Abstract
Neuropeptide Y (NPY) induced reentry of differentiated rat neonatal and adult cardiomyocytes into the cell cycle. NPY also induced differentiation of bone marrow-derived mesenchymal stem cells (MSC) into cardiomyocytes following transplantation into infarcted myocardium. Rat neonatal and adult cardiomyocytes were treated in vitro with vehicle, NPY, fibroblast growth factor (FGF; 100 ng/ml), or FGF plus NPY. DNA synthesis, mitosis, and cytokinesis were determined by immunocytochemistry. NPY-induced MSC gene expression, cell migration, tube formation, and endothelial cell differentiation were analyzed. Male rat green fluorescent protein-MSC (2 × 106), pretreated with either vehicle or NPY (10−8 M) for 72 h, were injected into the border zone of the female myocardium following left anterior descending artery ligation. On day 30, heart function was assessed, and hearts were harvested for histological and immunohistochemical analyses. NPY increased 5-bromo-2′-deoxy-uridine incorporation and promoted both cytokinesis and mitosis in rat neonatal and adult myocytes. NPY also upregulated several genes required for mitosis in MSC, including aurora B kinase, FGF-2, cycline A2, eukaryotic initiation factor 4 E, and stromal cell-derived factor-1α. NPY directly induced neonatal and adult cardiomyocyte cell-cycle reentry and enhanced the number of differentiated cardiomyocytes from MSC in the infarcted myocardium, which corresponded to improved cardiac function, reduced fibrosis, ventricular remodeling, and increased angiomyogenesis. It is concluded that a combined treatment of NPY with MSC is a novel approach for cardiac repair.
Keywords: deoxyribonucleic acid synthesis
fetal and neonatal cardiac myocytes are capable of undergoing DNA synthesis and cell division. However, their ability to divide diminishes progressively during postnatal development (2). Adult mammalian cardiomyocytes are considered terminally differentiated, incapable of proliferation and irreversibly withdrawn from the cell cycle soon after birth (28, 29). The permanent loss of cardiomyocytes following myocardial infarction (MI) often results in heart failure. Potential therapies could include stimulating the myocytes to divide and adding new cells via pharmacological and genetic manipulations or delivering stem cells to multiply and subsequently differentiate into cardiomyocytes (7). Recent reports have shown that adult cardiac myocytes can be induced to reenter into cell cycle with periostin (17), p38 MAP kinase inhibitor (8), cyclin D1/CDK4 (27), cyclin A2 (3), and transforming growth factor-β (4). Although the adult heart has a limited capacity for myocyte proliferation, increasing the number of remaining cardiomyocytes by activating their proliferative potential via extrinsic factors could result in the repair of damaged myocardium. Therefore, pharmacological manipulations may be important in enhancing proliferation of differentiated myocytes for cardiac repair.
Neuropeptide Y (NPY) is one of the most abundant neuropeptides present in the human peripheral and central nervous systems (1). It acts as a neurotransmitter, regulating various autonomic and endocrine functions (21). NPY is a 36-amino acid peptide that is released by sympathetic neurons surrounding the heart and blood vessels. The angiogenic activity of NPY has been demonstrated in a variety of models (16). NPY has been shown to cause angiogenesis with equal potency and efficacy as both basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) (22). However, its role in proliferation of cardiomyocyte derived from NPY-pretreated mesenchymal stem cells (MSC) and its therapeutic potential in stem cell-mediated therapy for MI has not been explored to date. NPY-pretreated MSC may trigger production of reparative growth factors.
Here, we have investigated whether NPY can trigger cell-cycle reentry in neonatal or adult cardiomyocytes and increase the proliferation of transplanted MSC that have already differentiated into cardiomyocytes within the infarcted myocardium. This would be a novel approach to promote angiomyogenesis and populate the infarcted myocardium via transplantation of NPY-pretreated MSC.
METHODS
Animal Experiments
All animal work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996) and was approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
In Vitro Study
Preparation of MSC and hypoxia/reoxygenation.
MSC primary culture was as follows. Bone marrow-derived stem cells (BMSCs) were isolated from male Sprague-Dawley (SD; 8-wk-old) rats according to previously published procedures from our laboratory (37). The animals were euthanized by cervical dislocation, and BMSCs were flushed out of tibias and femurs. After being washed, centrifuged cells were resuspended in normal culture medium (NM) to a final concentration of 5 × 105 viable cells per milliliter in a T75 flask. NM consisted of Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 10% (vol/vol) fetal bovine serum (FBS) and antibiotics. The T75 flask was kept in a humidified 5% CO2 incubator at 37°C for 72 h. Culture medium was changed every 3 to 4 days, and nonadherent cells were removed by changing the medium.
Passages 2–4 MSC were used in the study. To mimic the ischemic injury in vitro, the culture medium was immediately changed to hypoxic buffer containing (in mmol/l) 118 NaCl, 24 NaHCO3, 1.0 NaH2PO4, 2.5 CaCl2-2H2O, 1.2 MgCl2, 20 sodium lactate, 16 KCl, and 10 2-deoxyglucose (pH adjusted to 6.2), and dishes were incubated under hypoxic conditions of 1% O2-5% CO2-94% N2 at 37°C in a hypoxic incubator (O2/CO2 incubator-MCO-18M; Sanyo) for 8 h. Normal culture (5% CO2-95% room air) served as a control. Cells were reoxygenated by replacing the ischemic buffer with culture medium at 5% CO2 and 37°C for another 18 h as described previously (5).
Quantitative real-time PCR and ELISA for genes upregulation in NPY-pretreated MSC.
After pretreatment of MSC with vehicle or NPY (10−8 M) for 72 h, MSC were subjected to hypoxic conditions for 8 h. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the procedures recommended by the manufacturers. The mRNA level of aurora B kinase, FGF-2, cycline A2, eukaryotic initiation factor 4 E (eIF4E), stromal cell-derived factor-1α (SDF-1α), and GAPDH was examined by RT-PCR and quantitative real-time PCR (qPCR). Total RNA was isolated using TRIzol reagent (Invitrogen) followed by DNAse treatment and purification using RNeasy mini column kit (Qiagen). cDNA was synthesized using the SuperScript III RNase H-Reverse Transcriptase (Invitrogen) in a 20-μl reaction mixture. Rat sequence-specific oligonucleotide primers designed from published rat mRNA sequences (GenBank accession Nos. NM_022205 and AF209976, respectively) were as follows: aurora B, sense 5′-caatctggcctgaacacctt-3′ and antisense 5′-cccgagccaagtacacattt-3′; cycline A2, sense 5′-atgtcaaccccgaaaaagtg-3′ and antisense 5′-gggacgtgctcatcgtttat-3′; FGF-2, sense 5′-cggtacctggctatgaagga-3′ and antisense 5′-ccgttttggatccgagttta-3′; SDF-1α, sense 5′-agccagtcagcctgagctac-3′ and antisense 5′-taatttcgggtcaatgcaca-3′; eIF4E, sense 5′-caagcaaaccttcggttgat-3′ and antisense 5′-ctccccgtttgtttttctca-3′; GAPDH, sense 5′-atgggaagctggtcatcaac-3′ and antisense 5′-gtggttcacacccatcacaa-3′. qPCR was carried out on the iQ5 real-time system (Bio-Rad) with the iQ SYBR Supermix (Bio-Rad). The expression of each target mRNA relative to GAPDH under experimental and control conditions was calculated based on the threshold cycle (CT) as r = 2−Δ(ΔCT), where ΔCT = CT target − CT GAPDH and Δ(ΔCT) = ΔCT experimental − ΔCT control.
