Abstract
Hyperglycemia induces p38 MAPK-mediated renal proximal tubular cell (RPTC) apoptosis. The current study hypothesized that alteration of the Akt signaling pathway by hyperglycemia may contribute to p38 MAPK activation and development of diabetic nephropathy. Immunoblot analysis demonstrated a hyperglycemia-induced increase in Akt phosphorylation in diabetic kidneys at 1 mo, peaking at 3 mo, and dropping back to baseline by 6 mo. Immunohistochemical staining with anti-pAkt antisera localized Akt phosphorylation to renal tubules. Maximal p38 MAPK phosphorylation was detected concomitant with increase in terminal uridine deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive cells and caspase-3 activity in 6-mo diabetic kidneys. Exposure of cultured RPTCs to high glucose (HG; 22.5 mM) significantly increased Akt phosphorylation at 3, 6, and 9 h, and decreased thereafter. In contrast, p38 MAPK phosphorylation was detected between 9 and 48 h of HG treatment. Increased p38 MAPK activation at 24 and 48 h coincided with increased apoptosis, demonstrated by increased caspase-3 activity at 24 h and increased TUNEL-positive cells at 48 h of HG exposure. Blockade of p38 cascade with SB203850 inhibited HG-induced caspase-3 activation and TUNEL-positive cells. Overexpression of constitutively active Akt abrogated HG-induced p38 MAPK phosphorylation and RPTC apoptosis. In addition, blockade of the phosphatidylinositol-3 kinase/Akt pathway with LY294002 and silencing of Akt expression with Akt small interfering RNA induced p38 MAPK phosphorylation in the absence of HG. These results collectively suggest that downregulation of Akt activation during long-term hyperglycemia contributes to enhanced p38 MAPK activation and RPTC apoptosis. Mechanism of downregulation of Akt activation in 6-mo streptozotocin diabetic kidneys was attributed to decreased Akt-heat shock protein (Hsp) 25, Akt-p38 interaction, and decreased PTEN activity. Thus PTEN or Hsp25 could serve as potential therapeutic targets to modulate Akt activation and control p38 MAPK-mediated diabetic complications.
Keywords: diabetes mellitus, apoptotic cell death, renal inflammation
the serine/threonine kinase Akt regulates a number of cellular functions, including glucose metabolism, glycogen synthesis, protein synthesis, cell proliferation, cell hypertrophy, and cell death (42). Akt is an important mediator of insulin action. Upon insulin stimulation, Akt regulates uptake of glucose in muscle, adipocytes, the liver, and other tissues by promoting translocation of glucose transporters from intracellular stores to the plasma membrane. In addition, Akt has been identified as an essential gene required for maintenance of normal glucose homeostasis. Mice lacking this gene develop insulin resistance as seen in type 2 diabetes (8). Akt has been investigated for its role in hyperglycemia-induced renal glomerular hypertrophy and apoptosis. However, contributions of the Akt pathway to hyperglycemia-induced renal tubular damage in type 1 diabetes have not received much attention.
Diabetes is the leading cause of end-stage renal disease, and 10–21% of all people with diabetes have nephropathy, a frequent complication of both type 1 and type 2 diabetes (5, 45). However, mechanisms underlying the pathogenesis of diabetic nephropathy (DN) are not completely understood (5, 38, 45). Apoptotic cell death has been considered as an initiator of renal remodeling, including extracellular matrix accumulation, tubular cell hypertrophy and glomerular membrane expansion, and consequently progressive glomerulosclerosis and tubulointerstitial fibrosis (16, 20, 22, 28, 30). Therefore, renal proximal tubule cell (RPTC) apoptosis is proposed as one pathogenic mechanism of tubular atrophy, a hallmark of chronic renal disease and a histological predictor of clinical outcomes. In addition, apoptotic cell death due to hyperglycemia is attributed to renal inflammation and associated with generation of reactive oxygen or nitrogen species (ROS or RNS) (2, 32).
Signaling pathways such as the changes in mitogen-activated protein kinases (MAPKs) were recently found to play certain roles in renal cell death and the development of DN. Three MAPK subfamilies have been well characterized: ERK1/2, JNK, and p38 MAPK. Each of these MAPK subfamilies are shown to be involved in the pathogenesis of DN (11, 23, 26). The cellular response to stress, inflammation, an increase in inducible nitric oxide synthase activation, and vasoactive mediators such as endothelin-1 have been shown to activate p38 MAPK (13, 17, 37). In turn, p38 MAPK activation contributes to the pathogenesis of DN via stimulation of ROS/RNS and inflammatory and profibrotic factors such as TNF-α, transforming growth factor (TGF)-β, and plasminogen activator inhibitor-1 (PAI-1). However, signaling mechanisms contributing to hyperglycemia-induced p38 MAPK activation are not completely defined. We hypothesized that downregulation of Akt activation during hyperglycemia contributes to p38 MAPK-mediated RPTC apoptosis, fibrosis, and consequent DN. Therefore, the objectives of the present study were to determine 1) whether Akt/p38 MAPK expression/phosphorylation is altered during the development of DN; 2) if so, whether the altered Akt/p38 MAPK expression/phosphorylation and RPTC apoptosis are mediated by hyperglycemia; and 3) whether hyperglycemia-mediated Akt downregulation contributes to p38 MAPK activation and RPTC apoptosis. In the present study, we have addressed these issues in the kidneys of streptozotocin (STZ)-induced diabetic mice and in cultured human RPTCs exposed to high levels of glucose (HG).
MATERIALS AND METHODS
Anti-Akt, anti-phospho-p38 MAPK, anti-p38 antisera, PTEN, and p-PTEN, were obtained from Cell Signaling (Beverly, MA). Anti-phospho-Ser473-Akt was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A PAI-1 antibody was obtained from BD Transduction Laboratories. TGF-β antibody was obtained from Anaspec. An Anti-caspase-3 rabbit polyclonal antibody was obtained from Calbiochem (235412). A mouse isotype control antibody was obtained from Santa Cruz Biotechnology. Recombinant heat shock protein 27 (Hsp27) was utilized as a p38 MAPK substrate in an in vitro p38 MAPK assay and was obtained from Stressgen Biotechnologies (Victoria, BC). Histone H2B was utilized as an Akt substrate in an in vitro Akt kinase assay and was obtained from Boehringer Manheim (Indianapolis, IN). Protein A-Sepharose and glutathione-Sepharose were obtained from Pharmingen (San Diego, CA). pUse vector, pUseAktCA (myristoylated-constitutively active Akt), pKD-control small interfering RNA (siRNA), and pKD-Akt1 siRNA constructs were obtained from Upstate Biotechnology (Lake Placid, NY).
Diabetic mouse model.
FVB mice, originally obtained from Harlan Bioproducts for Science (Indianapolis, IN), were housed in the University of Louisville Research Resources Center at 22°C with a 12:12-h light-dark cycle. They had free access to a rodent diet and tap water. All animal procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care. Eight-week-old male mice were given a single intraperitoneal dose of STZ (150 mg/kg body wt; Sigma, St. Louis, MO) dissolved in sodium citrate buffer (pH 4.5). Previous studies (4, 5, 43) by our own group have successfully utilized the STZ-diabetic mouse model. Whole-blood glucose obtained from mouse tail vein was monitored using a Sure Step complete blood glucose monitor (LifeScan, Milpitas, CA). Mice with whole-blood glucose >220 mmol/l were considered diabetic. Mice that served as vehicle controls were given the same volume of sodium citrate (4, 5, 43) . When hyperglycemia was diagnosed on day 3 after STZ treatment, insulin was immediately given to a subgroup of diabetic mice, using a long-term insulin preparation (Humulin U, Eli Lilly, Indianapolis, IN) at a concentration of 2 U·mouse−1·day−1 to maintain the blood glucose levels at a range of 300–380 mmol/l for 2 wk to reduce the acute mortality of diabetic mice.
Urine protein assay.
Urine was collected from individual mice in metabolic cages during a 24-h period (Nalgene, Braintree Scientific, Braintree, MA). Mice had free access to a standard mouse diet. Albumin level in the urine was assayed with a Mouse Albumin ELISA Quantitation Kit (Bethyl Laboratories, Montgomery, TX). Urine was diluted to fit the working range of 7.8–500 ng/ml.
Kidney histopathology.
Kidneys removed from anesthetized mice were immediately cut in half and fixed in 10% formaldehyde in 0.1 mol/l PBS (pH 7.2), embedded in paraffin, and sectioned at 5 μm. Sections were stained with periodic acid-Schiff (PAS) to evaluate fibrosis, based on our previous study (52).
Isolation of mouse renal tubules.
