Abstract
The effects of aerobic exercise training (ET) on muscle sympathetic nerve activity (MSNA) and renal vascular responses to mental stress (MS) have not been determined in humans. We hypothesized that aerobic ET would reduce MSNA and renal vasoconstriction during MS. MSNA, mean arterial pressure (MAP), heart rate, renal blood flow velocity (RBFV), and peak oxygen uptake (V̇o2 peak) were recorded in 23 healthy adults. Fourteen subjects participated in 8 wk of aerobic ET, while nine subjects served as sedentary controls (Con). ET significantly increased V̇o2 peak (Δ18 ± 1%; P < 0.001) and decreased RBFV at rest (60 ± 4 to 48 ± 3 cm/s; P < 0.01), whereas Con did not alter V̇o2 peak or RBFV. ET did not alter resting MSNA (11 ± 1 to 9 ± 1 bursts/min) or MAP (84 ± 2 to 83 ± 2 mmHg), and these findings were similar in the Con group. MS elicited similar increases in MSNA (∼Δ2 bursts/min; P < 0.05), MAP (∼Δ15 mmHg; P < 0.001), and heart rate (∼Δ20 beats/min; P < 0.001) before and after ET, and the responses were not different between ET and Con. Likewise, MS elicited similar decreases in RBFV and renal vascular conductance before and after ET, and the responses were not different between ET and Con. Perceived stress levels during MS were similar before and after the 8-wk study in both ET and Con. In conclusion, ET does not alter MSNA and renal vascular responses to MS in healthy humans.
Keywords: autonomic control, blood pressure, mental arithmetic, muscle sympathetic nerve activity, renal vascular conductance
sympathoexcitation during mental stress has been implicated as a potential risk factor for several cardiovascular diseases, including hypertension (9), myocardial infarction (7, 26), and atherosclerosis (27, 36). Therefore, the methods for reducing sympathetic neural responses to mental stress are clinically relevant. It was recently reported that diet combined with aerobic exercise training decreased muscle sympathetic nerve activity (MSNA) during resting conditions and mental stress (34). Unfortunately, this study examined only obese women, and it was unclear whether the reductions of MSNA were due to diet, exercise training, or the combination of diet and exercise. Thus the influence of aerobic exercise training on MSNA responses to mental stress remains equivocal.
In addition to eliciting sympathoexcitation, mental stress consistently causes renal vasoconstriction in humans (13, 15, 21, 23, 33). Elevated renal vascular resistance has been associated with essential hypertension (28); thus it is important to study interventions that may blunt the reduction of renal vasoconstriction during mental stress. Aerobic exercise training has been shown to blunt the reduction of renal blood flow during mental stress in borderline hypertensive rats (19), yet no studies have examined the influence of aerobic exercise training on renal blood flow during mental stress in humans.
Therefore, the purpose of the present study was to determine the influence of aerobic exercise training on MSNA and renal blood flow responses to mental stress in men and women. We hypothesized that 8 wk of aerobic exercise training would reduce MSNA and renal vascular responses to mental stress. Reductions of MSNA and/or renal vasoconstriction during mental stress could have significant cardiovascular health benefits.
METHODS
Subjects.
Twenty-three healthy subjects (11 men and 12 women) participated in the study. All subjects were nonsmokers, nondiabetics, and were not taking any medications. All subjects were instructed to abstain from exercise, alcohol, and caffeine for 12 h before laboratory testing. The experimental protocol was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine, and all subjects gave written informed consent before the study. This study conformed to the provisions of the Declaration of Helsinki.
Experimental design.
Heart rate, mean arterial pressure (MAP), MSNA, and renal blood flow velocity were measured during 5 min of supine rest (baseline), 5 min of mental arithmetic (mental stress), and 5 min of supine rest (recovery) in all subjects before (Pre) and after (Post) the 8-wk training study. Subjects were randomly assigned to the exercise training group (n = 14; 8 men and 6 women) or sedentary control group (n = 9; 3 men and 6 women). Subjects in the exercise training group were permitted to select either running (n = 8; 4 men and 4 women) or stationary cycling (n = 6; 4 men and 2 women) as their exercise for the 8-wk training period. Peak oxygen uptake (V̇o2 peak) was also determined before and after the 8-wk study in all subjects.
