Abstract
Renal interstitial fibrosis is a major determinant of renal failure in the majority of chronic renal diseases. Transforming growth factor-β (TGF-β) is the single most important cytokine promoting renal fibrogenesis. Recent in vitro studies identified novel non-smad TGF-β targets including p21-activated kinase-2 (PAK2), the abelson nonreceptor tyrosine kinase (c-Abl), and the mammalian target of rapamycin (mTOR) that are activated by TGF-β in mesenchymal cells, specifically in fibroblasts but less in epithelial cells. In the present studies, we show that non-smad effectors of TGF-β including PAK2, c-Abl, Akt, tuberin (TSC2), and mTOR are activated in experimental unilateral obstructive nephropathy in rats. Treatment with c-Abl or mTOR inhibitors, imatinib mesylate and rapamycin, respectively, each blocks noncanonical (non-smad) TGF-β pathways in the kidney in vivo and diminishes the number of interstitial fibroblasts and myofibroblasts as well as the interstitial accumulation of extracellular matrix proteins. These findings indicate that noncanonical TGF-β pathways are activated during the early and rapid renal fibrogenesis of obstructive nephropathy. Moreover, the current findings suggest that combined inhibition of key regulators of these non-smad TGF-β pathways even in dose-sparing protocols are effective treatments in renal fibrogenesis.
Keywords: mTOR, rapamycin, imatinib
renal interstitial fibrosis, i.e., disorderly accumulation of extracellular matrix proteins in the renal interstitium, determines onset and progression of chronic renal failure in most renal diseases (1–3, 16). Transforming growth factor-β (TGF-β) has long emerged as a prominent master regulator of renal fibrogenesis in general, and specifically in obstructive nephropathy (5, 6, 9). This cytokine has pleiotropic actions which are cell type and context dependent. Among these diverse biological activities are growth arrest and apoptosis as well as contributions to mesenchymal transition in epithelial cells. In contrast, in endothelial cells TGF-β contributes to growth, migration, and angiogenesis. TGF-β is also an important regulator of (renal) fibroblasts. In these cells in vivo and in vitro, TGF-β induces proliferation and accelerates the transition into the myofibroblast phenotype. The latter cell type serves as the predominant source for interstitially accumulating matrix proteins in renal fibrosis.
The “canonical” pathway that is activated in most cell types by TGF-β involves serine/threonine phosphorylation of smad-2 and -3 which are substrates of the TGF-β receptor heterotetramer Alk5/TBRII (11). These receptor-activated smads (R-smads) translocate to the nucleus where they recognize regulatory smad binding elements and transcriptionally activate or repress target genes in association with smad-4 and in collaboration with other transcription factors (11). Although this TGF-β-smad pathway is widely expressed and mediates several different effects of TGF-β including cell cycle arrest and apoptosis in epithelial cells, it does not explain the variability of TGF-β responses observed in other cell types. To address that issue, we and others provided evidence for the existence of other cell type-specific TGF-β pathways. We previously showed that TGF-β activates p21-activated kinase-2 (PAK2) and c-Abl kinases in renal fibroblasts in vitro but not in renal tubular or mesangial cells and that inhibition of Abl activity reduces renal fibroblast proliferation in vitro and in the kidney in vivo (17). Moreover, in a renal fibrosis rodent model, administration of imatinib, an Abl inhibitor, reduces renal fibrogenesis (17). These latter studies also provide experimental evidence that Abl primarily contributes to fibroblast proliferation but is probably less required for transition to the myofibroblast phenotype. More recently, we further elaborated on this model of fibroblast-specific signaling by showing that TGF-β also activates the mammalian target of rapamycin complex-1 (mTORC1) specifically in fibroblasts through a pathway involving phosphatidyl-inositol-3′-kinase, protein kinase-B/Akt, and tuberin (PI3K → PKB/Akt → TSC2). In vitro, this pathway is inhibited by rapamycin and fibroblast proliferation is reduced (13).
In the present studies, we tested the hypothesis that this novel TGF-β pathway contributes importantly to renal fibrogenesis and that an inhibitor of mTORC1, rapamycin, will also reduce renal fibrosis and may be used together with imatinib in a dose-sparing protocol. Studies were performed in unilateral obstructive nephropathy in rats where renal fibrogenesis is rapid and TGF-β-dependent (10, 23).
MATERIALS AND METHODS
Animal Model
Male Sprague-Dawley rats (n = 6 per group; Harlan, Indianapolis, IN), 200 g body wt, underwent unilateral ureteral obstruction (UUO) surgery. Briefly, animals were anesthetized with 85/15 mg/kg im ketamine/xylazine. The abdomen was shaved, sterile prepped, and the abdominal cavity was accessed via a sagittal incision. The left ureter was prepped and two ligatures were placed around the proximal portion, ∼3 mm apart. Successful ligation of the ureter was judged by distal collapse and proximal expansion of the ureter and renal pelvis. The abdomen was closed with sutures and the skin wound was closed with clips. The studies adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Los Angeles Biomedical Research Institute.
Animals were randomly assigned to one of five groups following UUO surgery: group 1, animals treated with vehicle injections (control); group 2, 50 mg/kg per day imatinib; group 3, 1.5 mg/kg per day rapamycin; group 4, 50 mg/kg imatinib + 1.5 mg/kg per day rapamycin; group 5, 25 mg/kg imatinib + 0.5 mg/kg per day rapamycin. Treatments were commenced after the surgical ureteral ligation and given daily for 7 days by intraperitoneal injection.
