Abstract
Glomerular endothelial cells (GEC) are strategically situated within the capillary loop and adjacent to the glomerular mesangium. GEC serve as targets of metabolic, biochemical, and hemodynamic signals that regulate the glomerular microcirculation. Unequivocally, hyperglycemia, hypertension, and the local renin-angiotensin system partake in the initiation and progression of diabetic nephropathy (DN). Whether free fatty acids (FFA) and reactive oxygen species (ROS) that have been associated with the endothelial dysfunction of diabetic macrovascular disease also contribute to DN is not known. Since endothelial cells from different organs and from different species may display different phenotypes, we employed human GEC to investigate the effect of high glucose (22.5 mmol/l), FFA (800 μmol/l), and angiotensin II (ANG II; 10−7 mol/l) on the genesis of ROS and their effects on endothelial nitric oxide synthase (eNOS), cyclooxygenase-2 (COX-2), and the synthesis of prostaglandins (PGs). We demonstrated that high glucose but not high FFA increased the expression of a dysfunctional eNOS as well as increased ROS from NADPH oxidase (100%) and likely from uncoupled eNOS. ANG II also induced ROS from NADPH oxidase. High glucose and ANG II upregulated (100%) COX-2 via ROS and significantly increased the synthesis of prostacyclin (PGI2) by 300%. In contrast, FFA did not upregulate COX-2 but increased PGI2 (500%). These novel studies are the first in human GEC that characterize the differential role of FFA, hyperglycemia, and ANG II on the genesis of ROS, COX-2, and PGs and their interplay in the early stages of hyperglcyemia.
Keywords: diabetes, prostaglandins, reactive oxygen species, nitric oxide
diabetic nephropathy constitutes the leading cause of end-stage renal disease in the United States and Europe (8, 41). Studies of the biology of the human glomerular endothelium in diabetes are lacking. Most studies have relied on data from large vessels such as the aorta and from retinal endothelial cells utilized as surrogates of human glomerular endothelial cells (GEC) (6). This approach ignored that GEC may have responses to pathobiological factors that differ significantly compared with cells derived from other vessels. The fenestrated glomerular capillary endothelium is strategically situated at the interface between the blood compartment and the glomerular mesangium, without interposition of the glomerular basement membrane (28). In this location, GEC serve as direct sensors and targets of metabolic, biochemical, and hemodynamic signals that regulate the glomerular microcirculation (31). Due to abnormal autoregulation of the glomerular microcirculation, increased glomerular filtration rate (hyperfiltration) and increased glomerular intracapillary pressure (glomerular hypertension) characterize the early stages of diabetic nephropathy (32). The interplay among the hemodynamic and nonhemodynamic actions of angiotensin II (ANG II), reactive oxygen species (ROS), nitric oxide (NO), and prostaglandins (PGs) modulates the intrarenal microcirculation under physiologic and pathologic conditions including diabetes mellitus (16, 31).
Clinically and experimentally, the association between hyperglycemia and microvascular disease affecting the retina and the kidney is stronger than with macrovascular disease affecting large vessels (10, 34). Studies performed during the last decade suggested a link between hyperglycemia, increased levels of free fatty acids (FFA), insulin resistance, activation of the renin-angiotensin system, and oxidative stress (ROS) in the pathogenesis of diabetic vascular disease (6, 11, 33). To address these questions, we investigated the mechanisms whereby high glucose, FFA, ANG II, and ROS induce human GEC injury. The present study demonstrates that high glucose but not high FFA increased the expression of a dysfunctional endothelial nitric oxide synthase (eNOS) as well as increased superoxide production from NADPH oxidase and likely from uncoupled eNOS. ANG II also induced ROS from NADPH oxidase. High glucose and ANG II upregulated human GEC cyclooxygenase-2 (COX-2) via ROS and significantly increased the synthesis of prostacyclin (PGI2). On the other hand, FFA did not upregulate COX-2 but increased synthesis of PGI2 likely by stimulating constitutive COX-1. We propose that understanding the pathophysiological mechanisms of human GEC dysfunction depicted in our studies would provide insights into therapeutic strategies for preventing and/or arresting diabetic nephropathy.
RESEARCH DESIGN AND METHODS
Cell culture conditions.
