Abstract
Peptide YY (PYY) antisecretory effect on intestinal epithelia is well established, whereas less is known about its actions to influence colonic motility in conscious animals. We characterized changes in basal function and stimulated colonic motor function induced by PYY-related peptides in conscious mice. PYY3–36, PYY, and neuropeptide Y (NPY) (8 nmol/kg) injected intraperitoneally inhibited fecal pellet output (FPO) per hour during novel environment stress by 90%, 63%, and 57%, respectively, whereas the Y1-preferring agonists, [Pro34]PYY and [Leu31,Pro34]NPY, had no effect. Corticotrophin-releasing factor 2 receptor antagonist did not alter PYY3–36 inhibitory action. PYY and PYY3–36 significantly reduced restraint-stimulated defecation, and PYY3–36 inhibited high-amplitude distal colonic contractions in restrained conscious mice for 1 h, by intraluminal pressure with the use of a microtransducer. PYY suppression of intraperitoneal 5-hydroxytryptophan induced FPO and diarrhea was blocked by the Y2 antagonist, BIIE0246, injected intraperitoneally and mimicked by PYY3–36, but not [Leu31,Pro34]NPY. PYY3–36 also inhibited bethanechol-stimulated FPO and diarrhea. PYY3–36 inhibited basal FPO during nocturnal feeding period and light phase in fasted/refed mice for 2–3 h, whereas the reduction of food intake lasted for only 1 h. PYY3–36 delayed gastric emptying after fasting-refeeding by 48% and distal colonic transit time by 104%, whereas [Leu31,Pro34]NPY had no effect. In the proximal and distal colon, higher Y2 mRNA expression was detected in the mucosa than in muscle layers, and Y2 immunoreactivity was located in nerve terminals around myenteric neurons. These data established that PYY/PYY3–36 potently inhibits basal and stress/serotonin/cholinergic-stimulated propulsive colonic motor function in conscious mice, likely via Y2 receptors.
Keywords: PYY3–36, stress, serotonin, bethanechol
peptide yy (pyy) is a 36-amino-acid peptide that was first isolated from porcine gut as containing tyrosine (Y) residues at both amino and carboxyl terminals (59). PYY shares structural homology with neuropeptide Y (NPY) and pancreatic polypeptide (PP), and together these comprise the so called PP-fold superfamily (41). Gut PYY is produced by endocrine L cells in the small and large intestinal mucosa of mammals including humans (14). The peptide exists in two main bioactive forms, namely PYY1–36 and the truncated form, PYY3–36 (21). PYY and related members have distinct affinity to the five cloned receptors Y1, Y2, Y4, Y5, and Y6, which belong to the family of G protein-coupled receptors (31, 41).
The well-established biological actions of PYY through interactions with Y1 and/or Y2 receptors primarily relate to the inhibition of gastric and small intestinal motility, pancreatic and intestinal mucosa secretion, and intestinal blood flow (10, 41). The peptide administered peripherally delayed gastric emptying in various species including rodents, guinea pigs, dogs, and humans (8, 33, 54). PYY is physiologically released into the systemic circulation after a meal when nutrients reach the small intestine and is a part of the “ileal brake” that postprandially inhibits gastrointestinal motility (44, 50).
Interestingly, the highest concentration of PYY is found in the colon of humans, dogs, rats, and mice (14, 44). The gene transcripts for Y1, Y2, Y4, and Y5 and immunoreactivity for Y1 receptors have been detected in human and rat colon although with heterogeneity in expression between species (16, 19, 28, 70). The influence of PYY in colonic motor function has been largely performed in vitro in muscle strips (17, 27, 48, 55). Superfusion of PYY to colonic muscle strips of guinea pig inhibited the twitch contraction induced by electrical stimulation (55) but increased basal colonic contraction in vitro in rats (16, 17, 48), mice (27), and human (16). In vivo studies on propulsive colonic motor function have been scarce and also yielded divergent results. Initial studies showed that intravenous injection of PYY and NPY suppressed colonic motility in anesthetized cats (25, 37), whereas subsequent studies in rats show an increase or decrease in colonic motility (9, 62, 63). In animal studies, receptor subtypes involved in PYY or NPY actions on colonic motor function are still to be characterized. In addition, although several NPY and Y receptor genetic models have been developed in mice (34), surprisingly there is no report on the influence of PYY and related family members in colonic motor function in conscious mice.
Therefore, the objectives of the study were to characterize the actions of intraperitoneal injection of PYY-related peptides on propulsive colonic motility in conscious mice under basal or stress-stimulated conditions, including prototypic PYY/NPY agonists with differential affinity for Y-receptor subtypes, namely PYY (Y2/Y1/Y5), NPY (Y1/Y2/Y5), [Leu31, Pro34]NPY, [Pro34]PYY (preferential Y1 agonists), and PYY3–36 (Y2 agonist) (41, 49). To address whether PYY3–36-induced suppression of the colonic response to a novel environmental stress involves activation of corticotrophin-releasing factor receptor 2 (CRF2) inhibitory pathways (42), we used the CRF2 antagonist, astressin2-B (51). Next, we investigated whether PYY3–36 and PYY inhibitory action can modulate the prokinetic effect of cholinergic and serotonergic transmitters (22, 64) using exogenous serotonin (5-HT) precursor, 5-hydroxytryptophan (5-HTP) that mimics a state of endogenous 5-HT production and release (52) and the long-acting muscarinic agonist, bethanechol. The role of Y2 in mediating PYY inhibitory action was further assessed pharmacologically with the selective Y2 antagonist, BIIE0246 (3, 12), and potential site of action by assessing the expression of Y2 receptor at the gene and protein levels in the mice colon.
MATERIALS AND METHODS
Animals
Male adult C57BL/6 mice (7–12 wk old, body wt 21–30 g, Harlan, Indianapolis, IN) were in group housing (4/cage) and fed ad libitum with standard rodent chow (Prolab RMH 2500; PMI Nutrition International, Brentwood, MO) and water. All the experiments, except otherwise stated, were performed between 9:00 AM and 12:00 PM in freely fed mice. NIH guidelines were followed in all experimental procedures that were undertaken under the auspices of an OLAW Assurance of Compliance (A3002-01) and performed according to approved protocols (IACUC Committee of the VA Greater Los Angeles Healthcare System, numbers 05058-02 and 04056-06).
Substances
Mouse/rat/porcine PYY, mouse/rat/porcine PYY3–36, mouse/rat/human NPY, mouse [Leu31,Pro34] NPY, and astressin2-B were kindly provided by Dr. J. Rivier (Clayton Foundation Laboratories for Peptide Biology, The Salk Institute, La Jolla, CA), and mouse [Pro34]PYY was provided by Dr. J. Reeve (Peptidomics Core, CURE: Digestive Diseases Research Center, UCLA, Los Angeles, CA). Y2 antagonist, BIIE0246, was purchased from Tocris (Ellisville, MO), bethanechol chloride and 5-HTP [L-2-amino-3-(5-hydroxyindolyl) propionic acid] from Sigma Chemical (St. Louis, MO). Peptides, 5-HTP, and bethanechol in powder form were dissolved in saline, astressin2-B in sterile distilled water, and BIIE0246 in vehicle (10% dimethyl sulfoxide, 5% Tween-80 and 85% saline) immediately before the experiments. The volume of intraperitoneal injection was 0.1 ml/mouse.
Acute Stress
Novel environment.
Naive mice were taken out from their home cage (group housing) and placed singly in a clean blue-colored and semitransparent cubic box with a white semitransparent cover (monitor box: 30 × 30 × 20 cm; Sterilite, Townsend, MA) for 1 h.
Restraint stress.
Naive mice were placed singly into a tube (3 × 7 cm) modified from Falcon 50-ml plastic tube (Becton Dickinson, Franklin Lakes, NJ) with holes on it for adequate ventilation as in our previous studies (39).
Measurements of Gut Motor Function and Food Intake
Defecation monitoring and diarrhea score.
The number of fecal pellets excreted was monitored at 15-min intervals for a 1-h period during stress exposure and/or after compound injections. Diarrhea was recorded using 4 levels of scoring: 0, no diarrhea; 1, ≤1 watery nonshaped and/or loose pellets; 2, 2 watery nonshaped pellets; 3, ≥3 watery nonshaped pellets.
Distal colonic pressure recording by microtransducer.
