Abstract
Little is known about the effects of nitric oxide (NO) and the cyclic GMP (cGMP)/protein kinase G (PKG) system on Ca2+ signaling in vascular smooth muscle cells (VSMC) of resistance vessels in general and afferent arterioles in particular. We tested the hypotheses that cGMP-, Ca2+-dependent big potassium channels (BKCa2+) buffer the Ca2+ response to depolarization by high extracellular KCl and that NO inhibits adenosine diphosphoribose (ADPR) cyclase, thereby reducing the Ca2+-induced Ca2+ release. We isolated rat afferent arterioles, utilizing the magnetized microsphere method, and measured cytosolic Ca2+ concentration ([Ca2+]i) with fura-2, a preparation in which endothelial cells do not participate in [Ca2+]i responses. KCl (50 mM)-induced depolarization causes an immediate increase in [Ca2+]i of 151 nM. The blockers Nω-nitro-l-arginine methyl ester (of nitric oxide synthase), 1,2,4-oxodiazolo-[4,3-a]quinoxalin-1-one (ODQ, of guanylyl cyclase), KT-5823 (of PKG activation), and iberiotoxin (IBX, of BKCa2+ activity) do not alter the [Ca2+]i response to KCl, suggesting no discernible endogenous NO production under basal conditions. The NO donor sodium nitroprusside (SNP) reduces the [Ca2+]i response to 77 nM; IBX restores the response to control values. These data show that activation of BKCa2+ in the presence of NO/cGMP provides a brake on KCl-induced [Ca2+]i responses. Experiments with the inhibitor of cyclic ADPR 8-bromo-cyclic ADPR (8-Br-cADPR) and SNP + downstream inhibitors of PKG and BKCa2+ suggest that NO inhibits ADPR cyclase in intact arterioles. When we pretreat afferent arterioles with 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP; 10 μM), the response to KCl is 143 nM. However, in the presence of both IBX and 8-Br-cGMP, we observe a surprising doubling of the [Ca2+]i response to KCl. In summary, we present evidence for effects of the NO/cGMP/PKG system to reduce [Ca2+]i, via activation of BKCa2+ and possibly by inhibition of ADPR cyclase, and to increase [Ca2+]i, by a mechanism(s) yet to be defined.
Keywords: potassium channel, renal microcirculation, adenine diphosphoribose cyclase, chloride channel
for nearly 20 years, investigators have explored mechanisms by which nitric oxide (NO) causes vasodilatation and blunts vasoconstriction. Nearly all studies of the renal microcirculation have examined renal blood flow (RBF) in intact animals or arteriolar diameter changes in isolated vessels or other in vitro preparations. To our knowledge, no laboratory has studied the Ca2+ signaling events that occur as a consequence of exposure to NO or to the subsequent formation of cyclic GMP (cGMP) and activation of protein kinase G (PKG) in a renal resistance vessel.
The vasodilatory properties of the NO/cGMP/PKG system have been ascribed to stimulation or inhibition of numerous Ca2+ signaling pathways in vascular smooth muscle cells (VSMC). Inhibition of inositol trisphosphate receptor (IP3R), the Na+/Ca2+ exchanger in both forward and reverse modes, voltage-gated L-type channels, adenosine diphosphoribose (ADPR) cyclase, phosphodiesterase 3 (responsible for cAMP degradation), canonical transient receptor potential (TRPC)6, and myosin light chain (MLC) phosphatase have been reported (13, 14, 21, 40, 42, 48). NO/cGMP/PKG activates maxi or big Ca2+-activated K channels (BKCa2+) in nonrenal vessels (34, 39, 41). Changes in activity of Na+/Ca2+ exchange, BKCa2+, TRPC6, and L-type channels affect Ca2+ entry, whereas inhibition of IP3R and ADPR cyclase diminish Ca2+ mobilization. Alterations in Ca2+ sensitization occur in response to NO/cGMP/PKG but are not involved in cytosolic Ca2+ concentration ([Ca2+]i) levels per se.
A key candidate for NO-induced vasodilatation in many vascular beds has been activation of BKCa2+ and subsequent hyperpolarization (34, 39, 41). Evidence for the presence of BKCa2+ has been demonstrated by immunohistochemistry in isolated afferent arterioles (26) and physiologically in the blood-perfused juxtamedullary nephron preparation (19, 49). In single VSMC isolated from rabbit arcuate artery, the current amplitude of BKCa2+ at positive voltages is inhibited by iberiotoxin (IBX) (37). The vast majority of studies of nonrenal vessels suggest that cGMP and PKG facilitate activation of BKCa2+(30, 41). However, some have shown that 1,2,4-oxodiazolo-[4,3-a]quinoxalin-1-one (ODQ) inhibition of guanylyl cyclase fails to alter activation of BKCa2+ channels by the NO donor sodium nitroprusside (SNP), suggesting a direct effect of NO on mesenteric artery VSMC (29). Studies of afferent arteriolar diameter utilizing the blood-perfused juxtamedullary vascular model report that stimulation of BKCa2+ with the agonist NS-1619 causes an increase in baseline diameter and inhibits the vasoconstrictor effect of angiotensin II (ANG II). On the other hand, inhibition of BKCa2+ activity with tetraethylammonium reduces lumen diameter (49) but does not alter the response to ANG II (7). Experiments measuring changes in RBF with ANG II fail to show an effect of IBX, a specific inhibitor of BKCa2+, but when the channel is stimulated with the BKCa2+ agonist NS-1619 the vasoconstrictor response to ANG II is diminished (26). Thus controversy exists in defining the physiological functional role of BKCa2+ in the contractile responses in the renal microcirculation.
We designed our experiments to test the hypothesis that activation of BKCa2+ provides an important brake on depolarization-related increases in [Ca2+]i in afferent arterioles when components of the NO/cGMP/PKG system are active. An increase in [Ca2+]i is considered a major driving force for contraction of VSMC. We chose high extracellular KCl as the agent to increase [Ca2+]i in afferent arteriolar VSMC to simplify the model. Because G protein-coupled receptors are not stimulated, there should be no activation of IP3R, no receptor-operated Ca2+ entry or activation of TRPC channels, and no products of arachidonic acid metabolism. KCl-induced increases in [Ca2+]i alter VSMC Ca2+ sensitivity primarily at the level of MLC phosphorylation by modulation of MLC phosphatase activity and possibly by modulation of MLC kinase activity as well (38). However, because we are studying changes in [Ca2+]i, and not arteriolar contraction, the effect of NO on the MLC sensitivity to Ca2+ is not an issue. KCl does, of course, cause plasma membrane depolarization. We chose SNP as our source of NO because SNP is degraded inside cells to yield NO, thereby avoiding problems with spontaneous degradation in stock solutions or issues of diffusion time into the cell (1, 17). Because certain processes may contribute to or oppose the effects of the NO/cGMP/PKG system on Ca2+ signaling in VSMC, we employed a variety of pharmacological inhibitors to study these pathways.