Human GEC were purchased from Cell Systems (Kirkland, WA) and grown in CSC-Complete Media (Cell Systems) supplemented with 10% fetal calf serum (FCS; Cell Systems). All experiments were performed in cells passage 2 to 5, grown in six-well dishes or 25-cm2 flasks, and the experiments were performed in cells that were 80% confluent. For experiments performed in six-well dishes, the cells were seeded at a density of 1 × 105 cells/well and for experiments performed in flasks they were seeded at a density of 2.5 × 105 cells/flask. The cell culture media were changed daily until cells reached 80% confluency usually 48 h after seeding. Before all experiments, cells were fasted for 24 h in maintenance media (Cell Systems). The cells were incubated in media containing either 5 mmol/l glucose, 22.5 mmol/l glucose, 17.5 mmol/l + 5 mmol/l mannitol + glucose, or 5 mmol/l + 1 mmol/l glucose + fatty acid-free albumin (Sigma), and 800 μmol/l oleic acid (Sigma) for the specified time points as described in results. In other experiments, the GEC were stimulated with ANG II (10−7 M) for the specified time points described in results.
Western blot analysis.
Western blot analysis was performed as previously described (19, 20). Briefly, cell homogenates were washed once with phosphate-buffered saline and resuspended in 300 μl of homogenization buffer (50 mmol/l Tris·HCl, pH 7.6, 100 mmol/l NaCl, 2 mmol/l EDTA, 2 mmol/l EGTA, 1 mmol/l DTT, 1 mmol/l PMSF, 1% Triton X-100) and incubated on ice for 30 min. Thereafter, lysates were centrifuged for 30 min at 10,000 g at 4°C. Supernatants were collected and the protein content was determined by Bio-Rad. Thirty micrograms of protein were separated by SDS-PAGE (6% acrylamide gel) and transferred to a nitrocellulose membrane. Blots were incubated overnight with rabbit anti-murine polyclonal antibody to COX-2 (Cayman), eNOS (Cayman), or polyclonal antibody to actin (Santa Cruz Biotechnology). After being washed, the blots were incubated with goat anti-rabbit antibody (Santa Cruz Biotechnology) for 1 h and the signal was detected by luminol chemiluminescence.
ROS measurements.
Superoxide anion in intact cells was measured by chemiluminescence of lucigenin as previously described (18). Briefly, after incubation, cells were washed, trypsinized, resuspended in 1 ml Krebs buffer, and kept on ice until use. Cells were added to a cuvette containing 1 ml of phosphate buffer (pH 7.4) and lucigenin (5 μmol/l). Photon emission was measured every minute for 10 min in a Berthold FB12 Luminometer. A buffer blank was subtracted from each reading, and the production of O2− was adjusted for protein content as measured by Bio-Rad and expressed as cpm/mg protein.
In separate experiments, we measured the NADPH-stimulated production of superoxide anion in cell homogenates as we previously described (17). Human GEC exposed to either normal (5 mmol/l) or high glucose (22.5 mmol/l) for 48 h were washed three times with Hanks' balanced salt solution, trypsinized, resuspended in 300-μl homogenization buffer (50 mmol/l phosphate buffer, 0.01 mmol/l EDTA, 2 μmol/l leupeptin, 2 μmol/l pepstatin A, 1 mmol/l phenylmethylsulfonyl fluoride or phenylmethanesulfonyl fluoride, pH 7.4), and homogenized by sonication (15-s pulses × 3). NADPH-stimulated superoxide production was measured by using a chemiluminescence assay in a 50 mmol/l phosphate buffer, pH 7.4, containing 1.0 mmol/l EGTA, 5 μmol/l lucigenin, and 100 μmol/l of NADPH. The assay was initiated by addition of 20 μl homogenate and the production of superoxide anion was measured by chemiluminescence of lucigenin (17). Protein content in homogenates was measured by the Bio-Rad method and the production of superoxide was adjusted for protein content and expressed as cpm/mg protein.
PGs measurements.
PGE2, thromboxane B2, and the stable PGI2 metabolite 6-keto-PGF1α were measured by enzymatic immunoassay (EIA, Cayman) following the manufacturer's instructions and adjusted for cell protein content as assessed by Bio-Rad.
COX-2 mRNA expression.
COX-2 mRNA expression in human GEC was determined by RT-PCR as previously described (20). Total RNA was isolated by lysing the cells in TRIzol reagent (Life Technologies) and by precipitation in isopropyl alcohol. A 5-μg aliquot of total RNA was used for cDNA synthesis with the Superscript preamplification system (Life Technologies). An aliquot of cDNA was amplified using Taq polymerase in the presence of sense and antisense primers for human COX-2 and GAPDH.
NO measurements.