Mouse renal cortical tubules were separated from glomeruli and collected according to the method described by Takemoto et al. (44). Mice were anesthetized by an intraperitoneal injection of ketamine HCl/xylazine HCl followed by perfusion of the heart with a 30-ml suspension of 4.5-μm magnetic beads (Dynal, Lake Success, NY) in PBS with the vena cava cut. The cortex of excised kidneys was dissected, minced with a razor blade, and subjected to digestion with type IA collagenase (1 mg/ml) for 30 min at 37°C. The suspension was gently pressed through a 100-mm cell strainer, and the filtrate was passed through an additional strainer. Glomeruli were separated from the final filtrate with a magnetic particle concentrator (Dynal). The remaining tissue suspension enriched with cortical tubule fragments was examined for purity by visualization under a microscope. As needed, suspensions were replaced in the magnetic particle concentrator for additional separation of glomeruli from tubule suspensions. Individual cells resulting from trypsin digestion were removed by allowing settling of intact tubules and removal of the supernatant.
Microarray analysis.
The renal tissues from each of the five mice described above were individually processed for RNA isolation. The total RNA was isolated with TRIzol Reagent (Invitrogen, Carlsbad, CA) and purified with RNeasy columns (Qiagen, Valencia, CA). After the purification, aliquots of the RNA samples (2–5 μg) were converted to an α-[32P]dATP-labeled cDNA probe using a Moloney murine leukemia virus reverse transcriptase and the Atlas custom array specific cDNA synthesis primer mix (560 genes) and purified with Nucleospin columns (Clontech, Palo Alto, CA). The membranes were prehybridized with Expresshyb (Clontech) for 60 min at 68°C and then hybridized with labeled probes overnight. Hybridizations were performed in triplicate. The membranes were washed four times with 2× standard saline citrate (SSC) containing 1% SDS for 30 min followed by two washes with 0.1× SSC containing 0.5% SDS. The membranes were then exposed to a Molecular Dynamics Phosphoimage Screen (Sunnyvale, CA). The images were quantified densitomertrically by using Atlas Images v2.0 software (Clontech). The gene expression intensities were first corrected for the external background and then globally normalized with the sum of all genes on the array.
Western blot analysis and immunoprecipitation.
Kidneys were collected after anesthesia and frozen at −80°C, and tissue powder was prepared, as described before (47, 52). Kidney lysates and HK11 cell lysates were prepared in lysis buffer containing 1% (vol/vol) Nonidet P-40, 10% (vol/vol) glycerol, 137 mM NaCl, 20 mM Tris·HCl, pH 7.4, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 5 mM PMSF, 20 mM NaF, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and 1% (vol/vol) Triton X-100. Samples (50 μg total protein) were subjected to SDS-PAGE (12% gel). After SDS-PAGE, resolved proteins were transferred to nitrocellulose membranes and blocked with Tris-buffered saline with 0.1% Tween plus 5% nonfat milk powder. The nitrocellulose blots were incubated overnight with rabbit polyclonal pAktSer473 antibody, rabbit polyclonal pp38 MAPK (Thr 180/Tyr 182) antibody (1:500 in 5% BSA, Cell Signaling Technology), mouse monoclonal total Akt antibody (1:1,000) (Santa Cruz Biotechnology), rabbit polyclonal total p38 antibody, rabbit polyclonal antibody against an active form of caspase-3 (1:500, Sigma), and then with the horseradish peroxidase-conjugated anti-rabbit IgG (1:2,000) for 1 h. Bound antibody was visualized using the enhanced chemiluminescence Western blotting protocol. Whole kidney tissue lysate and renal tubular lysates were subjected to anti-Akt immunoprecipitation as previously described (35). Immunoprecipitates were immunoblotted with anti-Hsp25, anti-p38 MAPK, and anti-PTEN antisera.
Immunohistochemical analysis.
Immunohistochemical examination of pSer473Akt , pp38 MAPK, PAI-1, and TGF-β in kidneys was also carried out as previously described (43, 52).
Cell culture.
Human RPTCs (HK-11) immortalized by transduction with adenovirus 12-SV40 were kindly provided by Lorraine C. Racusen (Johns Hopkins University) and were maintained in DMEM/F12 supplemented with 5% FBS (Invitrogen). When cell populations reached 40–50% confluence, the cultures were exposed to d-glucose in a final concentration of 22.5 mM as HG according to our previous study (4) and exposed to 5.5 mM d-glucose as a control (LG). To exclude a hyperosmotic effect, we added identical concentrations of mannitol (5.5 mM glucose+17 mM mannitol; Sigma) in control cultures. After exposure for varying time periods, the monolayer cultures were washed with PBS and the cells were removed by trypsinization. In some experiments, the cells were grown on 8- or 24-well chamber slides, which were used for caspase-3 activity assay and terminal uridine deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay. For inhibitor studies, RPTCs were preincubated with SB203580 (final concentration 3 μM in double-distilled water; Calbiochem) for 1 h and then incubated for an additional 48 h in the presence of LG or HG medium.
TUNEL assay and analysis.
RPTCs were cultured on eight-well chamber slides and fixed in 1% paraformaldehyde. The slides were processed for a TUNEL assay using an ApopTag in situ detection kit (Intergen, Purchase, NY) according to our previous study (4).
In situ detection of apoptosis in kidney sections of mice from both groups was performed by TUNEL assay using an ApopTag Plus Peroxidase Apoptosis Detection Kit (Chemicon, Millipore, Billerica, MA). Kidney sections were analyzed for positively stained tubule cells by an observer blinded to the identity of the slides. Positively stained tubule cells on the outer edges of the sections and unattached cells in the tubule lumen were omitted from the analysis. Numerous, random visual fields were observed under a ×40 objective.
Caspase-3 enzymatic assay.
Cell pellets were resuspended in extract buffer, which contained 25 mmol/l HEPES buffer (pH 7.4), 5 mmol/l EDTA, 2 mmol/l DTT, 0.1% [(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 1.0 mmol/l PMSF, and 10 μg/ml aprotinin, and homogenized with ultrasound for 20 s. The homogenate was centrifuged at 20,000 g for 30 min. The supernatant was diluted with an assay buffer (50 mmol/l HEPES, 10 mmol/l DTT, 1 mmol/l EDTA, 100 mmol/l NaCl, 0.1% CHAPS, and 10% glycerol, pH 7.4) and incubated at 37°C with 200 μmol/l caspase-3 substrate I [N-Acetyl-Asp-Glu-Val-Asp-pNA (Ac-DEVD-pNA); Calbiochem, La Jolla, CA]. Cleavage of the substrate was monitored at 405 nm using a microplate reader and recorded in 10-min intervals for 2 h. The specific activity is expressed in picomoles per minute per gram of protein (4).
cDNA and Akt-siRNA transfections.
RPTCs were plated in DMEM media containing 5.5 mM d-glucose on six-well trays 1 day before performance of transfections to achieve 60% confluence. On the day of transfection, appropriate cells were washed with serum-free RPMI 1640 medium. One microgram of pUSE vector or pUSE-AktCA (constitutively active Akt construct) was transfected into these cells using Geneporter reagent according to the manufacturer's protocol (Gene Therapy Systems). Twenty-four hours after transfection, cells were washed and fresh DMEM supplemented with 5.5 or 22.5 mM glucose were added to the cells, and cells were further incubated at 37°C in 5% CO2. After 48 h, cells were lysed in 100 μl of Akt lysis buffer containing 20 mM Tris·HCl, pH 7.4, 150 nM NaCl , 1% Triton X-100 (vol/vol), 0.5% NP-40 (vol/vol), 1 mM EDTA, 1 mM EGTA, 20 mM sodium orthovanadate, p-10 μM nitrophenol phosphate, 20 mM NaF, 5 mM PMSF, 21 μg/ml aprotinin, and 5 μg/ml leupeptin, and proteins concentrations were determined. Protein lysates (100 μg) were subjected to SDS-PAGE and appropriate immunoblot analysis. Control and Akt1 siRNA, namely, pKD-control siRNA or pKD-Akt1siRNA (Upstate Biotechnology), were transfected in RPTCs according to the manufacturer's instructions (Cell Signaling). Four to eight hours after transfection, cells were lysed with Akt lysis buffer and subjected to anti-phospho-p38 MAPK, anti-p38 MAPK, anti-β-actin, anti-c-myc, and anti-active caspase-3 immunoblot analysis.
p38 MAPK and Akt kinase assay.
RPTCs were cultured in DMEM supplemented with 5.5 and 22.5 mM glucose for 3 and 48 h, and at the end of each time point RPTCs were lysed in Akt lysis buffer. Lysates were subjected to anti-Akt or anti-p38 MAPK immunoprecipitation. Immunoprecipitated Akt and p38 MAPK were subjected to an in vitro Akt kinase and p38 MAPK assay using histone H2B and recombinant Hsp27, respectively, as substrates as previously described (35).
Statistical analysis.
Results are expressed as means ± SD, unless otherwise specified. One-way ANOVA was used followed by the post hoc Bonferroni multiple comparisons to analyze the difference for groups with different times post-diabetes or -HG treatments. Some experiments were also analyzed by Student's t-test. Differences were considered to be significant at P < 0.05.
RESULTS
Development of DN in STZ-diabetic mice.
