Abstract
Heat stress (HS)-induced cardioprotection is associated with the activation of focal adhesion kinase (FAK) and protein kinase B (Akt) in neonatal rat ventricular myocytes (NRVMs), suggesting that stress-induced activation of survival pathways may be important in protecting intact hearts from irreversible injury. The purposes of this study were 1) to examine the subcellular signaling pathways activated by HS and ischemic preconditioning (IP) in intact hearts, 2) to determine whether HS and IP activate an integrated survival pathway similar to that activated by HS in cultured NRVMs, and 3) to determine whether HS and IP reduce lethal cell injury in perfused intact hearts. Adult rat hearts perfused in the Langendorff mode were subjected to 25 min of global ischemia and 30 min of reperfusion (I/R) either 24 h after whole animal HS or following a standard IP protocol. Myocardial signaling was analyzed using Western blot analysis, whereas cell death was assayed by measuring lactate dehydrogenase release into the perfusate and confirmed by light microscopy. Similar to NRVMs, HS performed in the whole animal 24 h before I/R increased phosphorylation of FAK at tyrosine-397 and protein kinase B (Akt) and resulted in protection from cell death. Using IP as a myocardial stress also resulted in an increased phosphorylation/activation of both FAK and Akt and resulted in reduced cell death in adult perfused rat hearts subjected to I/R. In conclusion, 1) myocardial stress caused by whole animal HS activates cytoskeletal-based survival signaling pathways in whole heart tissue and reduces lethal I/R injury and 2) IP activates the same stress-induced survival pathway and the activation correlates with the well-known cardioprotective effect of IP on lethal I/R injury.
Keywords: cytoskeleton, protection, survival signaling
prolonged periods of myocardial ischemia, if not relieved, inexorably result in cell death. In contrast, brief episodes of ischemia (termed “reversible ischemia”) result in a mild injury pattern that eventually reverts to normal upon the restoration of normal arterial blood flow. Despite many years of active research, the exact series of events underlying the transition from reversible to irreversible injury remains elusive. It is known that certain interventions are capable of modulating or delaying the onset of irreversible injury in experimental model systems such as hypothermia (2, 12, 13), calcium channel blockade (16, 22, 34), heat stress (HS) (17, 35), and ischemic preconditioning (IP) (15, 20, 26, 29). However, even in experimental model systems, the mechanism(s) responsible for protection are not fully understood. If any intervention is to have impact in the clinical arena and reduce the mortality from acute myocardial infarction, a better understanding of the mechanism of cardioprotection is critical.
IP provides the most dramatic and consistent protection against cell death, but the subcellular mechanism of protection has remained controversial. Although “classic” IP is induced by a brief episode of reversible ischemia followed by reperfusion, a wide range of pharmacological agents has been described that mimic the protective effect of IP including drugs that activate adenosine, α1-adrenergic, muscarinic, angiotensin, and bradykinin receptors. As a consequence of these studies, it has been suggested that the subcellular signaling pathways used by these receptors may underlie the mechanism of IP (3, 21, 27, 39). Although it is an attractive idea that a common subcellular signaling pathway may underlie cardioprotection, to date there is no unifying hypothesis to explain the diverse number of pharmacological agents capable of mimicking the cardioprotection of classic IP.
Focal adhesion kinase (FAK) is a nonreceptor protein tyrosine kinase that normally exists in nonmuscle cells at cell-to-matrix junctions known as focal adherens or focal adhesion junctions and is activated in response to cell adhesion, integrin clustering, and growth factor stimulation (25). FAK binds to integrins (cell surface receptors) as well as several intracellular proteins that are important in signal transduction including paxillin, tensin, p130CAS, talin, and vinculin (24, 30). FAK plays a critical homeostatic/survival role because it transduces extracellular matrix (ECM)-derived survival signals to the inside of the cell (4). This cell signaling complex (integrin/FAK/paxillin) has been shown to play an important role in maintaining cell survival/viability in nonmuscle cells (9–11, 23, 31, 32) and more recently in cultured neonatal rat ventricular myocytes (NRVMs) (35, 37).