METHODS
All studies were approved by and performed in compliance with the guidelines and practices of the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Preparation of fresh afferent arterioles.
We used the magnetized polystyrene microsphere-sieving technique as previously described in our laboratory (9) to isolate afferent arterioles (<20 μm in diameter) from 4.5- to 5-wk-old (80–110 g) male Sprague-Dawley rats maintained in the Chapel Hill colony. Phosphate-buffered saline (PBS) containing (in mM) 137 NaCl, 4.1 KCl, 0.66 KH2PO4, 3.4 Na2HPO4, 2.5 NaHCO3, 1.0 MgCl2, and 5 glucose was adjusted daily to pH 7.4 at 4°C and 23°C. The vessel segments in PBS containing 0.1% bovine serum albumin (BSA) were treated with collagenase type IV (Worthington, Lakewood NJ; 374 U/mg, 4–5 μg/ml) for 18 min at 34°C. Arterioles were loaded with fura-2 AM (3 μM) and 0.1% BSA for 55 min at 23°C in the dark. After arterioles were washed with PBS, the suspension was kept in Ca2+ (1.1 mM)-containing buffer on ice.
Measurement of cytosolic free calcium concentration.
We measured [Ca2+]i as previously described (9). To enhance immobilization of vessels to the coverslips, we coated the glass with CellTak (BD Bioscience, Bedford, MA). Afferent arterioles were identified by their morphology and measured external diameter of 15–20 μm. As well, we required visualization of microspheres in the lumen of the afferent arteriole or in the proximal branch of an interlobular artery from which it arose, to exclude the possibility that the vessel was an efferent arteriole. A segment of an afferent arteriole was centered in a small window of the optical field that was free of glomeruli or tubular fragments. There was heterogeneity of sampling sites along the vessel length.
The VSMC were excited alternately with light of 340- and 380-nm wavelength from a dual-excitation wavelength Delta-Scan equipped with dual monochronometers and a chopper [Photon Technology International (PTI), Birmingham, NJ]. After signals passed through a barrier filter (510 nm), fluorescence was detected by a photomultiplier tube. Signal intensity was acquired, stored, and processed by an IBM-compatible Pentium computer and Felix software (PTI). Background subtraction was performed in all studies. There was no interruption in the recording during the addition of reagents to the chamber. A video camera projected images of afferent arterioles onto a video monitor, permitting visualization of contraction of vessel segments.
We previously demonstrated that application of fura-2 and pharmacological agents on the abluminal side of the afferent arteriole results in no detectable contribution to the [Ca2+]i signal from endothelial cells (EC). Bradykinin (10−7-10−5 M) increases [Ca2+]i by 10–25 nM, which is not significantly different from baseline. Nω-nitro-l-arginine methyl ester (l-NAME) does not change the [Ca2+]i response to the endothelin-B (ETB) receptor agonist sarafotoxin in our preparation (8), to high KCl in isolated microperfused afferent arterioles (51), or to ANG II in afferent arterioles isolated with the agarose gel infusion technique (16). We do not know whether the microspheres (4.5-μm diameter) in our preparation, during their passage through arterioles and ending in glomeruli, damage EC.
Reagents.
Reagents were added to the bath as concentrated solutions to achieve a final desired concentration in the buffer. When vessels were pretreated with an agent, at least 2 min elapsed before addition of KCl. We purchased 8-bromo-cyclic ADPR (8-Br-cADPR), IBX, SNP and l-NAME from Sigma Aldrich (St. Louis, MO), 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP), KT-5823, and ODQ from CalBiochem (San Diego, CA), fura 2-AM from Molecular Probes (Eugene, OR), and magnetized microspheres (4.5 μm) from Spherotech (Libertyville, IL).
Rationale for pharmacological agents.
We used SNP to supply a fixed exogenous amount of NO. SNP is commonly used at a concentration of 100 μM to generate NO in vitro (52, 55). Measured levels of NO in EC are in the high nanomolar range (22). The extent to which SNP liberates NO in VSMC in our preparation is unknown but likely is in the micromolar range.
IBX (100 nM) is a specific inhibitor of the BKCa2+ channel (45). To evaluate actions of NO and the cGMP/PKG system independent of activation of BKCa2+, we pretreated arterioles with the inhibitor.
When we wished to examine the effects of cGMP independent of those due to NO itself, we employed 8-Br-cGMP (10 μM), which resists hydrolysis and is able to enter the cell (27).
To block the activation of the cGMP/PKG system, we used two agents. KT-5823 (1 μM) blocks the activation of PKG; ODQ (10 μM) antagonizes soluble guanylyl cyclase. Such intervention isolates the actions of NO independent of the cGMP/PKG system.
To eliminate the possibility of a contribution of endogenous NO, we used l-NAME (10 μM) to inhibit NO synthase and the formation of NO (5, 24, 36, 51).
We previously showed that the response of afferent arterioles to KCl depends in part on endogenous cADPR and the ryanodine receptor (RyR) to amplify Ca2+-induced Ca2+ release (CICR) (11). 8-Br-cADPR antagonizes the action of cADPR on the RyR (55). Because NO has been reported to inhibit ADPR cyclase in coronary VSMC, we wanted to compare the effects of inhibiting the enzyme versus blocking its product (55).
KCl was used at a concentration of 50 mM; higher concentrations produce only modest increments in the [Ca2+]i response. Furthermore, we are concerned that concentrations of 100 mM lower the extracellular Na+ concentration sufficiently to activate reverse-mode Na+/Ca2+ exchange.
In no case did any of the aforementioned reagents, alone or in combination, cause a statistical difference in baseline values of [Ca2+]i in afferent arterioles.
Statistics.
Individual arterioles were studied only once and then discarded; thus each recording originates from a separate vessel. At least six arterioles from a minimum of two rats per experiment on two different days were examined for each data set. Data are presented as means ± SE (Table 1). Group comparisons were evaluated by one-way ANOVA, with P < 0.05 considered statistically significant (GraphPad Prism Software, La Jolla, CA).