ELISA for VEGF release.
The concentration of VEGF was measured in the supernatants obtained from vehicle or NPY-pretreated MSC, each grown under 72-h normoxic or 8-h hypoxic conditions using commercially available ELISA kit (Calbiochem for VEGF) according to the manufacturer's instructions. All samples were treated in duplicate. The concentration was calculated as picograms per milliliter and subsequently expressed as picograms per 106 cells, based on previously obtained cell counts.
In vitro cell migration assay.
To investigate migration of MSC after stimulation with NPY, a chemotaxis assay was performed using a chemotaxis chamber. We performed a chemotaxis assay in vitro using transwells (5.0-μm pore size; Costar; Corning, Corning, NY) as described (37). MSC (1 × 105 cells/well) pretreated with vehicle, or NPY (10−8 M), or NPY together with small interfering RNA (siRNA) were exposed to either normoxic for 72 h or hypoxic conditions for 8 h, and then the respective cells were added to the upper wells; the lower wells contained 600 μl medium with SDF-1α (100 ng/ml). To determine the number of migrated cells, after 6 h incubation at 37°C, the cells that had migrated to the bottom chamber were counted randomly under a fluorescence microscope.
Western blotting for determining the role of CXCR4 in NPY-induced cell migration.
MSC were pretreated with vehicle, NPY (10−8 M), or NPY together with siRNA for 72 h and then subjected to hypoxic conditions for 8 h. MSC were then transferred into centrifuge tube and centrifuged at 300 g for 10 min at 4°C. The pellet was then sonicated in radioimmunoprecipitation assay (RIPA) buffer (containing protease inhibitors; Sigma) on ice. After centrifugation (13,000 rpm for 10 min), the supernatants were stored at −20°C for Western blot analysis. Forty micrograms of protein extract were separated by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane (37). The membrane was blocked with 5% milk in Tris-buffer saline solution (pH 7.6) containing 0.05% Tween 20 (TBS/T) and incubated with primary antibodies for CXCR4 (AnaSpec) overnight at 4°C. The membrane was then incubated for 1 h with the horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature and then washed and developed with the ECL plus kit (GE Healthcare). To determine the role of CXCR4 in NPY-induced cell migration, we used adenovirus-based siRNA constructs to express siRNA, to knockdown CXCR4 gene. The adenoviral transduction allowed us to introduce a stable cell line with CXCR4 knockdown by blasticidin selection. siRNA-CXCR4 top strand sequence was 5′-CACCGGATCAGCATCGATTCCTTCACGAATGAAGGAATCGATGCTGATCC-3′; the bottom strand sequence was 5′-AAAAGGATCAGCATCGATTCCTTCATTCGTGAAGGAATCGATGCTGATCC-3′. To evaluate transfection efficiency, we used the BLOCK-iT Adenoviral RNAi Expression System with EmGFP to deliver the specific double-stranded DNA oligonucleotides that encoded the target CXCR4 pre-siRNA. Briefly, 12 h before transduction, MSC were maintained in DMEM with 10% FBS, supplemented with glutamine and penicillin-streptomycin in 6-well plates. Adenoviral stock (100 μl; 2 × 105 TU/ml) was added, and the cells were incubated for 8 h. The adenoviral medium was removed, and the cells were cultured in DMEM with 10% FBS until their use.
In vitro tube formation assay.
A tube formation assay was performed with a tube formation assay kit (Chemicon) according to the manufacturer's instructions. Briefly, the solution of ECMatrix was thawed on ice overnight, mixed with 10 × diluents of ECMatrix, and placed in a 96-well tissue culture plate at 37°C for 1 h to allow the matrix solution to solidify. MSC pretreated with vehicle or NPY (10−8 M) were exposed to either normoxia for 72 h or hypoxic conditions for 8 h. They were then seeded (3 × 105 cells/well) on top of the solidified matrix solution and incubated at 37°C for 4 h. Cellular network structures were fully developed. Total capillary tube lengths were measured under an inverted light microscope at 200× magnification. Five independent fields were assessed for each well, and the average number of tubes was determined (24).
Identification of the differentiated endothelial cells.
After 72 h pretreatment with vehicle or NPY, adherent MSC were incubated with 2 μg/ml 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine-labeled acetylated low density lipoprotein (DiI-Ac-LDL; Biomedical Technologies) for 4 h and then fixed with 2% paraformaldehyde for 10 min. After being washed with PBS, the MSC were reacted with 10 μg/ml fluorescein Griffonia (Bandeiraea) simplicifolia lectin I (FGSL), isolectin B4 (FITC-lectin; Vector) for 1 h. FGSL binds specifically to endothelial cells (ECs). After nuclei staining with DAPI (Vector), the double-positive cells stained with DiI-Ac-LDL+ (red color)/FITC-lectin+ (green color) were identified as differentiated ECs with a fluorescent microscope. The number of double-positive cells per well was counted in 12 randomly selected fields.
Cardiomyocyte isolation and culture.
For positive control, primary cultures of neonatal rat myocytes were prepared as described previously (18). Briefly, ventricular cardiomyocytes from rat neonatal hearts 2 days old and adult (200–250 g) were isolated as described with minor modifications. After digestion of hearts with 0.14 mg/ml collagenase II (Invitrogen) and 0.55 mg/ml pancreatin (Sigma), cells were cultured in DMEM/F12 (Gibco) containing 3 mM Na-pyruvate, 0.2% BSA, 0.1 mM ascorbic acid, 0.5% insulin-transferrin-selenium (100×), penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM L-glutamine. Adult cardiomyocytes were cultured for 1 day in standard medium [DMEM; 25 mM HEPES, 5 mM taurine, 5 mM creatine, 2 mM L-carnitine (Sigma), 20 U/ml insulin (Gibco), 02% BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin]. Cells were pretreated in culture medium containing 2 mM L-glutamine. Neonatal and adult cardiomyocytes were initially cultured for 48 h in the presence of 20 μM cytosine β-D-arabinofuranoside (araC; Sigma) and 5% horse serum before stimulation to prevent proliferation of nonmyocytes. Subsequently, adult cardiomyocytes were incubated for additional 3 days with araC during stimulation. Bromodeoxyuridine [5-bromo-2′-deoxy-uridine (BrdU), 30 μM] was added for the last 3 days. Cells were then divided into various treatment groups as mentioned above. To determine the effects of NPY on proliferation of neonatal or adult rat cardiomyocytes in vitro, NPY (10−8 M) was added to the dishes. FGF, known to induce myocyte division, was used as a positive control (9). To detect additive effects of NPY and FGF on proliferation of neonatal and adult rat myocytes, FGF (100 ng/ml; R&D Systems) was added to the culture medium. Primary neonatal rat ventricular cardiomyocytes were quantified by counting with a hemocytometer after 4 and 7 days of NPY treatment. Corresponding samples were stained with an antibody (troponin I) directed against cardiomyocyte marker combined costaining with DAPI to determine the number of differentiated cardiomyocytes.
Immunofluorescence staining.
DNA synthesis was determined with incorporation of BrdU (30 μM; Sigma) into the DNA of dividing cells. Cell proliferation was analyzed by using the presence of the cell-cycle-associated nuclear protein BrdU. The BrdU labeling and detection kit I (Roche Diagnostics) was used for detection of BrdU-positive cells. For the inhibition of DNA polymerization, araC (20 μM; Sigma) was added to the dishes. Mitosis was determined by visualization of positive phosphorylated histone H3 (H3P) nuclei bound by its antibody. An antibody (Cat. No. A5102; Sigma-Aldrich) directed against aurora B kinase was used to visualize cytokinesis. Signals were visualized with secondary antibodies conjugated to donkey anti-rabbit (FITC; Jackson Immunoresearch). BrdU was stained using the 5-bromo-2′-deoxy-uridine labeling and detection kit I (Roche Applied Science). Nuclei were visualized with DAPI (Invitrogen). Fluorescent imaging was performed with an Olympus BX41 microscope (Olympus America, Melville, NY), equipped with epiflourescence, and images were recorded using a digital camera with MagnaFire 2.1 software.