Imatinib (Gleevec, Novartis Pharmaceuticals, East Hanover, NJ) was purchased from the Mayo Pharmacy in 100-mg capsules and solubilized in ddH2O. Particulate matter was removed by centrifugation at 2,500 g (×2) and the supernatant was lyophilized with 90% recovery. Reverse-phase high-pressure liquid chromatography and mass spectrometry indicated >99% purity of the lyophilized material. Imatinib was dissolved in sterile PBS for injection.
Rapamycin (Sirolimus, LC Laboratories, Woburn, MA) was dissolved in ethanol as a stock solution. For administration, the stock was diluted to 40 μg/0.1 ml in sterile PEG400/8% ethanol and then further diluted to 20 μg/0.1 ml in 10% Tween-80 for intraperitoneal injection. Vehicle control animals received equal amounts of this solvent.
Twelve hours after the last injection, animals were killed. The right (normal) kidney and the left kidney with obstructive nephropathy were obtained from each animal. Kidneys were decapsulated and 1-mm coronal slices were obtained and aliquoted for various assays.
Kinase Assays and Western Analysis
PAK2 activity assay and PAK2.
Kidneys were minced with a razor blade on ice and proteins were solubilized in kinase lysis buffer [50 mM Tris, pH 7.4, 5 mM EDTA, 250 mM NaCl, 0.1% Triton X-100, 50 mM NaF, 100 μM Na3VO4, and 1× complete protease inhibitor (Roche Applied Science, Indianapolis, IN)]. PAK2 was immunoprecipitated from tissue lysates (∼1.0 mg) with anti-PAK2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight; complexes were collected with protein-A agarose beads, washed twice in lysis buffer and twice in kinase buffer (25 mM Tris, pH 7.4, 10 mM MgCl2, 1 mM dithiothreitol), and then incubated in 50 μl of kinase buffer containing 5 μM ATP, 5 μg of myelin basic protein (Sigma), and 5 μCi [γ-32P]-ATP/μl. After 10 min at 37°C, the reaction was stopped by addition of 2× Laemmli buffer. Proteins were separated electrophoretically in SDS-PAGE gels and visualized by autoradiography. For determination of total PAK2, an aliquot of the tissue lysate (50 μg) was subjected to SDS-PAGE and Western analysis with anti-PAK2 (Cell Signaling Technology, Beverly, MA).
C-Abl activity assay and c-Abl.
c-Abl was immunoprecipitated overnight (antibody K-12, Santa Cruz Biotechnology) from tissue lysates prepared as described for PAK2. Immune complexes were collected with protein A-agarose beads, washed as above, and incubated in 40 μl of kinase buffer containing 5 μM ATP, 2 μg GST-Crk, and 0.5 μCi/μl [γ-32P]-ATP. After 5 min at 37°C, the reaction was stopped by addition of 40 μl 2× Laemmli buffer and assessed by SDS-PAGE and autoradiography. An aliquot (50 μg) of the tissue lysate was used to determine total c-Abl by Western transfer and blotting with anti-c-Abl (BD PharMingen, San Diego, CA).
Akt, TSC2, and P70S6K assays.
Phosphorylated and total Akt, tuberin (TSC2), as well as P70S6 kinase (P70S6K) were assessed by Western blot analysis. Briefly, 30 μg of tissue lysate in kinase lysis buffer were electrophoretically separated in SDS-PAGE and transferred to nitrocellulose. Membranes were blotted with commercially available anti-phospho- and anti-total Akt, TSC2, and P70S6K (Cell Signaling Technology, Danvers, MA). Quantitations were derived by densitometry.
pSmad2/3.
Phosphorylated smad2/3 was examined by Western blot analysis. Briefly, 40 μl of tissue lysates were electrophoresed in SDS-PAGE gels and transferred to nitrocellulose. Membranes were blotted with a rabbit antibody that recognizes both, phosphorylated smad2 and phospho-smad3 (Santa Cruz Biotechnology). Membranes were then stripped and reblotted with mouse anti-GAPDH for loading control (Fitzgerald Industries, Concord, MA). Quantitative data were derived by densitometry.
Quantitative Immunohistology
One-millimeter coronal sections were fixed with 4% neutral paraformaldehyde in PBS and embedded in paraffin. Four-micrometer sections were immunostained using commercially available antibodies and secondary reagents including goat anti-fibronectin (Santa Cruz Biotechnology), biotinylated donkey anti-goat IgG (Santa Cruz Biotechnology), and strept AB-Complex-HRP (Dako, Carpinteria, CA); mouse anti-α-SMA (Zymed Laboratories, South San Francisco, CA) and the above secondary reagents; and goat anti-collagen type III (Southern Biotechnology Associates, Birmingham, AL) and Alexa Fluor 488-conjugated donkey anti-goat IgG (Molecular Probes/Invitrogen, Carlsbad, CA). Digital histomorphometry was performed to quantitatively assess immunohistological findings. Twenty nonoverlapping cortical fields (corresponding to ∼90% of the cortex of each coronal section) were microphotographed with a ×20 magnifying objective in a Nikon Eclipse E400 microscope with a Nicon DXM1200 digital camera. In each digital image, the color-specific pixel number was counted with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA) and expressed as percent of the total pixel number in each image. For quantitative assessment of fibronectin, each image was digitally overlaid with a point grid and points overlapping fibronectin-positive interstitium were counted.
mRNA Encoding TGF-β and Selective Extracellular Matrix Proteins
Aliquot coronal sections were obtained in RNALater (Qiagen, Hilden, Germany) and total RNA was extracted with the TRIzol reagent and the manufacturer's proposed protocol (Invitrogen, Carlsbad, CA). Specific mRNAs encoding Col1A2, Col3A1, and fibronectin were measured by PCR using specific primers and optimized PCR protocols with coamplification of 18S rRNA in each sample as standard. Quantitative results were obtained by densitometry and calculation of the ratio of mRNA/18S rRNA densitometric units.
