Abstract
Atherosclerosis originates as focal arterial lesions having a predictable distribution to regions of bifurcations, branches, and inner curvatures where blood flow characteristics are complex. Distinct endothelial phenotypes correlate with regional hemodynamics. We propose that systemic risk factors modify regional endothelial phenotype to influence focal susceptibility to atherosclerosis. Transcript profiles of freshly isolated endothelial cells from three atherosusceptible and three atheroprotected arterial regions in adult swine were analyzed to determine the initial prelesional effects of hypercholesterolemia on endothelial phenotypes in vivo. Cholesterol efflux transporter ATP-binding cassette transporter A1 (ABCA1) was upregulated at all sites in response to short-term high-fat diet. Proinflammatory and antioxidative endothelial gene expression profiles were induced in atherosusceptible and atheroprotected regions, respectively. However, markers for endoplasmic reticulum stress, a signature of susceptible endothelial phenotype, were not further enhanced by brief hypercholesterolemia. Both region-specific and ubiquitous (ABCA1) phenotype changes were identified as early prelesional responses of the endothelium to hypercholesterolemia.
Keywords: microarray, hypercholesterolemia, Oil Red O, endoplasmic reticulum stress, unfolded protein response
atherosclerosis is a progressive disease prevalent at arterial branches, curvatures, and bifurcations. These sites are characterized by complex hemodynamics, collectively referred to as disturbed blood flow, and are associated with regional atherosusceptibility (40). Endothelial cells (ECs), which orchestrate adaptive arterial homeostasis, display heterogeneous phenotypes throughout the circulation depending on their location and the functions they perform (1, 2). Lipoproteins and the endothelium play critical roles in the onset of atherosclerosis through the regulation of trans-endothelial lipoprotein flux in the subintima, the surface expression of monocyte adhesion molecules, and the recruitment of monocytic precursors to intimal macrophage foam cells (55). Transendothelial permeability to lipoproteins is increased in regions of atherosusceptibility in normal animals where the endothelial phenotype is characterized by a steady-state balance of proinflammatory and antioxidative gene expression that primes the cells for the initiation of atherosclerosis (40). In regions of susceptibility, atherosclerotic risk factors may shift this balance toward lesion formation. However, the early responses of endothelial phenotype to hypercholesterolemia have not been addressed in vivo where the spatial geometry related to lesion susceptibility is retained. We proposed that systemic hypercholesterolemia may influence endothelial phenotype differentially in regions of atherosusceptibility compared with sites protected from the onset of atherogenesis.
Cellular and molecular changes in the arteries of hyperlipidemic animals have been studied extensively (52). In prelesional stages of atherogenesis, extracellular lipoprotein accumulation in the intima of the aortic arch (AA) of rabbits (53) and hamsters (36) precedes the recruitment of monocytes and the appearance of macrophage foam cells. Since the early classic studies of aging and atherosclerosis in swine by Luginbuhl and Jones (28), swine have been recognized to be an appropriate model for human atherosclerosis. Furthermore, hypercholesterolemic diet induces accelerated disease progression (5, 20, 45, 47). An earlier study in prelesional adult swine (41) suggested that site specificity is a more dominant determinant of endothelial phenotype than brief exposure (2 wk) to hypercholesterolemia. Here we investigate the interplay of systemic hyperlipidemia and endothelial phenotype at atherosusceptible and protected sites in adult swine during this prelesional period and report that, in contrast to distinct site-specific endothelial gene expression profile changes, hypercholesterolemia induces the universal upregulation of cholesterol efflux transporter ATP-binding cassette transporter A1 (ABCA1).
MATERIALS AND METHODS
Detailed methods are provided as supplementary information online. The research was conducted according to the Guide for the Care and Use of Laboratory Animals. The protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania and Food and Drug Administration, Office of Research, Laurel, MD.
Animals.
