Abstract
Very-low-density lipoprotein receptor (VLDLR) is a member of the low-density receptor family, highly expressed in adipose tissue, heart, and skeletal muscle. It binds apolipoprotein E-triglyceride-rich lipoproteins and plays a significant role in triglyceride metabolism. PPARγ is a primary regulator of lipid metabolism in adipocytes and controls the expression of an array of genes involved in lipid trafficking in adipocytes. However, it is not known whether VLDLR is also under the control of PPARγ. In this study, we investigated the role of PPARγ in the regulation of VLDLR expression and function in vivo and in vitro. During the differentiation of 3T3-L1 preadipocytes, the levels of VLDLR protein and mRNA increased in parallel with the induction of PPARγ expression and reached maximum in mature adipocytes. Treatment of differentiated adipocytes with PPARγ agonist pioglitazone upregulated VLDLR expression in dose- and time-dependent manners. In contrast, specific inhibition of PPARγ significantly downregulated the protein level of VLDLR. Induction of VLDLR is also demonstrated in vivo in adipose tissue of wild-type (WT) mice treated with pioglitazone. In addition, pioglitazone increased plasma triglyceride-rich lipoprotein clearance and increased epididymal fat mass in WT mice but failed to induce similar effects in vldlr−/− mice. These results were further corroborated by the finding that pioglitazone treatment enhanced adipogenesis and lipid deposition in preadipocytes of WT mice, while its effect in VLDLR-null preadipocytes was significantly blunted. These findings provide direct evidence that VLDLR expression is regulated by PPARγ and contributes in lipid uptake and adipogenesis.
Keywords: white adipose tissue, triglyceride, triglyceride-rich lipoproteins, triglyceride clearance
very-low-density lipoprotein receptor (VLDLR), a member of the low-density lipoprotein receptor (LDLR) family, is highly expressed in adipose tissue (35). In mammals, VLDLR shows a high degree of identity among different species with a 95% identity between the corresponding proteins (43). In neurons, VLDLR is a ligand for reelin and plays a crucial role in neuron migration during development (46). In addition, VLDLR binds apolipoprotein E-triglyceride-rich (apoE-TG rich) lipoproteins and mediates lipid uptake in peripheral tissues (33, 42, 44). Initially, Frykman et al. (15) reported that VLDLR-null (vldlr−/−) mice are leaner, with normal blood lipids. However, several studies have revealed an increase in plasma TGs at postprandial (16) and after long fasting (48). Later studies (34, 48) showed that VLDLR mediated the trans-cytosis of lipoprotein lipase (LPL) across endothelial cells and that plasma LPL activity in post-heparin plasma is significantly reduced in vldlr−/− mice. These studies suggested a possible link among decreased LPL, hypertriglyceridemia, and VLDLR deficiency. Furthermore, VLDLR seems to be linked to obesity, since its deficiency protects mice from obesity linked to high-fat feeding or leptin deficiency (17). In agreement with those results in mice, patients with VLDLR mutations have abnormally low body mass index (BMI < 18.5) compared with control subjects (5). Whether through the modulation of LPL activity or by endocytosis of lipoproteins, VLDLR seems to play a significant role in the delivery of VLDL-derived fatty acids (FAs) into adipose tissue.
Peroxisome proliferator-activated receptor-γ (PPARγ) is a member of the family of the steroid/thyroid/retinoid receptor superfamily of ligand-activated nuclear transcription factors (29, 45). PPARγ heterodimerizes with the retinoid X receptor (RXR) and binds to specific regions in the promoter of target genes (39). PPARγ is highly expressed in adipose tissue (45), where it serves as an essential regulator of adipocyte differentiation and maintenance of mature adipocyte phenotype (11). It is also the specific target for thiazolidinediones (TZDs), the antidiabetic agents that improve insulin sensitivity, glucose tolerance, and lipid homeostasis (47). In adipocytes, PPARγ regulates an array of genes involved in adipogenesis and lipid metabolism, including LPL, the enzyme required for TG hydrolysis, CD36, also called fatty acid translocase (FAT), fatty acid transport protein (FATP), and fatty acid-binding protein-4 (11, 13). Through the activation of these target genes, PPARγ agonists promote lipid retention in adipose tissue by directing TG-derived FAs mainly to adipose tissue and prevent their recycling to the circulation and to other organs (28).
Although VLDLR is highly expressed in adipocytes, it is not known whether its expression and function are regulated by PPARγ and whether it participates in mediating the proadipogenic role of PPARγ. In this study, we investigated the mechanism(s) of VLDLR regulation by PPARγ in isolated adipocytes and in mouse models.
EXPERIMENTAL PROCEDURES
Materials.
Pioglitazone was obtained from Cayman Chemical (Ann Arbor, MI). GW-9662, bovine insulin, dexamethasone, and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Sigma-Aldrich (St. Louis, MO). Bovine serum albumin (BSA) was from BioPharm laboratories (Alpine, UT). To make stock solutions, pioglitazone was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich), and bovine insulin was prepared in 0.1 N HCl (Fisher Scientific, Pittsburgh, PA) and IBMX in 0.35 M KOH (Fisher Scientific). 3T3-L1 cells were purchased from the American Tissue Culture Collection (Rockville, MD). Antibodies specific for VLDLR, LPL, PPARγ, and β-actin were from R&D Systems (Minneapolis, MN), Cell Application (San Diego, CA), Upstate (Lake Placid, NY), and Sigma-Aldrich, respectively.
Cell culture and differentiation and VLDLR expression.
3T3-L1 cells were grown in Dulbecco's modified Eagle medium (DMEM; GIBCO, Grand Island, NY) with 10% delipidated fetal calf serum (FCS). Differentiation of 3T3-L1 to mature adipocytes was performed as described previously (14, 40). Briefly, after 2 days of confluence, cells were induced by incubation in DMEM with 10% FCS and differentiation cocktail containing 0.5 mM IBMX, 1 μg/ml dexamethasone, and 10 μg/ml insulin for 48 h. Then, cells were incubated in DMEM with 10% delipidated FCS supplemented with 10 μg/ml insulin for another 6 days. Medium was replaced every 2 days. Cells were harvested at indicated days of differentiation to determine mRNA and protein levels of VLDLR, LPL, and PPARγ.
Effect of PPARγ activation and inhibition on VLDLR expression.
For time course experiment, the fully differentiated 3T3-L1 cells were incubated overnight with DMEM plus 0.2% serum-free BSA, and then the final 10 μM of pioglitazone or 0.1% DMSO was added respectively for different time periods. To test the effects of different doses of pioglitazone, fully differentiated cells were cultured in DMEM with 0.2% serum-free BSA, with DMSO, or with different doses of pioglitazone for 24 h. At the end of the experiments, cells were harvested in radioimmunoprecipitation assay buffer consisting of 50 mM Tris·HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 2 mM sodium fluoride, 2 mM EDTA, 0.1% SDS, and protease inhibitor cocktail for Western blot protein analysis. To test the effect of PPARγ inhibition on VLDLR expression, adipocytes were cultured in the presence of the PPARγ-specific antagonist GW-9662. Fully differentiated 3T3-L1 adipocytes were replaced by 0.2% serum-free BSA overnight, and then cells were treated with GW-9662 for 48 h for indicated doses.
Protein analysis by Western blot.
Measurement of protein concentration in cell lysate and Western blot analysis were performed as described elsewhere (22). Briefly, 60–80 μg of proteins were resolved by SDS-PAGE and then transferred onto nitrocellulose membranes (Amersham, Piscataway, NJ) and blocked in 5% nonfat dry milk in Tris-buffered saline with 0.1% Triton X-100. Proteins were probed with primary antibodies specific for VLDLR, LPL, PPARγ, and β-actin by incubation with horseradish peroxidase-conjugated secondary antibody. Blots were visualized with an ECL Plus (Pierce, Rockford, IL), and the membrane was exposed to Hyperfilm. Densitometric units were determined for each band by means of a ChemiDoc apparatus and Quantity One software (Bio-Rad, Hercules, CA). Relative density for each optic density was optimized to β-actin density.
Analysis of gene expression by quantitative PCR.
Total RNA was extracted using a Total RNA fatty and fibrous tissue pack (Bio-Rad) according to the manufacturer's protocol. Complementary DNA was synthesized from 1 μg of total RNA with iScript reverse transcriptase (Bio-Rad). Quantitative (q)PCR was performed using SYBR Green Supermix (Bio-Rad) with iTaqDNA polymerase (Bio-Rad) on Bio-Rad's IQ5 thermocylcer, as described earlier (20). Oligonucleotides (Table 1) were designed, optimized, and efficiently tested before use. For each primer set, annealing temperature was optimized, and a solitary product formed was confirmed through melt curve analysis in each PCR run. The PCR program includes 36 cycles of heat, denaturing at 95°C for 30 s and annealing at 52–62°C for 30 s, followed by melt curve cycle. qPCR data were obtained as CT values, where CT was defined as the threshold cycle of PCR at which products amplify exponentially. As internal control, β-actin expression was measured in parallel. The difference in the CT values (ΔCT) was derived from the specific gene tested and CT of control gene (β-actin) according to the equation 2CT actin − CT target gene(30). Final results are presented as relative expression (ΔCT).
Table 1.
