitometrically quantified and expressed as the ratios of the intensities of phosphoAkt to that of total Akt. The values are means ± SE, n = 3.
DISCUSSION
The results of this study show that rottlerin inhibited tumor development and increased apoptosis in the orthotopic model of pancreatic cancer. Rottlerin also stimulated apoptosis in PaCa cell lines.
Several lines of evidence indicate that rottlerin induced apoptosis in PaCa cells through interacting with Bcl-2 proteins. 1) The proapoptotic effects of rottlerin were not mediated by PKC-δ inhibition, as other inhibitors of PKC-δ do not have such effect. 2) Rottlerin directly interacts with mitochondria, resulting in cytochrome c release and mitochondrial depolarization. 3) Rottlerin displaces Bim and Puma from complexes with Bcl-xL. 4) Bim and Puma are necessary for rottlerin to cause apoptosis because their knockdown prevented induction of apoptosis by rottlerin. Taken together, these findings provide strong support for our hypothesis that rottlerin acts to cause apoptosis through disrupting the ability of prosurvival Bcl-2 proteins to sequester proapoptotic BH3-only proteins, Bim, and Puma.
The conclusion that proapoptotic effects of rottlerin are mediated through Bcl-2 proteins is further supported by the findings that rottlerin has actions similar to a known inhibitor of Bcl-2/Bcl-xL, BH3I-2′ (13, 18). BH3I-2′ is a mimetic of BH3 domain; it binds to the hydrophobic pocket of Bcl-2 and Bcl-xL, preventing their interaction with BH3-only proteins (13, 18). The effects of BH3I-2′ and rottlerin on apoptosis were not additive. Rottlerin and BH3I-2′ both directly affected mitochondria, resulting in cytochrome c release and loss of ΔΨm. Rottlerin and BH3I-2′ both disrupted complexes of Bcl-xL and BH3-only proteins. Proapoptotic effects of rottlerin and BH3I-2′ are mediated by BH3-only proteins.
Importantly, we found that effects of rottlerin and BH3I-2′ on the interaction of Bcl-xL with BH3-only proteins are not the same. Rottlerin prevented coimmunoprecipitation of Bcl-xL with Bim and Puma, but it had little effect on the complex of Bcl-xL with Bad. Differently, BH3I-2′ decreased the level of Bad, but not Bim and Puma, in the complex with Bcl-xL. The mechanisms underlying such differences in the interaction of rottlerin and BH3I-2′ with Bcl-xL remain to be determined.
Rottlerin stimulated not only cytochrome c release but also loss of ΔΨm in PaCa cells. Data from other cell types show that cytochrome c release and mitochondrial depolarization are mediated through different mitochondrial permeability systems (5). Cytochrome c is released through the Bax/Bak channels in the outer mitochondrial membrane, whereas mitochondrial depolarization is mediated by permeability transition pore (mPTP), which involves proteins of both inner and outer membranes. Bcl-2 proteins were shown to be involved in the regulation of both mPTP and Bax/Bak channels (2, 4), which explains the effect of rottlerin on both cytochrome c release and ΔΨm in PaCa cells.
In nontransformed cells, mitochondria are the major source of ATP production; therefore, mitochondrial depolarization leads to ATP depletion and necrosis. ATP depletion also prevents caspase activation, leading to the inhibition of apoptosis (10, 37, 47). Differently, in cancer cells, ATP production is mostly mediated through glycolysis (43–45) and is relatively independent on ΔΨm (19, 43, 44). In agreement with these considerations, we found that, in PaCa cells (differently from normal pancreatic acinar cells) (36, 39), rottlerin stimulated both apoptosis and necrosis.
Importantly, the results of in vivo experiments suggest that normal and cancer cells display different sensitivity to rottlerin. The doses of rottlerin we applied in vivo stimulated neither apoptosis nor necrosis in normal pancreas and liver. These same doses of rottlerin greatly potentiated apoptosis in pancreatic tumor. Although the mechanisms underlying these differences remain to be determined, the findings that low doses of rottlerin to preferentially stimulate apoptosis in cancer cells are important for possible rottlerin application in cancer treatment.
