Abstract
Urethral reflexes are important regulators of micturition, and impairment of urethral afferent neuronal function may disrupt coordinated bladder and urethral activity, thereby contributing to voiding dysfunction in lower urinary tract disorders. Chemical stimulation by intraurethral irritant solution perfusion was used to determine whether urethral afferent neuronal function is altered in diabetes mellitus (DM). Sprague-Dawley rats were studied 10 wk after streptozotocin injection to induce DM or vehicle alone. Escalating doses of capsaicin (0.1–30 μM) or acetic acid (0.01–1%; AA) were perfused intraurethrally while recording isovolumetric bladder activity, urethral perfusion pressure, and electromyography of the external urethral sphincter (EUS-EMG). Some rats were additionally treated with α-bungarotoxin, hexamethonium, or bilateral transection of the sensory branches of the pudendal nerves (PudSNx). Intraurethral capsaicin inhibited bladder contractions in six out of seven control rats but not in any of six DM rats. Low-frequency oscillations (LFOs) of intraurethral pressure were observed in five out of six control rats with capsaicin-induced bladder inhibition. In contrast, intraurethral AA inhibited bladder contractions and enhanced tonic EUS-EMG activity in six out of six control and five out of six DM rats. LFOs occurred in four out of six control and three of five DM rats with AA-induced bladder inhibition. Chemically induced bladder inhibition and LFOs were not prevented by α-bungarotoxin but were eliminated by PudSNx and hexamethonium. Finally, LFOs were followed by phasic EUS activity. These findings show that DM affects urethral afferent neurons differentially, compromising those expressing TRPV1 receptors. Urethral smooth muscle LFOs are neurogenically mediated and induce EUS activity, revealing the existence of a hitherto undescribed reflex pathway: a smooth-to-striated muscle urethra-to-urethra reflex.
Keywords: diabetes mellitus, urethra, capsaicin, acetic acid, bladder, external urethral sphincter
bladder dysfunction in patients with chronic diabetes mellitus (DM) (diabetic cystopathy) is characterized by impaired bladder sensation, increased bladder capacity, decreased bladder contractility, and increased residual urine. Diabetic cystopathy has been attributed primarily to peripheral polyneuropathy, involving autonomic and somatic sensory and motor nerves (11, 22, 34).
Normal urine storage and bladder emptying requires precise coordination between the smooth muscles of the bladder and urethra, and urethral innervation plays a prominent role in that coordination. In addition to efferent coordination of urethral motor events necessary for efficient voiding, activation of urethral afferents can evoke reflexes that augment (3, 8, 12, 13) or inhibit (5, 31) reflex bladder contractions. The urethra also plays an important role in urinary continence (8, 9, 13, 15). Thus impairment of urethral afferents in DM may disrupt the coordination between the bladder and urethra, compounding diabetic cystopathy and thereby worsening voiding dysfunction in DM (35).
Whereas previous studies have examined the effects of DM on the smooth and striated muscle of the urethra (32, 33), few studies have examined the possibility that DM may damage the innervation of the urethra (2, 23, 36). Several studies have demonstrated DM-induced damage to bladder afferent neurons, with Aδ and C fiber afferent neurons being injured and the responses to chemical stimulation with capsaicin and acetic acid being reduced (7, 10, 20, 27, 28, 30), and similar damage to urethral afferent neurons should be expected. Accordingly, the current study investigated chemical stimulation of urethral afferent neurons with capsaicin or dilute acetic acid to determine whether urethral afferent neuronal function is altered in DM.
MATERIALS AND METHODS
Rats and induction of DM.
Sixty-one adult female Sprague-Dawley rats (Charles River, Wilmington, MA) initially weighing 250–275 g were used (Table 1). DM was induced in isoflurane-anesthetized rats after 18 h of fasting by a single intraperitoneal injection of streptozotocin at 65 mg/kg in citrate buffer; control rats of the same age were injected with citrate buffer only. Blood glucose was monitored weekly with a FreeStyle Flash blood glucose meter (Abbott Diabetes Care, Alameda, CA). All DM rats had blood glucose levels of at least 300 mg/dl. The rats were allowed to survive for 10 wk after injection. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committees at the Duke University and Durham Veterans Affairs Medical Centers.
Table 1.