Statistical Analysis
Data are expressed as group means ± SE. Statistical comparisons of group means were performed with ANOVA followed by Newman-Keuls multicomparison test. A probability less than 5% (P < 0.05) was defined to reflect statistically significant differences.
RESULTS
TGF-β Expression and Smad2/3 Phosphorylation
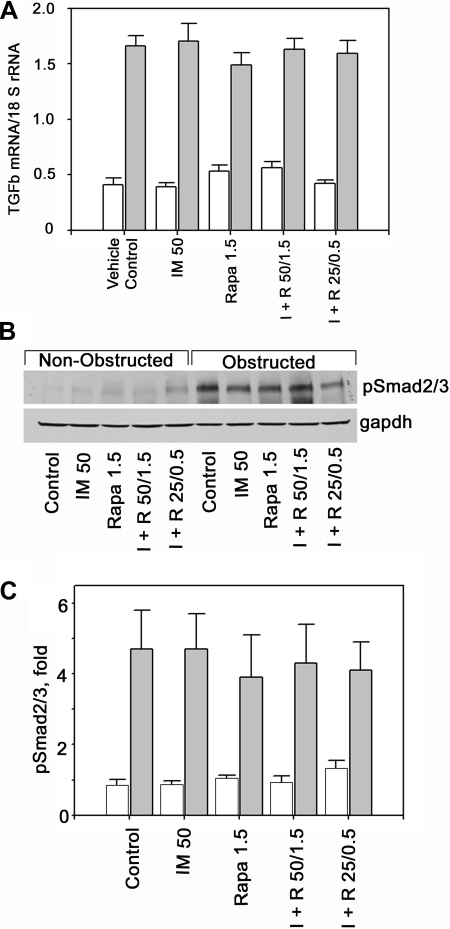
TGF-β expression was increased, about fourfold, in the left, obstructed kidneys compared with the contralateral control kidney (Fig. 1A). As expected, induction of TGF-β by obstructive nephropathy was not affected by either treatment and was similar in all five groups of animals (Fig. 1A). Similarly, levels of pSmad2/3 were increased in obstructed kidneys, about fivefold compared with the right, nonobstructed, control kidney (Fig. 1, B and C). As we showed previously, treatment with imatinib does not affect smad2/3-phosphorylation (17). Moreover, treatment with rapamycin alone or in combination with imatinib also does not affect smad2/3 phosphorylation (Fig. 1, B and C).
Fig. 1.
Transforming growth factor-β (TGF-β) expression and Smad2/3 phosphorylation. A: expression of TGF-β is increased in obstructed kidneys (gray bars) compared with contralateral, nonobstructed kidneys (open bars). This ∼4-fold increase is not affected by treatment with imatinib (IM or I) or rapamycin (Rapa or R) alone or in combination at 2 different dosages (n = 6 each). B: in obstructed kidneys smad2/3 phosphorylation is increased compared with nonobstructed kidneys. Smad2/3 phosphorylation is not changed by treatment with imatinib or rapamycin. C: quantitative depiction of pSmad2/3 in control (open bars) and obstructed kidneys (gray bars) in each of the 5 treatment groups (n = 4 each).
Activation of Noncanonical TGF-β Pathways in Obstructive Nephropathy
We previously reported that TGF-β activates PAK2 and its downstream target c-Abl in vitro in a number of different mesenchymal cells but not in any of several epithelial cell lines that were tested (18, 20). Moreover, these targets are stimulated in models of TGF-β-driven kidney and pulmonary fibrosis and, most importantly, inhibition of c-Abl with imatinib mesylate reduces the profibrogenic actions of TGF-β in vitro as well as in vivo (4, 17). More recently, our laboratories also demonstrated that the mTORC1 represents an additional cell type-specific pathway regulating the mesenchymal cell responses to TGF-β (13). Thus, the present studies were undertaken to determine 1) whether the mTORC1 pathway is also activated in vivo in obstructive nephropathy, a chronic renal disease with TGF-β-driven interstitial fibrosis; 2) whether rapamycin, a mTORC1 inhibitor, alone and in conjunction with imatinib mesylate can reduce renal fibrogenesis that is associated with UUO; and 3) whether a combination of both drugs each at a lower dose has renal anti-fibrogenic efficacy.