Table 1.
Summary of increases in cytosolic calcium concentration of afferent arterioles in response to high extracellular KCl and pharmacological agents
| Peak [Ca2+]i |
Plateau [Ca2+]i |
|||||
|---|---|---|---|---|---|---|
| Group | Mean ± SE | P* | P† | Mean ± SE | P* | P† |
| KCl | 151±3 (28) | 101±2 | ||||
| KCl + l-NAME | 126±21 (6) | NS | 97±20 | NS | ||
| KCl + ODQ | 124±15 (4) | NS | 90±16 | NS | ||
| KCl + KT-5823 | 105±10 (5) | NS | 77±8 | NS | ||
| KCl + IBX | 134±2 (5) | NS | 108±4 | NS | ||
| KCl + SNP | 77±8 (12) | <0.001 | 60±8 | <0.001 | ||
| KCl + SNP + IBX | 126±18 (10) | NS | <0.05 | 86±10 | NS | NS |
| KCl + SNP + KT-5823 | 54±1 (9) | <0.01 | NS | 45±1 | <0.001 | NS |
| KCl + SNP + IBX + KT-5823 | 75±3 (9) | <0.001 | NS | 60±2 | <0.001 | NS |
| KCl + SNP + ODQ | 66±6 (5) | <0.01 | NS | 47±5 | <0.001 | NS |
| KCl + SNP +8-Br-cADPR | 81±3 (11) | <0.01 | NS | 75±4 | NS | NS |
| KCl +8-Br-cGMP | 143±8 (9) | NS | 109±8 | NS | ||
| KCl +8-Br-cGMP + IBX | 340±27 (9) | <0.001 | 137±7 | <0.001 | ||
Values are means ± SE for no. of observations in parentheses. [Ca2+]i, cytosolic calcium concentration; l-NAME, Nω-nitro-l-arginine methyl ester; ODQ, 1,2,4-oxodiazolo-[4,3-a]quinoxalin-1-one; IBX, iberiotoxin; SNP, sodium nitroprusside; 8-Br-cADPR, 8-bromo-cyclic adenosine diphosphoribose; 8-Br-cGMP, 8-bromoguanosine 3′,5′-cyclic monophosphate.
P vs. KCl: ANOVA for “peak” P < 0.001, ANOVA for “plateau” P < 0.001;
P vs. KCl + SNP: ANOVA for “peak” P < 0.001, ANOVA for “plateau” P < 0.001; NS, not significant (P > 0.05).
RESULTS
Measured baseline [Ca2+]i for the entire data set was 110 ± 5 nM. In no experimental subgroup was baseline [Ca2+]i different from control baseline (109 ± 2 nM) or from the grand mean.
[Ca2+]i response to KCl.
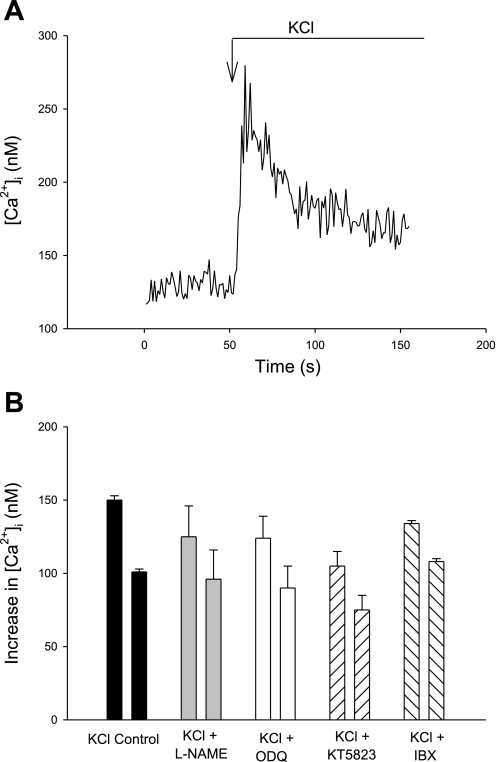
Afferent arterioles respond to KCl (50 mM) with a prompt peak increase of 151 ± 3 nM followed by a sustained plateau 50–75 s later of Δ101 ± 2 nM above baseline (n = 28, Fig. 1, Table 1).
Fig. 1.
Changes in cytosolic Ca2+ concentration ([Ca2+]i) of isolated afferent arterioles to KCl (50 mM). A: representative recording of [Ca2+]i response to KCl. B: summary data of increases in peak (left bar in each pair) and plateau (right bar in each pair) [Ca2+]i in afferent arterioles in response to KCl (50 mM) in afferent arteriolar vascular smooth muscle cells (VSMC) in the absence or presence of the inhibitors Nω-nitro-l-arginine methyl ester (l-NAME; nitric oxide synthase), 1,2,4-oxodiazolo-[4,3-a]quinoxalin-1-one (ODQ; soluble guanylyl cyclase), KT-5823 (protein kinase G), or iberiotoxin [IBX; Ca2+-dependent big potassium channel (BKCa2+)]. P = not significant (NS) vs. control for each agent, both peak and plateau. See Table 1 for P and n values.
Effect of interruption of NO/cGMP system on [Ca2+]i responses to KCl.
We showed previously (8) that bradykinin and l-NAME do not alter the [Ca2+]i response to sarafotoxin (ETB receptor agonist) or endothelin-1 in afferent arteriolar VSMC. In the presence of l-NAME, the peak response to KCl is Δ126 ± 21 nM and the plateau is 97 ± 20 nM [P not significant (NS) for both vs. control]. To further explore a possible role of endogenous NO in our afferent arterioles, we used two inhibitors of the cGMP/PKG system, ODQ and KT-5823. In the presence of KT-5823, the peak [Ca2+]i response to KCl is Δ105 ± 10 nM and the plateau is 77 ± 10 (P = NS vs. control). When arterioles are pretreated with ODQ, the [Ca2+]i response is Δ124 ± 15 nM (n = 6, P > 0.5 vs. control). These results further substantiate our assumption that EC are not physiologically active (producing endogenous NO to affect Ca2+ signaling) in our preparation of afferent arterioles (Fig. 1B).
Inhibition of BKCa2+ and KCl responses.
