Abstract
To test the hypothesis that differences in duodenal iron absorption may explain the variable phenotypic expression among HFE C282Y homozygotes, we have compared relative gene expression of duodenal iron transporters among C282Y homozygotes [hereditary hemochromatosis (HH)] with and without iron overload. Duodenal biopsy samples were analyzed using real-time PCR for expression of DMT1, FPN1, DCYTB, and HEPH relative to GAPDH from 23 C282Y homozygotes, including 5 “nonexpressors” (serum ferritin < upper limit of normal and absence of phenotypic features of hemochromatosis) and 18 “expressors.” Four subjects of wild type for HFE mutations without iron overload or liver disease served as controls. There was a significant difference in expression of DMT1 (P = 0.03) and DMT1(IRE) (P = 0.0013) but not FPN1, DCYTB, or HEPH between groups. Expression of DMT1(IRE) was increased among HH subjects after phlebotomy compared with untreated (P = 0.006) and nonexpressor groups (P = 0.026). A positive relationship was observed among all HH subjects regardless of phenotype or treatment status between relative expression of FPN1 and DMT1 (r = 0.5854, P = 0.0021), FPN1, and DCYTB (r = 0.5554, P = 0.0040), FPN1 and HEPH (r = 0.5100, P = 0.0092), and DCYTB and HEPH (r = 0.5400, P = 0.0053). In summary, phlebotomy is associated with upregulation of DMT1(IRE) expression in HH subjects. HFE C282Y homozygotes without phenotypic expression do not have significantly decreased duodenal gene expression of iron transport genes compared with HH subjects with iron overload. There is coordinated regulation between duodenal expression of FPN1 and DMT1, FPN1 and DCYTB, and FPN1 and HEPH and also DCYTB and HEPH in HH subjects regardless of phenotype.
Keywords: iron homeostasis, duodenal iron transporters, hemochromatosis, HFE gene, DMT1, ferroportin, gene expression, iron absorption
hereditary hemochromatosis (HH), an autosomal recessive disorder characterized by progressive accumulation of hepatic iron is one of the most common inherited diseases among Caucasians with an estimated prevalence of 1 in 250 and a heterozygote carrier rate of 8–10% (1, 3, 18, 22). If left untreated, irreversible organ damage, cardiomyopathy, diabetes mellitus, cirrhosis, and hepatocellular carcinoma may occur in the fourth or fifth decade of life. Most cases of hemochromatosis are due to a homozygous missense mutation in the HFE gene that results in a single amino acid substitution from cysteine to tyrosine (C282Y). Rarely, mutations in other genes involved in iron metabolism such as TFR2, HJV, and HAMP cause forms of hemochromatosis of varying severity collectively known as “non-HFE hemochromatosis” (16). There is incomplete penetrance of the C282Y mutation, and some studies have suggested that the majority of C282Y homozygotes may not develop end-organ damage (2, 4, 5, 24). It has been suggested that mutations in one or more disease-modifying genes may contribute to the variable phenotypic expression of HH (13–15).
Mammalian iron homeostasis is maintained primarily via regulation of dietary iron absorption. Absorption of inorganic iron in the small intestine is increased in response to a decrease in body iron stores. This process begins with the reduction of dietary ferric iron (Fe3+) in the duodenal lumen to ferrous (Fe2+) iron at the apical membrane of the mature duodenal enterocyte and is thought to be facilitated by the ferrireductase duodenal cytochrome b (DCYTB). Iron is then transported into the duodenal enterocyte by the divalent metal transporter-1 (DMT1). Once inside the enterocyte, iron is either complexed to ferritin for storage or directly transported across the basolateral membrane by ferroportin (FPN1), oxidized back to Fe3+ by the ferrioxidase hephaestin (HEPH), and then transported into the circulation by binding to the iron storage protein transferrin. Hepcidin, a small 25-amino acid circulating peptide, is now recognized to be the major regulator of iron absorption and exerts its effect by binding to and internalizing FPN1 into the enterocyte, resulting in reduced iron efflux from the enterocyte (8, 17).
