Abstract
In female mammals, increased ovarian estradiol (E2) secretion triggers GnRH release from neurons in the basal forebrain, which drives LH secretion from the pituitary and subsequently induces ovulation. However, the neural circuits that activate this preovulatory GnRH/LH surge remain unidentified. Neurotensin is expressed in neurons of the anteroventral periventricular nucleus (AVPV), a region thought to be critical for generating the preovulatory GnRH/LH surge. E2 induces neurotensin (Nts) gene expression in this region, and blockade of neurotensin signaling reduces the LH surge in the rat. We postulated that neurotensin signaling plays a similar role in generating the E2-induced GnRH/LH surge in mice. We used in situ hybridization (ISH) to determine whether E2 induces Nts expression in the mouse and found evidence to support this proposition. Next, we determined that the neurotensin receptor (Ntsr2) is present in many GnRH-expressing neurons. Since the kisspeptin gene (Kiss1) is expressed in the AVPV and is responsive to E2, we predicted that some neurons in this region express both Kiss1 and Nts; however, by double-label ISH, we observed no coexpression of the two mRNAs. We also postulated that Nts mRNA expression would increase in parallel with the E2-induced LH surge and that the central (icv) administration of neurotensin would stimulate LH secretion and activation of GnRH neurons but found no evidence to support either of these hypotheses. Together, these findings suggest that, although neurotensin neurons in the AVPV are targets for regulation by E2, neurotensin does not appear to play a direct role in generating the GnRH/LH surge in the mouse.
Keywords: gonadotropin-releasing hormone, luteinizing hormone, hypothalamus, brain, reproduction, estradiol, anteroventral periventricular
in rodents and other mammals, neurons in the ventral forebrain secrete gonadotropin-releasing hormone (GnRH), and these cells serve as the final common pathway by which the brain regulates gonadotropin secretion (15). During proestrus in the rat and mouse, a rising plasma level of estradiol (E2) activates GnRH neurons, which in turn triggers the preovulatory luteinizing hormone (LH) surge (5, 6, 13). The inductive effect of E2 on GnRH and LH secretion, so-called positive feedback, is both necessary and sufficient to stimulate ovulation (11, 37). Although positive feedback plays a critical role in governing estrous cycles, we have only a partial understanding of the underlying cellular and molecular mechanisms by which this occurs. E2 does not appear to act directly on GnRH neurons, since these cells do not express estrogen receptor-α (ERα)-the essential estrogen receptor isoform that controls the GnRH/LH surge (7, 16, 17). Instead, evidence suggests that E2 acts indirectly through another population of E-sensitive neurons, which relay the signal to GnRH neurons.
Several lines of evidence implicate neurotensin-producing cells in the anteroventral periventricular (AVPV) and medial preoptic nuclei (MPN) as targets for the action of E2 to trigger the preovulatory GnRH/LH surge. First, lesions of the AVPV/MPN area ablate the LH surge, ovulation, and estrous cycles in rats (39). Second, many neurons in this region express ERα and project to GnRH neurons (27, 33, 40), including neurons that express neurotensin (17–19). In the rat, E2 induces the expression of neurotensin (Nts) mRNA in the AVPV/MPN (2, 36). The administration of neurotensin directly into the medial preoptic area (where GnRH cell bodies reside) evokes LH secretion, whereas antiserum directed against neurotensin delivered into this same region blocks the LH surge (1, 4, 12). Finally, in the rat, GnRH neurons express mRNA for the neurotensin receptor 1 (Ntsr1), arguing that GnRH neurons may be direct targets for activation by neurotensin during the preovulatory GnRH/LH surge (36).
The goal of this study was to examine the role of neurotensin signaling in the regulation of GnRH/LH secretion in the female mouse. We tested several specific hypotheses related to the idea that neurons in the AVPV/MPN region of the hypothalamus mediate the positive feedback effect of E2 on GnRH neurons. First, we examined whether E2 induces expression of Nts mRNA in the AVPV/MPN in the mouse, as shown previously in the rat. Second, we used confocal microscopy to search for neurotensin-containing fibers in close apposition to GnRH cell bodies in the medial preoptic area (MPOA) and evaluated whether neurotensin receptors (Ntsr1 and Ntsr2) are expressed in GnRH neurons of the mouse. Third, since kisspeptin is also expressed in the AVPV and has been shown to play an important role in regulating GnRH secretion, we evaluated whether Nts is coexpressed with kisspeptin (Kiss1) mRNA in this area (14, 24, 35). Fourth, we sought to determine whether Nts mRNA is upregulated in association with the E2-induced GnRH/LH surge in mice. Finally, we assessed the ability of neurotensin to induce LH secretion by injecting the peptide into the cerebral ventricles of female mice with and without pretreatment with E2.
MATERIALS AND METHODS
Animals and surgeries.
Most animals used in this study were C57BL/6 mice purchased from Jackson Laboratory (Bar Harbor, ME). The mice used in the E2-induced LH surge paradigm (for examination of Nts mRNA expression) were a hybrid cross between the C57BL/6 and 129S1 strains. These animals were kept in a specific pathogen-free central animal housing facility at the University of Washington, where they were housed in groups of two to four with free access to water and standard rodent chow. The light cycle was set for 14:10-h light-dark, with lights on at 0400 and lights off at 1800. For immunohistochemistry studies, female young adult CF-1 mice from Charles River Laboratories (Wilmington, MA) were used. RNA microarray studies were performed with green fluorescent protein (GFP)-GnRH mice (C57BL6/DBA; provided by Dr. Peter H. Seeburg and Dr. Daniel J. Spergel, Max-Planck-Institute for Medical Research, Heidelberg, Germany). These animals were housed and bred in the central animal facility (Division of Laboratory Animal Resources) of the University of Kentucky Medical Center under controlled temperature with food and water available ad libitum. All animal care and techniques were conducted in accordance with the National Institutes of Health (NIH) Animal Care and Use Guidelines and with the approval of the appropriate Animal Care and Use Committees at the University of Washington and the University of Kentucky.