In Vivo Study
The purpose of these experiments was to evaluate the direct effects of NPY on the proliferation and differentiation of exogenously administered MSC into cardiomyocytes. Six groups of female SD rats (200–250 g) were used in these experiments postligation of the left anterior descending artery (LAD). These animals were randomly divided into six groups to receive treatment, as follows: 1) animals underwent a sham operation with loose suture around LAD (sham group), 2) animals underwent LAD ligation and only saline was injected (MI group), 3) MSC pretreated with vehicle were injected into the myocardium (MSC group) as a control, 4) MSC pretreated with NPY (10−8 M for 72 h) were injected into the myocardium (NPY group). BrdU (50 mg/kg) was injected (intraperitoneally) daily on days 1–7 to determine DNA synthesis. To study the direct effect of NPY on angiogenesis in vivo, an additional two groups were used to determine vascular density from sham and MI groups subjected to direct injection intramyocardially by either vehicle (20 μl saline) or NPY (7.2 ng/20 μl).
Surgical procedure for LAD occlusion.
A MI model was developed in female SD rats (200–250 g), as previously described (32). Briefly, isoflurane anesthesia was induced by spontaneous inhalation. The animals were mechanically ventilated with room air supplemented with oxygen (1.5 l/min) using a rodent ventilator (Model 683; Harvard Apparatus, South Natick, MA). Body temperature was carefully monitored with a probe (Cole-Parmer Instrument, Vernon Hill, IL) and was maintained at 37°C throughout the surgical procedure. The heart was exposed by left side limited thoracotomy, and the LAD was ligated with a 6-0 polyester suture 1 mm from the tip of the normally positioned left auricle. Male MSC (30 μl, 2 × 106) were injected into border area of the ischemic left ventricular (LV) wall at 10 min after LAD ligation. The chest was closed with 5-0 silk sutures. Approximately 10% of rats succumbed during surgical procedures.
Immunohistochemical analysis.
Immunohistochemical studies were performed on heart tissue at 4 wk after cell implantation. Heart tissue sections were harvested and fixed in 10% Formalin, and 5-μm-thick sections were prepared. DNA synthesis was determined with incorporation of BrdU into the DNA of dividing cells and cell proliferation by the presence of the cell-cycle-associated nuclear protein Ki67. Ki67 is expressed by cells in the G1, S, G2, and M phases and provides a means to estimate the percentage of cells in cycle at the time of death (34, 35). Incorporation of BrdU into DNA during S phase allows to obtain a history of cell division over time (34). Ki67 (Novocastra) and BrdU detection kit-I (Roche Diagnostics, Indianapolis) were used for studying DNA synthesis. The troponin I antibody (Sigma) was used to identify cardiomyocytes. Von Willebrand (vWF) antibody (Santa Cruz Biotechnology) and α-smooth muscle actin (SMA; Dako) were used to assess capillary and vascular density, respectively, whereas DAPI was used to identify nuclei. Fluorescent imaging was performed with an Olympus BX41 microscope (Olympus America) equipped with epiflourescence attachment, and images were recorded using a digital camera with MagnaFire 2.1 software. Fluorescence in situ hybridization (FISH) analysis of heart tissue samples was carried out using STAR*FISH Rat 12/Y Paints Protocol (CA-1631), as described previously (37). Confocal images were obtained with Leitz DMRBE fluorescence microscope equipped with a TCS 4D confocal scanning attachments (Leica).
Measurement of infarct size.
Fixed hearts were embedded in paraffin, and sections from apex, mid-LV, and base were stained with Masson Trichrome. An Olympus BX41 camera was used to obtain images of LV area on each slide using MagnaFire (Olympus) software. Fibrosis and total LV area of each image were measured using the Image-Pro Plus software (Media Cybernetics, Carlsbad, CA), and the percentage of the fibrotic area was calculated as shown: (fibrosis area/total LV area) × 100, as previously described (33).
Assessment of heart function.
Rats were anesthetized with pentobarbital sodium (40 mg/kg) by intraperitoneal injection. The right carotid artery was cannulated with a micro tip pressure transducer catheter (SPR-839; Millar Instruments) connected to MPCU-200 P-V signal conditioning hardware, which provided analog outputs of the time-varying ventricular pressure and volume signals for data acquisition. The inferior vena cava (IVC) was exposed, and IVC occlusion was performed by external compression. Hemodynamic changes and pressure-volume loops were recorded during steady state. LV systolic function was evaluated by maximum pressure, maximal change in pressure over time (dP/dtmax), minimum change in pressure over time (dP/dtmin), end-systolic pressure-volume relationship (ESPVR), and ejection fraction (EF) as previously described (35). Hemodynamic parameter analysis was carried out using Millar's PVAN software (Version 3.2).
Statistical Analysis
Experiments were performed in quadruplicate and repeated at least three times. Data are expressed as means ± SE. Statistical significance was assessed by one-way ANOVA followed by Bonferroni/Dunn testing. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Upregulation of Genes in NPY-pretreated MSC Under Hypoxic Conditions
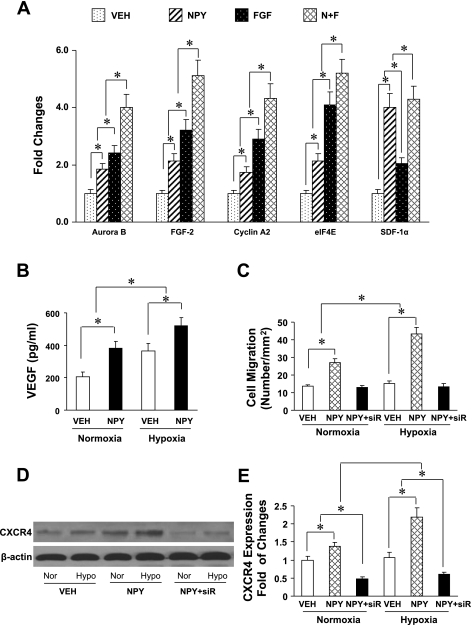
To determine the potential candidate factors for stimulation of cell proliferation, qPCR was performed to analyze NPY-pretreated MSC under hypoxic conditions (Fig. 1A). NPY significantly increased upregulation of aurora B, FGF-2, cycline A2, eIF4E, and SDF-1α compared with the vehicle-treated control group. The effect of NPY was similar to that of FGF, with the exception of the FGF group, which was more pronounced on aurora B, cyclin A2, and eIF4E than on SDF-1α. Combination of the FGF and NPY resulted in further increase of these factors. In addition, results from ELISA demonstrated that NPY-pretreated MSC produced significantly higher VEGF protein under normoxia compared with vehicle-treated control group and further increased after hypoxia (Fig. 1B).
Fig. 1.