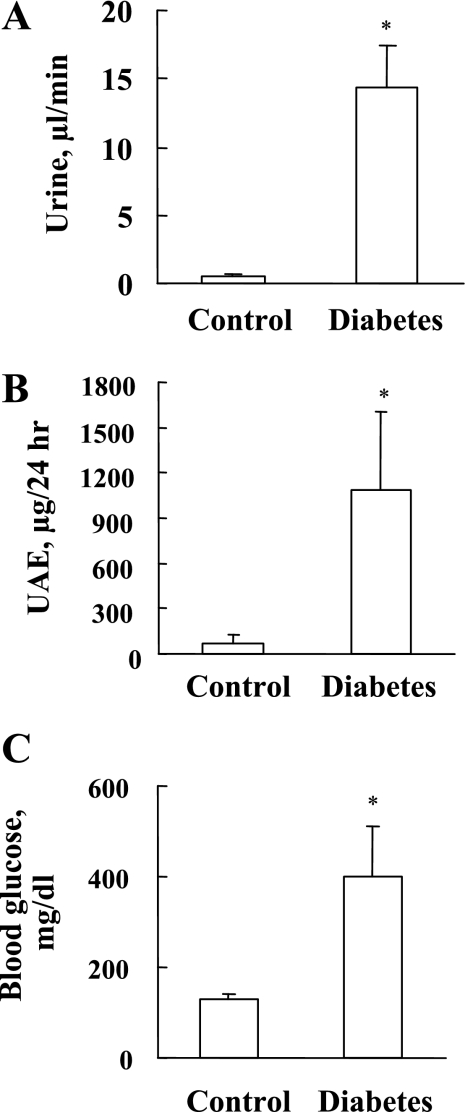
Mice were injected intraperitoneally with STZ or vehicle to induce hyperglycemia resulting from insulin depletion. As in previous studies (4, 5, 43), urine volume (Fig. 1A), urine albumin (Fig. 1B), and blood glucose levels in a fasting condition (Fig. 1C) were significantly increased in the diabetic group compared with the control group. These parameters suggested renal dysfunction due to the development of DN.
Fig. 1.
Development of diabetic nephropathy in streptozotocin (STZ)-diabetic mice. In kidneys from control and diabetic mice 6 mo after STZ treatment, urine volume (A), urine albumin for 24 h (B), and blood glucose (C) were measured before mice were killed. Values are means ± SD (n = 6). *P < 0.05 vs. control.
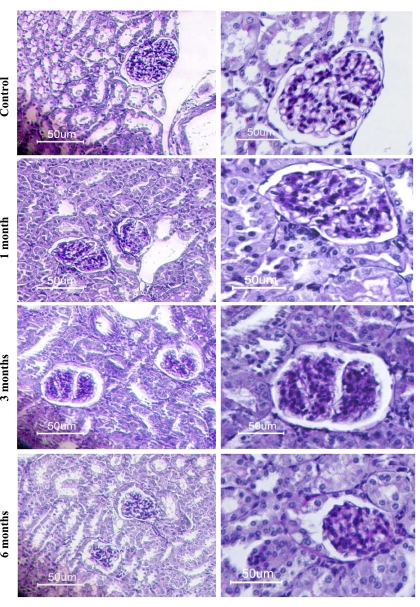
To further define the development of DN, diabetic and age-matched control kidneys were examined histochemically by PAS staining (Fig. 2). Compared with control, shrunken glomeruli were evident in the kidneys of diabetic mice at 6 mo after STZ treatment. A significant increase in PAS-positive material was detected in the diabetic glomeruli at 6 mo after STZ treatment, indicative of increased fibrosis (Fig. 2).
Fig. 2.
Renal fibrosis in diabetic mice. Periodic acid-Schiff (PAS) staining was performed on kidney sections of control and diabetic mice 1, 3, and 6 mo after STZ treatment. The images indicate glomeruli with advanced mesangial expansion. A significant increase in PAS-positive material was detected in the diabetic glomeruli at 6 mo after STZ treatment indicative of increased fibrosis. Magnification: ×10 (left) and ×40 (right).
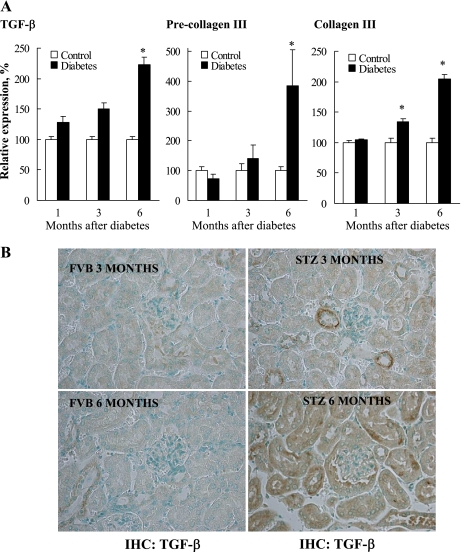
Consistent with the observation of histopathological changes in terms of renal fibrosis, expressions of fibrotic genes including TGF-β, pre-collagen III, and collagen III (Fig. 3) were significantly increased in 6-mo diabetic kidneys. In addition, kidney sections from 3- and 6-mo FVB and STZ mice were stained with antibodies against the profibrotic marker TGF-β. As shown in Fig. 3, TGF-β staining is increased in 6-mo STZ kidney sections compared with age-matched controls and 3-mo STZ kidney sections. In addition, the immunohistochemical data demonstrate increased TGF-β expression present in renal tubular cells. Together, the data demonstrate increased TGF-β transcript as well as protein levels in kidneys of diabetic mice.
Fig. 3.
Diabetes-induced transcriptional upregulation of profibrotic markers in the kidney. Renal tissues from mice in each group (5 mice/group) were individually processed for RNA isolation with TRIzol reagent and purified with RNeasy columns. A gene array was run using the Atlas custom array specific cDNA synthesis primer mix (560 genes) and purified with Nucleospin columns. The membranes were prehybridized with Expresshyb and then exposed to a Molecular Dynamics Phosphoimage Screen. The images were quantified densitomertrically by using Atlas Images v2.0 software for the selected profibrotic markers. Gene expression intensities were first corrected for the external background and then globally normalized with the sum of all genes on the array. *P < 0.05 vs. corresponding controls (A). Kidney slices from 3- and 6-mo FVB and STZ-diabetic mice were subjected to immunohistochemistry (IHC) with anti-transforming growth factor (TGF)-β antibody (B).
Akt and p38 phosphorylation profile in STZ-induced diabetic and age-matched control mice kidneys.
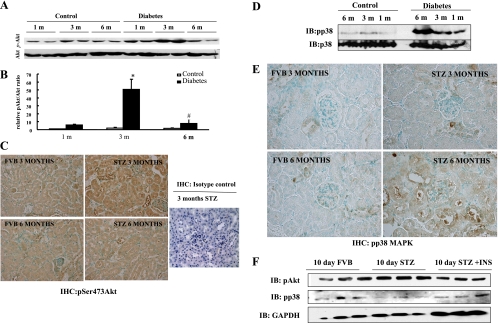
To determine the status of Akt Ser473 phosphorylation in the onset and progression of DN, kidneys were collected from mice at 1, 3, and 6 mo after treatment with STZ or vehicle. Western blot analysis of kidneys from STZ-diabetic mice revealed a time-dependent increase in Akt phosphorylation, starting at 1 mo, peaking at 3 mo, and decreasing to normal levels at 6 mo compared with age-matched control mice. Total Akt expression remained unchanged in all conditions (Fig. 4, A and B). To localize this increased Akt phosphorylation to a specific kidney cell type, kidney sections from control, 3-, and 6-mo diabetic mice were stained with phosphoSer473-Akt antibody. Figure 4C demonstrated increased phosphoSer473-Akt immunoreactivity was also present in renal tubules in 3-mo diabetic kidneys compared with their age-matched controls. This effect was markedly reduced in 6-mo diabetic kidneys. No immunoreactivity was detected in kidney sections stained with the isotype control antibody.
Fig. 4.
Akt and p38 MAPK phosphorylation profiles during the development of diabetic nephropathy in STZ-induced diabetic mice. Kidney lysates from control and diabetic mice 1, 3, and 6 mo after STZ treatment were subjected to Western blotting with anti-Akt and anti-phosho-Ser473-Akt antibodies (A and B) or anti-p38 and ant-phospho-Thr 180/Tyr 182 p38 antibodies (D). Six mice were used for the above study. Values are means ± SD. *P < 0.05 1-mo vs. 3-mo STZ. #P < 0.05 3- vs. 6-mo STZ kidney lysates. Kidney sections from 3- and 6-mo FVB and STZ-diabetic mice were subjected to immunohistochemistry with anti-phosphoSer473-Akt antibody (C) and phospho-p38 MAPK antibody (E). Three-month STZ-diabetic kidney slices were also immunostained with isotype control antibody (C). Renal tubular lysates from n = 3 each untreated FVB, 10-day STZ-treated, and 10-day STZ- and insulin-treated mice were subjected to phosphoSer473-Akt, phosphop38 MAPK, and GAPDH immunoblotting (IB; F).
