Abstract
Insulin resistance in skeletal muscle in obesity and T2DM is associated with reduced muscle oxidative capacity, reduced expression in nuclear genes responsible for oxidative metabolism, and reduced activity of mitochondrial electron transport chain. The presented study was undertaken to analyze mitochondrial content and mitochondrial enzyme profile in skeletal muscle of sedentary lean individuals and to compare that with our previous data on obese or obese T2DM group. Frozen skeletal muscle biopsies obtained from lean volunteers were used to estimate cardiolipin content, mtDNA (markers of mitochondrial mass), NADH oxidase activity of mitochondrial electron transport chain (ETC), and activity of citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD), key enzymes of TCA cycle and β-oxidation pathway, respectively. Frozen biopsies collected from obese or T2DM individuals in our previous studies were used to estimate activity of β-HAD. The obtained data were complemented by data from our previous studies and statistically analyzed to compare mitochondrial content and mitochondrial enzyme profile in lean, obese, or T2DM cohort. The total activity of NADH oxidase was reduced significantly in obese or T2DM subjects. The cardiolipin content for lean or obese group was similar, and although for T2DM group cardiolipin showed a tendency to decline, it was statistically insignificant. The total activity of citrate synthase for lean and T2DM group was similar; however, it was increased significantly in the obese group. Activity of β-HAD and mtDNA content was similar for all three groups. We conclude that the total activity of NADH oxidase in biopsy for lean group is significantly higher than corresponding activity for obese or T2DM cohort. The specific activity of NADH oxidase (per mg cardiolipin) and NADH oxidase/citrate synthase and NADH oxidase/β-HAD ratios are reduced two- to threefold in both T2DM and obesity.
Keywords: insulin resistance, β-oxidation, cardiolipin, reduced nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide ratio, trichloroacetic acid cycle
type 2 diabetes mellitus (T2DM) is characterized by insulin resistance in skeletal muscle that can be a result of combining a genetic predisposition with obesity and sedentary lifestyle (12, 34, 44). Insulin resistance in skeletal muscle in obesity or T2DM is associated with reduced muscle oxidative capacity (53, 60) and with reduced expression in a cluster of nuclear genes responsible for oxidative metabolism [peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and genes activated by PGC-1α] (17, 30, 33, 38). In our previous study, we found a significant reduction in the total activity of mitochondrial electron transport in the skeletal muscle in obese and T2DM individuals compared with lean and healthy controls (24). The content of mitochondria in human skeletal muscle depends strictly on the level of physical activity; it increases with exercise (14, 19–21) and decreases during the detraining period (8). The deficiency in the mitochondrial electron transport in skeletal muscle in T2DM and obesity could be a result of deficiency in mitochondria mass, deficiency in mitochondrial function, or both. The current study was undertaken to address these questions. Vastus lateralis needle biopsies were obtained from sedentary lean individuals before and after completion of a program of moderate-intensity aerobic exercise that could benefit study participants. Frozen tissue biopsies were used to estimate markers of mitochondrial mass [cardiolipin and mitochondrial DNA (mtDNA)], activity of mitochondrial electron transport chain (ETC), and activity of citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD), which are key enzymes of the TCA cycle and β-oxidation pathway, respectively. Frozen biopsies collected from obese or T2DM individuals in our previous studies (58, 59) were used to estimate activity of β-HAD. The obtained data were complemented by data from our previous studies and statistically analyzed to compare mitochondrial content and mitochondrial enzyme profile in the lean, obese, or T2DM cohort.
Several advanced techniques previously developed in our laboratory for analysis of mitochondrial function and content in skeletal muscle were applied in this study. We developed a unique, nondestructive procedure for analysis of activity of mitochondrial ETC in human skeletal muscle. This procedure is based on the use of the channel-forming peptide alamethicin that provides unrestricted access of NADH inside of mitochondria without any effects on the integrity of mitochondrial ETC (24, 48). Standard technique provides only ∼50% extraction of mitochondria from skeletal muscle (43). Our previously developed procedure allows the assessment of mitochondrial function in skeletal muscle without necessity of mitochondria isolation (48). We also developed a technique that allows us to accurately estimate content of mitochondria in the tissue. This technique is based on the quantifying the tissue cardiolipin, a specific lipid marker of inner mitochondrial membrane (47).
The combined data (from the current and our previous studies) show for the first time that the content of mitochondrial mass markers (cardiolipin, mtDNA) and activity of citrate synthase or β-HAD in middle-aged sedentary lean subjects is similar to obese or T2DM individuals. However, the average activity of ETC for lean group is significantly higher than corresponding activity for the obese or T2DM cohort. As a result, the NADH oxidase/cardiolipin (specific activity of NADH oxidase), NADH oxidase/citrate synthase, and NADH oxidase/β-HAD ratios are reduced significantly (2- to 3-fold) in both T2DM and obesity compared with sedentary lean subjects. We hypothesize that this specific mitochondrial profile (normal or excessive production of reducing equivalents by TCA cycle and β-oxidation and the deficiency in oxidative capacity of ETC) plus sedentary lifestyle can affect metabolite profile in skeletal muscle in T2DM or obesity by shifting it to increased NADH/NAD+ ratio and to accumulation of toxic byproducts of incomplete oxidation of glucose and lipids, and that, in turn, as was recently suggested, can lead to the development of skeletal muscle insulin resistance (37).
MATERIALS AND METHODS
Research volunteers.
The protocol was approved by the University of Pittsburgh Institutional Review Board. All participants had a screening medical history, physical examination, and screening laboratory tests. The inclusion criteria for all groups were described elsewhere (58, 59). The obese nondiabetic and obese type 2 diabetic groups were matched for age and body mass index. Clinical characteristics of the research volunteers are presented in Table 1.
Table 1.