Primers used for real-time RT-PCR
| Forward | Reverse | GenBank No. | |
|---|---|---|---|
| Mouse VLDLR | 5′-GATGATGACGCAGACTGTTC-3′ | 5′-CACTGGATCTCACTGGTAGG-3′ | NM_013703 |
| Mouse PPARγ | 5′-ATGGTTGACACAGAGATGC-3′ | 5′-GAATGCGAGTGGTCTTCC-3′ | NM_011146 |
| Mouse β-actin | 5′-AGGGAAATCGTGCGTGACAT-3′ | 5′-CGTTGCCAATAGTGATGACC-3′ | NM_007393 |
| Mouse LPL | 5′-TTGAGAAAGGGCTCTGCCTGAGTT-3′ | 5′-TCTTGCTGCTTCTCTTGGCTCTGA-3′ | NM_008509 |
VLDLR, very-low-density lipoprotein receptor; PPARγ, peroxisome proliferator-activated receptor-γ; LPL, lipoprotein lipase.
Isolation and differentiation of primary human and mouse preadipocytes.
Preadipocytes were prepared from human fat and epididymal fat of wild-type (WT) and vldlr−/− mice as described earlier (6). Biopsies of subcutaneous abdominal adipose tissue were obtained from the lower abdominal region outside the fascia superficialis of obese patients undergoing gastric bypass surgery. The Institutional Review Board at the University of Vanderbilt approved this protocol, and all subjects were given informed consent. Briefly, 1–2 g of white adipose tissue was minced and digested in Hank's buffered salt solution with 2% BSA containing 1 mg/ml collagenase type I (Sigma-Aldrich) at 37°C with shaking for 60 min. The digested material was filtered and centrifuged at 500 g for 10 min. The resulting pellets were resuspended in erythrocyte lysis buffer (Lonza, Wakersville, MD). After a wash with phosphate-buffered saline (PBS), the cells were suspended in DMEM-F12 with 10% FCS and used for cell culture at passage 2 to eliminate non-preadipocyte cell contamination (6). For differentiation of primary mouse preadipocytes, confluent cells were differentiated in DMEM-F12 with 10% FCS, 0.5 mM IBMX, 0.1 μM corticosterone, and 10 μg/ml insulin for 4 days and then maintained in culture in medium DMEM-F12 with 10% FCS, 10 μg/ml insulin, and 0.1 μM corticosterone (6). For primary human preadipocyte differentiation, cells were cultured over a period of 8–12 days in differentiation medium (FCS-free adipocyte medium supplemented with 10 μg/ml insulin, 1 nM triiodothyronine, and 100 nM hydrocortisone). Culture medium was changed every 3 days (6). Durations for mouse and human preadipocytes to reach maturity were ∼12 and 21 days, respectively. At maturity, experiments of time- and dose-dependent effects of pioglitazone on VLDLR and LPL protein levels in human adipocytes were examined as described for 3T3-L1 cells. First, 10 μM pioglitazone or 0.1% DMSO was added for indicated time periods. Second, increasing doses of pioglitazone were added and protein levels examined by Western blotting after a 24-h incubation. In addition, mRNA abundance of VLDLR and LPL was assayed in human and mouse preadipocytes and mature adipocytes with or without 24-h exposure to pioglitazone.
Assessment of adipogenesis in vitro with oil red O staining.
Preadipocytes of WT and vldlr−/− mice were differentiated as described above, with the exception that preadipocytes were differentiated without or with the addition of VLDL at a final concentration of 100 μg of TG per well. Our objective was to test the role of VLDLR in adipogenesis in the presence of a source of lipids, and VLDL particles are the natural ligands for VLDLR (44). Thus, we added VLDL particles and tested whether the expression of VLDLR regulates preadipocyte differentiation (8). VLDL particles were isolated from mouse plasma by ultracentrifugation as described earlier (23). For comparative purposes, additional preadipocyte cultures were differentiated in the presence of FAs (oleate) as an alternative source for lipids. Oleate was complexed to albumin (4:1) and was added to a final concentration 0.5 nM. On the indicated day, cells were stained with Oil red O (18). In brief, cell monolayers were washed three times with PBS and fixed with 10% formalin in PBS (pH 7.4) for 1 h. Then, cells were stained with freshly prepared 0.3% Oil red O in 60% isopropanol for 30 min. After being rinsed twice with distilled water, stained cells were viewed and photographed using a microscope with phase contrast optics and a digital camera. For quantification, cells were dried for 2 h at 37°C, followed by incubation with 100% isopropanol for 15 min at room temperature to extract lipid-bound dye. Solubilized stained lipids were quantified by spectrophotometer at 520 nm for absorbance. In addition, TG content in adipocytes was also tested enzymatically as described earlier (21).
Animals, diets, and experimental design.
To test whether PPARγ also regulates VLDLR expression in vivo, we compared the effects of PPARγ activator pioglitazone in WT and vldlr−/− mice. Mice used in this study were male vldlr−/− mice (Jackson Laboratory, Bar Harbor, ME) and WT control littermates of identical genetic background and similar age (14–18 wk). All experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. Mice were caged in an animal room with alternating 12:12-h light (7 AM to 7 PM) and dark (7 PM to 7 AM) cycles and were fed a high-fat diet (Harlan-Teklad Laboratory, Madison, WI) that contained 29.3% fat, 25.2% protein, and 28.8% carbohydrate by weight (diet no. TD 01151). In addition to high-fat feeding, each group of WT and vldlr−/− mice was randomly subdivided into two subgroups (n = 12 per group). Pioglitazone-treated mice (PIO) received 10 mg/kg body wt by oral gavage daily for 4 wk. while control mice (CON) received vehicle alone (0.25% of carboxymethylcellulose). Body weight and food intake was monitored weekly. To document progressive changes in plasma TG concentration, blood samples were collected weekly from the tail vein of overnight-fasted mice. Initially, a pilot experiment was designed to establish the dose-response effects of pioglitazone on blood lipids. Accordingly, WT and vldlr−/− mice were fed a high-fat diet and received graded amounts of pioglitazone (5, 10, 15 mg/kg body wt) by oral gavage, and blood lipids were tested weekly. Dosing of pioglitazone was based on previously published reports (41). At the end of the experimental period and after an overnight fast, blood was collected under anesthesia, and epididymal adipose tissues were harvested, weighed, and frozen for protein and RNA analysis. For Western blot, tissues were homogenized in ice-cold TES buffer (20 mM Tris, 1 mM EDTA, 250 mM sucrose) in Elvehjem pottery as described earlier (22). Supernatant was prepared from homogenate by centrifugation at 1,000 g for 20 min at 4°C. RNA extraction, Western blot, and qPCR analyses were performed as described above (20).
Plasma lipid and lipoprotein analysis.
After a 4-wk experimental period, blood was collected from fasted mice, and plasma was separated by centrifugation at 1,000 g for 20 min at 4°C. Lipoproteins were separated from pooled plasma of mice by ultracentrifugation in a preformed saline (KBr) density gradient as described by earlier (23). VLDL particles were collected according to their density (1.006 kg/l), and TG concentrations were determined by enzymatic assay kit from as described elsewhere.
Postprandial lipid clearance.
To assess postprandial lipolysis and TG clearance, animals were fasted overnight and received an intragastric gavage of olive oil (200 μl). Blood samples were collected from the tail vein at the indicated time, and plasma TG concentration was measured as described earlier (23).
Preparation of labeled TG emulsion and kinetics.
We studied the catabolism of fat emulsion in this study as a surrogate of chylomicrons. Lipid emulsion was labeled with [3H]-triolein (PerkinElmer Life Sciences, Waltham, MA) using the procedure described by Qi et al. (36). Previous studies have shown that TG-rich particles in commercial fat emulsions are cleared in a manner similar to postprandial chylomicrons (2). Briefly, labels were added to a small glass vial and slowly evaporated to dryness under N2. Five hundred microliters of a 5% diluted solution of Intralipid (Kabi Pharmacia, Clayton, NC) was added to the glass vial, mixed vigorously, and sonicated three times on ice for 30 s each using a Branson Sonifier Cell Disruptor (model W185; Branson Scientific, Plainview, NY) to incorporate labeled TGs (36) into the core of fat particles. Nonattached isotopes were separated from labeled emulsion by a two-step procedure. First, labeled TG particles were concentrated by gradient ultracentrifugation as described elsewhere (22). Labeled TG-rich particles, separated from free phospholipid in the emulsion, were harvested in the top 1 ml at a density [(d) <1.006 (23)]. In a second step, salt and unbound label were removed from labeled TG particles by size exclusion Sephadex G-25 PD-10 column (GE Healthcare, Piscataway, NJ) equilibrated with saline (NaCl 0.9%). For this, the column was first balanced with saline, and then the sample was added and eluted by gravimetry with saline. The resulting labeled TG-rich particles were resuspended in saline, stored at 4°C, and used the following day. To quantify the [3H]TG in the emulsion, lipids were extracted with a chloroform-methanol mixture and separated by TLC as described elsewhere (23). Spots of TGs were scraped off, eluted with methanol-chloroform (9:1, vol/vol), and radioactivity was counted as described previously (23). TGs in labeled emulsion were determined using enzymatic kits (23), and the specific activity of TG was determined.