Training protocol.
Exercise training consisted of running or cycling 4 times/wk for 8 wk. Subjects wore a Polar heart rate monitor (Polar S610; Polar Electro; New York, NY) during all exercising sessions to ensure that target heart rates were maintained. Training began by exercising for 20 min/day at a work rate sufficient to achieve 80% of subjects' maximum heart rate. Exercising times increased ∼5 min/wk until subjects reached 60 min of training per day by the end of the study. As training progressed, subjects ran faster or increased bike power output to maintain heart rate at 80% of maximum. In addition, subjects performed interval exercises twice a week after the first week of training. Intervals consisted of increasing exercise intensity throughout a given exercise period to obtain subjects' maximum heart rate with a recovery period between each high-intensity exercise bout. Runners performed one sprint interval and one hill interval per week. Subjects initially performed four intervals per day and were increased to seven per day as training progressed. Sprint intervals consisted of 30 s sprint, 1 min recovery, 1 min sprint, and 2 min recovery performed on a flat course. Hill intervals consisted of an uphill sprint and downhill recovery on a 0.1-mile course at 6% grade (weeks 2–5) or 10% grade (weeks 6–8). Stationary cyclists performed 2 days of bike intervals per week. Bike intervals consisted of increased power output for 30 s, 1 min recovery, 1 min increased power output, and 2 min recovery. All interval sessions were preceded and followed by 15 min of moderate exercise that elicited ∼60% of maximum heart rate.
Mental stress.
Mental stress was elicited by mental arithmetic. During mental stress, subjects repeatedly subtracted the number six or seven from a two- or three-digit number. Subjects answered verbally and were encouraged by the investigators to subtract as fast as possible. An investigator provided a new number to subtract from every 5–10 s. The subtraction number, six or seven, was randomized. Subjects were asked to rate their perceived stress following mental stress using a standard five-point scale of 0 (not stressful), 1 (somewhat stressful), 2 (stressful), 3 (very stressful), and 4 (very, very stressful) (2, 3). Subject-perceived stress levels were recorded after each mental stress trial.
Measurements.
Multifiber recordings of MSNA were measured directly by inserting a tungsten microelectrode into the peroneal nerve posterior to the fibular head. A reference electrode was inserted subcutaneously 2 to 3 cm from the recording electrode. Both electrodes were connected to a differential preamplifier and then to an amplifier where the nerve signal was band-pass filtered (700–2,000 Hz) and integrated at a time constant of 0.1 s to obtain a mean voltage display of nerve activity. Satisfactory recordings of MSNA were defined by spontaneous, pulse-synchronous bursts that did not change during arousal or stroking of the skin.
Duplex ultrasound (HDI 5000, ATL Ultrasound, Bothell, WA) was used to measure renal blood flow velocity. The renal artery was scanned using the anterior abdominal approach while the subject was lying supine. To scan the arteries, a curved-array transducer (2–5 MHz) with a 2.5-MHz pulsed-Doppler frequency was used. The probe insonation angle to the renal artery was <60°. The focal zone was set at the depth of the target artery. The transducer was held in the same place to record velocity tracings during each trial, and the data were obtained in the same phase of the respiratory cycle. Each cardiac cycle Doppler tracing was analyzed using the software of the ATL machine to obtain renal blood flow velocity measurements. A minimum of five heartbeats were averaged for each minute for the whole experimental protocol. The ratio of blood flow velocity and MAP was used as an index of renal vascular conductance.
Arterial blood pressure was measured using two techniques. Resting arterial blood pressure was measured three consecutive times (separated by ∼1-min intervals) using an automated sphygmomanometer and reported as a mean value. Beat-to-beat arterial blood pressure was recorded continuously via Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). The Finometer allowed us to determine precise changes in blood pressure during mental stress, whereas the sphygmomanometer allowed us to compare baseline arterial pressures. Arterial blood pressures are expressed as MAP. Heart rate was recorded using a three-lead electrocardiogram.