Neuropeptide Y (NPY)-pretreated mesenchymal stem cells (MSC) induced expression of specific genes under hypoxic conditions. To determine the effect of NPY on cardiomyocyte differentiation and proliferation, quantitative real-time PCR was performed using rat MSC (A). NPY-pretreated MSC significantly increases expression of cell cycle regulators including aurora B kinase, fibroblast growth factor-2 (FGF-2), cyclin A2, eukaryotic initiation factor 4 E (eIF4E), and stromal cell-derived factor-1α (SDF-1α). The expression of genes was further increased by treatment with FGF and NPY. The expression of genes was significantly increased in FGF-treated group for aurora B kinase, cyclin A2, and eIF4E, respectively, compared with NPY treatment alone, but was not increased further for SDF-1α. Veh, vehicle-pretreated MSCs; N + F, NPY plus FGF-pretreated MSC (n = 4 in each group). *P ≤ 0.05 was considered statistically significant. All values were expressed as means ± SE. B: ELISA results for release of VEGF in vehicle and NPY-pretreated MSC groups under normoxic, immediately after 8-h hypoxic conditions. *P ≤ 0.05 was considered statistically significant. All values are expressed as means ± SE (n = 6 for each group). C: quantitative data for chemotaxis experiments by using transwells. *P ≤ 0.05 was considered statistically significant. All values are expressed as means ± SE (n = 4 in each group). NPY + siR, MSC-treated with small interfering RNA (siRNA) targeting CXCR4. D: Western blot to verify CXCR4 expression in NPY-treated MSCs. CXCR4 upregulation by NPY was determined by Western blotting under condition of normoxic and hypoxic conditions (A). Quantification of CXCR4 expression in NPY-treated MSC was shown in E. Nor, normoxia; Hypo, hypoxia.
CXCR4 is Involved NPY-induced Cell Migration Under Hypoxic Conditions
MSC pretreated with vehicle, NPY (10−8M), or siRNA were exposed to normoxic or hypoxic conditions and then added to the upper wells. The lower wells contained 600 μl medium with SDF-1α (100 ng/ml) for determination of the number of migrated cells. After 6 h incubation at 37°C, migrated cells were counted. We observed that cell migration was significantly increased in NPY-pretreated MSC (27.1 ± 3.4 cells/mm2) under normoxia compared with vehicle-treated control group (14.2 ± 0.8 cells/mm2), and this effect further increased after exposure to hypoxic conditions (44.6 ± 3.3 cells/mm2 vs. 14.6 ± 1.2 cells/mm2; Fig. 1C). However, the increase in the number of migrated cells was completely blocked by siRNA targeting CXCR4 gene.
Western blot analyses were carried out to verify whether NPY induces CXCR4 upregulation in MSC and whether upregulated CXCR4 could be knocked down by siRNA. The results from Western blots demonstrated that there was significant upregulation of CXCR4 in NPY-treated MSC under normoxic conditions compared with vehicle-treated MSC, and further increase was observed after exposure to hypoxic conditions (Fig. 1, D and E). This upregulation was completely blocked by siRNA. In addition, cell migration induced by NPY-pretreatment was also blocked by siRNA, suggesting that CXCR4 was likely involved in NPY-induced cell migration.
Induction of Tube Formation by NPY
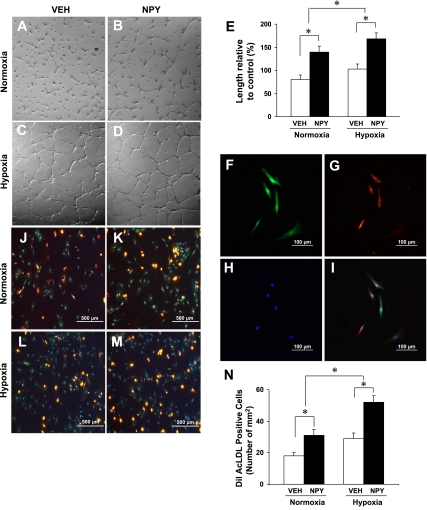
To examine whether NPY induces morphogenetic changes resembling capillary like-structure tube formation, MSC (3 × 105 cells/well) were seeded onto Matrigel-precoated wells and cultured in low-serum conditions (0.5% FCS) with or without NPY treatment. Our study demonstrated that the formation of these cellular networks is a dynamic process. MSC in the control group without NPY treatment exhibited small round shapes, isolated cells, and did not spread (Fig. 2A). When MSC pretreatment with NPY for 72 h, the cells were seeded onto Matrigel-precoated wells; they started migration and alignment, followed by the development of capillary tubes, sprouting of new capillaries, and finally the formation of the cellular networks. These cells became elongated, forming thin cords of interconnecting cells and branching to form a network of capillary-like structures (Fig. 2B). The number of tube-like structures was significantly increased in the NPY-treated group under normoxia compared with the vehicle-treated control group and was further increased in hypoxic conditions (Fig. 2, C–E).
Fig. 2.
NPY-pretreated MSC induced tube formation and differentiation. Tube formation was inspected under an inverted light microscope in vehicle-treated control group and NPY-treated group under normoxic (A and B) or hypoxic (C and D) condition. Quantitative data was shown in E (n = 5 in each group). *P ≤ 0.05 was considered statistically significant. All values were expressed as means ± SE. F–I: endothelial cells (ECs) differentiated from NPY-pretreated MSC. After NPY pretreatment for 72 h, MSC were differentiated into ECs, which were recognized as cells with spindle-shaped morphology and double-stained cells with FITC-lectin binding (F, green) and ,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine-labeled acetylated low density lipoprotein (DiI-Ac-LDL) uptake (G, red) identified under a fluorescent microscope. All nuclei were stained with 6-diamino-2-phenylindole (DAPI; H, blue). I: same area as in F–H except merged together. Magnification, ×200. The differentiated ECs derived from NPY-induced MSC were identified by double-staining with FITC-lectin and DiI-Ac-LDL. NPY significantly increased the number of double-positive cells under normoxic conditions (K) compared with vehicle control (J), and increased further after exposure to hypoxia (L and M). Quantitative assessment of double-positive cells was observed in N. All values were expressed as means ± SE (n = 6 for each group). *P ≤ 0.05 was considered statistically significant. Magnification, ×100.
NPY-induced Endothelial Cell Differentiation From MSC in Vitro Conditions
MSC were harvested from wild-type rats and cultured in 5% serum medium. After 72 h stimulation with NPY (10−8 M), differentiated ECs were recognized as cells with endothelial-like spindle-shaped morphology, and cells double-stained with FITC-lectin binding (Fig. 2F, green) and DiL-Ac-LDL uptake (Fig. 2G, red) were identified under a fluorescent microscope. All nuclei were stained with DAPI (Fig. 2H), and merged photographs were shown in Fig. 2I. Treatment with NPY significantly increased the number of DiI-Ac-LDL/FITC-lectin double-positive cells in normoxic conditions from 17.8 ± 0.8 [vehicle-pretreated MSCs (Veh) group; Fig. 2J] to 35.7 ± 2.5 cells/mm2 (NPY group; Fig. 4K), and this number of double-positive cells was further increased in hypoxic conditions from 29.4 ± 0.9 (Veh group; Fig. 2L) to 51.6 ± 1.9 cells/mm2 (NPY group; Fig. 2, M and N). The observations by fluorescent microscopy suggest that NPY enhanced MSC differentiation into endothelial cells.
Fig. 4.