Mice were fed the high-fat diet either alone (CON) or in combination with pioglitazone treatment (PIO) as described above. Then, mice were fasted for 4 h prior to receiving an intravenous injection through the tail vein of 100 μl of labeled emulsion containing 5 × 107 dpm [3H]TG and less than 100 μg of TG. Blood was withdrawn at the indicated time from the retroorbital plexus. At the end of the experiment, mice were placed under anesthesia, bled by cardiac puncture, and then perfused with 8 ml of saline to wash the cardiovascular system. Epididymal fat pads, heart, gastrocnemius muscle, and liver were excised, washed with saline, and frozen for radioactivity analysis. Lipids extracted from the plasma and tissues were separated by TLC (19), and TG-bound counts were determined and used to calculate radioactivity disappearance from the blood (23). To ensure that the bulk of the 3H label was bound to TG-rich particle in the blood, plasma from the last bleeding was pooled according to experimental groups, and lipoproteins were separated in a discontinuous density gradient as described elsewhere (23). Distribution of radioactivity was determined in each lipoprotein fraction after lipid extraction (23).
Disappearance curves of [3H]TG were generated by dividing the plasma radioactivity at each point by the radioactivity determined 0.5 min after injection. Clearance curves for each experiment were modeled separately with a monoexponential equation as described elsewhere (23). Fractional catabolic rates (FCR) of TG-rich lipoprotein were determined from the area under the curve, as described previously (23).
Preparation of labeled endogenous VLDL.
To study the clearance of endogenous TG-rich lipoproteins, we labeled VLDL in vivo according to the procedure of Aalto-Setala et al. (1). Since our objective was to test the effect of pioglitazone on the clearance of VLDL in WT and vldlr−/− mice, we chose a common tracer (VLDL of WT) to exclude any differences related to VLDL composition. Accordingly, WT mice were injected intravenously into the tail vein with 100 μCi of [3H]palmitate complexed with BSA as described elsewhere (1, 2). Then, blood was collected 60 min after the injection, and serum samples were ultracentrifuged to obtain the VLDL fraction (23). Lipids were separated by TLC as described above (19), and VLDL-bound radioactivity was counted to ensure that the bulk of the label was in the TG fraction. The removal of labeled VLDL was examined in vivo in WT and vldlr−/− mice fed either high-fat diet alone (CON) or high-fat diet and pioglitazone gavage (PIO). Mice were injected intravenously with ∼200,000 dpm of [3H]VLDL, and disappearance of radiolabeled VLDL was determined from blood samples drawn at the indicated time after the injection. Lipid extraction and separation were performed as described above. TG-bound counts were determined and used to calculate VLDL radioactivity disappearance from the blood (23). The radioactivity at each time point was measured and plotted as described above for fat emulsion.
Uptake of DiI- and [3H,125I]VLDL labeled by isolated adipocytes.
VLDL particles were isolated from mouse plasma by ultracentrifugation (23) and were labeled with the lipophilic fluorescent molecular probe DiI (1,1-dioleyl-3,3,3′,3′-tetramethylindocarbocyanine methanesulfonate) from Invitrogen (Eugene, OR) according to a previously published procedure (25). In addition, WT mouse VLDL were labeled with [3H]TG in vivo after intravenous injection of [3H]palmitate as described above. Then, [3H]TG-labled VLDL were iodinated in vitro with 125I as described previously (23). After labeling, free DiI and 125I were removed using size exclusion Sephadex G-25 PD-10 columns equilibrated with saline as described above. After passage, TG-rich lipoproteins were collected in the first fraction, while unbound labels and salt were retained in the column. Preadipocytes were isolated from WT and vldlr−/− mice and differentiated according to the procedure of Caserta et al. (6) described above. DiI-VLDL uptake was assayed as described by others (25). DiI-VLDL particles were added to adipocyte culture for a 3-h duration. Then, cells were washed three times with 2% BSA-PBS and fixed with 4% formalin. Fluorescence was visualized using a fluorescent microscope (Zeiss Axiolab HB50) as described previously (25). To examine whether uptake of VLDL-derived lipids occur through whole remnant particle uptake or as free fatty acid after lipolysis, [3H,125I]-double-labeled VLDL were added to adipocyte culture for 3 h. Cells were then washed three times rapidly with Tris buffer containing BSA (2 mg/ml) and then washed three times (5 min each wash) with Tris buffer (without BSA). The cells were detached and solubilized with 500 μl of 0.1 N NaOH for 30 min at room temperature with gentle shaking. Protein concentrations were determined as described elsewhere (23), and radioactivity corresponding to 125I was counted in a gamma counter. In addition, lipids were extracted from lysate aliquots as described elsewhere (19), and 3H radioactivity was measured in a beta counter.
Statistics.
Results are expressed as means ± SE, and significant differences between groups were assessed by Student's t-test, except for time course changes of plasma TG concentrations, for which repeated-measures analysis of variance and Student's t-test were used. Significance was set at P < 0.05.
RESULTS
VLDLR expression is increased with 3T3-L1 preadipocyte differentiation.
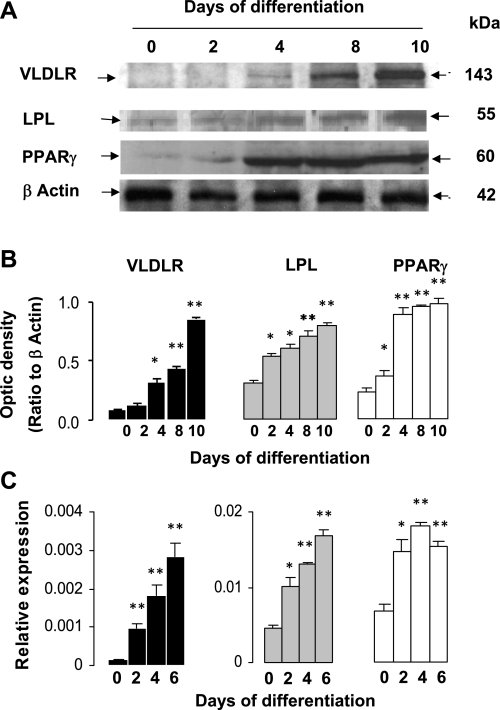
Although VLDLR is highly expressed in adipose tissue, it is not known whether its expression changes with adipocyte differentiation. Accordingly, we analyzed VLDLR protein at different stages of adipocyte differentiation (Fig. 1). VLDLR protein was not detected in either nondifferentiated or early-differentiated preadipocytes. However, protein levels quickly increased, reaching maximum levels in fully differentiated adipocytes (Fig. 1, A and B). Similarly to LPL, VLDLR protein and mRNA increased after the induction of PPARγ (Fig. 1, A–C).
Fig. 1.
Expression of very-low-density lipoprotein receptor (VLDLR), lipoprotein lipase (LPL), and peroxisome proliferator-activated receptor-γ (PPARγ) during adipogenesis. Representative blots (A), mean of optic density (B), and mRNA abundance (C), determined by qPCR in 3T3-L1 adipocytes at different times of differentiation (n = 4 per time point). Data of optical density are arbitrary units and were obtained after scan of bands as described in experimental procedures. Relative expression of mRNA genes represents ΔCT normalized to β-actin as described in experimental procedures. Data are means ± SE; n = 4 experiments performed in triplicate. Statistical differences between differentiated adipocytes and preadipocytes: *P < 0.01, **P < 0.001.
PPARγ agonist pioglitazone increases VLDLR expression in a time-dependent manner.
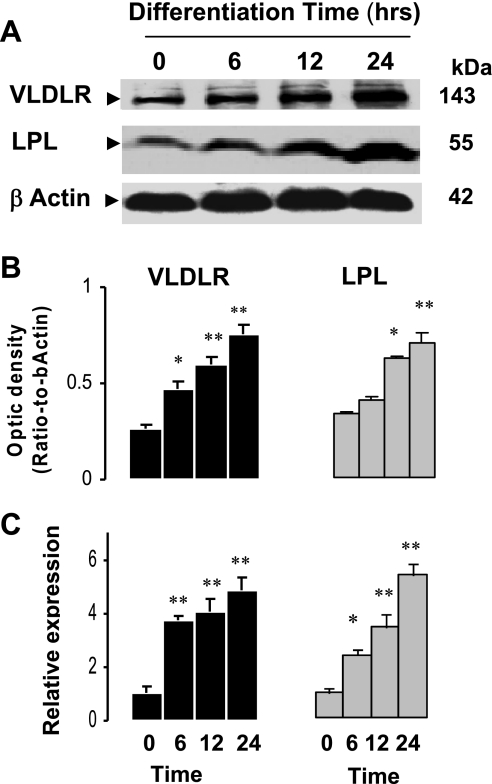
To investigate the effects of PPARγ ligand on VLDLR expression, differentiated adipocytes were exposed to pioglitazone (10 μM) for different times, and the expression of VLDLR was analyzed (Fig. 2). Time course experiment shows that pioglitazone gradually increased VLDLR protein (Fig. 2, A and B) and mRNA abundance (Fig. 2C), with maximum levels reached at 24 h (3.5- and 4.9-fold increases for protein and mRNA, respectively). For comparative purposes, we also tested the expression of LPL since it is a target gene of PPARγ and is upregulated by TZDs (38). Exposure to pioglitazone induced VLDLR and LPL protein and mRNA in a time-dependent manner by PPARγ activation (Fig. 2, A–C).
Fig. 2.
Time-dependent regulation of VLDLR expression by pioglitazone in 3T3-L1 adipocytes. Representative blots (A), mean of optic density (B), and mRNA abundance (C) of VLDLR and LPL in 3T3-L1 adipocytes treated with pioglitazone for indicated time periods. Experiments for time course effects of pioglitazone were generated by incubating fully differentiated 3T3-L1 adipocytes (day 9) in culture medium with pioglitazone for indicated time as described in experimental procedures. Relative expression of mRNA genes represents ΔCT normalized to β-actin as described in experimental procedures. Data are means ± SE; n = 4–5 experiments. Statistical differences between treated and nontreated adipocytes (t = 0): *P < 0.05, **P < 0.001.
