Abstract
A clinical observation in pediatric and adult intensive care units is that the incidence of multiple organ failure in pediatric trauma victims is lower than in adult patients. However, the molecular mechanisms are not yet defined. Recent experimental studies have shown that the nuclear peroxisome proliferator-activated receptor-γ (PPARγ) modulates the inflammatory process. In this study, we hypothesized that severity of liver injury may be age dependent and PPARγ activation may provide beneficial effects. Hemorrhagic shock was induced in anesthetized young (3–5 mo old) and mature male Wistar rats (11–13 mo old) by withdrawing blood to a mean arterial blood pressure of 50 mmHg. After 3 h, rats were rapidly resuscitated with shed blood. Animals were euthanized 3 h after resuscitation. In mature rats, liver injury appeared more pronounced compared with young rats and was characterized by marked hepatocyte apoptosis, extravasation of erythrocytes, and accumulation of neutrophils. The ratio between the antiapoptotic protein Bcl-2 and the proapoptotic protein BAX was lower, whereas activity of caspase-3, the executioner of apoptosis, was higher in liver of mature rats compared with young rats. Plasma alanine aminotransferase levels were not different between the two age groups. This heightened liver apoptosis was associated with a significant downregulation of PPARγ DNA binding in mature rats compared with young rats. Treatment with the PPARγ ligand ciglitazone significantly reduced liver apoptosis in mature rats. Our data suggest that liver injury after severe hemorrhage is age dependent and PPARγ activation is a novel hepatoprotective mechanism.
Keywords: hemorrhagic shock, apoptosis, BAX, Bcl-2, caspase-3
there is evidence that age is an important factor for the development of multiple organ dysfunction syndrome (MODS) in trauma victims with severe blood loss (27), since the incidence of MODS and mortality rate is much lower in children than that for adults with similar critical conditions (3). The liver with its important metabolic and homeostasis functions is among the most frequently affected organs after hemorrhage-induced hypotension in humans (12). Although the liver tolerates prolonged periods of diminished oxygen delivery, primary (hypoxic) or secondary (ischemia and reperfusion) hepatocellular injury may occur and is associated with metabolic changes, inflammation, and eventually cell death (13). With different degrees of extent, both necrotic and apoptotic processes have been demonstrated to be responsible for the marked liver impairment after hemorrhagic shock (13, 16, 21–24). However, the molecular mechanisms by which hemorrhage induces cell injury and death in the liver and whether these mechanisms may be age dependent have not been fully defined.
Peroxisome proliferator-activated receptor-γ (PPARγ) is a nuclear receptor that functions directly as a transcription factor to control gene regulation for glucose homeostasis and lipid metabolism (38). Although it is most highly expressed in adipose tissue, PPARγ is also found in hepatocytes, hepatic stellate cells, and macrophages (7). Several experimental studies have demonstrated that PPARγ is also an important regulator of the cell proinflammatory pathways (38). In this regard, natural (cyclopentenone prostaglandins) and synthetic PPARγ ligands (thiazolidinediones) have been reported to exert beneficial effects in hemorrhagic shock in young experimental animals (1, 5). We have also demonstrated that pharmacological activation of PPARγ reduces the systemic inflammatory response and lung injury secondary to severe hemorrhage (4, 39) and preserves organ structure and function in hepatic ischemia and reperfusion injury in young rats (19). To further support the role of PPARγ in inflammation, several studies including ours have reported that its expression and function may be altered by cytotoxic mediators. For example, inflammatory cytokines, such as tumor necrosis factor-α and interleukin-1β, decreased PPARγ expression in human hepatocytes (18). We have also demonstrated that the inflammatory response of septic shock is associated with downregulation of PPARγ expression in the bronchial epithelium and in the endothelium of thoracic aortas in rats (37). PPARγ expression and DNA binding are also markedly reduced in lungs of mice subjected to endotoxic shock and are associated with massive lung injury and neutrophil infiltration (17, 34). In support of our findings, it has been reported that PPARγ expression is also downregulated in the liver of rats subjected to sepsis by cecal ligation and puncture (30) or double-hit hemorrhage and sepsis (14). Recently, we have reported that age may also influence PPARγ expression and may represent an enhancing risk factor for lung injury in rats subjected to severe hemorrhage (39).
In this study we provide insight into the mechanism of age-dependent liver injury after severe hemorrhage. We demonstrated that the DNA binding activity of PPARγ is markedly downregulated after hemorrhagic shock in mature rats (11–13 mo old) compared with young animals (3–5 mo old). Our results also indicate that in mature animals apoptosis is a prominent feature of liver injury and may be regulated by PPARγ activation.
MATERIALS AND METHODS
Hemorrhagic shock model.