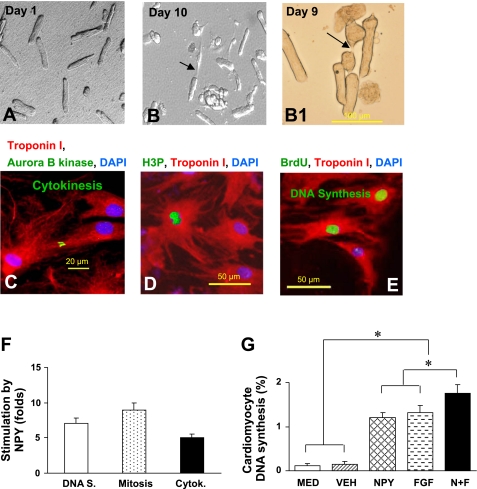
Effect of NPY on cell division and proliferation of rat adult cardiomyocytes. Adult cardiomyocytes in culture were photographed without treatment after attachment on day 1 (A). These cells were then treated with NPY (10−8 M) and pretreated with BrdU for 5 days. Several adult myocytes photographed in phase contrast were seen dividing after NPY treatment at days 9 and 10 (B and B1). Cells stained for aurora B kinase for cytokinesis (C), phosphorylated histone H3 (H3P) for mitosis (D), and BrdU for DNA synthesis (E) were observed. Color codes are given at top in C–E. Quantitative and count data for DNA synthesis (DNA S), mitosis, and cytokinesis (Cytok) are shown in F. Additive effect of NPY and FGF on DNA synthesis is shown in G. Data are means ± SE of 20 independent experiments; *P <0.05.
NPY Induces Proliferation of Neonatal and Adult Cardiomyocytes
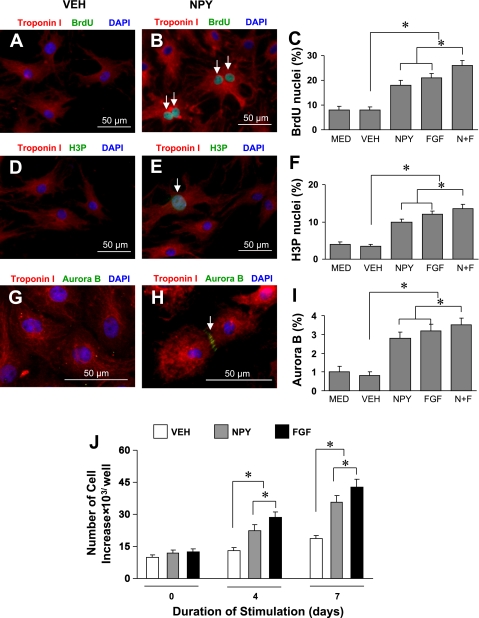
NPY-treated primary neonatal rat ventricular cardiomyocytes were analyzed for DNA synthesis (Fig. 3, A–C), mitosis (Fig. 3, D–F), cytokinesis (Fig. 3, G–I), and cell cycle activity (Fig. 3J) by fluorescent microscopy after 4 and 7 days. NPY induced a twofold increase in neonatal myocyte DNA synthesis to 18.3 ± 1.6% compared with nontreated myocytes, which was similar to the effect of FGF (21.5 ± 1.9%), a known stimulant for cell-cycle reentry of differentiated cardiomyocytes (4). Cumulative effects of NPY and FGF on cardiomyocyte DNA synthesis were the highest (26.6 ± 2.8%; Fig. 3C). A similar pattern was observed for cell mitosis (Fig. 3F) and cytokinesis (Fig. 3I). NPY significantly increased neonatal cardiomyocyte mitosis and cytokinesis to 10.2 ± 0.8% vs. 3.2 ± 0.3% and 2.7 ± 0.4% vs. 0.8 ± 0.2%, respectively, compared with vehicle treated myocytes. The effect of NPY was similar to that of FGF on neonatal cardiomyocyte mitosis and cytokinesis. The cumulative effect of NPY and FGF on cardiomyocyte mitosis (Fig. 3F) and cytokinesis (Fig. 3I) was the highest (14.2 ± 1.4% and 3.6 ± 0.3%, respectively). The proliferation rate of neonatal cardiomyocytes following NPY treatment was significantly higher, which could mimic the effects of FGF on cell proliferation than control group at days 4 and 7 (Fig. 3J).
Fig. 3.
Effect of NPY on cell division and proliferation of rat neonatal cardiomyocytes. Neonatal rat ventricular cardiomyocytes were stimulated by NPY (10−8 M) for 4 days. Cell cycle activity was detected by an antibody directed against 5-bromo-2′-deoxy-uridine (BrdU) for DNA synthesis (A for control, B for NPY-treated group, and C for quantitative data), phosphorylated histone H3 (H3P) for mitosis (D for control, E for NPY-treated group, and F for quantitative data), aurora B kinase for cytokinesis (G for control, H for NPY-treated group, and I for quantitative data). Cardiomyocyte proliferation was determined by cell count (J). Color codes are given at top. Data are means ± SE of 20 independent experiments. MED, not exposed to NPY; *P ≤ 0.05 was considered statistically significant.
NPY also induced proliferation of primary adult rat ventricular cardiomyocytes (Fig. 4, A–H). Primary adult rat ventricular cardiomyocytes were photographed without treatment after attachment on day 1 (Fig. 4A). These cultured cells were stimulated with NPY, labeled with BrdU for 5 days, and several adult myocytes were seen dividing (Fig. 4, B and B1). Cell cycle activity was determined by fluorescent microscopy after 10 days. NPY stimulated cytokinesis (Fig. 4C), mitosis (Fig. 4D), and DNA synthesis (Fig. 4E) in adult cardiomyocyte, and cell cycle activity was quantified using fluorescent microscopy (Fig. 4F). NPY induced 12-fold increase in adult cardiomyocyte DNA synthesis to 1.3 ± 0.2%, similar to FGF treatment (1.4 ± 0.2%; Fig. 4G). However, there was no statistical difference between the NPY and FGF groups.
Immunohistochemical Evidence for Cell Proliferation, Differentiation, and Angiogenesis In Vivo
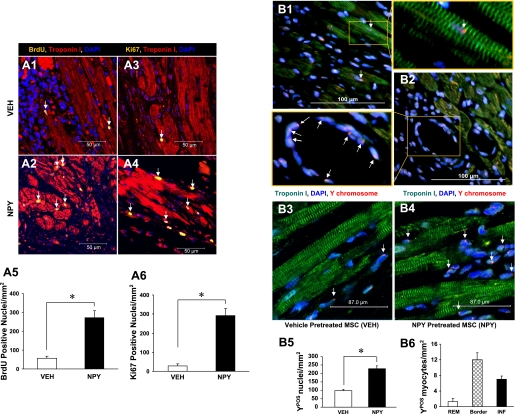
Cell proliferation was determined with BrdU and Ki67. The proliferation of newly formed myocytes from MSC was determined with BrdU, which was incorporated into the DNA of dividing cells and detected in their progeny (37). BrdU-positive nuclei could only indicate cell proliferation in cardiomyocytes and in noncardiomyocytes after MI. Therefore, this study focused on BrdU and troponin I double-positive cardiomyocytes. Additionally, Ki67, a nuclear protein expressed by cells G1, S, G2, and M, provided a means to estimate the percentage of cells in cycle at the time of death (35). Ki67- and BrdU-positive nuclei were primarily located in the damaged area of infarcted hearts (Fig. 5, A1–A4). There were significantly more BrdU- and Ki67-positive nuclei in the NPY group (Fig. 5A2 for BrdU and Fig. 5A4 for Ki67) compared with the vehicle treatment group (Fig. 5A1 for BrdU and Fig. 5A3 for Ki67). Quantitative analysis confirmed the number of BrdU- and Ki67-positive nuclei in the NPY group (271.2 ± 27.3/mm2 and 291.5 ± 38.6/mm2, respectively) as significantly higher than that in the vehicle-treated group (BrdU = 55.6 ± 10.1/mm2, Fig. 5A5; and Ki67 = 27.8 ± 12.2/mm2, Fig. 5A6). The presence of BrdU- and Ki67-positive nuclei at 30 days after cell implantation suggests that the regeneration of cardiomyocytes had occurred.