Activation and inhibition of PPARγ modulates the expression of VLDLR expression in a dose-dependent manner in 3T3-L1 adipocytes.
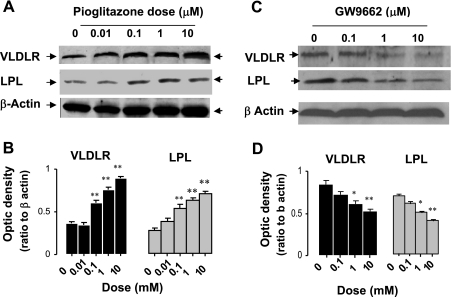
We tested the effects of graded doses of pioglitazone and noted dose-dependent increases in VLDLR protein level (Fig. 3, A and B). In addition, our results show that both VLDLR and LPL protein levels increased in tandem with the increases of pioglitazone dose. To test whether inhibition of PPARγ also downregulates VLDLR, we examined the expression of VLDLR in mature adipocytes exposed to graded concentration of PPARγ antagonist GW-9662 (Fig. 3, C and D). Induction of VLDLR expression with pioglitazone was significantly blunted by the addition of PPARγ antagonist.
Fig. 3.
Dose-dependent regulation of VLDLR expression by PPARγ agonist pioglitazone and antagonist GW-9962 in 3T3-L1 adipocytes. Representative blots (A) and mean of optic density (B), in 3T3-L1 adipocytes treated with pioglitazone for indicated doses. Representative blots (C) and mean of optic density (D) of VLDLR and LPL in 3T3-L1 adipocytes treated with GW-9962 for indicated time periods. Experiments for dose effects of pioglitazone and GW-9962 were generated by incubating fully differentiated 3T3-L1 adipocytes in culture medium with either pioglitazone or GW-9962 for indicated doses as described in experimental procedures. Western blots were performed from 60 (A and B) and 80 μg (C and D) of lysate proteins. Data are means ± SE; n = 5 experiments each performed in triplicate. Statistical differences between treated and nontreated adipocytes (t = 0): *P < 0.05; **P < 0.001.
Induction of PPARγ increases VLDLR expression in human and mouse adipocytes.
Preadipocytes isolated from human fat were differentiated in vitro, and VLDLR protein levels were tested at indicated times (Fig. 4A) and doses after exposure to pioglitazone (Fig. 4B). Similarly to 3T3 adipocytes, treatment of human adipocytes with PPARγ agonist pioglitazone upregulated VLDLR and LPL protein levels in dose- and time-dependent manners (Fig. 4, A and B). Abundance of mRNA of VLDLR and LPL was very low in preadipocytes of human (Fig. 4C) and mice (Fig. 4D) and increased in differentiated adipocytes without pioglitazone. Pioglitazone addition dramatically increased the mRNA levels of VLDLR and LPL in human and mouse adipocytes (Fig. 4, C and D). These data demonstrate that induction of VLDLR by pioglitazone occurs across species.
Fig. 4.
Time- and dose-dependent (A and B) effects of pioglitazone on VLDLR and LPL protein levels in human adipocytes. Effect of pioglitazone on VLDLR and LPL nRNA in adipocytes of human (C) and mouse (D). Preadipocytes were isolated from adipose tissue of human and WT mouse as described in experimental procedures. Condition of culture and differentiation are described in experimental procedures. Experiments to test time- and dose-dependent effects of pioglitazone were done as described for 3T3-L1 cells in legends of Figs. 2 and 3. For mRNA abundance, pioglitazone (10 μM) was added to culture medium of fully differentiated adipocyte, and 24 h later cells were harvested for mRNA extraction and gene expression. Data are means ± SE; n = 3 experiments performed in triplicate for preadipocytes (Preadipo) and adipocytes (Adipo) with (+) or without (−) pioglitazone treatment. Statistical differences between differentiated adipocytes and preadipocytes, *P < 0.001; a,bstatistical differences between pioglitazone-treated and nontreated adipocytes (t = 0): aP < 0.001, and bP < 0.05.
Pioglitazone-induced VLDLR expression modulates adipogenesis and lipid deposition in adipocytes.
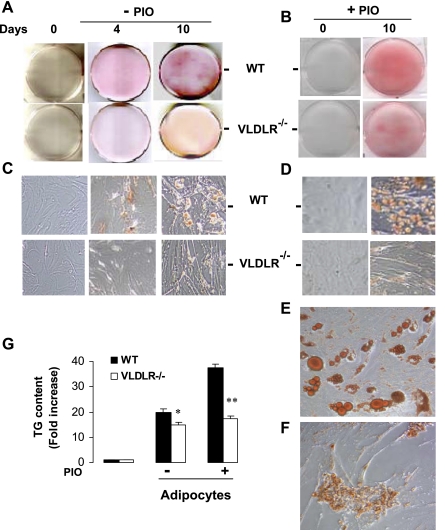
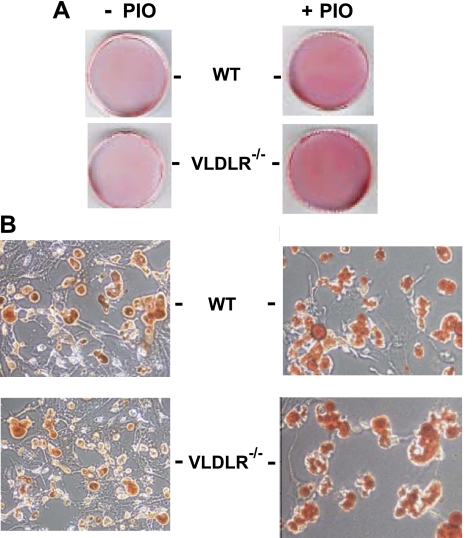
At an early stage of adipogenesis, induction of PPARγ stimulates an array of target genes involved in lipid uptake and retention in the adipocyte (45). Our data described here shows that VLDLR is also induced by PPARγ during preadipocyte differentiation. Consequently, we examined the effects of VLDLR expression on adipogenesis and lipid deposition in adipocytes. Preadipocytes isolated from adipose tissue of WT and vldlr−/− mice were cultured and differentiated in vitro. Lipid accumulation was estimated by Oil red O staining at different stages of adipocyte differentiation in the presence of VLDL and with or without pioglitazone (Fig. 5). In the presence of VLDL, Oil red O lipid staining increased with adipocyte differentiation but with a much greater rate in WT adipocytes than in VLDLR-null adipocytes (Fig. 5, A and C). Addition of pioglitazone increased the rate of differentiation of WT but had less effect in VLDLR-null adipocytes (Fig. 5, B and D). High magnification (x400) shows that pioglitazone treatment of WT preadipocytes resulted in the formation of multi- and monolocular cells, indicative of mature adipocyte phenotype. However, lipid accumulation and droplets in VLDLR-null adipocytes treated with pioglitazone were clearly smaller (Fig. 5, E and F). Analysis of TG content corroborated these observations and showed that after 10 days differentiation pioglitazone increased lipid content by 19 and 5% in WT and VLDLR-null adipocytes, respectively (Fig. 5G). Taken together, these results show that induction of PPARγ increased the rate of preadipocyte differentiation and lipid deposition in WT adipocytes. These effects were significantly blunted in VLDLR-null preadipocytes. FAs are another source of lipid that could be used for lipid synthesis during adipocyte differentiation. For comparative purposes, we cultured preadipocytes in the presence of oleate and examined lipid accumulation (Fig. 6). At day 10, Oil red O showed comparable lipid staining in WT and VLDLR-null adipocytes (Fig. 6A). Supplementation of medium with oleate and pioglitazone together increased lipid staining in WT and VLDLR-null adipocytes. At higher magnification (Fig. 6B), lipid droplets in WT and VLDLR-null adipocytes were comparable and increased in size after oleate and pioglitazone exposure. Analysis of TG content also confirmed the similarity of levels of lipid deposition in WT and VLDLR-null adipocytes (not shown). These data suggest that VLDLR is not required for FA-induced adipogenesis.
Fig. 5.
Representative Oil red O staining in preadipocytes and differentiated adipocytes of WT and vldlr−/− with or without pioglitazone treatment. Preadipocytes isolated from WT and vldlr−/− mice were differentiated in presence of VLDL and without (A and C) or with (B, D–F) pioglitazone treatment. Cells were then fixed and stained with Oil red O as described in experimental procedures. Photos were taken for whole plates (A and B) and for cells at indicated day of differentiation at initial magnifications ×200 (C and D) and ×400 (E and F). G: triglyceride (TG) content was measured in preadipocytes and differentiated adipocytes with or without pioglitazone treatment.
Fig. 6.
Representative Oil red O staining in adipocytes of WT and vldlr−/− differentiated in presence of oleate and with or without pioglitazone treatment. Preadipocytes isolated from WT and vldlr−/− mice were differentiated in presence of oleate (0.35 nM) and without or with pioglitazone (10 μM). Cells were then fixed and stained with Oil red O as described in experimental procedures. Photos were taken at day 10 of differentiation for whole plates (A) and for cells at initial magnifications ×400 (B).