Eleven gonadally intact male swine were raised to sexual maturity (6 mo old, ∼250 lbs) on a normal (standard commercial) diet. Six swine were maintained on the normal diet (NC), and five swine were fed an isocaloric high-fat (15%) and cholesterol (1.5%) diet (HC) for 2 wk.
Cell isolation.
At tissue harvest, ECs were isolated from three atherosusceptible and three atheroprotected regions by gentle scraping as described previously [Table 1 and Fig. S1 (Supplemental material for this article can be found on the American Journal of Physiology: Heart and Circulatory Physiology website.)] (40). Cells were transferred to lysis buffer for RNA extraction.
Table 1.
Lipid deposition in regions of arterial endothelial isolation
| No. of Arterial Regions With Positive Oil Red O Staining |
||||
|---|---|---|---|---|
| Arterial Regions of Endothelial Cell Isolation | Normal | Hypercholesterolemic | Fisher's exact P value | |
| Atherosusceptible regions | ||||
| AA | 1-cm2 susceptible region located at the inner-curve and lateral walls of the aortic arch | 1/6 | 5/5 | 0.013 |
| RB | 0.5 cm of proximal susceptible region of the renal artery | |||
| AbA | 1-cm2 susceptible region 2 cm distal to the branching of the renal artery | 1/4 | 5/5 | 0.048 |
| Atheroprotected regions | ||||
| C | 1-cm2 protected region in the straight section of the artery | 0/5 | 1/5 | 0.500 |
| DT | 1-cm2 protected region located from an undisturbed flow region of the dorsal descending thoracic aorta | 0/5 | 1/3 | 0.375 |
| RA | 1-cm-long protected region 1.5 cm distal to the renal artery ostium | 0/5 | 1/4 | 0.444 |
AA, aortic arch; RB, renal branch; AbA, abdominal aorta; C, carotid artery; DT desending thoracic aorta; RA, renal artery.
Gene expression and microarray analysis.
High-quality total RNA (100 ng) was linearly amplified (42) and hybridized to custom-printed porcine microarrays (ArrayExpress A-CBIL-16). Cy5-labeled sample mRNA was combined with Cy3-labeled common reference RNA that consisted of aRNA amplified from pooled total RNA from all regions. Microarrays were scanned with an Agilent DNA Microarray Scanner, and the images were analyzed with Agilent Feature Extraction Software (version 9.1).
Data preprocessing and print-tip loess normalization were performed using the Bioconductor marray package (version 1.12.0) for R (version 2.4.0). Differential gene expression was performed with PaGE 5.1.7 (http://www.cbil.upenn.edu/PaGE) (18). Genes with <25% false discovery rate were considered differentially expressed.
The complete MIAME compliant annotated study has been deposited in the public repository ArrayExpress (E-CBIL-43). Detailed MIAME compliant annotation and data for this study are also available for user-friendly querying at www.cbil.upenn.edu/RAD (RAD study_id=3608).
Quantitative real-time PCR.
cDNA was reverse transcribed from 0.5–1 μg total endothelial RNA. PCR primers for genes of interest are listed in Table S1. Quantitative real-time PCR was performed using LightCycler FastStart DNA Master SYBR Green I on a LightCycler System (Roche Applied Science, Indianapolis, IN). For each sample, gene expression was normalized to glyceraldehydes-3-phosphate dehydrogenase expression (GAPDH). Statistical significance of gene expression with HC treatment compared with NC controls was assessed with Student's t-test.
Immunohistochemistry.
Arterial tissue was frozen in optimum-cutting temperature compound (Sakura Finetek, Torrance, CA) immediately after harvest. Sections (8 μm) were stained for lipid deposition and the expression of ABCA1 protein using Oil Red O and an antibody against ABCA1 (clone ABH.10; ab18180; Abcam), respectively. ABCA1 was detected using the Cell and Tissue Staining Kit (HRP-DAB; R&D Systems, Minneapolis, MN) according to manufacturer's instructions. Primary antibody was omitted or replaced by mouse anti-KLH (12B4.G3.A8, Abcam; ab34607) in negative control slides. Statistical significance of the number of Oil Red O-positive sections with HC treatment compared with NC control samples was assessed with Fisher's exact test.