Animal usage
| Groups |
Chemical Only | BGT + Chemical | PudSNx + Chemical | Chemical + Hex | |
|---|---|---|---|---|---|
| Chemical | State | ||||
| CAP | Controls | 7 | 4 | 4 | 4 |
| DM | 6 | 4 | 4 | 0 | |
| AA | Controls | 6 | 4 | 4 | 0 |
| DM | 6 | 4 | 4 | 0 | |
| Total | 25 | 16 | 16 | 4 | |
CAP: capsaicin; AA: acetic acid; PudSNx, bilateral transection of the sensory branches of the pudendal nerves; BGT: α-bungarotoxin; Hex: hexamethonium.
Pressure and electromyography of the external urethral sphincter recordings.
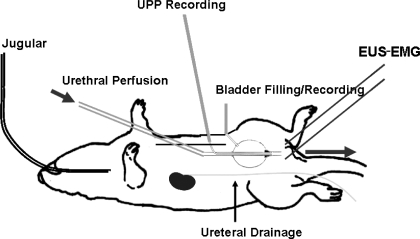
Rats were anesthetized with 1.2 g/kg urethane subcutaneously before surgery. One jugular vein was catheterized to allow intravenous injections. In rats undergoing treatment with the neuromuscular blocker α-bungarotoxin, the trachea was cannulated to permit mechanical respiration. A midline abdominal incision was made, the descending colon was gently cleared of feces, and the proximal part of the descending colon was loosely ligated to prevent further migration of feces that could compress the urethra. Both ureters were transected, the proximal ends were cannulated with PE-10 tubing, and the free ends of the cannulas were externalized to prevent contribution of urine formation to bladder volume. A bladder catheter to record bladder pressure was inserted through the bladder dome (Fig. 1), and bladder capacity was determined by slowly filling the bladder until voiding occurred. Following the bladder capacity measurement, a double-lumen urethral catheter for urethral perfusion and measurement of urethral perfusion pressure (UPP) was also inserted through the bladder dome as depicted in Fig. 1. The double-lumen urethral catheter consisted of internal PE-50 tubing for recording UPP and external PE-160 tubing for urethral perfusion. A cone-shaped plug fashioned from a 200-μl Eppendorf pipette tip and serving as the tip of the double-lumen urethral catheter was seated in the bladder neck. This set-up allows functional separation of the bladder and urethra without risk of damage to the innervation or blood supply of the bladder neck and urethra. The abdomen was closed in layers. Two fine polytetrafluoroethylene-coated silver electrodes were inserted periurethrally via a percutaneous approach to record electromyography of the external urethral sphincter (EUS-EMG) activity. These electrodes were connected through HZP high-impedance probes to P511 AC amplifiers (Grass-Telefactor, West Warwick, RI).
Fig. 1.
The isolated bladder-urethra preparation. The double-lumen urethral catheter consisted of external PE-160 tubing for urethral perfusion and internal PE-50 tubing for recording urethral perfusion pressure (UPP). A cone-shaped plug fashioned from a 200-μl Eppendorf pipette tip and serving as the tip of the double-lumen urethral catheter was seated in the bladder neck to separate the urethra from the bladder functionally. Separate PE-50 tubing was used for bladder filling and pressure recording.
The urethra was perfused with normal saline (0.9% NaCl) via the outer lumen of the urethral catheter at 0.075 ml/min. Saline was slowly infused in the bladder until rhythmic isovolumetric bladder contractions were induced, at which point bladder volume was increased by 50%. Pressure transducers were mounted at the height of the pubic symphysis. PowerLab hardware and Chart software (ADInstruments, Colorado Springs, CO) were used for data acquisition with a sampling rate of 1 kHz. Isovolumetric bladder pressure, UPP, and EUS-EMG activity were recorded following a 30-min postsurgical stabilization period.
Drugs and treatments.
Table 1 indicates the numbers of rats undergoing each type of treatment. Streptozotocin was dissolved in 0.1 M citrate buffer (pH 4.5). Capsaicin (M-2028; Sigma) was prepared in concentrations ranging from 0.1 to 30 μM in vehicle (1% ethanol in normal saline). Acetic acid was added to normal saline at concentrations ranging from 0.01 to 1%. Both capsaicin and acetic acid were given in escalating doses, and the responses were recorded over 30-min intervals.