V̇o2 peak was determined by maximal graded exercise test on a cycle ergometer (Lode, The Netherlands; workload increased 30 W every minute to fatigue). Oxygen consumption was measured continuously during the graded exercise test (True One 2400, Parvo Medics). V̇o2 peak was accepted as the highest value obtained during the last 30 s of the graded exercise test. All experimental procedures were replicated for each subject at the end of 8 wk.
Data analysis.
Sympathetic bursts were identified from the inspection of mean voltage neurograms displayed by a computer program (Peaks, ADInstruments). MSNA was expressed as burst frequency and total activity [the sum of individual burst area expressed in arbitrary units (AU)]. Resting variables were analyzed using a one-within (time, Pre vs. Post) one-between (group, exercise vs. control) repeated-measures ANOVA. Data during the mental stress trial were analyzed using a two-within (condition, mental stress trial × time, Pre vs. Post) one-between (group, exercise vs. control) repeated-measures ANOVA. Heart rate, MAP, and MSNA were averaged minute by minute during the 5-min mental stress trial, whereas renal blood flow measurements were averaged over the entire 5-min mental stress trial. Significance was considered at a P value of <0.05. Results are expressed as means ± SE.
RESULTS
Resting baseline values for the aerobic exercise training group and control group are presented in Table 1. Age, height, weight, and body mass index were similar between groups. Resting heart rate, MAP, MSNA, and renal blood flow velocity were similar between groups before the study. V̇o2 peak increased in the exercise training group (Δ18 ± 1%; P < 0.001) but did not in the control group (Δ1 ± 1%). Despite this significant training effect, aerobic exercise training did not alter resting heart rate, MAP, and MSNA (Table 1). However, heart rate at 120 W of the maximal-graded exercise test was significantly reduced after 8 wk of exercise training (Δ−9 ± 2 beats/min; P < 0.01); this reduction of heart rate during submaximal exercise was not observed in the control group (Δ0 ± 3 beats/min). In addition to the alteration of V̇o2 peak and heart rate, exercise training reduced both renal blood flow velocity and conductance at rest (Table 1).
Table 1.
Baseline subject characteristics for exercise training and control groups
| Exercise (n = 14) |
Control (n = 9) |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age, yr | 25±1 | — | 25±1 | — |
| Height, cm | 171±2 | — | 169±2 | — |
| Body mass, kg | 73±3 | 71±3 | 71±5 | 70±6 |
| Body mass index, kg/m2 | 25±1 | 24±1 | 25±2 | 25±2 |
| Heart rate, beats/min | 66±4 | 64±4 | 76±5 | 75±4 |
| MAP, mmHg | 84±2 | 83±2 | 85±3 | 82±2 |
| MSNA, bursts/min | 11±1 | 9±1 | 9±1 | 11±2 |
| RBFV, cm/s | 60±4 | 48±3* | 60±4 | 59±3 |
| RVC, units | 0.69±0.04 | 0.59±0.04* | 0.70±0.04 | 0.72±0.05 |
| V̇o2peak, ml·kg−1·min−1 | 38±2 | 45±2* | 31±2 | 31±2 |
Values are means ± SE; n, number of subjects. Pre, pretraining, Post, posttraining; MAP, mean arterial pressure; MSNA, muscle sympathetic nerve activity (n = 14 for exercise, n = 7 for control); RBFV, renal blood flow velocity (n = 10 for exercise, n = 7 for control); RVC, renal vascular conductance; V̇o2peak, peak oxygen uptake.
P < 0.05 vs. corresponding pretraining value.
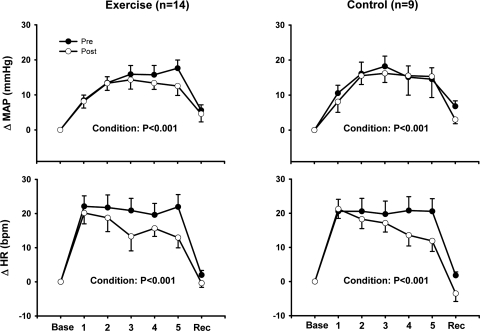
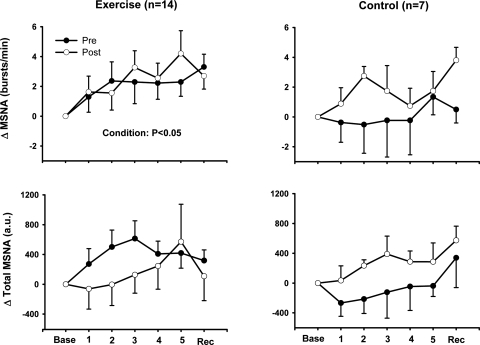
Mental stress increased heart rate and MAP in both the exercise training and control groups, and these increases were similar before and after the 8-wk study in both groups (Fig. 1). Heart rate and MAP returned to baseline levels during recovery in all trials. Mental stress elicited significant increases of MSNA in the exercise training group but not in the control group (Fig. 2). The increases in MSNA in the exercise training group were similar before and after the 8-wk exercise training regimen.