Several large-scale population screening studies have shown that a substantial proportion of C282Y homozygotes may not have phenotypic expression of iron overload or evidence of end-organ damage. The variable phenotypic expression of the homozygous C282Y genotype has been attributed to possible disease-modifying genes that may modulate intestinal iron absorption. However, previous studies examining the expression levels of duodenal iron transport genes in HH subjects have found conflicting results (7, 10, 12, 19, 23, 27–29). In addition, earlier reports have combined gene expression data from pre- and postphlebotomy subjects (7, 19, 28, 29); most importantly, no previous studies have included a cohort of C282Y homozygotes without iron overload (“nonexpressors”). We hypothesized that downregulation of duodenal iron transporter gene expression may explain the variable phenotypic expression among HFE C282Y homozygotes. Thus the goal of the present study was to examine whether 1) the variable penetrance of the C282Y mutation could be explained by differences in duodenal iron transporter gene expression, 2) phlebotomy therapy would increase expression of duodenal iron transporter genes, and 3) there is coordinated regulation of DMT1 and FPN1 expression and other iron transport genes across different phenotype groups.
METHODS
Subjects.
Subjects identified from the Iron Overload and Endoscopy clinics at the University of Washington Medical Center who agreed to have duodenal biopsies obtained via endoscopy for the purpose of this study or at the time of a clinically indicated endoscopy were enrolled in the study. A total of 29 unique duodenal biopsy specimens were obtained from 27 subjects; two subjects had biopsies before and after phlebotomy therapy and were therefore included in both the untreated and posttreatment groups. Twenty-three subjects were C282Y homozygotes, and four persons without HFE mutations, iron overload, or liver disease served as control subjects. Eighteen of the 23 C282Y homozygotes had phenotypic hemochromatosis [i.e., one or more of the following; an initial serum ferritin > the upper limit of normal (ULN, i.e., men > 300 μg/l, women > 200 μg/l) or increased hepatic iron concentration (i.e., > 2,000 μg/g dry wt)]. Nine specimens were obtained from subjects prior to phlebotomy treatment (mean serum ferritin 1,535 μg/l), and 11 specimens were obtained from iron-depleted subjects following phlebotomy treatment (mean serum ferritin 57 μg/l). Five subjects had a serum ferritin level < ULN, did not exhibit any phenotypic features of hemochromatosis, and were classified as nonexpressors (mean serum ferritin 118 μg/l). This study was approved by the institutional review board at the University of Washington, and written, informed consent was obtained from all subjects.
Sample collection.
Typically, five biopsy samples were obtained from the second portion of the duodenum by use of standard endoscopy forceps. Samples were immediately snap frozen in liquid nitrogen and stored at −70°C for subsequent RNA purification. Simultaneously, serum iron indexes were obtained for each patient, including serum iron, transferrin-iron saturation (TS), and serum ferritin through the local clinical laboratory.
RNA preparation and cDNA synthesis.
Total RNA was extracted by using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. RNA was then digested with DNase I (Roche) at 37°C for 1 h prior to cDNA synthesis. RNAs were washed in ethanol, resuspended in RNase-free water, then run on a Bioanalyzer 2100 (Agilent Technologies) to determine RNA quality and concentration. RNA quality was estimated from electrophoresis gel images, electropherograms, and the RNA Integrity Number (RIN) algorithm (20); samples below a RIN value of 3.0 were deemed degraded and excluded. Reverse transcription reactions of 1.5 μg RNA were performed with a Transcriptor First Strand cDNA Synthesis Kit (Roche) in 20-μl volume by using oligo(dT) primers following the protocol provided with the kit. The reactions are incubated for 1 h at 55°C followed by 5 min at 85°C to inactivate the reverse transcriptase. The cDNAs were brought to 300 μl volume in water (∼5 ng/μl of the original RNA) prior to gene expression analysis.
Quantitative real-time PCR.