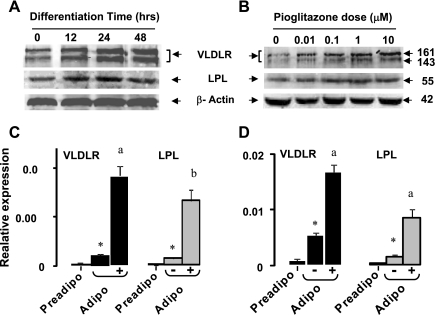
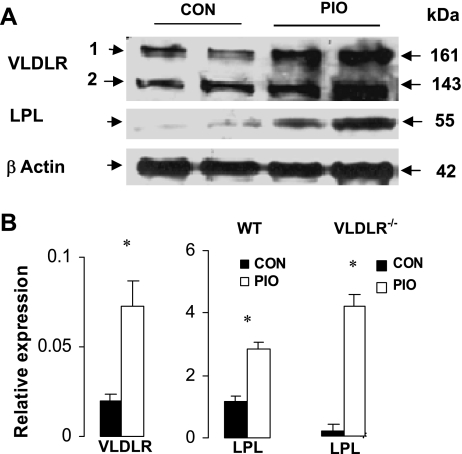
VLDLR expression is induced by PPARγ agonist in vivo.
We also questioned whether the induction of PPARγ in vivo enhances the expression of VLDLR. Accordingly, we measured the levels of VLDLR protein and mRNA in WT mice with or without pioglitazone treatment (Fig. 7). As described elsewhere (37), two isoforms of VLDLR protein were identified in mouse adipose tissue, and both were significantly increased (2-fold) after pioglitazone treatment (Fig. 7A). In WT mice, mRNA abundance of VLDLR and LPL in adipose tissue was three- and twofold higher after pioglitazone treatment (Fig. 7B). Furthermore, pioglitazone induced a robust increase of LPL mRNA in adipose tissue of vldlr−/− mice, suggesting that silencing the vldlr gene did not affect pioglitazone induction of other genes in adipose tissues.
Fig. 7.
Effect of pioglitazone on VLDLR expression in adipose tissue of WT mice. A: representative blot of VLDLR and LPL proteins in adipose tissue of WT mice fed high-fat diet with or without pioglitazone treatment. B: abundance of mRNA in adipose tissue of WT and vldlr−/−mice fed high-fat with or without pioglitazone treatment. Mice were ad libitum-fed high-fat diet and received daily oral gavage of vehicle (CON) or pioglitazone (PIO) at 10 mg/kg body wt. Data for qPCR are presented as relative expression of genes and represent ΔCT normalized to β-actin as described in experimental procedures. Data are means ± SE; n = 6 per group. Statistical differences between treated and nontreated: *P < 0.05, **P < 0.001.
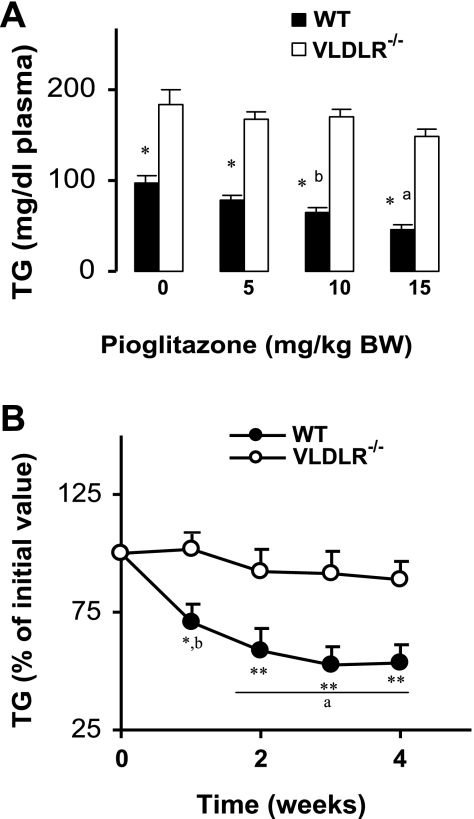
Plasma TG concentration is not reduced by pioglitazone in vldlr−/− mice.
We investigated the effects of graded amounts of pioglitazone on plasma TGs in WT and vldlr−/− mice (Fig. 8A). In WT mice, pioglitazone treatment decreased plasma TGs in a dose-dependent manner with a maximum reduction (−55%) at the highest dose (Fig. 8A). However, the effect of pioglitazone was less significant in vldlr−/− mice (−18% at the highest dose). Next, we studied the time course effect of a high dose of pioglitazone (10 mg/kg body wt) on plasma TG concentration (Fig. 8B). At the entrance of the feeding period (time 0), plasma TGs of vldlr−/− mice were 40% higher than in WT. Therefore, we expressed the levels of plasma TGs during the experimental period as a percentage of the initial values (Fig. 8B). Plasma TG concentration of WT mice was significantly reduced by pioglitazone treatment, reaching a stable level at 3 wk (−48%, P < 0.01). In vldlr−/− mice, pioglitazone had minor effects on plasma TG levels (Fig. 8B). Changes in total plasma TGs were also reflected in plasma VLDL-TG concentrations (Table 2), showing that pioglitazone treatment induced minor reductions of VLDL-TG in vldlr−/− mice (−8%) compared with robust reductions in WT mice (−37%).
Fig. 8.
Dose-dependent (A) and time course (B) changes of plasma TG in WT and vldlr−/− mice fed high-fat diet with or without pioglitazone. Mice were ad libitum-fed high-fat diet and received daily oral gavage of vehicle (CON) or pioglitazone (PIO). Blood samples were collected weekly, and TG concentrations were determined enzymatically as described in experimental procedures. Results are means ± SE; n = 10–12. Statistical differences between WT and VLDLR mice receiving the same treatment: *P < 0.05, **P < 0.001; a,bstatistical differences between treated and nontreated groups from the same genotype: aP < 0.05, bP < 0.01.
Table 2.
Body weights, final epididymal fats pad weights, and plasma parameters for WT and vldlr−/− mice without and with pioglitazone treatment
| CON |
PIO |
|||
|---|---|---|---|---|
| WT | vldlr−/− | WT | vldlr−/− | |
| Initial body weight, g | 27.2±0.7 | 21.4±0.6* | 27.8±0.6 | 21.9±0.8* |
| Final body weight, g | 31.6±0.5 | 23.4±0.4** | 34.6±0.6b | 24.9±0.7* |
| Epididymal fat pads, g | 0.80±0.04 | 0.43±0.05* | 1.32±0.05b | 0.54±0.05* |
| TG, mg/dl | 99.8±6.1 | 168±11** | 65.7±6.4b | 141±15** |
| VLDL-TG, mg/dl | 78.1±9.1 | 142.5±13.3* | 49.2±8.9a | 131±10.6** |
Values represent means ± SE; n = 9–12. TG, triglyceride. Statistical comparisons between wild-type (WT) and vldlr-null (vldlr−/−) mice fed the same diet:
P < 0.01,
P < 0.05;
statistical comparison between pioglitazone-treated (PIO) and nontreated (CON) mice of the same genotype:
P < 0.01,
P < 0.05.
Pioglitazone is less effective in increasing body weight and fat mass in vldlr−/− mice.
Compared with WT mice, vldlr−/− mice fed a high-fat diet gained significantly less body weight (2.0 ± 0.3 vs. 4.4 ± 0.5 g, P < 0.01), and their epididymal fat mass was ∼46% of WT fat mass mice (Table 2). Compared with the high-fat diet alone, addition of pioglitazone resulted in a higher body weight gain in WT (6.8 ± 0.5 g) than vldlr−/− mice (3.0 ± 0.3 g). In addition, pioglitazone treatment resulted in a bigger increase of epididymal fat mass of WT mice (0.52 ± 0.3 g vs. nontreated WT) than vldlr−/− mice (0.11 ± 0.2 g vs. nontreated vldlr−/−). These observations corroborate the in vitro data showing lower adipogenesis rates of VLDLR-null preadipocytes.
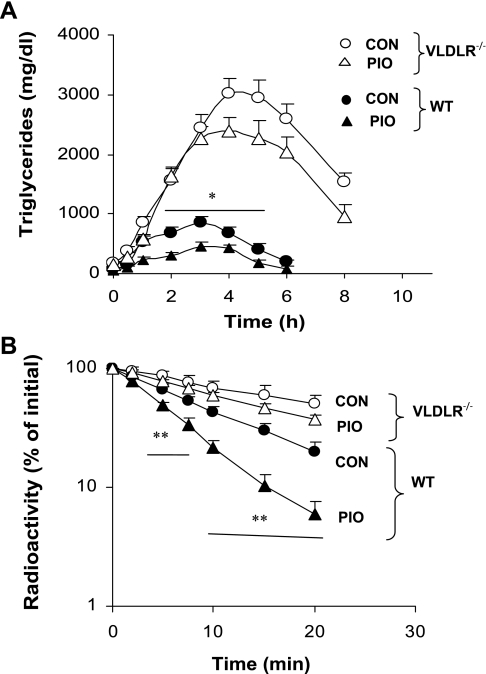
Pioglitazone did not increase clearance of postpostprandial TGs in vldlr−/− mice.