Distal colonic pressure recording was achieved by using a noninvasive miniature pressure transducer inserted into the distal colon as we recently described (20). Mice were briefly anesthetized with isoflurane (4% in O2), and a pressure transducer catheter (SPR-524 Mikro-Tip catheter; Millar Instruments, Houston, TX) lubricated with medical grade lubricant was introduced into the distal colon 2 cm past the anus and secured to the tail with tape. The colonic contractions were recorded in conscious mice after placing them in the restraint tube. The pressure transducer was connected to a preamplifier (model 600; Millar Instruments). The signal was then amplified using a transducer amplifier (TBM4; World Precision Instruments, Boca Raton, FL), acquired via a Micro1401 A/D interface (Cambridge Electronic Design, Cambridge, MA) and recorded using Spike 2 version 5 data acquisition software. Abdominal contractions were excluded by smoothing the original trace with a time constant of 2 s. The colonic contractile pressure changes were quantified by measuring the phasic component of the intraluminal pressure trace of the area under the curve (pAUC) using online quantifications for every minute. The phasic component of intracolonic pressure was extracted from the original trace by removing the direct current component with a time constant of 10 s from the 2-s smoothed original trace. In addition, the mean pAUC was calculated over the time periods of 0–15 min because the main contractile colonic pressure occurred usually during the first 20 min, as we recently reported under similar conditions (20). In each trace, the number of high-amplitude contractions (>25 mmHg) was calculated. We previously established that these contractions propagated in more than 50% of the cases in conscious mice under similar conditions of recording and bore characteristics of giant migrating contractions (20). The low-amplitude contractions were defined as amplitude comprised between 10 and 25 mmHg. The colonic motility results were expressed as frequency (number of contractions/h) for the first 15 min and 15–60-min periods as previously stated (20). A total of 439 contractions from 16 h of recording were analyzed.
Gastric and distal colonic transit measurements.
Gastric emptying of a solid nutrient meal and distal colonic transit were monitored simultaneously in conscious mice as in our previous studies (40). Overnight (16 h) fasted mice had free access to water and preweighed rodent chow for 1 h and then were briefly anesthetized with isoflurane to insert a 2-mm glass bead into the distal colon at 2 cm from the anus using a glass rod with a polished end lubricated with water to avoid tissue damage. Distal colonic transit was determined by monitoring the time when the glass bead was expelled (bead latency). The percentage of gastric emptying of the ingested meal was assessed 2 h after the end of 1-h refeeding as previously detailed (39), and fecal pellets were also counted for the 2-h postfeeding period.
Food intake.
Mice were trained in individual cages with a grid about 3 cm above the cage bottom 4 h/day for 3 days under similar conditions used for food intake tests. Food intake was monitored at 1, 2, and 4 h after intraperitoneal injection by measuring the difference between the preweighed standard chow and the weight of chow and spill at the end of each time point as in our previous studies (65).
Colonic Tissue Processing
RNA isolation.
The proximal and distal segments of the colon were dissected from naive mice and the mucosa separated from other layers. Total RNAs were extracted with a phenol-quanidine thiocyanate-chloroform method (RNA Bee from Tel-Test, Friendswood, TX).
RT-PCR.
First-strand oligo-dT-primed cDNA was synthesized from total RNA (5 μg) of each sample by thermostable SuperScript reverse transcriptase at 55°C for 1 h (Invitrogen, Carlsbad, CA). Oligonucleotide primers for Y2 transcripts were forward 5′-GTCAATACAGTAAGCCAAGTGAG and reverse 5′-AGGGCACCAAATGGCACAAGACC (423 bp; accession number GenBank D86238); and house keeping gene, S16 forward 5′-TGCGGTGTGGAGCTCGTGCTTGT and reverse 5′-GCTACCAGGCCTTTGAGATGGA (309 bp, GenBank accession number M11408). RT-PCR was performed in a final volume of 50 μl using a Red-Taq DNA Polymerase System (Sigma). The reaction mixture was performed under the following conditions: predenaturation at 94°C for 2 min and then cycling 34 times (92°C, 50 s; 59°C, 40 s; 72°C, 1 min 30 s) for amplification, followed by a 5-min elongation at 72°C (MyCycler; Bio-Rad Laboratories, Hercules, CA). The amplified PCR products were fractionated by electrophoresis in 1% agarose gel with ethidium bromide and detected under UV light. The gel images were acquired by Kodak EDAS 290 system. The band density of the RT-PCR products was measured using NIH Image program (Scion, Frederick, MD), and the Y2 band density was normalized to that of S16 from the same sample; results were expressed in corrected arbitrary units.
Whole-mount preparation of colon enteric plexus.
The proximal and distal colonic tissues were collected from naive mice, opened longitudinally along the mesenteric border, and processed as previously described (64). Briefly, the stretched tissue was fixed by immersion with 4% paraformaldehyde and 14% saturated picric acid in 0.1 M phosphate buffer (pH 7.4) overnight at 4°C. The submucosal layer with the submucosal plexus was peeled off as whole mount. In addition, another whole-mount preparation consisting of the serosa and longitudinal muscle layer with the myenteric plexus attached to its internal side (longitudinal muscle/myenteric plexus, LMMP) was obtained.
Immunohistochemistry for Y2 receptor in enteric plexus.
Free-floating submucosal and LMMP whole mounts of both proximal and distal colon from three naive mice were treated in 10% normal goat serum for 30 min and followed by incubation with polyclonal rabbit anti-Y2 serum diluted at 1:1,000 (Neuromics, Edina, MN) for 2 nights at 4°C. After a thorough rinse, the colon whole mounts were incubated in goat anti-rabbit IgG conjugated with FITC (1:100; Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. The tissues were mounted on slides and sealed by cover slides with anti-fading media (Vector Laboratories, Burlingame, CA). For the antibody specificity, controls were performed by preabsorption of primary antibody with the Y2-immunogenic peptide (TDSFSEATN-COOH; Neuromics) or omission of the primary antibody. For the preabsorption, the Y2 antibody was diluted at working titer (1:1,000), and 20 μg of Y2 immunogenic peptide was added with an antibody:antigen ratio at 1:20. The solution was incubated for 2 days at 4°C. The procedures for immunostaining were the same as above, except either the primary antibody incubation was replaced by preabsorbed or without primary antibody.
The whole mounts were observed by fluorescent microscopy (Axioscop II; Carl Zeiss, Jena, Germany). Images were acquired by a digital camera (Hamamatsu, Bridgewater, NJ) using the image acquisition system SimplePCI (Hamamatsu, Sewickley, PA).
Experimental Protocols
Effects of PYY3–36 and related peptides on novel environment-induced defecation.
The effective dose of PYY3–36 was selected on the basis of a dose-response study using intraperitoneal injection of the peptide at 0.8, 2.5, and 8 nmol/kg compared with intraperitoneal saline. In subsequent studies in naive mice, the related peptides, namely PYY, NPY, [Pro34]PYY, and [Leu31,Pro34]NPY were injected intraperitoneally at the equimolar effective dose of PYY3–36 (8 nmol/kg), and controls received intraperitoneal saline. Ten minutes after intraperitoneal injection, mice were placed in a novel environment (monitor box) for 1 h, and fecal pellet output (FPO) was monitored at 15-min intervals. To assess whether CRF2 receptor mediated the PYY3–36 inhibitory effect on stress-induced increased FPO, astressin2-B (30 μg/kg) or vehicle (sterile water) was injected intraperitoneally 10 min before the intraperitoneal injection of PYY3–36 (8 nmol/kg), and 10 min later mice were exposed to novel environment for 1 h. The dose of astressin2-B was based on our previous dose-response studies showing complete suppression of intraperitoneal urocortin-induced alterations of gut motor function in mice (51).
Effects of PYY3–36 and PYY on restraint stress-induced defecation and colonic contractile activity.
Mice were trained for 1 h/day for 3–4 days before the experiments by handling and placing them singly in the monitor box to avoid novel environment response in the control group. On the experimental day, mice were injected intraperitoneally with saline, PYY, or PYY3–36 (8 nmol/kg), and 10 min later they were placed individually in a restraint tube or the monitor box (controls) for 1 h. FPO was assessed for 1 h at 15-min intervals.
To measure distal colonic contractions, naive mice under brief anesthesia were equipped with a miniature transducer inserted into the distal colon and then injected intraperitoneally with PYY3–36 (8 nmol/kg) or saline. Ten minutes later, conscious mice were placed individually in a restraint tube, and changes in colonic intraluminal pressure were recorded for 1 h.
