Abstract
Angiotensin II (ANG II) stimulates proximal tubule (PT) sodium and water reabsorption. We showed that treating rats acutely with the angiotensin-converting enzyme inhibitor captopril decreases PT salt and water reabsorption and provokes rapid redistribution of the Na+/H+ exchanger isoform 3 (NHE3), Na+/Pi cotransporter 2 (NaPi2), and associated proteins out of the microvilli. The aim of the present study was to determine whether acute ANG II infusion increases the abundance of PT NHE3, NaPi2, and associated proteins in the microvilli available for reabsorbing NaCl. Male Sprague-Dawley rats were infused with a dose of captopril (12 μg/min for 20 min) that increased PT flow rate ∼20% with no change in blood pressure (BP) or glomerular filtration rate (GFR). When ANG II (20 ng·kg−1·min−1 for 20 min) was added to the captopril infusate, PT volume flow rate returned to baseline without changing BP or GFR. After captopril, NHE3 was localized to the base of the microvilli and NaPi2 to subapical cytoplasmic vesicles; after 20 min ANG II, both NHE3 and NaPi2 redistributed into the microvilli, assayed by confocal microscopy and density gradient fractionation. Additional PT proteins that redistributed into low-density microvilli-enriched membranes in response to ANG II included myosin VI, DPPIV, NHERF-1, ezrin, megalin, vacuolar H+-ATPase, aminopeptidase N, and clathrin. In summary, in response to 20 min ANG II in the absence of a change in BP or GFR, multiple proteins traffic into the PT brush-border microvilli where they likely contribute to the rapid increase in PT salt and water reabsorption.
Keywords: hypertension, captopril
angiotensin ii (ANG II), a potent vasoconstrictor and sodium-retaining hormone, is crucial for the regulation of sodium transport in the kidneys and therefore for blood pressure (BP) homeostasis. Strong evidence highlights the contribution of high ANG II levels to the development of cardiovascular and renal diseases (23, 37). Consequently, drugs affecting the renin-angiotensin system (RAS), and in particular angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers, are commonly used for the treatment of high BP.
The angiotensin type I receptor (AT1R) is responsible for the Na+-retaining effects of ANG II. The renal proximal tubule (PT) expresses AT1R on both the apical and basolateral membranes and ANG II is delivered via the general circulation or filtered at the glomerulus. In addition, the PT cells synthesize all the components necessary to produce and secrete ANG II into its lumen: angiotensinogen, renin, and ACE (14, 29). ANG II has been shown to increase PT sodium and water reabsorption, and ACE inhibitors and AT1R blockers decrease PT sodium and water reabsorption (10, 13). The sodium hydrogen exchanger isoform 3 (NHE3) is the main transporter mediating sodium reabsorption in the PT (20) and cultured kidney cell studies indicate that ANG II can rapidly increase NHE3 abundance and activity in the plasma membrane (9).
We recently investigated the molecular mechanisms responsible for the natriuretic effect of ACEI in vivo (17). In rats acutely infused with the ACEI captopril at a dose that blocks production of ANG II (both outside and inside the kidney) without lowering BP or glomerular filtration rate (GFR), PT flow rate rapidly increased coincident with a redistribution of NHE3 out of the body of the microvilli to the base of the microvilli (17). The PT sodium phosphate cotransporter 2 (NaPi2), another major sodium transporter essential for the reabsorption of filtered phosphate (24), also redistributed out of the microvilli during captopril, to a subapical vesicular compartment (17). The redistribution of these transporters was also accompanied by the coincident redistribution of the putative NHE3- and NaPi2-associated proteins NHE3 regulatory factor 1 (NHERF-1), myosin VI, megalin, dipeptidyl peptidase IV (DPPIV), and ezrin, as well as the vacuolar H+-ATPase β2 subunit, aminopeptidase N (APN; which degrades ANG III to ANG IV), myosin IIa, and clathrin out of the low-density microvilli-enriched membranes. These concerted responses of transporters, cytoskeleton, molecular motors, and angiotensin metabolism pathways likely work together to affect the captopril-mediated diuresis and natriuresis (17).
The goal of the present study was to determine whether acute ANG II infusion increases the abundance of PT NHE3, NaPi2, and associated proteins in the microvilli available for reabsorbing NaCl. The design aimed to isolate the effects of ANG II per se, distinct from potentially confounding effects of ANG II on BP, hemodynamics, or intrarenal generation of ANG II (29) by using a subpressor dose of ANG II and incorporating infusion of an ACEI along with the ANG II to block effects of ANG II on intrarenal RAS, as we previously reported (27). We found that in response to acute ANG II treatment, NHE3, NaPi2, and additional transporter-associated proteins redistributed into microvillar-enriched low-density membranes coincident with ANG II-stimulated salt and volume reabsorption.
EXPERIMENTAL PROCEDURES
Animal protocol.