Next, we determined the status of p38 MAPK phosphorylation in the kidneys of STZ-diabetic mice and age-matched control mice. Western blot analysis revealed a time-dependent increase in p38 MAPK phosphorylation peaking at 6 mo in diabetic kidneys compared with age-matched controls (Fig. 4D). Figure 4E demonstrated increased phospho-p38 MAPK immunoreactivity in renal tubules of 6-mo diabetic kidneys while remaining unaltered between 3-mo diabetic and respective control kidneys.
Next, to determine the effects of insulin treatment on hyperglycemia-induced Akt phosphorylation and p38 MAPK phosphorylation in renal tubules, FVB mice were untreated or treated with STZ with or without insulin for 10 days. Three mice for each condition were utilized. After 10 days, the mice were killed and their renal tubules were isolated. Renal tubular cell lysates (20 μg) were immunoblotted with phosphoSer473-Akt and phospho-p38 MAPK antisera. Ten-day STZ treatment induced Akt Ser473 phosphorylation, which was reduced to basal levels by insulin treatment. Thus hyperglycemia-induced Akt activation was restored to baseline by insulin treatment. In contrast, 10-day STZ treatment induced decreased p38 MAPK phosphorylation compared with the basal level, which was restored by insulin treatment (Fig. 4F).
Occurrence of inflammation and apoptotic cell death in the kidneys of 6-mo diabetic mice.
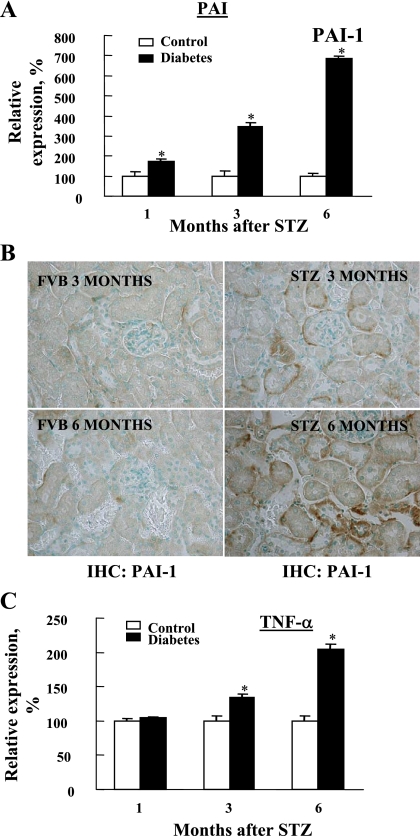
We next determined the status of renal inflammation as well RPTC apoptosis in control and diabetic mouse kidneys. To this end, we examined the transcriptional expression of inflammatory markers including PAI-1 (Fig. 5, A and B) and TNF-α (Fig. 5C) in control and 1-, 3-, and 6-mo diabetic kidneys. Both were significantly increased in a time-dependent manner similar to the fibrotic markers TGF-β and collagen III (Fig. 3). In addition, kidney sections from 3- and 6-mo FVB and STZ mice were stained with antibodies against PAI-1. We demonstrated enhanced PAI-1 staining in 6-mo STZ kidney sections compared with age-matched controls and in 3-mo STZ kidney sections. In addition, the immunohistochemistry data demonstrated increased PAI-1 expression in renal tubular cells as well. Therefore, both PAI-1 transcript and protein levels are increased in kidneys of 6-mo STZ-diabetic animals (Fig. 5, A and B).
Fig. 5.
Diabetes-induced transcriptional upregulation of inflammatory markers. Procedures including tissue harvest and RNA isolation for detecting the transcriptional levels of inflammatory makers such as plasminogen activator inhibitor-1 (PAI-1; A) and TNF-α (C) were performed as described in Fig. 3. B: kidney sections from 3- and 6-mo FVB and STZ-diabetic mice were subjected to immunohistochemistry with anti-PAI-1 antibody. Differences in the inflammatory markers between control and diabetes groups were significant. *P < 0.05 vs. corresponding controls.
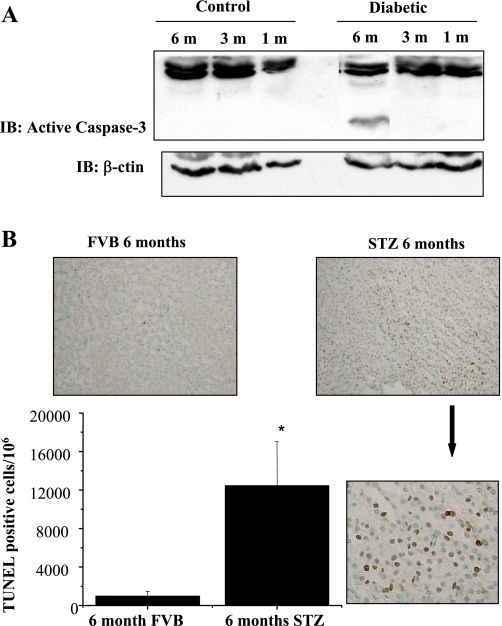
We next determined whether increase in PAI-1 and TNF-α mRNA expression in 6-mo diabetic kidneys correlated with an increase in apoptosis. Control and 1-, 3-, and 6-mo STZ-diabetic kidney lysates were subjected to active caspase-3 immunoblot analysis. Activation of caspase-3 was detected in the kidney lysates of 6-mo diabetic mice by appearance of a 17-kDa immunoreactive fragment of activated caspase-3 but not in age-matched control mice (Fig. 6A). In addition to active caspase-3 blots, control (FVB) and 6-mo STZ-diabetic kidney sections were analyzed for TUNEL-positive cells. A significant increase in TUNEL-positive staining was detected in renal tubular cells of 6-mo diabetic mice compared with their age-matched controls (Fig. 6B).
Fig. 6.
Activation of caspase-3 in 6-mo STZ-diabetic mice kidneys. A: kidney lysates from control and diabetic mice 1, 3, and 6 mo after STZ treatment were subjected to Western blotting with anti-actin and anti-active caspase-3 antisera. The activated form of caspase-3 was detected in 6-mo diabetic kidney lysates by Western blotting. Loading was normalized by β-actin immunoblotting. B: caspase-3 activation in 6-mo STZ-diabetic kidney lysates was confirmed by performing a terminal uridine deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay on 6-mo STZ-diabetic and age-matched control kidneys. Results demonstrate increased TUNEL positivity in 6-mo STZ-diabetic kidney sections compared with control kidneys. *P < 0.05 by Student's t-test.
Hyperglycemia-induced changes in Akt and p38 MAPK phosphorylation profiles in RPTCs.
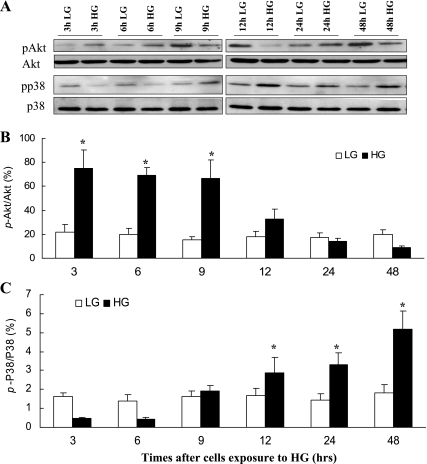
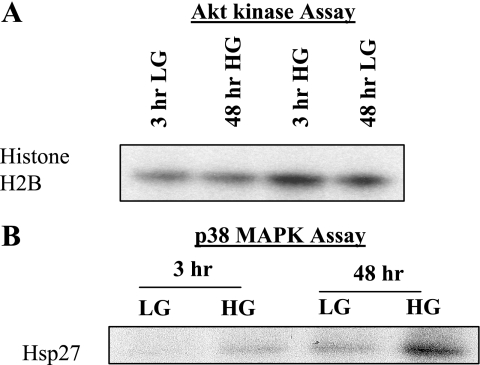
Having localized increased Akt Ser473 phosphorylation to the renal tubules of diabetic mice, we next extended our study to immortalized RPTCs cultured in LG (5.5 mM glucose+17 mM mannitol) and HG (22.5 mM glucose). Cell culture models provide a useful tool for performing gene modification studies. To validate our in situ results in our cell culture model, we treated RPTCs to LG/ HG conditions and determined the effect of HG on Akt and p38 MAPK phosphorylation. Figure 7, A and B, demonstrates that HG, but not LG, stimulated increased Akt phosphorylation in a time-dependant manner, peaking at 3 h and gradually decreasing from 12 to 48 h. In contrast, HG induced an increase in p38 MAPK phosphorylation between 12 and 48 h (Fig. 7, A and C). To determine whether Akt and p38 MAPK phosphorylation profiles correlated with Akt and p38 MAPK kinase activities, RPTCs exposed to LG/HG for 3 and 48 h were lysed and the lysates were subjected to in vitro Akt kinase and in vitro p38 MAPK kinase assays as previously described (35). RPTCs are actively growing cells; hence, as expected, Akt activity was higher in LG-treated RPTCs at 48 h compared with the 3-h time point (Fig. 8A, compare lanes 1 and 3). However, HG treatment for 3 h increased Akt activity compared with its 3-h LG control (Fig. 8A, compare lanes 3 and 1). Moreover, at 48 h Akt activity was downregulated compared with 3-h HG-treated cells (Fig. 8A, compare lanes 2 and 3). In contrast, HG failed to stimulate p38 MAPK activity at 3 h but induced enhanced p38 MAPK activity after 48 h of HG treatment (Fig. 8B, compare lanes 2 and 4). Thus, similar to our in situ data, we demonstrated contrasting Akt and p38 MAPK activation profiles in RPTCs exposed to LG and HG medium.
