Abstract
We examined the expression of tight junction (TJ) proteins in the cerebral cortex, cerebellum, and spinal cord of fetuses after maternal treatment with single and multiple courses of dexamethasone. Ewes received either single courses of four 6-mg dexamethasone or placebo injections every 12 h for 48 h between 104 and 107 days or the same treatment once a week between 76–78 and 104–107 days of gestation. TJ protein expression was determined by Western immunoblot analysis on tissue harvested at 105–108 days of gestation. Blood-brain barrier permeability has been previously quantified with the blood-to-brain transfer constant (Ki) with α-aminoisobutyric acid (39). After a single course of dexamethasone, claudin-5 increased (P < 0.05) in the cerebral cortex, occludin and claudin-1 increased in the cerebellum, and occludin increased in the spinal cord. After multiple dexamethasone courses, occludin and zonula occludens (ZO)-1 increased in the cerebral cortex, and occludin and claudin-1 increased in the cerebellum. Junctional adhesion molecule-A and ZO-2 expressions did not change. Linear regression comparing Ki to TJ proteins showed inverse correlations with claudin-1 and claudin-5 in the cerebral cortex after a single course and ZO-2 in the spinal cord after multiple courses and direct correlations with ZO-1 in the cerebellum and spinal cord after multiple courses. We conclude that maternal glucocorticoid treatment increases the expression of specific TJ proteins in vivo, patterns of TJ protein expression vary after exposure to single and multiple glucocorticoid courses, and decreases in blood-brain barrier permeability are associated with increases in claudin-1, claudin-5, and ZO-2 expression and decreases in ZO-1 expression. In utero glucocorticoid exposure alters the molecular composition of the barrier and affects fetal blood-brain barrier function.
Keywords: blood-brain barrier, dexamethasone, steroids
the blood-brain barrier is composed of a continuous layer of cerebrovascular endothelial cells connected by intercellular tight junctions (TJs) (4). TJs are composed of transmembrane and associated cytoplasmic proteins (22). The transmembrane proteins include occludin, claudins, and junctional adhesion molecules (JAMs). The proteins of the claudin family form the primary seal between adjacent endothelial cells, whereas occludin is an important support molecule that increases electrical resistance across the barrier and decreases paracellular permeability (22). JAMs regulate TJ formation and leukocyte and endothelial cell interactions (13). The associated cytoplasmic proteins zonula occuldens (ZO)-1 and ZO-2 stabilize TJs by connecting them to actin (22).
Maternally administered glucocorticoids have been widely used to accelerate fetal maturation (9, 12, 30, 34). The first Consensus Conference on the effects of glucocorticoids on fetal maturation recommended the use of glucocorticoids for women at risk of premature delivery (34). More recently, observations suggesting that the effects of glucocorticoids might not be sustained raised questions whether repeated courses would be more effective than a single course in women who remained at risk of premature delivery (10, 42). The second National Institutes of Health Consensus Panel recommended that weekly glucocorticoid courses should be reserved for controlled trial studies until the results of such studies were available (33). The panel also suggested that animal studies were needed to examine the pathophysiological effects of the potential risks and benefits of multiple courses of glucocorticoids on the fetus (33). Recent trials comparing repeated and single courses of glucocorticoids have demonstrated better short-term outcomes after repeated courses than single courses (11, 50). However, these trials raised concerns about lower birth weights (11, 50), smaller head circumferences (11), and the possibility of increases in the incidence cerebral palsy (49) after the repeated courses.
The ovine fetus has been widely used to investigate brain development (1, 19, 45). The neurodevelopment of the immature ovine brain is similar to that of premature infants with respect to the time course of neurogenesis, onset of cerebral sulcation, and detection of cortical auditory evoked potentials (1–3, 8). We have shown that a single course of maternal glucocorticoids reduces blood-brain barrier permeability in fetal sheep (44, 45) and that additional courses do not accentuate the decrease in permeability observed after treatment with a single course of glucocorticoids (39).
Glucocorticoids decrease endothelial cell monolayer permeability for sucrose, increase transendothelial resistance (24), and increase the expression of TJ proteins in vitro (17, 36). Although there is currently insight into the molecular structure of endothelial TJs, much less is known about their regulation in physiological and pathophysiological conditions (26). Moreover, there are no studies examining the effects of glucocorticoid treatment on the expression of TJ proteins in vivo in the brain of the fetus except for our recent work (31).
Given these considerations, we tested the following hypotheses: 1) maternal glucocorticoid treatment increases in vivo TJ protein expression in the preterm fetal brain, 2) the patterns of TJ protein expression are similar after exposure to single and multiple courses of maternal glucocorticoids, and 3) decreases in blood-brain barrier permeability correlate with increases in the expression of TJ proteins in the immature fetal brain. In addition, we examined the relative differences in the expression of TJ proteins in the cerebral cortex, cerebellum, and cervical spinal cord of the fetal brain under basal conditions.
MATERIALS AND METHODS
This study was conducted after approval by the Institutional Animal Care and Use Committees of The Warren Alpert Medical School of Brown University and Women & Infants' Hospital of Rhode Island and according to the National Institutes of Health Guidelines for the use of experimental animals.
The brain tissue samples for this study were obtained from animals enrolled in a series of studies to examine the effects of antenatal glucocorticoids on blood-brain barrier permeability, brain water content, placental 11β-hydroxysteroid dehydrogenase type 2 expression, and myosin isoform expression and contractile dynamics in fetal carotid arteries (20, 27, 39, 46). We have included the blood-brain transfer constants (Ki) that we (39) previously determined to correlate changes in barrier permeability with changes in TJ protein expression in the present study.
Animal preparation and experimental design.
All of the surgical and experimental procedures were performed for our blood-brain barrier permeability study (39). Details of the surgical preparation have been provided in our previous publications (39, 43). Briefly, surgery was performed under 1–2% halothane anesthesia on 29 mixed breed pregnant ewes at 99–101 days of gestation. Polyvinyl catheters were placed into a brachial vein and brachial artery of the fetuses.
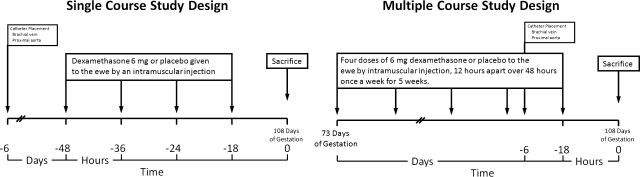
Ewes were assigned to one of four treatment groups: a single course of placebo (n = 6) or dexamethasone (n = 7) or five repeated courses of placebo (n = 7) or dexamethasone (n = 9). After recovery from surgery in the single course groups, ewes were given either four 6-mg intramuscular injections of dexamethasone (Fujsawa USA, Deerfield, IL; concentration = 4 mg/ml) or placebo (0.9% NaCl, 1.5 ml) every 12 h over 48 h. In the multiple course groups, ewes were given the same treatment once a week for 5 consecutive weeks starting at 76–78 days of gestation, as previously described (27, 39). After the ewes received the fourth course of glucocorticoids, the fetuses and ewes were catheterized at 99–101 days of gestation, which was comparable to the timing of surgery in the fetuses of the ewes that received the single course. The fifth and last course was started on the fourth day of recovery from surgery at 104–107 days of gestation (full term gestation: 145–150 days). The study designs for the single and multiple course treatment regimens are shown in Fig. 1.