Clinical characteristics of the research volunteers
| Lean (n = 9) |
Obese (n = 9) |
T2DM (n = 10) |
||||
|---|---|---|---|---|---|---|
| Pre-Int | Post-Int | Pre-Int | Post-Int | Pre-Int | Post-Int | |
| Age, yr | 47.1±2.2 | 42.4±2.7 | 44±3 | |||
| BMI, kg/m2 | 24.0±0.7 | 23.6±0.7 | 34.8±1.2 | 31.9±1.2 | 34.0±0.7 | 31.9±0.7*(P<0.001) |
| Weight, kg | 65.4±2.9 | 64.5±2.8 | 94.8±4.4 | 86.3±4.6*(P<0.01) | 99.5±3.8 | 92.4±3.4*(P<0.001) |
| Fat mass, kg | 20.3±1.3 | 18.7±0.7 | 40.2±1.7 | 33.1±1.9*(P<0.01) | 36.6±2.1 | 31.1±2.1*(P<0.001) |
| Fat-free mass, kg | 44.3±3.2 | 44.4±3.3 | 52.6±3.8 | 51.3±3.5 | 60.0±4.1 | 59.1±3.7 |
| V̇o2max, ml·kg FFM−1·min−1 | 52.7±1.7 | 53.2±2.1 | 47.6±2.5 | 52.4±2.2*(P<0.05) | 39.0±6.9 | 48.7±7.2*(P<0.01) |
| GINF, mg·kg FFM−1·min−1 | 12.2±2.8 | 13.8±1.1 | 5.7±0.8 | 8.7±1.1*(P<0.01) | 4.1±0.6 | 6.3±0.9 |
Values are means ± SE. T2DM, type 2 diabetes mellitus; Pre-Int, before intervention; Post-Int, after intervention; BMI, body mass index; V̇o2max, maximal aerobic capacity; FFM, fat-free mass; GINF, glucose infusion rate during euglycemic clamp.
Statistically significant.
Intervention.
The intervention lasted 16–20 wk after initial baseline assessments and was monitored by dietitians and exercise physiologists. Participants were asked to participate in four to six exercise sessions weekly, with at least one supervised session weekly. The physical activity intervention was moderate-intensity exercise at 60–70% of maximal heart rate for 30 min for the first 4 wk, increased to 40 min for the next 4 wk, and increasing intensity but not duration for the final 8 wk. Mostly, the exercise included using a stationary cycle, treadmill, or walking (58, 59). In the present study, for analysis of β-HAD, we also used along with frozen biopsies from lean individuals the frozen biopsies obtained from obese or T2DM participants in our previous studies (58, 59). In our previous study, the nutritional intervention was separate or concomitant to exercise and aimed at a target weight loss of ≥5% of baseline weight via a reduction in calorie intake of 500–1,000 kcal/day (58).
Metabolic assessments.
Euglycemic clamps were used to measure insulin sensitivity (59). Maximal aerobic capacity (V̇o2 max) was measured on a treadmill with a modified Bruce protocol (58).
Analysis of mitochondrial content and function.
Muscle biopsy samples of vastus lateralis muscle (15–25 mg wet wt) obtained by Bergstrom needle were analyzed on mtDNA (31) and cardiolipin (47) content, citrate synthase (48), β-HAD, and ETC NADH oxidase activities. Frozen samples (particulate and soluble homogenate fractions) prepared from biopsies collected from obese or T2DM individuals in our previous studies and stored in 25% glycerol at −80°C were used for analyses of activity of β-HAD. Activity of β-HAD was simultaneously estimated in the present study for the lean, obese, or T2DM group. The obtained data were complemented by data from our previous studies and statistically analyzed to compare mitochondrial content and mitochondrial enzyme profile in the lean, obese, or T2DM cohort. Activity of β-HAD was measured at 30°C in the reverse reaction by standard method adapted for higher sensitivity to our HPLC technique (48). Activity of rotenone-sensitive NADH:O2 oxidoreductase in total particulate fraction prepared from muscle homogenate was measured in the presence of alamethicin, as described previously (48). Creatine kinase activity was used as a marker of muscle fiber content in biopsy (48).
Reagents and equipment.
Tetraoleoyl cardiolipin (internal standard) was purchased from Avanti Polar Lipids (Alabaster, AL). HPLC grade chloroform, stabilized by 0.7% ethanol, was obtained from Fisher Scientific (Pittsburgh, PA). Other HPLC grade solvents and reagents were purchased from Sigma Chemical (St. Louis, MO). 1-Pyrenyldiazomethane was obtained from Molecular Probes (Eugene, OR). A Shimadzu high-performance liquid chromatograph (model LC-10AT vp) equipped with an autosampler (model SIL-10AD), a tray cooler, and a Shimadzu fluorescence detector (model RF-10Axl) was used for these studies. The analog signal of the detector was processed and stored in digital form with Shimadzu Class-VP software (Shimadzu Scientific Instruments, Columbia, MD).
Statistical analysis.
Data are presented as means ± SE unless otherwise indicated. To address the effects of intervention on insulin sensitivity and skeletal muscle parameters, a paired t-test was applied to all data. ANOVA was used to compare groups. Statistical significance was assumed a priori at P < 0.05.
RESULTS
Mitochondrial content and mitochondrial enzyme activities in the basal conditions.
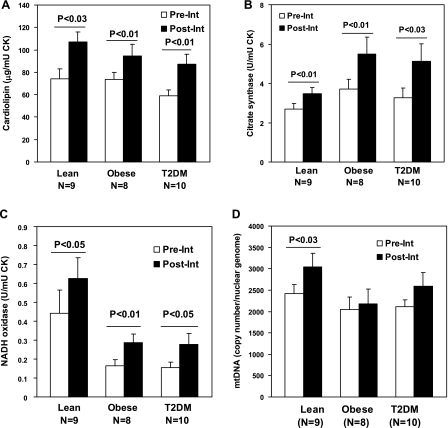
The biopsies were taken from sedentary nondiabetic lean subjects (current study) or from sedentary type 2 diabetic or nondiabetic obese subjects (58, 59). The clinical characteristics of the research volunteers are presented in Table 1. The content of mitochondria in the biopsy was assessed from analysis of mtDNA (31) and cardiolipin (47). Table 2 presents data from the current study on the lean subjects and data from previous studies on obese and T2DM subjects (58, 59). The statistical analysis of these data shows that there is no significant difference in the cardiolipin or mtDNA content between obese and healthy sedentary lean groups in the basal state before the intervention (Table 2). The T2DM group shows a tendency to reduced level of cardiolipin; however, it is not statistically significant. In conclusion, the presented data show that there is no significant decrement in the mitochondrial content in skeletal muscle in T2DM or obesity.
Table 2.
