Abstract
The recently discovered peptide apelin is known to be involved in the maintenance of insulin sensitivity. However, questions persist regarding its precise role in the chronic setting. Fasting glucose, insulin, and adiponectin levels were determined on mice with generalized deficiency of apelin (APKO). Additionally, insulin (ITT) and glucose tolerance tests (GTT) were performed. To assess the impact of exogenously delivered apelin on insulin sensitivity, osmotic pumps containing pyroglutamated apelin-13 or saline were implanted in APKO mice for 4 wk. Following the infusion, ITT/GTTs were repeated and the animals euthanized. Soleus muscles were harvested and homogenized in lysis buffer, and insulin-induced Akt phosphorylation was determined by Western blotting. Apelin-13 infusion and ITTs/GTTs were also performed in obese diabetic db/db mice. To probe the underlying mechanism for apelin's effects, apelin-13 was also delivered to cultured C2C12 myotubes. 2-[3H]deoxyglucose uptake and Akt phosphorylation were assessed in the presence of various inhibitors. APKO mice had diminished insulin sensitivity, were hyperinsulinemic, and had decreased adiponectin levels. Soleus lysates had decreased insulin-induced Akt phosphorylation. Administration of apelin to APKO and db/db mice resulted in improved insulin sensitivity. In C2C12 myotubes, apelin increased glucose uptake and Akt phosphorylation. These events were fully abrogated by pertussis toxin, compound C, and siRNA knockdown of AMPKα1 but only partially diminished by LY-294002 and not at all by l-NAME. We conclude that apelin is necessary for the maintenance of insulin sensitivity in vivo. Apelin's effects on glucose uptake and Akt phosphorylation are in part mediated by a Gi and AMPK-dependent pathway.
Keywords: insulin resistance, obesity, diabetes, hormones
insulin resistance, defined as a diminution in a cell, tissue, or organism's ability to take up glucose in response to insulin, is the pathophysiological hallmark of type 2 diabetes mellitus. Although insulin resistance is typically asymptomatic, it is independently and strongly associated with an increased risk of coronary disease (22), heart failure (15), and mortality (19). Insulin resistance is thus rapidly gaining in importance as a disease entity in the Western world. Unfortunately, despite the clear need for novel therapies for insulin resistance, our understanding of its pathogenesis and mechanisms remains incomplete.
Apelin is a peptide hormone recently identified as an endogenous ligand (37) for the Gi protein-coupled, angiotensin receptor-like receptor APJ (26, 31). The human preproapelin gene, located on chromosome Xq25-26.1, encodes a 77-amino acid preproprotein (20) that is cleaved to active forms that are 36, 17, 13, and 12 residues in length (37). Of these, the 36-amino acid isoform is the most widely expressed, although the shorter isoforms are more potent and more abundant in the circulation (38). Apelin has gained significant attention in recent years because it has been found to possess numerous distinct biological activities in a variety of organs, including the heart, brain, stomach, placenta, and breast (for review, see Ref. 25).
In addition to the above, recently, evidence that apelin is associated with insulin resistance has accumulated. For instance, apelin is upregulated by insulin (3, 9) and inhibits pancreatic insulin secretion (35). In clinical studies, apelin levels are increased in states of obesity (3, 12) and insulin resistance (24); moreover, reduction of body weight results in a coincident decline in apelin expression (4). Importantly, direct administration of apelin in preclinical animal models results in improved insulin sensitivity (7, 13), suggesting the potential for therapeutic applicability.
However, despite these advances, questions persist regarding apelin's relationship with chronic insulin-glucose homeostasis. For example, all studies to date have involved acute intraperitoneal or intravenous administration; therefore, it is unknown whether apelin exerts any longer-term impact on insulin sensitivity. Also, although preliminary studies (7) have identified signaling intermediates [e.g., AMP-activated protein kinase (AMPK), endothelial nitric oxide synthase (eNOS)] involved in apelin's regulation of glucose uptake, it remains unclear whether the underlying mechanisms for apelin's effects upon skeletal muscle cells are direct, indirect, or both.
To address these issues, we have 1) investigated apelin's effects on insulin sensitivity using a murine line deficient in apelin production and 2) utilized differentiated C2C12 myotubes to further explore apelin's influences at the cellular and intracellular level. We report that apelin-null mice have impaired insulin sensitivity; also, this effect is reversed by supplementation with exogenous apelin. Moreover, we find that apelin exerts a direct effect on glucose uptake and intracellular signaling in vitro. Given the prominence of type 2 diabetes and the metabolic syndrome in Western societies, these results carry significant implications regarding the search for novel therapies for insulin resistance.
RESEARCH DESIGN AND METHODS
Animals.
Generalized apelin-null (APKO) mice based on a 129Sv/J background were produced by homologous recombination in embryonic stem cells. Detailed methodology regarding construct, breeding, and husbandry has been published previously (33). Lack of apelin mRNA expression in various tissues, including brain, heart, liver, lung, and skeletal muscle, was confirmed by qRT-PCR (data not shown). For experiments involving APKO mice, mice from the parental wild-type (WT) strain (from both the parent colony and Jackson Laboratories, Bar Harbor, ME) were used as controls. Additionally, C57BL/KLS-leprdb/leprdb (db/db) mice were purchased from Jackson Laboratories. All experimental animals were male.
Animals were maintained on a normal chow diet (Purina LabDiet HMR3000) containing 26% protein, 14% fat, and 60% carbohydrate by calorie count, given free access to tap water, and housed in a room with a 12:12-h light-dark cycle and a temperature of 22°C. All protocols were approved by the Administrative Panel on Laboratory Animal Care at Stanford University and were performed in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care.