Fig. 1.
Diagram summarizing the single and multiple course study designs, catheterization, and timing of the experiments in this study.
Eighteen hours after the last dose of dexamethasone or placebo had been given to the ewe at 105–108 days (70%) of gestation, the cerebral cortex, cerebellum, and cervical spinal cord were obtained from the fetuses and snap frozen in liquid nitrogen. These brain regions were selected because they represent the major regions of the rostral and hindbrain, which exhibit different patterns of maturation (2). Furthermore, we (43) have previously shown that there is heterogeneity in the barrier permeability such that permeability is higher in the cerebellum and cervical spinal cord than in the cerebral cortex during development. Samples were stored at −80°C until the time of analysis.
Ki.
Ki values were measured as described in our previous studies (39, 43). Briefly, blood-brain barrier function was measured in the fetuses with [14C]α-aminoisobutyric acid (Dupont-New England Nuclear, Boston, MA). α-Aminoisobutyric acid is a synthetic small amino acid that can cross the blood-brain barrier and is not present in normal mammalian tissues. Brain vascular volume was determined by intravenous [3H]sucrose administration to the fetus just before the end of the experiment. All samples were corrected for background, sample spillover, and quenching. Previously reported Ki values from the cerebral cortex, cerebellum, and cervical spinal cord were used so that the Ki values that were measured in vivo could be compared with the expression of the TJ proteins in the same fetal sheep.
Protein extraction.
Tissues from the cerebral cortex, cerebellum, and cervical spinal cord were prepared as follows. The cell membrane fraction was extracted in Triton X-100-deoxycholate-SDS (100 mM NaCl, 1% Triton X-100, 0.5 sodium deoxycholate, 0.2% SDS, 2 mM EDTA, and 1 mM benzamidine) with 1% complete protease inhibitor cocktail (Sigma, St. Louis, MO) for occludin, claudin-1, claudin-5, and JAM-A analysis. The cytosolic cell fraction for ZO-1 and ZO-2 was extracted in urea buffer [6 M urea, 150 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 10 mM Tris (pH 8.0), and 1% Triton X-100] with 1% complete protease inhibitor cocktail. The total protein concentrations of the homogenates were determined with a BCA protein assay (Pierce, Rockford, IL). Aliquots of the extracted samples were stored at −80°C.
Western immunoblot detection and quantification of proteins.
Tissue samples were extracted and protein concentrations determined as described above. Fifty micrograms of total protein per well were fractionated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (0.2 μm, Bio-Rad Laboratories, Hercules, CA) using a semidry technique. Membranes were blocked with 10% nonfat milk for 1 h at room temperature and then washed in Tris-buffered saline with 0.1% Tween 20 (TBST) three times for 10 min per wash. Membranes were probed for occludin (catalog no. 33-1500, Zymed, San Francisco, CA), claudin-5 (catalog no. 35-2500, Zymed), and ZO-1 (catalog no. 33-9100, Zymed) with primary mouse monoclonal antibodies and for claudin-1 and ZO-2 with rabbit polyclonal antibodies (claudin-1: catalog no. 51-9000, Zymed; and ZO-2: catalog no. 71-1400, Zymed) at a dilution of 1:5,000. Immunoblots were incubated in primary antibody overnight at 4°C. Membranes were probed for JAM-A protein with rabbit polyclonal antibody (catalog no. 36-1700, Zymed) at a dilution of 1:10,000 for 1 h at room temperature. After incubation with the primary antibodies, immunoblots were washed in TBST three times for 10 min per wash and then incubated for 1 h at room temperature with goat anti-mouse (Zymed) or goat anti-rabbit (Alpha Diagnostic, San Antonio, TX) horseradish peroxidase-conjugated secondary antibodies at a dilution of 1:10,000 for all of the proteins that we examined. Immunoblots were again washed in TBST four times for 10 min per wash. Binding of the secondary antibody was detected with enhanced chemiluminescence (ECL plus, Western Blotting Detection reagents, Amersham Pharmacia Biotech, Piscataway, NJ) before an exposure to autoradiography film (Daigger, Vernon Hills, IL).
All experimental samples were normalized to a respective protein obtained from the same brain region of nonstudy fetal sheep at 70% of gestation. For example, the experimental cerebral cortical occludin samples were normalized to occludin protein extracted from the cerebral cortex of a single fetal sheep that had not exposed to the study interventions. For the purpose of this report, we refer to these samples as internal control samples.
As we have previously described (28, 31, 37, 40), these samples served as an internal control for the quality of loading, transfer of the samples, normalization of the cerebral cortical, cerebellar, and cervical spinal cord densitometric values, and to permit accurate comparisons among the different immunoblots (28, 31, 37). The same internal control sample was used for each specific TJ protein on all immunoblots. The experimental TJ protein autoradiographic densitometric values were expressed as a ratio to the internal control, thus facilitating normalized comparisons among the groups.
Each immunoblot included samples from the four treatment groups and three internal control samples. The internal control samples were included in three lanes as the first, middle, and last samples on each immunoblot. We calculated a coefficient of variation for the internal control samples on each immunoblot. The values for the experimental samples were accepted as valid only if the percent coefficient of variation for the internal control samples was <20% on the immunoblot. Molecular weight standards (Bio-Rad Laboratories) were included in each immunoblot. Uniformity in interlane loading was also established by Coomassie blue (Sigma) staining of the polyacrylamide gels, and uniformity of transfer to the polyvinylidene difluoride membranes was confirmed by Ponceau S staining (Sigma) (47).
As previously described (31), Madin-Darby canine kidney cell lysate (Canine Kidney Carcinoma, BD Biosciences, San Diego, CA) was used as a positive control for occludin, claudin-1, ZO-1, and ZO-2; the rat lung was used for claudin-5; and human colorectal adenocarcinoma cells (Caco-2, ProSci, Poway, CA) were used for JAM-A. Positive controls were used on the same immunoblot with the sheep brain samples to establish with certainty that the proteins that were identified by these antibodies in the sheep brain were the same as the known protein for these specific antibodies. Detection of the occludin, claudin-1, claudin-5, JAM-A, ZO-1, and ZO-2 bands at 65, 23, 23, 35, 225, and 160 kDa, respectively, was dependent on incubation with the primary antibody, the omission of which resulted in the absence of this signal.