Fig. 1.
Changes in heart rate (HR) and mean arterial pressure (MAP) during mental stress (MS) after 8 wk of exercise training or sedentary control. MS increased HR and MAP in both our exercise training and control groups, and these increases were similar before (Pre) and after (Post) the 8-wk training study in both groups. Base, baseline; Rec, recovery; bpm, beats/min. Values are reported as means ± SE; n, number of subjects.
Fig. 2.
Changes in muscle sympathetic nerve activity (MSNA) during MS after 8 wk of exercise training or sedentary control. MS increased MSNA burst frequency in our exercise training group, and these increases were similar before and after training. AU, arbitrary units. Values are reported as means ± SE; n, number of subjects.
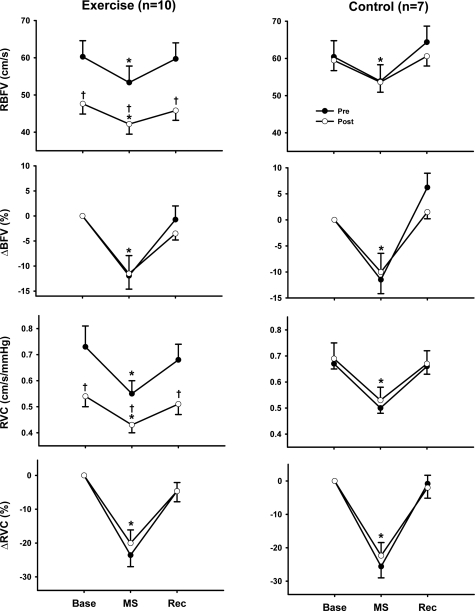
Figure 3 shows that mental stress elicited a consistent decrease in renal blood flow velocity during all trials, which returned to baseline levels during recovery. Whereas the changes in renal blood flow velocity were not influenced by the time (Pre vs. Post) or group (exercise training vs. control), exercise training decreased the absolute values of renal blood flow velocity during mental stress. Mental stress elicited a similar reduction of renal vascular conductance during all trials (Fig. 3).
Fig. 3.
Renal blood flow velocity (RBFV) and renal vascular conductance (RVC) during MS after 8 wk of exercise training or sedentary control. Aerobic exercise training significantly reduced RBFV at rest and during MS, but the magnitude of these responses (i.e., change in RBFV and RVC) was similar before and after the 8-wk study in both groups. RVC was calculated using MAP measurements obtained from the Finometer. There was only a small difference observed between blood pressure at rest obtained using a cuff and that of the Finometer. Values are reported as means ± SE; n, number of subjects. *P < 0.05 vs. baseline; †P < 0.05 vs. corresponding pretraining value.
Perceived stress levels during mental stress were similar before and after the 8-wk study in both the exercise training group (2.8 ± 0.2 vs. 2.7 ± 0.2 AU) and control group (3.0 ± 0.2 vs. 3.1 ± 0.2 AU).
DISCUSSION
This study examined the effects of aerobic exercise training on MSNA and renal blood flow responses to mental stress in humans. It is important to study nonpharmacological methods of reducing MSNA and/or renal vasoconstriction during mental stress, since mental stress is a well-recognized contributor to several cardiovascular diseases. The novel findings of this study are that aerobic exercise training does not alter MSNA and renal vascular responses to mental stress.