At euthanization, animals were lethally anesthetized with isoflurane. Selected animals were injected with insulin (2 U/kg) 30 min prior to euthanization. Five hundred to one thousand microliters of whole blood was obtained via ventricular cannulation and centrifuged at 13,200 rpm for 10 min. Two hundred- to two hundred fifty-microliter aliquots of plasma were then collected for insulin (Mercodia mouse ELISA kit; Alpco Diagnostics, Salem, NH), apelin (apelin-12 ELISA kit; Phoenix Pharmaceuticals, Burlingame, CA), and adiponectin (mouse ELISA kit; Alpco) measurement. Soleus muscles were isolated, harvested, snap-frozen in liquid nitrogen, and then stored at −80°C for downstream analysis.
Diet-induced insulin resistance.
Diet-induced insulin resistance was generated by feeding animals a 45% fat-by-kcal diet (TD.06415; Harlan-Teklad, Indianapolis, IN) as well as having them drink water containing 10% sucrose by weight, which has been well documented to increase body weight and worsen glucose tolerance in mice (28). Animals were maintained on this diet for 3 wk.
Insulin and glucose tolerance testing.
Insulin tolerance testing (ITT) was performed as described previously (44), with slight modifications. Animals were fasted for 6 h prior to testing. Tail-nick glucose samples were obtained at baseline and every 10 min for 1 h following intraperitoneal injection of insulin (1.0 U/kg; Eli Lilly, Indianapolis, IN). For diet-induced insulin-resistant mice, a dose of 1.25 U/kg was used. For db/db mice, a dose of 3.0 U/kg was used. Glucose tolerance testing (GTT) was performed identically to the ITTs, with the substitution of d-glucose (2 g/kg for all groups; Sigma-Aldrich, St. Louis, MO) for insulin.
Insulin suppression test.
An insulin suppression test based on the protocols of Taketomi et al. (36) and Terauchi et al. (39) was performed with slight modifications. Animals fasted for 6 h were administered an intraperitoneal injection of somatostatin (0.1 mg/kg; Sigma-Aldrich), glucose (1 g/kg), and insulin (0.5 U/kg). Steady-state plasma glucose (SSPG) levels were assessed at 80 min postinjection, using a glucometer to measure tail-nick blood samples. Steady-state plasma insulin (SSPI) levels were also determined from serum collected at 80 min with an insulin enzyme-linked immunoassay. SSPG and SSPI levels were not significantly different from those measured from blood collected at 60 and 70 min (data not shown).
Apelin infusion.
Apelin infusion studies were performed on APKO mice aged 12 wk as well as db/db mice aged 9 wk. Following anesthetic induction with isoflurane, osmotic infusion pumps (Alzet 2002; Durect, Cupertino, CA) containing 2 mg·kg−1·day−1 pyroglutamated apelin-13 (pGlu-apelin-13; American Peptide, Sunnyvale, CA) or normal saline were implanted subcutaneously along the scruff of the neck. After 2 wk the db/db groups were euthanized, and new pumps were reimplanted in the APKO groups for an additional 2 wk. At euthanization, serum was harvested for downstream analysis, as described above.
Western blotting.
Isolated soleus muscles were homogenized in lysis buffer (T-PER; Pierce Biotechnology, Rockford, IL) supplemented with protease (Halt Protease Inhibitor Cocktail; Pierce Biotechnology) and phosphatase (Phosphatase Inhibitor Cocktail II; Sigma-Aldrich) inhibitors and then spun at 13,200 rpm for 15 min at 4°C to obtain lysates. Cell lysates were subjected to Western blotting, as described previously (9). Samples were probed with antibodies directed against Akt and Ser473-phosphorylated Akt (Cell Signaling Technology, Danvers, MA). Quantification of band intensities was performed with ImageJ software.
Cell culture.
C2C12 myoblasts (American Type Tissue Collection, Manassas, VA) (42) were grown in low-glucose DMEM (Invitrogen, Carlsbad, CA) containing 20% FBS. To differentiate myoblasts into myotubes, the medium was switched to DMEM plus 10% horse serum when the cells reached 80% confluence and then incubated for 72 h. Differentiated myotubes were serum starved for 18 h and then exposed to insulin, the phosphoinositide 3-kinase (PI3K) inhibitor LY-204002 (EMD Biosciences, Gibbstown, NJ), pertussis toxin (PTX; EMD), the AMPK inhibitor compound C (EMD), Nω-nitro-l-arginine methyl ester (l-NAME) hydrochloride (Sigma-Aldrich), and/or pGlu-apelin-13 at the indicated doses and times.
Western blotting.
Following incubation, C2C12 myotubes were harvested in lysis buffer (M-PER; Pierce Biotechnology) supplemented with protease (Halt Protease Inhibitor Cocktail; Pierce Biotechnology) and phosphatase (Phosphatase Inhibitor Cocktail II; Sigma-Aldrich) inhibitors. Cell lysates were subjected to Western blotting, as described above. Samples were probed with antibodies directed against Akt, Ser473-phosphorylated Akt, Ser79-phosphorylated acetyl-CoA carboxylase (ACC), and GAPDH (Cell Signaling).
Glucose uptake.