In our most recent studies we have shown that HS, representing a cause of acute myocardial stress, causes the activation of a cytoskeletal-based survival pathway that includes integrin, FAK, phosphatidylinositol 3-kinase (PI3K), and Akt. Furthermore, the activation of the pathway reduced both oncotic and apoptotic cell death, and the inhibition/interruption of either FAK or PI3K (members of the pathway) resulted in an increased cell death that correlated with a reduced activation of Akt (37). However, these studies were conducted in cultured neonatal rat myocytes rather than intact, adult hearts where stress-induced signaling may be different and/or more complex. In addition, our previous studies used HS followed by 24 h of recovery and therefore did not test for protection immediately after the activation of the pathway. The purpose of the present study was to determine whether myocardial stress (HS and classic IP) activates the same survival pathways in intact adult hearts.
MATERIALS AND METHODS
All experiments reported here conformed to the standards in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1996). All animal protocols were approved by the Animal Investigation Committee at Wayne State University.
Perfused hearts.
Male Sprague-Dawley rats (200–250 g) were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). After the absence of a tail-flick response was confirmed, the chest was opened and heparin was injected into the inferior vena cava and allowed to circulate for 5 s. The hearts were rapidly removed and plunged into ice-cold buffered Krebs-Henseleit solution. Excess tissue was removed, and the aorta was cannulated and perfused in the Langendorff mode at a constant pressure of about 75 mmHg at 37°C. Control perfusate was a modified Krebs-Henseleit bicarbonate buffer containing (in mmol/l) 118 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 10 glucose, 2.5 CaCl2, and 2.5 NaHCO3, equilibrated with 95% O2-5% CO2. The coronary flow rate was monitored via the effluent and ranged from 10–15 ml/min. Any heart with a flow rate of <10 ml/min was excluded from further manipulation.
Experimental design/protocol.
Forty-four adult male Sprague-Dawley rats were used in these studies. Hearts were divided into three main groups: control, HS, and IP. For each main group, one subgroup was used for analysis of cell signaling and the other was used for analysis of cell death.
Whole body heat shock.
Anesthesia was administered to adult rats by intraperitoneal injection of pentobarbital sodium (50 mg/kg). Rats were then placed in a controlled environmental chamber under an infrared light while body temperature was monitored using a rectal probe. The rat remained under the light until the core temperature reached a stable temperature of 41°C for 15 min. Following the HS, rats were allowed to recover from anesthesia and recover to normal body temperature in the ambient conditions (room temperature). While recovering, rats were hydrated with an intraperitoneal bolus of normal saline (10 ml/kg). Control animals were subjected to anesthesia only. All rats were allowed to recover for 24 h, at which time they were reanesthetized with 50 mg/kg pentobarbital sodium before excision and harvest of the heart for Western blot analysis of signaling activity and/or analysis of cell injury.
Ischemic preconditioning.
IP was induced using a standard 2 × 5′ protocol; i.e., two 5-min episodes of ischemia each followed by a 5-min period of reflow before the onset of the sustained period of global ischemia.
Ischemia-reperfusion.
Global ischemia was induced by clamping the flow line and reducing coronary flow to 0 ml/min. The heart temperature was maintained at 37°C throughout the ischemic period by a heat-jacketed chamber and bathing the heart in perfusion buffer at 37°C. Following 25 min of global ischemia, flow was restored by unclamping the flow line and allowing the resumption of spontaneous coronary flow. Coronary flow was measured at 1-min intervals during the 30-min reperfusion phase, and the effluent was saved for analysis of cell death where indicated [lactate dehydrogenase (LDH) assay].
Western blot analysis.