Male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 225–275 g (3–5 mo) were included in the young group; rats weighing 500–700 g (11–13 mo old) were included in the mature group. The animals were anesthetized with thiopentone sodium (70 mg/kg), intraperitoneally (ip). Hemorrhagic shock was performed as previously described (39). The trachea was cannulated to facilitate respiration and temperature was maintained at 37°C by use of a homeothermic blanket. The right carotid artery was cannulated (PE-50 tubing) and connected to a pressure transducer for the measurement of mean arterial blood pressure (MABP). The right femoral artery was cannulated for withdrawal of blood. Upon completion of the surgical procedure, MABP was allowed to stabilize for 15 min. To facilitate hemorrhage animals received heparin (100 IU/kg). Hemorrhagic shock was then induced by withdrawing blood (∼0.5 ml/min) from the femoral artery into a reservoir until MABP stabilized at 50 mmHg. After this initial bleeding, additional small volumes of blood were withdrawn or retransfused as necessary to maintain MABP at 50 mmHg. At 3 h after hemorrhage, rapid resuscitation was performed by transfusing the shed blood over a 5-min period. If small volumes of blood were needed to be retransfused during the hypoperfusion period to maintain MABP at 50 mmHg, rapid resuscitation was performed by transfusing the remaining shed blood supplemented with Ringer lactate solution to a final volume of fluids equal to the initial total shed blood. Animals were divided in five groups: 1) in the Vehicle (0 mg)-Hemorrhagic Shock group, rats received vehicle (100% dimethyl sulfoxide) instead of the PPARγ ligand; 2) in the Ciglitazone (5 mg)-Hemorrhagic Shock group, rats received the PPARγ ligand at 5 mg/kg; 3) in the Ciglitazone (10 mg)-Hemorrhagic Shock group, rats received the PPARγ ligand at 10 mg/kg; 4) in the Ciglitazone (20 mg)-Hemorrhagic Shock group, rats received the PPARγ ligand at 20 mg/kg; 5) in the Sham group, rats served as control at time 0 and underwent similar surgical preparation but were not bled. The PPARγ ligand or vehicle was administered ip as a bolus at the beginning of resuscitation. Time-course experiments were performed in the Vehicle-Hemorrhagic Shock group. Animals were euthanized at 1, 2, and 3 h after the hemorrhage during the hypoperfusion period and at 15 and 30 min and at 1, 2, and 3 h after the transfusion. Plasma samples and livers were collected for the histological and biochemical studies described below.
Histopathological analysis.
Tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin and evaluated by a pathologist blinded to the experimental protocol.
MPO activity.
Myeloperoxidase (MPO) activity was determined as an index of neutrophil accumulation in lungs. It was defined as the quantity of enzyme degrading 1 μmol hydrogen peroxide/min at 37°C and expressed in units per 100 mg tissue (37).
Plasma ALT levels.
Blood was obtained by the carotid artery at the time of euthanasia for analysis of plasma alanine aminotransferase (ALT) as an index of hepatocellular injury. Measurements of ALT were made by using a diagnostic kit (Biotron Diagnostics, Hemet, CA).
Determination of apoptosis.
Cell death by apoptosis was evaluated by measurement of oligonucleosomal DNA fragments by a histochemical terminal deoxynucleotidyl transferase (TdT) nick-end labeling (TUNEL)-like staining (TdT-FragEL kit, Oncogene Research Products, Cambridge, MA) as previously described (9). Liver sections were permeabilized with protease K (2 mg/ml) in 10 mM Tris (pH 8) at room temperature for 20 min. Endogenous peroxidase was quenched with 3% H2O2 in methanol for 5 min. Sections were incubated with a reaction buffer composed by biotin 2′-deoxycitidine 5′-triphosphate (dCTP) and unlabeled dCTP and TdT enzyme in a humidified chamber at 37°C. In this assay TdT binds to exposed 3′-OH ends of DNA fragments and catalyzes the addition of biotin-labeled and unlabeled deoxynucleotides. Biotinylated nucleotides were then detected by use of a streptavidin-horseradish peroxidase conjugate and diaminobenzidine. To quantitate the degree of apoptosis, the numbers of apoptotic cells were counted by three independent observers blinded to the experimental protocol. The apoptotic index (number of stained hepatocyte nuclei/number of total hepatocyte nuclei) was calculated.
Subcellular fractionation and nuclear protein extraction.
Livers were homogenized in a buffer containing 0.32 M sucrose, 10 mM Tris·HCl, pH 7.4, 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM β-mercaptoethanol, 20 μM leupeptin, 0.15 μM pepstatin A, 0.2 mM PMSF, 50 mM NaF, 1 mM sodium orthovanadate, 0.4 nM microcystin. The homogenates were centrifuged (1,000 g, 10 min), and the supernatant (cytosol + membrane extract) was collected to evaluate BAX and Bcl-2 content and activity of caspase-3 as described below. The pellets were solubilized in Triton buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris·HCl, pH 7.4, 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 20 μM leupeptin A, 0.2 mM PMSF). The lysates were centrifuged (15,000 g, 30 min, 4°C), and the supernatant (nuclear extract) was collected to evaluate the DNA binding of PPARγ and activity of caspase-3.
Western blot analyses.
Cytosol content of BAX and Bcl-2 were determined by immunoblot analyses. Cytosol extracts were boiled in loading buffer (125 mM Tris·HCl, pH 6.8, 4% SDS, 20% glycerol, and 10% 2-mercaptoethanol), and 50 μg of protein were loaded per lane on an 8–16% Tris-glycine gradient gel. Proteins were separated electrophoretically and transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked with 5% nonfat dried milk in Tris-buffered saline (TBS) for 1 h and then incubated with primary antibodies against BAX or Bcl-2 for 1 h. The membranes were washed in TBS with 0.1% Tween 20 and incubated with secondary peroxidase-conjugated antibody. To control for variations in protein loading, each membrane was labeled with a primary antibody against β-actin. Immunoreaction was visualized by chemiluminescence. Densitometric analysis was performed with ImageQuant (Molecular Dynamics, Sunnyvale, CA).