RESULTS
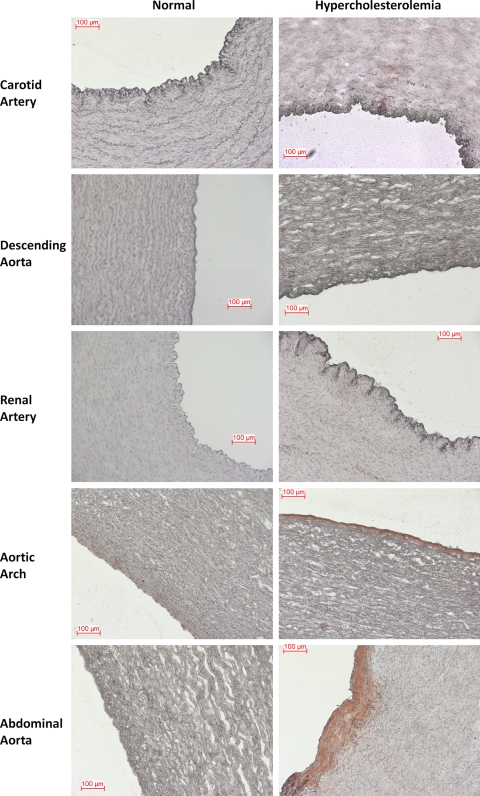
Hypercholesterolemia induced over a period of 2 wk resulted in significant increases in total cholesterol, HDL, and triglycerides in swine (Table S2). Oil Red O staining indicated increased lipid deposition in regions susceptible to atherosclerosis (Fig. 1), but there was no histological evidence of monocytic cell recruitment, edema, or macrophage foam cells. The number of samples showing lipid deposits with HC diet was significantly higher in atherosusceptible regions of AA and abdominal aorta (AbA) when compared with the same regions with NC diet (Table 1). However, atheroprotected regions [common carotid artery (C), descending thoracic aorta (DT), and renal artery (RA)] displayed no significant differences (Table 1). These data demonstrate that the tissue is prelesional and that the molecular changes in endothelial phenotype reported below predate an inflammatory response.
Fig. 1.
Oil Red O staining in multiple arterial regions of normal and hypercholesterolemic animals. Frozen sections from three atheroprotected (carotid, descending artery, and renal artery) and two atherosusceptible (aortic arch, abdominal aorta) samples were stained with Oil Red O, followed by hematoxylin, for the detection of lipid deposits and cell histology. Scale bar is 100 μm.
Endothelial ABCA1 is universally upregulated in response to HC diet.
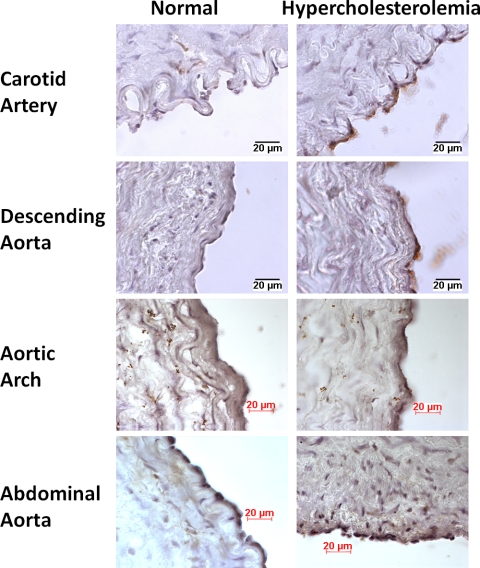
A limited number of endothelial genes were differentially expressed in response to short-term high-fat diet in atherosusceptible and atheroprotected regions compared with standard diet (Table 2). ABCA1 was the only endothelial gene that was upregulated in both atherosusceptible and atheroprotected regions in response to brief hypercholesterolemia. This gene encodes for a protein that functions as a cholesterol efflux pump in cellular lipid removal (24). Protective effects have been shown in mice overexpressing ABCA1, which resulted in decreased aortic atherosclerosis, increased high-density lipoprotein (HDL), and decreased plasma cholesterol (22). Mutations in this gene are associated with Tangier's disease and familial HDL deficiency (32). Quantitative real-time PCR confirmed that HC diet upregulated ABCA1 mRNA expression in the endothelium from all six arterial regions (Fig. 2). Heterogeneity in ABCA1 expression in different EC lines has been reported. ECs from human umbilical vein have higher ABCA1 expression compared with cells from human aorta (25). In the present study, we also observed differences in basal expression levels of ABCA1 in different regions in normal animals (Fig. 2) and different levels of induction in ABCA1 expression in each region (Fig. 2). Consistent with enhanced transcript expression, elevated ABCA1 protein expression was observed by immunohistochemistry in response to HC diet (Fig. 3).