Left ventricular myocardium was harvested for protein analysis using standard Western blot analysis as described previously (36, 37). Ventricular tissue was homogenized with lysis buffer containing 50 mM Tris·Cl (ph 8.0), 150 mM NaCl, 0.02% sodium azide, and 1% Triton X-100 and 1× protease inhibitor cocktail. Sixty micrograms of sample (as determined by BCA protein assay) were loaded to each lane and subjected to SDS-protein electrophoresis. Following electrophoresis, the proteins were transferred to nitrocellulose membranes, and the membranes were incubated overnight at 4°C with one of the following primary antibodies: 1) a rabbit anti-FAK (No. 06-543, Upstate Biotechnology) or a mouse anti-FAK (No. 610087, BD Biosciences), 2) a rabbit anti-FAKpy397 (No. 44-624G, Biosource), 3) rabbit anti-Akt and/or anti-phospho-Akt (Ser473; Nos. 9271 and 9172, Cell Signaling), or 4) a mouse anti-Akt (No. AM1011, ECM Biosciences). The membranes were then incubated with a secondary antibody, donkey anti-rabbit IgG (Santa Cruz) for 1 h at room temperature, and the final protein expression was detected using a standard horseradish peroxidase chemiluminescence system (Amersham; Arlington, IL). In some experiments, the membranes were incubated with fluorophore-labeled donkey anti-rabbit IgG (Invitrogen) or RDye 800CW conjugated goat anti-mouse IgG (LI-COR Biosciences), and the final protein expression was detected using the Odyssey infrared imaging system (LI-COR Biosciences). Signaling data are reported in arbitrary units and/or percent elevation over control protein expression as indicated.
Cell injury assay.
LDH release was used as an indication of cell death/lethal cell injury. LDH release was measured in the effluent collected at zero time (just before the onset of ischemia) and at 1, 3, 5, 10, 15, 20, and 30 min of reperfusion. LDH was assayed using a commercial kit (TOX7 kit, Sigma) and LDH release if expressed as international units per 30 min per gram wet heart weight. The presence of cell death and/or protection was confirmed by processing a separate group of hearts from each group for routine histological analysis. Hearts were fixed in buffered formalin, processed, embedded in paraffin, and stained with hematoxylin and eosin.
Statistics.
All LDH data are expressed as means ± SE [in international units released per 30 min of perfusion (reperfusion)]. All Western blot data are expressed as means ± SE (in arbitrary units). Statistically significant differences between groups were tested using a paired t-test analysis. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Effect of HS on protein expression and cell signaling.
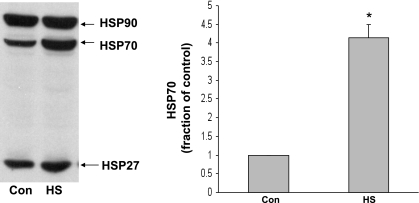
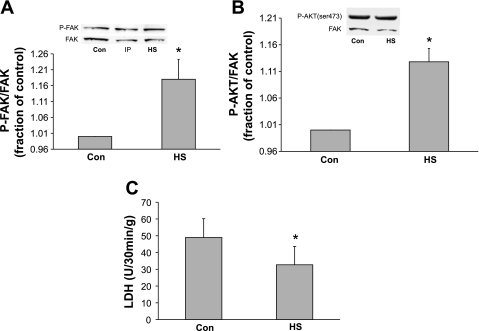
In the first series of experiments, we sought to determine whether whole body HS increases the expression of heat shock proteins and causes the activation of the cytoskeletal-based survival pathway previously identified in cultured NRVMs. As expected, HS caused an increase in expression of heat shock protein 27, 70, and 90 in left ventricular myocardium (Fig. 1). We next sought to determine the level of activation of FAK and Akt in whole hearts harvested from adult rats subjected to whole body HS. Confirming and extending our previous data from cultured NRVMs, we noticed that HS resulted in a significant activation of FAK (as measured by phosphorylation of FAK tyrosine residue 397) compared with non-heat shocked control hearts perfused for the same period of time before tissue sampling (P ≤ 0.04 vs. control heart tissue; Fig. 2A). Similar results were obtained when Akt signaling was examined (P ≤ 0.05 vs. control heart tissue; Fig. 2B).
Fig. 1.
Effect of whole body heat stress (HS) on heat shock protein (HSP) expression in adult rat heart ventricular myocardium. Left: induction of whole body heat shock (see materials and methods for details) in live animals resulted in a significant increase in expression of HSP27, -70, and -90 in the heart. Right: densitometry data for myocardial HSP70 expression (n = 4 rats; *P ≤ 0.01). Con, control.
Fig. 2.