We also sought to quantify the relative differences in TJ protein expression among the cerebral cortical, cerebellar, and cervical spinal cord brain regions. To evaluate the basal differences in protein expression among these brain regions, we examined the expression of each protein from the fetuses of ewes treated with the single course of placebo. In this segment of our study, each protein was normalized to an internal control sample obtained from the cerebral cortex of a single nonstudy fetal sheep to enable comparisons among the three brain regions to one standard on the same immunoblot.
Densitometric analysis.
Band intensities were analyzed with a Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD). All experimental samples were normalized to the respective internal control samples. We used the average of the three internal control values on each immunoblot to normalize the experimental values. The final values represented an average of the densitometry values obtained from the different immunoblots and are presented as a ratio to the internal control sample. We (28) have previously shown that this method correlates well with values that have been normalized as ratios to β-actin.
Statistical analysis.
All results are expressed as means ± SD. Two-way ANOVA was used to compare values among fetuses of ewes treated with single or multiple dexamethasone or placebo courses, where the factors were treatment (dexamethasone or placebo) and course frequency (single or multiple). If a significant difference was found by ANOVA, the Fischer least-significant-difference test was used to detect specific differences among the fetuses of ewes treated with dexamethasone or placebo and the fetuses of single and multiple course treatment regimens. One-way ANOVA was also used to detect differences among the three brain regions for each TJ protein examined. If a significant difference was found by ANOVA, the Fischer least-significant-difference test was used to detect specific differences among the brain regions. Least-squares linear regression analysis was used to compare the previously measured Ki values in the cerebral cortex, cerebellum, and cervical spinal cord, as the dependent variable, to the expression of each TJ protein in the same brain region, as the independent variable. P values of <0.05 were considered statistically significant.
RESULTS
The fetal sheep from which the regional brain samples were obtained for this study had pH, blood gas, and mean arterial blood pressure values within the physiological range for our laboratory and similar to values reported by others (48).
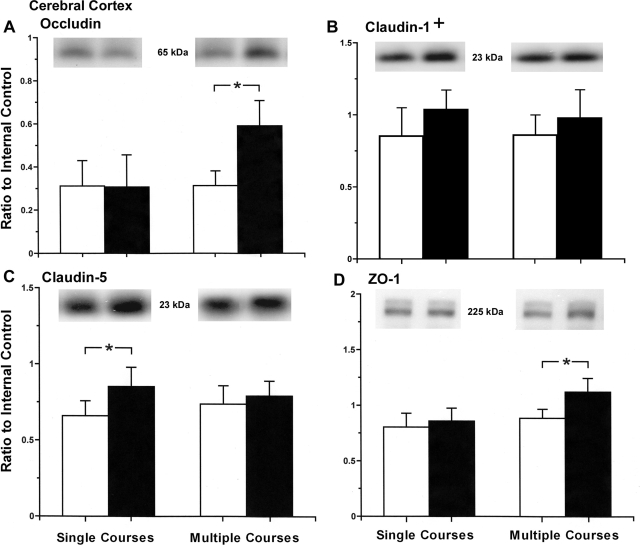
Maternal treatment with multiple courses of dexamethasone was associated with increases in occludin protein expression in the cerebral cortex, but a single course of dexamethasone did not affect occludin in the cerebral cortex (ANOVA, main effect for dexamethasone vs. placebo treatment, F = 9.39, P < 0.01; ANOVA, main effect for course frequency, single vs. multiple courses, F = 10.3, P < 0.01; Fig. 2A). There was an overall effect of maternal dexamethasone treatment on claudin-1 expression in the cerebral cortex of the fetal sheep (ANOVA, main effect for dexamethasone vs. placebo treatment, F = 5.19, P < 0.05; ANOVA, main effect for course frequency, F = 0.45, P = 0.51; Fig. 2B). However, specific significant differences were not detected (P > 0.05) by post hoc analyses among the fetuses of dexamethasone- and placebo-treated ewes in the single or multiple course treatment groups. Maternal treatment with a single course of dexamethasone was associated with increases in claudin-5 protein expression in the cerebral cortex, but treatment with multiple courses of dexamethasone did not affect claudin-5 in the fetus (ANOVA, main effect for dexamethasone vs. placebo treatment, F = 9.45, P < 0.01; ANOVA, main effect for course frequency, F = 0.06, P = 0.81; Fig. 2C). Maternal treatment with multiple courses of dexamethasone was associated with increases in ZO-1 protein expression in the cerebral cortex, but maternal treatment with a single course of dexamethasone did not affect ZO-1 expression in the fetus (ANOVA, main effect for dexamethasone vs. placebo treatment, F = 9.38, P < 0.01; ANOVA, main effect for course frequency, F = 13.02, P < 0.01; Fig. 2D). Maternal treatment with single and multiple courses of dexamethasone was not associated with changes in JAM-A or ZO-2 expression in the cerebral cortex of the fetal sheep (JAM-A: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 1.0, P = 0.33; ANOVA, main effect for course frequency, F = 0.83, P = 0.37; and ZO-2: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 0.85, P = 0.36; ANOVA, main effect for course frequency, F = 0.10, P = 0.76).
Fig. 2.
Tight junction protein expression in the cerebral cortex of ovine fetuses at 70% gestation of ewes exposed to single and multiple courses of placebo and dexamethasone. Top: representative Western immunoblots; bottom: bar graphs. A: occludin expression plotted as a ratio to the internal control for fetuses of single course placebo-treated ewes (n = 6), single course dexamethasone-treated ewes (n = 5), multiple course placebo-treated ewes (n = 5), and multiple course dexamethasone-treated ewes (n = 7) (ANOVA, main effect for treatment, F = 9.39, P < 0.01). B: claudin-1 expression for fetuses of single course placebo-treated ewes (n = 7), single course dexamethasone-treated ewes (n = 6), multiple course placebo-treated ewes (n = 6), and multiple course dexamethasone-treated ewes (n = 9) (+ANOVA, main effect for treatment, F = 5.125, P < 0.03). C: claudin-5 expression for fetuses of single course placebo-treated ewes (n = 6), single course dexamethasone-treated ewes (n = 7), multiple course placebo-treated ewes (n = 7), and multiple course dexamethasone-treated ewes (n = 9) (ANOVA, main effect for treatment, F = 9.45, P < 0.01). D: zonula occludens (ZO)-1 for fetuses of single course placebo-treated ewes (n = 5), single course dexamethasone-treated ewes (n = 7), multiple course placebo-treated ewes (n = 7), and multiple course dexamethasone-treated ewes (n = 8) (ANOVA, main effect for treatment, F = 9.38, P < 0.01). Open bars represent fetuses of placebo-treated ewes; closed bars represent fetuses of dexamethasone-treated ewes. Values are means ± SD. *P < 0.05.