Because BK Ca2+ are present in afferent arterioles and because activation of the channels with NO/cGMP or an increase in [Ca2+]i should lead to hyperpolarization and a subsequent reduction in [Ca2+]i entry, we tested the response to KCl in the presence of the specific inhibitor IBX (100 nM). IBX has no net effect on the peak or plateau [Ca2+]i response to KCl (Δ134 ± 2 and Δ108 ± 4 nM respectively; both P = NS; Figs. 1B and 2B, first 2 pairs of bars). Thus, under control conditions and in the absence of exogenous NO, there is no discernible activation of the BKCa2+ to alter [Ca2+]i responses during KCl-induced depolarization.
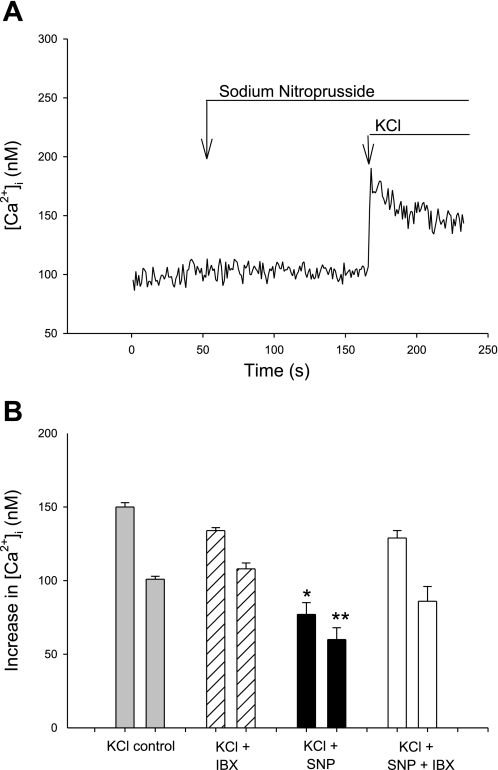
Fig. 2.
[Ca2+]i responses of afferent VSMC to KCl in the presence of sodium nitroprusside (SNP). A: representative tracing showing absence of an effect of SNP on baseline [Ca2+]i and reduced [Ca2+]i response to KCl in the presence of SNP. B: summary data of control peak and plateau [Ca2+]i responses to KCl vs. those with SNP and SNP + IBX. *P < 0.001 vs. peak, **P < 0.001 vs. plateau; peak bars are on left in each pair of bars.
[Ca2+]i responses to KCl in afferent arterioles pretreated with SNP to generate NO.
As noted above, SNP has no effect on baseline [Ca2+]i (109 ± 1 control vs. 114 ± 3 nM with SNP). In the presence of SNP, however, the peak [Ca2+]i response to KCl is reduced by ∼50% to Δ77 ± 8 nM and the plateau by ∼40% to Δ60 ± 2 nM (both P < 0.001; Fig. 2, Table 1). Thus exogenous NO exerts a pronounced inhibitory effect on the KCl-induced increase in [Ca2+]i.
Effect of iberiotoxin in presence of NO.
The peak increase in the [Ca2+]i response to KCl in the presence of IBX and SNP is Δ126 ± 18 nM, and the plateau is Δ86 ± 10 nM [P < 0.05 vs. SNP without IBX for peak and P = NS for plateau, but neither is different from control responses to KCl, P = NS; Fig. 2B]. These data show that exogenously administered NO can activate BKCa2+ to limit Ca2+ entry through voltage-gated Ca2+ channels (VGCC) activated by KCl-induced depolarization and reduce the overall [Ca2+]i response. The specific BKCa2+ inhibitor IBX negates this group of related processes.
Inhibition of activation of PKG.
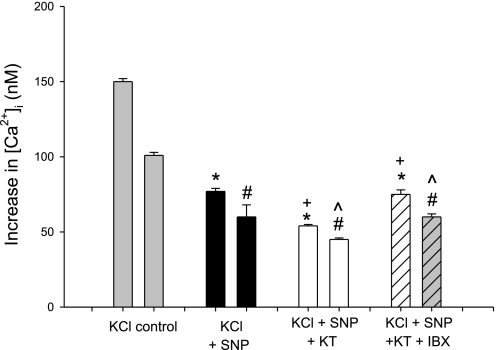
The peak [Ca2+]i response to KCl in the presence of SNP and KT-5823 is Δ54 ± 1 nM (P < 0.01 vs. control, P = NS vs. KCl + SNP; Fig. 3, Table 1). Addition of IBX to SNP and KT-5823 results in a peak [Ca2+]i response of KCl of 75 ± 3 nM (P < 0.01 vs. control, P = NS vs. KCl + SNP). These results suggest the presence of a PKG-dependent system that, when inhibited, is associated with a fall rather than the anticipated increase in the [Ca2+]i response to KCl. Moreover, this intriguing process is unmasked when BKCa2+ is inhibited with IBX, that is, the [Ca2+]i responses rise back to the values with SNP alone (P = 0.02). That plateau [Ca2+]i values fall suggests that Ca2+ entry is inhibited.
Fig. 3.
Summary data of peak (left) and plateau (right) [Ca2+]i responses to KCl: control vs. SNP, SNP + KT-5823 (KT), or SNP + KT-5823 and IBX. *P < 0.01 vs. peak control, #P < 0.01 vs. plateau control, +P = NS vs. SNP peak, ^P = NS vs. SNP plateau.
Experiments were conducted with ODQ to block the formation of cGMP, and the results were very similar to those with KT-5823 (Table 1). To further delineate the nature of the cGMP/PKG-dependent process, we performed experiments with exogenous 8-Br-cGMP.
[Ca2+]i response to KCl in presence of 8-Br-cGMP.