Effect of prior whole body HS on focal adhesion kinase (FAK) activation, Akt activation, and lactate dehydrogenase (LDH) release in adult rat myocardium. A: representative immunoblot showing phosphorylation of FAK at tyrosine-397 (indicative of activation) from lysates of control left ventricular (LV) tissue and LV heart tissue harvested from rats subjected to whole body HS. HS induced increased activation of FAK at time 0 (*P ≤ 0.028, Con vs. HS; n = 4 rats). Y-axis data are plotted as fraction of control LV levels. There was no difference in the total amount of FAK protein in any of the samples (lower portion of blot). B: hearts were subjected to HS or control conditions. Cell lysates were probed for phosphorylated (p-)Aktser473 to detect activated Akt. When compared with control hearts, hearts harvested from rats subjected to HS showed increased phosphorylation/activation of Akt (*P ≤ 0.007, Con vs. HS; n = 3 rats). Y-axis data are plotted as fraction of control LV levels. The amount of total FAK (to control for loading) present in each sample was not significantly different. C: adult hearts subjected to whole body heat shock 24 h prior were harvested and subjected to 25 min of global ischemia followed by 30 min of reperfusion and compared with hearts harvested from control rats (no HS). Y-axis values indicate the amount of LDH release, indicating loss of membrane integrity (dead cells). As expected, HS resulted in significant protection against oncotic cell death (*P ≤ 0.02; n = 5 rats). IP, ischemic preconditioning.
Effect of HS on cell injury: LDH release.
In cultured NRVMs, we have shown that HS results in the protection against oncotic cell death as measured by trypan blue exclusion (35, 37). It is well known that HS protects hearts from lethal ischemia-reperfusion injury. The current study was designed to confirm that HS would protect against ischemia-reperfusion in intact hearts subjected to global ischemia-reperfusion. Figure 2C confirms that whole body HS applied 24 h before harvest and perfusion resulted in significantly less cell death in response to 25 min of global ischemia followed by 30 min of reperfusion (P ≤ 0.05 HS vs. control; Fig. 2C).
Effect of IP on cell signaling.
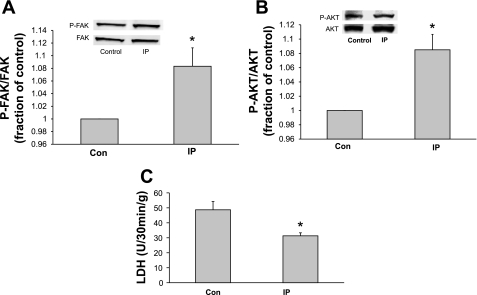
Previous studies have shown that IP is protective against cell death in isolated Langendorff-perfused adult rat hearts subjected to global ischemia-reperfusion (28, 38). However, the status of the cytoskeletal-based cell survival pathway in IP hearts is not known. To determine the effect of IP on FAK and Akt activity, hearts were subjected to a standard IP protocol in perfused rat hearts that consists of two 5-min episodes of global ischemia (at 37°C), each followed by a 5 min period of reperfusion (2 × 5′ protocol). Figure 3, A and B, shows that IP caused the activation of both FAK (a proximal member of the pathway) and Akt (distal member of the pathway) in whole adult rat hearts, confirming that myocardial stress, in this instance IP, is capable of activating the cell survival pathway.
Fig. 3.
Effect of IP on FAK activation, Akt activation, and LDH release in adult rat myocardium. LV lysates of control LV tissue and LV tissue harvested from rats subjected to standard IP without a sustained period of global ischemia were used for A and B. A: representative immunoblot showing phosphorylation of FAK at tyrosine-397 (indicative of activation). IP increased activation of FAK at time 0 (*P ≤ 0.028, Con vs. IP; n = 4 rats). Y-axis data are plotted as fraction of control LV levels. There was no difference in the total amount of FAK protein in any of the samples (lower portion of blot). B: LV tissue was probed for p-Aktser473 to detect activated Akt. When compared with control hearts, hearts harvested from rats subjected to IP showed increased phosphorylation/activation of Akt (*P ≤ 0.007, Con vs. IP; n = 3 rats). Y-axis data are plotted as fraction of control LV levels. The amount of total FAK (to control for loading) present in each sample was not significantly different. C: adult hearts were subjected to either control aerobic perfusion for 40 min or a standard 4 × 5′ IP protocol followed by 25 min of global ischemia and 30 min of reperfusion to induce cell death. Y-axis values indicate the amount of LDH release, indicating loss of membrane integrity (dead cells). As expected, IP resulted in significant protection against oncotic cell death. (*P ≤ 0.02 vs. control hearts; n = 5 rats).