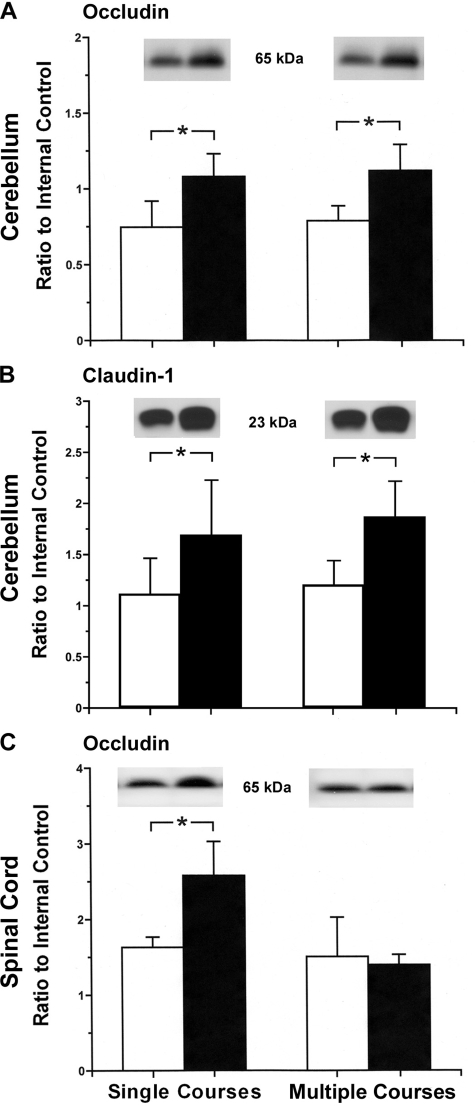
Maternal treatment with single and multiple courses of dexamethasone were associated with increases in occludin and claudin-1 protein expression in the cerebellum of the fetal sheep [occludin: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 27.54, P < 0.01; ANOVA, main effect for course frequency, F = 0.40, P = 0.54 (Fig. 3A); claudin-1: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 14.43, P < 0.01; ANOVA, main effect for course frequency, single vs. multiple courses, F = 0.67, P = 0.42 (Fig. 3B)]. Maternal treatment with single and multiple courses of dexamethasone was not associated with changes in claudin-5, JAM-A, ZO-1, or ZO-2 expression in the cerebellum (claudin-5: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 0.01, P = 0.99; ANOVA, main effect for course frequency, F = 0.28, P = 0.60; JAM-A: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 3.13, P = 0.09; ANOVA, main effect for course frequency, F = 1.35, P = 0.26; ZO-1: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 2.30, P = 0.14; ANOVA, main effect for course frequency, F = 0.01, P = 0.92; and ZO-2: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 0.92, P = 0.35; ANOVA, main effect for course frequency, F = 0.06, P = 0.80).
Fig. 3.
Tight junction protein expression in the cerebellum and cervical spinal cord of ovine fetuses at 70% gestation of ewes exposed to single and multiple courses of placebo and dexamethasone. Top: representative Western immunoblots; bottom: bar graphs. A: occludin expression in the cerebellum of fetuses of single course placebo-treated ewes (n = 6), single course dexamethasone-treated ewes (n = 6), multiple course placebo-treated ewes (n = 6), and multiple course dexamethasone-treated ewes (n = 6) (ANOVA, main effect for treatment, F = 27.54, P < 0.01). B: claudin-1 expression in the cerebellum of fetuses of single course placebo-treated ewes (n = 5), single course dexamethasone-treated ewes (n = 6), multiple course placebo-treated ewes (n = 5), and multiple course dexamethasone-treated ewes (n = 6) (ANOVA, main effect for treatment, F = 11.46, P < 0.01). C: occludin expression in the spinal cord of fetuses of single course placebo-treated ewes (n = 6), single course dexamethasone-treated ewes (n = 6), multiple course placebo-treated ewes (n = 6), and multiple course dexamethasone-treated ewes (n = 6) (ANOVA, main effect for treatment, F = 7.82, P < 0.01). Open bars represent fetuses of placebo-treated ewes; closed bars represent fetuses of dexamethasone-treated ewes. Values are means ± SD. *P < 0.05.
Maternal treatment with a single course of dexamethasone was associated with increases in occludin protein expression in the fetal cervical spinal cord, but treatment with multiple courses of dexamethasone did not affect occludin expression (ANOVA, main effect for dexamethasone vs. placebo treatment, F = 7.83, P < 0.05; ANOVA, main effect for the frequency of the treatment courses, e.g., single or multiple treatment courses, F = 19.11, P < 0.001; Fig. 3C). Maternal treatment with single and multiple courses of dexamethasone was not associated with changes in claudin-1, claudin-5, JAM-A, ZO-1, or ZO-2 expression in the cervical spinal cord of the fetal sheep (claudin-1: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 1.01, P = 0.33; ANOVA, main effect for course frequency, F = 3.80, P = 0.07; claudin-5: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 0.34, P = 0.56; ANOVA, main effect for course frequency, F = 3.31, P = 0.08; JAM-A: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 3.38, P = 0.08; ANOVA, main effect for course frequency, F = 2.03, P = 0.16; ZO-1: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 0.45, P = 0.51; ANOVA, main effect for course frequency, F = 0.0, P = 0.98; and ZO-2: ANOVA, main effect for dexamethasone vs. placebo treatment, F = 0.05, P = 0.82; ANOVA, main effect for course frequency, F = 0.30, P = 0.59).
Table 1 schematically summarizes the changes in TJ protein expression in the fetuses of ewes exposed to the single and multiple courses of dexamethasone compared with those of placebo-treated ewes.
Table 1.
Tight junction protein expression in fetuses of dexamethasone-treated ewes compared with placebo-treated ewes
| Cerebral Cortex |
Cerebellum |
Cervical Spinal Cord |
||||
|---|---|---|---|---|---|---|
| Tight Junction Protein | Single Course | Multiple Courses | Single Course | Multiple Courses | Single Course | Multiple Courses |
| Occludin | ↔ | ↑ | ↑ | ↑ | ↑ | ↔ |
| Claudin-1 | ↑ | ↑ | ↑ | ↑ | ↔ | ↔ |
| Claudin-5 | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ |
| JAM-A | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| ZO-1 | ↔ | ↑ | ↔ | ↔ | ↔ | ↔ |
| ZO-2 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
JAM, junctional adhesion molecule; ZO, zonula occludens. ↔, No change compared with fetuses of placebo-treated ewes from the same treatment course; ↑, increase compared with fetuses of placebo-treated ewes from the same treatment course.