Applied Biosystems predesigned gene expression assays containing both primers and fluorescent TaqMan probes were used for all expression assays. Primers and probes are designed to span intronic sequences to avoid amplification of any contaminant genomic DNA. The housekeeping gene glyceraldehyde phosphate dehydrogenase (GAPDH) was used for normalization of quantitative and qualitative RNA variation. ABI part numbers for the primer/probe sets are as follows: FPN1, Hs00205888_m1; DMT1, Hs00167206_m1; HEPH, Hs00207710_m1; DCYTB, Hs00227411_m1; GAPDH, Hs99999905_m1. Additionally we assessed DMT1 for iron response element (IRE) splice variants with two ABI custom-designed primer/probe sets. The first probe (IRE+) bound at the IRE site (nucleotides 1814–1818 GenBank accession no. AB004857). The second probe (IRE−) recognized the non-IRE-containing segment (nucleotides 1814–1820, GenBank accession no. AF064484).
Each 20-μl real-time PCR reaction contained 2.0 μl 10× PCR buffer without Mg2+, 2.8 μl 25 mM MgCl2 (3.5 mM final concentration), 0.4 μl ROX passive reference dye, 0.4 μl 10 mM dNTPs, 1.0 μl ABI primer/probe, and 0.16 μl (0.8 U) Fast Start Taq Polymerase (Roche), 8.24 μl H2O, and 5 μl of the cDNA template. All reactions were run in triplicate in 384-well plates on an ABI7900HT and, for inclusion in the data set, we required standard deviations of the triplicates to be <0.15 cycles. Additionally, we verified that the PCR efficiencies of the ABI assays were >95% and that the slopes of the linear portion of the amplification curves varied by <5%.
Statistical analysis.
Descriptive statistics of continuous variables were calculated and expressed as medians and interquartile range (IQR; 25th percentile-75th percentile). Sex was expressed as a proportion and compared between groups via Fisher's exact test. Statistical differences between continuous variables were assessed by the Kruskal-Wallis test followed by post hoc comparisons between groups using the Wilcoxon rank-sum tests in conjunction with the Holm step-down procedure to obtain P values adjusted for multiple comparisons. Duodenal levels of iron transport genes were expressed as a ratio of GAPDH and reported as median and IQR. Spearman rank correlation coefficients and linear regression analyses were performed to determine the relationship between DMT1, FPN1, DCYTB, and HEPH gene expression and the relationship of expression of these genes to serum ferritin and TS levels. All statistical analyses were performed with STATA 9.0 (College Station, TX) except the Wilcoxon rank-sum tests in conjunction with the Holm step-down procedure were performed with R statistical software (www.R-project.org).
RESULTS
Clinical and laboratory differences in the study population.
Clinical characteristics and serum iron studies of each patient group are shown in Table 1. All HH subjects (i.e., nonexpressors, untreated, and postphlebotomy groups) were homozygous for the C282Y mutation. Control subjects were wild type for both the C282Y and H63D mutations. The majority of subjects were male (66%); however, four of five subjects in the nonexpressor group (80%) were female. There was no significant difference in the sex or age of the groups. There were significant differences in total iron binding capacity (P = 0.02), TS (P = 0.005), and serum ferritin (P = 0.0003). The nonexpressor group, lacking features of phenotypic hemochromatosis, had significantly lower TS (P = 0.04) and serum ferritin (P = 0.003) compared with the untreated group.
Table 1.
Comparison of serum iron parameters between patient groups
| Variable | Control (n = 4) | Nonexpressor (n = 5) | Untreated (n = 9) | Postphlebotomy (n = 11) | P Value |
|---|---|---|---|---|---|
| Age, yr | 33 (26.5–54) | 36 (28–46) | 46 (35–52) | 54 (48–59) | 0.15 |
| Male sex | 3 (75) | 1 (20) | 7 (78) | 8 (73) | 0.15 |
| Serum iron, μg/dl | 118.5 (79–148) | 201 (133–229) | 229 (203–230) | 107.5 (92–232) | 0.06 |
| TIBC, μg/dl | 338.5 (309–352) | 267 (253–286) | 251 (249–305) | 315 (281–428) | 0.02 |
| Transferrin saturation, % | 33.5 (25–42) | 84 (53–86) | 92 (91–92) | 36 (21–80) | 0.005 |
| Serum ferritin, μg/l | 67.5 (20–194) | 55 (43–126) | 1,208 (824–1,399) | 23 (14–55) | 0.0003 |
Data are presented as the median (25th to 75th percentile) or n (%). Statistical significance was determined by Kruskal-Wallis test. “Nonexpressor” was defined as serum ferritin < upper limit of normal and absence of phenotypic features of hemochromatosis. TIBC, total iron binding capacity.