Table 2.
Effects of HC diet on endothelial gene expression
| Upregulated with HC diet |
Downregulated with HC diet |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene symbol | Gene identification | Confidence* | Gene symbol | Gene identification | Confidence* | |||||
| Atherosusceptible regions | ||||||||||
| ABCA1 | ATP-binding cassette, subfamily A, member 1 | 0.96 | NOV | Nephroblastoma overexpressed | 0.75 | |||||
| APOE | Apoliporotein E | 0.90 | SERPINI1 | Serpin peptidase inhibitor 1 | 0.75 | |||||
| LGALS3 | Galectin 3 | 0.80 | ||||||||
| SPARC | Acidic cysteine-rich secreted protein | 0.75 | ||||||||
| Atheroprotected regions | ||||||||||
| ABCA1 | ATP-binding cassette, subfamily A, member 1 | 0.99 | FNDC1 | Fibronectin type III domain containing 1 | 0.80 | |||||
| CYBB | NADPH oxidase heavy chain subunit | 0.87 | CYBRD1 | Cytochrome b reductase 1 | 0.80 | |||||
| PLTP | Plasma phospholipid transfer protein | 0.87 | ABLIM3 | Actin binding LIM protein family member 1 | 0.80 | |||||
| FOLR1 | Folate receptor 1 | 0.81 | ||||||||
| GLRX | Glutaredoxin | 0.81 | ||||||||
| CSK | C-SRC kinase | 0.77 | ||||||||
| CYBA | NADPH oxidase light chain subunit | 0.77 | ||||||||
| MAN2B1 | Mannosidase, α, class 2B, member 1 | 0.77 | ||||||||
| RNF128 | Ring finger protein 128 | 0.77 | ||||||||
| SCD | Stearoyl-CoA desaturase | 0.77 | ||||||||
| YKT6 | YKT6 v-SNARE protein | 0.77 | ||||||||
Estimated confidence based on PaGE permutation algorithm. 1.0 indicates 100% confidence that the gene is differentially expressed.
Fig. 2.
ATP-binding cassette transporter A1 (ABCA1) gene expression in normal and hypercholesterolemic animals. ABCA1 transcript expression was normalized to glyceraldehydes-3-phosphate dehydrogenase (GAPDH) in normal and hypercholesterolemic animals in six different regions. AA, aortic arch; AbA, abdominal aorta; RB, renal branch; C, common carotid artery; DT, descending thoracic aorta; RA, renal artery; NC, normocholesterolemia; HC, hypercholesterolemia. Data are presented as means ± SE. Significance was assessed by one-sided (for upregulation) t-test. *P < 0.05 and **P < 0.01.
Fig. 3.
ABCA1 protein expression in normal and hypercholesterolemic animals. Arteries were frozen in optimum-cutting temperature compound, sectioned, and stained with an antibody against ABCA1 antigen using standard immunohistochemistry techniques. Scale bar is 20 μm.