NO was measured with the NO-sensitive dye DAF-FM (22). Human GEC were exposed to either normal glucose (5 mmol/l) or high glucose (22.5 mmol/l) for 48 h, washed with fresh maintenance media (CSC), and treated with DAF-FM (5 μmol/l; Invitrogen) for 30 min. After treatment with DAF-FM for 30 min, the cells were washed and the cellular fluorescence was measured utilizing a fluorescent-inverted microscope and analyzed utilizing the NIH Image software.
RESULTS
High glucose increases the production of ROS in human GEC.
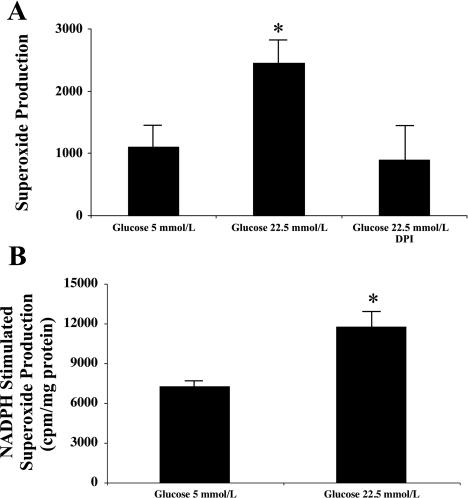
To determine the effects of high glucose on GEC production of superoxide anion, GEC were incubated in media containing normal glucose (5 mmol/l) or high glucose (22.5 mmol/l) for 48 h with and without the NADPH oxidase inhibitor DPI (1 μmol/l). As shown in Fig. 1, high glucose resulted in a significant increase in GEC production of ROS that was prevented by DPI demonstrating that high glucose increases ROS generation in human GEC via NADPH oxidase activation. In addition, we measured the NADPH-stimulated production of superoxide in homogenates from human GEC incubated for 48 h under normal- and high-glucose conditions. Similar to our measurements in intact cells, incubation under high-glucose conditions resulted in a significant increase in NADPH-stimulated production of superoxide anion (Fig. 1B).
Fig. 1.
High glucose increases the production of superoxide anion in human glomerular endothelial cells. A: human glomerular endothelial cells grown in 25-cm2 flasks and 80% confluent were incubated in normal glucose (5 mmol/l) or high glucose (22.5 mmol/l) for 48 h with and without the NADPH oxidase inhibitor DPI (1 μmol/l). Exposure to high glucose increased the production of O2− as assessed by chemiluminescence of lucigenin that was prevented by NADPH oxidase inhibition. O2− measurements were normalized for total cellular protein content (n = 6, *P < 0.05 vs. control and high glucose + DPI). B: human glomerular endothelial cells grown in 25-cm2 flasks and 80% confluent were incubated in normal glucose (5 mmol/l) or high glucose (22.5 mmol/l) for 48 h. NADPH-stimulated production of O2− was measured in cell homogenates. O2− measurements were normalized for total cellular protein content (n = 3, *P < 0.05 vs. 5 mmol/l glucose).
High glucose increases COX-2 expression in human GEC.
To determine the effects of high glucose on COX-2 expression and activity, human GEC were incubated in media containing either normal glucose (5 mmol/l) or high glucose (22.5 mmol/l) for 48 h. As shown in Fig. 2, A and B, high glucose increased COX-2 protein expression in GEC as assessed by Western blot. These changes in COX-2 protein expression were accompanied by concomitant increases in PGE2 and principally PGI2 (Fig. 2C). To control for changes in osmolality induced by high glucose, we performed additional experiments utilizing media-containing mannitol. As shown in Fig. 3, A and B, media-containing mannitol did not significantly increase COX-2 expression or PGI2 production: 5 mmol/l glucose: 132.7 ± 25 pg/mg; 22.5 mmol/l glucose: 2,239 ± 1,105* pg/mg; 5 mmol/l glucose + 17.5 mmol/l mannitol: 382.9 ± 213 pg/mg (*P < 0.05 vs. 5 mmol/l glucose and 5 mmol/l glucose + mannitol, n = 3), demonstrating that the effects of high-glucose concentration on COX-2 expression are not secondary to changes in osmolality induced by glucose.
Fig. 2.