Surgeries were performed in a decentralized facility that is maintained to be specifically pathogen free. Mice were anesthetized by inhaled isoflurane (5% mixed with oxygen; Abbott Laboratories, North Chicago, IL) delivered by a vaporizer (Veterinary Anesthesia Systems, Bend, OR). Ovariectomies and capsule implantations were performed as described by Dungan et al. (10). Capsules were constructed of Silastic (dimensions: 1.5 cm long, 1.0 mm inner diameter, 2.2 mm outer diameter; Dow-Corning, Midland, MI) sealed with silicone adhesive (VWR Scientific, West Chester, PA). The contents of the capsules differed by experiment and experimental group.
Immunohistochemistry.
Mice were deeply anaesthetized and perfused transcardially with PBS (0.1 M, pH 7.4), followed by 4% paraformaldehyde in PBS. Brains were removed and postfixed in fixative for 24 h at 4°C before 30-μm-thick coronal vibratome sections were collected. Sections were washed twice in Tris·HCl buffer (0.05 M, pH 7.6) for 15 min each and stored in cryoprotectant at −20°C until use.
After removal from storage, sections were washed in Tris·HCl buffer twice for 15 min each and incubated for 30 min in blocking solution (10% normal horse serum, 0.2% Triton X-100, and 0.1% Na-azide in Tris·HCl). One set of sections was exposed for 48 h at 4°C to a mixture of guinea pig anti-GnRH (GP6-4, 1:2,000; Jennes) and rabbit anti-neurotensin (220-5, 1:2,000; Jennes) in blocking solution. Sections were then rinsed in Tris·HCl buffer for 15 min and incubated for 1 h in a mixture of affinity-purified and cross-absorbed donkey anti-guinea pig IgG labeled with FITC (1:100; Jackson Laboratory) and donkey anti-rabbit IgG labeled with Alexa Fluor 594 (1:200; Invitrogen). After several washes in Tris·HCl buffer, sections were mounted onto slides, dried, and coverslipped with ProLong Gold antifade solution.
A second set of sections was incubated for 48 h at 4°C in goat anti-neurotensin receptor 2 (P-19, 1:500; Santa Cruz Biotechnology) in blocking solution, washed in Tris·HCl, and exposed for 30 min to biotinylated donkey anti-goat IgG (1:400; Jackson Laboratory). After several washes, sections were incubated for 45 min in peroxidase-conjugated streptavidin (1:500; Jackson Laboratory), diluted in Tris·HCl buffer, washed extensively, and amplified with biotin-tyramide for 6 min (PerkinElmer). After three washes, 20 min each, sections were incubated for 30 min in streptavidin-Alexa Fluor 594 (1:1,000; Invitrogen), washed extensively, and processed as above for GnRH. Sections were viewed with a Leica TCS NT confocal microscope using the “Sequential Scanning Mode.”
In situ hybridization.
PCR product from mouse hypothalamus cDNA amplified with the primer pairs described for each experiment was used as template for the transcription of each probe except for the Kiss1 and c-fos probes. The template for these probes was linearized plasmid containing Kiss1 or c-fos cDNA, respectively. Probes were prepared as described in Cunningham et al. (9) and Gottsch et al. (14). Fresh frozen brains were stored at −80°C until sectioning on a cryostat (Leica CM1900). Sections were cut to 20 μm thick, mounted on glass slides (VWR Scientific), and stored at −80°C until processing. Tissue was then processed as described previously (9).
Single- and double-label in situ hybridization (ISH) was performed as described previously (14, 30). The specificity of the hybridization signal was validated by hybridizing brain sections with a radiolabeled sense probe, which produced no specific signal (data not shown).
Regulation of Nts mRNA expression by E2.
Adult female mice were ovariectomized (OVX) and implanted with capsules filled with safflower oil (OVX + Sham; n = 5) or capsules filled with E2 dissolved in safflower oil (OVX + E2; n = 7). After 1 wk, blood was collected and the mice were euthanized. Brains were collected, frozen on dry ice, and subjected to ISH. We chose this paradigm on the basis of previous studies in rats demonstrating that exposure to chronic supraphysiological levels of E2 upregulates the expression of Nts in the medial preoptic region of the rat (2). Furthermore, our protocol of ovariectomy and chronic E2 treatment has been used in our own previous studies to demonstrate that E2 stimulates the expression of the Kiss1 gene in the AVPV of mice (33, 34). The published sequence for mouse Nts mRNA (NM 024435) was used to design the probe for Nts. The forward primer, including a clamp sequence and promoter for triiodothyronine (T3) RNA polymerase, was 5′-cagagatgcaattaaccctcactctaaagggagaaggcaagaggaagcaccgag -3′. The reverse primer, including a clamp sequence and promoter for T7 RNA polymerase, was 5′-ccaagcctctaatacgactcactatagggagacctttgtaccgagagatgaactagcag-3′. Data were analyzed by unpaired t-test for the effect of E2 treatment.
Neurotensin receptors in GnRH neurons.
We used double-label ISH to identify GnRH neurons that expressed mRNA for Ntsr1 (n = 9). All animals were OVX, implanted with a sham or E2-filled capsule as described above, and then euthanized 1 wk later. Brains were processed for double-label ISH, and every fifth section was hybridized with a radiolabeled Ntsr1 probe and GnRH probe labeled with digoxigenin (DIG). The number of GnRH-expressing neurons and the number of GnRH neurons overlaid by silver grains (representing neurotensin receptor mRNA) were counted. Data were converted to percentages for each animal, averaged, and analyzed by group by t-test. The GnRH probe was designed from the published rat mRNA sequence (NM 012767), as described by Irwig et al. (21). The published mRNA sequence for mouse Ntsr1 (NM 018766) was used to design the Ntsr1 mRNA probe. The forward primer, including a clamp sequence and promoter for T3 RNA polymerase, was 5′-ccaagccttcattaaccctcactaaagggagatgcccttatccctctc-3′. The reverse primer, including a clamp sequence and promoter for T7 RNA polymerase, was 5′-cagagatgcataatacgactcactatagggagagtcccgtggcaatttc-3′.