Effects of PYY3–36 or related peptides and Y2 antagonist on 5-HTP and bethanechol-induced defecation and diarrhea.
All the mice used in these experiments had been trained to be acquainted to the monitor box, thereby avoiding the influence of novelty stress. Mice were injected intraperitoneally with saline, PYY, PYY3–36, or [Leu31,Pro34]NPY (8 nmol/kg) and 10 min later with saline, 5-HTP (10 mg/kg), or bethanechol (5 mg/kg). In a subgroup, the Y2 receptor antagonist, BIIE0246 (5 mg/kg), or its vehicle (10% DMSO, 5% Tween-80 and 85% saline) was injected intraperitoneally 10 min before intraperitoneal injection of PYY (8 nmol/kg), and 10 min thereafter 5-HTP (10 mg/kg ip) or saline was injected. After 5-HTP or bethanechol injection, mice were placed individually in the monitor box for 1 h, and FPO and diarrhea were monitored every 15 min. The doses of 5-HTP and bethanechol were selected on the basis of previous studies showing maximal defecation or diarrhea in mice (46, 64) and that of Y2 antagonist on the basis of previous reports (45, 56).
Simultaneous monitoring of PYY3–36 effects on food intake and defecation.
Mice were trained to be maintained in individual cages with grid for 4 h/day, 3–4 days before the experiment. Food intake and FPO were monitored hourly for 4 h after the intraperitoneal injection of saline or PYY3–36 (8 nmol/kg), either before the onset of dark phase in nonfasted mice (6:00 PM) or around 9:00 AM in overnight fasted, but not water-deprived, mice.
Simultaneous monitoring of PYY and related peptides on gastric and distal colonic transit.
After an overnight fast, mice had free access to rodent chow for 1 h; then food and water were removed, and mice were injected intraperitoneally with saline, PYY, PYY3–36, or [Leu31,Pro34]NPY (8 nmol/kg). A glass bead was then inserted into the distal colon under brief anesthesia (∼2 min), and the time at which the bead was expelled was monitored. At 2 h postinjection, animals were euthanized, and gastric emptying of the ingested meal was monitored along with the cumulative FPO.
Y2 receptor expression in colon.
Nonfasted naive mice (n = 4) were euthanized, and the colon was harvested. The mucosa was separated from other layers in the proximal and distal colon, and tissue were processed for Y2 mRNA. In other naive nonfasted mice (n = 3), the proximal and distal colon was collected, and Y2 immunohistochemistry performed on submucosal and LMMP whole mounts.
Statistical Analysis
Results are expressed as means ± SE. Comparisons within multiple groups were performed using one-way ANOVA followed by an all pair-wise multiple-comparison test (Tukey's test). Comparisons between two groups were performed with Student's t-test. Correlations were analyzed by linear regression. Values of P < 0.05 were considered statistically significant.
RESULTS
PYY3–36 and Related Peptides Inhibited Acute Stress-Induced Propulsive Colonic Motor Function in Conscious Mice
Novel environment stress.
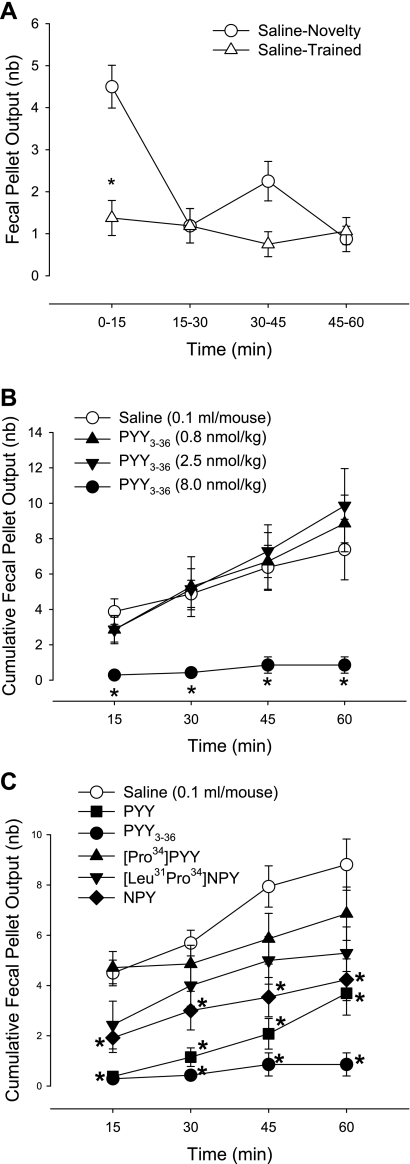
Naive mice injected intraperitoneally with vehicle and placed singly in a novel housing environment had increased FPO compared with mice with intraperitoneal vehicle acquainted to the monitoring boxes. The cumulative FPO at 15 and 60 min was 4.5 ± 0.5 and 8.8 ± 1.0 vs. 1.4 ± 0.4 and 4.4 ± 0.9 in trained mice, respectively (n = 16 in each group; P < 0.05), with a peak response at 15 min (Fig. 1A). Compared with trained mice, PYY3–36 injected intraperitoneally at 8 nmol/kg (30 μg/kg, n = 8) completely prevented FPO induced by novelty stress throughout the 1-h monitoring period (0.3 ± 0.2 and 0.9 ± 0.5 at 15 min and 1 h, respectively), whereas lower doses (0.8 or 2.5 nmol/kg) had no significant effect at any time point (Fig. 1B).
Fig. 1.
Intraperitoneal peptide YY (PYY) and PYY3–36 inhibit fecal pellet output (FPO) response to novelty environmental stress for 1 h in mice. A: novelty increased defecation in naive mice (n = 16) exposed singly to a novel environment (box) compared with trained mice (n = 16) accustomed singly to the box for 1 h/day for 3–4 days prior. B: dose-response of PYY3–36 on novelty stress-induced defecation. PYY3–36 was injected intraperitoneally 10 min before the stress (n = 7–8/group). C: effect of intraperitoneal injection of neuropeptide Y (NPY)/PYY agonists with different Y receptor selectivity on novelty stress-induced defecation. Peptides (8.0 nmol/kg ip) or saline were injected 10 min before the stress. FPO was monitored every 15 min for 1 h. Data are means ± SE of n = 7–13/group. *P < 0.05 vs. saline at the corresponding time.
PYY (8 nmol/kg, n = 13) injected intraperitoneally also blocked the 15-min peak FPO response (number/h) to a novel environment (0.5 ± 0.3) with defecation resuming thereafter although remaining significantly inhibited (4.6 ± 1.3) compared with the saline group (Fig. 1C). NPY (8 nmol/kg ip, n = 13) induced a lesser inhibitory effect than PYY3–36 both at the peak effective time (15 min) and 1 h (FPO: 1.9 ± 0.6 and 4.2 ± 0.8, respectively, n = 13). The Y1-preferring agonists, [Pro34]PYY and [Leu31,Pro34]NPY (8 nmol/kg, n = 7 in each group) had no significant effect compared with saline injection at any time point (Fig. 1C).
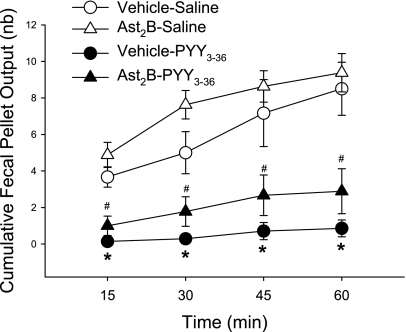
Involvement of CRF2 signaling pathways in PYY3–36 inhibitory action in novelty stress-induced FPO was assessed using the peptide CRF2 antagonist, astressin2-B as pretreatment. Astressin2-B (30 μg/kg ip) had a similar tendency to enhance FPO both in saline- and PYY3–36 (8 nmol/kg ip)-treated mice exposed to novelty stress, and there was no modification of PYY3–36 inhibitory effect on novelty stress-stimulated FPO (n = 6–9/group, Fig. 2).
Fig. 2.
Corticotrophin-releasing factor 2 is not involved in PYY3–36 inhibition of 1-h novelty stress-induced defecation. Astressin2-B (Ast2B, 30 μg/kg ip) or vehicle was injected 10 min before intraperitoneal injection of PYY3–36 (8 nmol/kg) or saline, and mice were exposed to novelty stress 10 min later. Data are means ± SE of n = 6–9/group. *P < 0.05 vs. vehicle-saline; #P < 0.05 vs. Ast2B-saline at the corresponding time.
Restraint stress.