Some rats received additional treatments to discriminate between mechanisms affecting UPP. The neuromuscular blocker α-bungarotoxin, which blocks EUS activity and thereby allows study of urethral smooth muscle in isolation, was dissolved in normal saline at 1 mg/ml and intravenously administered at 100 μg/kg to 16 rats that were subsequently artificially ventilated. Hexamethonium, which prevents binding of acetylcholine to nicotinic cholinergic receptors in autonomic ganglia, was freshly dissolved in normal saline and intravenously administered at 25 mg/kg to four rats (14). Finally, to determine the role of pudendal afferent neurons in the response of the urethra to chemical stimulation, the sensory branches of the pudendal nerves (PudSNx) were exposed via a posterior approach through the distal gluteus major muscles and bilaterally transected just proximal to its descent in the ischiorectal fossa in 16 rats under urethane anesthesia as previously described (17, 21, 25, 26).
Intravenous 0.2-ml injections of vehicle or drug were made over a period of ∼1 min followed by 0.1 ml normal saline to flush the catheter. All drugs were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analysis.
Data are presented as means ± SE. Student's t-test, Fisher's exact test, and two-way ANOVA were used for statistical analysis using Prism 4 (GraphPad, San Diego, CA). P < 0.05 was considered statistically significant.
RESULTS
General characteristics.
Blood glucose levels were 414.5 ± 9.8 mg/dl in DM rats and 99.3 ± 1.4 mg/dl in control rats (P < 0.0001). DM rats had a lower body mass at 10 wk postinjection (248.8 ± 4.5 g in DM vs. 325.8 ± 3.6 g in controls, P < 0.0001) and a higher bladder capacity (5.38 ± 0.22 ml in DM vs. 0.65 ± 0.05 ml in controls, P < 0.0001).
Effects of intraurethral chemical perfusion on bladder contractions and UPP.
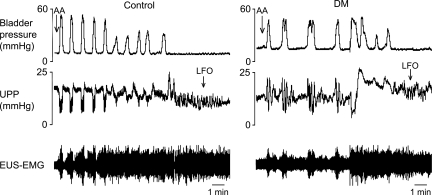
In both control and DM rats, contraction of the bladder was associated with relaxation of the urethra. During urethral relaxation, high-frequency oscillations (HFOs) of urethral pressure were present in both control and DM rats and were associated with phasic activity of the EUS (Fig. 2, A and B, for example of a control rat). Similar to previous results from this laboratory (35), the quality of HFOs was dramatically altered in DM rats vs. controls [P < 0.05 for overall DM effect on lowering HFO amplitude (P = 0.037) and on lowering frequency (P = 0.025) by 2-way ANOVA]. No effect was seen on HFO duration period.
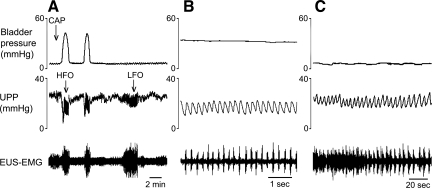
Fig. 2.
Response of bladder contractions and urethral activity to intraurethral administration of capsaicin in a control rat. Each panel shows isovolumetric bladder pressure (top), UPP (middle), and electromyography of the external urethral sphincter (EUS-EMG, bottom) recordings. A: cessation of bladder contractions and micturition-associated urethral relaxations and high-frequency oscillations (HFOs) following intraurethral administration of capsaicin (CAP). Note the appearance of urethral low-frequency oscillations (LFOs) following cessation of bladder activity. B and C compare, with different time bases, HFOs and LFOs, respectively. Numeric comparisons are found in Table 2.
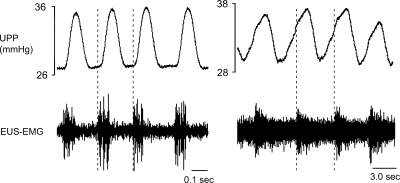
Addition of capsaicin to the intraurethral perfusate inhibited bladder contractions in six out of seven control rats at a median dose of 3 μM and consequently also eliminated urethral relaxations and HFOs (Fig. 2A). Unexpectedly, five out of the six control rats in which bladder contractions were inhibited by capsaicin also developed slower grouped oscillations of urethral pressure (0.14 ± 0.04 Hz) that we term low-frequency oscillations (LFOs; Fig. 2C). As far as we are aware, this is the first observation of such urethral activity, and it appears to be revealed only after isolated urethral irritation-induced inhibition of bladder contractions. LFOs lasted 2–9 min before spontaneously stopping, whereas bladder contractions remained inhibited for 30–60 min. As shown in Fig. 3, each LFO contraction was associated with an increase in EUS-EMG activity. However, whereas during HFOs the onset of each burst of EUS-EMG activity precedes and presumably induces a rise in UPP, the rise in UPP during LFOs precedes the onset of EUS-EMG activity. HFOs and LFOs are compared in more detail in Table 2.