Fig. 5.
NPY-pretreated MSC enhanced cardiomyocyte DNA synthesis and myogenesis in vivo study. Cardiomyocyte DNA synthesis was analyzed by continuous intraperitoneally injection of BrdU for a week. NPY induced adult cardiomyocyte DNA synthesis by visualization of BrdU and Ki67 antibody (A1–A4). A significantly increased number of BrdU (A5) and Ki67-positive nuclei (A6) was observed in NPY-treated group compared with control (*P ≤ 0.05). Color codes are given at top. Original magnification, ×200. *P ≤ 0.05 vs. control group (n = 6 for each group). All values were expressed as means ± SE. The Y chromosome-positive nuclei (arrow, red color) are shown in the differentiated cardiomyocytes (B1) and ECs (B2) in female hearts after transplantation of NPY-pretreated MSC (data is not shown for the other groups). Fluorescent in situ hybridization analysis for Y chromosome in vehicle-pretreated MSC group (B3) and NPY-pretreated MSC group (B4) is shown. Myocytes were identified by troponin I (green). All nuclei were identified by DAPI staining (blue; original magnification, ×630, confocal imaging). B5: quantitative data for number of Y chromosome-positive nuclei after different treatments. Quantitation of data for distribution of Y chromosome-positive myocytes in remote zone (Rem), border zone (Border), and infarcted area (Inf) was observed in B6. *P ≤ 0.05 vs. control group; n = 6 for each group. All values were expressed as means ± SE.
In addition, male MSC-derived Y chromosome-positive (Ypos) nuclei (arrow, red point) in the differentiated cardiomyocytes (Fig. 5B1), and ECs (Fig. 5B2), were seen in female hearts for the NPY-pretreated MSC group (data not shown for other groups), indicating that NPY also induced MSC differentiation into cardiomycytes and ECs. When compared with that in the vehicle-treated MSC group (Fig. 5B3), heart tissue in the NPY-pretreated MSC group revealed a higher number of Ypos nuclei as determined by FISH (Fig. 5B4). Quantitative data demonstrated that the NPY-treated MSC group had a significant increase in the number of Ypos nuclei compared with the vehicle-treated control group (Fig. 5B5). The engrafted Ypos cells (17.6 ± 3.6%) exhibited evidence of myocyte commitment (8.8 × 102 Ypos myocytes/106 cardiomyocyte nuclei), based upon costaining with troponin I in NPY-treated MSC group. A quantitative study of transplanted cells based on Y chromosome tracking showed that Ypos myocytes were more distributed in the border zone than in the infarcted area and were rarely observed in the remote zone of the left ventricle (Fig. 5B6). Additional clusters of Ypos cells (82.4 ± 5.3% of engrafted cell population) were located within the interstitial compartment. These cells were not positive for cardiac marker troponin I and were believed to be nondifferentiated MSC.
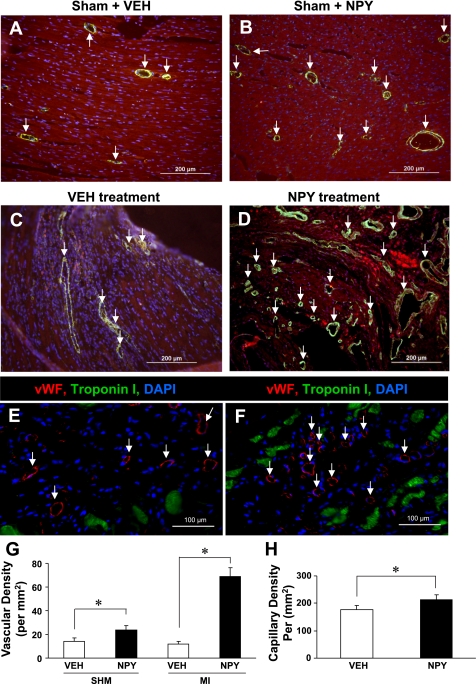
Results from the sham group (Fig. 6, A and B) demonstrated that vascular density, visualized by α-SMA staining, was significantly higher in the NPY group (24.6 ± 0.8 per mm2) compared with vehicle group (14.8 ± 0.6 per mm2). Interestingly, vascular density was further increased in the infarction zone (Fig. 6, C and D) in the NPY group (71.6 ± 2.4 per mm2) compared with vehicle group (12.5 ± 0.9 per mm2; P < 0.05; Fig. 6G).
Fig. 6.
NPY enhanced angiogenesis in vivo study. Results from vehicle or NPY direct injection into the hearts of sham group (A and B) and myocardial infarction (MI) group (C and D) demonstrated that vascular density visualized by α-smooth muscle actin and quantitative data (G). Myocytes were identified by troponin I (red). All nuclei were identified by DAPI staining (blue). *P ≤ 0.05 vs. control group; n = 6 for each group. All values were expressed as means ± SE. SHM, sham group. Capillary density was identified by Von Willebrand (vWF) antibody in vehicle-pretreated MSC group (E, white arrows) and NPY-pretreated MSC group (F, white arrows). H: quantitative analysis of capillary density with vWF antibody staining in the infarcted area. Color codes are given at top. *P ≤ 0.05 vs. Veh group; n = 6 for each group. All values were expressed as means ± SE.
When compared with the vehicle-treated MSC group (Fig. 6E), capillary density (as reflected by labeling of vWF, an EC marker) was significantly higher in the NPY-treated MSC group (Fig. 6F). Capillary density, detected at low-power magnification with vWF immunostaining, was 212.4 ± 20.0 cells/mm2 in NPY-treated MSC group and significantly higher than that found in the vehicle-treated MSC group (176.9 ± 15.2 cells/mm2; Fig. 6H).
NPY-pretreated MSC Reduces Fibrosis and Improves Cardiac Function After MI
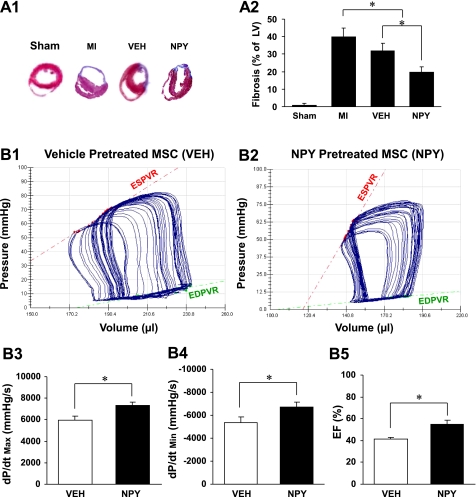
Figure 7A shows Masson-Trichome-stained LV sections from various treatment groups. Treatment with NPY resulted in remarkable reduction in fibrosis of the LV after MI compared with medium without cells (MI alone) or vehicle controls (Veh). Figure 7, A1 and A2, shows the percentage of fibrosis in hearts after permanent LAD occlusion and various treatments. Hearts receiving NPY-pretreated MSC exhibited significant reduction in fibrosis (20.3 ± 2.8%) compared with MI (39.8 ± 4.3%) and vehicle controls (Veh; 34.7 ± 4.1%; Fig. 7A2). In addition, there were significant differences between the MI and the vehicle-treated MSC group.
Fig. 7.