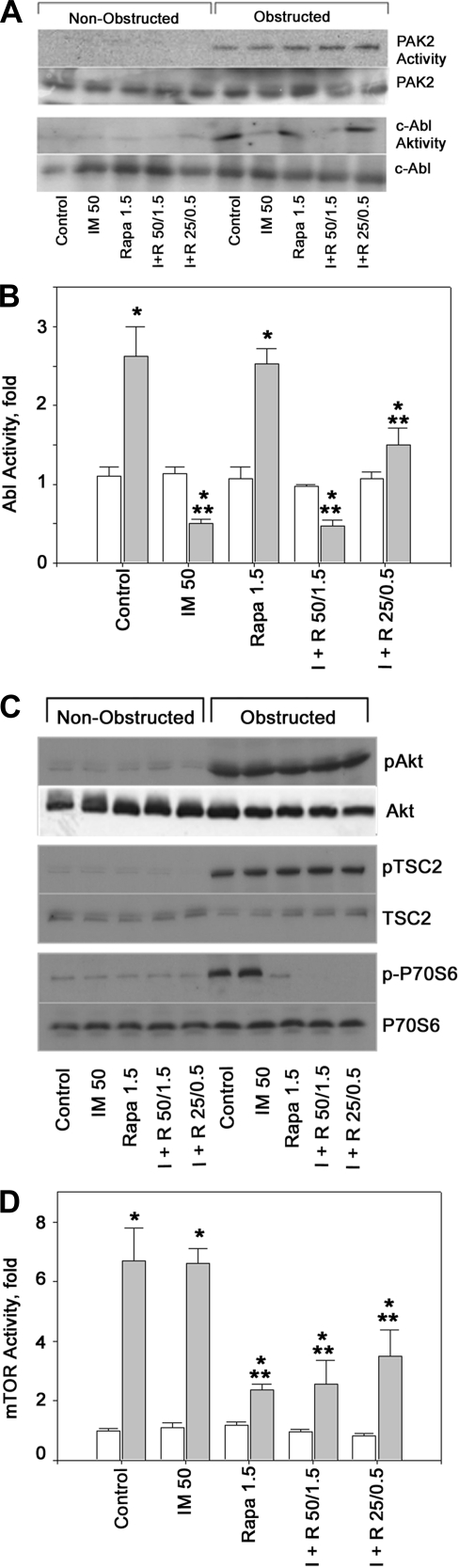
Control and UUO animals were treated with imatinib, rapamycin, or both and TGF-β non-smad pathway activation was assessed by examining PAK2 and c-Abl kinase activity. PAK2 is activated in obstructive nephropathy compared with control kidneys and is not affected either by imatinib or rapamycin (Fig. 2A). The lack of inhibition of PAK2 activation by imatinib confirms previous in vitro and in vivo studies from our laboratories (17, 18). Abl activity is also increased in obstructed compared with nonobstructed control kidneys (Fig. 2, A and B). Administration of imatinib at the higher dose (50 mg/kg) blocks Abl activation while rapamycin does not affect Abl activity. Of note, in animals receiving only a low dose of imatinib (25 mg/kg) albeit in combination with low-dose rapamycin, Abl is blocked less efficiently (Fig. 2B).
Fig. 2.
A: non-smad TGF-β targets p21-activated kinase-2 (PAK2) and c-Abl are activated in obstructive nephropathy. Kinase activity and levels of PAK2 and c-Abl in the right (nonobstructed) and the contralateral (obstructed) kidney were determined as described previously (17) and under materials and methods. Groups consisted of vehicle (control), imatinib (IM or I), rapamycin (Rapa or R), or I + R at the indicated drug concentrations (mg/kg). In obstructive nephropathy, both PAK2 and c-Abl are activated and PAK2 activation is not affected by any of the treatment regimens. At the higher dose (50 mg/kg), imatinib (IM) blocks Abl activation. At the lesser dose of 25 mg/kg, IM inhibition of Abl activity by imatinib is submaximal. B: quantitative assessment of Abl activity in right, control kidneys (open bars) and left, obstructed kidneys (gray bars); n = 3 each. *P < 0.05 vs. control kidneys. **P < 0.05 vs. obstructed kidneys in vehicle-treated rats. C: Akt phosphorylation and mammalian target of rapamycin (mTOR) signaling are induced in obstructive nephropathy. Akt phosphorylation is substantially increased in the obstructed kidney and, as expected, is not modified by treatment with imatinib or rapamycin. Similarly, TSC2 (a substrate of Akt and an inhibitor of mTORC1 which is inactivated by phosphorylation) is phosphorylated to a similar degree in unilateral ureteral obstruction (UUO) rats irrespective of treatment with imatinib (IM or I) or rapamycin (Rapa or R). In contrast, P70S6K, a downstream effector of mTORC1, is activated in obstructive nephropathy and not affected by IM, but very effectively reduced by Rapa even at the lower dose. Thus, mTORC1 is activated during obstructive nephropathy, presumably through TGF-β, and its activation is blocked by Rapa, 1.5 or 0.5 mg/kg. D: quantitative assessment of mTOR activity which was measured as the ratio of phosphorylated over total P70S6K in right, control kidneys (open bars) and left, obstructed kidneys (gray bars); n = 3 each. *P < 0.05 vs. control kidneys. **P < 0.05 vs. obstructed kidneys in vehicle-treated rats.