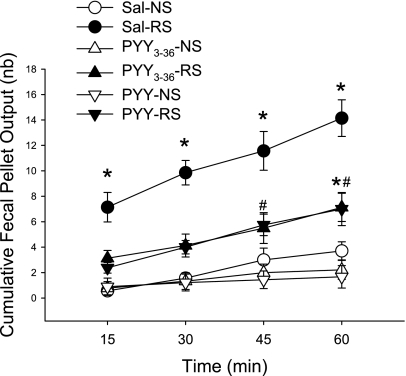
All mice used in this experiment had been trained and habituated to the monitor box and displayed only a nonstimulated FPO. In intraperitoneal saline-injected mice, restraint induced a peak response within the first 15 min (FPO/15 min: 7.1 ± 1.2 vs. nonrestraint, 0.6 ± 0.3, n = 7/group, P < 0.05), whereas, thereafter, there was a steady increment of ∼2 pellet/15 min resulting in 14.1 ± 1.4 FPO for the 1-h restraint period compared with 3.7 ± 0.7 in control group (Fig. 3). PYY3–36 and PYY (8 nmol/kg ip; n = 8/group) inhibited significantly the restraint stress-induced 15-min peak defecation by 52% and 67% and 1-h FPO by 50% and 50%, respectively (Fig. 3). In nonrestraint mice, both PYY and PYY3–36 values were similar to those of saline group (Fig. 3).
Fig. 3.
Intraperitoneal PYY and PYY3–36 inhibit 1-h restraint stress-induced FPO in mice. Peptides (8 nmol/kg) and saline (Sal) were injected 10 min before restraint stress (RS) or nonstress (NS) (mice were placed in the monitor box for 1 h, in which they were acquainted). Data are means ± SE of n = 7–9/group. *P < 0.05 vs. Sal-NS; #P < 0.05 vs. PYY-NS or PYY3–36-NS.
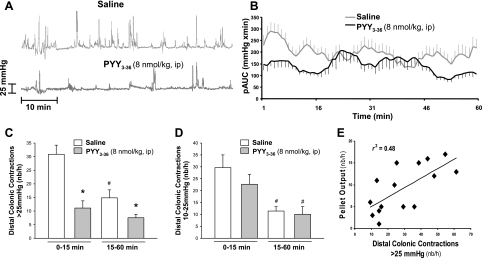
Conscious mice injected intraperitoneally with saline (n = 6) and placed in the restraint tubes displayed low- (10–25 mmHg) and high-amplitude (>25 mmHg) distal colonic contractions (Fig. 4A). Analysis of the first 15-min restraint period indicated a robust transient activation of distal colonic contractions (Fig. 4B). There was an increase in frequency (number/h) of contractions with high (30.9 ± 3.3) and low (29.7 ± 5.2) amplitude compared with those during the remaining 15–60-min period (14.8 ± 2.9 and 11.4 ± 1.9, respectively, P < 0.05; Fig. 4, C and D), which were similar to those previously reported in naive mice (20). PYY3–36 (8 nmol/kg, n = 9) injected intraperitoneally before restraint exposure reduced significantly the frequency (number/h) of high-amplitude contractions threefold during the first 15 min of restraint (11.1 ± 2.6 vs. 30.9 ± 3.3 in saline) and twofold during the subsequent 15–50-min period (7.6 ± 1.2 vs. 14.8 ± 2.9 in saline) (Fig. 4C). The frequency (number/h) of low-amplitude contractions did not change either during the 15-min period (PYY3–36: 22.6 ± 4.2 vs. saline: 29.7 ± 5.2) or the subsequent 45 min (PYY3–36: 10.1 ± 3.2 vs. saline: 11.4 ± 1.9; Fig. 4D). The pAUC of distal colonic motility during the first 15-min period was reduced by 46.5% (131.6 ± 19.2 vs. saline: 232.1 ± 26.3 mmHg × min, respectively, P < 0.05). The 1-h FPO monitored at the same time as the recording of distal intracolonic pressure was correlated to the number of high (r2 = 0.48, P < 0.05; Fig. 4E) but not to the low-amplitude contractions (r2 = 0.08, P > 0.05, data not shown).
Fig. 4.
Intraperitoneal PYY3–36 reduces distal colonic intraluminal pressure using a noninvasive miniature pressure transducer inserted into the distal colon in restrained conscious mice. After intraperitoneal injection of PYY3–36 (8 nmol/kg, n = 9) or saline (n = 6), mice were briefly anesthetized for insertion of the pressure transducer, and distal intracolonic pressure was recorded 10 min later for 1 h. A: representative raw trace during the 1-h recording in mice injected with intraperitoneal saline and PYY3–36. B: time course of the phasic component of intraluminal pressure trace of area under curve (pAUC; mmHg × min) over 1-h recording. C: mean of distal colonic contractions (number/h) with high amplitude (>25 mmHg) in the first 15 min and in 15–60 min. D: mean of distal colonic contractions (number/h) with low amplitude (10–25 mmHg) in the first 15 min and in 15–60 min. F: correlation between 1-h distal colonic contractions (number/h) with high amplitude (>25 mmHg) and FPO. Data are means ± SE. *P < 0.05 vs. saline; #P < 0.05 vs. 0–15 min of the same treatement.
PYY3–36-inhibited Colonic Motor Response to 5-HTP and Bethanechol
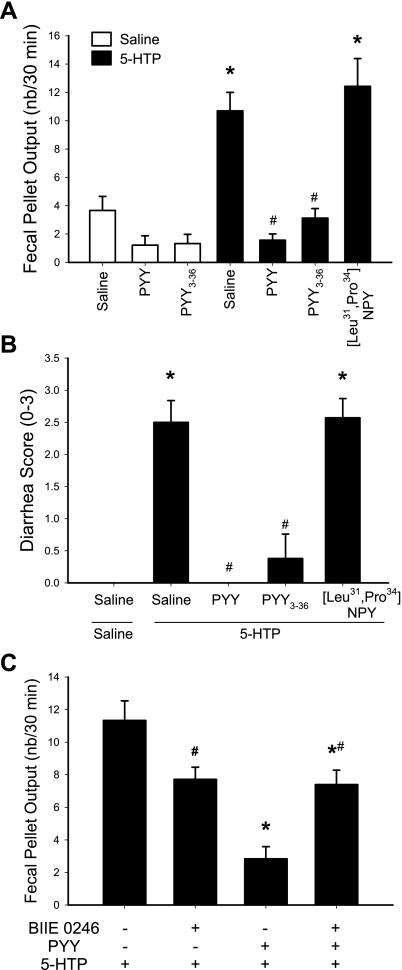
All mice used in these experiments had been acclimated to the monitor boxes to avoid the response to novelty stress. The intraperitoneal injection of 5-HTP (10 mg/kg) alone induced a peak stimulation of FPO within the first 15 min postinjection as we previously described (64). Therefore, results are presented as the cumulative first 30-min response. In saline-pretreated mice, 5-HTP induced a significant increase in FPO/30 min, reaching 10.7 ± 1.3 (n = 10) compared with 3.7 ± 1.0 in saline plus saline-treated group (n = 9, P < 0.05). Both PYY3–36 and PYY (8 nmol/kg ip) completely blocked the defecation response to 5-HTP (3.1 ± 0.7 and 1.6 ± 0.4, respectively, n = 8 or 7 in each group; P < 0.05 vs. saline plus 5-HTP), whereas the Y1-preferring agonist, [Leu31,Pro34]NPY, had no effect (number/30 min: 12.4 ± 2.0, n = 7; P < 0.05; Fig. 5A). All mice treated with 5-HTP developed diarrhea that occurred mostly within the first 15 min after the injection (Fig. 5B). PYY and PYY3–36 both abolished the diarrhea, whereas [Leu31,Pro34]NPY had no effect (Fig. 5B). The Y2 antagonist, BIIE0246 (5 mg/kg ip), completely prevented intraperitoneal PYY-induced blockade of the 30-min FPO increase (Fig. 5C) and diarrhea (data not shown) in response to intraperitoneal 5-HTP. BIIE0246 injected before 5-HTP resulted in a 32–35% significant reduction of FPO compared with vehicle plus 5-HTP group.
Fig. 5.