Markers of mitochondrial mass and total activity electron transport chain in vastus lateralis biopsy
| Basal State | Lean (n = 9) | Obese (n = 15) | T2DM (n = 10) |
|---|---|---|---|
| Total tissue CK, U/g wet wt | 5,655±388 | 5,808±315 | 6,198±316 |
| Cardiolipin, μg/mU CK | 78.2±9.5 | 76.0±6.4 | 58.6±5.5 (P=0.079) |
| CS, U/mU CK | 2.70±0.28 | 3.7±0.5*(P<0.02) | 3.28±0.49 |
| mtDNA (Rc) | 2,417±218 | 2,271±286 | 2,114±156 |
| Rotenone-sensitive NADH oxidase, U/mU CK | 0.44±0.12 | 0.18±0.02*(P<0.04) | 0.16±0.03*(P<0.03) |
Values are means ± SE. T2DM, type 2 diabetes mellitus; CK, creatine kinase; mtDNA, mitochondrial DNA; Rc, relative copy number of mtDNA per diploid nuclear genome; CS, citrate synthase. P values are for T2DM or obese vs. healthy lean sedentary individuals.
Statistically significant.
Comparative data analysis on activity of selected mitochondrial enzymes is presented in Table 2 and Figs. 1 and 2. A comparison of the lean, obese, or T2DM group shows that the total activity of citrate synthase is significantly higher in the obese vs. lean group (Table 2). Total biopsy activity of β-HAD that was simultaneously estimated in the present study for all three groups shows no differences between the groups (Fig. 2C). However, the total activity of mitochondrial ETC (rotenone-sensitive NADH oxidase) expressed per the same amount of tissue creatine kinase (1,000 U activity) is more than twofold higher in the biopsy obtained from lean sedentary subjects than in biopsies obtained from obese or T2DM subjects (Table 2). There is no statistically significant difference in NADH oxidase between obese and T2DM groups.
Fig. 1.
The markers of mitochondrial mass and enzyme activity in skeletal muscle biopsy from type 2 diabetes mellitus (T2DM), obese, or healthy lean sedentary individuals. Data represent means ± SE. The total content of cardiolipin (A), citrate synthase enzymatic activity (B), and rotenone-sensitive NADH oxidase enzymatic activity (C) in biopsy were normalized to the creatine kinase activity. D: mitochondrial DNA (mtDNA) presented as a relative copy number of mtDNA/diploid nuclear genome. Biopsies were analyzed before (Pre-Int) and after intervention (Post-Int). P values are for mitochondrial parameters Pre-Int vs. after Post-Int. CK, creatine kinase.
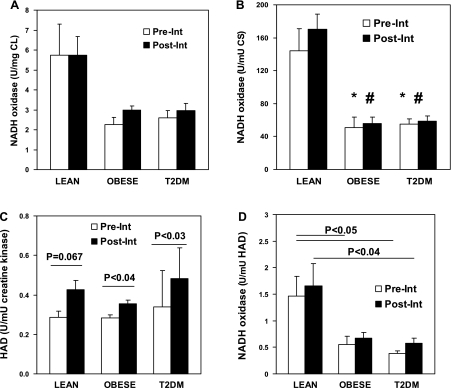
Fig. 2.
The ratios between activity of mitochondrial electron transport chain and cardiolipin, β-oxidation, and TCA cycle in skeletal muscle biopsy from T2DM, obese, or healthy lean sedentary individuals Pre-Int and Post-Int. Activity of β-hydroxyacyl-CoA dehydrogenase (HAD) in skeletal muscle biopsy from T2DM, obese, or healthy lean sedentary individuals Pre-Int and Post-Int (C). Total NADH oxidase activity in biopsy was normalized to total cardiolipin (CL) content (A), citrate synthase (CS; B), or β-HAD activity (D). Data represent means ± SE. NADH oxidase/CL, NADH oxidase/CS, or NADH oxidase/HAD for T2DM vs. lean and for obese vs. lean are statistically significant before and after interventions. *P < 0.05, lean vs. obese or T2DM (baseline); #P < 0.05, lean vs. obese or T2DM (Post-Int).
The ratio between cardiolipin, TCA cycle, β-oxidation, and ETC.
The ratios between activity of ETC and cardiolipin, citrate synthase, or β-HAD are presented in Fig. 2, A, B, and D. As can be seen in Fig. 2, the ratios between activity of mitochondrial NADH oxidase and cardiolipin (which can be defined as specific activity of NADH oxidase because cardiolipin is a marker of mitochondrial mass; Fig. 2A), citrate synthase (Fig. 2B), or β-HAD (Fig. 2D) are significantly higher for lean group than for obese or T2DM groups. There is approximately threefold difference for NADH oxidase/citrate synthase or NADH oxidase/β-HAD ratio in lean vs. obese or T2DM groups (Fig. 2, B and D).
In summary, the presented data show that in obesity or T2DM there is a significant deficiency in the activity of mitochondrial ETC, and also there is a significant disbalance between activity of ETC and activity of citrate synthase or β-HAD.
Response of skeletal muscle mitochondria to interventions.
The lean participants of this study completed a 16- to 20-wk exercise program, as described in materials and methods. This transition from sedentary lifestyle to regular moderate physical activity was sufficient to significantly increase mitochondrial content in vastus lateralis muscle. As can be seen in the Fig. 1, exercise intervention significantly increases cardiolipin and mitochondrial DNA content. Nevertheless, after completion of the exercise program, the changes in V̇o2 max, which is an index of physical fitness, were nonsignificant for lean, previously sedentary volunteers.
The lean subjects that participated in the exercise program showed significant increase in total activity of ETC (NADH oxidase), citrate synthase, and β-HAD calculated per 1,000 U of tissue creatine kinase (Figs. 1 and 2). However, the specific activity of ETC, citrate synthase, or β-HAD calculated relative to cardiolipin as a replacement index of mitochondrial mass remains the same after completion of the exercise program by lean participants (NADH oxidase; Fig. 2A). Accordingly, the completion of the exercise program by lean participants had no effect on the NADH oxidase/citrate synthase or NADH oxidase/β-HAD ratios that were still significantly (2- to 3-fold) higher than corresponding ratios for both T2DM and obese participants (Fig. 2, B and D).
In our previous study, T2DM or obese volunteers completed two programs for lifestyle modification; one was based on a caloric restriction, and another one, in addition to caloric restriction, included the exercise program that was similar to the exercise program that was applied to lean subjects in the current study. Both interventions were aimed to reach a weight loss of ≥5–10% of baseline weight (58, 59). It had been found that only the program that included the exercise induced the increase in cardiolipin content, citrate synthase, and mitochondria NADH oxidase activities in the biopsies obtained from obese or T2DM individuals (58, 59).