Effect of NPY on fibrosis and cardiac performance after MI. A1: 5-μm sections from heart slices staining with Masson-Trichome in various treatments. A2: percentage of fibrosis in hearts after permanent left anterior descending artery occlusion in various treatment groups. All values were expressed as means ± SE; n = 6 for each group. *P ≤ 0.05 was considered statistically significant. Left ventricular (LV) pressure-volume loops were obtained after 4 wk of administration of vehicle treatment (B1) and NPY treatment (B2), which showed differences in the end-systolic pressure-volume relation (ESPVR). The slope of the regression line is ventricular end-systolic elastance (red line). LV systolic [maximal change in pressure over time (dP/dtmax); B3] and diastolic [minimum change in pressure over time (dP/dtmin); B4] functions and ejection fraction (EF; B5) were measured by a pressure-volume conductance catheter system. Data are means ± SE; n = 6 in each group. *P ≤ 0.05 was considered statistically significant. EDPVR, end-diastolic pressure-volume relation.
Heart function was significantly improved in the NPY-treated group compared with the vehicle-treated group (Fig. 7, B1 and B2; data not shown for other groups). LV end-systolic elastance of the NPY-treated group was 2.3-fold greater (Fig. 7B2) than that of the vehicle-treated group (Fig. 7B1). End-systolic volume and end-diastolic volume of the vehicle-treated group were shifted rightward and showed a shallow ESPVR, indicating a character of dilated cardiomyopathy.
LV systolic function was determined by measuring dP/dtmax. dP/dtmax was significantly increased in the NPY-treated group (7,326 ± 436 mmHg/s) compared with the vehicle-treated group (5,952 ± 291 mmHg/s; Fig. 7B3). A similar pattern was observed in dP/dtmin, which reflects LV relaxation. dP/dtmin was notably improved in the NPY-treated group (−6,683 ± 461 mmHg/s) compared with the vehicle group (−5,348 ± 427 mmHg/s; Fig. 7B4). Additionally, EF, a load-dependent parameter, was higher in the NPY group (55.3 ± 3.8%) compared with the vehicle group (40.3 ± 4.1; Fig. 7B5).
DISCUSSION
Adult cardiomyocytes have generally been considered as terminally differentiated cells that have permanently withdrawn from the cell cycle (12, 15). Recent investigations have shown they possess a limited degree of cell cycle activity (14, 17). Although several investigators have reported regeneration of infarcted myocardium with BMSCs (26), homing of MSC to the infarction site and differentiation of MSC into cardiomyocytes is very limited for full cardiac functional recovery. The strategies to stimulate proliferation of differentiated cardiomyocytes from progenitor cells by pharmacological manipulations could be important in repopulating the infarcted myocardium. Our results reveal that NPY alone induced proliferation of neonatal and adult cardiomyocytes under in vitro conditions and NPY-pretreated MSC induced an additive effect on the repair of infarcted myocardium. Some of the newly formed vessels and cardiomyocytes had Ypos nuclei as determined by FISH (Fig. 5, B1 and B2), suggesting that NPY-pretreated MSC possibly undergoes angiomyogenesis. This would be a novel approach to promote MSC-based cardiac repair. However, the mechanisms underlying cardiomyocyte withdrawal from the cell cycle have not been studied extensively.
We have shown that MSC pretreatment with NPY under hypoxic condition induced pronounced upregulation of aurora B kinase, FGF-2, cycline A2, eIF4E, and SDF-1α compared with the control group. As discussed below, upregulation of these genes may play a crucial role in cell proliferation and differentiation into cardiomyocytes and ECs. Cyclin A2 has been reported to mediate cardiomyocyte mitosis in the postmitotic myocardium and to regulate both G1/S and G2/M in cultured mammalian cell lines (3). In addition, cyclin A2 induces cardiac regeneration after MI and prevents heart failure. FGF-2 is known to induce myocyte division (9). Exogenous FGF caused a 62% increase in the overall number of dividing cells (10). SDF-1α and its G protein-coupled receptor CXCR4 are also implicated in cardiogenesis (25). Aurora B kinase is a key regulator of chromosome segregation during mitosis (11). It has been reported that inhibition of aurora B kinase activity results in cytokinesis defects (11). eIF4E, a factor in the regulation of cell cycle and differentiation (36), is the mRNA cap-binding protein, which controls the rate-limiting reaction for translational initiation (18). The overexpression of eIF4E is responsible for initiating the translation of many polypeptides, including the angiogenic and growth-promoting factors (38), which may alter cellular morphology and enhance proliferation (6). Phosphorylation of eIF4E occurs in response to growth factor stimulation, and a positive correlation between eIF4E phosphorylation and the rate of protein synthesis exists in many cell types, including cardiomyocytes (20). One of the greatest attributes of NPY-pretreated MSC is their potential to supply growth factors and cytokines to repair tissue. In this study, BMSCs were extracted from SD transgenic male rats expressing green fluorescent protein (GFP). Several studies have failed to document MSC differentiation into cardiomyocytes in the infarcted heart. A significant number of these studies have relied solely on GFP to track cell fate following transplantation, with continued presence serving as an indication of engraftment. However, GFP labeling is known to diminish, which would silence the transgenic construct as cells proliferate and differentiate. Continued detection would, therefore, be obscured as differentiation continues (31). Therefore, the NPY-pretreated MSC from male SD rats employed in this study were able to trigger the expression of cell proliferation and migration-related genes in the myocardium of female SD rats and acquire the characteristics of cardiomyocytes and endothelial cells as revealed by FISH analysis. In our study, although we did not find direct evidence that NPY stimulated MSC differentiation into cardiomyocytes in vitro, cardiomyocytes derived from MSC in vivo were confirmed by colocalization of Y chromosome nuclei with cardiac specific marker (troponin I). In addition to NPY, it is known that transplanted MSC in the cardiac microenvironment may differentiate into cardiomyocytes in vivo. More recent studies suggest that the transplanted MSC may interact with paracrine factors released from local tissue and may facilitate the regenerative process (23).
CXCR4 is the receptor for a chemokine, CXCL12 (SDF-1). The SDF-1/CXCR4 axis has been confirmed to be involved in stem/progenitor cell migration and repair of MI (37). A previous study from our laboratory has demonstrated that CXCR4 overexpression on MSC leads to enhanced in vivo mobilization and engraftment of MSC into the ischemic area, where these cells promote angiogenesis and alleviate early signs of LV remodeling (37). The current study has shown that NPY induced CXCR4 upregulation on MSC. Hence, this increase in CXCR4 expression by NPY suggests a possible role for CXCR4 receptors in new vessel formation in the repair of infarcted myocardium. The mechanisms responsible for the salutary effects of NPY on postinfarction seem complicated, probably reflecting the multiple functions that NPY performs. The biological effects of NPY, such as cell proliferation, angiogenesis, and cardiomyocyte regeneration, play a significant role in postinfarction changes that can prevent heart failure via the paracrine pathway. NPY has been revealed to be angiogenic with equal potency and efficacy as bFGF and VEGF (22). VEGF and bFGF are the most potent angiogenic factors that stimulate migration, proliferation, and differentiation of ECs (30). Angiogenesis begins with activation of ECs, followed by their migration, proliferation, and differentiation into capillary tubes (19). We demonstrated that pretreated MSC-engrafted heart tissue had much less infarct scar and reduced ventricular dilation, indicating that one beneficial mechanism of NPY involves attenuation of pathological ventricular remodeling. In this study, we revealed that NPY exhibited stimulatory effects on the EC differentiation from MSC as confirmed by double staining of Dil-Ac-LDL and FITC-lectin. The treatment of MSC with NPY adds new ECs augmenting angiogenesis and, thus, leading to attenuated LV remodeling against MI.