Intraperitoneal PYY-induced suppression of colonic secretory motor response to 5-hydroxytryptophan (5-HTP) through Y2 receptor in conscious mice. A: 30-min FPO after PYY, Y1 agonist, [Leu31,Pro34]NPY, and Y2 agonist, PYY3–36 (8 nmol/kg), or saline were injected intraperitoneally 10 min before 5-HTP (10 mg/kg ip); n = 7–10/group. *P < 0.05 vs. saline-saline; #P < 0.05 vs. saline-5-HTP. B: diarrhea score from the same mice in A. *P < 0.05 vs. saline-saline; #P < 0.05 vs. saline-5-HTP. C: PYY-induced suppression of 30-min stimulated defecation induced by intraperitoneal 5-HTP in mice, with reversal by the Y2 antagonist, BIIE0246. BIIE0246 (5 mg/kg) or vehicle was injected intraperitoneally at 20 min before and PYY3–36 (8 nmol/kg) 10 min before 5-HTP (10 mg/kg) or saline in mice (n = 6–10/group). Data are means ± SE *P < 0.05 vs. vehicle-saline-5-HTP; #P < 0.05 vs. vehicle-saline-5-HTP.
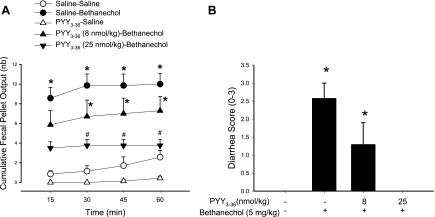
Bethanechol injected (5 mg/kg ip) in saline-pretreated mice induced diarrhea and increased significantly FPO in the first 15 min compared with saline plus vehicle (8.6 ± 1.1 vs. 0.9 ± 0.3 pellets, n = 7 in each group; P < 0.05, Fig. 6). The FPO reached a plateau response at 30 min (Fig. 6A). PYY3–36 (8 and 25 nmol/kg ip) pretreatment resulted in a dose-related suppression of bethanechol-induced stimulation of propulsive colonic motor function (pellets/15 min: 5.9 ± 1.4 and 3.5 ± 0.6, respectively, n = 7 or 8 in each group), and there was a 100% inhibition of FPO and diarrhea response at 1 h postinjection with the highest dose of PYY3–36 (Fig. 6).
Fig. 6.
Dose-related action of intraperitoneal PYY3–36 on bethanechol-induced stimulation of FPO (A) and diarrhea (B) in mice. PYY3–36 (8 and 25 nmol/kg) or saline was injected intraperitoneally 10 min before intraperitoneal bethanechol chloride (5 mg/kg) or saline. FPO was monitored every 15 min for 1 h. Data are means ± SE of n = 7/group. *P < 0.05 vs. saline-saline; #P < 0.05 vs. saline-bethanechol.
Time Course of PYY3–36 Inhibitory Effects on Food Intake and Gut Motor Function
Food intake and FPO.
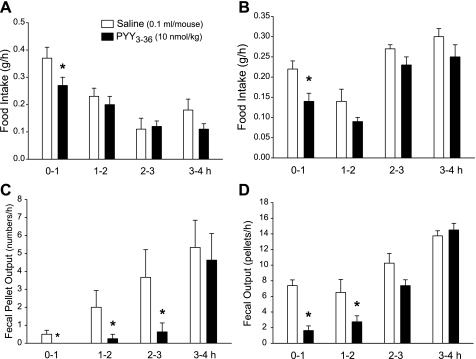
PYY3–36 (8 nmol/kg) injected intraperitoneally inhibited food intake response to an overnight fast by 27.0% only for the first hour compared with saline and no longer during the subsequent 3-h period (Fig. 7A). By contrast, the peptide significantly inhibited FPO/h monitored simultaneously by 100%, 87.5%, and 82.8% during the first 3 h postinjection, respectively, with a return to control values during the fourth hour (Fig. 7C). Likewise, PYY3–36 injected (8 nmol/kg ip) in freely fed mice at the onset of the dark phase significantly reduced food intake by 36.4% during the first hour only, whereas there was a significant 77.9% and 57.7% inhibition of FPO during the first and second hour periods postinjection, respectively (Fig. 7, B and D).
Fig. 7.
Time course of PYY3–36-induced inhibition of food intake (A and B) and fecal output (C and D) monitored simultaneously hourly for 4 h in fasted/refed mice (A and C) or in response to dark phase in freely fed mice (B and D). Data are means ± SE of n = 8/group. *P < 0.05 vs. saline.
Gastric and distal colonic transit.
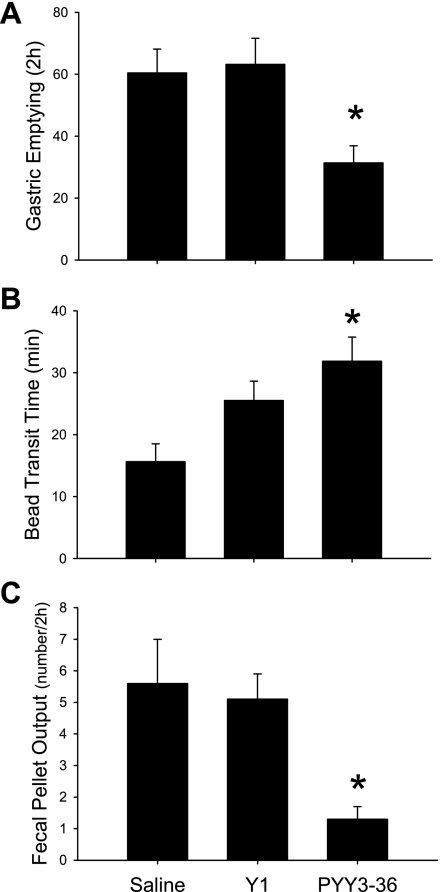
In overnight-fasted and 1-h freely refed mice, PYY3–36 injected intraperitoneally (8 nmol/kg) reduced by 48% the amount of food emptied from the stomach (Fig. 8A) and slowed the distal colonic transit time as shown by the 104% increase in the time at which the intracolonic bead was expelled compared with saline (Fig. 8B). By contrast, [Leu31,Pro34]NPY had no significant effect on gastric and colonic transit under the same conditions (Fig. 8, A and B). There was also a significant 77% reduction of 2-h FPO induced by PYY3–36 that was not observed with the Y1-preferring agonist (Fig. 8C).
Fig. 8.
Intraperitoneal PYY3–36 inhibits gastric emptying of solid food (A), distal colonic transit (B), and defecation (C) monitored simultaneously in fasted/refed mice. Peptides, Y1 agonist [Leu31,Pro34]NPY (Y1), PYY3–36 (8 nmol/kg), or saline was injected intraperitoneally after 1-h refeeding in mice fasted overnight. Data are means ± SE of n = 7–8/group; *P < 0.05 vs. saline.
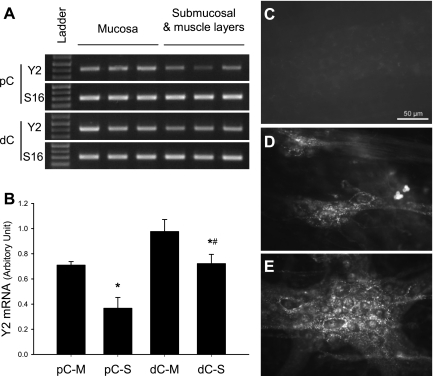
Y2 Receptor Expression in Mouse Colon
Y2 receptor transcript was detected by RT-PCR both in the mucosa layer and submucosa plus muscle layers with a higher signal in the mucosa than in the other layers in both the proximal and distal colon, as well as a higher signal in the distal than proximal colon (Fig. 9, A and B). Immunofluorescent staining revealed the localization of Y2 receptor in the submucosal (Fig. 9D) and myenteric plexi (Fig. 9E) in whole-mount submucosal layer and LMMP preparations of both proximal and distal colon. The labeling was found mainly in nerve terminals shown as varicosity around neurons (Fig. 9, D and E) and running between ganglia. Incubation of whole-mount preparation of proximal and distal colon with primary antiserum preadsorbed with the immunogenic Y2 receptor peptide resulted in complete disappearance of immunostaining (Fig. 9C), as well as when the Y2 antibody was omitted (data not shown).
Fig. 9.
Expression of Y2 receptor at the gene and protein levels in proximal and distal colon of naive mice. A: representative gel images of Y2 mRNA (423 bp) detected by RT-PCR in both proximal colon (pC) and distal colon (dC) of mice. S16 is the housekeeping gene (309 bp). B: semiquantitative analysis of Y2 mRNA gel bands performed by NIH imaging. M, mucosal layer; S, layers including smooth muscles and enteric plexi; n = 4; *P < 0.05 vs. pC-M and dC-M, respectively; #P < 0.05 vs. pC-S. C: primary antibody control by preabsorption with immunogenic peptide in myenteric plexus of the distal colon. D and E: representative photomicrographs of Y2 immunoreactivity in the distal colon submucosal (D) and myenteric (E) plexus. Scale bar = 50 μm.