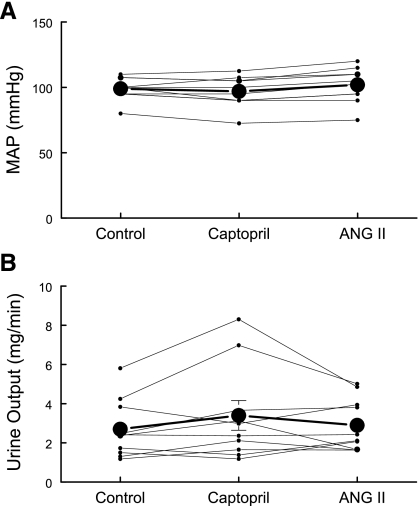
All animal experiments were approved by the University of Southern California Keck School of Medicine Institutional Animal Care and Use Committee and were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments were performed on male Sprague-Dawley rats (300–350 g body wt) that were kept under diurnal light conditions and had free access to food and water. Rats were anesthetized intraperitoneally with Inactin (Sigma; 100 mg/kg). During surgery, body temperature was maintained thermostatically at 37°C, BP was measured via a polyethylene catheter (PE-50) placed in the carotid artery, and euvolemia was maintained by continuous infusion of BSA-saline (4% BSA in 0.9% saline) into the jugular vein (via PE-50) at 50 μl/min. The left ureter was cannulated with a Surflo IV catheter (Terumo) for measurement of urine output. At the completion of surgery, the animals were allowed to recover for >60 min with continuing infusion of BSA-saline before treatment. Two treatment groups were compared: 1) captopril (12 μg/min) added to the BSA-saline infusate for 20 min before death, 2) ANG II treated: following 20-min captopril infusion (12 μg/min), ANG II (20 ng·kg−1·min−1) was coinfused with captopril (12 μg/min) in the BSA-saline for an additional 20 min before death. Throughout the equilibration and treatment periods, urine was collected at 10-min intervals, urine volume was determined gravimetrically, and BP was continuously collected. Kidneys were collected for subcellular fractionation or fixed for imaging. Figure 1 summarizes results collected from animals in treatment group 2 during control, captopril, and ANG II + captopril treatment periods.
Fig. 1.
Effects of captopril and ANG II infusion protocols on mean arterial pressure (MAP) and urine output. Rats were sequentially infused with 4.0% BSA in 0.9% saline (50 μl/min) for >30 min, then with captopril (12 μg/min) for 20 min, and then with ANG II + captopril (20 ng·kg−1·min−1 ANG II and 12 μg/min captopril). A: MAP was measured continuously at the carotid (mmHg). B: urine was collected continuously in 10-min intervals and output rate was averaged over the treatment period (mg/min), n = 10, individual traces and means ± SE.
Homogenization and subcellular fractionation on sorbitol gradients.
Sorbitol density gradient fractionation is an empirical approach that enriches, as opposed to separates, membrane domains and populations based on density. We previously characterized the distribution of various membrane markers in renal cortex separated on these gradients (44, 45). In brief, basolateral (sodium pump) and apical (alkaline phosphatase) plasma membranes are enriched in low-density fractions 4–6, while intermicrovillar cleft markers (megalin, clathrin) are found spread between fractions 6 and 10. Endosomal (rabs) and lysosomal (β-hexosaminidase) markers are enriched in higher-density fractions 7–12.
The procedure for subcellular fractionation of the renal cortex membranes has been described in detail previously (39). After the kidneys were collected, renal cortices were dissected, diced, suspended in ∼5 ml isolation buffer (5% sorbitol, 0.5 mM disodium EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 9 μg/ml aprotinin, and 5 mM histidine/imidazole buffer, pH 7.5), homogenized for 10 min at a low setting with an Ultra-Turrax T25 (IKA-Labortechnik), and then centrifuged at 2,000 g for 10 min. The supernatant was saved, the pellet was rehomogenized in 5 ml isolation buffer, recentrifuged, and the two supernatants were pooled (So). Then, 4 ml of So were mixed with 6 ml of 87.4% sorbitol buffer, loaded between two hyperbolic sorbitol gradients, and centrifuged at 100,000 g for 5 h in a swinging bucket rotor. Twelve fractions were collected from the top, diluted with isolation buffer, pelleted by centrifugation (250,000 g for 1.5 h), resuspended in 1 ml of isolation buffer, and stored in aliquots at −80°C, pending assays.
Immunoblot analysis and antibodies.
As described (17), to assess the density distribution patterns of proteins, a constant volume of each fraction was assayed. Samples were denatured in SDS-PAGE sample buffer for 30 min at 37°C, resolved on 7.5% SDS-polyacrylamide gels according to Laemmli (16), and transferred to polyvinylidene difluoride membranes (Millipore Immobilon-P). Blots were probed with the following antibodies: NHE3-C00 against NHE3, Mc-NaPi2 against NaPi2 (both 1:2,000; McDonough laboratory), anti-myosin VI (1:2,000; Proteus Biosciences), R-1046 against NHERF-1 (1:3,000; E. Weinman, Univ. of Maryland), anti-gp105 against DPPIV (1:1,000) and 459 against megalin (1:5,000; both provided by M. Farquhar, UCSD), BT561 against myosin IIa (1:2,000; Biomedical Technologies), MAS 401 against the heavy chain of clathrin (1:200; Harlan Sera-Lab), sc-15360 against APN (1:100; Santa Cruz Biotechnology), sc-6409 against ezrin (1:1,000; Santa Cruz Biotechnology), and A1565eF1 against the β2-subunit of the vacuolar H+-ATPase (1:100; D. Brown, Harvard University). Polyclonal primary antibodies were detected with Alexa 680-labeled goat anti-rabbit or Alexa 680-labeled donkey anti-goat secondary antibody (Molecular Probes), and polyclonal chicken anti-β2 primary antibody was probed with monoclonal mouse anti-chicken secondary antibody (Sigma) followed by detection with Alexa 680-labeled goat anti-mouse secondary antibody (Molecular Probes). Monoclonal antibodies were detected with Alexa 680-labeled goat anti-mouse secondary antibody (Molecular Probes). Signals were detected and quantitated with the Odyssey Infrared Imaging System (Li-COR, Lincoln, NE) and accompanying Li-COR software.
Statistical analysis of density gradient patterns.