TGF-β-directed fibroblast proliferation is regulated by a number of Smad and non-Smad effectors. Given that the mTOR pathway had been shown to play critical roles in the fibroblast response to TGF-β in vitro (13), we determined whether a similar pathway is activated in vivo in obstructive nephropathy. As illustrated in Fig. 2C, ureteral obstruction causes phosphorylation of Akt and tuberin (TSC2), two upstream regulators of mTOR. Similarly, the mTORC1 substrate, P70S6 kinase, is also phosphorylated in obstructed compared with control kidneys indicating activation of mTORC1 (Fig. 2C). Neither imatinib nor rapamycin affects Akt or tuberin phosphorylation. Imatinib, even at the high dose, does not affect P70S6K phosphorylation and, hence, mTORC1 activity (Fig. 2, C and D). In contrast, mTORC1 is blocked by rapamycin at the high (1.5 mg/kg) and even at the low dose (0.5 mg/kg) as assessed by phosphorylation of the mTORC1 substrate, P70S6K (Fig. 2, C and D). Although the low dose of rapamycin was coadministered with low-dose imatinib, the latter has no effect on P70S6K activity (Fig. 2, C and D), and conversely, the former has no effect on Abl activity (Fig. 2, A and B). Overall, these findings show in vivo activation of two noncanonical TGF-β pathways, namely PAK2 → Abl and Akt → TSC2 → mTORC2 → P70S6K, each of which is targeted separately as well as in combination by an inhibitory drug, imatinib or rapamycin, respectively, and outcome with respect to renal fibrogenesis was examined.
Effects of Abl and/or mTORC1 Inhibition on Renal Fibrosis
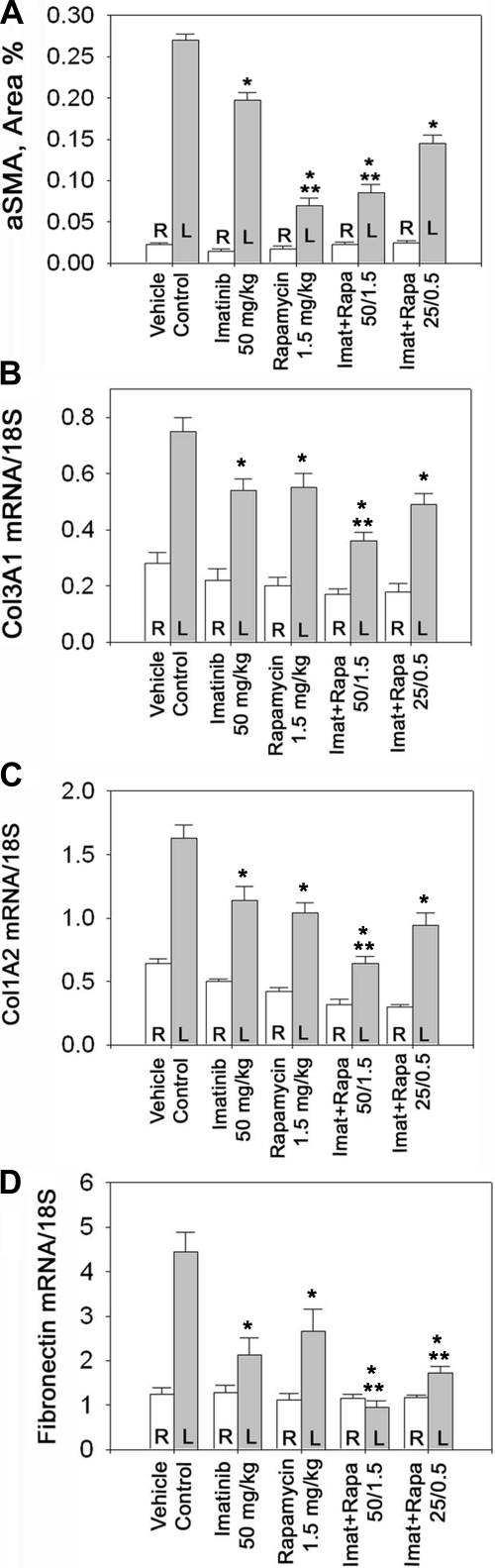
A hallmark of fibrogenic disorders is the presence of activated fibroblasts and myofibroblasts. As myofibroblast differentiation is often defined by the acquisition of contractile stress fibers that are composed of cytoplasmic actins, α-smooth muscle actin (α-SMA) was used as a marker of interstitial myofibroblasts. In obstructive nephropathy, the number of interstitial myofibroblasts increases ∼12-fold (Fig. 3A). This rise in the myofibroblast number is moderately but significantly reduced by high-dose imatinib, similar to what we reported previously (17). However, high-dose rapamycin was appreciably more effective than imatinib. The combination of both drugs each at their higher dose provides no further reduction in myofibroblast number beyond the effect of rapamycin alone (Fig. 3A). A combination of imatinib and rapamycin, each at their lower dose, reduces interstitial myofibroblasts more effectively than high-dose imatinib but less effectively compared with high-dose rapamycin. These data suggest that the contribution of the mTORC1 pathway to fibroblast proliferation may dominate that of the Abl pathway in vivo. Moreover, a low-dose combination of imatinib and rapamycin induces a substantial, albeit not maximal, reduction in myofibroblast number.
Fig. 3.
A: quantitative assessment of myofibroblast accumulation by immunoreactive interstitial α-smooth muscle actin (α-SMA) in UUO rats. Animals were treated as described in Fig. 1 legend and the expression of interstitial α-SMA was determined. L (gray bars) indicates left, obstructed kidney; R (open bars) indicates right, nonobstructed kidney; n = 6 each. *P < 0.05 vs. control. **P < 0.05 vs. imatinib, 50 mg/kg. B–D: matrix gene expression in UUO rats. mRNA levels encoding Col3A1 (B), Col1A2 (C), and fibronectin (D) in the left (obstructed) kidney (gray bars) and the right (nonobstructed) kidney (open bars); n = 6 each. *P < 0.05 vs. control. **P < 0.05 vs. 50 mg/kg imatinib or 1.5 mg/kg rapamycin.