DISCUSSION
The present study demonstrates that mouse PYY3–36 injected intraperitoneally completely suppressed the stimulated colonic propulsive response to the stress of a novel housing environment or restraint and the secretory-motor responses to peripheral injection of 5-HTP and cholinergic agonist (bethanechol) as assessed by monitoring defecation, diarrhea, distal colonic contractions, or distal colonic transit in conscious mice. PYY3–36 injected intraperitoneally also inhibited basal defecation occurring during the nocturnal feeding period and the refeeding following an overnight fast. To our knowledge, the present findings provide the first evidence of an inhibitory action of PYY3–36 on propulsive colonic motor function in conscious mice. There was only one in vivo study that reported reduced basal colonic myoelectrical activity in conscious rats after intravenous injection of PYY as assessed by the decrease in the total number of spike bursts compared with controls (9). In our study, PYY3–36 injected intraperitoneally significantly reduced the frequency of high-amplitude contractions in the distal colon throughout the 1-h restraint conditions in conscious mice monitored using a novel noninvasive manometric method (20). We recently characterized these high-amplitude contractions to be giant migrating contractions (20) that were found in the present study to be correlated with fecal output. By contrast, other studies in anesthetized rats and rabbits showed that intravenous injection of NPY or PYY increased colonic contractile activity (38, 63). The existing and present data may indicate that the state of the animal conditions, conscious vs. anesthetized, influences PYY- and NPY-induced colonic motility alterations, resulting in an inhibitory or stimulatory effect, respectively.
We compared the inhibition of colonic motor function induced by intraperitoneal PYY3–36 with its well-established inhibitory effects on upper gut transit and food intake (4, 8, 30, 66). We found that PYY3–36 injected intraperitoneally at 8 nmol/kg resulted in a more robust suppression of propulsive colonic than gastric motor function or food consumption as monitored simultaneously in conscious mice. This was shown by the greater magnitude (78%-58%) inhibition of defecation occurring during the first 2 h of the nocturnal free feeding, whereas the decrease in food intake was 36% and lasts only the first hour after PYY3–36 injection. Moreover, concurrent monitoring of distal colonic transit, defecation, and gastric emptying of solid food in conscious mice showed that intraperitoneal PYY3–36 induced a higher percentage of inhibition of distal colonic transit (104%) and defecation (77%) than gastric emptying (48%). In addition, there was a long-lasting suppression of giant migrating contractions in the distal colon induced by intraperitoneal PYY3–36 throughout the 1-h restraint period compared with saline group. The intraperitoneally effective dose (8 nmol/kg) at which PYY3–36 completely suppressed basal- and stress-stimulated colonic motor function was lower that the reported dose (50 μg/kg, about 12 nmol/kg) inducing maximal activation of medullary brain neurons and reduction of dark phase food intake in mice (23, 66).
We used a pharmacological approach to ascertain the role of Y2 receptors in mediating PYY3–36 inhibitory actions on the colon. Convergent data support such a contention. First, binding studies indicate that PYY3–36 is a relatively selective agonist for the Y2 receptor (13, 31, 41). Second, differential results were obtained with prototypic NPY/PYY agonists with differential binding affinity to the Y2 receptors when peptides were injected intraperitoneally at the effective equimolar dose of PYY3–36. In particular, we showed that the preferential Y1 agonists, [Leu31,Pro34]NPY or [Pro34]PYY (41, 47), were unable to elicit any significant changes in distal colonic transit and defecation during the refeeding response after a fast and in the stimulated defecation induced by 5-HTP and a novel environment. In contrast, PYY that displays Y2/Y1/Y5 receptor affinity (31, 41, 57) inhibited defecation stimulated by exposure to a novel environment, restraint, and peripheral injection of 5-HTP. NPY, which also displays binding affinity to the Y2 receptor (31, 47), significantly inhibited the colonic response to novel environment although less prominently than PYY or PYY3–36, which display PYY-preferring profile (31, 47) with enhanced Y2 receptor effect in many tissues (18). Third, the highly selective nonpeptide Y2 antagonist, BIIE0246 (3, 12), injected intraperitoneally blocked PYY-induced defecation in response to 5-HTP. There was a 32–35% decrease in fecal output after BIIE0246 treatment in 5-HTP-treated mice, which could reflect a partial agonistic effect. Fourth, we found that PYY and PYY3–36 blocked 5-HTP- and bethanechol-induced diarrhea, whereas [Leu31,Pro34]NPY under the same conditions was not able to affect the diarrhea response to 5-HTP. Moreover, the selective Y2 antagonist, BIIE0246 (12), blocked PYY-induced suppression of 5-HTP-related diarrhea. Taken together, these data suggest that the activation of Y2 receptor is the predominant Y receptor subtype involved in PYY- and PYY3–36-induced suppression of basal- and stress-stimulated colonic motor function and diarrhea induced by muscarinic and serotonergic activation in conscious mice.
Previous studies in mice established that the decrease in gastric emptying and food intake induced by intraperitoneal injection of PYY3–36 is Y2 mediated, respectively, through vagal-dependent and -independent recruitment of specific brain circuitries (23, 58, 66). Nonaka et al. (43) have reported that PYY3–36 crosses the blood-brain barrier by nonsaturable process, and PYY3–36 injected intraperitoneally at a similar dose as used in the present experiment induced neuronal activation in some brainstem nuclei of mice (23). However, in the present study, functional and neuroanatomical evidence supports that the suppression of colonic function induced by intraperitoneal PYY3–36 may be exerted through a direct effect on Y2 receptor expressed in the colon. Previous in vitro studies using preparations of mouse colonic mucosa with intact submucosal plexus innervation showed that PYY3–36 inhibited electrogenic ion secretion stimulated by vasoactive intestinal peptide, and the response was completely abolished by the Y2 antagonist, BIIE0246, in wild-type mice and not observed in colonic mucosal tissue from Y2 knockout mice (27). Additionally, in this isolated colonic mucosa/submucosal plexus tissue preparation, the Y2 receptor antisecretory action is neurally mediated predominantly through modulation of submucosal enteric transmission by prejunctional Y2 receptor and, to a lesser extent, Y2 receptors on noncholinergic submucosal neurons (26, 27). Consistent with the functional evidence of the presence of Y2 receptor in mice colon, we showed the expression of Y2 receptors at the gene and protein levels that was not established before in the mice colon. In particular, we found that Y2 receptor mRNA expression in mucosa and submucosa/muscle layers displayed a more prominent density in the distal than proximal colon. Immunohistochemistry of colonic whole mount revealed Y2 labeling in nerve fibers and terminals forming varicosities around enteric neurons along with a suggestive localization within enteric neurons. The Y2 immunolabeling appears specific in that it was no longer observed with the Y2 antibody preabsorbed with the immunogenic peptide or omitting the Y2 antibody. In addition, previous studies established that the same kind of Y2 antibody stained a population of dorsal root ganglia neurons and superficial laminae nerve endings in spinal cord of wild-type mice, and such a staining is not present in Y2 knockout mice (6). In further support of Y2-mediated peripheral action is the prevention of PYY inhibitory effect on 5-HTP-stimulated defecation and diarrhea by the peripherally restricted Y2 antagonist, BIIE0246 (68), along with existing evidence that the central action of NPY and related peptides on colonic motor function is largely stimulatory through Y1 receptor activation (60).
The peripheral mechanisms through which PYY3–36 suppressed stress-stimulated colonic motor function were investigated in the context of known transmitters involved in the colonic motor response to stress. CRF1 and CRF2 receptors are expressed in mice proximal and distal colon (42). Immunohistochemistry studies revealed the presence of CRF1 and CRF2 receptors on neurons and neuronal fibers of colonic myenteric and submucosal plexi in rats and guinea pigs (7, 32, 36, 69). CRF1 receptor-mediated activation of colonic myenteric neurons contributes to stress-related defecation response, whereas activation of CRF2 receptors by intraperitoneal injection of the endogenous CRF2 ligand, urocortin 2, blocked novel environment stress-induced defecation in rodents (42). However, the CRF2 antagonist, astressin2-B, injected intraperitoneally, at a dose previously established to completely prevent gut motor alterations induced by intraperitoneal urocortin 1 in mice (51), did not alter the magnitude of PYY3–36 inhibitory effect on novel environment-induced defecation. These data indicate that activation of Y2 receptors by intraperitoneal PYY3–36 does not recruit CRF2 inhibitory signaling pathways in the colon under stress.