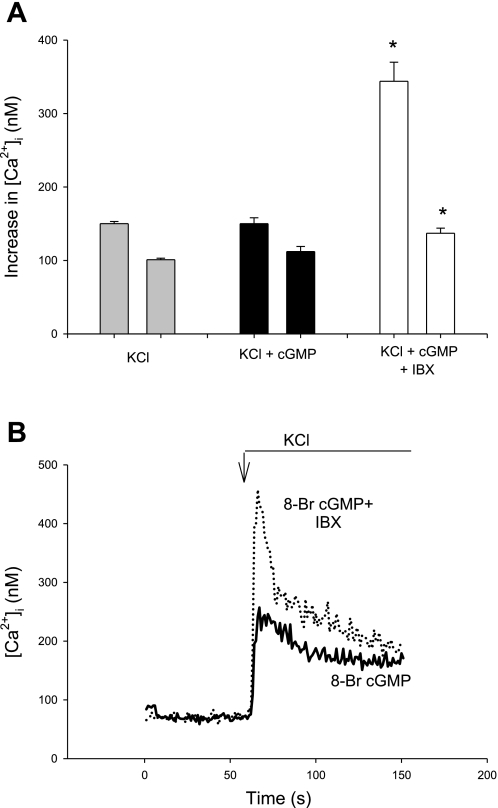
Exposure of afferent arteriolar VSMC to cGMP could result in multiple effects, such as stimulation of BKCa2+ or inhibition of voltage-gated L-type channels. In both cases, one would expect cGMP to decrease the [Ca2+]i response to KCl. This does not occur, however, when KCl stimulates VSMC pretreated with 8-Br-cGMP (10 μM). Neither the peak [Ca2+]i response to KCl of Δ143 ± 8 nM nor the plateau response of 109 ± 8 nM in the presence of 8-Br-cGMP is different from control KCl responses (Fig. 4A, Table 1). The data do not exclude, however, the possibility that equal and opposite mechanisms are operative, that is, a combination of pathways inhibited by and stimulated by cGMP.
Fig. 4.
[Ca2+]i responses of afferent arteriolar VSMC to KCl (50 mM) in the absence or presence of 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP). A: summary data showing that the peak and plateau [Ca2+]i responses to KCl are not changed in the presence of 8-Br-cGMP but that addition of IBX doubles the peak response and increases the plateau response by 26%. *P < 0.001, peak and plateau. Plateau bars are on right in each pair of bars. B: representative tracings illustrating the enhanced [Ca2+]i response to KCl in the presence of cGMP and IBX.
8-Br-cGMP plus IBX.
When arterioles are treated with both 8-Br-cGMP and IBX, the peak [Ca2+]i response to KCl is more than doubled, increasing to Δ340 ± 27 (P < 0.001 vs. 8-Br-cGMP without IBX; Fig. 4). The plateau [Ca2+]i response is increased by 25%. These data strongly support the idea that there is a cGMP-dependent pathway that enhances the [Ca2+]i response to KCl in afferent arterioles. We are unaware of any currently identified process in which NO or cGMP increases [Ca2+]i in VSMC. These unusual results raise the question of whether Ca2+ entry via activation of a Ca2+-, cGMP-dependent Cl− channel (ClCa2+, cGMP) is a possible candidate for this process (vide infra).
ADPR cyclase and NO.
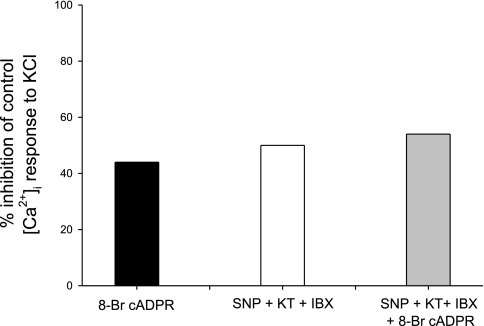
We designed this protocol to test the action of NO, independent of activation of BKCa2+ and other downstream consequences of cGMP/PKG signaling. By pretreating afferent arterioles with both KT-5823 and IBX, we avoid these other Ca2+-related mechanisms (Fig. 5). Under these conditions, the peak [Ca2+]i response to KCl is Δ75 ± 3 nM (P = NS vs. SNP alone, P < 0.001 vs. control). Although NO has been implicated in the activation of BKCa2+ independent of cGMP/PKG, the presence of IBX eliminates this possibility (29). When 8-Br-cADPR is added to SNP, the peak [Ca2+]i response to KCl is Δ81 ± 3 (P = NS vs. both SNP and SNP + KT-5823 + IBX), demonstrating that the specific cADPR inhibitor causes no further inhibition in the presence of NO. Thus NO alone, in the absence of other downstream effects, inhibits the [Ca2+]i response to KCl by ∼45%, a value very similar to that of the effect of 8-Br-cADPR on KCl-induced [Ca2+]i responses in our previous studies (11). Because inhibition of ADPR cyclase (and formation of cADPR) diminishes mobilization of Ca2+ via the RyR from the sarcoplasmic reticulum, one would expect a greater effect on initial peak rather than plateau values of [Ca2+]i. It is generally accepted that mobilization accounts for at least half of peak [Ca2+]i responses, whereas entry is largely responsible for the plateau phase. This is indeed the case for this group of experiments (Table 1).
Fig. 5.
Percent inhibition of peak [Ca2+]i responses to KCl in afferent arteriolar VSMC. 8-Bromo-cyclic ADPR (8-Br-cADPR) inhibits the response by 44% (see Ref. 11). KT-5823 and IBX block downstream effects of the NO donor SNP, demonstrating the inhibitory effect of NO alone. No further inhibition occurs with addition of 8-Br-cADPR to KCl + SNP.
Table 1 summarizes the peak and plateau values for increase in [Ca2+]i from baseline values and P values based on ANOVA for each data set.
DISCUSSION
We designed the present study to test the hypothesis that activation of BKCa2+ provides an important brake on increases in [Ca2+]i in VSMC of afferent arterioles that are depolarized by high extracellular KCl. Our new data demonstrate the importance of BKCa2+ in the global [Ca2+]i response to KCl and uncover some of the complexities of the NO/cGMP/PKG system in Ca2+ signaling in this important renal resistance arteriole.
The traditional view of the role of EC-produced NO is that NO diffuses out of the EC plasma membrane through the VSMC cell membrane and into the VSMC cytosol. More recently, it has been shown that aquaporin-1 transports NO across EC and VSMC membranes, facilitating the rate of transfer compared with simple diffusion (18). Once transported, NO may interact directly or via the cGMP/PKG signaling system with a variety of channels or enzymes, the net result of which leads to some reversal of vasoconstriction, a major fraction of which is due to a reduction in [Ca2+]i. Key candidates for counteracting a vasoconstrictor-induced rise in [Ca2+]i are reduced Ca2+ entry secondary to activation of BKCa2+ channels and/or inhibition of voltage-gated L-type channels and diminished Ca2+ mobilization caused by inhibition of ADPR cyclase. cADPR is required for optimal operation of the RyR to produce CICR. To explore the integrated effects of NO/cGMP on afferent arteriolar VSMC, we elected to use 50 mM KCl to cause membrane depolarization and to initiate Ca2+ signaling cascades. By so doing, we avoid consideration of participation of IP3R activation, receptor-operated Ca2+ mechanisms, and arachidonic acid metabolites.