Fig. 3.
The temporal relationship of HFOs and LFOs to associated EUS-EMG activity. Although HFOs (left) are caused by preceding high frequency bursts of EUS activity, LFOs (right) precede and thus apparently cause bursts of EUS activity. Note the marked difference in time scale.
Table 2.
Characteristics of preirritation LFOs and HFOs induced by intraurethral administration of chemical irritants in rats with bladder inhibition
| Treatment | Groups | Frequency, Hz |
Amplitude, mmHg |
Durations, s |
|||
|---|---|---|---|---|---|---|---|
| LFO | HFO | LFO | HFO | LFO | HFO | ||
| CAP | Control | 0.14±0.04§ (n=5) | 4.85±0.19 (n=6) | 6.7±1.2 (n=5) | 7.6±2.0 (n=6) | 288±12§ (n=5) | 30.8±6.0 (n=6) |
| DM | (n=0) | 4.57±0.16 (n=6) | (n=0) | 2.55±0.62 (n=6) | (n=0) | 21.0±4.4 (n=6) | |
| AA | Control | 0.21±0.07§ (n=4) | 4.77±0.17 (n=6) | 5.6±1.6 (n=4) | 6.9±2.8 (n=6) | 390±93*(n=4) | 26.6±4.8 (n=6) |
| DM | 0.19±0.07*(n=3) | 3.72±0.53 (n=5) | 6.5±2.6 (n=3) | 3.7±1.0 (n=5) | 280±20*(n=3) | 28.9±3.5 (n=5) | |
Values are means ± SE. LFO and HFO, low-frequency and high-frequency oscillations, respectively.
Indicates P < 0.05 as compared to HFO;
Indicates P < 0.001 as compared to HFO.
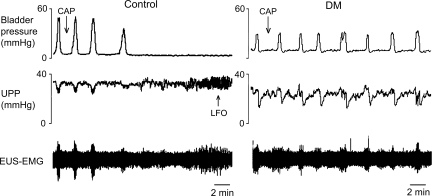
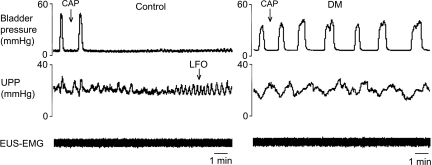
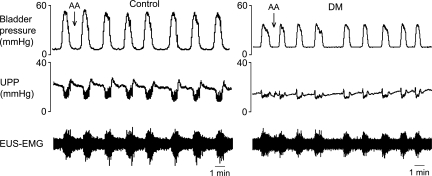
Interestingly, whereas capsaicin inhibited bladder contractions and thereby urethral relaxation and HFOs in six out of seven control rats, it was without effect (0 out of 6) in DM rats (P = 0.005; Fig. 4). LFOs were observed in five out of the six control rats with capsaicin-inhibited bladder contractions, but not in any DM rats (P = 0.015; Fig. 4).
Fig. 4.
Different responses of bladder contractions and urethral activity to intraurethral administration of capsaicin in control rats and rats with diabetes mellitus (DM). Intraurethral capsaicin inhibited isovolumetric bladder contractions in control rats but not in DM rats. LFOs were seen only in control rats.
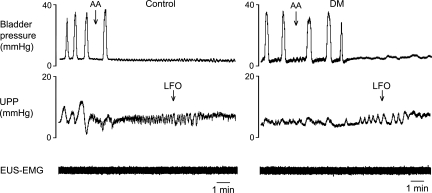
Addition of acetic acid to the intraurethral perfusate eliminated bladder contractions in all six control rats and five out of six DM rats (P = 1.0; Fig. 5). The median effective concentration was 0.10% in control rats and 0.07% in DM rats (P = 0.337). LFOs developed in four out of six control rats and three out of five DM rats (P = 1.0) in which bladder contractions were inhibited following acetic acid exposure.
Fig. 5.
Responses of bladder contractions and urethral activity to intraurethral administration of dilute acetic acid (AA) in control and DM rats. AA inhibited isovolumetric bladder contractions in both controls and DM rats. LFOs were seen in both the control (left) and DM (right) rat after bladder contractions ceased.
Mechanisms of bladder inhibition and LFOs.