In summary, our data showed that rottlerin has a potent proapoptotic and antitumor activity in pancreatic cancer. The proapoptotic effect of rottlerin is mediated by the disruption of the complexes between prosurvival Bcl-2 proteins and BH3-only proteins Puma and Bim. Thus rottlerin represents a promising novel agent for pancreatic cancer treatment.
GRANTS
This study was supported by the Hirshberg Foundation for Pancreatic Cancer Research, the NIH grants CA119025 (to A. Gukovskaya.), NCCAM AT003960 (to S. Pandol), and the Department of Veterans Affairs Merit Award (to A. Gukovskaya).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26: 1324–1337, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagci EZ, Vodovotz Y, Billiar TR, Ermentrout GB, Bahar I. Bistability in apoptosis: roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys J 90: 1546–1559, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya S, Mandal D, Saha B, Sen GS, Das T, Sa G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem 282: 15954–15964, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 8: 705–711, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ 13: 1396–1402, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Clark AS, West KA, Blumberg PM, Dennis PA. Altered protein kinase C (PKC) isoforms in non-small cell lung cancer cells: PKCdelta promotes cellular survival and chemotherapeutic resistance. Cancer Res 63: 780–786, 2003 [PubMed] [Google Scholar]
- 7.Crane CH, Ben-Josef E, Small W., Jr Chemotherapy for pancreatic cancer. N Engl J Med 350: 2713–2715; author reply 2713–2715, 2004 [PubMed] [Google Scholar]
- 8.Duchen MR. Roles of mitochondria in health and disease. Diabetes 53, Suppl 1: S96–S102, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Edderkaoui M, Hong P, Vaquero EC, Lee JK, Fischer L, Friess H, Buchler MW, Lerch MM, Pandol SJ, Gukovskaya AS. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol 289: G1137–G1147, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res 57: 1835–1840, 1997 [PubMed] [Google Scholar]
- 11.Eibl G, Reber HA, Wente MN, Hines OJ. The selective cyclooxygenase-2 inhibitor nimesulide induces apoptosis in pancreatic cancer cells independent of COX-2. Pancreas 26: 33–41, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Eibl G, Takata Y, Boros LG, Liu J, Okada Y, Reber HA, Hines OJ. Growth stimulation of COX-2-negative pancreatic cancer by a selective COX-2 inhibitor. Cancer Res 65: 982–990, 2005 [PubMed] [Google Scholar]
- 13.Feng WY, Liu FT, Patwari Y, Agrawal SG, Newland AC, Jia L. BH3-domain mimetic compound BH3I-2′ induces rapid damage to the inner mitochondrial membrane prior to the cytochrome c release from mitochondria. Br J Haematol 121: 332–340, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 199: 93–98, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Guha S, Eibl G, Kisfalvi K, Fan RS, Burdick M, Reber H, Hines OJ, Strieter R, Rozengurt E. Broad-spectrum G protein-coupled receptor antagonist, [d-Arg1,d-Trp5,7,9,Leu11]SP: a dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res 65: 2738–2745, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Guha S, Rey O, Rozengurt E. Neurotensin induces protein kinase C-dependent protein kinase D activation and DNA synthesis in human pancreatic carcinoma cell line PANC-1. Cancer Res 62: 1632–1640, 2002 [PubMed] [Google Scholar]
- 18.Hao JH, Yu M, Liu FT, Newland AC, Jia L. Bcl-2 inhibitors sensitize tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by uncoupling of mitochondrial respiration in human leukemic CEM cells. Cancer Res 64: 3607–3616, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hockenbery DM. A mitochondrial Achilles' heel in cancer? Cancer Cell 2: 1–2, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Hotz HG, Reber HA, Hotz B, Yu T, Foitzik T, Buhr HJ, Cortina G, Hines OJ. An orthotopic nude mouse model for evaluating pathophysiology and therapy of pancreatic cancer. Pancreas 26: e89–e98, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hudson TS, Hartle DK, Hursting SD, Nunez NP, Wang TT, Young HA, Arany P, Green JE. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res 67: 8396–8405, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kamo N, Muratsugu M, Hongoh R, Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol 49: 105–121, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 8: 1348–1358, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7: 1166–1173, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Kowaltowski AJ, Vercesi AE, Fiskum G. Bcl-2 prevents mitochondrial permeability transition and cytochrome c release via maintenance of reduced pyridine nucleotides. Cell Death Differ 7: 903–910, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, Blackwood M, Stoner GD. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res 61: 6112–6119, 2001 [PubMed] [Google Scholar]
- 28.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17: 525–535, 2005 [DOI] [PubMed] [Google Scholar]
- 28a.Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology 133: 1637–1648, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Mizuta T, Shimizu S, Matsuoka Y, Nakagawa T, Tsujimoto Y. A Bax/Bak-independent mechanism of cytochrome c release. J Biol Chem 282: 16623–16630, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Ni H, Ergin M, Tibudan SS, Denning MF, Izban KF, Alkan S. Protein kinase C-delta is commonly expressed in multiple myeloma cells and its downregulation by rottlerin causes apoptosis. Br J Haematol 121: 849–856, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem 274: 19115–19123, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 5: 897–907, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, Decker T. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood 100: 3741–3748, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Jr, Shimosegawa T, Pandol SJ. PKC-δ and -ε regulate NF-kappaB activation induced by cholecystokinin and TNF-α in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 287: G582–G591, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J Biol Chem 276: 37986–37992, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Stefanelli C, Bonavita F, Stanic I, Farruggia G, Falcieri E, Robuffo I, Pignatti C, Muscari C, Rossoni C, Guarnieri C, Caldarera CM. ATP depletion inhibits glucocorticoid-induced thymocyte apoptosis. Biochem J 322: 909–917, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sultana A, Tudur Smith C, Cunningham D, Starling N, Tait D, Neoptolemos JP, Ghaneh P. Systematic review, including meta-analyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer 96: 1183–1190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapia JA, Jensen RT, Garcia-Marin LJ. Rottlerin inhibits stimulated enzymatic secretion and several intracellular signaling transduction pathways in pancreatic acinar cells by a non-PKC-delta-dependent mechanism. Biochim Biophys Acta 1763: 25–38, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Tillman DM, Izeradjene K, Szucs KS, Douglas L, Houghton JA. Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer Res 63: 5118–5125, 2003 [PubMed] [Google Scholar]
- 41.Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology 125: 1188–1202, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem 279: 34643–34654, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Warburg O. The Metabolism of Tumors London, UK: London Constable, 1930 [Google Scholar]
- 44.Warburg O. On the origin of cancer cells. Science 123: 309–314, 1956 [DOI] [PubMed] [Google Scholar]
- 45.Warburg OP, Neigelein K. E. Metabolism of carcinoma cells. Biochem Z 152: 309–344, 1924 [Google Scholar]
- 46.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol 17: 617–625, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Yazbec M, Nam KJ, Vaquero EC, Gukovskaya AS, Pandol SJ. The protein kinase C delta inhibitor rottlerin greatly stimulates apoptosis in pancreatic cancer cells. Gastroenterology 124: A103, 2003 [Google Scholar]
- 47.Zamaraeva MV, Sabirov RZ, Maeno E, Ando-Akatsuka Y, Bessonova SV, Okada Y. Cells die with increased cytosolic ATP during apoptosis: a bioluminescence study with intracellular luciferase. Cell Death Differ 12: 1390–1397, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Nimmer P, Rosenberg SH, Ng SC, Joseph M. Development of a high-throughput fluorescence polarization assay for Bcl-x(L). Anal Biochem 307: 70–75, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.