Measurement of activity of caspase-3.
Activity of caspase-3 was measured by the cleavage of the fluorogenic tetrapeptide-amino-4-methylcoumarine conjugate (DEVD-AFC) as described (33). Cytosol and nuclear extracts and substrates (50 μM) were combined in the caspase reaction buffer (100 mM HEPES, 10% sucrose, 5 mM dithiothreitol, 0.1% CHAPS, pH 7.25). AFC liberation was monitored over 30 s with a Perkin-Elmer fluorimeter using 400-nm excitation and 505-nm emission wavelength. Fluorescence units were converted to picomoles of AFC by using a calibration curve generated with free AFC. Data are given as DEVD-fmk-inhibitable AFC generation.
EMSA.
Electrophoretic mobility shift assays (EMSAs) were performed as previously described (39). An oligonucleotide probe corresponding to PPARs consensus sequence (5′-GAA AAC TAG GTC AAA GGT CA-3′) was labeled with γ-[32P]ATP using T4 polynucleotide kinase and purified in Bio-Spin chromatography columns (Bio-Rad, Hercules, CA). Ten micrograms of nuclear protein were preincubated with EMSA buffer (12 mM HEPES pH 7.9, 4 mM Tris·HCl pH 7.9, 25 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 50 ng/ml poly[d(I-C)], 12% glycerol vol/vol, and 0.2 mM PMSF) on ice for 10 min before addition of the radiolabeled oligonucleotide for an additional 10 min. The specificity of the binding reactions was determined by coincubating duplicate nuclear extract samples with 100-fold molar excess of respective unlabeled oligonucleotides (competitor assays). Supershift assays, to further determine the specificity of binding, were performed by coincubating samples with antibodies corresponding to PPARα, PPARβ/δ, or PPARγ (data not shown). Protein-nucleic acid complexes were resolved by using a nondenaturing polyacrylamide gel consisting of 5% acrylamide (29: 1 ratio of acrylamide-bisacrylamide) and run in 0.5× TBE (45 mM Tris·HCl, 45 mM boric acid, 1 mM EDTA) for 1 h at constant present (30 mA). Gels were transferred to Whatman 3M paper, dried under a vacuum at 80°C for 1 h, and exposed to photographic film at −70°C with an intensifying screen. Densitometric analysis was performed with ImageQuant (Molecular Dynamics, Sunnyvale, CA).
Materials.
The primary antibodies directed at BAX and Bcl-2 and the oligonucleotide for PPARs were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The primary antibody directed at β-actin was obtained from Abcam (Cambridge, MA). All other chemicals were from Sigma-Aldrich (St. Louis, MO).
Data analysis.
All values in the figures and text are expressed as means ± SE of n observations (n = 3–12 animals for each group). The results were examined by analysis of variance with individual comparisons performed by t-test.
RESULTS
Severity of liver injury is higher in mature rats.
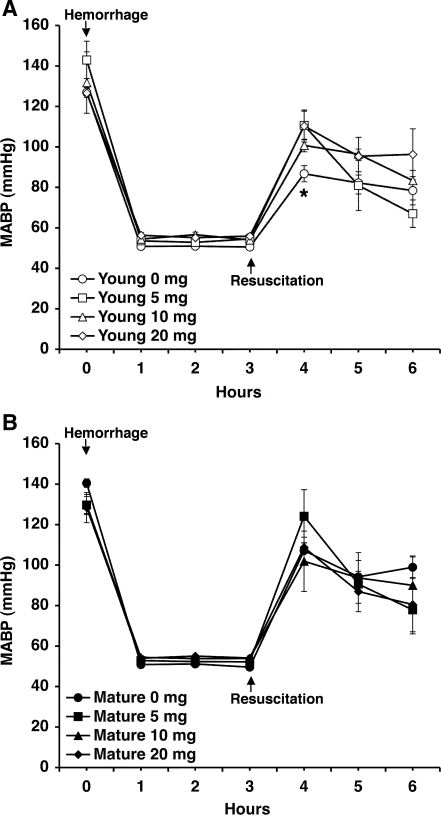
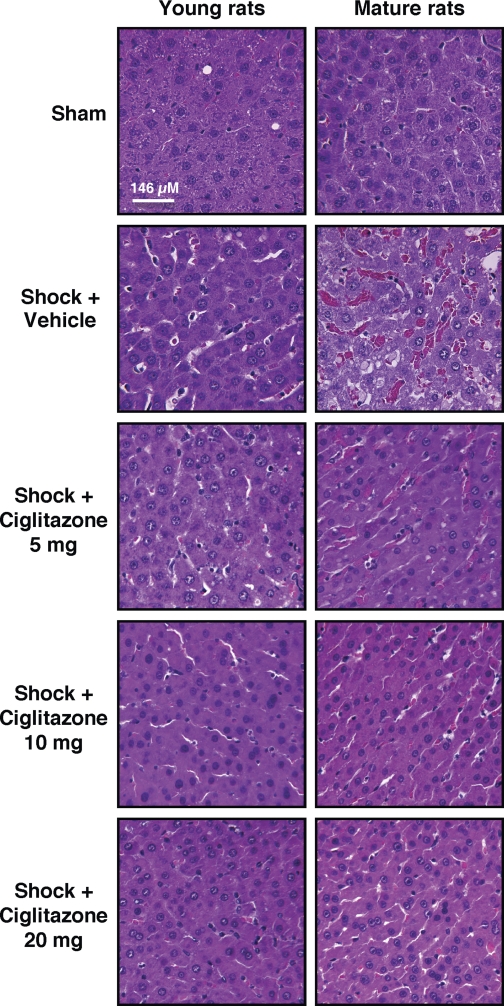
Hemorrhagic shock was induced by pressure-controlled hemorrhage to maintain MABP at 50 mmHg for 3 h, followed by rapid resuscitation. As shown in Fig. 1, after an initial increase at blood retransfusion, MABP progressively declined in a similar fashion in both vehicle-treated young and mature animals, thus suggesting that a similar degree of pressure-controlled low flow was achieved in both groups. Following hemorrhage (3 h) and resuscitation (3 h), a scarce interstitial edema and a few infiltrated cells were observed in the liver of vehicle-treated young rats at histological analysis (Fig. 2). Vehicle-treated young rats also had a modest increase in plasma levels of ALT (Fig. 3A). On the contrary, livers of vehicle-treated mature rats had far more hepatocellular