Matrix Accumulation
Collagen type I, type III, and fibronectin are important extracellular matrix proteins that contribute the bulk of interstitially accumulating fibrous matrix in renal fibrosis in general, and obstructive nephropathy specifically. The expression of these matrix proteins is each several fold increased in obstructive nephropathy compared with control kidneys (Fig. 3, B–D). The mRNA levels encoding col3A1 and col1A2 as well as fibronectin are substantially reduced by high doses of either imatinib or rapamycin (Fig. 2, B–D) consistent with their effects on myofibroblast number. Moreover, a further reduction beyond the effects of each of the two drugs alone is achieved when both drugs are combined at their high dose. A combination of both drugs each at a substantially lower dose is approximately as effective as treatment with maximal concentrations of either drug alone (Fig. 3, B–D).
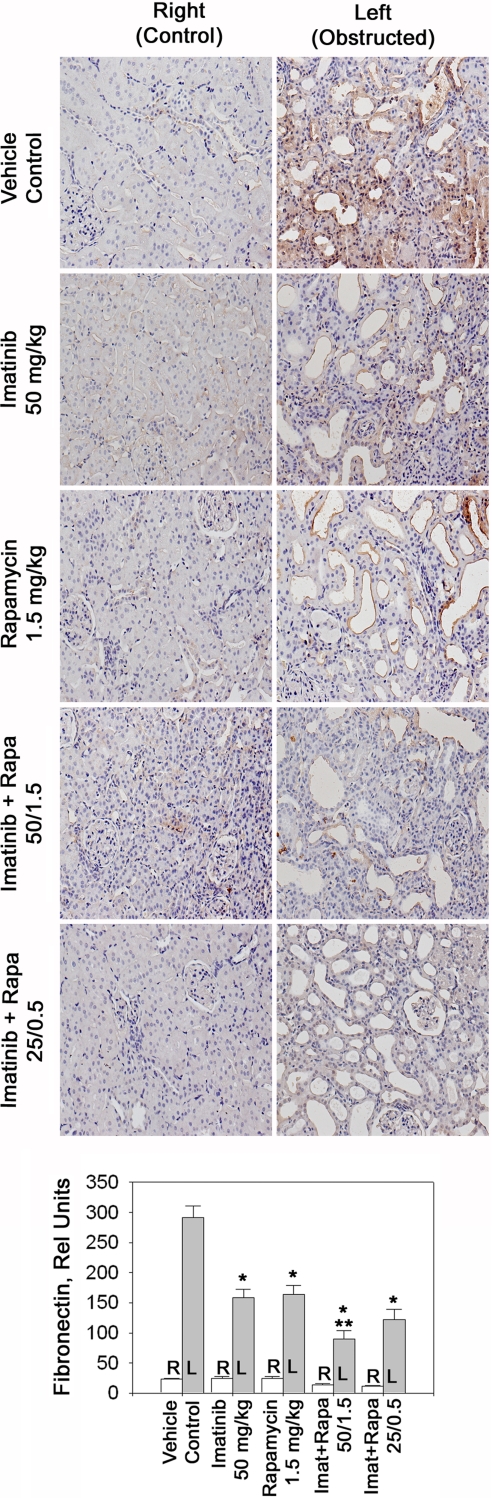
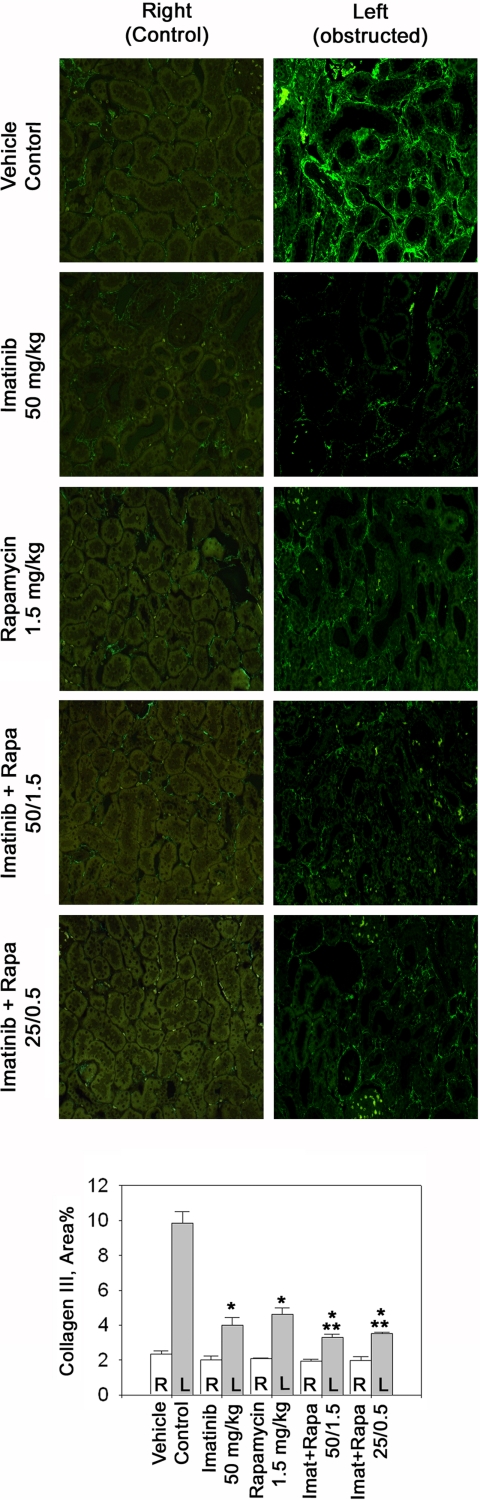
Interstitial matrix accumulation is not only a function of increased gene transcription but also of reduced matrix protein degradation. Thus, we also examined accumulated levels of renal interstitial collagen type III and fibronectin (Figs. 4 and 5). In obstructed compared with control kidneys, interstitial collagen type III increased ∼5-fold (Fig. 4) and fibronectin levels were increased 15-fold compared with control kidneys (Fig. 5). High-dose imatinib as well as rapamycin each reduced collagen III and fibronectin levels by ∼50–60% (Figs. 4 and 5). Both compounds given in combination had an additive effect and tended to reduce collagen III further and reduced fibronectin significantly more than either drug alone (Figs. 4 and 5). The low-dose combination tended to be less effective than both drugs given together at their high dose but tended to be more effective than high-dose rapamycin or imatinib alone.