Fig. 7.
Hyperglycemia-induced changes in Akt and p38 MAPK phosphorylation profiles in renal proximal tubular cells (RPTCs). RPTCs were incubated in DMEM/F12 medium containing either 5.5 mM d-glucose/17 mM mannitol (LG) or 22.5 mM d-glucose (HG) for 3–48 h. RPTC lysates were subjected to immunoblot analysis with anti-phoshoSer473Akt or anti-Akt antibodies (A and B) or anti-phosphop38 or anti-p38 antibodies (A and C). HG induced Akt and p38 MAPK phosphorylation in a time-dependent manner. Values are means ± SD obtained from at least 3 separate experiments. *P < 0.05 vs. corresponding LG groups.
Fig. 8.
Contrasting Akt kinase and p38 MAPK activities in RPTCs cultured with HG for 3 and 48 h. RPTCs were incubated in DMEM/F12 medium containing either 5.5 mM d-glucose/17 mM mannitol (LG) or 22.5 mM d-glucose (HG) for 3 or 48 h. RPTC lysates were subjected to anti-Akt or anti-p38 MAPK immunoprecipitation. Akt and p38 MAPK immunoprecipitates were subjected to an in vitro kinase assay utilizing histone H2B and Hsp27 as a substrate, respectively. A: similar to the Akt phosphorylation profile, Akt activity was detected after 3 h of HG treatment followed by a decrease in Akt activity at 48 h. B: in contrast, p38 MAPK activity was detected at 48 h of HG treatment.
p38 MAPK pathway contributes to hyperglycemia-induced renal proximal tubular apoptosis.
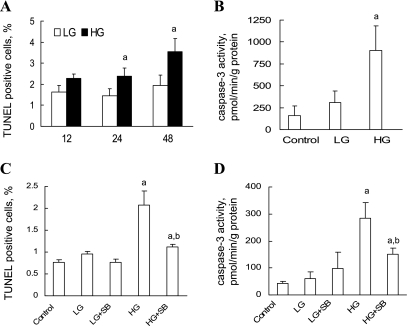
Next, we determined the effects of HG treatment on RPTC survival. RPTCs exposed to LG and HG were subjected to a TUNEL assay. As shown in Fig. 9A, exposure of human RPTCs to HG for 24 and 48 h induced a significant increase in TUNEL-positive cells. To determine whether HG-induced caspase-3 mediated RPTC apoptosis, caspase-3 activation was measured by biochemical assay as described in materials and methods. Results show that exposure of RPTCs to HG for 24 h caused a significant increase in caspase-3 activity (Fig. 9B).
Fig. 9.
The p38 MAPK pathway contributes to hyperglycemia-induced renal proximal tubular apoptosis. The effect of HG on RPTC apoptosis was examined by TUNEL staining (A and C) and caspase-3 activation (B and D). A: RPTCs were incubated in DMEM/F12 medium containing either 5.5 mM d-glucose/17 mM mannitol (LG) or 22.5 mM d-glucose (HG) for 12, 24, and 48 h followed by determination of TUNEL-positive cells. B: RPTCs were exposed to LG and HG for 24 h, and caspase-3 activity was determined. C: RPTCs were exposed to LG and HG in the presence and absence of p38 MAPK inhibitor SB203580 (3 μM final concentration) for 24 h, and cells were subjected to TUNEL assay. D: RPTCs were exposed to LG and HG in the presence and absence of p38 MAPK inhibitor SB203580 (3 μM final concentration) for 24 h, and caspase-3 activity was determined. Values are means ± SD obtained from at least 3 separate experiments with samples in triplicate. aP < 0.05 vs. corresponding LG group. bP < 0.05 vs. HG.
To determine the contributions of HG-induced p38 MAPK activation to RPTC apoptosis, RPTCs were cultured in LG or HG in the presence and absence of 3 μM SB203580 for 24 h and cells were subjected to a TUNEL assay and caspase-3 activity assay. As shown in Fig. 9, C and D, blockade of p38 MAPK activation significantly inhibited the HG-induced increase in TUNEL-positive cells and caspase-3 activity, thus promoting RPTC survival in HG. The results demonstrate a role for p38 MAPK activation in mediating HG-induced RPTC apoptosis.
Blockade of phosphatidylinositol-3 kinase/Akt pathway stimulates p38 MAPK phosphorylation.
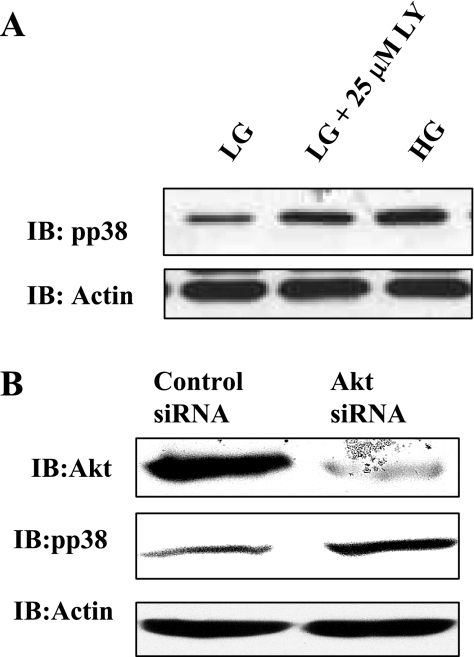
Next, we determined the effects of pharmacologically inhibiting phosphatidylinositol-3 kinase (PI-3K), a known upstream positive regulator of Akt, with LY294002 or by silencing Akt expression on basal p38 MAPK phosphorylation in RPTCs. RPTCs were incubated with LG and HG medium for 24 h. In addition, to block the PI-3K/Akt pathway, RPTCs were cultured in LG medium and incubated with 25 μM LY294002 for 24 h. RPTC lysates generated were immunoblotted with phospho-p38 MAPK antibody. Protein load was normalized by immunoblotting the membranes with anti-actin antibody. As expected, HG treatment for 24 h resulted in increased phospho-p38 MAPK immunoreactivity (Fig. 10A). However, blockade of the PI-3K/Akt pathway enhanced phospho-p38 MAPK levels without altering β-actin levels in all conditions (Fig. 10A). In addition, RPTCs were transfected with control siRNA or Akt siRNA for 24 h. Cell lysates generated were immunoblotted with anti-Akt antibody to document effective gene silencing. In addition, blots were stripped and reprobed with anti-phospho-p38 MAPK and β-actin antisera. Figure 10B demonstrates that Akt expression was effectively silenced in Akt siRNA-transfected cells but not in control siRNA-transfected cells. Moreover, silencing Akt expression resulted in increased phospho-p38 MAPK levels without alteration of β-actin levels among various conditions (Fig. 10B). These results collectively show that blockade of the Akt pathway leads to increased p38 MAPK phosphorylation in RPTCs.
Fig. 10.
Blockade of phosphatidylinositol-3 kinase (PI-3K)/Akt pathway stimulates p38 MAPK phosphorylation. A: RPTCs were incubated with LG and HG medium for 24 h in the presence and absence of PI-3K inhibitor LY294002 (LY; 25 μM). RPTC lysates were immunoblotted with anti-phospho-p38 MAPK and anti-β-actin antisera. HG treatment for 24 h induces p38 MAPK phosphorylation in RPTCs. Blockade of the PI-3K pathway, a known upstream regulator of Akt, induces p38 MAPK phosphorylation in the absence of HG. B: RPTCs were transfected with control small interfering RNA (siRNA) or Akt siRNA for 24 h. Cell lysates generated were immunoblotted with anti-Akt antibody to document effective gene silencing. In addition, blots were stripped and reprobed with anti-phospho-p38 MAPK and β-actin antisera.
Overexpression of constitutively active akt inhibits hyperglycemia-induced p38 activation and RPTC apoptosis.