Expression of DMT1, FPN1, HEPH, and DCYTB.
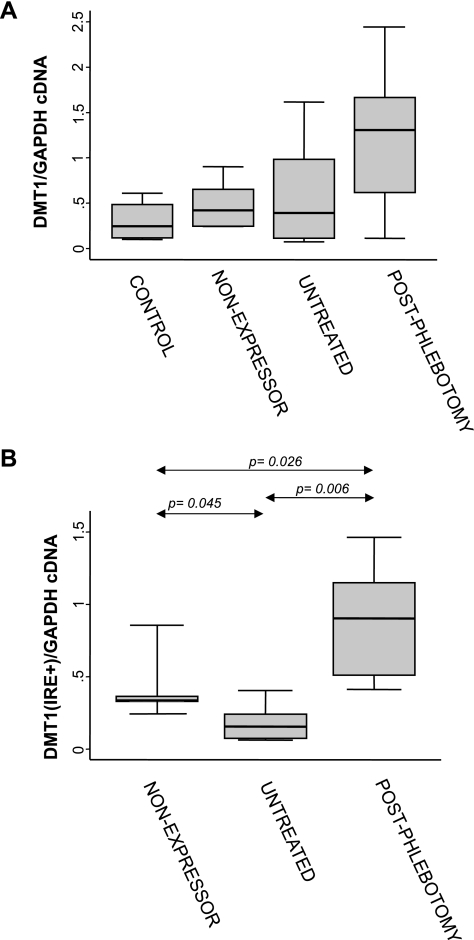
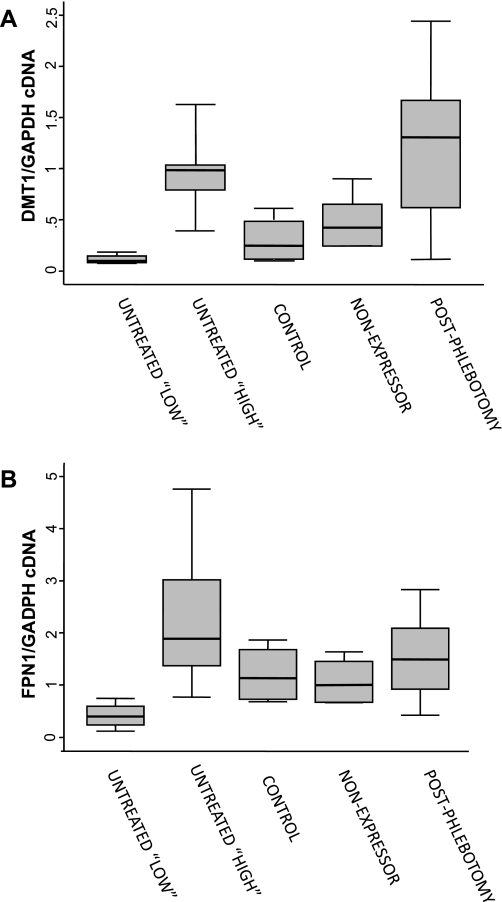
We measured the expression of four genes by real-time PCR: FPN1, DMT1, HEPH, and DCYTB. Expression was normalized to GAPDH mRNA levels. There was a significant difference in expression of DMT1 across groups (P = 0.03) with the highest expression levels in the phlebotomized HH group (Fig. 1A). There were no significant differences in expression of FPN1, DCYTB, or HEPH between groups. To determine the role of the IRE in DMT1 expression, custom primer/probe sets were also created to differentiate the IRE bound or non-IRE splice variant. There was a significant difference between groups in relative expression of DMT1(IRE) (P = 0.0013) but not the non-IRE splice variant (P = 0.23, data not shown). A significant increase in DMT1(IRE) expression was observed in the postphlebotomy HH group compared with both nonexpressors (2.7-fold; P = 0.026) and untreated subjects (5.9-fold; P = 0.006). Median DMT1(IRE) expression was significantly lower among the overall untreated HH group compared with nonexpressors (2.2-fold; P = 0.045) (Fig. 1B). Further examination of the DMT1 expression data from the untreated HH subjects appeared to suggest the presence of two subgroups: those with low DMT1 expression (n = 4) and those with high DMT1 expression (n = 5) (see Fig. 2). Supporting the existence of these two subgroups is the fact that subjects in the “low DMT1/FPN1” group had significantly lower median serum ferritin values [median 838.5 (IQR, 498–1208)] compared with the “high DMT1/FPN1” group [median 1399 (IQR, 824–3630)] (P = 0.05). When we divided the untreated group into the two subgroups and compared gene expression across all groups, there were significant differences in relative expression of DMT1 (P = 0.0026) (Fig. 2A), FPN1 (P = 0.035) (Fig. 2B), and DCYTB (P = 0.041) (data not shown). Expression of both DMT1 and FPN1 was lowest in the untreated “low DMT1” patient subgroup.