injury than did young animals; architectural alterations were characterized by marked hemorrhagic and neutrophil infiltration (Fig. 2). Interestingly, vehicle-treated mature rats were found to have similar elevation in plasma ALT levels compared with vehicle-treated young rats (Fig. 3A). Treatment with the PPARγ ligand ciglitazone, at doses of 5, 10, or 20 mg/kg, ameliorated MABP in the first hour after reperfusion in young rats only (Fig. 1), whereas it reduced liver architectural derangement in both young and adult rats (Fig. 2). In young rats, plasma ALT levels were significantly decreased after treatment with ciglitazone at the highest dosage only. However, despite the improved liver histology, ciglitazone treatment did not affect levels of plasma ALT in adult rats (Fig. 3A).
Fig. 1.
Mean arterial blood pressure (MABP) after hemorrhage and resuscitation in young (A) and mature (B) rats treated with vehicle (i.e., 0 mg/kg) or ciglitazone (5, 10, or 20 mg/kg ip). Arrows indicate initiation of hemorrhage and resuscitation, respectively. Drug treatment was given at the time of resuscitation. Data are means ± SE of 7–10 rats for each time point. *P < 0.05 of vehicle-treated vs. ciglitazone-treated rats at the first hour after reperfusion.
Fig. 2.
Representative histology photomicrographs of liver sections stained with hematoxylin and eosin. Normal liver architecture was observed in a sham young and a sham mature rat. Following hemorrhage (3 h) and resuscitation (3 h), scarce architectural alterations were observed in a vehicle-treated young rat. Extensive red and inflammatory cell infiltration was observed in a vehicle-treated mature rat. In young or mature rats treated with ciglitazone (5, 10, 20 mg/kg ip) amelioration of liver structure was observed. Drug treatment was given at the time of resuscitation. Magnification ×100; 1 cm = 146 μm.
Fig. 3.
Effect of treatment with vehicle (i.e., 0 mg/kg) or ciglitazone (5, 10, or 20 mg/kg ip) on plasma alanine aminotransferase (ALT; A), liver myeloperoxidase activity (MPO, B) and percentage of hepatic apoptotic cells in young and mature rats following hemorrhage (3 h) and resuscitation (3 h). Each data point represents mean ± SE of 3–9 rats for each group. *P < 0.05 vs. sham value of the same age group. #P < 0.05 vs. young rats. †P < 0.05 vs. vehicle-treated rats (i.e., 0 mg/kg) of the same age group.
Liver neutrophil infiltration is higher in mature rats.
Activity of MPO, an enzyme constituent of neutrophil, increased in the liver after hemorrhage and resuscitation in vehicle-treated young and mature rats compared with sham rats. The degree of neutrophil infiltration was significantly more pronounced in the vehicle-treated mature group compared with young rats. Treatment with ciglitazone significantly reduced MPO activity in the liver of both age groups in a dose-independent manner. However, in ciglitazone-treated adult animals the degree of MPO reduction was significantly lower compared with that of ciglitazone-treated young rats (Fig. 3B).
Liver apoptosis is more prominent in mature rats.
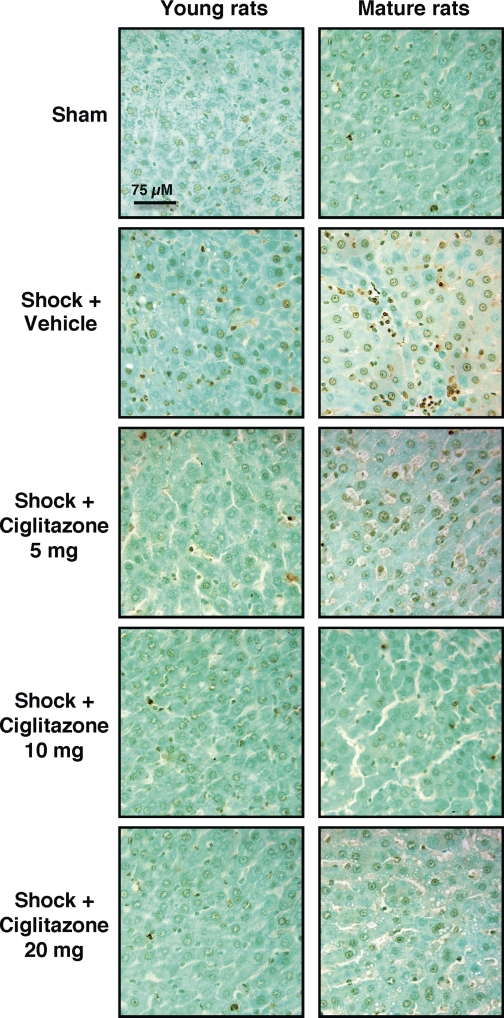
To further characterize the divergent organ injury in young and mature rats, we evaluated liver apoptosis. Using a “TUNEL-like” staining as an indicator of the DNA fragmentation that occurs as a result of apoptosis (9), we found that in vehicle-treated mature rats there was a larger number of apoptotic cells compared with vehicle-treated young rats. Treatment with ciglitazone significantly reduced the number of TUNEL-positive cells in the livers of both age groups (Figs. 3B and 4).