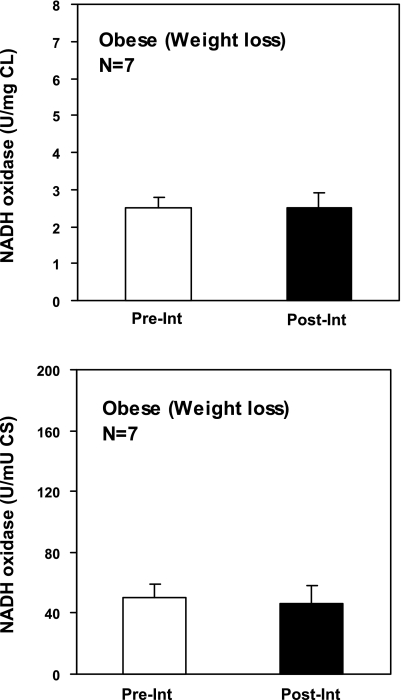
In the current study, we used particulate and soluble fractions prepared from the biopsies obtained from the obese or T2DM individuals who participated in the study that included combined intervention (diet plus exercise) to additionally measure the activity of β-HAD. As can be seen in Fig. 2C, according to the previous data, the intervention program that included exercise significantly increased activity of β-HAD in vastus lateralis muscle of obese or T2DM individuals. We used these data and the previously published data on NADH oxidase, cardiolipin, and citrate synthase to calculate the ratios between NADH oxidase/citrate synthase or NADH oxidase/β-HAD and to compare it with corresponding ratios for lean volunteers. As can be seen in Fig. 2, the caloric restriction plus exercise intervention does not improve the balance between activity of NADH oxidase and citrate synthase or β-HAD. Additional calculations performed for estimation of specific activity of NADH oxidase (NADH oxidase/cardiolipin) in the caloric restriction study also show that nether caloric restriction alone nor caloric restriction plus exercise has an ability to increase the reduced specific activity of mitochondrial NADH oxidase in vastus lateralis muscle of obese or T2DM individuals (Fig. 2 and 3). The calculation of NADH oxidase/citrate synthase ratio for the obese cohort by using data from a previous study (58, 59) and comparing it with the corresponding ratio in the current study for lean subjects shows that the caloric restriction alone also does not improve the balance between NADH oxidase and citrate synthase in vastus lateralis muscle of obese individuals (Fig. 3).
Fig. 3.
The ratios between CL, TCA cycle, and activity of mitochondrial electron transport chain in skeletal muscle biopsy from obese individuals before and after completion of weight loss program only. The rotenone-sensitive NADH oxidase enzymatic activity was measured Pre-Int and Post-Int. Total NADH oxidase activity in biopsy wass normalized to total CL content or to total CS activity. Data represent means ± SE.
DISCUSSION
The primary goal of the presented study was to compare mitochondrial content and activity of selected mitochondrial enzymes in skeletal muscle of biopsies obtained from lean, obese, or obese T2DM individuals in the basal state. Frozen tissue samples obtained by needle biopsy from research volunteers were homogenized, and the homogenate was separated by high-speed centrifuging to collect the total particulate and soluble fractions. Total particulate fraction that contains >95% of tissue mitochondria was used for analysis of cardiolipin and activity of mitochondrial ETC (rotenone-sensitive NADH:O2 oxidoreductase). Both particulate and soluble fractions were used for analysis of citrate synthase and β-HAD because of partial release of these soluble mitochondrial matrix enzymes from mitochondria due to tissue freezing.
Mitochondrial content in human vastus lateralis muscle in obesity and T2DM.
There is little choice for nonenzymatic markers to quantitate mitochondrial content in tissue homogenates or total particulate fractions that are complex mixtures of proteins and subcellular organelles. Mitochondrial DNA or cardiolipin can be considered as suitable markers. Previously, we found decreased mtDNA in obesity and diabetes (46). Mitochondrial DNA is increased significantly in human skeletal muscle during adaptation to high-intensity physical activity (41). Also, in nonobese elderly individuals, a moderate-intensity physical activity intervention induced a significant increase in muscle mtDNA content along with the increase in activity of citrate synthase and ETC (27). As can be seen in Table 2, the content of mtDNA increases significantly in vastus lateralis of lean sedentary study participants after completion of an exercise program. However, it should be taken into consideration that mitochondrial nucleoid contains a variable number of DNA copes (∼2–10 copies) that makes mtDNA content only a semiquantitive indicator for mitochondrial mass. In fact, Menshikova et al. (29) observed that the adaptation to moderate-intensity exercise in obesity occurs without an increase in muscle mtDNA content. Additionally, the mtDNA content in skeletal muscle does not always reflect the state of mitochondrial biogenesis. Combined weight loss plus exercise intervention in obese or T2DM cohort significantly increases content of cardiolipin, activity of NADH oxidase, citrate synthase (58, 59), or β-HAD (Fig. 1 and 2). Taken together, it is a clear indication of the activation of mitochondrial biogenesis induced by intervention. However, the same intervention is unable to induce the increase in mtDNA in these obese or T2DM study participants (Fig. 2) (58, 59). A more reliable marker for mitochondrial mass is mitochondrial phospholipid cardiolipin. Cardiolipin is a phospholipid that is specific to the inner mitochondrial membrane (18). This phospholipid is a basic structural component of mitochondria that accounts for ∼15% of total lipids in inner mitochondrial membrane (10). Cardiolipin is a sensitive marker of mitochondrial content and mitochondrial biogenesis. Cardiolipin content in vastus lateralis obtained from the highly trained lean volunteers is ∼1.0 mg/g wet wt, which is more than twofold higher than the cardiolipin content in biopsies obtained from sedentary volunteers in the present study (28). Cardiolipin content in vastus lateralis biopsy is increased after any intervention that includes exercise (28, 29, 58). Conversely, cardiolipin decreases along enzymes after muscle inactivity induced by denervation in animals (62). It is increased independently on concomitant caloric restriction, and this increase is proportional to the increase in mitochondrial activity of ubiquinol oxidase (respiratory complexes III plus IV) (28). Inner membrane in skeletal muscle mitochondria, which forms densely packed mitochondrial cristae, contributes >80% of the total mitochondrial mass (1, 16). The surface area of inner mitochondrial membrane depends in part on the phospholipid content, and we suggest that the content of cardiolipin as a basic structural component of inner mitochondrial membrane reflects the surface area and accordingly the mass of inner mitochondrial membrane. Our method for analysis of cardiolipin provides information not only on the total content of cardiolipin in biopsy but also on the content of cardiolipin molecular species (47). The analysis of distribution of molecular species of cardiolipin allows for the monitoring of any disturbances in the cardiolipin biosynthesis (52). We use cardiolipin as a replacement for mitochondrial mass to estimate mitochondrial content in biopsy and to calculate specific activity (activity per unit of mitochondrial mass) of mitochondrial enzymes.