In VSMC of afferent arterioles and interlobular arteries, although BKCa2+ is highly expressed, its physiological function is poorly understood (26). Conceptually, operation of this channel should counteract an increase in [Ca2+]i and depolarization that follows agonist stimulation and therefore could be important in the control of renal vascular tone. Although the channel is typically viewed as Ca2+ dependent, it is also voltage sensitive and activated by NO/cGMP/PKG. The weight of evidence supports activation of BKCa2+ by PKG rather than NO per se (30, 34, 39, 44). Patch clamp studies in renal artery EC show strong voltage and cGMP dependence of BKCa2+. In the absence of exogenous cGMP or at neutral voltage, high micromolar concentrations of Ca2+ are required to open the channel (4). Depolarization enhances the open probability. The resting membrane potential in rat afferent arterioles is between −40 and −65 mV, depending on the experimental preparation (25, 53). High extracellular KCl will, of course, depolarize the membrane. Thus assessment of the role of BKCa2+ in any experimental model must take into account four variables: membrane potential, [Ca2+]i, cGMP concentration, and PKG activity. In our preparation, the afferent arterioles are not pressurized; the global [Ca2+]i does not rise above ∼400 nM, and the supply of NO/cGMP is exogenous. We acknowledge that [Ca2+]i may be higher in certain intracellular microenvironments.
The extent of depolarization caused by KCl (50 mM) in an afferent arteriolar VSMC is unknown. The results of the control experiments with KCl and IBX in the absence of SNP or 8-Br-cGMP are not different from those with KCl alone. Thus it appears that the increase in [Ca2+]i and the level of depolarization following addition of KCl to afferent arteriolar VSMC is insufficient to activate BKCa2+. Our data clearly show, however, that in the presence of putative physiological concentrations of cGMP, BKCa2+ has a prominent braking effect on the increase in [Ca2+]i initiated by KCl in afferent arterioles.
To explore the contribution of the cGMP/PKG system to KCl-induced [Ca2+]i signaling in afferent arterioles independent of BKCa2+, we treated the vessels with 8-Br-cGMP (10 μM) in the presence of IBX. 8-Br-cGMP does not alter the control [Ca2+]i response to KCl. When arterioles are pretreated with 8-Br-cGMP plus IBX, however, the [Ca2+]i response to KCl nearly doubled, suggesting that the cGMP/PKG system of VSMC has both stimulatory and inhibitory components to [Ca2+]i signaling. These are surprising and novel findings.
To our knowledge, there is no previously described Ca2+ signaling pathway that responds to stimulation with cGMP to increase [Ca2+]i in VSMC. Although the NO/cGMP/PKG system has been shown to activate skeletal muscle RyR1 via S-nitrosylation and cardiac RyR2 through S-nitrosoglutathione, it has no effect on caffeine stimulation of the RyR in pulmonary and aortic VSMC (21, 32, 47). Our results with 8-Br-cGMP in the presence of IBX are in the opposite direction of the traditional view that the cGMP/PKG system causes a decrease in [Ca2+]i.
It is well accepted that L-type VGCC provide a major pathway for Ca2+ entry in afferent arterioles. cGMP has been reported to inhibit L-type Ca2+ channel activity in portal vein and cultured aortic VSMC (2, 40). To date, there are no studies that examine the specific effects of cGMP/PKG on VGCC in arteriolar VSMC. If cGMP/PKG were a major pathway for reducing [Ca2+]i, one would anticipate reversal of SNP inhibition of [Ca2+]i responses to KCl in the presence of KT-5823 or ODQ. Instead, we observe the opposite effect—that is, blockade of cGMP/PKG with KT-5823 or ODQ inhibits the [Ca2+]i response to KCl in afferent arterioles, supporting the concept that there is a cGMP/PKG-dependent process that increases [Ca2+]i.
Although some investigators believe that Ca2+ stimulation of Ca2+-activated Cl− channels (ClCa2+) causes sufficient depolarization to activate VGCC, we (12) and others (43) note that inhibitors of this classic ClCa2+ fail to alter the response to ANG II in afferent arterioles. Many of the drugs utilized to inhibit ClCa2+ are nonspecific and, depending on concentration, may activate BKCa2+(15, 35). The classic ClCa2+ is voltage dependent and requires ∼650 nM [Ca2+]i at a voltage of −80 mV and 300 nM at +100 mV for activation (reviewed in Ref. 31). It is an open question whether there is sufficient depolarization and high enough [Ca2+]i to immediately activate the ClCa2+ after agonist stimulation of afferent arteriolar VSMC.
A cGMP-dependent Ca2+-activated Cl− channel (ClCa2+, cGMP) has been identified according to its effects on vasomotion and electrophysiological characteristics in small mesenteric arterial VSMC (3, 27, 33, 36). In contrast to the classic ClCa2+, Ca2+, cGMP has an EC50 for [Ca2+]i of ∼74 nM, is activated by 3–6 μM cGMP, is voltage- and time independent, is not inhibited by niflumic acid or by DIDS (<200 μM), and is inhibited by 2–6 μM Zn2+ (28, 36). Subsequently, electrophysiological evidence for a ClCa2+, cGMP has been found in a variety of vessels such as renal, cerebral, and superior mesenteric arteries and femoral and portal veins (28). The low IC50 for Ca2+ suggests that the channel may be partially activated at rest and contribute to maintenance of membrane potential.
Our study raises the question of a possible role of ClCa2+, cGMP in Ca2+ signaling in afferent arteriolar VSMC. Zn2+ (3 μM) consistently inhibits the responses to KCl in the present study (data not shown), but because Zn2+ also inhibits voltage-gated L-type channels, we cannot conclude that inhibition of a ClCa2+, cGMP is occurring (46). Future studies designed to explore a functional role for ClCa2+, cGMP in Ca2+ signaling in VSMC are required.