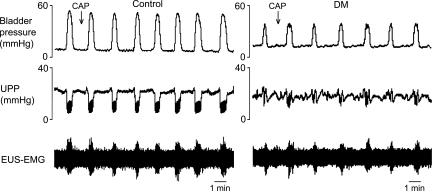
To determine whether LFOs arose from skeletal or smooth muscle contraction, we investigated the effect of systemic α-bungarotoxin in both control and DM rats. α-Bungarotoxin had no effect on bladder activity and did not affect the ability of capsaicin (Fig. 6) or acetic acid (Fig. 7) to eliminate bladder contractions and induce LFOs in control animals and control and DM animals, respectively. As previously seen without neuromuscular blockade, following α-bungarotoxin, capsaicin still only induced inhibition of bladder contractions and emergence of LFOs in control animals, whereas acetic acid did so for both control and DM animals. Thus neither bladder inhibition nor LFOs depended on activity of the skeletal muscle EUS.
Fig. 6.
Effect of neuromuscular blockade by α-bungarotoxin on the responses to capsaicin in control and DM rats. α-Bungarotoxin was administered iv before iu administration of capsaicin. α-Bungarotoxin blocked EUS-EMG activity but did not alter the effects of capsaicin on smooth muscle activity in either control or DM rats. Once again, capsaicin inhibited bladder contractions and resulted in subsequent LFOs in control rats (left), but not in DM rats (right).
Fig. 7.
Effect of neuromuscular blockade by α-bungarotoxin on the responses to dilute acetic acid in control and DM rats. α-Bungarotoxin was administered iv before iu administration of acetic acid. α-Bungarotoxin blocked EUS-EMG activity but did not alter the effects of acetic acid on smooth muscle activity in either control or DM rats. Once again, acetic acid inhibited bladder contractions and resulted in subsequent LFOs in both control rats (left) and DM rats (right).
To determine whether pudendal nerve afferent fibers were involved in the generation of LFOs, indicating a urethra-to-urethra reflex, we investigated the effect of PudSNx in both control and DM rats. PudSNx eliminated the ability of both capsaicin (Fig. 8) and acetic acid (Fig. 9) to either inhibit bladder contractions or induce LFOs.
Fig. 8.
Pudendal afferent signaling is essential for intraurethral capsaicin-induced inhibition of bladder contraction. Bilateral transection of the sensory branches of the pudendal nerves (PudSNx) caused loss of inhibition of bladder contractions induced by intraurethral capsaicin (30 μM) in control rats (left) and was without effect in DM (i.e., capsaicin was still unable to inhibit bladder activity; right). These data support a role for pudendal afferents in conferring the capsaicin effect of bladder activity inhibition.
Fig. 9.
Pudendal afferent signaling is essential for intraurethral acetic acid-induced inhibition of bladder contraction. After PudSNx, acetic acid did not inhibit bladder contractions in control rats (left) or DM rats (right) as it had previously in pudendal intact animals, again indicating that such inhibition was mediated via the sensory branch of the pudendal nerve.
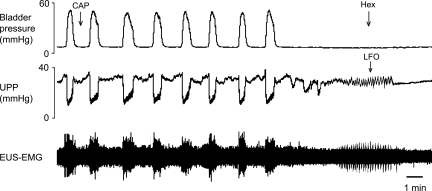
To determine whether LFOs were myogenic (originating in the urethral smooth muscle) or neurogenic (dependent on peripheral nerve activity and spinal or supraspinal sites), the autonomic ganglionic blocker hexamethonium was administered intravenously to four control rats during capsaicin-induced LFOs. Hexamethonium eliminated the LFOs in all four rats (Fig. 10), indicating that LFOs are neurally mediated.
Fig. 10.
Role of autonomic efferent innervation in the genesis of LFOs. Intravenous administration of hexamethonium (Hex) at a dose that blocks autonomic ganglionic transmission (25 mg/kg) eliminated the LFOs in a control rat following intraurethral capsaicin.
DISCUSSION
Chemical stimulation of urethral afferent neurons inhibits isovolumetric bladder contractions in control rats.