RNA microarray methods.
Regularly cycling young adult female mice (2–3 mo old) were OVX 2 wk prior to the experiments. On the day of experiment, the animals were given one subcutaneous injection of 17β-estradiol (10 μl of 12 μg/ml in sesame oil) at 0900. Mice were anesthetized and decapitated immediately after the injection or 1 or 24 h after the injection. The forebrains of the mice were rapidly removed, and coronal sections of 150-μm thickness were prepared with a vibratome (Leica) in artificial cerebrospinal fluid (aCSF; 118 mM NaCl, 3 mM KCl, 11 mM d-glucose, 10 mM HEPES, 25 mM NaHCO3, 0.5 mM CaCl2, 6 mM MgCl2, pH 7.4), which was preequilibrated with 95% O2-5% CO2 for 15 min. Slices were transferred to the above oxygenated solution at room temperature.
Brain slices were transferred to a 35-mm tissue culture dish with cover glass bottom, held in place with a nylon grid, and superfused with gassed patch-clamp buffer. Slices were viewed with a fluorescence microscope (Zeiss Axioskop; Zeiss, Göttingen, Germany) first under bright-field illumination with a 4× Achroplan objective and then with a 40× water immersion Achroplan objective. Under fluorescence illumination, GFP-fluorescent GnRH neurons were approached with a patch pipette that was filled with 1 μl of aCSF containing 1 U RNase inhibitor SUPERase (Ambion) before the plasma membrane was penetrated and negative pressure applied to harvest the cytoplasm. Subsequently, the pipette content was expelled into a 0.5-ml sterile plastic centrifuge tube and stored at −80°C until RNA isolation. An average of ten GnRH-GFP neurons were collected from each mouse brain. A total of five sets of 30 neurons (150 neurons) were collected. The RNA samples were stored at −80°C.
All experiments were performed using Affymetrix Mouse 430 2.0 oligonucleotide arrays (Affymetrix, Santa Clara, CA) at the University of Kentucky DNA Microarray Core Facility. Total RNA from each sample was processed according to the instructions of the manufacturer, scanned on an Affymetrix GeneChip scanner, and analyzed with Affymetrix MAS 5.0 array analysis software.
Nts mRNA expression in Kiss1 neurons.
Ten adult female mice were OVX and implanted with a capsule containing E2 dissolved in safflower oil (mean level of serum estradiol = 149.5 ± 17.1 pg/ml). All animals in this experiment were treated with E2 to enhance the expression of both Nts and Kiss1 mRNAs. Double-label ISH was performed with a DIG-labeled Kiss1 probe and radiolabeled Nts probe on every fifth tissue section. Linearization of the Kiss1-containing plasmid with HindIII, followed by transcription with T7 RNA polymerase, resulted in a 409-bp segment of the mouse Kiss1 mRNA in the antisense orientation, as described previously (14). All Kiss1-expressing cells were counted, and the percentage of Kiss1-expressing cells overlaid with silver grains (indicating Nts mRNA expression) was determined.
Regulation of Nts mRNA expression during the E2-induced LH surge.
Animals were subjected to a regimen of ovariectomy and E2 treatment to induce a daily surge in LH levels. The protocol devised by Christian et al. (8) was used to induce a predictably timed surge of LH release in adult female mice. On the morning of day 0, mice were OVX, and a capsule containing 0.625 μg of E2 suspended in sesame seed oil was immediately implanted under the skin, as described above. Each animal was assigned to a morning (AM) or evening (PM) group. For animals in the AM group, on the morning of day 3, between 0700 and 0800, blood was collected from the orbital sinus while the animals were anesthetized with isoflurane (n = 7). All animals in the PM group were anesthetized with isoflurane between 1830 and 1930 (30–90 min after lights out) on the evening of day 3, and blood was collected from the orbital sinus (n = 7). Immediately after blood collection, all animals were euthanized by cervical dislocation, and the brains were collected. Data were analyzed by two-way ANOVA, and significant differences between groups were identified by Fisher's protected least significant difference (PLSD) test.
Effect of centrally administered neurotensin on LH secretion.
Adult female mice were OVX and implanted with a capsule containing only safflower oil (sham) or E2 dissolved in safflower oil (E2). Seven days after the surgeries, each mouse was given an intracerebroventricular (icv) injection of aCSF with 0.1% BSA alone (vehicle), 1 nmol of neurotensin (Sigma-Aldrich) dissolved in 3 μl of vehicle, or 1 nmol of galanin-like peptide (GALP; Phoenix Pharmaceuticals, Belmont, CA) dissolved in vehicle, creating six groups: Sham + Vehicle (n = 6), Sham + GALP (n = 6), Sham + neurotensin (n = 8), E2 + Vehicle (n = 5), E2 + GALP (n = 7), and E2 + neurotensin (n = 8). Previous studies have demonstrated that, in female mice, doses of GALP of 0.5 and 1.2 nmol induce a small but statistically significant rise in serum LH (23). Because we expected icv neurotensin treatment to have a similar effect in this experiment, we included a GALP-treated group as a positive control. All icv injections were conducted by the freehand method, as described previously (14). For each icv injection, the animal was anesthetized for ∼3 min while a total volume of 3 μl of solution was slowly injected with a Microliter syringe (Hamilton, Reno, NV). The needle was held in place for 1 min after the injection to allow the solution to disperse and minimize backflow through the needle track. Sera and brains were collected 20 min after the injection. A Thermalert TH5 (Physitemp, Clifton, NJ) with an attached rectal probe was used to measure the body temperature of each mouse (while awake) just prior to injection and again just prior to sera collection. Sera were assayed for LH, and brains were frozen and stored at −80°C. Data were analyzed by two-way ANOVA, and Fisher's PLSD test was used to identify significant differences between groups.