MSC transplantation is a promising strategy in the treatment of MI. However, the exact timing for transplanting cells remains controversial because the infarct area appears to be swollen at 15 h after MI and softens at 24–48 h. Granulation tissue outlines the infarct at 3 to 4 days, and scar tissues mature at 2 to 3 mo. The ideal time point for cell delivery should be when the local microenvironment is less hostile for the transplanted MSC. Jiang CY et al. (13) reported that MSC transplantation at 1 wk post-MI exerted the best effects on cardiac function, antiapoptosis, and angiogenesis. However, many patients suffer acute MI due to thrombosis or other factors, which highlights the urgent need for immediate treatment. Our study demonstrated that pharmacological manipulation of MSC before transplantation with NPY is a novel, albeit less studied, concept in heart cell-based therapy, which may have important clinical significance.
Conclusion
Our data demonstrate that NPY contributes to myocardial repair by enhancing the number of differentiated cardiomyocytes via transplantation of NPY-pretreated cells into the infarcted myocardium. NPY exhibited stimulating effects on endothelial differentiation of MSCs in both in vitro and in vivo studies. NPY directly induces neonatal and adult cardiomyocyte cell cycle reentry. This presents a novel approach to cell-based cardiac repair by using NPY to accelerate the angiomyogenesis by transplanted MSC.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-089824 and HL-081859 (to Y. Wang) and R37-HL-074272 and HL-087246 (to M. Ashraf), and Veterans Affairs Grant BX000263 and Shriners Hospital for Children Grants Nos. 8570 and 8640 (to A. Balasubramaniam).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Christian Paul, Mahyar Pourriahi, and Ambreesh Chawla for technical assistance.
REFERENCES
- 1.Anonymous. NPY and cohorts in human disease. Proceedings of the 8th International NPY Meeting April 22–26, 2006 St. Petersburg, Florida, USA Peptides 28: 197–483, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Brooks G, Poolman RA, McGill CJ, Li JM. Expression and activities of cyclins and cyclin-dependent kinases in developing rat ventricular myocytes. J Mol Cell Cardiol 29: 2261–2271, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Cheng RK, Asai T, Tang H, Dashoush NH, Kara RJ, Costa KD, Naka Y, Wu EX, Wolgemuth DJ, Chaudhry HW. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res 100: 1741–1748, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Dai P, Nakagami T, Tanaka H, Hitomi T, Takamatsu T. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Mol Biol Cell 18: 2264–2273, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai Y, Ashraf M, Zuo S, Uemura R, Dai YS, Wang Y, Haider H, Li T, Xu M. Mobilized bone marrow progenitor cells serve as donors of cytoprotective genes for cardiac repair. J Mol Cell Cardiol 44: 607–617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene 23: 3189–3199, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Engel FB. Cardiomyocyte proliferation: a platform for mammalian cardiac repair. Cell Cycle 4: 1360–1363, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA 103: 15546–15551, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev 19: 1175–1187, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franciosi JP, Bolender DL, Lough J, Kolesari GL. FGF-2-induced imbalance in early embryonic heart cell proliferation: a potential cause of late cardiovascular anomalies. Teratology 62: 189–194, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Glotzer M. The molecular requirements for cytokinesis. Science 307: 1735–1739, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Haley SA, Zhao T, Zou L, Klysik JE, Padbury JF, Kochilas LK. Forced expression of the cell cycle inhibitor p57Kip2 in cardiomyocytes attenuates ischemia-reperfusion injury in the mouse heart. BMC Physiol 8: 1–13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang CY, Gui C, He AN, Hu XY, Chen J, Jiang Y, Wang JA. Optimal time for mesenchymal stem cell transplantation in rats with myocardial infarction. J Zhejiang Univ Sci B 9: 630–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajstura J, Leri A, Castaldo C, Nadal-Ginard B, Anversa P. Myocyte growth in the failing heart. Surg Clin North Am 84: 161–177, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Katz EB, Steinhelper ME, Delcarpio JB, Daud AI, Claycomb WC, Field LJ. Cardiomyocyte proliferation in mice expressing α-cardiac myosin heavy chain-SV40 T-antigen transgenes. Am J Physiol Heart Circ Physiol 262: H1867–H1876, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Kitlinska J. Neuropeptide Y in neural crest-derived tumors: effect on growth and vascularization. Cancer Lett 245: 293–302, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13: 962–969, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Makhlouf AA, McDermott PJ. Increased expression of eukaryotic initiation factor 4E during growth of neonatal rat cardiocytes in vitro. Am J Physiol Heart Circ Physiol 274: H2133–H2142, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Matou S, Colliec-Jouault S, Galy-Fauroux I, Ratiskol J, Sinquin C, Guezennec J, Fischer AM, Helley D. Effect of an oversulfated exopolysaccharide on angiogenesis induced by fibroblast growth factor-2 or vascular endothelial growth factor in vitro. Biochem Pharmacol 69: 751–759, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Morley SJ. Signal transduction mechanisms in the regulation of protein synthesis. Mol Biol Rep 19: 221–231, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Morris MJ, Pavia JM. Increased endogenous noradrenaline and neuropeptide Y release from the hypothalamus of streptozotocin diabetic rats. Brain Res 1006: 100–106, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Movafagh S, Hobson JP, Spiegel S, Kleinman HK, Zukowska Z. Neuropeptide Y induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. FASEB J 20: 1924–1926, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA 106: 14022–14027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol 157: 1703–1711, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics 4: 590–601, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res 77: 525–533, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Tamamori-Adachi M, Hayashida K, Nobori K, Omizu C, Yamada K, Sakamoto N, Kamura T, Fukuda K, Ogawa S, Nakayama KI, Kitajima S. Down-regulation of p27Kip1 promotes cell proliferation of rat neonatal cardiomyocytes induced by nuclear expression of cyclin D1 and CDK4. Evidence for impaired Skp2-dependent degradation of p27 in terminal differentiation. J Biol Chem 279: 50429–50436, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Tamamori-Adachi M, Ito H, Nobori K, Hayashida K, Kawauchi J, Adachi S, Ikeda MA, Kitajima S. Expression of cyclin D1 and CDK4 causes hypertrophic growth of cardiomyocytes in culture: a possible implication for cardiac hypertrophy. Biochem Biophys Res Commun 296: 274–280, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Tamamori-Adachi M, Ito H, Sumrejkanchanakij P, Adachi S, Hiroe M, Shimizu M, Kawauchi J, Sunamori M, Marumo F, Kitajima S, Ikeda MA. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res 92: e12–e19, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Tomanek RJ, Zheng W, Yue X. Growth factor activation in myocardial vascularization: therapeutic implications. Mol Cell Biochem 264: 3–11, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Toth ZE, Shahar T, Leker R, Szalayova I, Bratincsak A, Key S, Lonyai A, Nemeth K, Mezey E. Sensitive detection of GFP utilizing tyramide signal amplification to overcome gene silencing. Exp Cell Res 313: 1943–1950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Ahmad N, Wang B, Ashraf M. Chronic preconditioning: a novel approach for cardiac protection. Am J Physiol Heart Circ Physiol 292: H2300–H2305, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol 37: 1041–1052, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Haider H, Ahmad N, Zhang D, Ashraf M. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol 41: 478–487, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol 40: 736–745, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Willis AE. Translational control of growth factor and proto-oncogene expression. Int J Biochem Cell Biol 31: 73–86, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol 44: 281–292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou S, Wang GP, Liu C, Zhou M. Eukaryotic initiation factor 4E (eIF4E) and angiogenesis: prognostic markers for breast cancer. BMC Cancer 6: 1–12, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]