To determine glucose uptake, C2C12 myotubes in culture were treated with insulin, pGlu-apelin-13, LY-294002, compound C, and/or l-NAME as indicated. Cells were then incubated in Krebs-Ringer bicarbonate-HEPES (KRBH) buffer (130 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.3 mM MgSO4, 25 mM HEPES, pH 7.4) containing 1% fatty acid-free bovine serum albumin (Sigma-Aldrich), 2-deoxyglucose (0.5 mM; Sigma-Aldrich), and [1,2-3H]2-deoxy-d-glucose (100 μM; GE Healthcare, Piscataway, NJ) for 10 min at 37°C. The cells were washed with ice-cold KRBH buffer, harvested in 100 μl of lysis buffer (Pierce Biotechnology), and then added to scintillation vials containing 5 ml of scintillation fluid. Radioactive counts were determined with a scintillation counter (LS6500; Beckman Coulter, Fullerton, CA). Counts were converted to moles of glucose taken up and normalized to the protein concentration of the lysates. To account for nonspecific deoxyglucose uptake, counts were measured in the presence of 20 μM cytochalasin B (Sigma-Aldrich) and subtracted from each determination to obtain specific uptake.
RNA interference of AMPKα1.
Molecular inhibition of AMPKα1 was accomplished with RNA interference based on the methods of Lee et al. (21). Five-nanomole aliquots of an AMPKα1 (NM_ 001013367) siRNA, a cyclophilin B (NM_011149) siRNA used as a positive control, and a nontargeting siRNA were purchased from Dharmacon (Lafayette, CO) and rehydrated in 250 μl of DNase/RNase-free water. C2C12 cells were grown in low-glucose DMEM plus 20% FBS to 60% confluence in six-well plates. For each well, at the time of transfection, the medium was aspirated and replaced by 800 μl of reduced serum medium (Opti-MEM; Invitrogen). Five microliters (i.e., 0.1 nmol) of siRNA was diluted in 95 μl of Opti-MEM and then combined with 5 μl of lipofectamine 2000 reagent (Invitrogen) prediluted in 95 ml of Opti-MEM. The siRNA-lipofectamine mixture (total volume 200 μl) was allowed to equilibrate at room temperature for 30 min and then added to each well. Transfections involving AMPKα1 and nontargeting control siRNAs were allowed to proceed for 4 h, after which the medium was replaced by DMEM-20% FBS. Twenty-four hours later, transfected cells were exposed to pGlu-apelin-13 for 2 h, harvested in lysis buffer, and subjected to Western blotting for Ser473-phosphorylated and nonphosphorylated Akt, as described above.
To determine transfection efficiency, cells were transfected with cyclophilin B siRNA and harvested in Trizol reagent (Invitrogen). RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA) and cDNA conversion performed using a SuperScript First-Strand cDNA synthesis kit (Invitrogen). Real-time quantitative PCR (qRT-PCR) for the mouse cyclophilin B gene (Mm00478295_m1) and 18S ribosomal RNA (4352339E), furnished by Applied Biosystems (Foster City, CA), was then performed as described previously (9). Confirmation of AMPKα1 knockdown was performed by Western blotting of transfected C2C12 lysates with an antibody directed against AMPKα (Cell Signaling).
Statistics.
For ITTs and GTTs, statistical analysis of individual curves was performed with Student's unpaired t-tests of areas under the curve. For the remaining experiments, statistical comparisons between groups were performed using two-way ANOVA. A P value of <0.05 was considered statistically significant. All statistical analyses were performed with SPSS (Chicago, IL).
RESULTS
Apelin-null mice.
To characterize the effects of apelin on insulin sensitivity, a line of mice deficient in the apelin gene (APLN−/−; APKO) was created. In general, APKO mice were viable and fertile, and no gross or histological abnormalities were observed in any major organs ≤12 wk of age. Notably, no differences in body weight vs. WT were evident at 12 wk (Table 1).
Table 1.
Phenotypic characteristics of APKO and wild-type mice aged 8 wk
| APKO Mice | Wild-Type Mice | |
|---|---|---|
| BW, g | 27.0±1.2 | 26.5±0.71 |
| AUC ITT, mg·min−1·dl−1 | 237±27* | 174±14 |
| AUC GTT, mg·min−1·dl−1 | 954±36† | 753±45 |
| Insulin, pg/ml | 0.70±0.033* | 0.55±0.098 |
| Adiponectin, μg/ml | 64±3.3* | 80±8.0 |
| Fed glucose, mg/dl | 83±11 | 76±11 |
| Fasting glucose, mg/dl | 76±14 | 68±11 |
| SSPG, mg/dl | 150±20† | 86±6.6 |
Values are means ± SE. APKO, generalized apelin-null; BW, body weight; AUC, area under the curve; ITT, insulin tolerance test; GTT, glucose tolerance test; SSPG, steady-state plasma glucose.
P < 0.05 vs. wild type;
P < 0.01 vs. wild type. For SSPG, n = 6–9/group; for all other studies, n = 8–12/group.
APKO mice are insulin resistant.
To assess for differences in glucose and insulin handling, 8-wk APKO and age-matched WT mice were subjected to glucose and insulin tolerance testing (Fig. 1, A and B, and Table 1). Whereas WT mice exhibited normal responses to glucose and insulin loading, the APKO mice demonstrated an abnormal response to both. Additionally, consistent with an insulin-resistant phenotype, APKO mice had significantly increased insulin levels as well as decreased adiponectin levels (Table 1). Interestingly, serum glucose was also increased, albeit not significantly. To supplement these data, we also measured SSPG levels during an insulin suppression test. Compared with WT, SSPG levels were significantly greater in the APKO mice (Table 1). Notably, steady-state plasma insulin levels were similar between groups [0.58 ± 0.12 vs. 0.57 ± 0.10 pg/ml for APKO vs. WT, respectively, P = not significant (NS)]. Taken together, these results suggest that APKO mice are systemically insulin resistant but not overtly diabetic.