High glucose increases the expression and activity of cyclooxygenase-2 (COX-2) in human glomerular endothelial cells. Human glomerular endothelial cells grown in 6-well dishes and 80% confluent were incubated in normal glucose (5 mmol/l) or high glucose (22.5 mmol/l) media for 48 h. Western blot analysis was performed in 30 μg of cell homogenate. A: representative Western blots for COX-2 and actin, which was used to control for loading. B: densitometry data analysis performed after reprobing the blot with an α-actin antibody (n = 6, *P < 0.05 vs. 5 mmol/l glucose). C: glucose-stimulated COX-2 expression was accompanied by concomitant increases in PGI2 and PGE2 but not thromboxane-A2 in human glomerular endothelial cells. Prostaglandin measurements were normalized for total cellular protein content (n = 6 to 9, *P < 0.05 vs. control).
Fig. 3.
Mannitol does not increase COX-2 expression in human glomerular endothelial cells. Human glomerular endothelial cells were incubated in normal glucose (5 mmol/l), high glucose (22.5 mmol/l), or mannitol (5 mmol/l glucose + 17.5 mmol/l mannitol) for 48 h. Western blot analysis was performed utilizing 30 μg of cell homogenate. A: representative Western blots for COX-2 and actin, which was used to control for loading. Lane 1: 5 mmol/l glucose; lane 2: 22.5 mmol/l glucose; lane 3: 5 mmol/l glucose + 17.5 mmol/l mannitol. B: densitometry data analysis performed after reprobing the blot with an α-actin antibody (n = 3, *P < 0.05 vs. 5 mmol/l glucose). C: high glucose media increased COX-2 mRNA expression without modifying the expression of the housekeeping gene GAPDH (n = 3).
To determine whether transcriptional mechanisms were involved, GEC were incubated in media containing either 5 mmol/l glucose or 22.5 mmol/l glucose for 48 h and COX-2 mRNA expression was assessed by RT-PCR. As shown in Fig. 3C, high-glucose incubation resulted in significant increases in COX-2 mRNA expression demonstrating that high glucose increases COX-2 expression via transcriptional mechanisms.
ROS mediate COX-2 induction in response to glucose.
To determine the role of ROS as mediators of COX-2 induction in response to glucose, human GEC were incubated in media containing a 22.5 mmol/l concentration of glucose with and without the NADPH oxidase inhibitor DPI (1 μmol/l). At the end of the incubation period, COX-2 expression was measured by Western blot. As shown in Fig. 4, high glucose increased COX-2 expression in GEC that was reduced by NADPH oxidase inhibition, suggesting that ROS play a role in COX-2 expression in human GEC in response to glucose.
Fig. 4.
NADPH oxidase inhibition prevents the effects of glucose on COX-2 expression. Human glomerular endothelial cells grown in 6-well dishes and 80% confluent were incubated in normal glucose (5 mmol/l), high glucose (22.5 mmol/l), and high glucose (22.5 mmol/l) + DPI (1 μmol/l) for 48 h. Western blot analysis was performed in 30 μg of cell homogenate. NADPH oxidase inhibition with DPI significantly prevented the increases in COX-2 expression stimulated by glucose (n = 3, *P < 0.05 vs. 5 mmol/l glucose, #P < 0.05 vs. 22.5 mmol/l glucose).
Oleic acid does not increase superoxide anion production in GEC.
To determine the effect of oleic acid on superoxide anion production, GEC were incubated on normal-glucose media (5 mmol/l) for 48 h and treated with oleic acid (800 μM) and 1 μM fatty acid-free albumin for 6 h. As shown in Fig. 5A, oleic acid did not increase the production of O2− under these conditions. We used a concentration of oleic acid and albumin, which would result in a FFA fraction (the fraction unbound to albumin) similar to that found in diabetics.
Fig. 5.
Oleic acid does not increase superoxide production or increases COX-2 expression in human glomerular endothelial cells. Human glomerular endothelial cells grown in 6-well dishes and 80% confluent were incubated in normal glucose (5 mmol/l), high glucose (22.5 mmol/l), or normal glucose (5 mmol/l) + oleic acid (800 μmol/l). Western blot analysis was performed in 30 μg of cell homogenate. A: high glucose but not oleic acid increased the production of superoxide anion in human glomerular endothelial cells. B: representative Western blots for COX-2 and actin, which was used to control for loading. Lane 1: 5 mmol/l glucose; lane 2: 22.5 mmol/l glucose; lane 3: 5 mmol/l glucose + 800 μmol/l oleic acid. C: densitometry data analysis performed after reprobing the blot with an α-actin antibody (n = 3, *P < 0.05 vs. 5 mmol/l glucose).