Fig. 1.
Total (A) or iron responsive element (IRE)-bound (B) divalent metal transporter 1 (DMT1) mRNA levels in duodenal biopsy samples from nonexpressing, untreated, and postphlebotomy hemochromatosis subjects and control subjects. Values are a ratio of DMT1/GAPDH. Median (horizontal lines), interquartile range (25th percentile-75th percentile) (boxes), and upper and lower adjacent values (vertical lines) are shown. Statistically significant differences were observed between groups (Kruskal-Wallis test; A, P = 0.03; B, P = 0.0013). P values for post hoc pairwise comparisons are indicated (Wilcoxon rank-sum test in conjunction with the Holm step-down procedure to obtain P values adjusted for multiple comparisons).
Fig. 2.
DMT1 mRNA levels (A) and ferroportin (FPN1) mRNA levels (B) in duodenal biopsy samples from untreated “high” and untreated “low” subgroups compared with nonexpressing and postphlebotomy hemochromatosis subjects and control subjects. Values are a ratio of the gene of interest to GAPDH cDNA. Median (horizontal lines), interquartile range (25th percentile-75th percentile) (boxes), and upper and lower adjacent values (vertical lines) are shown. Statistically significant differences were observed between groups (Kruskal-Wallis test; A, P = 0.0026; B, P = 0.035).
Relationship between DMT1, FPN1, HEPH, and DCYTB gene expression and correlation to levels of serum iron parameters.
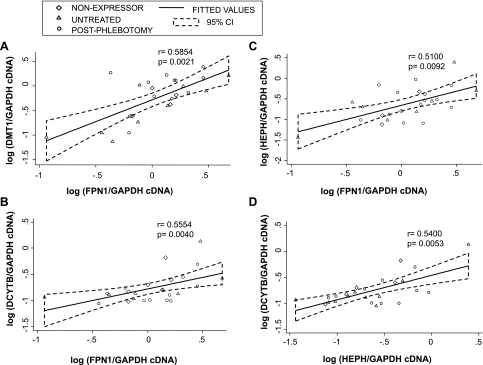
Spearman rank correlation coefficients were calculated to investigate associations among DMT1, FPN1, HEPH, and DCYTB gene expression. There was a strong positive relationship between the following genes in all groups except the iron-depleted phlebotomy-treated HH subjects (see Fig. 3): DMT1 and FPN1 expression (all: r = 0.5980, P = 0.0006; HH only: r = 0.5854, P = 0.0021), between FPN1 and HEPH (all: r = 0.4438, P = 0.0159; HH only: r = 0.5100, P = 0.0092); between DCYTB and HEPH (all: r = 0.5064, P = 0.0051; HH only: r = 0.5400, P = 0.0053) and between FPN1 and DCYTB (all: r = 0.4778, P = 0.0088; HH only: r = 0.5554, P = 0.0040)
Fig. 3.
Correlation between gene expression of iron transport genes in hemochromatosis subjects. A: DMT1 vs. FPN1. B: DCYTB vs. FPN1. C: HEPH vs. FPN1. D: HEPH vs. DCYTB. Plotted values are a ratio of GAPDH cDNA on a logarithmic scale. Regression lines and 95% confidence intervals (95% CI) are shown for all subjects. Spearman rank correlation was used to calculate P and r values.