Fig. 4.
Representative photomicrographs of in situ terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) method of liver sections. Sections of sham young or mature rats revealed only a few apoptotic nuclei. Following hemorrhage and resuscitation a scarce staining for apoptosis was observed in liver sections from young rats. In mature rats a dark staining was observed in several clustered cell groups. In young or mature rats treated with ciglitazone (5, 10, 20 mg/kg ip) reduction of staining was observed. Magnification ×400; 1 cm = 75 μm. A similar pattern was seen in n = 3–9 different tissue sections in each experimental group.
Expression of apoptotic proteins is altered and activation of caspase 3 is increased in mature rats.
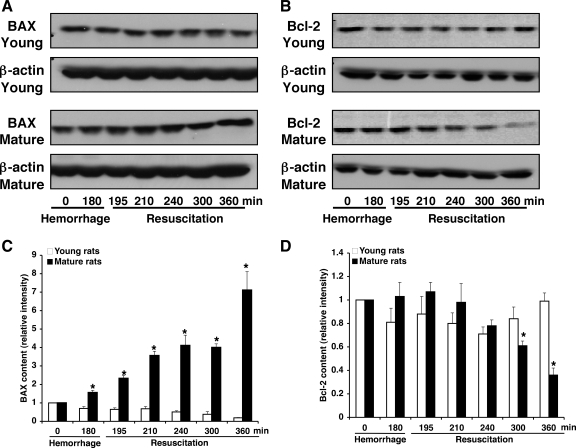
Since apoptotic death is regulated by a delicate balance of several proapoptotic and antiapoptotic modulators (25), we evaluated the liver expression of the proapoptotic protein BAX and the antiapoptotic Bcl-2. In a time-course study, Western blot analysis revealed that liver expression of BAX steadily increased after resuscitation in vehicle-treated mature rats, whereas it progressively declined in vehicle-treated young animals. On the contrary, liver expression of Bcl-2 declined in a time-dependent manner after resuscitation in vehicle-treated mature rats, whereas it was stably maintained in vehicle-treated young animals (Fig. 5). Thus the ratio of Bcl-2 to BAX, as an indicator of cell survival, was significantly lower in the vehicle-treated mature group compared with vehicle-treated young animals, which had a balanced ratio (Fig. 6A). Treatment with ciglitazone significantly increased the ratio of Bcl-2/BAX to basal levels in the mature group (Fig. 6A).
Fig. 5.
Western blot analyses for BAX (A) and Bcl-2 (B) in liver cytosol extracts of young and mature vehicle-treated rats. Fifty micrograms of protein were loaded for each lane. Equal loading was confirmed by β-actin immunoblotting. Image analysis of expression of BAX (C) and Bcl-2 (D) was determined by densitometry. Fold increase was calculated vs. respective sham value (time 0) set to 1.0. Results are representative of 3 separate time-course experiments. *P < 0.05 vs. young rats.
Fig. 6.
Effect of treatment with vehicle (i.e., 0 mg/kg) or ciglitazone (5, 10, or 20 mg/kg ip) on Bcl-2/BAX ratio (A), as a survival indicator, and catalytic activity of caspase-3 (B) in young and mature rats at the end of the resuscitation period. Each data point represents mean ± SE of 3 rats for each group. *P < 0.05 vs. sham value of the same age group. #P < 0.05 vs. young rats. †P < 0.05 vs. vehicle-treated rats (i.e., 0 mg/kg) of the same age group.
Because caspase-3 plays a key role in the execution of apoptosis (10), we investigated whether this enzyme activity could be also altered. Consistent with the modest apoptosis and the normal ratio of Bcl-2/BAX, in vehicle-treated young animals there was no significant elevation in caspase-3 activity at the end of the resuscitation period compared with basal levels. On the contrary, caspase-3 activity was significantly augmented in vehicle-treated rats at the end of the resuscitation. Treatment with ciglitazone significantly decreased caspase-3 activity in both age groups (Fig. 6B). These data suggest that apoptosis may be responsible for liver injury in mature animals during severe hemorrhage.
Liver activation of PPARγ is reduced in mature rats.
Since PPARγ is highly abundant in the liver, where it functions as a modulator for the lipid and glucose homeostasis (7), we also evaluated changes of PPARγ activation in this organ. As shown in Fig. 7, A and B, in the liver of both sham rats (i.e., at time 0) there was a constitutive DNA binding activity of PPARγ that was more pronounced in the vehicle-treated mature rats. In the liver of vehicle-treated young rats, DNA binding of PPARγ was maintained or slightly increased after hemorrhage, declined 30 min after resuscitation, and rose again at later time points of resuscitation. On the contrary, in the liver of vehicle-treated mature rats activation of PPARγ was downregulated below basal levels at all time points of observation after hemorrhage and resuscitation, with a maximum decline at 30 min after resuscitation. Treatment with ciglitazone restored PPARγ DNA binding to basal levels in the mature group (Fig. 7C).