The comparison of the data from the present study on the lean sedentary volunteers and data from our two previous studies on obese and T2DM participants (58, 59) shows that the distribution of molecular forms of cardiolipin for lean sedentary, obese, or T2DM was the same (data not shown). The predominant molecular form is tetralinoleoyl cardiolipin, which accounted for ∼80% of cardiolipin in human skeletal muscle (47, 52). In the basal conditions, there is no significant deficiency in the mitochondrial mass in insulin-resistant obese subjects assessed by analysis of cardiolipin (Table 1 and Fig. 1). This is in accordance with previous findings that showed the preservation of a high level of citrate synthase in skeletal muscle in obesity but not in T2DM (24). The T2DM group showed a tendency to reduce the level of cardiolipin; however, this trend does not reach the statistical significance for the present number of study participants, although in our first study on mitochondria in obesity and T2DM, the trend in the slight decrease in mitochondria mass for participants from the T2DM group (estimated from citrate synthase activity) had reached statistical significance (24). The absence of effects of obesity/insulin resistance on mitochondrial content in biopsy suggests that the reduction in mitochondrial mass in skeletal muscle and changes in mitochondrial morphology that linked to T2DM (32, 42) can be secondary to this condition.
Mitochondrial enzyme profile in obesity and T2DM.
Measurements of activity of mitochondrial enzymes require eliminating a permeability barrier for mitochondrial substrates created by inner mitochondrial membrane. This especially concerns activity of NADH oxidase because of the lack of NADH transporter in mitochondria to cross inner membrane. The standard approach to eliminating the permeability barrier in mitochondria is based on exposing mitochondria to detergents. It effectively allows measurements of activity of separate respiratory complexes and matrix enzymes (5). However, it prevents analysis of activity of whole ETC because detergents disturb the interaction between respiratory complexes in inner mitochondrial membrane. Previously, we developed a detergent-free assay for estimation of activity of whole mitochondrial ETC directly from analysis of oxidation of NADH (24). The assay includes a channel-forming peptide alamethicin that provides unrestricted access of NADH inside of mitochondria without any effects on the integrity of mitochondrial ETC. This assay provides information on the real oxidative capacity of mitochondrial ETC contrary to respiration technique (6) that measures maximal oxygen consumption in the state of mitochondrial ATP synthesis (state 3) or in the presence of uncouplers and TCA cycle substrates. The respiration or ATP synthesis in mitochondria is controlled mostly by specific transporters that deliver ADP, phosphate, and other substrates into mitochondria (57, 63, 64). The flux control of respiration by ETC is relatively weak, and human skeletal mitochondria have approximately threefold reserves in the capacity of ETC over the respiratory capacity (25). Maximal respiration by human skeletal muscle mitochondria in the presence of alamethicin and NADH is three- to fourfold higher than respiration with pyruvate in state 3 (13). This difference is a result of direct substrate delivery (NADH) to respiratory chain in the presence of alamethicin.
The alamethicin-based assay was used for measurements of activity of rotenone-sensitive NADH:O2 oxidoreductase that represent activity of whole mitochondrial ETC in vastus lateralis biopsy. As can be seen in Table 2, in the basal condition, the activity of ETC in both T2DM and obese groups is reduced significantly relative to the lean sedentary group. In our previous study (24), we discovered similar reduction in total NADH oxidase activity in skeletal muscle biopsies obtained from T2DM participants; however, the decrease in activity of NADH oxidase for the obese group was not as significant as in the current study. We suggest that we have more homogeneous selection of the participants for the obese insulin-resistant group and for the lean sedentary group in the current study. Also, in our first study, the trend in the slight decrease in mitochondrial mass for participants from the T2DM group (estimated from citrate synthase activity) had reached statistical significance (24).
A calculation of specific NADH oxidase activity (per mg of tissue cardiolipin) shows that skeletal muscle mitochondria in obesity or T2DM have significantly reduced activity of ETC (Fig. 1). At the same time, in these conditions there is no deficiency in activity of citrate synthase or β-HAD (Table 2 and Figs. 1 and 2). As a result, there is significant disbalance (3-fold) between activity of ETC and citrate synthase or β-HAD, the key enzymes of TCA cycle and β-oxidation, respectively. This disbalance cannot be explained by insulin resistance itself. As can be seen in Fig. 2, the abnormally low ratio between activity of ETC and activity of citrate synthase or β-HAD retains even after completion the exercise plus caloric restriction program and significant improvement in insulin sensitivity. We also calculated the mitochondrial ETC/citrate synthase activity ratio from the data obtained in our previous study on the obese group that completed only the weight loss program (without exercise) (58). This intervention significantly improved insulin sensitivity. As follows from data presented in Figs. 2B and 3, the significant difference between lean and obese in the mitochondrial NADH oxidase/citrate synthase ratio is retained after caloric restriction (weight loss program) despite an improvement in insulin sensitivity. This disbalance also cannot be explained exclusively by sedentary lifestyle. As can be seen in Fig. 1, both sedentary lean and obese subjects have the same amount of mitochondria (cardiolipin) in vastus lateralis, and both groups respond to exercise or exercise plus caloric restriction by increasing the mitochondrial mass. The mitochondrial mass in skeletal muscle is a function of physical activity (14, 17, 19–21). The same basal level of cardiolipin in skeletal muscle of lean and obese subjects is an independent indicator that these two groups of study volunteers have similar levels of everyday physical activity before the interventions. Nevertheless, the obese group shows significant reduction in activity of NADH oxidase and significant disbalance between ETC and TCA cycle or β-oxidation pathway.