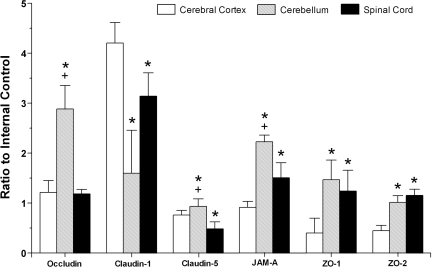
We also examined the relative expression of each specific TJ protein in the cerebral cortex, cerebellum, and cervical spinal cord in fetuses of ewes exposed to the single course of placebo to determine if there were any differences in TJ protein expressions among these brain regions under basal conditions (Fig. 4). Occludin expression was higher in the cerebellum than in the cerebral cortex and cervical spinal cord (ANOVA, main effects, F = 59.47, P < 0.01). In contrast, claudin-1 expression was lower in the cerebellum and cervical spinal cord than in the cerebral cortex (ANOVA, main effects, F = 27.51, P < 0.01). Claudin-5 expression was higher in the cerebellum than in the cerebral cortex and the cervical spinal cord and higher in the cerebral cortex than in the cervical spinal cord (ANOVA, main effects, F = 18.15, P < 0.01). Expression of JAM-A was higher in the cerebellum than in the cerebral cortex and the cervical spinal and higher in the cervical spinal cord than in the cerebral cortex (ANOVA, main effects, F = 62.83, P < 0.01). Expressions of ZO-1 and ZO-2 were higher in the cerebellum and the cervical spinal cord than in the cerebral cortex (ZO-1: ANOVA, main effects, F = 11.84, P < 0.01; and ZO-2: ANOVA, main effects, F = 50.83, P < 0.01).
Fig. 4.
Bar graph representing tight junction protein expression in the cerebral cortex, cerebellum, and spinal cord of fetuses at 70% gestation of ewes exposed to the single course placebo treatment for occludin (ANOVA, main effect for brain regions, F = 59.47, P < 0.01), claudin-1 (ANOVA, main effect for brain regions, F = 27.51, P < 0.01), claudin-5 (ANOVA, main effect for brain regions, F = 18.15, P < 0.01), junctional adhesion molecule (JAM)-A (ANOVA, main effect for brain regions, F = 62.83, P < 0.01), ZO-1 (ANOVA, main effect for brain regions, F = 11.84, P < 0.01), and ZO-2 (ANOVA, main effect for brain regions, F = 50.83, P < 0.01). *P < 0.05 vs. the cerebrum; +P < 0.05 vs. the spinal cord.
The results of the least-squares linear regression analyses comparing blood-brain barrier permeability measured with α-aminoisobutyric acid (Ki; in μl·g brain−1·min−1) to the TJ proteins expressed as the ratio to the internal control protein in the cerebral cortex, cerebellum, and cervical spinal cord in the fetuses of ewes treated with single and multiple courses of placebo and dexamethasone are shown in Table 2. Ki values demonstrated inverse linear correlations with claudin-1 and claudin-5 expressions in the cerebral cortex of the fetal sheep after maternal treatment with single courses of dexamethasone and placebo but not with occludin, JAM-A, ZO-1, or ZO-2. Ki values did not demonstrate correlations with occludin, claudin-1, claudin-5, JAM-A, ZO-1, or ZO-2 in the cerebral cortex of the fetal sheep after maternal treatment with multiple courses of dexamethasone and placebo. Ki values did not demonstrate correlations with occludin, claudin-1, claudin-5, JAM-A, ZO-1, or ZO-2 protein expressions in the cerebellum of the fetal sheep after maternal treatment with single courses of dexamethasone and placebo. Ki values demonstrated a direct linear correlation with ZO-1 but did not show correlations with occludin, claudin-1, claudin-5, JAM-A, or ZO-2 expressions in the cerebellum of the fetal sheep after maternal treatment with multiple courses of dexamethasone and placebo.
Table 2.
Least-squares linear regression analyses comparing blood-brain barrier permeability measured with α-aminoisobutyric acid with tight junction proteins expressed as the ratio to the internal control protein in the cerebral cortex, cerebellum, and cervical spinal cord in fetuses of ewes treated with single and multiple courses of placebo and dexamethasone
| Cerebral Cortex |
Cerebellum | Cervical Spinal Cord |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single Course |
Multiple Courses |
Single Course |
Multiple Courses |
Single Course |
Multiple Courses |
|||||||||||||
| Tight Junction Protein | r | n | P value | r | n | P value | r | n | P value | r | n | P value | r | n | P value | r | n | P value |
| Occludin | −0.11 | 12 | 0.73 | −0.07 | 14 | 0.81 | 0.23 | 11 | 0.50 | −0.02 | 11 | 0.95 | −0.36 | 11 | 0.27 | 0.02 | 12 | 0.95 |
| Claudin-1 | −0.73* | 12 | 0.01* | −0.04 | 15 | 0.90 | 0.30 | 11 | 0.36 | −0.10 | 11 | 0.78 | −0.46 | 11 | 0.15 | 0.07 | 12 | 0.83 |
| Claudin-5 | −0.65* | 12 | 0.02* | 0.03 | 16 | 0.91 | 0.18 | 11 | 0.59 | −0.03 | 11 | 0.92 | −0.17 | 11 | 0.62 | 0.07 | 12 | 0.82 |
| JAM-A | −0.06 | 10 | 0.87 | −0.06 | 10 | 0.87 | −0.62 | 10 | 0.06 | 0.20 | 11 | 0.56 | −0.26 | 11 | 0.44 | 0.12 | 12 | 0.72 |
| ZO-1 | 0.10 | 11 | 0.77 | −0.30 | 15 | 0.30 | −0.19 | 11 | 0.58 | 0.73 | 11 | 0.01 | 0.37 | 11 | 0.26 | 0.66* | 11 | 0.03* |
| ZO-2 | −0.40 | 11 | 0.22 | 0.06 | 15 | 0.82 | −0.29 | 11 | 0.38 | −0.46 | 11 | 0.16 | −0.22 | 11 | 0.51 | −0.61* | 11 | 0.04* |
Significant correlation.
Ki values did not demonstrate correlations with occludin, claudin-1, claudin-5, JAM-A, ZO-1, or ZO-2 protein expressions in the cervical spinal cord of the fetal sheep after maternal treatment with single courses of dexamethasone and placebo. Ki values demonstrated a direct linear correlation with ZO-1, an inverse correlation with ZO-2, and did not show correlations with occludin, claudin-1, claudin-5, or JAM-A protein expressions in the cervical spinal cord after maternal treatment with multiple courses of dexamethasone and placebo.
DISCUSSION
The purpose of our study was to examine the effects of single and multiple courses of maternally administered glucocorticoids in doses similar to those used in the clinical setting on TJ protein expression in brain regions of midgestation fetal sheep. The neurodevelopment of the sheep fetus at 70% gestation is comparable with that of the preterm human between ∼25 and 29 wk of gestation (1). The novel findings of our study are as follows. First, maternal treatment with glucocorticoids is associated with increases in select TJ proteins in vivo in the brain of fetal sheep. Second, the responses of specific TJ proteins in the fetal brain vary with respect to maternal exposure to single and multiple course glucocorticoid regimens. Third, there are marked differences in the relative expression of TJ proteins among brain regions in the fetal sheep brain under basal conditions. Fourth, the response of specific TJ proteins varies among the different brain regions as a function of the two maternal glucocorticoid regimens. Finally, changes in blood-brain barrier permeability measured with α-aminoisobutyric acid (Ki) correlate with changes in the expression of some of the TJ proteins.