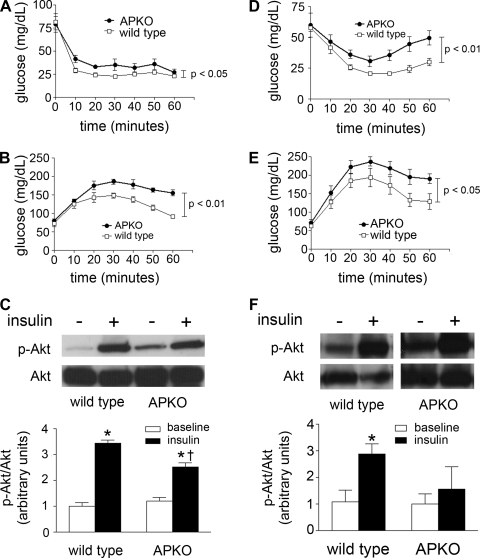
Fig. 1.
A and B: insulin (1 U/kg) and glucose (2 g/kg) tolerance tests for generalized apelin-null (APKO) mice; n = 9–14 animals/group. C: representative Western blots of soleus muscle lysates from wild-type and APKO mice at baseline and with insulin stimulation, probing for phosphorylated Akt (p-Akt). Quantification and normalization of band intensity to native Akt is depicted below. *P < 0.05 vs. baseline, †P < 0.05 vs. insulin-treated wild type; n = 3–4 animals/group. D and E: insulin (1.25 U/kg) and glucose (2 g/kg) tolerance tests for APKO mice with diet-induced insulin resistance; n = 6–7 animals/group. F: representative Western blots of soleus lysates from diet-induced insulin-resistant wild-type and APKO mice, probing for p-Akt and normalizing to native Akt. *P < 0.05 vs. baseline; n = 4 animals/group. For all experiments, results are expressed as means ± SE within each group.
In addition to the above studies, soleus muscles were isolated from WT and APKO mice treated with and without insulin. Akt phosphorylation was determined by Western blotting (Fig. 1C). In the WT animals, Akt phosphorylation was increased 3.4-fold by insulin. However, in the APKO group, insulin-stimulated Akt phosphorylation was decreased significantly compared with WT, with only a 2.1-fold increase relative to baseline.
High-fat, high-sucrose diet exacerbates insulin resistance in APKO mice.
To lend further support to apelin's role in maintaining insulin sensitivity, we performed ITTs and GTTs in 8-wk APKO and WT mice exposed to a high-fat-chow, high-sucrose-drinking water diet for 3 wk. As with the baseline studies, the APKO mice responded abnormally to insulin relative to WT [area under the curve (AUC) 250 ± 16 vs. 177 ± 6.9 mg·min−1·dl−1 for APKO vs. WT, respectively, P < 0.01; Fig. 1D]. Similarly, the APKO mice had an exaggerated response to the glucose challenge (AUC 1,120 ± 54 vs. 907 ± 66 mg·min−1·dl−1 for APKO vs. WT, respectively, P < 0.05; Fig. 1E). Finally, skeletal muscle lysates derived from APKO mice showed significantly decreased insulin-stimulated Akt phosphorylation compared with WT (Fig. 1F).
Restoration of apelin to knockout mice leads to reversal of the phenotypic features of insulin resistance.
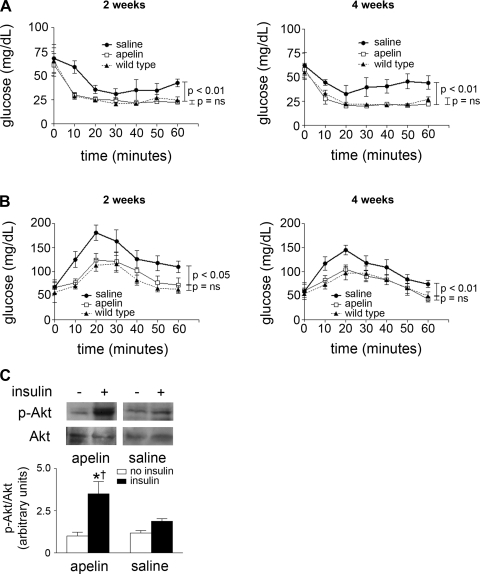
We next administered 4-wk infusions of either pGlu-apelin-13 or saline to APKO mice. The dose (2 mg·kg−1·day−1) was selected because, in our prior experience (1), this was the amount found to be sufficient to result in significant changes in the cardiovascular system. Apelin levels were not significantly different in the apelin-treated APKO mice than in age-matched WT mice (4.3 ± 0.44 vs. 4.0 ± 0.69 ng/ml, respectively, P = 0.72), confirming that this dose produced physiological levels of apelin in the APKO mice.
At baseline, no differences in ITTs or GTTs were seen between saline- and apelin-treated APKO mice (Table 2). However, at 2 and 4 wk, the apelin-treated APKO mice were significantly more responsive to insulin and glucose than the saline group (Fig. 2). Notably, the insulin and glucose response curves were indistinguishable from a matched, untreated WT group. Additionally, at euthanization, adiponectin levels were significantly increased and insulin significantly decreased in apelin-treated mice, whereas glucose levels were nonsignificantly reduced (Table 3). Concomitant with these features, insulin-induced Akt phosphorylation was augmented significantly in the apelin-treated group relative to saline (Fig. 2C). Finally, no differences in body weight were observed between the groups at any time point.
Table 2.
ITT and GTT results of APKO mice treated with a 4-wk infusion of apelin or saline
| Apelin-Treated APKO Mice | Saline-Treated APKO Mice | |
|---|---|---|
| AUC ITT baseline | 200±15 | 202±27 |
| AUC GTT baseline | 821±13 | 831±66 |
| AUC ITT 2 wk | 167±9.1† | 251±28 |
| AUC GTT 2 wk | 573±68* | 804±49 |
| AUC ITT 4 wk | 153±10† | 243±25 |
| AUC GTT 4 wk | 486±29† | 647±27 |
Values are means ± SE and in mg·min−1·dl−1.