Stimulation of urethral nociceptors by intraurethral perfusion of capsaicin in control rats has previously been shown to produce complete inhibition of bladder contractions (6, 13). We confirmed that finding in this study, and additionally showed that stimulation of urethral afferent neurons with acetic acid similarly inhibited bladder contractions. The simplest explanation for these results is that stimulation of either TRPV1+ (and thus capsaicin-sensitive) urethral afferent neurons or TRPV1−, acid-sensitive urethral afferent neurons, activates an inhibitory urethral-to-bladder reflex. It was shown by Conte et al. (6) that bilateral transection of the pudendal nerves prevents the capsaicin-stimulated inhibitory reflex. Similarly, we have shown here that selective PudSNx prevented the reflex with both capsaicin and acetic acid stimulation, confirming that urethral afferent neurons traveling in the pudendal nerves, whether TRPV1+ neurons or TRPV1−, acid-sensing neurons (or both), are responsible for the urethra-to-bladder inhibitory reflex. It is known that rat nociceptors can contain either TRPV1, ASIC, or non-ASIC, non-TRPV1 (NANT) proton-sensing ion channels, but they can also contain all three types of proton-sensing ion channels (19). The current study design does not allow us to discriminate between the possibilities that DM selectively targets urethral TRPV1+ neurons for neuropathic destruction and spares the other urethral TRPV1−, ASIC+, NANT+ afferent neurons, or that DM reduces the expression of TRPV1 in these afferents (a phenotype shift; see Ref. 24).
An additional possibility is that afferents that are involved in an excitatory urethra-to-bladder reflex are desensitized by capsaicin in control animals and, in both control and DM animals, damaged by acetic acid. Such a reflex, which was also followed by inhibition of bladder activity following intraurethral capsaicin, has been described previously using a similar technique (13). For this possibility to be responsible for the observed results in this manuscript, it is necessary to assume that basal activity from these afferents during the filling phase of the micturition cycle is not only modulatory but it is necessary to induce micturition. Otherwise, desensitization or damage of these afferents might modulate but would not be expected to eliminate bladder contractions. Moreover, with an excitatory scenario, PudSNx would necessarily result in bladder inhibition, which did not occur.
Furthermore, it should be noted that both TRPV1- and ASIC-like channels have been described in bladder urothelium (18). It is possible that changes in urethral urothelial proton sensors may be responsible or contribute to the results of this study.
Most peptidergic afferent nerve fibers in the urethra are removed by interruption of the pelvic and hypogastric nerves via bilateral pelvic ganglionectomy (1). Therefore, the urethral afferents traveling in the pudendal nerve and forming the afferent limb of the urethra-to-bladder inhibitory reflex must be nonpeptidergic TRPV1+ neurons or must represent only a small proportion of urethral peptidergic terminals.
Chemical stimulation of urethral afferent neurons with capsaicin yields very different effects in control and DM rats.
TRPV1 and ASIC channels are proton sensors of nociceptive afferents (24). Both capsaicin (TRPV1 agonist) and acetic acid (TRPV1 and ASIC agonist) have been utilized heavily in the literature as standard lower urinary tract irritants, and both types of proton sensors have been demonstrated in the urinary tract (4, 16, 18).
Whereas intraurethral perfusion of both capsaicin and acetic acid inhibited bladder contractions in control rats, only acetic acid had this effect in DM rats. The absence of the TRPV1−-mediated inhibitory urethral-to-bladder reflex in DM rats provides convincing evidence not only that urethral afferent neuropathy and/or phenotypic change occurs in DM, but that it occurs selectively in the TRPV1 receptor-expressing urethral afferent neurons. Proton sensitivity that is not dependent on TRPV1 receptors appears to remain functional following DM, at least up to the 10 wk duration of our study.
The inhibition of bladder contractions induced by chemical stimulation of urethral afferent neurons is associated with the emergence of LFOs.
Shortly after the inhibitory urethra-to-bladder reflex is evoked with either capsaicin or acetic acid, oscillations of urethral pressure begin in most rats which persist for an average of ∼6 min. Because their frequency is ∼35 times lower than the “HFOs” of urethral pressure attending phasic EUS activity (Table 2), we refer to them as “LFOs.”
LFOs appear to induce EUS activity, whereas HFOs are induced by EUS activity. The induction of EUS activity by LFOs is suggested by the phase lag between LFOs and bursts of tonic EUS-EMG activity (Fig. 3); their independence from EUS activity, quite different from HFOs, is shown by the loss of EUS activity but not LFOs following α-bungarotoxin treatment (Figs. 6 and 7). The relationship between LFOs and ensuing EUS activity suggests that chemical stimulation of the urethra induces a hitherto unknown reflex pathway, in this case an excitatory urethral smooth muscle-to-urethral striated muscle reflex. It is indeed interesting to speculate that these results may provide evidence of a physiological role of the well-documented pudendal-to-pudendal reflex in the rat (5, 29), with increases in urethral smooth muscle tension driving pudendal afferents and resulting in an EUS response.