To determine the effects of oleic acid on COX-2 expression, human GEC were exposed to oleic acid for 6 h (Fig. 5, B and C). Although oleic acid did not induce the expression of COX-2, it produced a fivefold increase in the production of PGI2: 5 mmol/l glucose: 15.1 ± 1.5 ng/mg; 22.5 mmol/l glucose: 33 ± 2.7* ng/mg; 5 mmol/l glucose + oleic acid: 80.7 ± 8.3# ng/mg (n = 3, *P < 0.05 vs. 5 mmol/l glucose; #P < 0.05 vs. 5 mmol/l glucose and 22.5 mmol/l glucose).
High glucose and eNOS.
Studies by others (12, 33, 38) showed that high glucose is associated with reductions in eNOS bioactivity. We therefore performed a series of experiments to determine the effects of high-glucose concentrations on eNOS dimerization, a well-established indicator of eNOS uncoupling and reduced enzymatic bioactivity (47). Human GEC were incubated under high-glucose conditions for 48 h and eNOS expression was assessed by Western blot under reducing conditions and without boiling of the samples before loading. As shown in Fig. 6, A and B, incubation of human GEC on high-glucose conditions resulted in significant increases in total eNOS expression accompanied by a concomitant increase in eNOS monomers expression, suggesting that high glucose induces eNOS uncoupling in human GEC. To determine whether these changes in eNOS protein expression were accompanied by changes in NO production, we measured the production of NO under low- and high-glucose conditions utilizing the NO-sensitive fluorescent dye (DAF-FM). As shown in Fig. 6, the incubation of human GEC to high-glucose conditions resulted in significant reductions in the basal production of NO.
Fig. 6.
High glucose increases endothelial nitric oxide synthase (eNOS) expression but reduces NO production in human glomerular endothelial cells. Human glomerular endothelial cells grown in 6-well dishes and 80% confluent were incubated in normal-glucose media (5 mmol/l) and high-glucose media (22.5 mmol/l) for 48 h. Western blot analysis was performed utilizing 30 μg of cell homogenate. A: representative Western blots for eNOS dimers and monomers from human glomerular endothelial cells exposed to normal- and high-glucose media. B: densitometry data analysis for eNOS dimers and monomers (n = 3, *P < 0.05 vs. 5 mmol/l glucose; n = 3, *P < 0.05 vs. control). C: incubation in a high-glucose media results in a significant reduction in the endothelial production of NO (n = 6, *P < 0.05 vs. 5 mmol/l glucose).
ANG II increases O2− production in GEC.
To determine the effects of ANG II on O2− production in endothelial cells from the glomerular microcirculation, human GEC were incubated with ANG II (10−7 mol/l) for 30 and 60 min. As shown in Fig. 7A, ANG II increased O2− concentration as assessed by chemiluminescence of lucigenin in human GEC after 30 min but not after 60 min (not shown). These increases in O2− were prevented by NADPH oxidase inhibition with DPI (Fig. 7).
Fig. 7.
ANG II increases the production of superoxide anion and increases COX-2 expression in human glomerular endothelial cells. Human glomerular endothelial cells grown in 25-cm2 or 6-well dishes and 80% confluent were stimulated with ANG II (10−7 mol/l) with and without the NADPH oxidase inhibitor DPI (1 μmol/l) for 30 min (superoxide) or 4 h (COX-2). A: exposure to ANG II increased the production of O2− as assessed by chemiluminescence of lucigenin that was prevented by NADPH oxidase inhibition. O2− measurements were normalized for total cellular protein content (n = 6, *P < 0.05 vs. control and high glucose + DPI). B: representative Western blots for COX-2 and actin, which was used to control for loading. Western blot analysis was performed in 30 μg of cell homogenate. C: densitometry data analysis performed after reprobing the blot with an α-actin antibody (n = 3, *P < 0.05 vs. 5 mmol/l glucose).
ROS mediate COX-2 expression in response to ANG II in human GEC.
To determine the role of ROS as mediators of COX-2 induction in response to ANG II in endothelial cells, human GEC were incubated with ANG II (10−7 mol/l) for 6 h with and without the NADPH oxidase inhibitor DPI (1 μmol/l). This time point was chosen based on pilot experiments designed to assess the peak stimulation of COX-2 in response to ANG II. As shown in Fig. 7, B and C, ANG II increased COX-2 expression in human GEC accompanied by a concomitant increase in the production of PGI2: control: 101 ± 25 ng/ml vs. ANG II: 295.7 ± 77* ng/ml (n = 3, *P < 0.05 vs. control). NADPH oxidase inhibition with DPI prevented the ANG II-stimulated increase in COX-2 expression (Fig. 7) and PGI2 production: ANG II + DPI: 88.1 ± 22 ng/ml (n = 3, *P < 0.05 vs. ANG II).