The importance of the role of cADPR in Ca2+ signaling VSMC and in endothelial cells has been summarized in a recent review (56). A growing body of literature demonstrates a critical role for cADPR in the Ca2+ response to angiotensin, endothelin, and superoxide generation (8, 10, 56). The ADPR cyclase of VSMC differs from the cyclase of sea urchin eggs, Aplysia, and HL-60 cells by being inhibited rather than stimulated by NO and Zn2+ (6, 55). There is convincing evidence for a direct inhibitory effect of NO on ADPR cyclase activity in membrane fragments of coronary VSMC and airway smooth muscle cells (54, 55). Because the active site of ADPR cyclase is ectocellular, NO need only exit the EC to initiate S-nitrosylation of the enzyme (23). Independence from entry into the VSMC plasma membrane, and from a requirement for formation of cGMP, might predict early responses to inhibition by NO in vivo, but the concentration of NO needed for S-nitrosylation is not yet known. A study with gaseous NO in the isolated, perfused hydronephrotic kidney shows an ODQ-insensitive component to ANG II constriction at concentrations of >1 μM NO (50). Whether this vasorelaxant effect of NO occurs because of inhibition of ADPR cyclase is not yet known.
We find that the [Ca2+]i response of afferent arterioles to KCl in combination with SNP is not different from that achieved when both KT-5823 and IBX are blocking downstream effects of cGMP and of BKCa2+ or different from the response to KCl in the presence of the cADPR inhibitor 8-Br-cADPR. Under both of these experimental conditions, it is the peak rather than the plateau response that is largely affected, suggesting an inhibition of mobilization of [Ca2+]i from the SR. Thus our data are compatible with a direct effect of NO on ADPR cyclase activity in afferent arterioles. We previously showed (11) that the [Ca2+]i response of afferent arterioles to KCl involves CICR and that blockade of cADPR with 8-Br-cADPR diminishes the response by ∼45%. Further studies are needed to explore the effects of NO on the ADPR cyclase pathway in VSMC of resistance vessels. Because the VSMC cyclase differs from that of many other cell types and because at least two ADPR cyclases have been identified in mammals (CD38 and CD157), differentiation of the distribution of enzymatic subtypes in EC and VSMC presents a particular challenge.
In summary, our study of Ca2+ signaling in afferent arteriolar VSMC provides new information showing that KCl-mediated increases in [Ca2+]i, in the presence of exogenous NO or cGMP, strongly activate BKCa2+, suggesting an important role for this channel when intact renal resistance vessels are stimulated with depolarizing agonists. Our data are also suggestive of an inhibitory role of NO on ADPR cyclase to block the formation of cADPR and its effect on the function of the RyR. A surprising finding is that a yet to be defined PKG-dependent pathway appears to be responsible for an increase in [Ca2+]i. Future studies need to be designed to utilize physiological depolarizing and vasoconstrictor agonists such as ANG II to further investigate the role of the NO/cGMP/PKG system in arteriolar VSMC.
GRANTS
This work was supported in part by an award from the Thomas H. Maren Foundation and by National Heart, Lung, and Blood Institute Research Grant HL-02334.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Al-Sa'doni HH, Khan IY, Poston L, Fisher I, Ferro A. A novel family of S-nitrosothiols: chemical synthesis and biological actions. Nitric Oxide 4: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Blatter LA, Wier WG. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium 15: 122–131, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Boedtkjer DM, Matchkov VV, Boedtkjer E, Nilsson H, Aalkjaer C. Vasomotion has chloride-dependency in rat mesenteric small arteries. Pflügers Arch 457: 389–404, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Brakemeier S, Eichler I, Knorr A, Fassheber T, Kohler R, Hoyer J. Modulation of Ca2+-activated K+ channel in renal artery endothelium in situ by nitric oxide and reactive oxygen species. Kidney Int 64: 199–207, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Cavarape A, Endlich N, Assaloni R, Bartoli E, Steinhausen M, Parekh N, Endlich K. Rho-kinase inhibition blunts renal vasoconstriction induced by distinct signaling pathways in vivo. J Am Soc Nephrol 14: 37–45, 2003 [DOI] [PubMed] [Google Scholar]
- 6.de Toledo FG, Cheng J, Liang M, Chini EN, Dousa TP. ADP-ribosyl cyclase in rat vascular smooth muscle cells: properties and regulation. Circ Res 86: 1153–1159, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Fallet RW, Bast JP, Fujiwara K, Ishii N, Sansom SC, Carmines PK. Influence of Ca2+-activated K+ channels on rat renal arteriolar responses to depolarizing agonists. Am J Physiol Renal Physiol 280: F583–F591, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellner SK, Arendshorst W. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 292: F175–F184, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fellner SK, Arendshorst WJ. Angiotensin II Ca2+ signaling in rat afferent arterioles: stimulation of cyclic ADP ribose and IP3 pathways. Am J Physiol Renal Physiol 288: F785–F791, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Fellner SK, Arendshorst WJ. Angiotensin II, reactive oxygen species, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 289: F1012–F1019, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Fellner SK, Arendshorst WJ. Voltage-gated Ca2+ entry and ryanodine receptor Ca2+-induced Ca2+ release in preglomerular arterioles. Am J Physiol Renal Physiol 292: F1568–F1572, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fellner SK, Arendshorst WJ. Angiotensin II-stimulated Ca2+ entry mechanisms in afferent arterioles: role of transient receptor potential canonical channels and reverse Na+/Ca2+ exchange. Am J Physiol Renal Physiol 294: F212–F219, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Furukawa K, Ohshima N, Tawada-Iwata Y, Shigekawa M. Cyclic GMP stimulates Na+/Ca2+ exchange in vascular smooth muscle cells in primary culture. J Biol Chem 266: 12337–12341, 1991 [PubMed] [Google Scholar]
- 14.Grassi C, D'Ascenzo M, Azzena GB. Modulation of Cav1 and Cav2.2 channels induced by nitric oxide via cGMP-dependent protein kinase. Neurochem Int 45: 885–893, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Greenwood IA, Leblanc N. Overlapping pharmacology of Ca2+-activated Cl− and K+ channels. Trends Pharmacol Sci 28: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Helle F, Hultstrom M, Skogstrand T, Palm F, Iversen BM. Angiotensin II-induced contraction is attenuated by nitric oxide in afferent arterioles from the nonclipped kidney in 2K1C. Am J Physiol Renal Physiol 296: F78–F86, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Herrera M, Garvin JL. Novel role of AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol 292: F1443–F1451, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension 48: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11,12-EET analogs involves PP2A activity and Ca2+-activated K+ channels. Microcirculation 15: 137–150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen JC, Aalkjaer C, Nilsson H, Matchkov VV, Freiberg J, Holstein-Rathlou NH. Activation of a cGMP-sensitive calcium-dependent chloride channel may cause transition from calcium waves to whole cell oscillations in smooth muscle cells. Am J Physiol Heart Circ Physiol 293: H215–H228, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ji J, Benishin CG, Pang PK. Nitric oxide selectively inhibits intracellular Ca++ release elicited by inositol trisphosphate but not caffeine in rat vascular smooth muscle. J Pharmacol Exp Ther 285: 16–21, 1998 [PubMed] [Google Scholar]