P < 0.05 vs. saline-treated mice,
P < 0.01 vs. saline-treated mice; n = 5–7/group.
Fig. 2.
A and B: insulin (1 U/kg) and glucose (2 g/kg) tolerance tests for generalized APKO mice treated with pyroglutamated apelin-13 or saline for 2 (A) and 4 wk (B). A matched group of wild-type mice was also monitored during the experiment; n = 5–7 animals/group. Results are expressed as means ± SE within each group. C: representative Western blots of soleus muscle lysates from wild-type and APKO mice at baseline and with insulin, probing for p-Akt. Normalization of band intensity to native Akt is depicted at bottom. *P < 0.05 vs. baseline, †P < 0.05 vs. insulin-treated saline mice; n = 3–4 animals/group. For all experiments, results are expressed as means ± SE within each group.
Table 3.
Phenotypic characteristics of APKO mice treated with a 4-wk infusion of apelin or saline
| Apelin-Treated APKO Mice | Saline-Treated APKO Mice | |
|---|---|---|
| BW baseline, g | 27.0±0.86 | 25.9±1.1 |
| BW study end, g | 27.8±1.2 | 28.6±0.87 |
| Insulin, pg/ml | 0.60±0.073* | 0.73±0.027 |
| Adiponectin, μg/ml | 67±0.94* | 61.6±2.1 |
| Fasting glucose, mg/dl | 46±4.4 | 62±11 |
Phenotypic characteristics of APKO mice treated with a 4-wk infusion of apelin or saline.
P < 0.05 vs. saline-treated mice.
Apelin ameliorates insulin resistance in db/db mice.
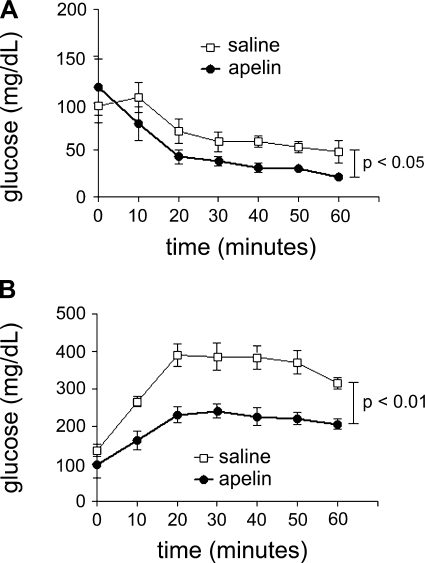
To evaluate whether apelin could modulate established insulin resistance, we administered a 2-wk infusion of pGlu-apelin-13 to 6-wk C57BL/KLS-leprdb/leprdb (db/db) mice. At the end of the infusion, apelin-treated db/db mice had improved insulin sensitivity, as determined by ITT and GTT (Fig. 3 and Table 4). Consistent with their insulin-resistant phenotype, both groups required a significantly greater dose of insulin (3 U/kg) to achieve a demonstrable hypoglycemic response. Additionally, apelin-treated mice had significantly decreased insulin levels as well as increased adiponectin levels (Table 4).
Fig. 3.
Insulin (A; 3 U/kg) and glucose (B; 2 g/kg) tolerance tests for obese C57BL/KLS-leprdb/leprdb (db/db) mice treated with pyroglutamated apelin-13 or saline for 2 wk. For all experiments, n = 5–7 animals/group. Results are expressed as means ± SE within each group.
Table 4.
Phenotypic characteristics of db/db mice treated with a 2-wk infusion of apelin or saline
| Apelin-Treated db/db Mice | Saline-Treated db/db Mice | |
|---|---|---|
| BW baseline, g | 26.4±0.53 | 26.2±0.48 |
| BW study end, g | 33.2±0.88 | 33.9±0.93 |
| AUC ITT, mg·min−1·dl−1 | 290±3* | 420±46 |
| AUC GTT, mg·min−1·dl−1 | 1,230±79† | 2,020±89 |
| Insulin, pg/ml | 6.0±0.77* | 9.7±1.9 |
| Adiponectin, μg/ml | 63±5.2* | 51±3.8 |
| Fasting glucose, mg/dl | 117±30 | 127±14 |
Values are means ± SE.
P < 0.05 vs. saline-treated mice,
P < 0.01 vs. saline-treated mice; n = 10–13/group.
Apelin increases glucose uptake and activates Akt in C2C12 myotubes.
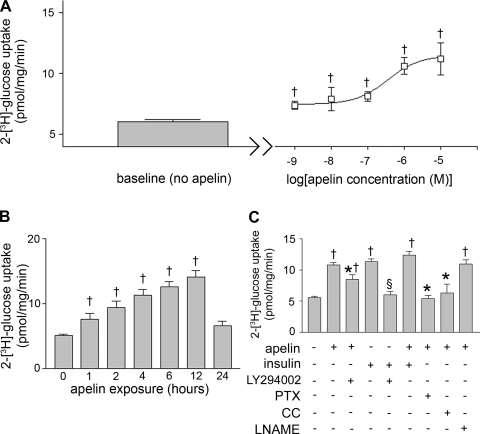
Because skeletal muscle is conventionally believed to underlie the majority of peripheral glucose uptake (34), we next sought to further examine apelin's effects in an in vitro model of skeletal muscle. Accordingly, we explored whether apelin affected glucose uptake in cultured C2C12 skeletal myotubes (Fig. 4). Incubation with pGlu-apelin-13 increased glucose uptake in a dose- and time-dependent manner (EC50 = 352 nM). Also, apelin-induced glucose uptake was significantly reduced by PTX, LY-294002, and compound C. However, apelin did not significantly affect insulin-stimulated glucose uptake. Interestingly, the LY-294002-mediated reduction in apelin-stimulated glucose uptake was only partial, suggesting the possibility of additional PI3K-independent mechanisms for glucose uptake. Notably, l-NAME did not significantly change apelin-induced glucose uptake.