PudSNx prevents the capsaicin- and acetic acid-induced inhibitory urethra-to-bladder reflex that is necessary to demonstrate LFOs; we therefore cannot tell whether the pudendal nerve carries the afferent fibers of the urethra-to-urethra reflex as well as those of the urethra-to-bladder reflex.
LFOs depend on ganglionic transmission, as shown by their interruption with hexamethonium treatment (Fig. 10), indicating that they are not myogenic in origin. That they were only observable when the bladder is quiescent suggests a function not associated with micturition and of uncertain significance. Although it is unlikely that pudendal nociceptors play an active role in normal lower urinary tract (LUT) activity, that reflexes they evoke can be compromised by DM suggests that these and other LUT reflexes affected by afferent neuropathy may play a more complex role in diabetes-induced LUT dysfunction than once imagined.
Thus the current study suggests that, in DM, either TRPV1+ pudendal afferents that drive the inhibitory urethra-to-bladder reflex are selectively destroyed or that TRPV1 receptors are selectively downregulated in these afferents. The current study did not allow for discrimination of these two possibilities. Additionally, that the inhibitory periods were followed only for 30–60 min does not allow us to say with any certainty that we were not simply desensitizing an excitatory reflex using capsaicin in control animals and that acetic acid simply destroyed afferents, resulting in the same effect. As stated previously, however, the results of PudSNx do not support the latter scenario. Future studies should include histological examination of urethral afferent fields to determine whether there are decreases in the extent of afferent innervation following DM, suggesting selective neuropathic lesion, or whether there is selective loss of TRPV1 expression.
In conclusion, these findings suggest that DM compromises urethral afferents differentially, selectively affecting those afferent nociceptive neurons expressing TRPV1 receptors. Urethral smooth muscle LFOs are neurogenically mediated and induce associated EUS activity. They suggest the existence of a hitherto undescribed reflex pathway: a smooth-to-striated muscle urethra-to-urethra reflex.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-61391 and a Veterans Affairs Merit Review.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Alm P, Zygmunt PK, Iselin C, Larsson B, Uvelius B, Werner S, Andersson KE. Nitric oxide synthase-immunoreactive, adrenergic, cholinergic, and peptidergic nerves of the female rat urinary tract: a comparative study. J Auton Nerv Syst 56: 105–114, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Andersen JT, Bradley WE. Early detection of diabetic visceral neuropathy. An electrophysiologic study of bladder and urethral innervation. Diabetes 25: 1100–1105, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Andersson KE. Bladder activation: afferent mechanisms. Urology 59: 43–50, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Avelino A, Cruz F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn-Schmiedeberg's Arch Pharmacol 373: 287–299, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Cheng CL, Chen JJ, Peng CW, de Groat WC. Reflexes evoked by electrical stimulation of afferent axons in the pudendal nerve under empty and distended bladder conditions in urethane-anesthetized rats. J Neurosci Methods 150: 80–89, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte B, Maggi CA, Meli A. Vesico-inhibitory responses and capsaicin-sensitive afferents in rats. Naunyn Schmiedebergs Arch Pharmacol 339: 178–183, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Dail WG, Evan AP, Gerritsen GC, Dulin WE. Abnormalities in pelvic visceral nerves. A basis for neurogenic bladder in the diabetic Chinese hamster. Investigat Urol 15: 161–166, 1977 [PubMed] [Google Scholar]
- 8.de Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor MB, Yoshimura N, Roppolo JR. Neural control of the urethra. Scand J Urol Nephrol Suppl 35–43: 106–125, 2001 [DOI] [PubMed] [Google Scholar]
- 9.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res 152: 59–84, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Faerman I, Glocer L, Celener D, Jadzinsky M, Fox D, Maler M, Alvarez E. Autonomic nervous system and diabetes. Histological and histochemical study of the autonomic nerve fibers of the urinary bladder in diabetic patients. Diabetes 22: 225–237, 1973 [DOI] [PubMed] [Google Scholar]