Effect of centrally administered neurotensin on c-fos mRNA expression in GnRH neurons.
Animals were treated as described above for the icv injection of neurotensin. Due to capacity limitations within the assay, five individual animals from each of the six groups were randomly selected to undergo double-label ISH for c-fos and GnRH. The GnRH probe was visualized with DIG as described above, and the 1,352-bp c-fos probe was labeled with 33P as described by Finn et al. (13). Data were analyzed by two-way ANOVA and Fisher's PLSD test was used to identify significant differences between groups.
Radioimmunoassays.
Serum levels of E2 and LH were measured at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA). E2 was measured with a double-antibody kit (Diagnostics Systems Laboratories, Webster, TX), which had a sensitivity of 10 pg/ml, an intra-assay coefficient of variation of 5.7%, and an interassay coefficient of variation of 11.6%. LH was measured by using a modified two-site sandwich immunoassay with monoclonal antibodies against bovine LH and the human LHβ subunit. The sensitivity of the LH assay was 0.04 ng/ml, the intra-assay coefficient of variation was 3.6%, and the interassay coefficient of variation was 9.2%.
RESULTS
Neurotensin-positive contacts on GnRH-positive cells.
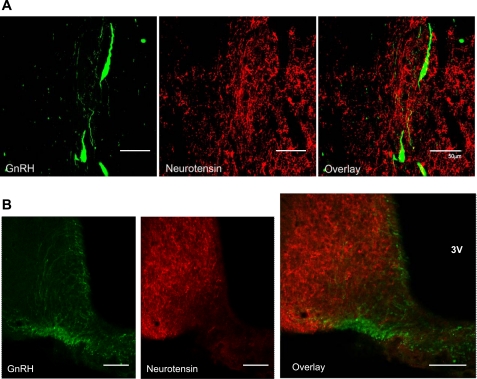
We posited that if neurotensin played a direct role in regulating GnRH secretion, then axons containing neurotensin should project to GnRH neurons. Using double-label fluorescent immunohistochemistry, we found that ∼50% of GnRH neurons in the preoptic area were apposed by fibers containing neurotensin (Fig. 1A). However, we observed few neurotensin-containing fibers in the median eminence (Fig. 1B).
Fig. 1.
Double-label immunohistochemistry for gonadotropin-releasing hormone (GnRH; green) and neurotensin fibers (red) in the medial preoptic area (MPOA) (scale bars, 50 μm; A) and median eminence (scale bars, 100 μm; B). 3V, 3rd ventricle.
Neurotensin receptors in GnRH neurons.
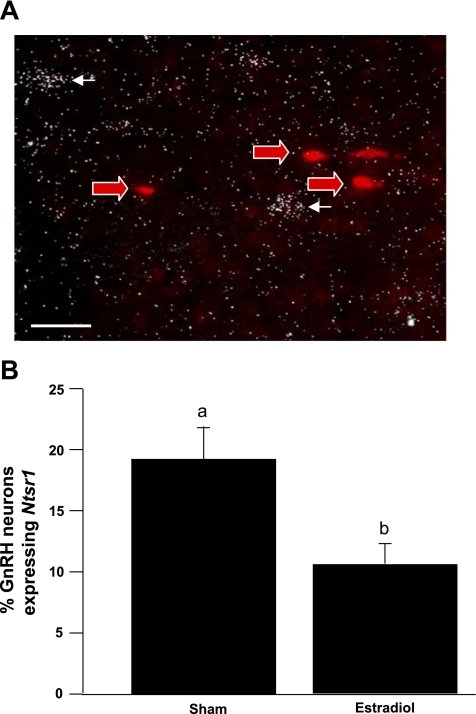
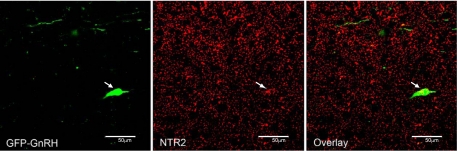
Although we observed neurotensin-containing fiber contacts on GnRH neurons, it does not necessarily follow that neurotensin signaling occurs at those contacts. Therefore, to determine whether neurotensin directly influences GnRH neuronal activity, we used double-label ISH to evaluate the expression of the neurotensin receptor genes Ntsr1 and Ntsr2 in GnRH neurons. Although expression of Ntsr1 mRNA is apparent in the general region of GnRH neurons, we observed very little coexpression between the two mRNAs (Fig. 2A). Furthermore, the percentage of GnRH neurons expressing Ntsr1 was decreased in mice treated with E2 compared with sham-treated mice (Fig. 2B). We also used mice engineered to express GFP under the control of the GnRH promoter to isolate mRNA from individual GnRH neurons in hypothalamic slices. Quantitative RT-PCR was performed on the extracts from the GnRH cells and used for microarray analysis. Consistent with our observations from ISH, we detected no Ntsr1 mRNA in any of the GnRH cells examined. In contrast, we detected Ntsr2 in each microarray sample. We proceeded to employ double-label IHC to visualize colocalization of GnRH and Ntsr2 proteins (Fig. 3). Approximately 75% of all identifiable GnRH neurons appeared to contain Ntsr2.
Fig. 2.
A: photomicrograph of GnRH and neurotensin receptor 1 (Ntsr1) in situ hybridization (ISH) in the rostral hypothalamus. Red arrows indicate neurons expressing GnRH mRNA. White arrows indicate neurons expressing Ntsr1 mRNA. Scale bar, 100 μm. B: number of GnRH cells coexpressing Ntsr1 mRNA in Sham- or estradiol-treated mice. Different letters indicate significant difference, P < 0.05. Data are expressed as means ± SE.
Fig. 3.