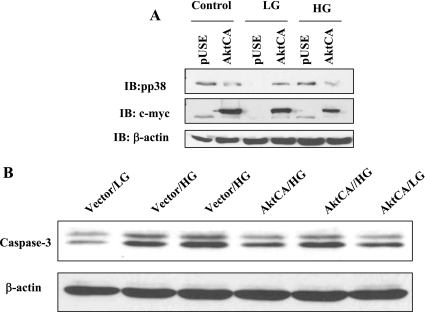
Next, to conclusively demonstrate a role for Akt activation in regulating p38 MAPK activity, we transfected RPTCs with a vector- or c-myc-tagged constitutively active Akt construct (AktCA) for 24 h. Transfected cells were then subjected to control (5 mM d-glucose, no mannitol) or LG (5 mM d-glucose ± 17.5 mM mannitol) or HG (22.5 mM d-glucose) medium for 48 h. Cell lysates were subjected to immunoblot analysis with anti-phospho-p38 MAPK, c-myc, and anti-actin and anti-active caspase-3 immunoblot analysis. Successful transfection of AktCA in RPTCs was documented by c-myc immunoblotting (Fig. 11A). Figure 11, A and B, demonstrates that overexpression of AktCA (constitutively active Akt) but not vector (pUse) in HK-11 cells for 48 h inhibited HG-induced-p38 MAPK phosphorylation in these cells. Equal protein loading was documented by β-actin immunoblotting. In addition, Fig. 11C demonstrates basal active caspase-3 immunoreactivity in LG-treated RPTCs, which is markedly increased in HG-treated RPTCs. Furthermore, overexpression of AktCA markedly inhibits HG-induced enhanced active caspase-3 immunoreactivity in RPTCs.
Fig. 11.
Akt overexpression inhibits p38 MAPK activation and RPTC apoptosis. RPTCs were transfected with pUse vector or c-myc-tagged constitutively active Akt (AktCA) construct for 24 h. Transfected cells were then subjected to control (no mannitol) or LG (5 mM d-glucose ± 17.5 mM mannitol) or HG (22 mM d-glucose) medium for 48 h. Cell lysates were subjected to immunoblot analysis with anti-pp38 MAPK, c-myc, and anti-β-actin (A). B: anti-active caspase-3 immunoblot analysis. *P < 0.05 by Student's t-test.
Diabetes-induced changes in Akt-Hsp25, Akt-p38 MAPK, and Akt-PTEN interactions in control and diabetic kidneys or renal tubules.
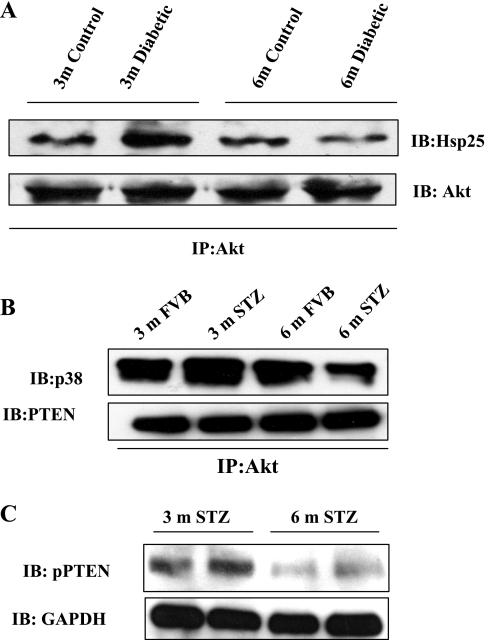
We have previously demonstrated in human neutrophils that Akt-Hsp27 interaction is critical to Akt activation and neutrophil apoptosis (35, 50). To determine whether changes in Akt-Hsp25 interactions in 3- and 6-mo STZ-diabetic kidneys correlated with changes in Akt phosphorylation status, we performed Akt immunoprecipitation studies in 3- and 6-mo FVB and STZ-diabetic kidney tissue homogenates. Figure 12A demonstrates that, indeed, Akt-Hsp25 interaction was enhanced in 3-mo STZ kidney homogenates compared with 6-mo STZ kidney homogenates and the appropriate age-matched controls.
Fig. 12.
Diabetes-induced changes in Akt-Hsp25, Akt-p38 MAPK, and Akt-PTEN interactions in control and diabetic kidneys or renal tubules. Three- and 6-mo FVB and STZ-diabetic kidney homogenates were subjected to anti-Akt immunoprecipitation and immunoblotted with anti-Hsp25 and anti-Akt antisera. A: representative immunoblot from 2 independent experiments performed. B: renal tubular lysates from 3- and 6-mo FVB and STZ-diabetic mice were subjected to anti-Akt immunoprecipitation and immunoblotted with anti-p38 MAPK and anti-PTEN antisera. C: renal tubular lysates from 3- and 6-mo STZ-diabetic mice were immunoblotted with phospho-PTEN and GAPDH antisera.
In our previous studies, we have demonstrated a protective arm of p38 MAPK signaling when associated with Akt (50). Thus renal tubular lysates from 3- and 6-mo FVB and STZ-treated mice were subjected to anti-Akt immunoprecipitation followed by immunoblot analysis with p38 MAPK. Figure 12B, top demonstrates that STZ treatment for 3 mo induced enhanced association of Akt-p38 MAPK (a time point at which Akt Ser473 phosphorylation is high), while 6-mo STZ treatment resulted in dissociation of Akt and p38 MAPK (a time point at which Akt Ser473 phosphorylation is decreased and p38 MAPK phosphorylation is increased).
PTEN is a negative regulator of Akt, and therefore we assessed whether association of the Akt-PTEN complex was altered in the renal tubules of FVB mice and those treated with STZ for 3 and 6 mo by performing Akt immunoprecipitation studies. Figure 12B, bottom demonstrates that the Akt-PTEN association was unaltered in renal tubules of 3- and 6-mo STZ-treated mice.
PTEN phosphorylation is associated with decreased PTEN activity (3). Conversely, unphosphorylated PTEN associates with the p85 subunit of PI-3K and inactivates Akt (33). Furthermore, PTEN activation has been documented to promote p38 MAPK activation (39). Thus we evaluated phospho-PTEN levels in renal tubular lysates of 3- and 6-mo STZ-treated mice to establish whether PTEN is the master regulator that controls contrasting Akt and p38 phosphorylation profiles in diabetes. PTEN phosphorylation was documented in renal tubular lysates from 3-mo STZ-treated mice, a time point at which pSer473Akt levels are high. PTEN phosphorylation was markedly attenuated in the renal tubules of 6-mo STZ-treated mice, a time point at which Akt phosphorylation is inhibited and phospho-p38 levels are markedly enhanced (Fig. 12C).
DISCUSSION
In the present study, we demonstrated a temporal relationship between activation status of prosurvival serine/threonine kinase Akt and prodeath kinase p38 MAPK in cultured RPTCs, renal tubules from control and diabetic mice treated with or without insulin, and in kidneys of STZ-diabetic mice compared with their age-matched controls. Downregulation of Akt activation during hyperglycemia coincided with increased p38 MAPK activation, caspase-3 activation, inflammatory and fibrotic markers, and renal dysfunction. In addition, inhibition of the PI-3K/Akt pathway with LY294002 or silencing Akt expression in cultured human RPTCs with Akt siRNA promoted p38 MAPK phosphorylation in the absence of hyperglycemia. Furthermore, overexpression of constitutively active Akt during hyperglycemia inhibited p38 MAPK phosphorylation and caspase-3 activation. Our current study clearly demonstrates that overexpression of Akt confers protection to RPTCs exposed to hyperglycemia by inhibiting proapoptotic p38 MAPK signaling. Therefore, pharmacological induction of Akt activation could provide therapeutic options to control p38 MAPK-mediated diabetic complications.
Using an in vitro diabetic model, we demonstrated in cultured RPTCs that Akt phosphorylation was significantly increased after 3–9 h of HG exposure, followed by a decrease between 12 and 48 h. In contrast, p38 MAPK phosphorylation was detected between 9 and 48 h of HG treatment. Decreased Akt phosphorylation and increased p38 MAPK phosphorylation between 9 and 48 h of HG treatment coincided with increased RPTC apoptosis as measured by TUNEL assay and caspase-3 activity. Furthermore, the role of insulin in regulating the contrasting effects of hyperglycemia on Akt and p38 MAPK phosphorylation was tested in isolated renal tubules from FVB, 10-day STZ-, and 10-day STZ- and insulin-treated mice. Insulin treatment decreased hyperglycemia-induced pSer473Akt to basal levels and increased p38 MAPK phosphorylation to the basal level. HG induces RPTC apoptosis in HK-2 and LLC-PK1 cells (2, 15, 28, 31, 46, 47). Decreased Bcl-2 and increased Bax expression was identified as a mechanism of HG-induced RPTC apoptosis. These studies suggest that hyperglycemia-induced RPTC apoptosis is mediated by a mitochondrial-dependent pathway. However, a contribution of signaling cascades mediating p38 MAPK activation, mitochondrial dysfunction, and RPTC death is unclear. While ceramide-induced p38 MAPK activation has been shown to negatively regulate Akt activation, effects of hyperglycemia-induced p38 MAPK activation on Akt activity has not been investigated previously (21). Since our results demonstrated that decreased Akt activity coincided with increased p38 MAPK activity, we determined effects of maintaining sustained Akt activity on p38 MAPK activation and RPTC apoptosis during hyperglycemia. Overexpression of constitutively active Akt in RPTCs in the presence of HG prevented p38 MAPK activation and caspase-3 activity in RPTCs. The present study suggests that downregulation of Akt activation during hyperglycemia may contribute to p38 MAPK activation and RPTC apoptosis. Recently, several studies have indicated the involvement of p38 MAPK activation in diabetic interstitial and tubular hypertrophy and fibrosis (1, 7, 9, 13). Most remarkably, increased p38 MAPK expression was found mainly in the tubules (1, 7, 13, 37), which coincided with localization of TGF-β (12) and correlated with tubulointerstitial lesions (37), suggesting the role of p38 MAPK activation in the induction of TGF-β and eventually renal fibrosis. To support this notion, the present study documented increases in p38 activity and increases in inflammatory and fibrotic markers in STZ-treated diabetic mouse kidneys such as TNF-α, PAI-1, TGF-β, pre-collagen III, and collagen III expression. These increases in p38 MAPK phosphorylation and inflammatory and fibrotic markers in the 6-mo STZ-diabetic kidneys coincided with increased active caspase-3 immunoreactivity. Thus identifying signaling pathways that lead to downregulation of p38 activation may provide therapeutic options to control p38 MAPK-mediated diabetic complications.