Fig. 4.
A: [2-3H]glucose uptake in cultured C2C12 myotubes at baseline (left) and with gradually increasing doses of pyroglutamated apelin-13 (right). Samples were incubated for 2 h. †P < 0.05 vs. baseline. B: [2-3H]glucose uptake in C2C12 myotubes with increasing exposure times to apelin (1 μM). †P < 0.05 vs. 0 min. C: [2-3H]glucose uptake in C2C12 myotubes treated with apelin (1 μM), insulin (100 nM), LY-294002 (1 mM), pertussis toxin (PTX; 10 ng/ml), compound C (CC; 1 μM), and/or Nω-nitro-l-arginine methyl ester (l-NAME; 1 mM). †P < 0.01 vs. baseline; *P < 0.05 vs. apelin alone; §P < 0.01 vs. insulin. Results are expressed as means ± SE of 9–13 independent experiments.
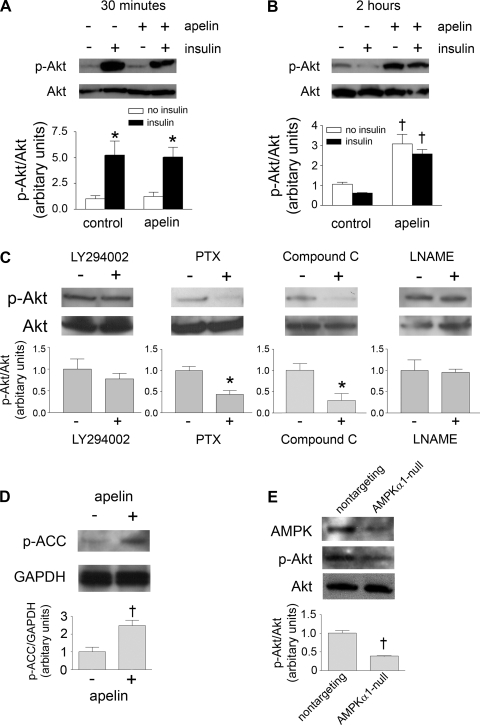
Akt phosphorylation was also evaluated in C2C12 myotubes exposed to apelin and insulin (Fig. 5, A–C). Apelin did not have an appreciable effect on Akt phosphorylation after 30 min. However, in concert with the glucose uptake results, apelin induced a direct increase in Akt phosphorylation independent of insulin signaling after a 2-h exposure. This increase was abrogated by compound C and PTX. However, LY-294002 only partially inhibited apelin-induced Akt phosphorylation, whereas l-NAME did not have any noticeable effect.
Fig. 5.
A and B: p-Akt as determined by Western blotting of C2C12 lysates exposed to apelin and/or insulin for 30 min (A) and 2 h (B). *P < 0.05 vs. control no insulin; †P < 0.01 vs. control no insulin. C: p-Akt as determined by Western blotting of apelin-treated C2C12 lysates exposed to LY-294002, PTX, compound C, and l-NAME. *P < 0.05 vs. apelin alone. D: acetyl-CoA carboxylase phosphorylation (p-ACC) as determined by Western blotting of C2C12 lysates exposed to apelin for 2 h. †P < 0.05 vs. control. E: Akt phosphorylation of lysates derived from C2C12 cells transfected with siRNAs directed against AMPKα1 and a scrambled sequence. †P < 0.01 vs. scramble. For A, B, C, and E, normalization of band intensity to native Akt is depicted below each blot. For D, normalization to GAPDH is depicted below the blot. All results are expressed as means ± SE of 3–5 independent experiments.
To confirm activation of AMPK downstream of apelin, we evaluated ACC phosphorylation in C2C12 myotubes exposed to pGlu-apelin-13 for 2 h (Fig. 5D). As assessed by Western blotting, ACC phosphorylation was increased in the apelin-treated cells, suggesting involvement of AMPK. In addition to assaying AMPK activation, we also inhibited AMPK expression using RNA interference (Fig. 5E). Compared with C2C12 cells treated with a scrambled siRNA sequence, AMPK-negative cells had significantly reduced apelin-induced Akt phosphorylation. These findings indicate that apelin activates Akt in an AMPK-dependent manner.
DISCUSSION
Although initial investigative attention into the apelin/APJ system focused on its neurological and cardiovascular properties, it has recently become apparent that apelin is also involved in the maintenance of insulin sensitivity. Our study supports this assertion and complements previous work involving direct injection of apelin into animals (7, 13). Through this study we have also contributed the following new observations. 1) Mice globally deficient in apelin expression are insulin resistant and have decreased skeletal muscle Akt phosphorylation, 2) long-term delivery of apelin to APKO and db/db mice improves their insulin sensitivity, 3) apelin increases glucose uptake and Akt phosphorylation in differentiated skeletal myotubes, and 4) apelin's effects on these phenomena are at least partially mediated by a pathway involving Gi and AMPK.
Although others (7, 13) have reported the acute effects of apelin on insulin sensitivity in vivo, the use of apelin-null mice allows us to investigate what impact apelin may have in the chronic setting. To complement this strategy, our study is also the first to evaluate the effects of a prolonged infusion of apelin on insulin sensitivity [although Higuchi et al. (13) did administer daily apelin injections over 2 wk, the recent finding that the half-life of apelin-13 in serum is roughly 8 min (16) suggests that this regimen was inadequate to accurately assess apelin's effects on a chronic basis]. We report that the acute insulin-sensitizing effect of apelin persists up to 4 wk without evidence of tolerance or other time-dependent changes in its actions.