Effect of IP on cell injury: LDH release.
After we confirmed that IP activates both FAK and Akt, it was important to determine whether IP would protect against ischemia-reperfusion in intact hearts subjected to global ischemia-reperfusion. Figure 3C confirms that the standard IP 2 × 5′ protocol resulted in significantly less cell death in response to 25 min of global ischemia followed by 30 min of reperfusion.
Effect of HS and IP on proline-rich tyrosine kinase 2 and ILK activity.
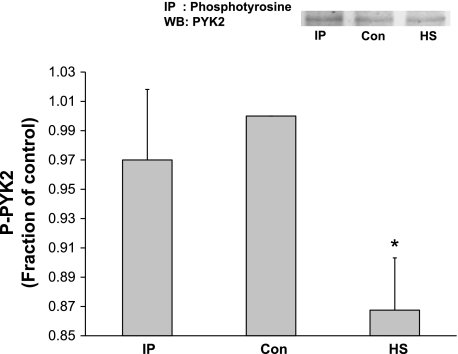
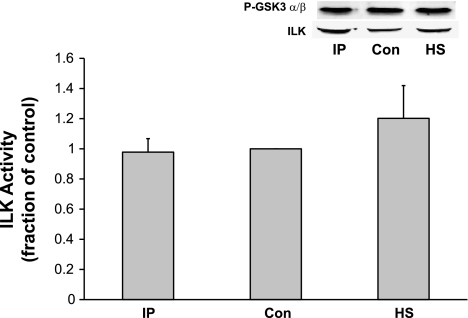
Proline-rich tyrosine kinase 2 (PYK2) has been shown to serve as a scaffolding protein for phosphoinositol-dependent kinase 1 (PDK1) in myocytes (7). PDK1 can phosphorylate/activate Akt at Thr308 and perhaps Ser473 and therefore could be responsible for the Akt activation we measured in response to IP. Alternatively, it is possible that IP directly activates integrin-linked kinase (ILK), which in turn activates/phosphorylates Akt. Figure 4 shows that neither IP nor HS caused a significant activation/tyrosine phosphorylation of PYK2. Interestingly, HS caused a significant inactivation of PYK2 activity. Similarly, to investigate the role of ILK in the activation of Akt, ILK activity was estimated by measuring GSK3-α/β phosphorylation on Western blot analysis. Figure 5 shows that ILK activity was not significantly increased by either IP or HS.
Fig. 4.
Effect of HS and IP on proline-rich tyrosine kinase 2 (PYK2) activity. Adult hearts were subjected HS or IP. LV extracts were immunoprecipitated with anti-phosphotyrosine (PY20) antibody followed by probing for PYK2. Neither HS nor IP had a significant stimulatory effect of PYK2 phosphorylation. Interestingly, HS caused an unanticipated significant reduction in PYK2 activation compared with control LV tissue. Y-axis data are plotted as arbitrary units of density. Representative blot is shown. *P ≤ 0.05 vs. control; n = 4 rats. WB, Western blot.
Fig. 5.
Effect of HS and IP on integrin-linked kinase (ILK) activity. Adult hearts were subjected HS or IP. LV extracts were immunoprecipitated with anti-ILK antibodies followed by an in vitro kinase assay using GSK3 fusion protein as a substrate (see materials and methods for details). Activation of ILK was measured by probing for anti-phospho-GSK3-α/β (Ser21/9). Total ILK protein present in each lane was detected using anti-ILK antibody after stripping the blot. Neither HS nor IP had a significant stimulatory effect of ILK phosphorylation. Y-axis data are plotted as arbitrary units of density. Representative blot is shown. P = not significant; n = 4 rats.