Fig. 4.
Levels of fibronectin in obstructive nephropathy. Representative sections of control and obstructed kidneys stained with anti-fibronectin antibody. Animals were treated as indicated and fibronectin expression was determined by immunohistochemistry. The bar graph displays quantification of immunoreactive renal interstitial fibronectin; n = 6 each. *P < 0.05 vs. control. **P < 0.05 vs. 50 mg/kg imatinib or 1.5 mg/kg rapamycin.
Fig. 5.
Non-smad TGF-β signaling is required for in vivo collagen III accumulation. Representative immunofluorescence sections of control and UUO kidneys stained with anti-collagen III. The bar graph displays quantification of immunoreactive renal interstitial collagen III; n = 6 each. *P < 0.05 vs. control. **P < 0.05 vs. 50 mg/kg imatinib or 1.5 mg/kg rapamycin.
DISCUSSION
TGF-β is the single most important cytokine inducing and accelerating parenchymal organ (and renal) fibrogenesis. The mechanisms of action have been unraveled during the past two decades and smad2 and especially smad3 were thought to be the only or major profibrogenic mediators downstream of TGF-β (14, 15). Clearly, smads play important roles in renal fibrogenesis and comprise an important pathway in epithelial cells including tubular cells. However, the involvement of TGF-β in renal fibrogenesis has become more complex with the discovery of cell-specific pathways downstream of the TGF-β receptor complex (4, 13, 17, 18).
These non-smad targets are primarily associated with fibroproliferative disorders under TGF-β control. The most upstream component currently identified in this cascade is phosphatidylinositol-3′-kinase (PI3K) (19). This enzyme represents a branchpoint in the mesenchymal cell and fibroblast response to TGF-β leading to activation of PAK2 and c-Abl (Fig. 6) (4, 18). Moreover, inhibition of Abl activity with imatinib reduces fibroblast proliferation and renal fibrogenesis in vivo without reducing phospho-smad2/3 levels and, hence, independent of the canonical TGF-β-smad pathway (17).
Fig. 6.
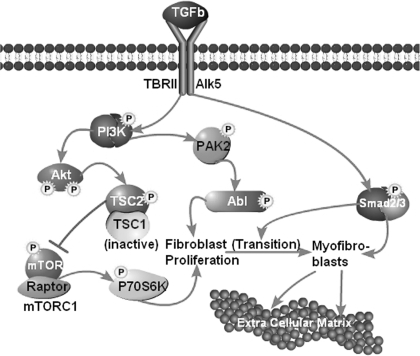
Working model of TGF-β actions in renal interstitial fibrogenesis. The canonical pathway through smad2/3 contributes primarily to the transition of fibroblasts into myofibroblasts and to the transcriptional activation of various extracellular matrix proteins in myofibroblasts as well as in epithelial and nonepithelial glomerular and tubular cells. The noncanonical (smad-independent) pathways are initiated by activation of PI3K which is a branch point for the activation of PAK2-Abl. The latter induces fibroblast proliferation, thereby increasing the number of myofibroblast precursors. PI3K also activates Akt. One of its downstream targets is TSC2 which becomes phosphorylated and thereby inactivated. Active (nonphosphorylated) TSC2 functions as a GTPase-activating protein (GAP) protein converting mTORC1-activating Rheb-GTP to Rheb-GDP. Thus, active TSC2 is an inhibitor of mTORC1, and loss of TSC2 activity by phosphorylation increases mTORC1 activity. This activates downstream substrates including P70S6 kinase, a translational activator of many proteins including cell cycle proteins and hence, proliferation. The Akt → mTORC1 → P70S6K branch pathway contributes also to fibroblast proliferation. Thus, in fibroblasts, the noncanonical and canonical TGF-β pathways closely collaborate: the noncanonical pathway induces fibroblast proliferation and the canonical smad2/3 pathway induces transition of the increased number of fibroblasts into active myofibroblasts and acts in these latter cells as transcriptional activator of multiple extracellular matrix proteins as well as regulators of matrix accumulation.