At present, it is unknown whether apelin affects insulin sensitivity by secondarily influencing the systemic environment of insulin resistance (e.g., altering hormone secretion, lipolysis, inflammation, etc.) or by a primary effect on individual cells. Recently, Dray et al. (7) demonstrated that apelin increased glucose uptake in isolated soleus muscles. They postulated that apelin's effects were mediated by the serine-threonine kinase AMPK in a pathway also involving eNOS. To support this, they determined that apelin-induced glucose uptake was impaired in both eNOS (−/−) mice and mice deficient in AMPK activity. Although these data are compelling, they do not exclude a possible juxtacrine mechanism involving both endothelial cells and myocytes. Our data suggest that apelin can indeed alter glucose uptake in skeletal myocytes in the absence of endothelial cells. Interestingly, the finding that l-NAME does not inhibit apelin-induced glucose uptake and Akt phosphorylation in C2C12 myotubes (despite endogenous expression of eNOS in these cells) lends further support to this possibility.
Little is known about potential intracellular signaling events connecting apelin with glucose uptake. Apelin has been shown to decrease cAMP levels by adenylate cylase inhibition (10), presumably via Gi. Interestingly, it has long been appreciated that Gi is involved in the regulation of glucose uptake; hepatocyte- and adipocyte-specific deletion of Gi results in insulin resistance (30), whereas hepatocyte-, adipocyte-, and myocyte-specific Gi overexpression results in improved insulin sensitivity (5). These results, coupled with our findings with pertussis toxin (a potent Gi inhibitor), argue that the apelin/APJ system increases glucose uptake and insulin sensitivity through a Gi-mediated mechanism.
The finding that apelin-induced glucose uptake and Akt phosphorylation are sensitive to compound C suggests the involvement of AMPK. That Gi stimulation could result in AMPK activation, however, is arguably counterintuitive to the established literature. It has been appreciated for some time that adrenergic stimulation via Gs, be it by exercise (32) or pharmacological means (29, 43), increases AMPK activity by phosphorylation of the Thr172 residue, a finding that ostensibly conflicts with our observations. However, the studies supporting this pathway have largely been confined to adipocytes and hepatocytes (17); to our knowledge, findings related to myocytes have not yet been reported. Notably, PKA activation mediated by forskolin was recently shown to inhibit AMPK activation in COS cells, mouse embryonic fibroblasts, and INS-1 cells (a β-cell line) by phosphorylation of the α-Ser485/491 residue (14). Thus, it would be of great interest to investigate these respective mechanisms in myocytes and evaluate for potential tissue-specific differences in their significance relative to each other.
Also at issue is the extent to which insulin signaling participates in apelin's regulation of glucose uptake. It is well known that insulin stimulates glucose uptake via PI3K and Akt (6, 18), although it is less certain whether apelin-induced glucose uptake traverses the same pathway. Our observations that 1) apelin deletion results in an abnormal response to an insulin challenge and 2) apelin activates Akt phosphorylation support the involvement of insulin signaling; however, since both glucose uptake and Akt phosphorylation are only partially inhibited by pharmacological PI3K inhibition, the presence of an additional mechanism is suggested. Importantly, because it has been demonstrated to increase both glucose uptake (11, 27) and Akt phosphorylation (23), AMPK represents a prime candidate for this mechanism. The inhibition of these events in our experiments also provides further evidence of AMPK's involvement. That said, the relative contributions of AMPK and PI3K toward apelin-induced glucose uptake and Akt phosphorylation remain undetermined.
Although our data suggest that apelin and insulin do not have a significant additive effect with respect to glucose uptake, Dray et al. (7) reported that apelin and insulin are synergistic in this regard. The apparent discrepancy could be explained by the fact that Dray et al. (7) performed their experiment ex vivo, whereas our work was conducted in vitro. These disparate circumstances suggest that apelin might secondarily influence the extracellular environment (perhaps by altering eNOS activity in endothelial cells) to affect insulin sensitivity in muscle. The fact that l-NAME, an eNOS inhibitor, had no demonstrable effect on apelin-induced glucose uptake and Akt phosphorylation in C2C12 cells would support this possibility. It should also be mentioned that, consistent with Dray et al. (7), the combination of apelin plus insulin trended toward a noticeable, albeit nonsignificant, increase in glucose uptake vs. apelin alone. Although this trend was not observed with Akt phosphorylation, it remains possible, albeit unlikely, that the lack of an apelin-insulin interaction in our data could represent a statistical artifact. Notably, it has been reported that AMPK activation is inhibited by insulin/PI3K signaling in both in vivo and in vitro settings (2, 8, 41, 43), ostensibly arguing against a synergistic effect.
It should be noted that apelin's effects on insulin sensitivity and glucose uptake are not necessarily mediated solely by its putative actions in muscle. It is plausible, even probable, that other tissues contribute toward the insulin resistance phenotype in the APKO mice. For example, APKO mice have decreased adiponectin levels, suggesting the involvement of adipose tissue. Interestingly, both ligand (3, 38, 40) and receptor (40) are expressed by adipocytes, lending additional support to this possibility. Further investigation into apelin's tissue-specific effects on insulin sensitivity and their systemic impact is thus warranted.