Fig. 7.
A: representative autoradiograph of electrophoretic mobility shift assay for time-course peroxisome proliferator-activated receptor-γ (PPARγ) DNA binding in young and mature rats following hemorrhage and resuscitation. B: image analysis of PPARγ DNA binding determined by densitometry of the time-course experiments. C: effect of treatment with vehicle (i.e., 0 mg/kg) or ciglitazone (5, 10, or 20 mg/kg ip) on PPARγ DNA binding in young and mature rats at the end of the resuscitation period. Each data point represents mean ± SE of 3 rats for each group. *P < 0.05 vs. sham value of the same age group. #P < 0.05 vs. young rats. †P < 0.05 vs. vehicle-treated rats (i.e., 0 mg/kg) of the same age group.
DISCUSSION
Biological aging is a fundamental process that represents the major risk factor with respect to the development of cancer, neurodegenerative, and cardiovascular diseases in vertebrates. However, the role of age in the progress and outcome of MODS, including liver injury, in acute critical conditions of severe hemorrhage has not been explored. Our study provides evidence that the severity of liver injury during hemorrhagic shock is age dependent and may be counterregulated by activation of the nuclear receptor PPARγ. Specifically, we have demonstrated that in young animals PPARγ activation in the liver is maintained and is associated with a scarce scattered hepatic necrosis and apoptosis following hemorrhage and resuscitation. Contrary to this finding, DNA binding of the receptor is markedly downregulated in mature rats and is associated with extensive liver apoptosis. This age-dependent PPARγ regulation also correlates to a diverse expression of proapoptotic members of the Bcl-2 family and diverse activation of the effector caspase-3. Treatment with ciglitazone, a synthetic PPARγ ligand, ameliorates hepatic injury by repressing the apoptotic process in mature animals.
Aging-related changes in physiological functions may not be evident under basal conditions; however, when an organ is under stress, these changes may contribute to the severity organ injury. In our experimental setting, the amount of blood loss and, therefore, the degree of hypoperfusion may also contribute to the severity of the inflammatory response. To avoid this variability, we adopted a model of pressure-controlled hemorrhage (20), which allowed maintaining a low constant MABP (50 mmHg for 3 h) to induce hypoperfusion. In both age groups, blood replacement appeared to partially restore MABP. Although it has been reported that age-related cardiovascular changes in aging animals do not alter mass perfusion rate in tissues under basal conditions (6), we cannot rule out that blood flow distribution in major organs might differ during hemorrhage and contribute to injury. Nevertheless, our results provide evidence that in the experimental population (i.e., 11–13 mo old) age may be an independent risk factor of liver failure in hemorrhagic shock, supporting the clinical findings of MODS incidence observed in humans >55 years old (27). Treatment with ciglitazone ameliorated MABP during early resuscitation in the young group only. Similar beneficial effects of PPARγ ligands on MABP, but not other hemodynamic parameters, were found in hemorrhagic shock in experimental young animals (4, 39). However, whether and to what extent treatment with ciglitazone results in improved organ blood flow deserves further investigation.
There is emerging evidence that the inflammatory response results in marked downregulation of nuclear hormone receptors involved in the lipid and glucose metabolism. We have previously demonstrated that the onset of systemic inflammation and lung injury is preceded by lung downregulation of PPARγ expression in septic rats (37) and endotoxemic mice (17, 34). Other laboratories also support the findings that PPARγ is downregulated in the liver in rats subjected to sepsis by cecal ligation and puncture (30) or double-hit hemorrhage and sepsis (14). Inflammatory cytokines also downregulate the expression of PPARγ and other coactivators in human hepatoma cell lines (18).
Moreover, we have previously reported data for significant variation in PPARγ expression in aged animals. We demonstrated that, under normal physiological conditions, lung expression of PPARγ was a function of the aging process and was maximally expressed at 2–5 mo age, whereas it declined in mature rats at the age of 11–13 mo old. The degree of PPARγ expression was also markedly reduced in lungs of mature rats subjected to hemorrhage and resuscitation and was associated with a more severe inflammatory response compared with young animals (39). Similarly to these previous finding, in our present study we have demonstrated that in the liver the degree of PPARγ downregulation is also a function of the aging process and correlates with the severity of organ injury during hemorrhagic shock in mature rats. However, contrary to our previous findings that treatment with the PPARγ ligand, the cyclopentenone prostaglandin 15-deoxy-Δ-prostaglandin J2, ameliorated lung injury in young but not in mature animals (39), in our present study we observed that ciglitazone was able to increase PPARγ activation and to reduce liver apoptosis in mature rats. Of note, we also found that mature rats had a marked constitutive DNA binding activity of PPARγ compared with young animals. Therefore, it is plausible to hypothesize that the liver of mature rats has a sufficient receptor reserve, which can be still recruited by exogenous ligands. In contrast, the marked decrease of PPARγ in the lung of mature rats hampers the ability of specific ligands to enable efficient DNA binding of PPARγ (39). In support to our hypothesis, Shin et al. (29) have previously reported that, in the liver of old mice following hepatic ischemia and reperfusion, loss of the nuclear expression of the isoform PPARγ1 is secondary to the receptor retention in the cytosol and treatment with the specific ligand rosiglitazone increases its nuclear translocation. Thus taken together these data suggest that constitutive activation of the receptor may exhibit a tissue specific pattern in the mature rats and may depend on the metabolic organ function. Furthermore, organ injury can differ in young and mature rats depending on the level of activation of the PPARγ pathway, which may additionally alter the expression of survival regulators.