The mitochondrial enzyme profile specific for obesity or T2DM can be reproduced in an animal model. Our recent data show that high-fat diet induces similar changes in rat liver mitochondria (increase in citrate synthase activity and β-HAD and decrease in activity of ETC) (26). We can hypothesize that obesity that is associated with increased mass of body fat and increased fat production in the liver has long-term consequences for the mitochondrial function and can produce a specific imprinting in skeletal muscle metabolism. However, it is not clear what is an immediate cause of dysfunction of mitochondrial ETC in obesity. It can be the result of excessive delivery of fatty acid. A local or generalized low-grade inflammation that is currently seen to be associated with obesity also can be a cause. Calorie restriction in our studies was unable to restore normal mitochondrial function in obese individuals. This can indicate an imprinting of “obese metabolic pattern”, or there is also a possibility that interventions that were used in our studies are not restrictive enough to significantly reduce the delivery of fatty acids into skeletal muscle or to reduce inflammation over that relatively short period of time of the study.
A possible metabolic role of mitochondrial enzyme disbalance in the development of insulin resistance in human skeletal muscle.
Insulin resistance in skeletal muscle is linked to mitochondrial metabolism and to mitochondrial fat oxidation (40, 65). An intracellular accumulation of lipid intermediates (acyl-CoAs, diacylglycerols, or some acyl-carnitines) can adversely affect insulin-mediated glucose transport in skeletal muscle (35, 36). The attenuation of the delivery of long-chain fatty acids into mitochondria by raising malonyl-CoA levels and inhibiting carnitine palmitoyltransferase I activates skeletal muscle insulin-mediated glucose oxidation in cultured human myotubes (7). This model suggests that the intracellular concentration of toxic byproducts of incomplete fat oxidation can be reduced by increasing the mitochondrial respiration during physical activity, moderately restricting food intake, or both, which in turn leads to improvement of insulin sensitivity in obese or T2DM individuals (2, 15, 54, 58, 59).
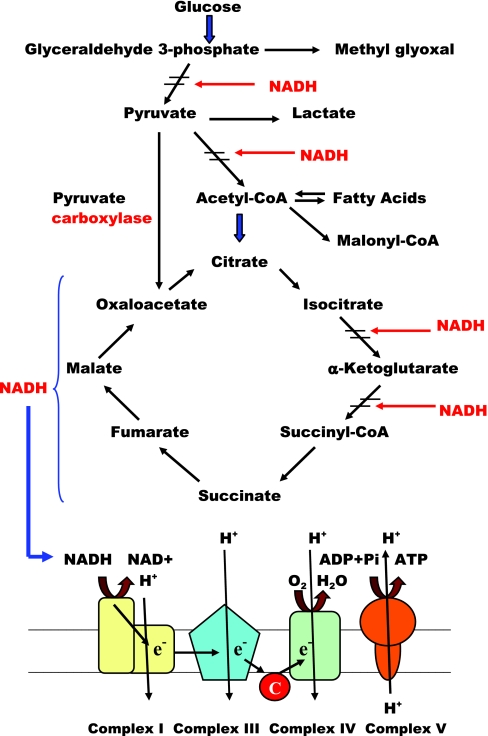
We hypothesize that the disbalance between mitochondrial electron transport and TCA cycle is a prime defect that predisposes human skeletal muscle to the development of insulin resistance. This defect can increase sensitivity of the insulin-mediated glucose metabolism in skeletal muscle to adverse effects of lack of physical activity and excessive caloric intake. In combination with sedentary lifestyle, this defect can shift skeletal muscle metabolism to accumulation of products of incomplete oxidation of glucose (lactate, methylglyoxal) or fatty acids (acyl-CoAs, hydroxyacyl-CoAs). The insufficient rate of NADH oxidation accompanied by high production of reducing equivalents in TCA cycle and by β-oxidation can render skeletal muscle of insulin-resistant subjects into the state with abnormally high intracellular NADH/NAD ratio or the state of “reductive stress” (22, 66). High NADH/NAD ratio can drive excessive production of muscle lactate due to accelerated reduction of pyruvate and slow down the pyruvate oxidation in pyruvate dehydrogenase reaction (Fig. 4). Also, it can increase the production of methylglyoxal, which has an ability to suppress insulin signaling (22, 45). Inhibition of pyruvate dehydrogenase reaction by high NADH can also divert more pyruvate into oxaloacetate through pyruvate carboxylase. Availability of oxaloacetate and high activity of citrate synthase can provide an accelerated production of citrate from fatty acid-derived acetyl-CoA. From the other side, the consumption of citrate in TCA cycle can be decreased due to inhibition of NAD-dependent isocitrate dehydrogenase by high NADH (Fig. 4). With these considerations, we can expect an increased production of citrate by skeletal muscle mitochondria in obesity and T2DM. Also, we can expect increased citrate transport from mitochondria into the cytoplasm, where it serves in biosynthetic pathways as a precursor of acetyl-CoA, malonyl-CoA, and diacyl- and triacylglycerols. The increased content of malonyl-CoA in the muscle tissue can be an indirect indicator of increased citrate leak into the cytoplasm. Indeed, the recent study from Bandyopadhyay et al. (3) indicates the increased malonyl-CoA levels in skeletal muscle from obese and type 2 diabetic subjects. The steady-state concentration of malonyl-CoA can also depend on the rate of acetyl-CoA carboxylase reaction and on the rate of malonyl-CoA decarbolxylation (49). Synthesis of malonyl-CoA by acetyl-CoA carboxylase is activated by citrate (50), and we can suggest that the rate of citrate delivery is an important factor that controls the concentration of malonyl-CoA in cytoplasm.
Fig. 4.
Potential effects of the disbalance between mitochondrial electron transport, TCA cycle, and β-oxidation on skeletal muscle metabolomics in obesity and type 2 diabetes (scheme). In the state of decreased contractile activity and excessive caloric intake, the suppressed ability of mitochondrial electron transport chain to oxidize NADH accompanied by excessive activity of CS in TCA cycle or HAD in β-oxidation pathway can lead to abnormally high steady-state concentration of intramitochondrial and cytoplasmic NADH. The increased concentration of NADH can impair substrate oxidation of 2 key enzymes that control oxidation of glucose and fatty acids, pyruvate dehydrogenase complex (PDC) and HAD, respectively. Thus the defect in mitochondrial electron transport chain can lead to general slowdown of basal metabolism in skeletal muscle, and perhaps it can result in decreased rate of ATP generation during contractile activity. Inhibition of PDC can divert more pyruvate to oxaloacetate and to citrate that can leak from mitochondria to cytoplasm. Excessive cytoplasmic citrate can be a precursor for acetyl-CoA and acyl-CoAs. Accumulation of array of metabolite byproducts and the products of incomplete oxidation of fatty acids and glucose in skeletal muscle of individuals adopting sedentary lifestyle and overeating could be a major cause of insulin resistance in skeletal muscle.