To test the effect of the induction of VLDLR by PPARγ agonist on the clearance of TG-rich lipoproteins originating in the intestine, we performed an oral fat gavage after 4-wk treatment with pioglitazone (Fig. 9A). In WT mice, pioglitazone treatment reduced the peak of plasma TGs detected after few hours of lipid gavage in nontreated mice (Fig. 9A). Compared with WT mice, plasma TG levels were dramatically increased in nontreated vldlr−/− mice after oil gavage. Pioglitazone treatment in vldlr−/− mice induced a modest but not significant reduction of TG at 4–6 h after gavage. TG concentration remained about four times higher in vldlr−/− treated mice compared with WT treated mice (Fig. 9A). Since food intake (data not shown) and lipid absorption were not altered by VLDLR deletion (17), we examined the clearance of labeled fat emulsion in WT and vldlr−/− with or without pioglitazone treatment. In WT mice, pioglitazone treatment significantly increased the decay of TG-labeled fat emulsion in plasma (Fig. 9B). In contrast to its effect in WT mice, pioglitazone did not change significantly the decay of labeled emulsion in vldlr−/− mice (Fig. 9B). Consequently, the fractional catabolic rates (FCR) of [3H]TG were increased by 40% in pioglitazone-treated WT mice (0.137 ± 0.01 pool/h) compared with the nontreated WT mice (0.094 ± 0.016 pool/h), whereas it was not significantly different in vldlr−/− mice treated and nontreated with pioglitazone (0.071 ± 0.01 vs. 0.067 ± 0.05 pool/h for treated and nontreated mice, respectively). Analysis of tissue radioactivity revealed that lipid uptake was smaller in adipose tissue, heart, and skeletal muscle of vldlr−/− mice compared with WT mice fed the high-fat diet alone (Table 3). In WT mice, treatment with pioglitazone increased lipid uptake in adipose tissue (145%), heart (37%), and skeletal muscle (61%) compared with mice without treatment. In vldlr−/− mice, the effect of pioglitazone was smaller in adipose tissue (62%) but comparable to WT in heart (28%) and skeletal muscle (56%). Pioglitazone treatment increased lipid uptake in liver by 45 and 37% in WT and vldlr−/− mice, respectively. These results show that pioglitazone treatment targeted mainly adipose tissue. Lipid uptake in adipose tissue was about onefold higher in WT than in vldlr−/− mice without pioglitazone. With pioglitazone treatment, the induction of lipid uptake in WT mice was threefold higher than that of vldlr−/− mice.
Fig. 9.
Clearance of TGs after oil gavage (A) and labeled emulsion (B) in WT and vldlr−/− mice. Mice were ad libitum fed high-fat diet and received daily oral gavage of vehicle (CON) or pioglitazone (PIO). A: time course change of plasma TGs was tested following gastric gavage of olive oil. B: decay of labeled TG in plasma was assayed after injection of labeled emulsion in WT and vldlr−/− mice. Labeling TGs in fat emulsion and turnover studies were performed as described in experimental procedures. Results are means ± SE; n = 6 per group. Statistical differences between treated and nontreated groups from the same genotype: *P < 0.05, **P < 0.001.
Table 3.
Distribution of radiolabeled lipids following injection of [3H]TG emulsion into CON and PIO WT and vldlr−/− mice
| %Injected Dose |
||||
|---|---|---|---|---|
| CON |
PIO |
|||
| WT | vldlr−/− | WT | vldlr−/− | |
| Adipose tissue | 9.1±0.8 | 4.2±0.6* | 22.3±1.2a | 6.8±0.4** |
| Heart | 14.6±1.1 | 8.1±0.9* | 20.1±0.6b | 10.4±0.7* |
| Skeletal muscle | 5.4±0.6 | 2.3±0.5* | 8.7±0.9b | 3.6±0.5*a |
| Liver | 4.2±0.8 | 6.8±1.0 | 6.1±0.7b | 9.3±0.9*b |
Values represent means ± SE; n = 6. Statistical comparisons between WT and vldlr−/− mice fed the same diet:
P < 0.01,
P < 0.05;
statistical comparison between PIO and CON mice of the same genotype:
P < 0.01,
P < 0.05.
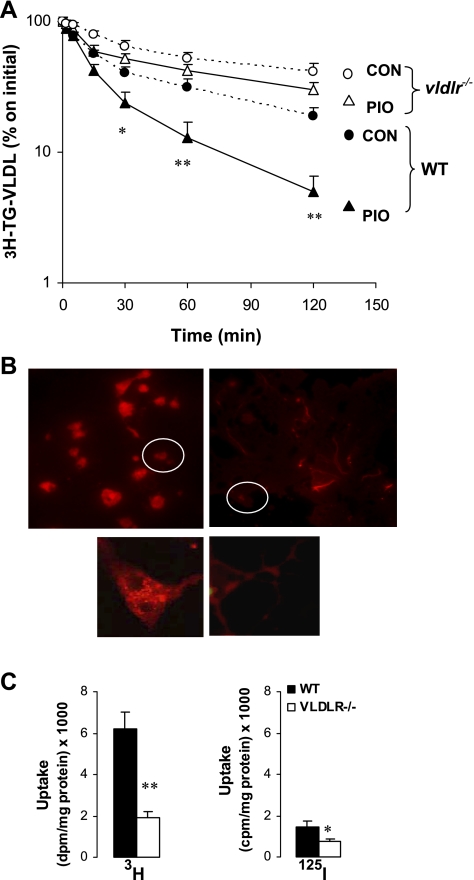
VLDLR is required for pioglitazone-inducted VLDL clearance.
We also examined the clearance of endogenous labeled VLDL (Fig. 10A). With high-fat diet, disappearance of [3H]TG VLDL from plasma of vldlr−/− mice was ∼50% lower than that of WT mice. The decay of [3H]TG VLDL was considerably enhanced in WT mice treated with pioglitazone (>2.4-fold increase); however, this effect was significantly blunted in vldlr−/− mice. These results demonstrate that VLDLR expression and induction by PPARγ is crucial for the clearance of TG-rich lipoproteins in postprandial (intestine origin) as well fasting (hepatic origin) states.
Fig. 10.
Clearance (A) and adipocyte uptake (B and C) of labeled VLDL in WT and vldlr−/− mice with or without pioglitazone treatment. Mice were ad libitum-fed high-fat diet and received daily oral gavage of vehicle (CON) or pioglitazone (PIO). VLDL particles from WT mice were radiolabeled in vivo with [3H]palmitate or in vitro with fluorescent probe DiI as described in experimental procedures. A: plasma decay of [3H]TG-labeled VLDL was performed in vivo in WT and vldlr−/− mice after iv injection of labeled VLDL. Results are means ± SE; n = 6. Statistical differences between treated and nontreated groups from the same genotype: *P < 0.05, **P < 0.001. B: uptake of 1,1-dioleyl-3,3,3′,3′-tetramethylindocarbocyanine methanesulfonate (DiI)-VLDL and [3H,125I]VLDL particles were examined in vitro in differentiated adipocytes as described in experimental procedures. Three experiments were performed each in triplicate, and representative photos of adipocytes with DiI-VLDL at original magnification ×200 and ×400 are presented. C: uptake of 3H and 125I are by adipocytes.
Defective VLDL lipid uptake was also demonstrated at the level of isolated adipocyte (Fig. 10B). After incubation with DiI-VLDL, fluorescent lipids derived from VLDL were located inside WT adipocytes, while less DiI label was situated in VLDLR-null adipocytes. Delivery of lipids to the adipocyte may occur either as FFA liberated after the action of LPL or as whole remnant particles. To examine the role of VLDLR in these pathways, we incubated adipocytes with [3H-TG,125I]-double-labeled VLDL (Fig. 10C). Irrespective of the genotype, the amount of 125I radioactivity was significantly lower than [3H]TG. In addition, there were about three- and twofold reductions in 3H and 125I radioactivity, respectively, in VLDLR-null adipocytes compared with WT adipocytes. Measurement of 125I-labeled proteins represents the uptake of whole remnant particle, while that of 3H corresponds to total uptake, including whole particle and labeled FFA. These data suggest that VLDLR participates in both pathways, but only a small part of lipids is delivered to adipocytes through the whole particle uptake.
DISCUSSION
In this study, we demonstrated that stimulation of PPARγ by pioglitazone increased VLDLR mRNA and protein in cultured adipocytes. Induction of VLDLR by PPARγ activation was also reproduced in vivo in adipose tissue of control WT mice treated with pioglitazone. Furthermore, pioglitazone increased fat mass, enhanced in vivo clearance of TG-rich lipoproteins, and stimulated in vitro adipogenesis of isolated WT preadipocytes. Absence of VLDLR significantly blunted PPARγ induction of TG clearance in vivo and adipogenesis in isolated preadipocytes and was less effective in alleviating diet-induced hypertriglyceridemia. These findings demonstrate that VLDLR is upregulated by PPARγ and contributes significantly to the lipid uptake and adipogenesis process.