ANG II and eNOS.
To determine the effects of ANG II on eNOS dimerization, human GEC were stimulated with ANG II for 30 min and eNOS expression was assessed by Western blot under reducing conditions and without boiling of the samples before loading. As shown in Fig. 8, A and B, and in contrast with our findings in human GEC exposed to high glucose, stimulation with ANG II did not modify eNOS dimerization in human GEC. Similar findings were observed with up to 24-h incubation with ANG II (not shown).
Fig. 8.
ANG II does not modify eNOS expression in human glomerular endothelial cells. Human glomerular endothelial cells grown in 6-well dishes and 80% confluent were incubated in normal-glucose media (5 mmol/l), with and without ANG II (10−7 mol/l) for 30 min. Western blot analysis was performed utilizing 30 μg of cell homogenate. A: representative Western blots for eNOS dimers and monomers from human glomerular endothelial cells exposed to vehicle or ANG II (10−7 mol/l). B: densitometry data analysis for eNOS dimers and monomers (n = 3, P = not significant vs. control).
DISCUSSION
Hyperglycemia, hypertension, dyslipidemia, and increased activation of the local renin-angiotensin system are major factors in the development and progression of diabetic microvascular (1, 2) and macrovascular disease (14, 30, 33, 46). Clinical and experimental studies unequivocally demonstrated that diabetic nephropathy is fostered by both hemodynamic (hypertension) (13, 32) and metabolic factors (hyperglycemia and dyslipidemia) (2, 4, 34).
Under normal physiologic conditions, renal autoregulatory mechanisms protect the glomeruli and prevent increases in systemic blood pressure from being fully transmitted to the glomerular capillaries (3, 26). Hyperglycemia impairs normal autoregulation because it reduces preglomerular resistances (42). It is likely that in genetically predisposed individuals these effects of hyperglycemia are accentuated and may explain the increased glomerular intracapillary pressure and glomerular filtration rate (hyperfiltration) that is already present during the early stages of diabetic nephropathy (9, 29).
Endothelial dysfunction is one of the hallmarks of diabetic vascular disease (33). The glomerular endothelium is strategically situated at the interface between the blood compartment and the glomerular mesangium, without interposition of the glomerular basement membrane (27). In this location, the GEC serve as targets of metabolic, biochemical, and hemodynamic signals that regulate the glomerular microcirculation (28).
Recent studies by Du et al. (6) provided evidence that increased production of ROS originating in part from mitochondrial dysfunction may be a critical pathogenetic mechanism of both micro (retinal) and macro (aortic) vascular complications of diabetes. This group showed that high FFA as well as high glucose can increase endothelial ROS production. The downstream consequences of increased ROS included oxidative damage of eNOS synthase and PGI2 synthase, resulting in reductions in the production of two critical autacoids endowed with vasodilatory, antithrombotic, and anti-inflammatory properties. Of note was the finding that endothelial susceptibility to hyperglycemia and FFA induced oxidative stress differed between retinal and aortic endothelium: hyperglycemia was more injurious to retinal vessels, whereas FFA induced more injury to aortic endothelium (6). Furthermore, recent clinical trials demonstrated that intensive glycemic control in type II diabetics does not prevent major cardiovascular events (ACCORD and ADVANCE) (10, 34) but reduces new or worsening nephropathy and new onset microalbuminuria (ADVANCE) (34).
To the best of our knowledge, the effects of hyperglycemia and of FFA on human GEC have not been investigated. Moreover, given their special location and function, there is no reason to a priori assume that human GEC will have identical responses to high glucose, FFA, or ANG II compared with retinal or aortic endothelial cells. To directly address these questions, we investigated the role of high glucose, high FFA, and of ANG II in the genesis of ROS in human GEC as well as the effect of ROS on eNOS and COX-2, two enzymes that play major roles in glomerular physiology and pathology (5, 20, 36).