Excess of NADH also can interfere with β-oxidation at the level of β-HAD. This can lead to accumulation of the products of incomplete oxidation of fatty acids. Finally, the sustained high level of NADH can increase the production of intracellular hydrogen peroxide in NAD(P)H-oxidase reaction (55). Thus, the selective defect of mitochondrial electron transport can explain many metabolic abnormalities linked to insulin resistance: increased excretion of muscle lactate (9), accumulation of methylglyoxal (4), diacylglycerols (23), malonyl-CoA (50), and products of incomplete oxidation of fatty acids (56), and also excessive reactive oxygen species markers (61).
Combined weight loss plus exercise intervention significantly increases mitochondrial biogenesis in vastus lateralis muscle in obesity or T2DM as it follows from increase in cardiolipin, citrate synthase β-HAD, or NADH oxidase (Figs. 1 and 2). However, the increase in NADH oxidase is not sufficient to restore the normal balance between ETC and enzymes of TCA cycle or β-oxidation pathway (Fig. 2). We cannot exclude a possibility of a counterbalancing effect of caloric restriction on mitochondria. It should be taken to account that mitochondrial ETC includes proteins encoded in mitochondrial genome contrary to enzymes of TCA cycle, β-oxidation, or cardiolipin biosynthesis that solely need expression of nuclear-encoded mitochondrial genes. Additionally, skeletal muscle mitochondria in T2DM or obesity are unable to efficiently replicate mtDNA in response to exercise plus caloric restriction (Fig. 2). It can be an effect of caloric restriction; however, it is tempting to connect the “resistance” in the replication of mtDNA with reduced activity of ETC in T2DM and obesity. The simultaneous decrease in the activity of ETC with hampered replication of mtDNA can be explained by disturbed balance in the interaction of mtDNA with mitochondrial transcription factor A (TFAM). TFAM is an abundant structural protein that is essential for stability of mitochondrial genome and for compaction of mtDNA copies into mitochondrial chromosome (nucleoid). TFAM is also critical for both replication and transcription of mtDNA (39, 51). We hypothesize that an excessive generation of active byproducts of oxidative metabolism, including oxoaldehydes, can affect interaction of mtDNA with TFAM in mitochondrial chromosome and attenuate the transcription of peptides essential for respiratory complexes. The previously shown reduction in the expression of nuclear-encoded mitochondrial genes in T2DM and obesity can be a secondary event as a response to insufficient syntheses of ETC subunits encoded in mitochondrial genome (11, 30, 33, 38).
In conclusion, the presented data indicate that insulin resistance is a condition that is marked with a deficiency of ETC and disbalance between ETC, β-oxidation, and TCA cycle. Sustained exercise program or calorie restriction improves insulin resistance in human skeletal muscle (58, 59). We hypothesize that this improvement can be a result of sustained reduction in muscle NADH/NAD ratio due to increased mitochondrial respiration or reduced fatty acid delivery. Finding an appropriate intervention(s) to restore the normal enzyme profile and conceivably normalize NADH/NAD ratio in skeletal muscle mitochondria could be an important accomplishment in the treatment of T2DM and obesity by making intensive blood glucose control more safe and beneficial.
GRANTS
Funding support for this study was provided primarily by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5-R01-DK-49200-11 and additionally by the University of Pittsburgh General Clinical Research Center (Grant 5-M01-RR-00056) and the Obesity and Nutrition Research Center (ONRC; Grant P-30-DK-462). E. V. Menshikova was supported by a pilot grant from ONRC (107325). K. Azuma was supported by a mentor-based fellowship grant from the American Diabetes Association.
DISCLOSURES
No conflicts of interest are delcared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the valuable contributions of the research coordinator Carol Kelley, Dr. Zofia Radikova, the intervention staff of nutritionists and exercise physiologists of the Pittsburgh Obesity and Nutrition Center, and the staff of the University of Pittsburgh General Clinical Research Center for their help in carrying out the clinical investigation. We also express our appreciation to the research volunteers who participated in these studies. We are grateful to Dr. Orian Shirihai, Dr. Nigel Turner, and Prof. Kent Sahlin for help and advice.