There is indisputable evidence that the activity of myenteric neurons is essential for induction of motility patterns associated with propulsive motor function (67). Indirect evidence indicates that PYY3–36 inhibitory action on colonic motor function may involve modulation of serotonergic and muscarinic enteric transmission. A direct action on colonic smooth muscles is unlikely based on a report that, in an isolated mouse colonic longitudinal smooth muscle preparation, PYY3–36 and NPY exclusively elicit a Y2-mediated tetrodotoxin-resistant excitatory contractile response (27). We previously characterized in conscious mice that the rapid onset defecation and diarrhea induced by intraperitoneal 5-HTP involves a 5-HT4 receptor-dependent activation of colonic cholinergic and nitrergic myenteric neurons (64). Restraint stress in mice also activates colonic myenteric neurons; the stimulation of motility is atropine sensitive (20), and defecation response is blocked by 5HT4 antagonists (53). The prokinetic effect of 5-HT4 receptor activation in mice is suggested to be linked with presynaptic induction or strengthening of excitatory neurotransmission (35). In the present study, we showed Y2 receptor labeling on colonic myenteric/submucosal nerve terminals around enteric neurons, consistent with a presynaptic site of action for the Y2-mediated inhibitory effects. Supportive of such a contention, electrophysiological studies indicate that PYY and Y2 agonist exert profound presynaptic inhibition of cholinergic transmission in myenteric neurons of guinea pig colon (5). Lastly, we found that PYY3–36 suppressed the bethanechol-induced diarrhea and increased fecal output in mice. Although the defecation and diarrhea score induced by bethanechol and 5-HTP were of similar magnitude, a threefold higher dose of PYY3–36 was required to achieve full blockade. These data suggest that Y2 inhibitory mechanisms have a higher sensitivity toward 5-HT4 than muscarinic receptor-initiated stimulation of colonic secretory motor function. In addition, the data indicate that PYY3–36 is able to interfere with muscarinic receptor activation of secretory motor processes, which are known to be mediated by both enteric and direct action on colonic epithelial cells (11). However, it cannot be ruled out that PYY- or PYY3–36-induced suppression of colonic motility also encompasses a more complex neural circuitry involving a primary action on sensory afferents, bearing similarity with that established in small intestinal transit inhibition (61).
In PYY3–36 doses ranging from 0.8 to 8.0 nmol, the inhibition of stimulated defecation induced by novel environment was not dose related, as shown by the lack of effect of 10-fold or threefold lower doses than the maximal effective dose. Likewise, other reports showed that PYY3–36 injected intraperitoneally at 3–4 doses ranging from 5 to 240 nmol/kg did not result in a clear dose-response suppression of food intake in mice (1, 24, 66), indicative that PYY3–36 may induce all-or-none actions to some extent. The intraperitoneal effective dose most likely results in supraphysiological PYY levels in the circulation, as postprandial plasma concentration of PYY in rats varies from 12 to 93 pmol and postprandial levels are reproduced by the intravenous infusion of PYY at 50–100 pmol/kg/h in rat (29, 44). However, it cannot be ascertained whether PYY3–36 at 8 nmol/kg injected intraperitoneally results in colonic concentrations similar to those induced by local postprandial release of PYY, which is highly expressed in colonic L cells (44), along with NPY released by sympathetic nerve stimulation in the colon near Y2 receptors (70). Although the pathophysiological significance of these observations still remains to be established, it can be speculated that the postprandial colonic release of PYY (44) may play a role in dampening the gastrocolic peristaltic reflex. Of interest are also clinical studies showing that patients with chronic idiopathic slow-transit constipation display an increase of PYY cells in the colon, which was commented to be potentially one of the causes of the disease (15).
In conclusion, using pharmacological approaches we demonstrated that intraperitoneal administration of PYY3–36 or PYY exerts Y2 receptor-mediated sustained inhibitory effects on basal- and stress-stimulated colonic propulsive motor function in conscious mice. We found that Y2 activation resulted in the suppression of high-amplitude, but not low-amplitude, contractions in the distal colon that we previously characterized to be giant migrating contractions related to the colonic propulsion and defection (20). PYY3–36 also inhibited defecation and diarrhea induced by exogenous activation of serotonergic and muscarinic receptors. The inhibitory effect on colonic transit and defecation was of greater magnitude and duration compared with that of gastric emptying or food intake monitored simultaneously. The demonstration of gene expression of Y2 receptor in both proximal and distal colonic mucosal and submucosal and muscle layers, along with the Y2 immunoreactivity in the submucosal and myenteric plexi, suggests a direct action in the colon that may involve modulation of enteric neuronal transmission although reflex circuitry cannot be ruled out. Peripheral injection of PYY3–36 in mice may provide a new model of constipation-like disorders to investigate underlying mechanisms of decreased bowel motor function. The mice and human colon contain the highest expression of PYY compared with other gut segments (2). It remains to be established whether the PYY-Y2 receptor signaling has relevance in modulating the gastrocolic reflex and chronic idiopathic slow-transit constipation associated with increased colonic PYY cells (15) or other functional pathology linked with alterations of colonic secretory motor function.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases R01 grant DK-57238 (Y. Taché), Center grant DK 41301 (Peptidomics core and Animal Model core), and DK078676 (M. Million), and VA Senior Career Scientist Award (Y. Taché).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Mrs. Honghui Liang for expert technical assistance, Dr. Joseph Reeve (Peptidomics Core, CURE, UCLA, Los Angeles, CA) for the generous supply of [Pro34]PYY, and Dr. Jean Rivier (Salk Institute, Peptide Laboratories, La Jolla, CA) for the generous gift of the other peptides.