As described previously (26, 27), two-way ANOVA was employed to determine whether there was a significant difference in the overall density distribution pattern of a protein. The repeated factors were treatment (captopril or ANG II + captopril) and fraction. If the interaction between treatment and fraction was found to be significant (P < 0.05), then it was concluded that the treatment had a significant effect. After significance was established, the location of the difference in the pattern was assessed by unpaired two-tailed Student's t-test assuming equal variance with Bonferroni adjustments for multiple comparisons. Data are expressed as means ± SE. Differences were regarded significant at P < 0.05.
Confocal microscopy.
Two series of experiments were analyzed. In the initial series, kidneys were rapidly perfusion fixed via the dorsal aorta with 4% paraformaldehyde solution containing 0.1 M sodium cacodylate at pH 7.2 without controlling for a possible increase in arterial pressure. Tissue blocks were trimmed from the cortex and postfixed in 4% paraformaldehyde solution containing 0.1 M sodium cacodylate, pH 7.4 for 3 h. The tissue blocks were cryoprotected overnight in 2.3 M sucrose in PBS, mounted on holders, and frozen in liquid nitrogen. These specimens were used for thin (0.8 μm) cryosections. Two sections of each kind of tissue were collected on the same charged object glass, side by side, to ensure comparable treatments. Sections were rinsed with PBS, incubated with 0.05 M NH4Cl, and blocked by incubation in PBS containing 0.05 M glycine and 0.1% skim milk powder. Sections were then incubated for 60 min at room temperature in a mixture of polyclonal anti-NHE3-C00 (1:25) and monoclonal anti-villin (1:50; Immunotech), diluted in blocking medium, rinsed, incubated for 60 min with a mixture of AlexaFluor 568-conjugated goat anti-rabbit IgG (1:100; Molecular Probes) and AlexaFluor 488-conjugated goat anti-mouse IgG (1:150; Molecular Probes), and mounted with DAKO fluorescence mounting medium (DAKO Danmark, Glostrup, Denmark). The sections were analyzed and recorded using Leica TSC SP2-inverted confocal laser-scanning microscope (Leica Microsystems, Heidelberg, Germany). Some immunofluorescence images were merged with the Nomarski differential interference contrast (DIC) images to reveal the distribution of the fluorophore as related to the tissue structure.
In a second series, kidneys were perfusion-fixed via the dorsal aorta with PLP fixative (2% paraformaldehyde, 75 mM lysine, and 10 mM Na-periodate, pH 7.4), at a rate that did not alter BP (measured via carotid cannula), and then postfixed in PLP for another 2–3 h. The fixed tissue was cryoprotected by incubation overnight in 30% sucrose in PBS, embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA), and frozen in liquid nitrogen. Cryosections (5 μm) were cut and transferred to Fisher Superfrost Plus-charged glass slides and air dried. For immunofluorescence labeling, the sections were rehydrated in PBS, followed by a 10-min wash in 50 mM NH4Cl in PBS, then with 1% SDS in PBS for 5 min for antigen retrieval. Dual labeling was performed by incubating with polyclonal antiserum NHE3-C00 (1:100) or McNaPi2 (1:50) and monoclonal anti-villin (1:100; Immunotech, Chicago, IL). The sections were then incubated with a mixture of FITC-conjugated goat anti-rabbit (Cappel Research Products, Durham, NC) and Alexa 568-conjugated goat anti-mouse (Molecular Probes) secondary antibodies diluted 1:100 for 1 h, mounted in Prolong Antifade containing the nuclear dye DAPI (Molecular Probes). Slides were viewed with a Zeiss LSM 510 microscope with DIC overlay.
RESULTS
Effects of ANG II plus captopril on mean arterial pressure and urine output.
Neither captopril infusion (12 μg/min) for 20 min nor coinfusion of ANG II (20 ng·kg−1·min−1) + captopril (12 μg/min) for 20 min significantly altered mean arterial pressure: control = 99 ± 3 mmHg, captopril = 97 ± 4 mmHg, ANG II+ captopril = 102 ± 4 mmHg, n = 10 (Fig. 1A). In our preceding paper using this protocol (17), we determined that proximal tubular flow rate was significantly increased above baseline during captopril infusion, an indicator of decreased tubular fluid reabsorption, while significantly reduced below baseline during ANG II + captopril infusion, a marker for increased tubular fluid reabsorption. Nonetheless, urine output, measured gravimetrically, was not significantly altered over this short time course: control = 2.7 ± 0.5 mg/min, captopril = 3.4 ± 0.8 mg/min, ANG II + captopril = 2.9 ± 0.4 mg/min, n = 10 (Fig. 1B), indicating that there was a downstream change in fluid reabsorption.
Effect of ANG II on subcellular distribution of NHE3 and associated proteins.