DISCUSSION
The present results extend the conclusions of our previous studies defining a cytoskeletal-based cell survival pathway present in myocardium (35, 37) in several important ways. First, the current study demonstrates that HS activates the critical pathway members FAK and Akt in intact hearts. Our previous studies in cultured rat ventricular myocytes provided convincing evidence that myocardial stress, specifically HS, resulted in a selective activation of the survival pathway as well as protection from lethal cell injury. However, the studies needed to be extended to whole hearts to validate the presence of the pathway outside of cultured myocyte systems. Second, the current study shows that the pathway is activated in adult heart tissue and is not unique to neonatal heart tissue. Third, and most important, the present study demonstrates that the activation of the survival pathway occurs in response to a different type of myocardial stress, reversible ischemia and reperfusion. This report used a well-known and reproducible form of acute cardioprotection known as IP. IP resulted in the activation of both FAK and Akt in whole heart tissue (activation was measured before the onset of lethal ischemia-reperfusion injury). As expected, the IP-induced activation of the survival pathway correlated with the expected IP-induced cardioprotection.
IP and lethal injury.
It is well known that IP protects myocardium against subsequent lethal ischemia-reperfusion injury. Many mechanisms have been hypothesized to explain the protective role of IP including the reduction of energy utilization, the inhibition of the mitochondrial F1F0-ATPase, the reduction in lactate accumulation/intracellular calcium, and the activation of several cellular signaling pathways (19). However, the precise mechanism of cardioprotection is not known. In support of the present study, it has been suggested that the induction of a general cell stress response may be all that is necessary for cardioprotection (1). The results of the present study show for the first time that IP activates the same subcellular signaling proteins that significantly reduce cell death in cultured NRVMs and support the notion that myocardial stress activates a generalized cell survival/protection pathway.
Role of myocardial stress in activation of a cell survival pathway.
Our previous studies have suggested that a cytoskeletal-based cell survival pathway may play an important role in cardioprotection by showing that HS causes an activation of the pathway and increased cardioprotection, whereas the inhibition of the pathway (inhibition of FAK or PI3K) resulted in an increased cell death (35, 37). However, theses studies were conducted in cultured neonatal myocytes using a nonischemic form of cardiac stress. Therefore, one of the goals of the present study was to investigate whether the survival pathway was present in intact, adult hearts and in a more clinically relevant form of stress, ischemia-reperfusion.
If cytoskeletal-based signaling is important in cell survival, then myocardial stress should result in the assembly/activation of the docking protein p130cas into a membrane/cytoskeletal-based signaling complex. HS does indeed result in increased p130cas in the membrane fraction (data not shown). This result coupled with the data from our previous study (35) showing that HS both enhanced the interaction between integrin and paxillin and increased the localization of paxillin in the membrane fraction suggests that stress results in the assembly of a signaling complex localized to the membrane fraction consisting of at least integrin, FAK, p130cas, and paxillin. Although not specifically interrogated in this study, it is likely that talin and vinculin are also localized in the membrane-based signaling complex (6).
Possible role of other signaling molecules.
PYK2 has been shown to serve as a scaffolding protein for PDK1 in myocytes, and PDK1 can phosphorylate Akt at Thr308 and perhaps Ser473 (7). Furthermore, it is possible that HS and/or IP activate ILK (a serine-threonine protein kinase) that may in turn directly phosphorylate Akt and therefore account for the measured cardioprotective effect. Therefore, we investigated whether HS- or IP-induced protection may in part be mediated through PYK2 and/or ILK activation. However, the results from Figs. 4 and 5 show that neither HS nor IP significantly increased PYK2 or ILK activity, arguing against a major role for these proteins in the cardioprotective effect of HS in our model system.
Other evidence supporting a protective role of FAK-PI3K-Akt pathway activation.