Cross talk between proapoptotic and antiapoptotic kinases regulates cell fate. Interplay between Akt and p38 MAPK pathways has been documented in various cell types (21, 35). However, cross talk between Akt and p38 MAPK cascades and its contributions to diabetes-and hyperglycemia-induced RPTC apoptosis have not been demonstrated previously. Our studies identified a direct role for p38 MAPK in inducing RPTC apoptosis, as pharmacological inhibition of p38 MAPK with SB203580 significantly inhibited HG-induced RPTC apoptosis. Furthermore, Akt was identified as a negative regulator of p38 MAPK activation, as overexpression of constitutively active Akt in RPTCs inhibited HG-induced p38 MAPK activation in these cells. It is known that p38 MAPK activation in response to HG contributes to hypertrophy of RPTCs. For example, exposure of renal tubular cells (LLC-PK1 cells) to 25 mM glucose for 24 and 72 h significantly increased p38 MAPK phosphorylation at both time points with significant cellular hypertrophy only at 72 h (13). However, this study did not determine the effect of HG on LLC-PK1 apoptosis, but a reduction in cell numbers with increased protein synthesis, indicative of hypertrophy, was presented (13).
Another important finding of the present study was the induction of Akt phosphorylation in 1-mo STZ-diabetic kidneys, with maximal phosphorylation occurring in 3 mo, followed by a decrease in Akt phosphorylation after 6 mo of STZ treatment compared with age-matched control kidneys. Akt activation in 1- and 3-mo STZ-diabetic kidneys may provide an adaptive response of renal cells to hyperglycemia. As such, no caspase-3 activity was detected in 1- and 3-mo STZ-diabetic kidneys, while a decrease in Akt phosphorylation in 6-mo STZ-diabetic kidneys coincided with increased active caspase-3 immunoreactivity and increased TUNEL positivity compared with aged-matched control kidneys. These results suggest that Akt activation in 1- and 3-mo STZ-diabetic kidneys may play a critical role in inhibiting caspase-3 activation. Similar to our ex vivo studies, we demonstrated that HG-enhanced Akt phosphorylation at 3 h, followed by a time-dependent decrease in in vitro cultured RPTCs. Recently, Clodfelder-Miller et al. (10) showed increased Akt phosphorylation in the brains of mice as early as 3 days after administration of STZ. Akt is an important cell survival kinase and has been shown to regulate cell fate in response to physiological or pathophysiological stresses (14, 19); however, the role of Akt signaling in the regulation of RPTC apoptosis, by modulating p38 MAPK activation in response to diabetes or HG, has not been documented previously.
Akt has been shown to regulate mesangial cell proliferation (41) and to regulate nitric oxide production, which contributes to glomerular hyperfiltration in early diabetic kidneys (48). The protective role of Akt against oxidant (H2O2)-induced apoptosis in RPTCs has been documented (19). The role of Akt in insulin signaling has been extensively investigated (14, 18, 39). However, direct effects of HG on Akt signaling, as seen in diabetes, are poorly understood. In situ renal tubular cells are continually exposed to various stresses and alterations in the ionic content of the extracellular medium at their apical surface; therefore, these cells may have established strong defense mechanisms against these stresses. Among cellular stresses, oxidative stress is a major consequence of hyperglycemia under diabetic conditions (2, 32), and Akt activation has been shown to serve as an initial survival response to cellular stress (29). To support the in vivo finding, an early increase in Akt phosphorylation was also confirmed in the RPTCs exposed to HG. Interestingly, the increased Akt phosphorylation seen between 3 and 6 h of HG treatment returned to control levels by 24 h and to even lower than control levels at 48 h, which is consistent with a recent study in cultured retinal cells which also showed that Akt phosphorylation dropped below control levels in response to HG at 96 h (12). In addition, Akt has been shown to be a substrate of caspase-3 (49). However, total Akt expression was unaltered by 48-h HG treatment. Thus it is quite unlikely that Akt activity is regulated by caspase-3 activation.
In our in vivo study, we demonstrate Akt phosphorylation in whole kidney lysates from 1- and 3-mo STZ-diabetic mice, while Akt phosphorylation was decreased to baseline in the kidneys of 6-mo diabetic mice. In agreement with our studies, Lloberas et al. (24) demonstrated increased Akt phosphorylation in the kidneys of 4-mo STZ-diabetic Sprague-Dawley rats, while Zdychová et al. (51) demonstrated a decrease in Akt phosphorylation in the kidneys of 1-mo STZ-diabetic male Wistar rats compared with their age-matched controls. This difference between our study and two studies noted above may be attributed to the fact that different species of animals (mice vs. rats) were used in these studies (51). Moreover, Qi et al. (33) have demonstrated differential susceptibility to STZ-induced diabetic nephropathy in various strains of mice.
The novel finding of the present study is that modulation of Akt activity during hyperglycemia regulates p38 MAPK activation and RPTC apoptosis. We demonstrated that continual activation of Akt by transfecting RPTCs with a constitutively active Akt construct inhibited p38 MAPK phosphorylation in the presence of HG and prevented caspase-3 activation. In addition, inhibition of the PI-3K/Akt pathway with LY294002 or silencing Akt expression in cultured RPTCs with Akt siRNA promoted p38 MAPK phosphorylation in the absence of hyperglycemia. These data collectively suggest that downregulation of Akt activation during sustained hyperglycemia contributes to p38 MAPK phosphorylation and HG-induced RPTC cell death.
We have previously shown in human neutrophils that Akt exists in a complex with p38 MAPK, MAPKAPK-2 (MK2), and Hsp27 (35, 36). In addition, we demonstrated that activation of the p38 MAPK pathway is required for Akt activation, as p38 MAPK downstream target MK2 acts as PDK2 for Akt, phosphorylating Akt on Ser473. Furthermore, we showed that Akt-Hsp27 interaction was critical to Akt activation, as Hsp27 acts as a scaffolding protein for Akt (50). As opposed to what was previously believed for p38 MAPK, it has now become clear that p38 MAPK can serve a proapoptotic as well as a prosurvival role depending upon its upstream activator (25, 27). It has been documented that MKK6-mediated p38 MAPK activation induces a protective signal while MKK3-mediated p38 MAPK activity promotes cell death. In human neutrophils, we have demonstrated a protective arm of p38 MAPK when associated with Akt and Hsp27 (50). In the current study, we documented the association of p38 MAPK and Hsp25 with the Akt signal complex in the kidneys of 3-mo FVB mice, which was markedly enhanced after 3-mo STZ treatment, coinciding with increased Akt phosphorylation. Furthermore, we documented the dissociation of p38 MAPK and Hsp25 from the Akt signal complex in 6-mo STZ-diabetic kidneys, coinciding with decreased Akt phosphorylation and increased p38 MAPK phosphorylation. Thus, similar to neutrophils, it is possible that disruption of Akt-Hsp25 and Akt-p38 MAPK interaction in 6-mo STZ-kidney and renal tubular lysates downregulates Akt activation by disrupting the MKK6-p38MAPK-Akt-Hsp25 signaling complex and promoting assembly of a MKK3-p38 MAPK-Hsp25 prodeath signaling pathway.
PTEN activity contributes to downregulation of Akt activity and upregulation of p38 activation in certain cell types (3, 40). Thus we next investigated whether PTEN is the master regulator that controls contrasting Akt and p38 phosphorylation profiles in diabetes. We demonstrated that while Akt-Hsp25 and Akt-p38 MAPK associations were altered in the kidneys of 6-mo STZ-diabetic mice, the Akt-PTEN association remained unaltered. However, we demonstrated that PTEN phosphorylation was increased in 3-mo STZ-diabetic kidneys and was markedly inhibited after 6 mo of STZ treatment. Collectively, these results suggest that PTEN activation and dissociation of Akt-Hsp25 and Akt-p38 MAPK interaction, in 6-mo diabetic kidneys, may contribute to contrasting Akt and p38 MAPK activation profiles.