Several studies have attempted to determine whether aerobic exercise training reduces catecholamine responses to mental stress (1, 4, 5, 30, 31). In general, aerobic exercise training does not appear to influence catecholamines responses to mental stress, although one study reported that aerobic exercise training tended to reduce cardiovascular and catecholamine responses to mental stress (1). More recently, the effects of diet and aerobic exercise on sympathetic neural responses to mental stress were examined in obese women (34). Interestingly, diet and aerobic exercise not only reduced resting MSNA but also reduced MSNA responses to mental stress. Unfortunately, the study was restricted to obese women, and the experimental design did not allow the authors to definitively determine whether the reductions of MSNA were primarily due to the diet or the exercise training. The aim of the present study was to determine whether aerobic exercise training alters sympathetic neural responses to mental stress by conducting a well-controlled study in which aerobic exercise training was the primary, and only, intervention employed.
Our results indicate that aerobic exercise training does not alter MSNA responses to mental stress. These findings are in agreement with previous studies examining catecholamine responses to mental stress and exercise training (1, 4, 5, 30, 31). Differences between our findings and Tonacio et al. (34) are likely related to differences in subject demographics (obese women vs. healthy young men and women) and the experimental design (diet combined with aerobic exercise vs. only aerobic exercise). Nevertheless, our data indicate that aerobic exercise training does not alter sympathetic neural responses to mental stress in healthy men and women.
Aerobic exercise training did not alter resting MSNA in the present study. Wallin et al. (35) have reported a significant positive correlation between MSNA and renal norepinephrine spillover in humans. This finding indicates that in healthy humans, resting renal sympathetic nerve activity (RSNA) is comparable with or proportional to MSNA. If this relation is maintained following exercise training, this would suggest that RSNA was not changed in the present study because MSNA was not altered by exercise training. This finding would diminish the likelihood of an increase in RSNA as a possible factor in mediating the change in renal blood flow velocity and conductance after exercise training. However, this does not preclude the possibility of greater neurotransmitter release in the kidney to mediate lower renal vascular conductance following exercise training.
Additionally, it is relevant to note that the influence of aerobic exercise training on resting MSNA appears to be highly dependent on the preexisting health condition of an individual. Aerobic exercise training has been reported to decrease resting MSNA in patients with heart failure (6, 10, 25), hypertension (16), and acute myocardial infarction (22). In contrast, most (11, 24, 29, 32), but not all (12), studies performed in subjects without cardiovascular disease report that aerobic exercise training does not alter resting MSNA. Patients with cardiovascular disease (6, 10, 16, 22, 25) have much higher levels of resting MSNA compared with subjects without cardiovascular disease (11, 12, 24, 29, 32), suggesting that reductions of MSNA with aerobic exercise training may be partly dependent on the initial levels of MSNA at rest. Our data support this concept as our young, healthy subjects (with the low levels of MSNA at rest) demonstrated that aerobic exercise training did not alter resting MSNA.
Previous studies examining renal vascular responses to aerobic exercise training used whole body clearance of p-aminohippuate (14, 20) or plasma and urinary clearance of [131I]hippuran (8) to estimate renal plasma flow. However, these studies have led to conflicting results regarding the effects of aerobic exercise training on resting renal vascular flow and conductance. To our knowledge, the present study is the first to use ultrasound Doppler techniques to determine the effects of aerobic exercise training on renal blood flow velocity in humans. Our results indicate that aerobic exercise training leads to renal vasoconstriction. We recognize that the lower absolute resting renal blood flow velocity and conductance reported in the present study could be viewed as a negative consequence of exercise training. However, it is important to note that the kidneys are overperfused at rest with a very low arterial-venous oxygen difference. Therefore, the apparent decrease in renal blood flow reported in the present study might not be of physiological significance because arterial-venous oxygen difference can be increased. Furthermore, reduced blood flow to the kidneys may facilitate a more rapid redistribution of blood flow to skeletal muscle. The mechanisms mediating these changes at rest are unknown. Further studies are needed to address this important finding.