We also investigated the relationship between DMT1 and FPN1 expression with serum ferritin and TS levels. There was no correlation between serum ferritin or TS levels and either total DMT1 or DMT1(IRE) in any group. FPN1 expression was not significantly associated with level of TS in any group but was significantly associated with serum ferritin in untreated subjects (r = 0.8214, P = 0.0234, data not shown). Serum ferritin and TS were also positively correlated to each other in the overall HH cohort (r = 0.6521; P = 0.0014, data not shown).
DISCUSSION
The present study is, to our knowledge, the first to examine the duodenal gene expression of mucosal iron transporters among HH subjects with and without phenotypic expression. Early studies examining the expression of duodenal iron transport genes in HH subjects were limited by the failure to analyze treated and untreated HH subjects separately (7, 19, 28, 29). Our goal was to examine whether lack of phenotypic expression in C282Y homozygous HH subjects (i.e., the nonexpressor group) could be explained by decreased expression of duodenal iron transport genes DMT1, FPN1, DCYTB, and HEPH. We did not observe decreased expression of duodenal iron transporter genes in subjects without phenotypic HH (nonexpressors) compared with the overall group of untreated HH subjects. By contrast, we observed that duodenal DMT1 and FPN1 gene expression among “nonexpressors” was similar to controls and at an intermediate level between the untreated subjects with mild to moderate iron overload (low DMT1/FPN1 group) and those with marked iron overload (high DMT1/FPN1 group). Phlebotomized HH subjects had higher expression of DMT1/DMT1(IRE) compared with all other groups. There was no significant difference between HH groups in the expression of HEPH or DCYTB. Similar to previous reports we observed a strong positive relationship between the expression of FPN1 and DMT1 (23, 26, 29), FPN1 and DCYTB (29), FPN1 and HEPH (29), and DCYTB and HEPH (10, 29) in all HH groups that had not been treated by phlebotomy, suggesting the presence of coordinated regulation of these genes in the absence of phlebotomy therapy, which may be disrupted because of the demands of erythropoiesis after iron depletion.
The most interesting finding in the present study was the unexpected observation of two subgroups within untreated HH subjects with iron overload that were distinguished by statistically significant differences in levels of serum ferritin and DMT1 and FPN1 gene expression: a group with mild-moderate levels of iron overload based on serum ferritin levels < 1,000 μg/l characterized by significant downregulation of DMT1 and FPN1 and a distinct group with markedly increased body iron stores and significantly increased duodenal DMT1 and FPN1 expression. On the basis of this observation, we propose the novel hypothesis for the variable phenotype of HFE-associated HH. We speculate that among nonexpressors, there is compensatory increase in hepcidin expression early in life that allows the maintenance of a “normal setpoint” in the level of expression of duodenal iron transport genes to levels similar to normal individuals. However, among those who have phenotypic expression of HH, downregulation of DMT1 and FPN1 (possibly via induction of hepcidin expression in the liver) is accomplished at some point, allowing maintenance of body iron stores at a mild-moderate level (i.e., low DMT1/FPN1 group). This compensatory response would be absent in those who develop progressive and continued iron overload and is due to failure to downregulate DMT1 and FPN1 in response to body iron stores (i.e., high DMT1/FPN1 group). The differences between the low DMT1/FPN1 and high DMT1/FPN1 groups could be due to genetic or environmental factors such as the presence of modifying genes or excess alcohol consumption, which was recently shown to downregulate hepcidin production in the liver (11).
Several other findings in our study extended and clarified findings of previously published reports on gene expression of duodenal iron transporters. One previous study also found that levels of DMT1 expression but not FPN1 were significantly increased in postphlebotomized HH subjects compared with untreated HH subjects or control subjects (12); cumulatively, these data corroborate early studies showing that duodenal iron uptake is increased in response to increased erythropoiesis after phlebotomy (21, 25). Recent studies of gene expression of duodenal iron transport genes in untreated HH subjects have found variable results; one group reported significantly increased expression of DMT1 and FPN1 between untreated HH subjects and controls (26), whereas others failed to observe a difference between untreated HH subjects and normal subjects (23). We speculate that these discordant findings may have been because of variable numbers of subjects with mild-moderate (“low” DMT1/FPN1) vs. marked iron overload (“high” DMT1/FPN1) within these studies, which may have led to divergent findings in the rate of duodenal expression of DMT1 and FPN1 among untreated HH subjects.