In summary, we report that apelin is necessary for the maintenance of systemic insulin sensitivity in vivo. Further in vitro experiments suggest the existence of a primary intracellular Gi and AMPK-dependent pathway that may underlie apelin's effects on glucose uptake and Akt phosphorylation. Our data provide additional insight into the influence of apelin on insulin-glucose homeostasis and raise the enthusiasm for exploiting the apelin/APJ pathway for therapeutic benefit in states of insulin resistance.
GRANTS
This study was funded in part by National Institutes of Health Grants 5R01-DK-071333 (P. S. Tsao), 5-R01-HL-077676 (T. Quertermous), 5-R01-DK-071309 (G. M. Reaven), and 1-K08-DK-080463 (P. Yue).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Ashley EA, Powers J, Chen M, Kundu R, Finsterbach T, Caffarelli A, Deng A, Eichhorn J, Mahajan R, Agrawal R, Greve J, Robbins R, Patterson AJ, Bernstein D, Quertermous T. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res 65: 73–82, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett 505: 348–352, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpene C, Audigier Y, Saulnier-Blache JS, Valet P. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146: 1764–1771, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Castan-Laurell I, Vitkova M, Daviaud D, Dray C, Kovacikova M, Kovacova Z, Hejnova J, Stich V, Valet P. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol 158: 905–910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JF, Guo JH, Moxham CM, Wang HY, Malbon CC. Conditional, tissue-specific expression of Q205L G alpha i2 in vivo mimics insulin action. J Mol Med 75: 283–289, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buleon M, Cani PD, Attane C, Guigne C, Carpene C, Burcelin R, Castan-Laurell I, Valet P. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab 8: 437–445, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Gamble J, Lopaschuk GD. Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism 46: 1270–1274, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Glassford AJ, Yue P, Sheikh AY, Chun HJ, Zarafshar S, Chan DA, Reaven GM, Quertermous T, Tsao PS. HIF-1 regulates hypoxia- and insulin-induced expression of apelin in adipocytes. Am J Physiol Endocrinol Metab 293: E1590–E1596, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta 1452: 25–35, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47: 1369–1373, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Heinonen MV, Purhonen AK, Miettinen P, Paakkonen M, Pirinen E, Alhava E, Akerman K, Herzig KH. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept 130: 7–13, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Higuchi K, Masaki T, Gotoh K, Chiba S, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology 148: 2690–2697, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281: 36662–36672, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Ingelsson E, Arnlov J, Lind L, Sundstrom J. Metabolic syndrome and risk for heart failure in middle-aged men. Heart 92: 1409–1413, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japp AG, Cruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, Sharma S, Neilson I, Webb DJ, Megson IL, Flapan AD, Newby DE. Vascular effects of apelin in vivo in man. J Am Coll Cardiol 52: 908–913, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kimball SR, Siegfried BA, Jefferson LS. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J Biol Chem 279: 54103–54109, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem 271: 31372–31378, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288: 2709–2716, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem 74: 34–41, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Lee YM, Lee JO, Jung JH, Kim JH, Park SH, Park JM, Kim EK, Suh PG, Kim HS. Retinoic acid leads to cytoskeletal rearrangement through AMPK-Rac1 and stimulates glucose uptake through AMPK-p38 MAPK in skeletal muscle cells. J Biol Chem 283: 33969–33974, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lempiainen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation 100: 123–128, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem 282: 20351–20364, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Li L, Yang G, Li Q, Tang Y, Yang M, Yang H, Li K. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes 114: 544–548, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal 17: 415–426, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65–77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J 18: 1909–1911, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab 273: E1107–E1112, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Messier C, Whately K, Liang J, Du L, Puissant D. The effects of a high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in C57BL/6 mice. Behav Brain Res 178: 139–145, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Moule SK, Denton RM. The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett 439: 287–290, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Moxham CM, Malbon CC. Insulin action impaired by deficiency of the G-protein subunit G ialpha2. Nature 379: 840–844, 1996 [DOI] [PubMed] [Google Scholar]
- 31.O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136: 355–360, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem 277: 32571–32577, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Sheikh AY, Chun HJ, Glassford AJ, Kundu RK, Kutschka I, Ardigo D, Hendry SL, Wagner RA, Chen MM, Ali ZA, Yue P, Huynh DT, Connolly AJ, Pelletier MP, Tsao PS, Robbins RC, Quertermous T. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol 294: H88–H98, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322: 223–228, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Sörhede Winzell M, Magnusson C, Ahrén B. The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept 131: 12–17, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Taketomi S, Ikeda H, Ishikawa E, Iwatsuka H. Determination of overall insulin sensitivity in diabetic mice, KK. Horm Metab Res 14: 14–18, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251: 471–476, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 99: 87–92, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, Inukai K, Asano T, Kaburagi Y, Ueki K, Nakajima H, Hanafusa T, Matsuzawa Y, Sekihara H, Yin Y, Barrett JC, Oda H, Ishikawa T, Akanuma Y, Komuro I, Suzuki M, Yamamura K, Kodama T, Suzuki H, Koyasu S, Aizawa S, Tobe K, Fukui Y, Yazaki Y, Kadowaki T. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat Genet 21: 230–235, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept 132: 27–32, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem 267: 2864–2867, 1992 [PubMed] [Google Scholar]
- 42.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270: 725–727, 1977 [DOI] [PubMed] [Google Scholar]
- 43.Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J Biol Chem 278: 43074–43080, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Yue P, Arai T, Terashima M, Sheikh AY, Cao F, Charo DN, Hoyt G, Robbins RC, Ashley EA, Wu J, Yang PC, Tsao PS. Magnetic resonance imaging of progressive cardiomyopathic changes in the db/db mouse. Am J Physiol Heart Circ Physiol 292: H2106–H2118, 2007 [DOI] [PubMed] [Google Scholar]