It is well documented that mental stress elicits renal vasoconstriction (13, 15, 21, 23, 33), yet the influence of exercise training on renal vasoconstriction during mental stress has not been previously examined in humans. However, aerobic exercise training has been shown to blunt the reduction of renal blood flow during psychological stress in borderline hypertensive rats (19). We hypothesized that aerobic exercise training would reduce renal vasoconstriction during mental stress. Contrary to our original hypothesis, aerobic exercise training did not alter changes in renal blood flow velocity or renal vascular conductance during mental stress. This response occurred despite lower absolute values of renal blood flow velocity.
In the current study, Doppler ultrasound could not accurately measure renal artery diameter. Thus we are unable to be certain that mental stress did not elicit changes in renal diameter. However, evidence suggests that pharmacological-mediated renal vasoconstriction (18) and vasodilation (17) do not alter diameter of the renal artery. Furthermore, the vessel we examined was a conduit and not a resistance vessel. Therefore, it is unlikely that changes in renal artery diameter during mental stress influenced the results of the study. However, if differences in renal diameter do occur as the result of exercise training, this could have affected our results and subsequent interpretation.
The training period (i.e., 8 wk) and stressor (i.e., 5 min of mental arithmetic) used in the present study are commonly employed in studies examining the influence of exercise training and/or stress on neural control of the cardiovascular system. However, these interventions are relatively short in duration compared with the stress experienced over a lifetime. It is likely that chronic levels of physical activity and stress contribute importantly to neural and renal responses. The balance between chronic physical activity and stress may be a key factor in the development of cardiovascular disease. Future studies should examine neural and renal responses to stress and exercise in individuals with cardiovascular complications. Based on the ability of aerobic exercise training to reduce resting MSNA in patients with cardiovascular disease (6, 10, 16, 22, 25) and obesity (34), it is possible that aerobic training would reduce sympathoexcitation and renal vasoconstriction during mental stress in patients with cardiovascular diseases.
In conclusion, this study examined the effects of aerobic exercise training on sympathetic neural and renal blood flow responses to mental stress in humans. Our results demonstrate that aerobic exercise training does not alter MSNA or renal vascular responses to mental stress in young, healthy individuals.
GRANTS
This project was supported by National Institutes of Health Grants DC-006549 and P01-HL-077670.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Charity Sauder, Jonathan Cook, Justin Shaw, Damian Dyckman, Matthew Kearney, Heather Kemp, and Amber Morgan for technical assistance.
REFERENCES
- 1.Blumenthal JA, Fredrikson M, Kuhn CM, Ulmer RL, Walsh-Riddle M, Appelbaum M. Aerobic exercise reduces levels of cardiovascular and sympathoadrenal responses to mental stress in subjects without prior evidence of myocardial ischemia. Am J Cardiol 65: 93–98, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol 454: 373–387, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter JR, Ray CA, Cooke WH. Vestibulosympathetic reflex during mental stress. J Appl Physiol 93: 1260–1264, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Claytor RP. Stress reactivity: hemodynamic adjustments in trained and untrained humans. Med Sci Sports Exerc 23: 873–881, 1991 [PubMed] [Google Scholar]
- 5.Cleroux J, Peronnet F, de Champlain J. Sympathetic indices during psychological and physical stimuli before and after training. Physiol Behav 35: 271–275, 1985 [DOI] [PubMed] [Google Scholar]
- 6.de Mello Franco FG, Santos AC, Rondon MU, Trombetta IC, Strunz C, Braga AM, Middlekauff H, Negrao CE, Pereira Barretto AC. Effects of home-based exercise training on neurovascular control in patients with heart failure. Eur J Heart Fail 8: 851–855, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Deanfield JE, Shea M, Kensett M, Horlock P, Wilson RA, de Landsheere CM, Selwyn AP. Silent myocardial ischaemia due to mental stress. Lancet 2: 1001–1005, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Dengel DR, Brown MD, Reynolds TH, 4th, Supiano MA. Effect of aerobic exercise training on renal responses to sodium in hypertensives. Med Sci Sports Exerc 38: 217–222, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand 177: 275–284, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail 9: 630–636, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Gardenghi G, Rondon MU, Braga AM, Scanavacca MI, Negrao CE, Sosa E, Hachul DT. The effects of exercise training on arterial baroreflex sensitivity in neurally mediated