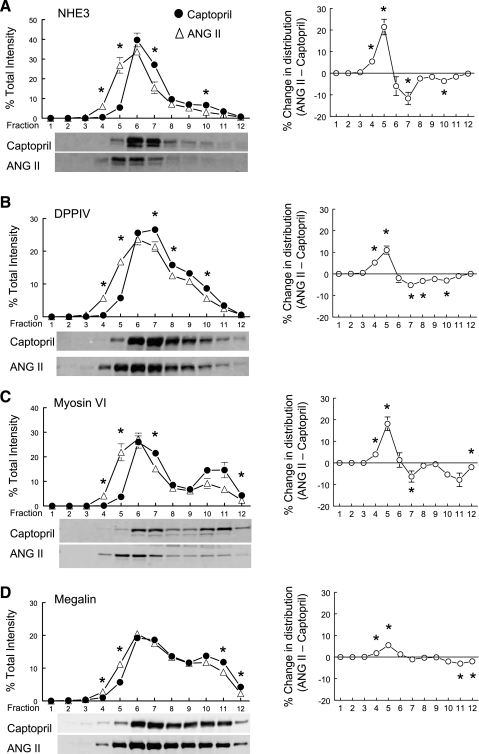
We previously showed that acute treatment with the ACE inhibitor captopril resulted in the redistribution of PT NHE3 along with its associated proteins DPPIV and myosin VI to the base of the microvilli (17), coincident with a decrease in PT reabsorption. In a related paper, we observed that captopril provoked a redistribution of distal convoluted tubule NCC from apical to subapical cytoplasmic vesicles and that 20 min ANG II + captopril restored NCC to baseline levels in the apical membrane (27). Based on these observations, we hypothesized that ANG II + captopril infusion, in the absence of a change in BP, would stimulate redistribution of NHE3 and NHE3-associated proteins into the microvilli, where they would be positioned for reabsorption. Figure 2A illustrates that, compared with captopril-treated kidneys, ANG II + captopril shifts the distribution of NHE3 toward lower-density membranes enriched in microvillar markers, supporting our hypothesis. Likewise, the NHE3 regulator DPPIV (11), the atypical molecular motor myosin VI (42), and, to a lesser extent, the endocytic receptor megalin (3) redistribute into lower-density membranes (Fig. 2, B, C, D). The difference in the distribution pattern across the gradient is plotted for each protein in the column on the right (Fig. 2).
Fig. 2.
Effects of acute ANG II infusion on the density distribution of proximal tubule microvillar proteins: Na+/H+ exchanger isoform 3 (NHE3; A), dipeptidyl peptidase IV (DPPIV; B), myosin VI (C), and megalin (D). Comparison of protein density distribution between 20-min captopril infusion (12 μg/min; ·, n = 5) and 20-min ANG II (20 ng·kg−1·min−1 ANG II and 12 μg/min captopril; ▵). Immunoreactivity in each fraction is expressed as a percentage of total signal in all 12 fractions. A–D, right: summarize the difference in the density distribution between ANG II- and captopril-treated groups. Values are means ± SE. *P < 0.05 compared with corresponding captopril fraction, unpaired Student's t-test after ANOVA. Shown below A–D are representative immunoblots of corresponding protein from typical experiments with NHE3 detected at 83 kDa, DPPIV at 89 kDa, myosin VI at 145 kDa, and megalin at 345 kDa.
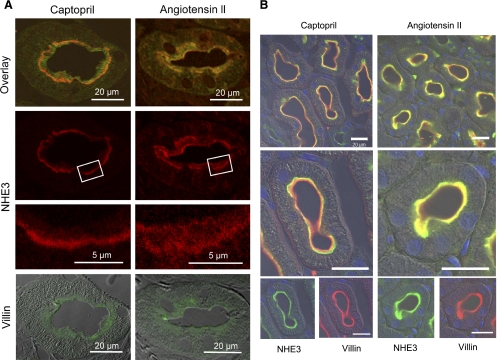
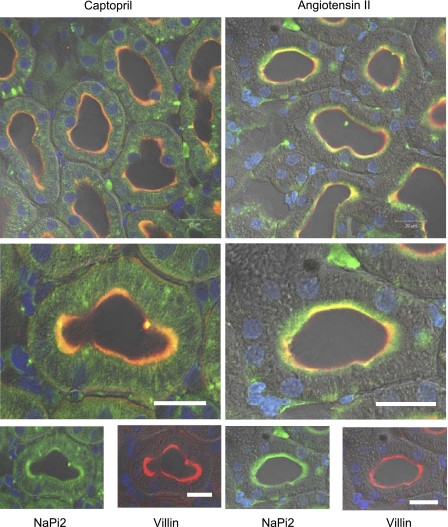
The localization of NHE3 was directly assessed by confocal immunofluorescence analysis in two series. In both, tissue slices from captopril- and ANG II + captopril-treated kidneys were placed side-by-side on the same slide for identical staining and visualizing, and in both the actin bundling protein villin was used as a marker for the PT microvilli. As shown in Fig. 3A, from the first series of experiments, following 20 min captopril (left), NHE3 (red) appears at the base of the microvilli as a thin band (low-magnification image) which is sharply limited (higher-magnification image). In the overlay image, a yellow band indicates overlapping of NHE3 (red) and villin (green) fluorophores at the basal part of the microvilli while green zone toward the lumen indicates lack of red fluorophore-labeled NHE in the villi after captopril treatment. After subsequent infusion of ANG II + captopril (Fig. 3A, right), NHE3 appears throughout the body of the microvilli overlapping with villin staining (yellow), but much of the NHE3 remained at the base of the microvilli. In this series, we found that the NHE3 distribution in the PT microvilli of different animals infused with ANG II + captopril was somewhat variable, leading us to suspect that the elevated perfusion pressure may interfere with the redistribution. Therefore, a second series was conducted in which the rate of infusion of fixative was controlled to prevent an elevation in BP (see experimental procedures). Confocal microscopy from this series made on 5-μm sections demonstrates that after captopril the images are similar to the previous series (note that the labeling colors are reversed; Fig. 3B, left) and that after ANG II + captopril infusion fluorophores for NHE3 (green) and villin (red) are overlapping (yellow) in the whole body of microvilli (Fig. 3B, right), supporting the hypothesis that elevated perfusion pressure blunts the redistribution of NHE3 into the microvilli in response to ANG II infusion. Taken together, the direct assay of NHE3 distribution in the microvilli by microscopy aids in interpreting the sorbitol density gradient redistribution, specifically, supporting the conclusion that the redistribution of proteins to low-density membranes during ANG II + captopril reflects movement of these proteins from the base into the body of the microvilli. We did not examine the distribution of all the associated proteins by confocal microscopy because our previous experience indicates that these only partially retract from the microvilli, for example in response to high-salt diet (43).
Fig. 3.