We (39, 44, 45) have shown that maternal treatment with glucocorticoids reduces blood-brain barrier permeability in the ovine fetus. Furthermore, maternal treatment with glucocorticoids at 70% gestation results in decreases in barrier permeability in fetuses of ewes after both single and multiple courses of glucocorticoids, but treatment with multiple courses does not accentuate the decreases in permeability observed after the single course of treatment (39). The site of action of glucocorticoids on the blood-brain barrier in the fetus (39, 44, 45) is most likely on the capillary endothelial cells because physiological hydrocortisone concentrations have been shown to increase transendothelial resistance and decrease permeability in cerebral capillary endothelial cells (24). The main structures responsible for the action of glucocorticoids are most likely the intercellular TJs of the cerebrovascular endothelium in the fetal blood-brain barrier because glucocorticoids in vitro upregulate the endothelial cell expression of several TJ proteins (16, 17, 29, 36). Dexamethasone upregulates occludin and ZO-1 in a rat brain immortalized endothelial cell line (36); hydrocortisone upregulates occludin in a murine immortalized cerebral capillary endothelial cell line (17), occludin and claudin-5 (but not claudin-1) in a human immortalized model of the blood-brain barrier (16), and occludin, claudin-5, and ZO-1 in primary cultures of porcine brain capillary endothelial cells (29). In addition, glucocorticoid-related increases in TJ protein expression are associated with increases in transendothelial electrical resistance and decreases in paracellular permeability in vitro (16, 17, 29, 36). Furthermore, in vivo treatment of adult mice with dexamethasone for 4 days resulted in increased occludin immunostaining that was specific to the brain endothelium (18). Even though glucocorticoids are widely used to treat women at risk for premature labor (34), there have been no previous studies examining the influence of this maternal treatment on the regulation of TJ proteins in vivo in the brain of the fetus except for our recent work (31). Consistent with previous studies (16, 17, 29, 36), we observed that maternal treatment with a single course of glucocorticoids increased occludin expression in the cerebellum and cervical spinal cord, claudin-1 expression in the cerebellum, and claudin-5 expression in the cerebral cortex, and multiple courses increased occludin expression in the cerebral cortex and cerebellum, claudin-1 expression in the cerebellum, and ZO-1 expression in the cerebral cortex of fetal sheep at 70% gestation. The increases in claudin-1 that we observed have not been previously reported after exposure of the brain endothelium to glucocorticoids (16, 17, 29, 36). Similar to findings in a model of the human blood-brain barrier (16), increases in TJ proteins in the fetal brain also suggest that TJs are molecular targets of glucocorticoids in the fetus after treatment of the mother.
In contrast to findings in the present study, we (31) have previously found decreases in the expression of the upper band of ZO-1 and that claudin-5 expression was not increased in the cerebral cortex of the fetal sheep at 80% gestation after maternal treatment with a single course of the same glucocorticoid treatment. We are uncertain of the reasons for the apparent discrepancies in the response to maternal glucocorticoids between this and our former work; however, in the present study, we detected one band for ZO-1 and the fetal sheep were examined at an earlier time in gestation than in our previous report (31). It is also important to point out that we used the same antibodies to ZO-1 and claudin-5 in this and our former study. Therefore, the apparent discrepancies in the response to maternal glucocorticoids between the two studies were not the result of the use of different antibodies for ZO-1 and claudin-5 between the studies.
In the contrast to our original hypothesis, the responses of the TJ proteins in the fetal brain varied as a function of maternal exposure to single and multiple courses of glucocorticoids. With regard to the two maternal treatment regimens, an important consideration is that the fetuses of ewes treated with multiple courses were exposed to glucocorticoids both beginning at a much more immature stage of development and over a more prolonged time interval than those exposed to single courses. Therefore, the different responses of the TJ proteins to glucocorticoid exposure after single and multiple courses could be related to exposure at an earlier time in gestation (hence, a time when the brain was more immature) (2) and also to a longer duration of exposure. We anticipated that changes in the TJ protein expression would have been similar after maternal treatment with both single and multiple courses of glucocorticoids as the effects of these treatments on barrier permeability were similar (39). However, increases in TJ protein expression were expected after both courses as barrier permeability decreased after both treatments (39), and changes in TJ protein expression have been reported in conjunction with in vivo changes in barrier permeability (26). Although it would also have been of interest to examine the fetal sheep after a single course of maternal treatment at a time in gestation that was commensurate with the onset of the studies in the multiple course treatment regimen, studies of fetal sheep at this very early time in gestation are not feasible.
We observed major differences in the expression patterns of the transmembrane and associated cytoplasmic proteins among the cerebral cortex, cerebellum, and cervical spinal cord in the fetal brain under basal conditions. The overall distribution patterns of occludin, claudin-5, and JAM-A were somewhat similar, as the expression of these proteins appeared higher in the cerebellum than in the other brain regions examined. In contrast, claudin-1 expression was highest in the cerebral cortex. The patterns for ZO-1 and ZO-2 were similar, as the expression appeared higher in the cerebellum and cervical spinal cord than in the cerebral cortex. To our knowledge there is very little information regarding relative differences in TJ protein expression among different brain regions under basal conditions. Hirakawa et al. (23) reported regional differences in blood-nerve barrier function and TJ protein expression within the dorsal route ganglion of the peripheral nervous system. Silwedel and Foerster (41) established immortalized cerebral and cerebellar murine microvascular endothelial cell lines and demonstrated that, under basal conditions, claudin-5 was higher in the cerebellar than cerebral cell line, whereas occludin expression was higher in the cerebral than in cerebellar cell line. Consistent with these findings, we found higher claudin-5 expression in the cerebellum than in the cerebral cortex but lower claudin-1 and higher occludin expression in the cerebellum than in the cerebral cortex. Therefore, our findings, together with these reports (23, 41), support the contention that there are differences in the expression of the major TJ proteins among brain regions under basal conditions in the normal brain.
The single and multiple maternal courses also had differential effects on specific TJ proteins among the different brain regions examined. The single courses increased both occludin and claudin-5 expression in the cerebellum but only claudin-5 in the cerebral cortex and only occludin in the spinal cord, whereas the multiple courses increased occludin and ZO-1 in the cerebral cortex and occludin and claudin-5 expression in the cerebellum but did not affect the spinal cord. Therefore, the cerebellum appears to be the region that was the most sensitive to maternal glucocorticoid treatment because both the single and multiple courses increased two of the major transmembrane proteins in this region. In this study, we examined a treatment that reinforces the barrier (29). On the other hand, Silwedel and Foerster (41) examined the effects of an inflammatory agent that disrupts barrier function. They found that TNF-α treatment had differential effects on endothelia from the cerebrum and cerebellum because the barrier function was affected to a greater extent in the cerebellar than cerebral endothelium, and TNF-α treatment resulted in decreases in claudin-1, claudin-3, claudin-5, and occludin mRNA levels in the cerebellar endothelium (41). Brooks et al. (5) showed that inflammatory pain is associated with regionally specific molecular and functional regulation of the blood-brain barrier. Hence, taken together with our findings, it appears that treatments that reinforce (29, 39, 44, 45) or damage the barrier (5, 41) have differential effects on the barrier endothelium in different brain regions. Nonetheless, it should be pointed out that in our former work (39) we did not find statistically significant reductions in the barrier permeability of the specific brain regions in which we measured the TJ protein expression; rather, we found an overall decrease in permeability after both single and multiple courses of glucocorticoids.