Double-label immunohistochemistry for GnRH (green) and neurotensin receptor 2 (NTR2; red) in the MPOA. White arrow indicates a cell expressing protein for both GnRH and Ntsr2. Scale bars, 50 μm. GFP, green fluorescent protein.
Regulation of Nts mRNA expression by E2.
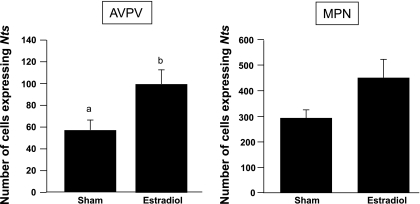
If neurotensin participates in mediating the positive effects of E2 on GnRH neurons, it is reasonable to expect E2 to upregulate the expression of Nts mRNA in the AVPV/MPN. To evaluate this possibility, we compared the expression of Nts between OVX animals with and without E2 treatment.
The average serum level of E2 was <10 pg/ml for the OVX group and 149.5 ± 17.1 pg/ml for the OVX + E2 group. The number of cells expressing Nts mRNA in the AVPV of the OVX + E2 group was significantly higher than that in the OVX + Sham group (P < 0.05; Fig. 4). There was a trend for increased numbers of Nts cells in the MPN with E2 treatment, but this did not reach statistical significance (P = 0.07; Fig. 4). As expected, LH levels were higher in the OVX + Sham group (1.9 ± 0.2 ng/ml) than in the OVX + E2 group (0.08 ± 0.01 ng/ml).
Fig. 4.
Number of cells expressing neurotensin (Nts) mRNA in the anteroventral periventricular (AVPV) and medial preoptic nuclei (MPN) of Sham- or estradiol-treated mice. Different letters indicate significant difference, P < 0.05. Data are expressed as means ± SE.
Regulation of Nts mRNA during the E2-induced LH surge.
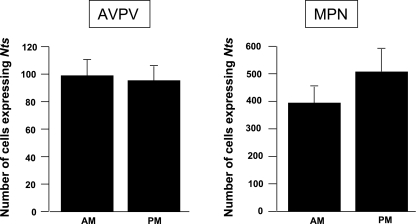
We further reasoned that if neurotensin signaling has a role in facilitating the generation of E2-induced LH surges, then the expression of Nts mRNA might be upregulated at the time of the surge. Animals were subjected to a regimen of ovariectomy and E2 treatment to induce a daily surge in LH levels (n = 7), and levels of Nts mRNA (based on cell counts and grains/cell) were compared between the AM (when LH levels were low) and PM (when LH levels were elevated). Data were analyzed by Student's t-test. Neither the number of Nts-expressing cells (Fig. 5) nor the grains per cell reflecting Nts mRNA content per cell (data not shown) were statistically different between the AM and PM time points. Although the average number of Nts-expressing cells in the MPN was higher at the PM time point, this trend was not statistically significant.
Fig. 5.
Number of cells expressing Nts mRNA in the AVPV and MPN of female mice in the morning (AM) and evening (PM). No significant differences were observed. Data are expressed as means ± SE.
Coexpression of Nts mRNA and Kiss1 mRNA in the AVPV.
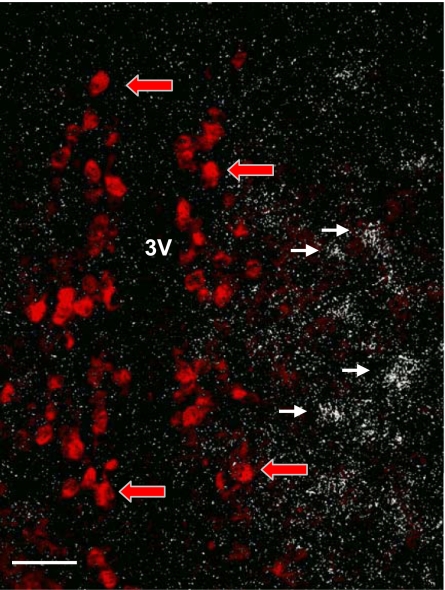
Since Nts and Kiss1 are expressed in the AVPV and both have been implicated for a role in the regulation of GnRH secretion (in the rat), we postulated that Kiss1 and Nts are coexpressed in the same population of neurons. Using double-label ISH, we found that, although cells expressing Kiss1 and Nts mRNAs were apparent in the AVPV region, there was little (or no) coexpression (Fig. 6). Kiss1 mRNA was found to be located medially, close to the third ventricle, whereas Nts mRNA was observed more laterally.
Fig. 6.
Photomicrograph of Kiss1 and Nts ISH in the rostral hypothalamus. Red arrows indicate neurons expressing Kiss1 mRNA. White arrows indicate neurons expressing Nts mRNA. Few, if any, neurons express both mRNAs. Scale bar, 100 μm.
Effect of centrally administered neurotensin on LH secretion.
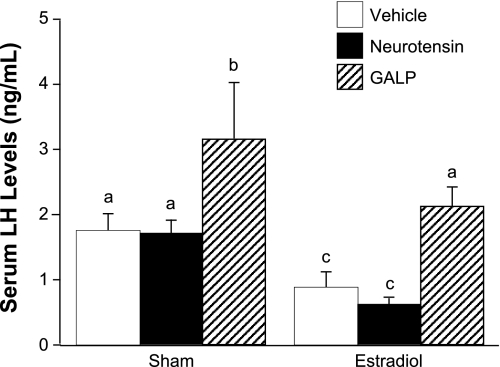
To test whether neurotensin can stimulate GnRH/LH secretion, we measured circulating LH levels after injecting neurotensin or vehicle into the lateral ventricle of OVX, E2-treated mice. As positive controls, we also measured the effect of GALP on LH release and the effect of neurotensin on body temperature. Among the sham-treated animals, the Sham + Vehicle and Sham + neurotensin groups had equivalent LH levels. The Sham + GALP group had significantly elevated serum LH compared with both Sham + Vehicle and Sham + neurotensin groups (P < 0.01). Likewise, LH levels in the E2 + Vehicle and E2 + neurotensin groups were similar, whereas the mean LH level of the E2 + GALP group was significantly increased (P < 0.01). LH data are shown in Fig. 7. Neurotensin significantly depressed body temperature compared with vehicle treatment, regardless of hormone status (P < 0.01; data not shown), indicating the efficacy of the treatment to induce a known biological response.