Indirect immunofluorescence microscopy of NHE3 redistribution in the proximal tubule was conducted in rats infused acutely with captopril (left) or ANG II (right) in distinct series conducted without controlling arterial pressure (A) or with controlling arterial pressure (B) as described in experimental procedures. In both series, a kidney sample from each group was placed on the same slide and processed identically for detection of NHE3 and villin. A: anti-NHE3 was detected with AlexaFluor 568-conjugated goat anti-rabbit secondary antibody and anti-villin with AlexaFluor 488-conjugated goat anti-mouse secondary antibody. B: anti-NHE3 was detected with FITC-conjugated goat anti-rabbit secondary antibody and anti-villin with Alexa 568-conjugated goat anti-mouse secondary and bar is 20 μm.
Effect of ANG II on subcellular distribution of NaPi2 and associated proteins.
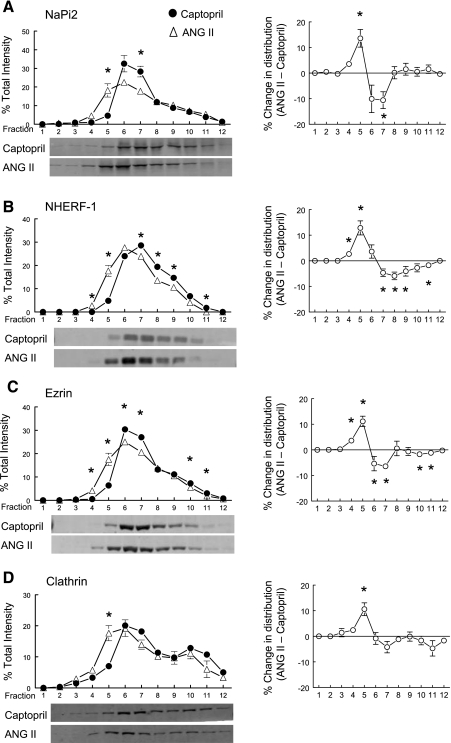
In addition to NHE3, the PT sodium phosphate cotransporter NaPi2 redistributes out of the microvilli to subapical vesicular locations in response to a variety of stimuli including parathyroid (PTH), acute hypertension, and captopril (17, 18, 41). Therefore, we hypothesized that NaPi2 would redistribute into the microvilli when infused with ANG II + captopril. As shown in Fig. 4A, ANG II + captopril significantly increased the percentage of total NaPi2 in low-density microvilli-enriched membranes, supporting our hypothesis. This redistribution was coincident with the redistribution of the NaPi2 regulator NHERF-1 (35) (Fig. 4B) as well as the actin tethering protein ezrin (Fig. 4C), known to anchor the NaPi2-NHERF-1 complex to the cytoskeleton (21, 30). NaPi2 is known to be internalized during PTH treatment via clathrin-coated vesicles (32) and it appears that even clathrin enriches in low-density membranes during ANG II stimulation.
Fig. 4.
Effects of acute ANG II infusion on the density distribution of proximal tubule microvillar proteins: NaPi2 (A), NHE3 regulatory factor 1 (NHERF-1; B), ezrin (C), and clathrin (D). Comparison of protein density distribution between 20-min captopril infusion (12 μg/min; ·, n = 5) and 20-min ANG II (20 ng·kg−1·min−1 ANG II and 12 μg/min captopril; ▵). Immunoreactivity in each fraction is expressed as a percentage of total signal in all 12 fractions. A–D, right: summarize the difference in the density distribution between ANG II- and captopril-treated groups. Values are means ± SE. *P < 0.05 compared with corresponding captopril fraction, unpaired Student's t-test after ANOVA. Shown below A–D are representative immunoblots of corresponding protein from typical experiments with NaPi2 detected at 82 kDa, NHERF-1 at 50 kDa, ezrin at 69 kDa, and clathrin at 193 kDa.
Direct assessment of NaPi2 by immunofluorescence in slices of kidneys from captopril-treated and ANG II + captopril-treated, stained, and processed side by side (Fig. 5) demonstrates that there is less NaPi2 in the microvilli after 20-min captopril treatment compared with the amount of NaPi2 colocalized with villin after adding ANG II to the infusate for 20 min. Some NaPi2 (green without red villin) staining is evident right below the brush border, indicating that not all of the NaPi2 returns fully to the brush border after 20-min ANG II. These results are consistent with the hypothesis that captopril provokes the retraction of NaPi2 to cytoplasmic vesicles localized in heavier-density regions of the sorbitol gradients and that ANG II infusion provokes rapid recruitment of NaPi2 back to the microvilli, which are localized to lighter-density membranes on the sorbitol density gradient.
Fig. 5.
Indirect immunofluorescence microscopy of Na+/Pi cotransporter 2 (NaPi2) redistribution in the proximal tubule of rats infused acutely with captopril (left) or ANG II (right) and perfusion fixed without elevating arterial pressure as described in experimental procedures. A kidney sample from each group was placed on the same slide and processed identically for detection of NaPi2 and villin. Anti-NaPi2 was detected with FITC-conjugated goat anti-rabbit secondary antibody and anti-villin with Alexa 568-conjugated goat anti-mouse secondary. Bar is 20 μm.