We examined the correlations between changes in blood-brain barrier permeability measured with α-aminoisobutyric acid (Ki) and the changes in the expression of the TJ proteins in the fetal sheep after maternal treatment with single and multiple courses of the placebo and dexamethasone. After single courses, decreases in barrier permeability were associated with increases in the amounts of claudin-1 and claudin-5 but not occludin. These findings would tend to support the contention that the increases in these TJ proteins in the fetal brain were of functional significance. These findings are of particular importance as claudins are thought to form the primary “seal” of the TJ, whereas occludin acts as an additional support structure (22). Our findings in the ovine fetus also support the concept that this commonly used maternal treatment (34) alters both the functional and molecular characteristics of the fetal blood-brain barrier (16, 29, 39).
On the other hand, after multiple maternal courses of placebo and dexamethasone, there was a direct linear correlation between barrier permeability and increases in ZO-1 protein expression in both the cerebellum and cervical spinal cord. Although these findings are counterintuitive to the findings that would have been expected, e.g., decreases in barrier permeability would correlate with higher ZO-1 protein expression, these findings are consistent with changes in barrier permeability and TJ protein expression previously reported in a model of inflammatory pain (25, 26). In the studies by Huber et al. (25, 26), inflammatory pain resulted in increases in barrier permeability, decreases in occludin, and increases in ZO-1 expression. Although we cannot be certain of the reason for these changes, as previously suggested, the increase in ZO-1 expression after maternal treatment with multiple courses of glucocorticoids could relate to transcriptional increases in ZO-1 and/or a change in the phosphorylation of ZO-1 (16–18, 26, 29). The latter mechanism could be a compensatory mechanism by which the cell attempts to recruit more TJ proteins from the cytoplasm to the plasma membrane (26). Although we did not find an increase in ZO-2 expression after maternal treatment with glucocorticoids, contrary to findings with ZO-1, as expected decreases in barrier permeability in the cervical spinal cord were associated with increases in ZO-2 expression.
In this study, we measured the expression of TJ proteins but did not report on their immunohistochemical localization in the endothelium as we had limited frozen tissue. The localization of the TJ proteins within the endothelium is an important factor that also influences the permeability after treatment with glucocorticoids (7, 35). Therefore, although decreases in barrier permeability showed correlations with increases in the expression of some of the TJ proteins, localization of the TJ proteins to periphery of cell borders also contributes to increases in transendothelial electrical resistances and decreases in permeability (7, 35).
The molecular basis of TJ protein regulation by maternal glucocorticoid treatment cannot be determined by our experiments. However, glucocorticoids have been reported to reinforce the barrier via signal transduction pathways originating from stimulation of the endothelial intracellular glucocorticoid receptor in the brain microvasculature, which acts as a responsive element controlling the expression of glucocorticoid target genes and their corresponding TJ proteins (6, 14–18, 21, 29). Glucocorticoids have been shown to regulate specific response elements on the promoters of both occludin and claudin-5 genes (6, 21). Therefore, it remains probable that similar mechanisms were responsible for the increased claudin-5 expression in the cerebral cortex and occludin in the cerebellum and cervical spinal cord in the fetal brain after maternal glucocorticoid treatment, as glucocorticoid receptors have been identified by 70% gestation in the fetal sheep brain (38).
In summary, maternal treatment with glucocorticoids regulates the expression of molecular components of the TJ in the brain microvasculature of the fetus. TJ proteins exhibit differential expression in the cerebral cortex, cerebellum, and cervical spinal cord under basal conditions. The expression of TJ proteins vary as a function of different maternal treatment regimens. Reductions in blood-brain barrier permeability are associated with increases in claudin-1, claudin-5, and ZO-2 and with decreases in ZO-1 expression.
Perspectives
Maternal glucocorticoid therapy is widely used to treat pregnant women in premature labor. The relatively low-dose treatment regimen that was given to the ewes in our study was similar to that used in the clinical setting. Our findings may be interpreted to suggest that this extensively used treatment affects the functional (39) and molecular properties of the blood-brain barrier in the fetus. The findings that maternal glucocorticoids reduce blood-brain barrier permeability (39, 44, 45) and affect the molecular composition of the TJs in the brain microvasculature of the fetus suggests that this maternal treatment could improve the functional and molecular vascular integrity in the premature human fetus, thereby providing protection from brain injury such as intraventricular hemorrhage (32). Nonetheless, multiple courses of glucocorticoids could also potentially be detrimental to the developing brain (49). In addition, it is important to emphasize that our findings are the first to suggest that the maternal glucocorticoid treatment alters the molecular composition of the barrier in the fetus.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants R01-HD-34618 and 1-R01-HD-057100.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol 21: 582–589, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol 135: 249–262, 1969 [DOI] [PubMed] [Google Scholar]
- 3.Bernhard CG, Kolmodin GM, Meyerson BA. On the prenatal development of function and structure in the somesthetic cortex of the sheep. Prog Brain Res 26: 60–77, 1967 [DOI] [PubMed] [Google Scholar]
- 4.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 40: 648–677, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks TA, Nametz N, Charles R, Davis TP. Diclofenac attenuates the regional effect of lambda-carrageenan on blood-brain barrier function and cytoarchitecture. J Pharmacol Exp Ther 325: 665–673, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Burek M, Forster CY. Cloning and characterization of the murine claudin-5 promoter. Mol Cell Endocrinol 298: 19–24, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Colgan OC, Collins NT, Ferguson G, Murphy RP, Birney YA, Cahill PA, Cummins PM. Influence of basolateral condition on the regulation of brain microvascular endothelial tight junction properties and barrier function. Brain Res 1193: 84–92, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Cook CJ, Williams C, Gluckman PD. Brainstem auditory evoked potentials in the fetal sheep, in utero. J Dev Physiol 9: 429–439, 1987 [PubMed] [Google Scholar]