In the patient in state of shock, ALT is the most specific marker of hepatocellular injury. Serum ALT concentrations typically rise abruptly and return to normal within days after hemodynamic stability is restored (31). However, levels of this enzyme cannot predict the different mode of cell death in the liver. This enzyme is bound to cytoplasm and leaks into the blood when the hepatocytes cell membranes are damaged, as it happens in hepatocellular necrosis. On the other hand, apoptosis may occur in the absence of significant transaminases increase (8). In our present study, plasma ALT values were similar in both age groups of rats, suggesting the occurrence of a similar degree of necrosis. However, a significant amount of TUNEL-positive cells showed distinct nuclear staining, characteristic of apoptotic hepatocytes, in mature rats. Only a few TUNEL-positive cells were found, on the contrary, in young rats. In time-course studies, we observed that this event was preceded by activation of the mitochondrial apoptotic pathway.
The cellular threshold for mitochondrial apoptosis is highly regulated by the Bcl-2 family members. Some proteins, such as Bcl-2 itself, are potent repressors of apoptosis, whereas other members, such as BAX, function as proapoptotic molecules, which lead to activation of the apoptotic executors, including caspase-3 (25). In our study, the increased expression of BAX and activation of caspase-3 well paralleled the decrease of Bcl-2 expression in the liver of mature rats. Our data are in agreement with previous reports, which have indicated that hepatocytes isolated from old animals are more sensitive to oxidative stress that targets the mitochondria than hepatocytes isolated from young animals (36). Liver tissue in aged organisms has also been shown to have more susceptibility to heat stress (11), increased reactive oxygen species production (35), and delayed regeneration after injury (26). In our study, treatment with ciglitazone normalized the ratio between BAX and Bcl-2 and inhibited apoptosis, as revealed by decreased activity of caspase-3 and TUNEL staining.
Although the exact mechanism for PPARγ favorably altering early indicators of apoptosis cannot be determined from the present experiments, production of proinflammatory mediators, and changes in neutrophil infiltration and reactive oxygen species are known to play a major role in the initiation phase of apoptosis (15). We have previously demonstrated that PPARγ ligands ameliorate liver injury and reduce the proinflammatory response in young models of hepatic ischemia and reperfusion injury (19). Similarly, other laboratories have reported that pioglitazone, another thiazolidinedione, significantly inhibited hepatic apoptosis following ischemia and reperfusion. The protective effect was associated with downregulation of the local expression of several potent proinflammatory cytokines, chemokine adhesion molecules, and neutrophil accumulation (2). PPARγ ligands have also inhibitory effects on generation of reactive oxygen species and lipid peroxidation in the liver (28, 32). Consistent with these previous findings, in the present study we have demonstrated that ciglitazone reduced liver neutrophil infiltration in both age groups. Similar beneficial effects of PPARγ ligands on tissue leukosequestration have been found in hemorrhagic shock in experimental young animals (4, 39). Thus the protective effect of PPARγ ligands may be attributed to control of inflammation.
In conclusion, our study provides evidence that apoptosis is a significant pathogenetic factor of hepatocellular damage that occurs after hemorrhage and resuscitation in mature animals. This cellular death event must be distinguished for the appropriate therapeutic regimen to be applied to aging patients recovering from severe blood loss. In this regard, our findings raise the important prospect that PPARγ participates in the regulation of liver injury by functioning as a repressing factor of the apoptotic process.