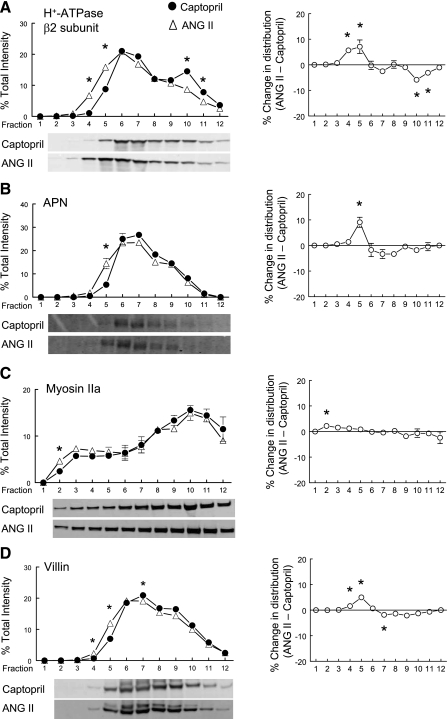
Effects of ANG II on distribution of vacuolar H+-ATPase β2, APN, myosin IIa, and villin.
In addition to NHE3, NaPi2, and their associated proteins, we previously identified, using a proteomic approach, additional proteins that redistribute to higher-density membranes following acute ACE inhibition (17). We aimed to determine whether subsequent ANG II infusion would provoke redistribution of these same proteins back into low-density membranes enriched in brush-border markers. The β2 subunit of the vacuolar H+-ATPase, found in the PT microvilli (25), redistributes into higher-density membranes with captopril treatment (17) and into lower-density membranes in response to ANG II infusion (Fig. 6A). The physiological significance of this redistribution from higher-to-lower density membranes is not readily apparent; however, it indicates that localization of the H+-ATPase in the microvilli is dependent on ANG II and that ACEI treatment will decrease H+-ATPase in the microvilli. APN, a metallohydrolase expressed in the brush border of PT cells, redistributes to heavier-density membranes with acute captopril treatment (17) and redistributes to lighter-density membranes with 20-min ANG II + captopril treatment (Fig. 6B). APN metabolizes ANG III to ANG IV and ANG III has actions similar to ANG II (1), so if APN redistribution to the microvilli activates the enzyme it will decrease ANG III and its activation of AT1 receptors and, thus, potentially limit ANG II-like responses. Myosin IIa is a conventional class II nonmuscle myosin heavy chain (28) expressed primarily in the podocytes but is also detected in the PT brush border (2), where its function is undefined. When ANG II + captopril is infused, there is a slight redistribution of myosin IIa redistributes into lower-density membranes (Fig. 6C). Similarly, there is a very slight but significant redistribution of the actin core bundling protein villin (Fig. 6D).
Fig. 6.
Effects of acute ANG II infusion on the density distribution of proximal tubule microvillar proteins: vacuolar H+-ATPase β2 (A), aminopeptidase N (APN; B), myosin IIa (C), and villin (D). Comparison of protein density distribution between 20-min captopril infusion (12 μg/min; ·, n = 5) and 20-min ANG II (20 ng·kg−1·min−1 ANG II and 12 μg/min captopril; ▵). Immunoreactivity in each fraction is expressed as a percentage of total signal in all 12 fractions. A–D, right: summarize the difference in the density distribution between ANG II- and captopril-treated groups. Values are means ± SE. *P < 0.05 compared with corresponding captopril fraction, unpaired Student's t-test after ANOVA. Shown below A–D are representative immunoblots of corresponding protein from typical experiments with vacuolar H+-ATPase β2 subunit detected at 57 kDa, APN at 110 kDa, myosin IIa detected at 227 kDa, and villin detected at 95 kDa.
DISCUSSION
Circulating ANG II levels are increased when extracellular fluid levels are reduced, whether or not there is a change in BP. The PT is a well-characterized target of ANG II action (6, 31) and NHE3 transport activity has been reported to be increased by ANG II treatment in cultured cells (9). Previous in vivo studies of the effects of ANG II infusion on PT transporters (33, 38) are complicated by the fact that the ANG II infusion protocols usually raise BP (7), which is known to depress PT Na+ reabsorption (44), making it difficult to dissect out the effects of ANG II alone. This study aimed to investigate molecular mechanisms that could account for the acute actions of ANG II to increase Na+ and H2O reabsorption in the PT, independent of changes in BP. The dose of ANG II was empirically adjusted to minimize changes in BP and GFR (19). In addition, the ACEI captopril was coinfused to minimize intrarenal generation of ANG II (14, 47). With this protocol, we previously demonstrated captopril infusion alone significantly increased PT flow rate ∼15% above control and that subsequent addition of ANG II (20 ng·kg−1·min−1) to the captopril infusate immediately (<3 min) caused PT flow rate to decrease to 10% below control baseline levels (17). Thus, this nonpressor dose of ANG II chosen for systemic infusion can rapidly and significantly increase PT Na+ and H2O reabsorption. While there are many genomic effects of long-term treatment with ANG II (4), the 20-min ANG II infusion time is very unlikely to change the pool size of the proteins under investigation even if transcription and translation rates are changed.
The first set of confocal microscopy experiments showed a certain variation in the distribution of NHE3 after ANG II + captopril infusion. The perfusion pressure was suspected to cause this variation because it was not specifically determined. Therefore, we controlled the pressure in the second series of experiments. Confocal microscopy made from these specimens showed less or no variation in the distribution of NHE3. Perfusion fixation gives preferable results over surface fixation because it maintains open lumens with well-defined microvilli (22). However, if fixative is perfused at a rate high enough to raise BP and renal perfusion pressure, different ultrastructural changes can appear and NHE3 and NaPi2 may be retracted from the body of the microvilli, independent of prior treatment with captopril or ANG II + captopril. This response to elevated BP, which we previously characterized (41), is not unexpected and appears to occur before tissue fixation sets in, perhaps as a pressure wave hits the kidney before the fixative arrives. This observation established for us the critical importance of maintaining fixative perfusion pressure at a rate that does not elevate arterial pressure (recorded coincidently during perfusion) to obtain an accurate distribution of proteins in the pressure-sensitive PT. Perfusion pressure does not appear to be a significant issue with imaging the Na+-Cl− cotransporter in the distal convoluted tubule which is obviously responsive to captopril and ANG II in experiments in which the perfusion pressure was not controlled (27).