- 9.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol 173: 322–335, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database Syst Rev : CD003935, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet 367: 1913–1919, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Doyle LW, Kitchen WH, Ford GW, Rickards AL, Lissenden JV, Ryan MM. Effects of antenatal steroid therapy on mortality and morbidity in very low birth weight infants. J Pediatr 108: 287–292, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci 117: 19–29, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Felinski EA, Antonetti DA. Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Curr Eye Res 30: 949–957, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Felinski EA, Cox AE, Phillips BE, Antonetti DA. Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp Eye Res 86: 867–878, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forster C, Burek M, Romero IA, Weksler B, Couraud PO, Drenckhahn D. Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol 586: 1937–1949, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forster C, Silwedel C, Golenhofen N, Burek M, Kietz S, Mankertz J, Drenckhahn D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol 565: 475–486, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forster C, Waschke J, Burek M, Leers J, Drenckhahn D. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J Physiol 573: 413–425, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 99: 248–256, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hai CM, Sadowska G, Francois L, Stonestreet BS. Maternal dexamethasone treatment alters myosin isoform expression and contractile dynamics in fetal arteries. Am J Physiol Heart Circ Physiol 283: H1743–H1749, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Harke N, Leers J, Kietz S, Drenckhahn D, Forster C. Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Mol Cell Endocrinol 295: 39–47, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173–185, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Hirakawa H, Okajima S, Nagaoka T, Kubo T, Takamatsu T, Oyamada M. Regional differences in blood-nerve barrier function and tight-junction protein expression within the rat dorsal root ganglion. Neuroreport 15: 405–408, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, Galla HJ. Hydrocortisone reinforces the blood-brain properties in a serum free cell culture system. Biochem Biophys Res Commun 247: 312–315, 1998 [PubMed] [Google Scholar]
- 25.Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP. Blood-brain barrier tight junctions are altered during a 72-h exposure to λ-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol 283: H1531–H1537, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol 280: H1241–H1248, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Kerzner LS, Stonestreet BS, Wu KY, Sadowska G, Malee MP. Antenatal dexamethasone: effect on ovine placental 11β-hydroxysteroid dehydrogenase type 2 expression and fetal growth. Pediatr Res 52: 706–712, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kim CR, Sadowska GB, Petersson KH, Merino M, Sysyn GD, Padbury JF, Stonestreet BS. Effects of postnatal steroids on Na+/K+-ATPase activity and α1- and β1-subunit protein expression in the cerebral cortex and renal cortex of newborn lambs. Reprod Fertil Dev 18: 413–423, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kroll S, El-Gindi J, Thanabalasundaram G, Panpumthong P, Schrot S, Hartmann C, Galla HJ. Control of the blood-brain barrier by glucocorticoids and the cells of the neurovascular unit. Ann NY Acad Sci 1165: 228–239, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50: 515–525, 1972 [PubMed] [Google Scholar]
- 31.Malaeb SN, Sadowska GB, Stonestreet BS. Effects of maternal treatment with corticosteroids on tight junction protein expression in the cerebral cortex of the ovine fetus with and without exposure to in utero brain ischemia. Brain Res 1160: 11–19, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ment LR, Oh W, Ehrenkranz RA, Philip AG, Duncan CC, Makuch RW. Antenatal steroids, delivery mode, and intraventricular hemorrhage in preterm infants. Am J Obstet Gynecol 172: 795–800, 1995 [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health Consensus Conference Antenatal Corticosteroids Revisited: Repeat Courses. NIH Consens Statement 17: 1–10, 2000 [PubMed] [Google Scholar]
- 34.National Institutes of Health Consensus Development Panel Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 273: 413–418, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Nitz T, Eisenblatter T, Psathaki K, Galla HJ. Serum-derived factors weaken the barrier properties of cultured porcine brain capillary endothelial cells in vitro. Brain Res 981: 30–40, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Romero IA, Radewicz K, Jubin E, Michel CC, Greenwood J, Couraud PO, Adamson P. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett 344: 112–116, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Ron NP, Kazianis JA, Padbury JF, Brown CM, McGonnigal BG, Sysyn GD, Sadowska GB, Stonestreet BS. Ontogeny and the effects of corticosteroid pretreatment on aquaporin water channels in the ovine cerebral cortex. Reprod Fertil Dev 17: 535–542, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Rose JC, Kute TE, Winkler L. Glucocorticoid receptors in sheep brain tissues during development. Am J Physiol Endocrinol Metab 249: E345–E349, 1985 [DOI] [PubMed] [Google Scholar]
- 39.Sadowska GB, Patlak CS, Petersson KH, Stonestreet BS. Effects of multiple courses of antenatal corticosteroids on blood-brain barrier permeability in the ovine fetus. J Soc Gynecol Investig 13: 248–255, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sadowska GB, Stopa EG, Stonestreet BS. Ontogeny of connexin 32 and 43 expression in the cerebral cortices of ovine fetuses, newborns, and adults. Brain Res 1255: 51–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silwedel C, Forster C. Differential susceptibility of cerebral and cerebellar murine brain microvascular endothelial cells to loss of barrier properties in response to inflammatory stimuli. J Neuroimmunol 179: 37–45, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Stiles AD. Prenatal corticosteroids–early gain, long-term questions. N Engl J Med 357: 1248–1250, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Stonestreet BS, Patlak CS, Pettigrew KD, Reilly CB, Cserr HF. Ontogeny of blood-brain barrier function in ovine fetuses, lambs, and adults. Am J Physiol Regul Integr Comp Physiol 271: R1594–R1601, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Stonestreet BS, Petersson KH, Sadowska GB, Pettigrew KD, Patlak CS. Antenatal steroids decrease blood-brain barrier permeability in the ovine fetus. Am J Physiol Regul Integr Comp Physiol 276: R283–R289, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Stonestreet BS, Sadowska GB, McKnight AJ, Patlak C, Petersson KH. Exogenous and endogenous corticosteroids modulate blood-brain barrier development in the ovine fetus. Am J Physiol Regul Integr Comp Physiol 279: R468–R477, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Stonestreet BS, Watkins S, Petersson KH, Sadowska GB. Effects of multiple courses of antenatal corticosteroids on regional brain and somatic tissue water content in ovine fetuses. J Soc Gynecol Investig 11: 166–174, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Tseng YT, Yano N, Rojan A, Stabila JP, McGonnigal BG, Ianus V, Wadhawan R, Padbury JF. Ontogeny of phosphoinositide 3-kinase signaling in developing heart: effect of acute β-adrenergic stimulation. Am J Physiol Heart Circ Physiol 289: H1834–H1842, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Unno N, Wu WX, Wong CH, Bennett PR, Shinozuka N, Nathanielsz PW. Prostaglandin regulation of fetal plasma adrenocorticotropin and cortisol concentrations in late-gestation sheep. Biol Reprod 58: 514–519, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Malone F, Caritis SN, Mercer B, Harper M, Rouse DJ, Thorp JM, Ramin S, Carpenter MW, Gabbe SG. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med 357: 1190–1198, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, Caritis SN, Miodovnik M, Mercer B, Thorp JM, Moawad A, O'Sullivan MJ, Ramin S, Carpenter MW, Rouse DJ, Sibai B, Gabbe SG. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol 195: 633–642, 2006 [DOI] [PubMed] [Google Scholar]