It is possible, if not likely, that a combination of genetic and environmental differences between these untreated HH patient subgroups may underlie their ability to regulate iron transport genes in response to body iron stores, independently or by modulating hepcidin expression. In support of this hypothesis, Milet et al. (15) screened 592 C282Y homozygotes with known serum iron studies for single nucleotide polymorphisms (SNPs) in 10 genes involved in hepcidin regulation (including HAMP and FPN1). These authors identified an allele in the bone morphogenic protein 2 (BMP2) in 13% of their cohort that had significantly lower serum ferritin levels (47 and 87%) lower compared with the other two alleles at this locus in their cohort. They also found other SNPs in the HJV and BMP4 genes that had additive effects with the BMP2 allele resulting in even lower mean serum ferritin levels. Thus it appears there are now several candidate genes that may modify disease expression in HH.
The results of the present study support the hypothesis that iron stores and erythropoietic demand for iron are the key signals that modulate expression of iron transporters and iron absorption, possibly via hepcidin. Moreover, iron stores modulate hepcidin levels independent of HFE, since in Hfe-knockout mice hepcidin levels increase in response to iron accumulation, further supporting the idea that hepcidin regulation of iron transporter gene expression occurs through a signal from iron stores independent of HFE genotype (6, 9). Our data of DMT1 expression and that of others (12, 23) in some untreated HH subjects are in agreement with the hypothesis that hepcidin expression may be induced once a threshold level of iron loading has been attained.
We recognize that the present study has several important limitations. We did not have detailed information about alcohol consumption in our HH subjects and, more importantly, did not have serum or urine for hepcidin measurements or DNA to identify the presence of polymorphisms in other iron-related genes, which could have suggested possible mechanisms for the differences in duodenal gene expression among the various HH groups. Our hypothesis would also have been strengthened if we had liver tissue for determination of hepcidin gene expression. Furthermore the sample size in the present study was relatively small increasing the risk of a type 2 statistical error. We encourage other investigators to reproduce our results in a larger sample and to study concomitant hepcidin gene expression in liver tissue to verify these results.
In summary, the present study shows that there is coordinated positive regulation of FPN1 with DMT1, DCYTB, and HEPH of DCYTB with HEPH among normal subjects as well as HH subjects regardless of phenotype. Iron depletion via phlebotomy treatment in HH subjects resulted in significantly increased DMT1(IRE) gene expression compared with untreated and nonexpressor HH subjects. HFE C282Y homozygotes without phenotypic expression do not have significantly decreased duodenal gene expression of iron transport genes compared with HH subjects with iron overload.
GRANTS
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK02957, the Hepatology Research Gift Account at the University of Washington, and the Liver Center of Excellence Research Fund at Virginia Mason Medical Center.
ACKNOWLEDGMENTS
The authors thank Stuart Raaka for laboratory assistance with this study.