REFERENCES
- 1.Adams SH, Won WB, Schonhoff SE, Leiter AB, Paterniti JR., Jr Effects of peptide YY[3–36] on short-term food intake in mice are not affected by prevailing plasma ghrelin levels. Endocrinology 145: 4967–4975, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Arantes RM, Nogueira AM. Distribution of enteroglucagon- and peptide YY-immunoreactive cells in the intestinal mucosa of germ-free and conventional mice. Cell Tissue Res 290: 61–69, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Balasubramaniam A. Neuropeptide Y (NPY) family of hormones: progress in the development of receptor selective agonists and antagonists. Curr Pharm Des 9: 1165–1175, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Browning KN, Lees GM. Inhibitory effects of NPY on ganglionic transmission in myenteric neurones of the guinea-pig descending colon. Neurogastroenterol Motil 12: 33–41, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Brumovsky P, Stanic D, Shuster S, Herzog H, Villar M, Hokfelt T. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J Comp Neurol 489: 328–348, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chang J, Hoy JJ, Idumalla PS, Clifton MS, Pecoraro NC, Bhargava A. Urocortin 2 expression in the rat gastrointestinal tract under basal conditions and in chemical colitis. Peptides 28: 1453–1460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chelikani PK, Haver AC, Reidelberger RD. Comparison of the inhibitory effects of PYY(3–36) and PYY(1–36) on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 287: R1064–R1070, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G, Galmiche JP. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol Gastrointest Liver Physiol 275: G1415–G1422, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci 133: 76–85, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Diener M, Knobloch SF, Bridges RJ, Keilmann T, Rummel W. Cholinergic-mediated secretion in the rat colon: neuronal and epithelial muscarinic responses. Eur J Pharmacol 168: 219–229, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F, Engel W, Eberlein W, Rudolf K. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol 384: R3–R5, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Dumont Y, Cadieux A, Pheng LH, Fournier A, St Pierre S, Quirion R. Peptide YY derivatives as selective neuropeptide Y/peptide YY Y1 and Y2 agonists devoided of activity for the Y3 receptor sub-type. Brain Res Mol Brain Res 26: 320–324, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Ekblad E, Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides 23: 251–261, 2002 [DOI] [PubMed] [Google Scholar]
- 15.El Salhy M, Suhr O, Danielsson A. Peptide YY in gastrointestinal disorders. Peptides 23: 397–402, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ferrier L, Segain JP, Bonnet C, Cherbut C, Lehur PA, Jarry A, Galmiche JP, Blottiere HM. Functional mapping of NPY/PYY receptors in rat and human gastro-intestinal tract. Peptides 23: 1765–1771, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Ferrier L, Segain JP, Pacaud P, Cherbut C, Loirand G, Galmiche JP, Blottiere HM. Pathways and receptors involved in peptide YY induced contraction of rat proximal colonic muscle in vitro. Gut 46: 370–375, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goumain M, Voisin T, Lorinet AM, Ducroc R, Tsocas A, Roze C, Rouet-Benzineb P, Herzog H, Balasubramaniam A, Laburthe M. The peptide YY-preferring receptor mediating inhibition of small intestinal secretion is a peripheral Y(2) receptor: pharmacological evidence and molecular cloning. Mol Pharmacol 60: 124–134, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Goumain M, Voisin T, Lorinet AM, Laburthe M. Identification and distribution of mRNA encoding the Y1, Y2, Y4, and Y5 receptors for peptides of the PP-fold family in the rat intestine and colon. Biochem Biophys Res Commun 247: 52–56, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Gourcerol G, Wang L, Adelson DW, Larauche M, Taché Y, Million M. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol Gastrointest Liver Physiol 296: G992–G1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR., Jr Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul Pept 51: 151–159, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther 307: 460–467, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab 1: 159–168, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3–36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology 145: 2585–2590, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom PM. Mechanisms involved in colonic vasoconstriction and inhibition of motility induced by neuropeptide Y. Acta Physiol Scand 129: 549–556, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Hyland NP, Cox HM. The regulation of veratridine-stimulated electrogenic ion transport in mouse colon by neuropeptide Y (NPY), Y1 and Y2 receptors. Br J Pharmacol 146: 712–722, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyland NP, Sjoberg F, Tough IR, Herzog H, Cox HM. Functional consequences of neuropeptide Y Y2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br J Pharmacol 139: 863–871, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackerott M, Larsson LI. Immunocytochemical localization of the NPY/PYY Y1 receptor in enteric neurons, endothelial cells, and endocrine-like cells of the rat intestinal tract. J Histochem Cytochem 45: 1643–1650, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Jin H, Cai L, Lee K, Chang TM, Li P, Wagner D, Chey WY. A physiological role of peptide YY on exocrine pancreatic secretion in rats. Gastroenterology 105: 208–215, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Kamiji MM, Inui A. Neuropeptide y receptor selective ligands in the treatment of obesity. Endocr Rev 28: 664–684, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Keire DA, Bowers CW, Solomon TE, Reeve JR., Jr Structure and receptor binding of PYY analogs. Peptides 23: 305–321, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Kimura T, Amano T, Uehara H, Ariga H, Ishida T, Torii A, Tajiri H, Matsueda K, Yamato S. Urocortin I is present in the enteric nervous system and exerts an excitatory effect via cholinergic and serotonergic pathways in the rat colon. Am J Physiol Gastrointest Liver Physiol 293: G903–G910, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lin HC, Zhao XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology 110: 1491–1495, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides 38: 189–200, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol 289: G1148–G1163, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Gao N, Hu HZ, Wang X, Wang GD, Fang X, Gao X, Xia Y, Wood JD. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol 494: 63–74, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundberg JM, Tatemoto K, Terenius L, Hellstrom PM, Mutt V, Hokfelt T, Hamberger B. Localization of peptide YY (PYY) in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proc Natl Acad Sci USA 79: 4471–4475, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangel AW, Fitz JG, Taylor IL. Modulation of colonic motility by substance P, cholecystokinin and neuropeptide Y. Peptides 12: 1063–1067, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol 556: 221–234, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther 301: 611–617, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev 50: 143–150, 1998 [PubMed] [Google Scholar]
- 42.Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Taché Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol 292: R1429–R1438, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3–36 in the mouse. J Pharmacol Exp Ther 306: 948–953, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Onaga T, Zabielski R, Kato S. Multiple regulation of peptide YY secretion in the digestive tract. Peptides 23: 279–290, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Ortiz AA, Milardo LF, Decarr LB, Buckholz TM, Mays MR, Claus TH, Livingston JN, Mahle CD, Lumb KJ. A novel long-acting selective neuropeptide Y2 receptor polyethylene glycol-conjugated peptide agonist reduces food intake and body weight and improves glucose metabolism in rodents. J Pharmacol Exp Ther 323: 692–700, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Osinski MA, Seifert TR, Cox BF, Gintant GA. An improved method of evaluation of drug-evoked changes in gastric emptying in mice. J Pharmacol Toxicol Methods 47: 115–120, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Parker E, Van Heek M, Stamford A. Neuropeptide Y receptors as targets for anti-obesity drug development: perspective and current status. Eur J Pharmacol 440: 173–187, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Pheng LH, Perron A, Quirion R, Cadieux A, Fauchere JL, Dumont Y, Regoli D. Neuropeptide Y-induced contraction is mediated by neuropeptide Y Y2 and Y4 receptors in the rat colon. Eur J Pharmacol 374: 85–91, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Pheng LH, Regoli D. Receptors for NPY in peripheral tissues bioassays. Life Sci 67: 847–862, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, Tosetti C, Poggioli G, Morselli Labate AM, Monetti N. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology 105: 733–739, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem 45: 4737–4747, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Sanger GJ, Banner SE, Smith MI, Wardle KA. SB-207266: 5-HT4 receptor antagonism in human isolated gut and prevention of 5-HT-evoked sensitization of peristalsis and increased defaecation in animal models. Neurogastroenterol Motil 10: 271–279, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Sanger GJ, Yoshida M, Yahyah M, Kitazumi K. Increased defecation during stress or after 5-hydroxytryptophan: selective inhibition by the 5-HT(4) receptor antagonist, SB-207266. Br J Pharmacol 130: 706–712, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 28: 166–170, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawa T, Mameya S, Yoshimura M, Itsuno M, Makiyama K, Niwa M, Taniyama K. Differential mechanism of peptide YY and neuropeptide Y in inhibiting motility of guinea-pig colon. Eur J Pharmacol 276: 223–230, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3–36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol 17: 452–457, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Silva AP, Cavadas C, Grouzmann E. Neuropeptide Y and its receptors as potential therapeutic drug targets. Clin Chim Acta 326: 3–25, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Tatemoto K, Mutt V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature 285: 417–418, 1980 [DOI] [PubMed] [Google Scholar]
- 60.Tebbe JJ, Tebbe CG, Mronga S, Ritter M, Schafer MK. Central neuropeptide Y receptors are involved in 3rd ventricular ghrelin induced alteration of colonic transit time in conscious fed rats (Abstract). BMC Gastroenterol 5: 5, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep 8: 367–373, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Wager-Page SA, Ghazali B, Anderson W, Veale WL, Davison JS. The peripheral modulation of duodenal and colonic motility in rats by the pancreatic polypeptide-fold family: neuropeptide Y, peptide YY, and pancreatic polypeptide. Peptides 14: 153–160, 1993 [DOI] [PubMed] [Google Scholar]
- 63.Wager-Page SA, Raizada E, Veale W, Davison JS. Peripheral modulation of duodenal and colonic motility and arterial pressure by neuropeptide Y, neuropeptide Y fragment 13–36, peptide YY, and pancreatic polypeptide in rats: cholinergic mechanisms. Can J Physiol Pharmacol 71: 768–775, 1993 [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Martinez V, Kimura H, Taché Y. 5-Hydroxytryptophan activates colonic myenteric neurons and propulsive motor function through 5-HT4 receptors in conscious mice. Am J Physiol Gastrointest Liver Physiol 292: G419–G428, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Martinez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol 281: R1401–R1410, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Whited KL, Tso P, Raybould HE. Involvement of apolipoprotein A-IV and cholecystokinin1 receptors in exogenous peptide YY3 36-induced stimulation of intestinal feedback. Endocrinology 148: 4695–4703, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol 24: 149–158, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Wultsch T, Painsipp E, Thoeringer CK, Herzog H, Sperk G, Holzer P. Endogenous neuropeptide Y depresses the afferent signaling of gastric acid challenge to the mouse brainstem via neuropeptide Y type Y2 and Y4 receptors. Neuroscience 136: 1097–1107, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan PQ, Million M, Wu SV, Rivier J, Taché Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol Motil 19: 923–936, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, Halm ST, Halm DR. Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: desensitization via the Y2-neuropeptide receptor. Am J Physiol Gastrointest Liver Physiol 297: G278–G291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]