Fig. 7.
Serum LH levels in sham- and estradiol-treated mice after administration of vehicle, galanin-like peptide (GALP), or neurotensin. Different letters indicate significant difference, P < 0.05. Data are expressed as means ± SE.
Effect of centrally administered neurotensin on c-fos expression in GnRH neurons.
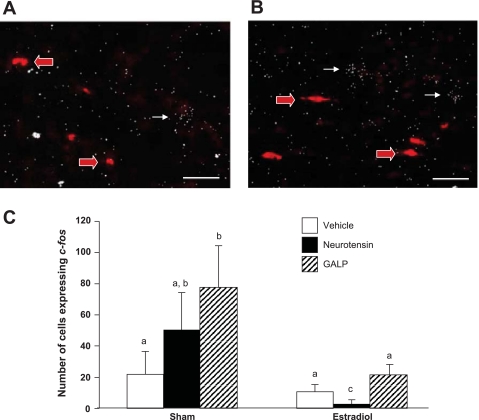
We sought to determine whether neurotensin treatment influenced the expression of immediate early gene c-fos. The expression of c-fos mRNA or protein is used as a marker of neuronal activity in GnRH neurons (13). We found no significant difference in the number of GnRH cells expressing c-fos among any of the groups (data not shown). However, we did observe differences between the number of non-GnRH c-fos-positive cells in the AVPV and periventricular nucleus (Fig. 8). Among sham-treated animals, the number of cells that expressed c-fos was not different between the Sham + Vehicle and the Sham + neurotensin groups. The Sham + GALP group had significantly more c-fos-expressing neurons than vehicle-treated animals (P < 0.05). Among the E2-treated groups, GALP had no significant effect upon c-fos expression. Interestingly, the E2 + neurotensin group had significantly fewer cells that expressed c-fos (P < 0.05).
Fig. 8.
Photomicrographs of GnRH and c-fos mRNA ISH in ovariectomized + sham mice treated with neurotensin (A) and GALP (B). Red arrows indicate cells expressing GnRH. White arrows indicate cells expressing c-fos. Scale bars, 100 μm. C: number of cells expressing c-fos mRNA in the AVPV/periventicular nucleus continuum. Different letters indicate significant difference, P < 0.05. Data are expressed as means ± SE.
DISCUSSION
Our aim was to characterize the role of neurotensin signaling in mediating the positive feedback effects of E2 on GnRH neurons and the GnRH/LH surge in the female mouse. First, we examined the pattern of expression of Nts mRNA and peptide in the hypothalamus. We found that Nts mRNA is expressed in the AVPV and MPN and that the number of Nts-expressing cells in the AVPV increased in the presence of E2. These observations are in accord with previous studies in rats and one study in mice (2, 3, 36, 38). We also examined neurotensin peptide in the MPOA and determined that neurotensin-positive fibers are closely associated with GnRH neuronal cell bodies, in agreement with previous work by Hoffman (19). We cannot state with any certainty that the neurotensin fibers surrounding GnRH neurons originate from Nts-expressing somas of the AVPV/MPN. However, Nts neurons in the AVPV/MPN express ERα, and many ERα-positive neurons project from the AVPV/MPN to GnRH neurons (17, 40). Together, these observations indicate that Nts neurons in the AVPV are stimulated by E2, and these neurons may communicate directly with GnRH neurons to influence GnRH release. In contrast to reports in rats, we found no evidence that neurotensin-containing fibers contact GnRH neuron terminals in the median eminence (22). This observation highlights the microanatomical differences between mice and rats and also suggests that the neurotensin system may have different roles in the two species. Thus, in mice, we sought to confirm other observations on the neurotensin system from the rat.
We evaluated the presence of neurotensin receptors in GnRH neurons as evidence that neurotensin signaling can communicate directly with GnRH neurons. First, we searched for expression of Ntsr1 in GnRH neurons, because this receptor has the highest affinity for neurotensin. Furthermore, Smith and Wise (36) had demonstrated in the rat that some GnRH neurons express Ntsr1. Double-label ISH for Ntsr1 and GnRH mRNAs revealed that very few GnRH neurons express mRNA for this receptor. Moreover, in corroboration of the findings of Smith and Wise (36), treatment with E2 decreased the percentage of GnRH-expressing cells that expressed Ntsr1. Single-cell RT-PCR in GnRH neurons from OVX mice confirmed a paucity of Ntsr1 mRNA in GnRH neurons. After finding little evidence for direct binding of neurotensin to GnRH neurons via Ntsr1, we turned to the lower-affinity receptor Ntsr2. Cell-specific RT-PCR demonstrated that the majority of GnRH neurons do express mRNA for Ntsr2 and, based upon our immunohistochemical findings, that mRNA is translated into protein. Thus, it seems likely that neurotensin neurons communicate with GnRH neurons via Ntsr2.
Neurotensin cells are not the only population of neurons in the AVPV/MPN region that respond to E2 and influence GnRH neurons. Kisspeptin, a product of the Kiss1 gene, is a potent stimulator of GnRH release (14, 31). Kisspeptin and neurotensin neurons share several characteristics besides their common residence in the AVPV. First, both transcripts are upregulated by E2 in rats and mice (2, 33, 35, 38). Second, in the rat, the number of Nts and Kiss1 mRNA-expressing cells peaks in coincidence with the preovulatory LH surge (35, 36). Third, neurotensin- and kisspeptin-containing fibers are found adjacent to GnRH neurons (Ref. 19 and the present study). On the basis of these observations, we wondered whether Nts and Kiss1 were expressed in the same population of neurons. We found no significant coexpression of Nts and Kiss1 mRNA in the AVPV. Kiss1 expression in the AVPV was concentrated in the medial/dorsal aspect, whereas Nts cells were located primarily in the rostral/ventral region and extending more laterally from the third ventricle. Nevertheless, we did observe many instances of close apposition of Nts and Kiss1 cells, suggesting that these populations may communicate with one another. Indeed, neurotensin receptors can be found in the AVPV, reinforcing the notion that communication may occur between Nts and Kiss1 neurons in the AVPV (Ref. 32 and our unpublished observations).