REFERENCES
- 1.Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR, Leiendecker-Foster C, Speechley M, Snively BM, Holup JL, Thomson E, Sholinsky P. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 352: 1769–1778, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Beutler E. Penetrance of haemochromatosis. Gut 52: 610–611, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler E, Felitti V, Gelbart T, Ho N. The effect of HFE genotypes on measurements of iron overload in patients attending a health appraisal clinic. Ann Intern Med 133: 329–337, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Beutler E, Felitti VJ. The clinical penetrance of hereditary hemochromatosis. Hepatology 37: 711, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G–> A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 359: 211–218, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet 361: 669–673, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Byrnes V, Barrett S, Ryan E, Kelleher T, O'Keane C, Coughlan B, Crowe J. Increased duodenal DMT-1 expression and unchanged HFE mRNA levels in HFE-associated hereditary hemochromatosis and iron deficiency. Blood Cells Mol Dis 29: 251–260, 2002 [DOI] [PubMed] [Google Scholar]
- 8.De Dominico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell 18: 2569–2578, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gehrke SG, Herrmann T, Kulaksiz H, Merle U, Bents K, Kaiser I, Riedel HD, Stremmel W. Iron stores modulate hepatic hepcidin expression by an HFE-independent pathway. Digestion 72: 25–32, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gleeson F, Ryan E, Barrett S, Russell J, Kelleher B, Crowe J. Duodenal Dcytb and hephaestin mRNA expression are not significantly modulated by variations in body iron homeostasis. Blood Cells Mol Dis 35: 303–308, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem 281: 22974–22982, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kelleher T, Ryan E, Barrett S, Sweeney M, Byrnes V, O'Keane C, Crowe J. Increased DMT1 but not IREG1 or HFE mRNA following iron depletion therapy in hereditary haemochromatosis. Gut 53: 1174–1179, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee P, Gelbart T, West C, Halloran C, Beutler E. Seeking candidate mutations that affect iron homeostasis. Blood Cells Mol Dis 29: 471–487, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Lee PL, Gelbart T, West C, Halloran C, Felitti V, Beutler E. A study of genes that may modulate the expression of hereditary hemochromatosis: transferrin receptor-1, ferroportin, ceruloplasmin, ferritin light and heavy chains, iron regulatory proteins (IRP)-1 and -2, and hepcidin. Blood Cells Mol Dis 27: 783–802, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Milet J, Dehais V, Bourgain C, Jouanolle AM, Mosser A, Perrin M, Morcet J, Brissot P, David V, Deugnier Y, Mosser J. Common variants in the BMP2, BMP4, and HJV genes of the hepcidin regulation pathway modulate HFE hemochromatosis penetrance. Am J Hum Genet 81: 799–807, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson JE, Kowdley KV. Non-HFE hemochromatosis: genetics, pathogenesis, and clinical management. Curr Gastroenterol Rep 7: 71–80, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Phatak PD, Sham RL, Raubertas RF, Dunnigan K, O'Leary MT, Braggins C, Cappuccio JD. Prevalence of hereditary hemochromatosis in 16031 primary care patients. Ann Intern Med 129: 954–961, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Rolfs A, Bonkovsky HL, Kohlroser JG, McNeal K, Sharma A, Berger UV, Hediger MA. Intestinal expression of genes involved in iron absorption in humans. Am J Physiol Gastrointest Liver Physiol 282: G598–G607, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7: 3, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith PM, Godfrey BE, Williams R. Iron absorption in idiopathic haemochromatosis and its measurement using a whole-body counter. Clin Sci 37: 519–531, 1969 [PubMed] [Google Scholar]
- 22.Steinberg KK, Cogswell ME, Chang JC, Caudill SP, McQuillan GM, Bowman BA, Grummer-Strawn LM, Sampson EJ, Khoury MJ, Gallagher ML. Prevalence of C282Y and H63D mutations in the hemochromatosis (HFE) gene in the United States. JAMA 285: 2216–2222, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Stuart KA, Anderson GJ, Frazer DM, Powell LW, McCullen M, Fletcher LM, Crawford DH. Duodenal expression of iron transport molecules in untreated haemochromatosis subjects. Gut 52: 953–959, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waalen J, Nordestgaard BG, Beutler E. The penetrance of hereditary hemochromatosis. Best Pract Res Clin Haematol 18: 203–220, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Weintraub LR, Conrad ME, Crosby WH. Regulation of the intestinal absorption of iron by the rate of erythropoiesis. Br J Haematol 11: 432–438, 1965 [DOI] [PubMed] [Google Scholar]
- 26.Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 120: 1412–1419, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 120: 1412–1419, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Zoller H, Pietrangelo A, Vogel W, Weiss G. Duodenal metal-transporter (DMT-1, NRAMP-2) expression in patients with hereditary haemochromatosis. Lancet 353: 2120–2123, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Zoller H, Theurl I, Koch RO, McKie AT, Vogel W, Weiss G. Duodenal cytochrome b and hephaestin expression in patients with iron deficiency and hemochromatosis. Gastroenterology 125: 746–754, 2003 [DOI] [PubMed] [Google Scholar]