Previous studies have supported a role for PI3K-Akt signaling in the cardioprotection including that associated with IP (5, 14, 18). However, the novel information from this study is the link between the activation of FAK with the downstream activation of the PI3K-Akt effector pathway. A recent study from Hakim et al. (8) showed that FAK was very important to cell survival in response to acute stress. In this study mice deficient in ventricular FAK from 13 wk postnatal onward were generated using the cre-lox system. Both in vitro and in vivo model systems were used to determine the effect of myocyte-specific deletion of FAK on cell injury. The loss of FAK resulted in increased cell death (25% to 60% control vs. FAK deficient) in response to 2 h of hypoxia/1 h of reoxygenation in cultured myocytes. Furthermore, cardiac-restricted deletion of FAK exacerbated ischemia-reperfusion-induced myocardial infarction (14% vs. 36% control vs. FAK deficient). The loss of FAK activity would prevent the activation of the survival pathway in response to stress, and therefore, it would be predicted that hearts and/or myocytes would suffer larger infarcts and/or more cell death compared with control tissue for a given duration of ischemia-hypoxia. Even though this study did not attempt to activate FAK, the authors concluded that targeted FAK activation might protect against stress-dependent cardiac remodeling. Other studies have implicated FAK in the survival of normal and cancer cells and even in tumor progression (33). Therefore, the present results are consistent with a growing body of literature implicating an important role for FAK in cell survival.
Summary and conclusions.
Data from our previous studies showed that HS in cultured NRVMs is associated with the activation of a series of linked signaling molecules starting at the focal adhesion complex (costamere equivalent in cultured myocytes) and resulting in the activation of the well-known cardioprotective protein Akt, whereas the inhibition of FAK with FAK-related nonkinase or inhibition of PI3K with wortmannin inhibited the activation of Akt and reduced HS-induced protection. The current study extends these results into whole adult hearts and for the first time shows that brief episodes of ischemia-reperfusion activate the same pathway and provide significant cardioprotection against global ischemia-reperfusion injury. These results continue to support the hypothesis that myocardial stress activates a cytoskeletal-based survival pathway that may play an important role in the protection against acute ischemia-reperfusion injury in ventricular myocardium.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-84405-A1 (to R. S. Vander Heide).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Alireza-Tousi A, Halcox JP, Henderson B. Stressing the obvious? Cell stress and cell stress proteins in cardiovascular disease. Cardiovasc Res 74: 19–28, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Angell WW, Rikkers L, Dong E, Jr, Shumway NE. Organ viability with hypothermia. J Thorac Cardiovasc Surg 58: 619–624, 1969 [PubMed] [Google Scholar]
- 3.Bogoyevitch MA, Parker PJ, Sugden PH. Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-epsilon is a major isotype present, and it is activated by phorbol esters, epinephrine and endothelin. Circ Res 72: 757–767, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Chan PC, Lai JF, Cheng CH, Tang MJ, Chiu CC, Chen HC. Suppression of ultraviolet irradiation-induced apoptosis by overexpression of focal adhesion kinase in Madin-Darby canine kidney cells. J Biol Chem 274: 26901–26906, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Fujio Y, Nguyen T, Wencker D, Kitsis R, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giancotti FG, Ruoslahti E. Integrin signaling. Science 285: 1028–1032, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Sabri A, Elouardighi H, Rybin V, Steinberg S. Alpha1-adrenergic receptors activate AKT via a Pyk2/PDK-1 pathway that is tonically inhibited by novel protein kinase C isoforms in cardiomyocytes. Circ Res 99: 1367–1375, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Hakim ZS, DiMichele LA, Rojas M, Meredith D, Mack CP, Taylor JM. FAK regulates cardiomyocyte survival following ischemia/reperfusion. J Mol Cell Cardiol 46: 241–248, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauck CR, Hsia DA, Schlaepfer DD. The focal adhesion kinase—a regulator of cell migration and invasion. IUBMB Life 53: 115–119, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hauck CR, Hsia DA, Schlapfer DD. Focal adhesion kinase facilitates platelet-derived growth factor-BB-stimulated ERK2 activation required for chemotaxis, migration of vascular smooth muscle cells. J Biol Chem 275: 41092–41093, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res 90: 1282–1289, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Jones RN, Hill ML, Reimer KA, Wechsler AS, Jennings RB. Effect of hypothermia on the rate of myocardial ATP and adenine nucleotide degradation in total ischemia (Abstract). Fed Proc 39: 1111, 1980 [Google Scholar]