Smith and Wise's (36) observation that Nts mRNA expression in the AVPV/MPN peaks just before the onset of the preovulatory LH surge in intact female rats supports a role for neurotensin signaling in the generation of the LH surge. We sought to demonstrate a similar phenomenon in a mouse model. We used a paradigm of ovariectomy and E2 treatment to induce LH surges in female mice and then examined Nts mRNA expression in the AVPV and MPN (8). In this paradigm, the AM time point mimicked conditions of the negative feedback effect of E2 on GnRH/LH secretion, whereas the PM time point mimicked conditions of the positive feedback effect of E2 on LH (28). We posited that if neurotensin signaling participated in the generation of the LH surge, then we would observe increased numbers of Nts-expressing cells at the PM time point compared with the AM time point. Contrary to our prediction, we found that the numbers of Nts-expressing neurons did not differ between groups. One possible reason for this outcome is that all mice in our experiment were exposed to the same level of E2, unlike Smith and Wise's experiments (36), which were conducted in intact cycling rats. Together with our observation that exposure to chronic high levels of E2 resulted in an increase in Nts expression in the AVPV, these findings suggest that Nts is responsive to E2 but not to circadian cues. It may be that Nts mRNA transcription is stimulated by E2, but a circadian signal is required to release the peptide.
If neurotensin stimulates GnRH neurons to produce a surge in GnRH/LH secretion, we would expect that delivery of neurotensin to the brain would elicit an increase in serum LH levels. Previous work in rats has tendered equivocal results on the ability of centrally administered neurotensin to elicit gonadotropin secretion. McCann and Vijayan (26) found that injection of the peptide into the third ventricle of long-term OVX rats significantly decreases levels of circulating LH, an effect that was confirmed by Motta and Martini (29). On the other hand, Akema et al. (1) reported no effect on LH when neurotensin was microinjected into the MPOA of OVX rats, yet they found that when rats were treated with estrogen to induce an LH surge, neurotensin amplified the E2-induced rise in LH. In dispute, Ferris et al. (12) reported a significant increase in LH levels following injection of neurotensin into the MPOA of OVX rats. These confusing and apparently contradictory results may reflect differences in methodology (i.e., dose and site of injection). Considering the extent of neurotensin expression in the hypothalamus, it is conceivable that, when administered into the diencephalon, this peptide could have variable effects depending on which population of neurons is exposed. We administered neurotensin into the lateral cerebral ventricles to determine whether the peptide would stimulate LH secretion in this species. Although the treatment produced a significant drop in body temperature (confirming its delivery and efficacy, at least into the hypothalamic sites involved in temperature regulation), we observed no effect on LH levels in the presence or absence of E2. We did observe an increase in LH levels following GALP treatment, suggesting that the injection technique was sufficient to provoke a response by a known secretagogue of GnRH. Since neurotensin and its receptors are widely expressed throughout the hypothalamus, it is possible that injections of neurotensin directly into the MPOA of mice would stimulate LH secretion, as has been observed in rats. This would alter perspectives on the matter but remains to be examined.
We next sought to determine whether the icv neurotensin injections activated GnRH neurons by examining c-fos mRNA expression in this population. The immediate early gene c-fos is an indicator of transcriptional activation in neurons, and c-fos mRNA and protein expression in GnRH neurons is induced in association with GnRH secretion and the downstream LH response (13, 20). We observed that icv neurotensin treatment had no discernible effect on the colocalization of c-fos and GnRH mRNAs, consistent with the finding that LH levels remained unchanged in this treatment group compared with the vehicle-treated group. We also found that whereas GALP injection significantly increased LH secretion, it did not affect c-fos expression in GnRH neurons. This outcome was surprising because previous studies in rats have demonstrated that GALP (delivered icv) induces Fos protein expression in GnRH neurons (25). Moreover, studies in mice show that blockade of GnRH receptors prevents GALP-induced LH secretion, indicating that GALP stimulates LH secretion through a GnRH-dependent mechanism (23). Our study did show that GALP treatment increased the overall number of c-fos-expressing cells in the rostral periventricular region in OVX Sham-treated mice; however, neurotensin did not have a significant effect on the number of c-fos cells in Sham-treated animals. E2 treatment abrogated the stimulatory effect of GALP while revealing an inhibitory effect of neurotensin on c-fos expression. These results invite further study of the interactions of GALP and neurotensin with GnRH neurons, but in any case our observations do not support a role for neurotensin in stimulating GnRH release.
In summary, we have demonstrated that Nts mRNA expression is induced by E2 in the AVPV/MPN of the female mouse. Combined with our observations that neurotensin neurons appeared to make contact with GnRH neurons and that neurotensin receptors were present in GnRH neurons, we predicted that neurotensin signaling played a role in the generation of the E2-induced LH surge of the mouse. However, we found that injection of neurotensin into the cerebral ventricles had no effect on circulating levels of LH. Moreover, we saw no change in Nts expression as a function of the E2-induced LH surge. Thus, although the evidence suggests that Nts neurons participate in the generation of the preovulatory LH surge (particularly in the rat), further studies are required to better understand the precise role of neurotensin signaling in regulating GnRH secretion in the mouse.
GRANTS
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/NIH through cooperative agreement U54-HD-12629 (to the University of Washington Center for Research in Reproduction and Contraception), the Marie Curie Outgoing International Fellowship of the European Union 7th Framework Programme, and NICHD/NIH Grants R01-HD-27142, AG-25047, and RR-015592. Dr. Heather Dungan Lemko is currently a postdoctoral researcher at the University of Texas Southwestern Medical Center.