GRANTS
These studies were supported by National Institutes of Health (NIH) Grant R01AI075212-01A1 (M. J. Rane), American Heart Association Grant 0335278N (M. J. Rane), NIH Grant K01 DK080951 (M. T. Barati), American Diabetes Association Grants 02-07-JF-02 and 05-07-02 (L. Cai), and Juvenile Diabetes Research Foundation Grant 5-2006-382 (L. Cai).
DISCLOSURES
No conflicts of interest have been declared by the authors.
Supplementary Material
REFERENCES
- 1.Adhikary L, Chow F, Nikolic-Paterson DJ, Stambe C, Dowling J, Atkins RC, Tesch GH. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia 47: 1210–1222, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J 17: 908–910, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Birle D, Bottini N, Williams S, Huynh H, deBelle I, Adamson E, Mustelin T. Negative feedback regulation of the tumor suppressor PTEN by phosphoinositide-induced serine phosphorylation. J Immunol 169: 286–291, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 51: 1938–1948, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 54: 1829–1837, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Caramori ML, Mauer M. Diabetes and nephropathy. Curr Opin Nephrol Hypertens 12: 273–282, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Brahmbhatt S, Gupta A, Sharma AC. Duration of streptozotocin-induced diabetes differentially affects p38-mitogen-activated protein kinase (MAPK) phosphorylation in renal and vascular dysfunction. Cardiovasc Diabetol 4: 3, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury I, Chaqour B. Regulation of connective tissue growth factor (CTGF/CCN2) gene transcription and mRNA stability in smooth muscle cells. Involvement of RhoA GTPase and p38 MAP kinase and sensitivity to actin dynamics. Eur J Biochem 271: 4436–4450, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Clodfelder-Miller B, De Sarno P, Zmijewska AA, Song L, Jope RS. Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3. J Biol Chem 280: 39723–39731, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Borst MH, Prakash J, Melenhorst WB, van den Heuvel MC, Kok RJ, Navis G, van Goor H. Glomerular and tubular induction of the transcription factor c-Jun in human renal disease. J Pathol 213: 219–228, 2007 [DOI] [PubMed] [Google Scholar]
- 12.el-Remessy AB, Bartoli M, Platt DH, Fulton D, Caldwell RB. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci 118: 243–252, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fujita H, Omori S, Ishikura K, Hida M, Awazu M. ERK and p38 mediate high-glucose-induced hypertrophy and TGF-β expression in renal tubular cells. Am J Physiol Renal Physiol 286: F120–F126, 2004 [DOI] [PubMed] [Google Scholar]
- 14.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, Dunger DB, Barford D, Umpleby AM, Wareham NJ, Davies HA, Schafer AJ, Stoffel M, O'Rahilly S, Barroso I. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304: 1325–1328, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harwood SM, Allen DA, Raftery MJ, Yaqoob MM. High glucose initiates calpain-induced necrosis before apoptosis in LLC-PK1 cells. Kidney Int 71: 655–663, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ishii N, Ogawa Z, Suzuki K, Numakami K, Saruta T, Itoh H. Glucose loading induces DNA fragmentation in rat proximal tubular cells. Metabolism 45: 1348–1353, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Kang SW, Adler SG, Lapage J, Natarajan R. p38 MAPK and MAPK kinase 3/6 mRNA and activities are increased in early diabetic glomeruli. Kidney Int 60: 543–552, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54: 1692–1697, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kaushal GP, Liu L, Kaushal V, Hong X, Melnyk O, Seth R, Safirstein R, Shah SV. Regulation of caspase-3 and -9 activation in oxidant stress to RTE by forkhead transcription factors, Bcl-2 proteins, and MAP kinases. Am J Physiol Renal Physiol 287: F1258–F1268, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kelly DJ, Stein-Oakley A, Zhang Y, Wassef L, Maguire J, Koji T, Thomson N, Wilkinson-Berka JL, Gilbert RE. Fas-induced apoptosis is a feature of progressive diabetic nephropathy in transgenic (mRen-2)27 rats: attenuation with renin-angiotensin blockade. Nephrology 9: 7–13, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Oh JE, Kim SW, Chun YJ, Kim MY. Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer Lett 260: 88–95, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem 259: 67–70, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Lin CL, Wang FS, Kuo YR, Huang YT, Huang HC, Sun YC, Kuo YH. Ras modulation of superoxide activates ERK-dependent fibronectin expression in diabetes-induced renal injuries. Kidney Int 69: 1593–1600, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyo JM. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17: 1395–1404, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Ma FY, Tesch GH, Flavell RA, Davis RJ, Nikolic-Paterson DJ. MKK3-p38 signaling promotes apoptosis and the early inflammatory response in the obstructed mouse kidney. Am J Physiol Renal Physiol 293: F1556–F1563, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Maestroni A, Tentori F, Meregalli G, Gabellini D, Asnaghi V, Ruggieri D, Zerbini G. Inhibition of MAP-kinase cascade normalizes the proliferation rate of fibroblasts from patients with type 1 diabetes and nephropathy. J Diabetes Complications 19: 291–296, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Martindale JJ, Wall JA, Martinez-Longoria DM, Aryal P, Rockman HA, Guo Y, Bolli R, Glembotski CC. Overexpression of mitogen-activated protein kinase kinase 6 in the heart improves functional recovery from ischemia in vitro and protects against myocardial infarction in vivo. J Biol Chem 280: 669–676, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morais C, Westhuyzen J, Pat B, Gobe G, Healy H. High ambient glucose is effect neutral on cell death and proliferation in human proximal tubular epithelial cells. Am J Physiol Renal Physiol 289: F401–F409, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Nishino T, Pusey CD, Domin J. Elevated Akt phosphorylation as an indicator of renal tubular epithelial cell stress. J Biol Chem 277: 33943–33949, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Obrosova IG, Julius UA. Role for poly(ADP-ribose) polymerase activation in diabetic nephropathy, neuropathy and retinopathy. Curr Vasc Pharmacol 3: 267–283, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Ortiz A, Ziyadeh FN, Neilson EG. Expression of apoptosis-regulatory genes in renal proximal tubular epithelial cells exposed to high ambient glucose and in diabetic kidneys. J Investig Med 45: 50–56, 1997 [PubMed] [Google Scholar]
- 32.Pacher P, Obrosova IG, Mabley JG, Szabo C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem 12: 267–275, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Rabinovsky R, Pochanard P, McNear C, Brachmann SM, Duke-Cohan JS, Garraway LA, Sellers WR. p85 Associates with unphosphorylated PTEN and the PTEN-associated complex. Mol Cell Biol 29: 5377–5388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem 276: 3517–3523, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278: 27828–27835, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Hara A, Yamahana J, Okumura T, Takasawa K, Takeda S, Yoshimura M, Kida H, Yokoyama H. Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am J Kidney Dis 45: 54–65, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16, Suppl 1: S30–S33, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Schinner S, Barthel A, Dellas C, Grzeskowiak R, Sharma SK, Oetjen E, Blume R, Knepel W. Protein kinase B activity is sufficient to mimic the effect of insulin on glucagon gene transcription. J Biol Chem 280: 7369–7376, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Shen YH, Zhang L, Gan Y, Wang X, Wang J, LeMaire SA, Coselli JS, Wang XL. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem 281: 7727–7736, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Sheu ML, Ho FM, Chao KF, Kuo ML, Liu SH. Activation of phosphoinositide 3-kinase in response to high glucose leads to regulation of reactive oxygen species-related nuclear factor-kappaB activation and cyclooxygenase-2 expression in mesangial cells. Mol Pharmacol 66: 187–196, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res 90: 1243–1250, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Song Y, Wang J, Li Y, Du Y, Arteel GE, Saari JT, Kang YJ, Cai L. Cardiac metallothionein synthesis in streptozotocin-induced diabetic mice, and its protection against diabetes-induced cardiac injury. Am J Pathol 167: 17–26, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorp ML. Diabetic nephropathy: common questions. Am Fam Physician 72: 96–99, 2005 [PubMed] [Google Scholar]
- 46.Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Frumento G, Berruti V, Gandolfo MT, Garibotto G, Deferran G. Taurine prevents apoptosis induced by high ambient glucose in human tubule renal cells. J Investig Med 50: 443–451, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Salvatore F, Berruti V, Gandolfo MT, Garibotto G, Deferrari G. Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J Am Soc Nephrol 15, Suppl 1: S85–S87, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Nagase S, Koyama A. Stimulatory effect of IGF-I and VEGF on eNOS message, protein expression, eNOS phosphorylation and nitric oxide production in rat glomeruli, and the involvement of PI3-K signaling pathway. Nitric Oxide 10: 25–35, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Widmann C, Gibson S, Johnson GL. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem 273: 7141–7147, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem 282: 21598–21608, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zdychova J, Vesela J, Kazdova L, Komers R. Renal activity of Akt kinase in experimental type 1 diabetes. Physiol Res 57: 709–715, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol 165: 2033–2043, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.