Using the parallel techniques of subcellular fractionation on density gradients and confocal microscopy, this study demonstrated that acute ANG II infusion provoked the rapid redistribution of both NHE3 and NaPi2 into the body of the apical microvilli. In addition, many PT proteins known to associate with these transporters including DPPIV, myosin VI, megalin, NHERF-1, ezrin, and clathrin, as well as H+-ATPase β subunit, APN and myosin IIa and villin redistributed to low-density microvilli-enriched membranes with ANG II infusion. We cannot say whether the redistribution of myosin IIa reflects an effect of ANG II on podocytes or epithelial cells. The slight, albeit significant redistribution of the actin bundling protein villin to lower-density fractions was not evident by confocal microscopy (Figs. 3 and 5). Perhaps the villin redistribution reflects an increased attachment of the actin core to the transporters in the microvillar membranes necessitated by ANG II-stimulated trafficking; alternatively, it may reflect a small generalized effect of ANG II on membrane density. Given those caveats, these results indicate that 1) the localization of this set of proteins in the body of the PT microvilli is augmented by ANG II infusion, 2) the rapid decrease in PT flow rate with ANG II that we previously reported (17) is, at least in part, due to the increase in sodium transporters in the body of the microvilli, and 3) that systemic inhibition of ANG II production with an ACEI, a common therapy for hypertension, would likely reduce the expression of these proteins in the microvilli and coincidently reduces PT Na+ and volume reabsorption (17, 40).
NHE3 and NaPi2 are coordinately regulated in vivo by many stimuli including acute hypertension (41), PTH (46), high-salt diet (43), ACE inhibition (40), and this study adds ANG II to the list. This coordinated regulation indicates that changes in PT sodium transport are a function of not just a decrease in transport via NHE3 but also via NaPi2 activity (and other yet to be identified regulated cotransporters). The coordination also suggests that common trafficking mechanism(s) may be moving the NHE3 and NaPi2 together. In fact, both are reported to interact with NHERF-1 and become tethered to the cytoskeleton via ezrin (8, 21). However, a number of observations indicate that common trafficking mechanisms are unlikely: 1) in the NHERF-1 knockout mouse, microvillar NaPi2 expression and activity are depressed while NHE3 appears normal (36), 2) NHE3 and NaPi2 are trafficked to different destinations by natriuretic stimuli: NHE3 to the base of the microvilli and NaPi2 to subapical endosomes, as evident in the captopril panels (Figs. 3 and 5), and 3) we recently discovered that renal cortical NHE3 and NaPi2, along with their associated proteins, are segregated into distinct membrane domains: NHE3, DPPIV, and myosin VI to lipid rafts and NaPi2, NHERF-1, and ezrin more enriched in nonrafts. However, it is still possible that there are common trafficking signals and mechanisms that anchor NHE3 and NaPi2 in their final destinations in the microvilli.
This nonpressor protocol for investigating the effects of ANG II in the PT is ideal for examining the signaling connection between ANG II and the increase in sodium reabsorption. Many questions remain to be addressed in this important area. Regarding the trafficking per se, it is not clear what event(s) initiates the traffic of transporters (NHE3 from the base of the microvilli, NaPi2 from subapical stores) into the microvilli. Potential mechanisms would include phosphorylation or dephosphorylation of one of the transporter-associated proteins such as NHERF-1 (34), or of the molecular motors such as myosin VI or myosin IIa (42), or of the transporters themselves (15). Regarding the change in sodium transport, it is not at all clear why reabsorption is lower when NHE3 is at the base of the microvilli and greater when NHE3 is in the body of the microvilli. Perhaps an answer lies with association with DPPIV, known to interact with and increase NHE3 transport, or with the vacuolar H+-ATPase, which is known to have a similar distribution with NHE3 at the base of the microvilli (5). Recent studies demonstrate that genetic removal of the AT1R specifically from the PT significantly lowers basal BP (12), increasing the significance of addressing these unanswered questions.