REFERENCES
- 1.Bachmann E, Lenaz G, Perdue J, Orme-Johnson N, Green D. The membrane systems of the mitochondrion. Arch Biochem Biophys 121: 73–87, 1967 [DOI] [PubMed] [Google Scholar]
- 2.Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F, Laakso M, Ferrannini E, EGIR-RISC Study Group Physical activity and insulin sensitivity: the RISC study. Diabetes 57: 2613–2618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55: 2277–2285, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Beisswenger PJ, Howell SK, Smith K, Szwergold BS. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochim Biophys Acta 1637: 98–106, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol 65: 97–117, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50: 790–796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouzraki K, Austin R, Rune A, Lassman M, Garcia-Roves P, Berger J, Krook A, Chibalin A, Zhang B, Zierath J. Malonyl coenzyme-A decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes 57: 1508–1516, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Chi MM, Hintz CS, Coyle EF, Martin WH, 3rd, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol Cell Physiol 244: C276–C287, 1983 [DOI] [PubMed] [Google Scholar]
- 9.Choi CS, Kim YB, Lee FN, Zabolotny JM, Kahn BB, Youn JH. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol Endocrinol Metab 283: E233–E240, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Daum G. Lipids of mitochondria. Biochim Biophys Acta 822: 1–42, 1985 [DOI] [PubMed] [Google Scholar]
- 11.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab 294: E607–E614, 2008 [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88: 787–835, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Fernström M, Bakkman L, Tonkonogi M, Shabalina IG, Rozhdestvenskaya Z, Mattsson CM, Enqvist JK, Ekblom B, Sahlin K. Reduced efficiency, but increased fat oxidation, in mitochondria from human skeletal muscle after 24-h ultraendurance exercise. J Appl Physiol 102: 1844–1849, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Freyssenet D, Berthon P, Denis C. Mitochondrial biogenesis in skeletal muscle in response to endurance exercises. Arch Physiol Biochem 104: 129–141, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster B, Katsiaras A, Kelley D. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52: 2191–2197, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Harmon H, Hall J, Crane F. Structure of mitochondrial cristae membranes. Biochim Biophys Acta 344: 119–155, 1974 [DOI] [PubMed] [Google Scholar]
- 17.Heilbronn L, Gan S, Turner N, Campbell L, Chisholm D. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab 92: 1467–1473, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta 1113: 71–133, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Holloszy JO. Adaptations of skeletal muscle mitochondria to endurance exercise: a personal perspective. Exerc Sport Sci Rev 32: 41–432004 [DOI] [PubMed] [Google Scholar]
- 20.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol 209: 2265–2275, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med Sci Sports Exerc 35: 95–104, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxid Redox Signal 9: 931–942, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Itani S, Ruderman N, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Kudin A, Vielhaber S, Elger CE, Kunz WS. Differences in flux control and reserve capacity of cytochrome c oxidase (COX) in human skeletal muscle and brain suggest different metabolic effects of mild COX deficiencies. Mol Biol Rep 29: 89–92, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Menshikova E, Huang W, Toledo F, Goodpaster B, O'Doherty R, Ritov V. Disbalance between electron transport and citric acid cycle in muscle and liver mitochondria characterizes insulin resistance in obesity and type 2 diabetes mellitus (Abstract A616). American Diabetes Association 69th Scientific Sessions, edited by Butler PC, New Orleans, LA, 2009 [Google Scholar]
- 27.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61: 534–540, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE. Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol 103: 21–27, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab 288: E818–E825, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P. Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 31: 1302–1310, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Miller FJ, Rosenfeldt FL, Zhang C, Linnane AW, Nagley P. Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: lack of change of copy number with age. Nucleic Acids Res 31: e61, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56: 1592–1599, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Morino K, Petersen K, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White M, Bilz S, Sono S, Pypaert M, Shulman G. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morino K, Petersen K, Shulman G. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55: S9–S15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muoio DM, Koves TR. Lipid-induced metabolic dysfunction in skeletal muscle. Novartis Found Symp 286: 24–38, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Muoio D, Newgard C. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9: 193–205, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Patti M, Butte A, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker E, Goldfine A, Mun E, DeFronzo R, Finlayson J, Kahn C, Mandarino L. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohjoismäki JL, Wanrooij S, Hyvärinen AK, Goffart S, Holt IJ, Spelbrink JN, Jacobs HT. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res 34: 5815–5828, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pospisilik A, Knauf C, Joza N, Benit P, Orthofer M, Cani P, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn C, Kroemer G, Rustin P, Burcelin R, Penninger J. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131: 476–491, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Puntschart A, Claassen H, Jostarndt K, Hoppeler H, Billeter R. mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Physiol Cell Physiol 269: C619–C625, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Rabol R, Boushel R, Dela F. Mitochondrial oxidative function and type 2 diabetes. Appl Physiol Nutr Metab 31: 675–683, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen H, Andersen A, Rasmussen U. Optimization of preparation of mitochondria from 25–100 mg skeletal muscle. Anal Biochem 252: 153–159, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Rhodes CJ. Type 2 diabetes—a matter of beta-cell life and death?. Science 307: 380–384, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Riboulet-Chavey A, Pierron A, Durand I, Murdaca J, Giudicelli J, Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes 55: 1289–1299, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Ritov VB, Menshikova EV, Kelley DE. Analysis of cardiolipin in human muscle biopsy. J Chromatogr B Analyt Technol Biomed Life Sci 831: 63–71, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Ritov VB, Menshikova EV, Kelley DE. High-performance liquid chromatography-based methods of enzymatic analysis: electron transport chain activity in mitochondria from human skeletal muscle. Anal Biochem 333: 27–38, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol Endocrinol Metab 276: E1–E18, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Saha AK, Vavvas D, Kurowski TG, Apazidis A, Witters LA, Shafrir E, Ruderman NB. Malonyl-CoA regulation in skeletal muscle: its link to cell citrate and the glucose-fatty acid cycle. Am J Physiol Endocrinol Metab 272: E641–E648, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88: 611–638, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Schlame M, Rua D, Greenberg M. The biosynthesis and functional role of cardiolipin. Prog Lipid Res 39: 257–288, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83: 166–171, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O'Carroll SM, O'Leary VB, Kirwan JP. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 104: 1313–1319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, Utsumi H, Nawata H. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med 37: 115–123, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Su X, Han X, Mancuso DJ, Abendschein DR, Gross RW. Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry 44: 5234–5245, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Tager J, Groen A, Wanders R, Duszynski J, Westerhoff H, Vervoorn R. Control of mitochondrial respiration. Biochem Soc Trans 11: 40–43, 1983 [DOI] [PubMed] [Google Scholar]
- 58.Toledo FG, Menshikova EV, Azuma K, Radiková Z, Kelley CA, Ritov VB, Kelley DE. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57: 987–994, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Toledo F, Menshikova E, Ritov V, Azuma K, Radikova Z, DeLany J, Kelley D. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 56: 2142–2147, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray GA, Smith SR. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes 56: 720–727, 2007 [DOI] [PubMed] [Google Scholar]
- 61.West I. Radicals and oxidative stress in diabetes. Diabet Med 17: 171–180, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Wicks KL, Hood DA. Mitochondrial adaptations in denervated muscle: relationship to muscle performance. Am J Physiol Cell Physiol 260: C841–C850, 1991 [DOI] [PubMed] [Google Scholar]
- 63.Wilson D. Factors affecting the rate and energetics of mitochondrial oxidative phosphorylation. Med Sci Sports Exerc 26: 37–43, 1994 [PubMed] [Google Scholar]
- 64.Winkler-Stuck K, Kirches E, Mawrin C, Dietzmann K, Lins H, Wallesch C, Kunz W, Wiedemann F. Re-evaluation of the dysfunction of mitochondrial respiratory chain in skeletal muscle of patients with Parkinson's disease. J Neural Transm 112: 499–518, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Wredenberg A, Freyer C, Sandström ME, Katz A, Wibom R, Westerblad H, Larsson NG. Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochem Biophys Res Commun 350: 202–207, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10: 179–206, 2008 [DOI] [PubMed] [Google Scholar]