In line with previous reports (16, 48), VLDLR deficiency increased plasma TG levels and reduced the turnover rate of TG-rich lipoproteins. In addition, our results demonstrate that pioglitazone activation of PPARγ enhanced TG-rich lipoprotein clearance and reduced plasma TGs in WT mice but failed to reproduce similar effects in vldlr−/− mice. Expression and induction of VLDLR by PPARγ is important to mediate the hypotriglyceridemic effect of pioglitazone. VLDLR impacts TG-rich lipoprotein catabolism at different levels. First, VLDLR is required for optimal functioning of LPL either by increasing LPL and TG-rich lipoprotein interactions at the capillary surface (33) or by serving as helper for the transcytosis of LPL (34). Second, VLDLR binds and internalizes TG-rich lipoprotein particles through specific binding of apolipoprotein E, as has been shown in isolated cells (44). The lack of pioglitazone effects on TG-rich lipoprotein catabolism in vldlr−/− mice seems to be related directly to VLDLR expression, since pioglitazone increased LPL expression in WT and vldlr−/− mice (Fig. 6). Furthermore, the activity of post-heparin plasma LPL, lower in nontreated vldlr−/− mice than in WT mice, was also increased by pioglitazone (results not shown). However, post-heparin plasma activity may not be synonymous with the activity of LPL at its site of action on the surface of the endothelial cell. It is possible that the absence of VLDLR contiguous to LPL results in a significant alteration in the activity of this enzyme at its site of action. It was previously suggested that the presence of VLDLR next to LPL at the surface of the endothelial cell is important for optimal action of LPL (44). Alternatively, we questioned whether the lack of pioglitazone effect in vldlr−/− mice could be linked to alteration of the expression of low-density lipoprotein receptor-related protein-1 (LRP1). LRP1 is recognized as a receptor for remnant TG-rich lipoproteins in liver (31) and is expressed also in adipose tissue (24). Specific inactivation of LRP1 in adipose tissue increased blood TGs, reduced TG clearance, and reduced obesity in mice (24), effects that are similar to those induced by VLDLR deficiency. We examined the expression of LRP1 in adipose tissue, and the results showed similar induction by pioglitazone in WT and vldlr−/− mice (not shown). Furthermore, the expression of low-density lipoprotein receptor (LDLR), the other member of this receptor family, was not altered in adipose tissue of vldlr−/− mice. Although LDLR binds VLDL remnants, its expression in adipose tissue is low, and its main function as receptor for cholesterol-rich particles like LDL is reported especially in hepatocytes and macrophages (24). Taken together, these results suggest that vldlr expression is in fact important for mediating the effect of pioglitazone on TG clearance and lipid uptake in adipose tissue. These results suggest the possibility that LRP1 and VLDLR exert a complementary function in adipose tissue, and in collaboration with LPL they enhance the clearance of lipids derived from TG-rich lipoproteins. Our results are thus in agreement with previous findings by Espirito Santo et al. (12), showing that VLDLR deficiency further enhanced hypertriglyceridemia in LDLR-LRP double-knockout mice fed a high-fat diet or in the postprandial state. These results suggest that these receptors contribute individually to TG-rich catabolism, but their roles become more evident with expansion of the TG-rich lipoprotein pool, such as with high-fat feeding or in the immediate postprandial period (12). In addition to LRP, other proteins have been identified as modulators of LPL activity. For instance, the endothelial cell protein glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein-1 (GPIHBP1) binds LPL and chylomicrons and facilitates TG lipolysis (4). The role of GPIHBP1 as a modulator of TG-rich lipoprotein lipolysis has been demonstrated in cell and animal models (4) and more recently in patients with chylomicronemia (3). Like VLDLR and LPL, GPIHBP1 is also a target gene for PPARγ (10). However, it is not known whether interaction of LPL and GPIHBP1 involves VLDLR also. The possibility of cooperative work among these proteins needs further investigation for clarification.
Consistent with previous findings, our results show that deficiency of VLDLR is associated with slower catabolism of TG-rich lipoproteins and smaller adipose tissue (15). Furthermore, our present studies provide additional evidence enforcing the role of VLDLR in mediating the proadipogenic effects of PPARγ. Pioglitazone induction of PPARγ greatly increased the rate of preadipocyte differentiation and lipid deposition in WT adipocytes. However, deficiency of VLDLR greatly reduced pioglitazone-induced adipogenesis in isolated preadipocytes. This effect was noticed when VLDL particles were used as a source of lipids and not with FAs, suggesting that the interaction receptor-ligand is the determining factor. In addition, induction of adipose tissue expansion by pioglitazone was reduced in vldlr−/− mice despite significant increases of FATP and CD36 (data not shown), proteins known to facilitate FA uptake (22) and under the control of PPARγ (13). These results suggest that the reduction of TG-rich lipoprotein lipolysis in vldlr−/− mice limits the availability of FFA in the vascular capillary bed. In this situation, induction of CD36 and FATP expression by pioglitazone in adipocytes does not translate into higher FA uptake, and thus resulting in slower adipogenesis rates. Analysis of radioactivity distribution showed that uptakes of [3H]TG-derived lipids was ∼50% lower in adipose tissue, heart, and muscle of vldlr−/− compared with WT mice fed high-fat alone. Pioglitazone treatment in WT mice increased the contribution of these tissues in lipid uptake, but the effects were clearly stronger in adipose tissue (+170%) compared with skeletal muscle (+70%) and heart (+35%). The contribution of liver in [3H]TG uptake was slightly higher in vldlr−/− mice fed the high-fat diet compared with WT mice. Pioglitazone increased liver uptake in both genotypes, but the uptake in liver of vldlr−/− remained higher than that of WT mice. Expression of PPARγ in liver is very low, and the difference between vldlr−/− and WT is possibly related to higher endocytosis through hepatic LDLR (31) independently of PPARγ activation. It is known that PPARγ is highly expressed in adipose tissue compared with heart and skeletal muscles, a situation that renders this tissue highly sensitive to PPARγ agonists. Laplante et al. (27) have shown that thiazolidinedione treatment in rats increased the uptake of lipids derived from TG-rich lipoproteins selectively in adipose tissue. Our results in WT mice are in agreement with those reports and highlight the primary role of adipose tissue in the uptake of lipids. Absence of VLDLR significantly diminishes the ability of adipose tissue for the uptake of TG-rich lipoprotein-derived lipids. These results suggest that VLDLR in peripheral tissue, especially in adipose tissue, contributes significantly to the clearance of lipids derived from VLDL and chylomicrons in mice. These in vivo results are also consistent with the in vitro data. Incubation of labeled VLDL with isolated adipocytes showed that VLDLR deficiency significantly reduced the uptake of lipids (Fig. 10B). Although the uptake of 125I was a small fraction compared with that of [3H]TG, it was also reduced in vldl−/− mice (Fig. 10C). These data suggest that VLDLR participates in TG-rich lipoprotein catabolism at multiple levels. VLDLR influences the rate of uptake of VLDL-derived FAs through the modulation of LPL activity (48) and also serves as a membrane receptor for the binding and endocytosis of the whole remnant particles (43, 44). These pathways have been reported as a route of delivery of lipids in heart of C57BL/6 WT mice (2), and VLDLR could be also playing a similar role as in adipose tissue.
In vldlr−/− mouse, delayed TG-rich lipoprotein catabolism and diminished lipid delivery to target organs is also associated with resistance to high-fat diet-induced obesity (15). Furthermore, feeding chow diet generated similar results (15, 16) but with less magnitude than high-fat diet. Because adipose tissue is the body's major energy store, alteration of adipose tissue mass often induces changes in the energy balance. There is evidence that nutrients and metabolic signals in adipocytes are integrated in metabolic and hormonal responses that act in the brain and other organs to adjust whole body energy balance. In mice, alteration of the expression of genes that promote lipid storage (7) or mobilization (32) triggers an adjusted response at the level of thermogenesis, energy expenditure, and/or metabolic responses (26). Our present results, in addition to previous investigations, (16) point to the fact that VLDLR expression and treatment with pioglitazone play a direct role in lipid homeostasis, especially at the level of the adipocyte. At present, it is not known whether energy expenditure and/or adipokine production are altered in vldlr−/− mice compared with WT mice. However, clearance of blood glucose and uptake in organs were significantly enhanced in vldlr−/− mice fed either chow or high-fat diets in addition to lower blood insulin levels (unpublished data). It is possible that a switch of energy substrate in vldlr−/− mice alters energy expenditure of the whole body that induces reduction of adiposity. This scenario confers to vldlr a possible role in the metabolic regulatory network that controls energy balance and obesity as described for other proteins in adipocyte (20,26,32).
In humans, VLDLR deficiency was reported in families with autosomal recessive cerebellar hypoplasia, and interestingly, 50% of affected patients were underweight with a body mass index (BMI) under 18.5 (5). In addition, analysis of common vldlr variants in the CLEAR study subjects conducted by the University of Washington and the Veterans Affairs Puget Sound Health Care System (Seattle, WA) identified a subpopulation of subjects with a single nucleotide polymorphism (named SNP 1226; rs1454626) located in the 5′-flanking region of VLDLR that was significantly associated with lower BMI (9). However, it is too early to establish a final link between low expression or dysfunctional VLDLR and low BMI in human. Further studies are needed to validate these observations and to test whether the catabolism of TG-rich lipoproteins is also linked to VLDLR expression in human. Then, treatments with PPARγ agonists could be addressed in light of the present findings.
In summary, this study demonstrates that PPARγ increases VLDLR mRNA expression in adipocytes derived from both humans and mice, as well as in differentiated 3T3L1 adipocytes. Significant reduction in VLDLR expression was also noticed with PPARγ inhibition. These results are supported by in vivo data showing significant increases of VLDLR mRNA and protein in adipose tissue of wild type mice treated with PPARγ agonist. In addition, the data provide evidence supporting involvement of VLDLR in mediating the proadipogenic effects of PPARγ. In WT mice, pioglitazone induction of PPARγ increased TG-rich lipoprotein clearance, enhanced fat mass, and increased lipid deposition in isolated adipocytes. However, these effects related to PPARγ induction are clearly blunted in vldlr−/− mice.
GRANTS
This work was supported by Development Award no. AHA0730356N from the American Heart Association (T. Hajri), Development Grant no. 1047089103 from the Department of Surgery, Vanderbilt University (T. Hajri), and National Institute of Diabetes and Digestive and Kidney Diseases Grant no. DK-070860-01S1 (N. N. Abumrad).