- 11.Gaballa MA, Peppel K, Lefkowitz RJ, Aguirre M, Dolber PC, Pennock GD, Koch WJ, Goldman S. Enhanced vasorelaxation by overexpression of beta 2-adrenergic receptors in large arteries. J Mol Cell Cardiol 30: 1037–1045, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Gustafson KJ, Creasey GH, Grill WM. A catheter based method to activate urethral sensory nerve fibers. J Urol 170: 126–129, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Jung SY, Fraser MO, Ozawa H, Yokoyama O, Yoshiyama M, De Groat WC, Chancellor MB. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol 162: 204–212, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Kakizaki H, Fraser MO, de Groat WC. Reflex pathways controlling urethral striated and smooth muscle function in the male rat. Am J Physiol Regul Integr Comp Physiol 272: R1647–R1656, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol 287: F434–F441, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi H, Yoshiyama M, Zakoji H, Takeda M, Araki I. Sex differences in the expression profile of acid-sensing ion channels in the mouse urinary bladder: a possible involvement in irritative bladder symptoms. BJU Int In press [DOI] [PubMed] [Google Scholar]
- 17.Kontani H, Shiraoya C. Sex difference in urethral response to electrical stimulation of efferent nerves in the pudendal sensory branch of rats. Int J Urol 9: 586–596, 2002 [PubMed] [Google Scholar]
- 18.Kullmann FA, Shah MA, Birder LA, de Groat WC. Funtional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296: F892–F901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leffler A, Mönter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 139: 699–709, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Maggi CA, Barbanti G, Santicioli P, Beneforti P, Misuri D, Meli A, Turini D. Cystometric evidence that capsaicin-sensitive nerves modulate the afferent branch of micturition reflex in humans. J Urol 142: 150–154, 1989 [DOI] [PubMed] [Google Scholar]
- 21.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol 248: 532–549, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Mitsui T, Kakizaki H, Kobayashi S, Morita H, Matsumura K, Koyanagi T. Vesicourethral function in diabetic patients: association of abnormal nerve conduction velocity with vesicourethral dysfunction. Neurourol Urodyn 18: 639–645, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Mumtaz FH, Sullivan ME, Thompson CS, Dashwood MR, Naseem KM, Bruckdorfer KR, Mikhailidis DP, Morgan RJ. Alterations in the nitric oxide synthase binding sites and non-adrenergic, non-cholinergic mediated smooth muscle relaxation in the diabetic rabbit bladder outlet: possible relevance to the pathogenesis of diabetic cystopathy. J Urol 162: 558–566, 1999 [PubMed] [Google Scholar]
- 24.Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Molecular Pain 4: 9–25, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacheco P, Camacho MA, Garcia LI, Hernandez ME, Carrillo P, Manzo J. Electrophysiological evidence for the nomenclature of the pudendal nerve and sacral plexus in the male rat. Brain Res 763: 202–208, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Peng CW, Chen JJJ, Cheng CL, Grill W. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol 294: R660–R672, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Pinna C, Bolego C, Puglisi L. Effect of substance P and capsaicin on urinary bladder of diabetic rats and the role of the epithelium. Eur J Pharmacol 271: 151–158, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, Kubo K, Kumon H, Groat WC, Yoshimura N. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J Urol 168: 1259–1264, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Song L, Schmidt BJ, Shefchyk SJ. Pelvic and pudendal reflexes in the in vitro neonatal rat preparation. Brain Res 675: 165–170, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Steers WD, Mackway-Gerardi AM, Ciambotti J, de Groat WC. Alterations in neural pathways to the urinary bladder of the rat in response to streptozotocin-induced diabetes. J Auton Nerv Syst 47: 83–94, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Thor KB, Muhlhauser MA. Vesicoanal, urethroanal, and urethrovesical reflexes initiated by lower urinary tract irritation in the rat. Am J Physiol Regul Integr Comp Physiol 277: R1002–R1012, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Torimoto K, Fraser MO, Hirao Y, De Groat WC, Chancellor MB, Yoshimura N. Urethral dysfunction in diabetic rats. J Urol 171: 1959–1964, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol 173: 1027–1032, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: relationship to autonomic neuropathy detected by sympathetic skin response. J Urol 157: 580–584, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Yang Z, Dolber PC, Fraser MO. Diabetic urethropathy compounds the effects of diabetic cystopathy. J Urol 178: 2213–2219, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, Nassar R, Dolber PC, Fraser MO. Voltage-dependent potassium currents of urethral afferent neurons in diabetes mellitus. Brain Res 1217: 132–138, 2008 [DOI] [PubMed] [Google Scholar]