GRANTS
Funding for this study was provided by the National Institutes of Health (Grants R01 GM-067202 and R01 AG-27990) to Dr. Basilia Zingarelli.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Abdelrahman M, Collin M, Thiemermann C. The peroxisome proliferator-activated receptor-γ ligand 15-deoxyΔ12,14 prostaglandin J2 reduces the organ injury in hemorrhagic shock. Shock 22: 555–561, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Akahori T, Sho M, Hamada K, Suzaki Y, Kuzumoto Y, Nomi T, Nakamura S, Enomoto K, Kanehiro H, Nakajima Y. Importance of peroxisome proliferator-activated receptor-gamma in hepatic ischemia/reperfusion injury in mice. J Hepatol 47: 784–792, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Calkins CM, Bensard DD, Moore EE, Bensard DD, Partrick DA, McIntyre RC, Harken AH. The injured child is resistant to multiple organ failure: a different inflammatory response? J Trauma 53: 1058–1063, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the IKK/NF-κB pathway following hemorrhagic shock. Crit Care Med 36: 2849–2857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collin M, Abdelrahman M, Thiemermann C. Endogenous ligands of PPARγ reduce the liver injury in haemorrhagic shock. Eur J Pharmacol 486: 233–235, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol 85: 1813–1822, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Everett L, Galli A, Crabb D. The role of hepatic peroxisome proliferator-activated receptors (PPARs) in health and disease. Liver 20: 191–199, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Eum HA, Cha YN, Lee SM. Necrosis and apoptosis: sequence of liver damage following reperfusion after 60 min ischemia in rats. Biochem Biophys Res Commun 358: 500–505, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graf D, Bode JG, Häussinger D. Caspases and receptor cleavage. Arch Biochem Biophys 462: 162–170, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Haak JL, Buettner GR, Spitz DR, Kregel KC. Aging augments mitochondrial susceptibility to heat stress. Am J Physiol Regul Integr Comp Physiol 296: R812–R820, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV. Outcome after hemorrhagic shock in trauma patients. J Trauma 45: 545–549, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Helling TS. The liver and hemorrhagic shock. J Am Coll Surg 201: 774–783, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Higuchi S, Wu R, Zhou M, Ravikumar TS, Wang P. Downregulation of hepatic cytochrome P-450 isoforms and PPAR-γ: their role in hepatic injury and proinflammatory responses in a double-hit model of hemorrhage and sepsis. J Surg Res 137: 46–52, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol 290: G1083–G1088, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Jaskille A, Koustova E, Rhee P, Britten-Webb J, Chen H, Valeri CR, Kirkpatrick JR, Alam HB. Hepatic apoptosis after hemorrhagic shock in rats can be reduced through modifications of conventional Ringer's solution. J Am Coll Surg 202: 25–35, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kaplan JM, Cook JA, Hake PW, O'Connor M, Burroughs TJ, Zingarelli B. 15-Deoxy-Δ12,14-prostaglandin J2 (15D-PGJ2), a peroxisome proliferator activated receptor γ ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock 24: 59–65, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kim MS, Sweeney TR, Shigenaga JK, Chui LG, Moser A, Grunfeld C, Feingold KR. Tumor necrosis factor and interleukin 1 decrease RXRα, PPARα, PPARγ, LXRα, and the coactivators SRC-1, PGC-1α, and PGC-1β in liver cells. Metabolism 56: 267–279, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Zingarelli B, Lentsch AB. Peroxisome proliferator-activated receptor-γ protects against hepatic ischemia/reperfusion injury in mice. Hepatology 47: 215–224, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Majde JA. Animal models for hemorrhage and resuscitation research. J Trauma 54: S100–S105, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43: S31–S44, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Matot I, Cohen K, Pappo O, Barash H, Abramovitch R. Liver response to hemorrhagic shock and subsequent resuscitation: MRI analysis. Shock 29: 16–24, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Mongan P, Capacchione West S J, Karaian J, Dubois D, Keneally R, Sharma P. Pyruvate improves the redox status and decreases indicators of hepatic apoptosis during hemorrhagic shock in swine. Am J Physiol Heart Circ Physiol 283: H1634–H1644, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Paxian M, Bauer I, Rensing H, Jaeschke H, Mautes AE, Kolb SA, Wolf B, Stockhausen A, Jeblick S, Bauer M. Recovery of hepatocellular ATP and “pericentral apoptosis” after hemorrhage and resuscitation. FASEB J 17: 993–1002, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Reed JC. Double identity for proteins of the Bcl-2 family. Nature 387: 773–776, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Sanz N, Diez-Fernandez C, Alvarez AM, Fernandez-Simon L, Cascales M. Age-related changes on parameters of experimentally-induced liver injury and regeneration. Toxicol Appl Pharmacol 154: 40–49, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Sauaia A, Moore FA, Moore EE, Norris JM, Lezotte DC, Hamman RF. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma 45: 291–301, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Sener G, Sehirli AO, Gedik N, Dulger GA. Rosiglitazone, a PPARγ ligand, protects against burn-induced oxidative injury of remote organs. Burns 33: 587–593, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Shin T, Kuboki S, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Pritts TA, Lentsch AB. Activation of peroxisome proliferator-activated receptor-γ during hepatic ischemia is age-dependent. J Surg Res 147: 200–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqui AM, Cui X, Wu R, Dong W, Zhou M, Hu M, Simms HH, Wang P. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-γ. Crit Care Med 34: 1874–1882, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Tarantino G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J Gastroenterol 13: 4669–4672, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomita K, Azuma T, Kitamura N, Nishida J, Tamiya G, Oka A, Inokuchi S, Nishimura T, Suematsu M, Ishii H. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology 126: 873–885, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Vanags DM, Pörn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem 271: 31075–31085, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Vish MG, Mangeshkar P, Piraino G, Denenberg A, Hake PW, O'Connor M, Zingarelli B. Proinsulin c-peptide exerts beneficial effects in endotoxic shock in mice. Crit Care Med 35: 1348–1355, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Yen TC, King KL, Lee HC, Yeh SH, Wei YH. Age-dependent increase of mitochondrial DNA deletions together with lipid peroxides and superoxide dismutase in human liver mitochondria. Free Radic Biol Med 16: 207–214, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Chong E, Herman B. Age-increases in the activity of multiple caspases in Fisher 344 rat organs. Exp Gerontol 37: 777–789, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Zingarelli B, Sheehan M, Hake PW, O'Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-γ ligands, 15-deoxy-Δ12,14-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol 171: 6827–6837, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-γ is a new therapeutic target in sepsis and inflammation. Shock 23: 393–399, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Zingarelli B, Hake PW, O'Connor M, Burroughs TJ, Wong HR, Solomkin JS, Lentsch AB. Lung injury after hemorrhage is age dependent: role of peroxisome proliferator-activated receptor γ. Crit Care Med 37: 1978–1987, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]