PI3K also activates Akt which can engage multiple downstream signaling substrates and pathways. These include tuberin [tuberous sclerosis complex-2 (TSC2)] which binds to hamartin (TSC1). The latter contributes stability and prolongs the half-life of TSC2 within this complex. The function of TSC2 has recently been unraveled: it acts as a GTPase-activating protein (GAP) specifically for Rheb (Ras homolog enriched in brain) (8). GTP-Rheb activates mTORC1 which plays important roles in cell proliferation (22). The active (nonphosphorylated) TSC1/2 complex converts Rheb-GTP to Rheb-GDP and, hence, reduces mTORC1 activity. By this indirect mechanism, active TSC2 blocks mTORC1. Phosphorylation of TSC2 inactivates this molecule and, hence, reduces its ability to block mTORC1. Huang and associates (7) showed very recently that the activated TSC1/2 complex is a negative regulator of mTORC1 but an activator of mTORC2. Rapamycin which directly inhibits raptor within mTORC1 inhibits the activity of this complex but not of mTORC2.
TGF-β activates mTORC1 in fibroblasts but not in most epithelial cells (13). Given the downstream activity of mTORC1 to contribute to cell growth and cell cycle activation, this pathway also contributes to the proliferation of fibroblasts which occurs in obstructive nephropathy as shown in the present and previous studies from this laboratory (17). Prabhu and co-workers (12) recently demonstrated cooperation between mTORC1 and Abl in chronic myelogenous leukemia cells most likely through the formation of a translationally active complex enhancing expression of cyclin D3. Thus, it is possible that TGF-β-activated mTORC1 and Abl have both independent as well as dependent functions in renal fibrogenesis. A cartoon depicting a working model of TGF-β signaling in fibroblasts as it is relevant to renal fibrogenesis is presented in Fig. 6.
We previously showed that the Abl inhibitor, imatinib, reduces renal fibrogenesis through inhibiting fibroblast proliferation in rats with unilateral obstructive nephropathy (17). In these previous studies, comparably high doses of imatinib were used, namely a total of 750 mg/kg over 7 days. In the present studies, we show that lesser doses are sufficient (350 mg/kg over 7 days as the presently used “high dose” of imatinib). The present findings that administration of the mTORC1 inhibitor rapamycin also reduces renal fibrogenesis confirm previous results by Wu and associates (21) in the same experimental model of obstructive nephropathy. Since in addition to Abl mTORC1 activation by TGF-β also contributes to fibrogenesis which can be reduced by rapamycin virtually calls for combination therapy with both imatinib and rapamycin. As each of the two drugs has substantial side effects, these may be reduced if a combination with drug-spearing dosing is used. Indeed, the current findings indicate that the use of imatinib and rapamycin, each at a low dose, has utility in the therapy of fibrogenesis in experimental chronic renal disease.
GRANTS
The present studies were supported, in part, by the National Institutes of Health Grant DK-63360 (to R. Hirschberg) and Public Health Services Grants GM-54200 and GM-55816 from the National Institutes of General Medical Sciences and the Mayo Foundation (to E. B. Leof).
DISCLOSURES
There are no disclosures by any of the authors.
REFERENCES
- 1.Bohle A, Strutz F, Muller GA. On the pathogenesis of chronic renal failure in primary glomerulopathies: a view from the interstitium. Exp Nephrol 2: 205–210, 1994 [PubMed] [Google Scholar]
- 2.Bohle A, Wehrmann M, Bogenschutz O, Batz C, Muller CA, Muller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract 187: 251–259, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Bohle A, Wehrmann M, Mackensen-Haen S, Gise H, Mickeler E, Xiao TC, Muller C, Muller GA. Pathogenesis of chronic renal failure in primary glomerulopathies. Nephrol Dial Transplant 9, Suppl 3: 4–12, 1994 [PubMed] [Google Scholar]
- 4.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 114: 1308–1316, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty NG, O'Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290: F4–F13, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Fukuda K, Yoshitomi K, Yanagida T, Tokumoto M, Hirakata H. Quantification of TGF-β1 mRNA along rat nephron in obstructive nephropathy. Am J Physiol Renal Physiol 281: F513–F521, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol 28: 4104–4115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17: 1829–1834, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneto H, Ohtani H, Fukuzaki A, Ishidoya S, Takeda A, Ogata Y, Nagura H, Orikasa S. Increased expression of TGF-beta1 but not of its receptors contributes to human obstructive nephropathy. Kidney Int 56: 2137–2146, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev 19: 2783–2810, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Prabhu S, Saadat D, Zhang M, Halbur L, Fruehauf JP, Ong ST. A novel mechanism for Bcr-Abl action: Bcr-Abl-mediated induction of the eIF4F translation initiation complex and mRNA translation. Oncogene 26: 1188–1200, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimi RA, Andrianifahanana M, Wilkes MC, Edens M, Kottom TJ, Blenis J, Leof EB. Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-beta. Cancer Res 69: 84–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts AB, Russo A, Felici A, Flanders KC. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann NY Acad Sci 995: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev 17: 19–27, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Taft JL, Nolan CJ, Yeung SP, Hewitson TD, Martin FI. Clinical and histological correlations of decline in renal function in diabetic patients with proteinuria. Diabetes 43: 1046–1051, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J 19: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Wilkes MC, Leof EB. Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J Biol Chem 281: 27846–27854, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Wilkes MC, Mitchell H, Penheiter SG, Dore JJ, Suzuki K, Edens M, Sharma DK, Pagano RE, Leof EB. Transforming growth factor-beta activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res 65: 10431–10440, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Wilkes MC, Murphy SJ, Garamszegi N, Leof EB. Cell-type-specific activation of PAK2 by transforming growth factor beta independent of Smad2 and Smad3. Mol Cell Biol 23: 8878–8889, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int 69: 2029–2036, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Noble NA, Miller DE, Border WA. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int 45: 916–927, 1994 [DOI] [PubMed] [Google Scholar]