DISCLOSURES
The authors have nothing to disclose.
ACKNOWLEDGMENTS
We are grateful for the technical assistance provided by Adrian Centers at the University of Kentucky and by Dr. Michelle Gottsch, Dr. Amy Oakley, Dr. Jodi Downs, and Janessa Lawhorn at the University of Washington. Hormone measurements were performed by the Specialized Cooperative Centers Program in Reproduction and Infertility Research Ligand Assay and Analysis Core at the University of Virginia (Charlottesville, VA).
REFERENCES
- 1.Akema T, Praputpittaya C, Kimura F. Effects of preoptic microinjection of neurotensin on luteinizing hormone secretion in unanesthetized ovariectomized rats with or without estrogen priming. Neuroendocrinology 46: 345–349, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Alexander MJ, Dobner PR, Miller MA, Bullock BP, Dorsa DM, Leeman SE. Estrogen induces neurotensin/neuromedin N messenger ribonucleic acid in a preoptic nucleus essential for the preovulatory surge of luteinizing hormone in the rat. Endocrinology 125: 2111–2117, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Alexander MJ, Kiraly ZJ, Leeman SE. Sexually dimorphic distribution of neurotensin/neuromedin N mRNA in the rat preoptic area. J Comp Neurol 311: 84–96, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Alexander MJ, Mahoney PD, Ferris CF, Carraway RE, Leeman SE. Evidence that neurotensin participates in the central regulation of the preovulatory surge of luteinizing hormone in the rat. Endocrinology 124: 783–788, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 104: 1247–1255, 1979 [DOI] [PubMed] [Google Scholar]
- 6.Caligaris L, Astrada JJ, Taleisnik S. Release of luteinizing hormone induced by estrogen injection into ovariectomized rats. Endocrinology 88: 810–815, 1971 [DOI] [PubMed] [Google Scholar]
- 7.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 149: 5328–5334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102: 15682–15687, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham MJ, Scarlett JM, Steiner RA. Cloning and distribution of galanin-like peptide mRNA in the hypothalamus and pituitary of the macaque. Endocrinology 143: 755–763, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27: 12088–12095, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferin M, Tempone A, Zimmering PE, Van de Wiele RL. Effect of antibodies to 17beta-estradiol and progesterone on the estrous cycle of the rat. Endocrinology 85: 1070–1078, 1969 [DOI] [PubMed] [Google Scholar]
- 12.Ferris CF, Pan JX, Singer EA, Boyd ND, Carraway RE, Leeman SE. Stimulation of luteinizing hormone release after stereotaxic microinjection of neurotensin into the medial preoptic area of rats. Neuroendocrinology 38: 145–151, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Finn PD, Steiner RA, Clifton DK. Temporal patterns of gonadotropin-releasing hormone (GnRH), c-fos, and galanin gene expression in GnRH neurons relative to the luteinizing hormone surge in the rat. J Neurosci 18: 713–719, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145: 4073–4077, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Herbison A. Gonadotropin-Releasing Hormone (GnRH) Neuronal Systems New York: Academic, 2005 [Google Scholar]
- 16.Herbison AE, Robinson JE, Skinner DC. Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology 57: 751–759, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 50: 283–298, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Herbison AE, Theodosis DT. Neurotensin-immunoreactive neurons in the rat medial preoptic area are oestrogen-receptive. J Neuroendocrinol 3: 587–589, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Hoffman GE. Organization of LHRH cells: differential apposition of neurotensin, substance P and catecholamine axons. Peptides 6: 439–461, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Hoffman GE, Lee WS, Smith MS, Abbud R, Roberts MM, Robinson AG, Verbalis JG. c-Fos and Fos-related antigens as markers for neuronal activity: perspectives from neuroendocrine systems. NIDA Res Monogr 125: 117–133, 1993 [PubMed] [Google Scholar]
- 21.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80: 264–272, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Jennes L, Stumpf WE, Kalivas PW. Neurotensin: topographical distribution in rat brain by immunohistochemistry. J Comp Neurol 210: 211–224, 1982 [DOI] [PubMed] [Google Scholar]
- 23.Kauffman AS, Buenzle J, Fraley GS, Rissman EF. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm Behav 48: 141–151, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146: 4431–4436, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto H, Noguchi J, Takatsu Y, Horikoshi Y, Kumano S, Ohtaki T, Kitada C, Itoh T, Onda H, Nishimura O, Fujino M. Stimulation effect of galanin-like peptide (GALP) on luteinizing hormone-releasing hormone-mediated luteinizing hormone (LH) secretion in male rats. Endocrinology 142: 3693–3696, 2001 [DOI] [PubMed] [Google Scholar]
- 26.McCann SM, Vijayan E. Control of anterior pituitary hormone secretion by neurotensin. Ann NY Acad Sci 668: 287–297, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144: 2055–2067, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol 21: 327–333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motta M, Martini L. Neurotensin inhibits LH release. Proc Soc Exp Biol Med 168: 62–64, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29: 11859–11866, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29: 48–69, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Sarret P, Perron A, Stroh T, Beaudet A. Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J Comp Neurol 461: 520–538, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146: 3686–3692, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146: 2976–2984, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26: 6687–6694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MJ, Wise PM. Neurotensin gene expression increases during proestrus in the rostral medial preoptic nucleus: potential for direct communication with gonadotropin-releasing hormone neurons. Endocrinology 142: 3006–3013, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96: 219–226, 1975 [DOI] [PubMed] [Google Scholar]
- 38.Watters JJ, Dorsa DM. Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci 18: 6672–6680, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiegand SJ, Terasawa E, Bridson WE. Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology 102: 1645–1648, 1978 [DOI] [PubMed] [Google Scholar]
- 40.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52: 271–280, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]