syncope patients. Eur Heart J 28: 2749–2755, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Seravalle G, Calhoun DA, Mancia G. Physical training and baroreceptor control of sympathetic nerve activity in humans. Hypertension 23: 294–301, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Hayashi N, Someya N, Endo MY, Miura A, Fukuba Y. Vasoconstriction and blood flow responses in visceral arteries to mental task in humans. Exp Physiol 91: 215–220, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kohno K, Matsuoka H, Takenaka K, Miyake Y, Nomura G, Imaizumi T. Renal depressor mechanisms of physical training in patients with essential hypertension. Am J Hypertens 10: 859–868, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Kuipers NT, Sauder CL, Carter JR, Ray CA. Neurovascular responses to mental stress in the supine and upright postures. J Appl Physiol 104: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE, Rondon MU. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension 49: 1298–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Manoharan G, Pijls NH, Lameire N, Verhamme K, Heyndrickx GR, Barbato E, Wijns W, Madaric J, Tielbeele X, Bartunek J, De Bruyne B. Assessment of renal flow and flow reserve in humans. J Am Coll Cardiol 47: 620–625, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Marraccini P, Fedele S, Marzilli M, Orsini E, Dukic G, Serasini L, L'Abbate A. Adenosine-induced renal vasoconstriction in man. Cardiovasc Res 32: 949–953, 1996 [PubMed] [Google Scholar]
- 19.McCoy DE, Steele JE, Cox RH, Wiley RL, McGuire GJ. Swim training alters renal and cardiovascular responses to stress in borderline hypertensive rats. J Appl Physiol 75: 1946–1954, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, Esler MD. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension 18: 575–582, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Middlekauff HR, Nguyen AH, Negrao CE, Nitzsche EU, Hoh CK, Natterson BA, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Impact of acute mental stress on sympathetic nerve activity and regional blood flow in advanced heart failure: implications for 'triggering' adverse cardiac events. Circulation 96: 1835–1842, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Mimura J, Yuasa F, Yuyama R, Kawamura A, Iwasaki M, Sugiura T, Iwasaka T. The effect of residential exercise training on baroreflex control of heart rate and sympathetic nerve activity in patients with acute myocardial infarction. Chest 127: 1108–1115, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer JB, Jr, Wolff HG, Winter OS. Studies in renal circulation during periods of life stress and accompanying emotional reactions in subjects with and without essential hypertension; observations on the role of neural activity in regulation of renal blood flow. J Clin Invest 29: 1227–1242, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray CA. Sympathetic adaptations to one-legged training. J Appl Physiol 86: 1583–1587, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton-Chalfen S, Hestrin L, Bietendorf J, Berman DS. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med 318: 1005–1012, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 99: 2192–2217, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Ruilope LM, Lahera V, Rodicio JL, Carlos Romero J. Are renal hemodynamics a key factor in the development and maintenance of arterial hypertension in humans? Hypertension 23: 3–9, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Sheldahl LM, Ebert TJ, Cox B, Tristani FE. Effect of aerobic training on baroreflex regulation of cardiac and sympathetic function. J Appl Physiol 76: 158–165, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Sinyor D, Peronnet F, Brisson G, Seraganian P. Failure to alter sympathoadrenal response to psychosocial stress following aerobic training. Physiol Behav 42: 293–296, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Sothmann MS, Hart BA, Horn TS. Sympathetic nervous system and behavioral responses to stress following exercise training. Physiol Behav 51: 1097–1103, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Svedenhag J, Wallin BG, Sundlof G, Henriksson J. Skeletal muscle sympathetic activity at rest in trained and untrained subjects. Acta Physiol Scand 120: 499–504, 1984 [DOI] [PubMed] [Google Scholar]
- 33.Tidgren B, Hjemdahl P. Renal responses to mental stress and epinephrine in humans. Am J Physiol Renal Fluid Electrolyte Physiol 257: F682–F689, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Tonacio AC, Trombetta IC, Rondon MU, Batalha LT, Kuniyoshi FH, Laterza MC, Suzuki PH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrao CE. Effects of diet and exercise training on neurovascular control during mental stress in obese women. Braz J Med Biol Res 39: 53–62, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol 491: 881–887, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med 325: 1551–1556, 1991 [DOI] [PubMed] [Google Scholar]