- 13.Jones RN, Reimer KA, Hill ML, Jennings RB. Effect of hypothermia on changes in high-energy phosphate production and utilization in total ischemia. J Mol Cell Cardiol 14: 123–130, 1982 [DOI] [PubMed] [Google Scholar]
- 14.Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 19: 5800–5810, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloner RA, Shook T, Przyklenk K, Davis VG, Junio L, Matthews RV, Burstein S, Gibson CM, Poole WK, Cannon CP, McCabe CH, Braunwald E, Investigators Previous angina alters in-hospital outcome in TIMI 4. A clinical correlate to preconditioning. Circulation 91: 37–47, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Lamping KA, Christensen CW, Pelc LR, Warltier DC, Gross GJ. Effects of nicorandil and nifedipine on protection of ischemic myocardium. J Cardiovasc Pharmacol 6: 536–542, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation 96: 4343–4348, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Matsui T, Tao J, del Monte F, Lee K, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murry CE, Reimer KA, Long JB, Jennings RB. Preconditioning with ischemia protects ischemic myocardium (Abstract). Circulation 72, Suppl III: 119, 1985. 4006123 [Google Scholar]
- 21.Prasad MR, Jones RM. Enhanced membrane protein kinase C activity in myocardial ischemia. Basic Res Cardiol 87: 19–26, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Reimer KA, Jennings RB. Verapamil in two reperfusion models of myocardial infarction. Temporary protection of severely ischemic myocardium without limitation of ultimate infarct size. Lab Invest 51: 655–666, 1984 [PubMed] [Google Scholar]
- 23.Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature 380: 538–540, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20: 6459–6472, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Schaller MD, Hildebrand JD, Parsons JT. Complex formation with focal adhesion kinase: a mechanism to regulate activity and subcellular localization of Src kinases. Mol Biol Cell 10: 3489–3505, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schott RJ, Rohmann S, Braun ER, Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res 66: 1133–1142, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Stable S, Parker PJ. Protein kinase C. Pharmalogic Ther 51: 71–95, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res 71: 112–125, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Thornton JD, Striplin S, Liu GS, Swafford A, Stanley AW, Van Winkle DM, Downey JM. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol Heart Circ Physiol 259: H1822–H1825, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol 2: E231–E236, 2000 [DOI] [PubMed] [Google Scholar]
- 31.van de Water B, Houtepen F, Huigsloot M, Tijdens IB. Suppression of chemically induced apoptosis but not necrosis of renal proximal tubular epithelial (LLC-PK1) cells by focal adhesion kinase (FAK). J Biol Chem 276: 36183–36193, 2001 [DOI] [PubMed] [Google Scholar]
- 32.van de Water B, Nagelkerke JF, Stevens JL. Dephosphorylation of focal adhesion kinase (FAK) and loss of focal contacts precede caspase-mediated cleavage of FAK during apoptosis in renal epithelial cells. J Biol Chem 274: 13328–13337, 1999 [DOI] [PubMed] [Google Scholar]
- 33.van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol 73: 597–609, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Vander Heide RS, Jennings RB, Reimer KA. A new calcium channel antagonist, Ro-5967, limits infarct size in a canine model of ischemia and reperfusion (Abstract). J Mol Cell Cardiol 25, Suppl III: S17, 1993 [Google Scholar]
- 35.Wei H, Campbell W, Vander Heide RS. Heat shock-induced cardioprotection activates cytoskeletal-based cell survival pathways. Am J Physiol Heart Circ Physiol 291: H638–H647, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Wei H, L'Ecuyer T, Vander Heide RS. Effect of increased expression of cytoskeletal protein vinculin on ischemia-reperfusion injury in ventricular myocytes. Am J Physiol Heart Circ Physiol 284: H911–H918, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Wei H, Vander Heide RS. Heat stress activates AKT via focal adhesion kinase-mediated pathway in neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol 295: H561–H568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss RG, de Albuquerque CP, Vandegaer K, Chacko VP, Gerstenblith G. Attenuated glycogenolysis reduces glycolytic catabolite accumulation during ischemia in preconditioned rat hearts. Circ Res 79: 435–446, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol Heart Circ Physiol 266: H1145–H1152, 1994 [DOI] [PubMed] [Google Scholar]