We found that exposure of human GEC to high glucose resulted in a onefold increase in O2− production accompanied by a significant increase in expression of a dysfunctional eNOS, since it was predominantly uncoupled as evidenced by a threefold increase in the monomeric eNOS isoform. Moreover, we found that the exposure of human GEC to high glucose results in significant reductions in the production of NO. These results suggest that hyperglycemia unfolds a vicious circle in which ROS induce oxidative uncoupling of the eNOS dimmers, a process that is known to lead to further production of O2− by the eNOS monomers (47). On the other hand, FFA did not increase ROS and had no effect on eNOS in human GEC. ANG II increased ROS but under the conditions of our experiments, it did not result in eNOS uncoupling likely due to the short duration of the burst in ROS production (25). An important difference between human GEC and aortic and retinal endothelial cells (6) is that increased ROS had no deleterious effect on the synthesis of PGI2 which in fact increased in response to both high glucose and ANG II. FFA also increased the production of PGI2 that most likely originated from constitutive COX 1 since in contrast with glucose and ANG II, FFA did not upregulate the expression of COX-2 in human GEC.
We also demonstrated that increased expression of human GEC COX-2 in response to either ANG II or glucose was mediated by ROS since it could be prevented with DPI. Of note, NADPH oxidase inhibition was much more effective in preventing COX-2 expression in response to ANG II than in response to high glucose, suggesting that under high-glucose conditions other mechanisms also participate in COX-2 expression. We speculate that other pathways activated by glucose may also mediate COX-2 expression such as JNK and P38 MAP kinase (44). A limitation of our studies is that they were performed in vitro. As others also showed (6), COX-2 expression is evident under normal-glucose conditions in cells grown in vitro. This increased basal expression of COX-2 is likely the result of the activation of pathways leading to COX-2 induction when cells are grown in vitro. However, our studies also demonstrate further increase in COX-2 expression after high-glucose exposure and ANG II. Moreover, the glomerular and cortical expression of COX-2 is increased in animal models of diabetes, suggesting that COX-2 plays an important role in the pathogenesis of diabetic nephropathy (23, 24, 45).
We interpret the upregulation of eNOS and COX-2 as critical counterregulatory mechanisms endowed with both friend and foe actions. In the case of NO, the superoxide formed within diabetic tissue and from uncoupled eNOS may overwhelm the bioactivity of NO. Under these conditions, the increased production of PGI2 would be compensatory particularly in antagonizing the actions of ANG II. Clinically, the exquisite sensitivity of the diabetic kidney glomerular filtration rate, which falls in response to COX inhibition, would support this contention (21). At the same time, however, PGI2 may reduce vascular tone of preglomerular resistances thereby impairing autoregulation of intrarenal flows and pressures and increasing the vulnerability of the diabetic kidney to hypertension.
Diabetic nephropathy secondary to type 2 diabetes is the most common cause of end-stage renal disease and it occurs in ∼20 to 40% of diabetics (8). A relatively long period of insulin resistance characterized by normoglycemia but increased levels of FFA precedes the development of frank hyperglycemia (37, 39). During this period of the natural history of diabetic nephropathy, however, vascular disease affecting large vessels progressively ensues driven by the abnormal metabolic environment, which includes high circulating FFA (7). This is supported by the clinical observation that NIDDM patients often present to the consult with early nephropathy but advanced macrovascular disease affecting the aorta and coronary vessels.
The cellular and molecular mechanisms by which hemodynamic and metabolic insults translate to structural and functional abnormalities leading to the initiation and progression of diabetic nephropathy have been and continue to be increasingly delineated. This includes the role of advanced glycosylation end products (40, 43), activated protein C (15), as well as the contribution of the activation of critical intracellular signaling pathways and transcription factors, that promote the production/release of cytokines, chemokines, and growth factors, which amplify diabetic renal damage of the glomerular endothelium, podocytes, and glomerular mesangium (35).
The studies reported here are the first in human GEC that characterize the differential role of FFA, hyperglycemia, and ANG II on the genesis of ROS, COX-2, and PGs and their potential interplay under high-glucose conditions.
GRANTS
These studies were supported by a Veterans Affairs Merit Review Award (E. A. Jaimes), a National Institutes of Health Research Grant (DK-063972 to E. A. Jaimes), and by research funds from the South Florida Veterans Affairs Research Foundation (L. Raij).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We are thankful for the excellent technical support of J. Nigro and the secretarial for support of K. Pilkerton.