GRANTS
This work was supported by a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01-DK-34316. Histology and microscopy were performed with the help of the Cell and Tissue Imaging Core of the University of Southern California Research Center for Liver Diseases (NIDDK Grant R01-DK-34316). K. Pihakaski-Maunsbach was supported by the Danish National Research Foundation (Grundforskningsfonden).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Ardaillou R. Active fragments of angiotensin II: enzymatic pathways of synthesis and biological effects. Curr Opin Nephrol Hypertens 6: 28–34, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Arrondel C, Vodovar N, Knebelmann B, Grunfeld JP, Gubler MC, Antignac C, Heidet L. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol 13: 65–74, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Biemesderfer D, Nagy T, DeGray B, Aronson PS. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem 274: 17518–17524, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Braam B, Allen P, Benes E, Koomans HA, Navar LG, Hammond T. Human proximal tubular cell responses to angiotensin II analyzed using DNA microarray. Eur J Pharmacol 464: 87–94, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 82: 2114–2126, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension 15: 451–458, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Du Cheyron D, Chalumeau C, Defontaine N, Klein C, Kellermann O, Paillard M, Poggioli J. Angiotensin II stimulates NHE3 activity by exocytic insertion of the transporter: role of PI 3-kinase. Kidney Int 64: 939–949, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+-H+ exchange and Na+/HCO3− cotransport in the rabbit proximal tubule. Proc Natl Acad Sci USA 87: 7917–7920, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girardi AC, Knauf F, Demuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Gurley SB, Parsons KK, Haase VH, Snouwaert JN, Koller BH, Thu HL, Coffman TC. Attenuated salt sensitivity in mice with tissue-specific deletion of AT1A angiotensin receptors from renal proximal tubule. Hypertension 50: e99, 2007 [Google Scholar]
- 13.Harris PJ, Navar LG, Ploth DW. Evidence for angiotensin-stimulated proximal tubular fluid reabsorption in normotensive and hypertensive rats: effect of acute administration of captopril. Clin Sci (Lond) 66: 541–544, 1984 [DOI] [PubMed] [Google Scholar]
- 14.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 17.Leong PK, Devillez A, Sandberg MB, Yang LE, Yip DK, Klein JB, McDonough AA. Effects of ACE inhibition on proximal tubule sodium transport. Am J Physiol Renal Physiol 290: F854–F863, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Leong PK, Yang LE, Lin HW, Holstein-Rathlou NH, McDonough AA. Acute hypotension induced by aortic clamp vs. PTH provokes distinct proximal tubule Na+ transporter redistribution patterns. Am J Physiol Regul Integr Comp Physiol 287: R878–R885, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Leong PK, Zhang Y, Yang LE, Holstein-Rathlou NH, McDonough AA. Diuretic response to acute hypertension is blunted during angiotensin II clamp. Am J Physiol Regul Integr Comp Physiol 283: R837–R842, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol Renal Physiol 277: F447–F453, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Mahon MJ. Ezrin promotes functional expression and parathyroid hormone-mediated regulation of the sodium-phosphate cotransporter 2a in LLC-PK1 cells. Am J Physiol Renal Physiol 294: F667–F675, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Maunsbach AB. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res 15: 242–282, 1966 [DOI] [PubMed] [Google Scholar]
- 23.Mitchell KD, Braam B, Navar LG. Hypertensinogenic mechanisms mediated by renal actions of renin-angiotensin system. Hypertension 19: I18–I27, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Murer H, Hernando N, Forster L, Biber J. Molecular mechanisms in proximal tubular and small intestinal phosphate reabsorption (plenary lecture). Mol Membr Biol 18: 3–11, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Paunescu TG, Da Silva N, Marshansky V, McKee M, Breton S, Brown D. Expression of the 56-kDa B2 subunit isoform of the vacuolar H+-ATPase in proton-secreting cells of the kidney and epididymis. Am J Physiol Cell Physiol 287: C149–C162, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Sandberg MB, Maunsbach AB, McDonough AA. Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291: F503–F508, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl− cotransporter to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta 1496: 3–22, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol 296: F1067–F1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA 99: 11470–11475, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson SC, Deng A, Wead L, Richter K, Blantz RC, Vallon V. An unexpected role for angiotensin II in the link between dietary salt and proximal reabsorption. J Clin Invest 116: 1110–1116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traebert M, Roth J, Biber J, Murer H, Kaissling B. Internalization of proximal tubular type II Na-Pi cotransporter by PTH: immunogold electron microscopy. Am J Physiol Renal Physiol 278: F148–F154, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Turban S, Beutler KT, Morris RG, Masilamani S, Fenton RA, Knepper MA, Packer RK. Long-term regulation of proximal tubule acid-base transporter abundance by angiotensin II. Kidney Int 70: 660–668, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Voltz JW, Brush M, Sikes S, Steplock D, Weinman EJ, Shenolikar S. Phosphorylation of PDZ1 domain attenuates NHERF-1 binding to cellular targets. J Biol Chem 282: 33879–33887, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Weinman EJ, Boddeti A, Cunningham R, Akom M, Wang F, Wang Y, Liu J, Steplock D, Shenolikar S, Wade JB. NHERF-1 is required for renal adaptation to a low-phosphate diet. Am J Physiol Renal Physiol 285: F1225–F1232, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Weinman EJ, Cunningham R, Wade JB, Shenolikar S. The role of NHERF-1 in the regulation of renal proximal tubule sodium-hydrogen exchanger 3 and sodium-dependent phosphate cotransporter 2a. J Physiol 567: 27–32, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens 12: 205S–213S, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Xu L, Dixit MP, Chen R, Dixit NM, Collins JF, Ghishan FK. Effects of angiotensin II on NaPi-IIa cotransporter expression and activity in rat renal cortex. Biochim Biophys Acta 1667: 114–121, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Leong PK, Chen JO, Patel N, Hamm-Alvarez SF, McDonough AA. Acute hypertension provokes internalization of proximal tubule NHE3 without inhibition of transport activity. Am J Physiol Renal Physiol 282: F730–F740, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Yang LE, Leong PK, McDonough AA. Reducing blood pressure in SHR with enalapril provokes redistribution of NHE3, NaPi2, and NCC and decreases NaPi2 and ACE abundance. Am J Physiol Renal Physiol 293: F1197–F1208, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol 287: F896–F906, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol 16: 2890–2896, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Yang LE, Sandberg MB, Can AD, Pihakaski-Maunsbach K, McDonough AA. Effects of dietary salt on renal Na+ transporters' subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Magyar CE, Norian JM, Holstein-Rathlou NH, Mircheff AK, McDonough AA. Reversible effects of acute hypertension on proximal tubule sodium transporters. Am J Physiol Cell Physiol 274: C1090–C1100, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Mircheff AK, Hensley CB, Magyar CE, Warnock DG, Chambrey R, Yip KP, Marsh DJ, Holstein-Rathlou NH, McDonough AA. Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1004–F1014, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol Renal Physiol 276: F711–F719, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Boron WF. Role of endogenously secreted angiotensin II in the CO2-induced stimulation of HCO3 reabsorption by renal proximal tubules. Am J Physiol Renal Physiol 294: F245–F252, 2008 [DOI] [PubMed] [Google Scholar]