DISCLOSURES
No conflicts of interest are reported by the author(s).
REFERENCES
- 1.Aalto-Setala K, Fisher EA, Chen X, Chajek-Shaul T, Hayek T, Zechner R, Walsh A, Ramakrishnan R, Ginsberg HN, Breslow JL. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. Journal of Clinical Investigation 90: 1889–1900, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Metab 284: E331–E339, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Beigneux AP, Franssen R, Bensadoun A, Gin P, Melford K, Peter J, Walzem RL, Weinstein MM, Davies BS, Kuivenhoven JA, Kastelein JJ, Fong LG, Dallinga-Thie GM, Young SG. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase [see comment]. Arteriosclerosis, Thrombosis & Vascular Biology 29: 956–962, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigneux AP, Weinstein MM, Davies BS, Gin P, Bensadoun A, Fong LG, Young SG. GPIHBP1 and lipolysis: an update. Current Opinion in Lipidology 20: 211–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boycott KM, Flavelle S, Bureau A, Glass HC, Fujiwara TM, Wirrell E, Davey K, Chudley AE, Scott JN, McLeod DR, Parboosingh JS. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. American Journal of Human Genetics 77: 477–483, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caserta F, Tchkonia T, Civelek VN, Prentki M, Brown NF, McGarry JD, Forse RA, Corkey BE, Hamilton JA, Kirkland JL. Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. Am J Physiol Endocrinol Metab 280: E238–E247, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Chen HC, Ladha Z, Smith SJ, Farese RV., Jr Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am J Physiol Endocrinol Metab 284: E213–E218, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Chiba T, Nakazawa T, Yui K, Kaneko E, Shimokado K. VLDL induces adipocyte differentiation in ApoE-dependent manner. Arteriosclerosis, Thrombosis & Vascular Biology 23: 1423–1429, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Crawford DC, Nord AS, Badzioch MD, Ranchalis J, McKinstry LA, Ahearn M, Bertucci C, Shephard C, Wong M, Rieder MJ, Schellenberg GD, Nickerson DA, Heagerty PJ, Wijsman EM, Jarvik GP. A common VLDLR polymorphism interacts with APOE genotype in the prediction of carotid artery disease risk. Journal of Lipid Research 49: 588–596, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Davies BS, Waki H, Beigneux AP, Farber E, Weinstein MM, Wilpitz DC, Tai LJ, Evans RM, Fong LG, Tontonoz P, Young SG. The expression of GPIHBP1, an endothelial cell binding site for lipoprotein lipase and chylomicrons, is induced by peroxisome proliferator-activated receptor-gamma [erratum appears in Mol Endocrinol 12: 2766, 2008]. Molecular Endocrinology 22: 2496–2504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20: 649–688, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Espirito Santo SM, Rensen PC, Goudriaan JR, Bensadoun A, Bovenschen N, Voshol PJ, Havekes LM, van Vlijmen BJ. Triglyceride-rich lipoprotein metabolism in unique VLDL receptor, LDL receptor, and LRP triple-deficient mice. Journal of Lipid Research 46: 1097–1102, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem 274: 3970–3977, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Frost SC, Lane MD. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem 260: 2646–2652, 1985 [PubMed] [Google Scholar]
- 15.Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci USA 92: 8453–8457, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goudriaan JR, Espirito Santo SM, Voshol PJ, Teusink B, van Dijk KW, van Vlijmen BJ, Romijn JA, Havekes LM, Rensen PC. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res 45: 1475–1481, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Goudriaan JR, Tacken PJ, Dahlmans VE, Gijbels MJ, van Dijk KW, Havekes LM, Jong MC. Protection from obesity in mice lacking the VLDL receptor. Arterioscler Thromb Vasc Biol 21: 1488–1493, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5: 19–27, 1975 [DOI] [PubMed] [Google Scholar]
- 19.Hajri T, Ferezou J, Lutton C. Total parenteral nutrition stimulates hepatic cholesterol synthesis in the rat. Biochimica et Biophysica Acta 1258: 188–194, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56: 1872–1880, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. Journal of Clinical Investigation 109: 1381–1389, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajri T, Ibrahimi A, Coburn CT, Knapp FF, Jr, Kurtz T, Pravenec M, Abumrad NA. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J Biol Chem 276: 23661–23666, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Hajri T, Khosla P, Pronczuk A, Hayes KC. Myristic acid-rich fat raises plasma LDL by stimulating LDL production without affecting fractional clearance in gerbils fed a cholesterol-free diet. J Nutr 128: 477–484, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Hofmann SM, Zhou L, Perez-Tilve D, Greer T, Grant E, Wancata L, Thomas A, Pfluger PT, Basford JE, Gilham D, Herz J, Tschop MH, Hui DY. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. Journal of Clinical Investigation 117: 3271–3282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JG, Keshava C, Murphy AA, Pitas RE, Parthasarathy S. Fresh mouse peritoneal macrophages have low scavenger receptor activity. Journal of Lipid Research 38: 2207–2215, 1997 [PubMed] [Google Scholar]
- 26.Kopecky J, Flachs P, Bardova K, Brauner P, Prazak T, Sponarova J. Modulation of lipid metabolism by energy status of adipocytes: implications for insulin sensitivity. Annals of the New York Academy of Sciences 967: 88–101, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Laplante M, Festuccia WT, Soucy G, Gelinas Y, Lalonde J, Berger JP, Deshaies Y. Mechanisms of the depot specificity of peroxisome proliferator-activated receptor gamma action on adipose tissue metabolism. Diabetes 55: 2771–2778, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Laplante M, Festuccia WT, Soucy G, Gelinas Y, Lalonde J, Deshaies Y. Involvement of adipose tissues in the early hypolipidemic action of PPARγ agonism in the rat. Am J Physiol Regul Integr Comp Physiol 292: R1408–R1417, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell 123: 993–999, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta CT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Mahley RW, Huang Y. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing [comment]. Journal of Clinical Investigation 117: 94–98, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nature Genetics 26: 474–479, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Niemeier A, Gafvels M, Heeren J, Meyer N, Angelin B, Beisiegel U. VLDL receptor mediates the uptake of human chylomicron remnants in vitro. Journal of Lipid Research 37: 1733–1742, 1996 [PubMed] [Google Scholar]
- 34.Obunike JC, Lutz EP, Li Z, Paka L, Katopodis T, Strickland DK, Kozarsky KF, Pillarisetti S, Goldberg IJ. Transcytosis of lipoprotein lipase across cultured endothelial cells requires both heparan sulfate proteoglycans and the very low density lipoprotein receptor. J Biol Chem 276: 8934–8941, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Oka K, Ishimura-Oka K, Chu MJ, Sullivan M, Krushkal J, Li WH, Chan L. Mouse very-low-density-lipoprotein receptor (VLDLR) cDNA cloning, tissue-specific expression and evolutionary relationship with the low-density-lipoprotein receptor. Eur J Biochem 224: 975–982, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Qi K, Seo T, Al-Haideri M, Worgall TS, Vogel T, Carpentier YA, Deckelbaum RJ. Omega-3 triglycerides modify blood clearance and tissue targeting pathways of lipid emulsions. Biochemistry 41: 3119–3127, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Sakai J, Hoshino A, Takahashi S, Miura Y, Ishii H, Suzuki H, Kawarabayasi Y, Yamamoto T. Structure, chromosome location, and expression of the human very low density lipoprotein receptor gene. J Biol Chem 269: 2173–2182, 1994 [PubMed] [Google Scholar]
- 38.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 15: 5336–5348, 1996 [PMC free article] [PubMed] [Google Scholar]
- 39.Schoonjans K, Watanabe M, Suzuki H, Mahfoudi A, Krey G, Wahli W, Grimaldi P, Staels B, Yamamoto T, Auwerx J. Induction of the acyl-coenzyme A synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. J Biol Chem 270: 19269–19276, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Sena-Esteves M, Saeki Y, Camp SM, Chiocca EA, Breakefield XO. Single-step conversion of cells to retrovirus vector producers with herpes simplex virus-Epstein-Barr virus hybrid amplicons. J Virol 73: 10426–10439, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiomi T, Tsutsui H, Hayashidani S, Suematsu N, Ikeuchi M, Wen J, Ishibashi M, Kubota T, Egashira K, Takeshita A. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 106: 3126–3132, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Tacken PJ, Teusink B, Jong MC, Harats D, Havekes LM, van Dijk KW, Hofker MH. LDL receptor deficiency unmasks altered VLDL triglyceride metabolism in VLDL receptor transgenic and knockout mice. J Lipid Res 41: 2055–2062, 2000 [PubMed] [Google Scholar]
- 43.Takahashi S, Sakai J, Fujino T, Miyamori I, Yamamoto TT. The very low density lipoprotein (VLDL) receptor–a peripheral lipoprotein receptor for remnant lipoproteins into fatty acid active tissues. Mol Cell Biochem 248: 121–127, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, Yamamoto T, Nakai T. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem 270: 15747–15754, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annual Review of Biochemistry 77: 289–312, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97: 689–701, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Wahli W, Braissant O, Desvergne B. Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more. Chem Biol 2: 261–266, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Yagyu H, Lutz EP, Kako Y, Marks S, Hu Y, Choi SY, Bensadoun A, Goldberg IJ. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem 277: 10037–10043, 2002 [DOI] [PubMed] [Google Scholar]