- 22.Lantoine F, Iouzalen L, Devynck MA, Millanvoye-Van Brussel E, David-Dufilho M. Nitric oxide production in human endothelial cells stimulated by histamine requires Ca2+ influx. Biochem J 330: 695–699, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HC. Structure and enzymatic functions of human CD38. Mol Med 12: 317–323, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez B, Moreno C, Salom MG, Roman RJ, Fenoy FJ. Role of guanylyl cyclase and cytochrome P-450 on renal response to nitric oxide. Am J Physiol Renal Physiol 281: F420–F427, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: actions of angiotensin II. Am J Physiol Renal Physiol 273: F307–F314, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Magnusson L, Sorensen CM, Braunstein TH, Holstein-Rathlou NH, Salomonsson M. Renovascular BKCa channels are not activated in vivo under resting conditions and during agonist stimulation. Am J Physiol Regul Integr Comp Physiol 292: R345–R353, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Matchkov VV, Aalkjaer C, Nilsson H. A cyclic GMP-dependent calcium-activated chloride current in smooth-muscle cells from rat mesenteric resistance arteries. J Gen Physiol 123: 121–134, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matchkov VV, Aalkjaer C, Nilsson H. Distribution of cGMP-dependent and cGMP-independent Ca2+-activated Cl− conductances in smooth muscle cells from different vascular beds and colon. Pflügers Arch 451: 371–379, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Mistry DK, Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol 124: 1131–1140, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nara M, Dhulipala PD, Ji GJ, Kamasani UR, Wang YX, Matalon S, Kotlikoff MI. Guanylyl cyclase stimulatory coupling to KCa channels. Am J Physiol Cell Physiol 279: C1938–C1945, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand 177: 119–147, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Pauvert O, Marthan R, Savineau J. NO-induced modulation of calcium-oscillations in pulmonary vascular smooth muscle. Cell Calcium 27: 329–338, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res 88: 810–815, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Peng W, Hoidal JR, Farrukh IS. Regulation of Ca2+-activated K+ channels in pulmonary vascular smooth muscle cells: role of nitric oxide. J Appl Physiol 81: 1264–1272, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Piper AS, Greenwood IA, Large WA. Dual effect of blocking agents on Ca2+-activated Cl− currents in rabbit pulmonary artery smooth muscle cells. J Physiol 539: 119–131, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piper AS, Large WA. Single cGMP-activated Ca2+-dependent Cl− channels in rat mesenteric artery smooth muscle cells. J Physiol 555: 397–408, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prior HM, Yates MS, Beech DJ. Functions of large conductance Ca2+-activated (BKCa), delayed rectifier (KV) and background K+ channels in the control of membrane potential in rabbit renal arcuate artery. J Physiol 511: 159–169, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol 288: C769–C783, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 265: C299–C303, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Ruiz-Velasco V, Zhong J, Hume JR, Keef KD. Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circ Res 82: 557–565, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Sampson LJ, Plane F, Garland CJ. Involvement of cyclic GMP and potassium channels in relaxation evoked by the nitric oxide donor, diethylamine NONOate, in the rat small isolated mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol 364: 220–225, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Sandner P, Kornfeld M, Ruan X, Arendshorst WJ, Kurtz A. Nitric oxide/cAMP interactions in the control of rat renal vascular resistance. Circ Res 84: 186–192, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Steendahl J, Holstein-Rathlou NH, Sorensen CM, Salomonsson M. Effects of chloride channel blockers on rat renal vascular responses to angiotensin II and norepinephrine. Am J Physiol Renal Physiol 286: F323–F330, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Stockand JD, Sansom SC. Mechanism of activation by cGMP-dependent protein kinase of large Ca2+-activated K+ channels in mesangial cells. Am J Physiol Cell Physiol 271: C1669–C1677, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Stockand JD, Sansom SC. Role of large Ca2+-activated K+ channels in regulation of mesangial contraction by nitroprusside and ANP. Am J Physiol Cell Physiol 270: C1773–C1779, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Sun HS, Hui K, Lee DW, Feng ZP. Zn2+ sensitivity of high- and low-voltage activated calcium channels. Biophys J 93: 1175–1183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun J, Yamaguchi N, Xu L, Eu JP, Stamler JS, Meissner G. Regulation of the cardiac muscle ryanodine receptor by O2 tension and S-nitrosoglutathione. Biochemistry 47: 13985–13990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi S, Lin H, Geshi N, Mori Y, Kawarabayashi Y, Takami N, Mori MX, Honda A, Inoue R. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol 586: 4209–4223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Troncoso Brindeiro CM, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol 295: F171–F178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trottier G, Triggle CR, O'Neill SK, Loutzenhiser R. Cyclic GMP-dependent and cyclic GMP-independent actions of nitric oxide on the renal afferent arteriole. Br J Pharmacol 125: 563–569, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uhrenholt TR, Schjerning J, Vanhoutte PM, Jensen BL, Skott O. Intercellular calcium signaling and nitric oxide feedback during constriction of rabbit renal afferent arterioles. Am J Physiol Renal Physiol 292: F1124–F1131, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Borrego-Conde LJ, Falck JR, Sharma KK, Wilcox CS, Umans JG. Contributions of nitric oxide, EDHF, and EETs to endothelium-dependent relaxation in renal afferent arterioles. Kidney Int 63: 2187–2193, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Takeya K, Aaronson PI, Loutzenhiser K, Loutzenhiser R. Effects of amiloride, benzamil, and alterations in extracellular Na+ on the rat afferent arteriole and its myogenic response. Am J Physiol Renal Physiol 295: F272–F282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White TA, Walseth TF, Kannan MS. Nitric oxide inhibits ADP-ribosyl cyclase through a cGMP-independent pathway in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 283: L1065–L1071, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Yu JZ, Zhang DX, Zou AP, Campbell WB, Li PL. Nitric oxide inhibits Ca2+ mobilization through cADP-ribose signaling in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 279: H873–H881, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Zhang AY, Li PL. Vascular physiology of a Ca2+ mobilizing second messenger—cyclic ADP-ribose. J Cell Mol Med 10: 407–422, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]