REFERENCES
- 1.Anonymous Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney Int 47: 1703–1720, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Anonymous Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 317: 703–713, 1998 [PMC free article] [PubMed] [Google Scholar]
- 3.Aukland K, Oien AH. Renal autoregulation: models combining tubuloglomerular feedback and myogenic response. Am J Physiol Renal Fluid Electrolyte Physiol 252: F768–F783, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Bonadonna RC, Cucinotta D, Fedele D, Riccardi G, Tiengo A. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care 29: 2701–2707, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Breyer MD, Harris RC. Cyclooxygenase 2 and the kidney. Curr Opin Nephrol Hypertens 10: 89–98, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest 116: 1071–1080, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 365: 1415–1428, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Gall MA, Nielsen FS, Smidt UM, Parving HH. The course of kidney function in type 2 (noninsulin-dependent) diabetic patients with diabetic nephropathy. Diabetologia 36: 1071–1078, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14–E22, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Hoshiyama M, Li B, Yao J, Harada T, Morioka T, Oite T. Effect of high glucose on nitric oxide production and endothelial nitric oxide synthase protein expression in human glomerular endothelial cells. Nephron Exp Nephrol 95: e62–e68, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hostetter TH, Rennke HG, Brenner BM. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med 72: 375–380, 1982 [DOI] [PubMed] [Google Scholar]
- 14.Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, Cowan LD, Gray RS, Welty TK, Go OT, Howard WJ. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the Strong Heart Study. Arterioscler Thromb Vasc Biol 20: 830–835, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Johnson CS, Carretero OA. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest 87: 1656–1663, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol 24: 1031–1036, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Jaimes EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int 54: 775–784, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Jaimes EA, Nath KA, Raij L. Hydrogen peroxide downregulates IL-1-driven mesangial iNOS activity: implications for glomerulonephritis. Am J Physiol Renal Physiol 272: F721–F728, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Jaimes EA, Tian RX, Pearse D, Raij L. Upregulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int 68: 2143–2153, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Khan KN, Stanfield KM, Harris RK, Baron DA. Expression of cyclooxygenase-2 in the macula densa of human kidney in hypertension, congestive heart failure, and diabetic nephropathy. Ren Fail 23: 321–330, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl 38: 3209–3212, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Komers R, Lindsley JN, Oyama TT, Anderson S. Cyclooxygenase-2 inhibition attenuates the progression of nephropathy in uninephrectomized diabetic rats. Clin Exp Pharmacol Physiol 34: 36–41, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest 107: 889–898, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem 278: 12094–12100, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michael AF, Keane WF, Raij L, Vernier RL, Mauer SM. The glomerular mesangium. Kidney Int 17: 141–154, 1980 [DOI] [PubMed] [Google Scholar]
- 29.Mogensen CE, Andersen MJ. Increased kidney size and glomerular filtration rate in early juvenile diabetes. Diabetes 22: 706–712, 1973 [DOI] [PubMed] [Google Scholar]
- 30.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O'Leary DH, Genuth S. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 348: 2294–2303, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996 [DOI] [PubMed] [Google Scholar]
- 32.O'Bryan GT, Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol 17: 93–100, 1997 [PubMed] [Google Scholar]
- 33.O'Driscoll G, Green D, Rankin J, Stanton K, Taylor R. Improvement in endothelial function by angiotensin converting enzyme inhibition in insulin-dependent diabetes mellitus. J Clin Invest 100: 678–684, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC., 3rd From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57: 1439–1445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raij L, Baylis C. Glomerular actions of nitric oxide. Kidney Int 48: 20–32, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963 [DOI] [PubMed] [Google Scholar]
- 38.Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol 288: F1144–F1152, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Shafrir E, Raz I. Diabetes: mellitus or lipidus? Diabetologia 46: 433–440, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes 40: 1328–1334, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Stewart JH, McCredie MR, Williams SM, Jager KJ, Trpeski L, McDonald SP. Trends in incidence of treated end-stage renal disease, overall and by primary renal disease, in persons aged 20–64 years in Europe, Canada and the Asia-Pacific region, 1998–2002. Nephrology (Carlton) 12: 520–527, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early [corrected] type 1 diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol 14: 530–537, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Vlassara H, Striker LJ, Teichberg S, Fuh H, Li YM, Steffes M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci USA 91: 11704–11708, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamawaki H, Saito K, Okada M, Hara Y. Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. Am J Physiol Cell Physiol 295: C1510–C1517, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Yar AS, Menevse S, Alp E, Helvacioglu F, Take G. The effects of resveratrol on cyclooxygenase-1 and cyclooxygenase-2 mRNA and protein levels in diabetic rat kidneys. Mol Biol Rep In press [DOI] [PubMed] [Google Scholar]
- 46.Zheng L, Hodis HN, Buchanan TA, Li Y, Mack WJ. Effect of antihypertensive therapy on progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Am J Cardiol 99: 956–960, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817–826, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]