Liver X receptor α (LXRα) controls the expression of the ABC superfamily of membrane transporters, including ABCA1 (58). LXRα targets stearoyl-CoA desaturase (SCD) and plasma phospholipid transfer protein (PLTP) that were also upregulated (Table 2) in atherosusceptible regions, suggesting that the mechanism of ABCA1 upregulation may be through increased expression of LXRα. Consistently, hypercholesterolemic diet induced 14 and 27% increase in LXRα expression in AA and DT, respectively, but the difference did not reach statistical significance (Fig. S2).
In contrast to ABCA1, induced gene expression profiles were regionally distinct at susceptible and protected sites.
Proinflammatory gene expression is induced in atherosusceptible regions.
In atherosusceptible sites (AA, renal branch, AbA), HC diet induced the expression of four genes and suppressed the expression of two genes when compared with NC diet (Table 2). Four of these genes are functional in endothelial inflammation. Galectin 3 (LGALS3) , a chemoattractant for monocytes and macrophages (49), was upregulated with HC diet. Galectin 3 induction by cholesterol treatment has been observed in cultured ECs consistent with our results (37). Secreted protein acidic and rich in cysteine (SPARC), which plays a role in leukocyte migration (23) and increases EC permeability (15), was also upregulated with HC diet. Downregulation of serpin peptidase inhibitor 1 (SERPINI1), an anti-inflammatory gene (39), and nephroblastoma overexpressed (NOV), which promotes EC survival (27), were also noted. Upregulation of galectin 3 and SPARC as well as downregulation of SERPINI1 and NOV indicate a proinflammatory endothelial gene expression profile primed for monocyte recruitment in atherosusceptible regions with HC treatment. However, monocyte accumulation in subendothelial regions of atherosusceptible sites was not observed by histological staining, consistent with a previous study that also did not detect monocytes in the subendothelium with 2 wk of hypercholesterolemic diet in rabbits (53).
Antioxidative gene expression is induced in atheroprotected regions.
In atheroprotected sites (C, DT, RA), HC diet induced the expression of 11 genes and suppressed the expression of 3 genes when compared with the NC diet (Table 2). Several genes related to endothelial redox balance were upregulated by HC diet. Genes encoding the light chain and heavy chain subunits of the superoxide-generating endothelial NADPH oxidase were upregulated. In counterbalance, however, several antioxidant genes were also induced in these regions. Glutaredoxin transcript, which has been shown to provide antioxidant protection in atherosclerotic endothelium (38), was upregulated. Folate receptor 1 (FOLR1), which has a high affinity for folic acid (19), had increased expression in response to HC diet. Upregulated FOLR1 provides antioxidant protection since folic acid increases the bioavailability of endothelial nitric oxide synthase cofactor BH4, resulting in increased nitric oxide production and decreased intracellular reactive oxygen species (ROS; see Ref. 34). SCD mRNA was also induced in response to HC diet. SCD protein desaturates stearic acid and converts it into oleic acid, which quenches endothelial ROS (31) and inhibits the expression of proinflammatory cytokines and adhesion molecules. Furthermore, PLTP, which has been shown to prevent endothelial dysfunction by delivering antioxidant vitamin E from lipoproteins to ECs, was also upregulated (10). Collectively, HC diet induced an antioxidative endothelial gene expression profile in atheroprotected regions.
Endothelium is not activated in response to brief hypercholesterolemia.
Early atherogenesis is characterized by an inflammatory response in ECs resulting in the expression of adhesion molecules for monocytes (26). In a previous study, we showed no difference in the expression of endothelial adhesion molecules vascular cell adhesion molecule (VCAM) 1 and E-selectin with 2-wk HC diet in swine, indicating that endothelium is not yet activated in these regions (41). However, we recently demonstrated the partial activation of unfolded protein response (UPR) pathway in response to the presence of endoplasmic reticulum (ER) stress in ECs from atherosusceptible regions in normal swine aorta (7). We therefore studied the effects of a 2-wk HC diet on the gene expression of ER stress marker HSPA5 and UPR signaling molecule ATF6α and transcription factor XBP1 in the endothelium from the atherosusceptible AA and atheroprotected DT (29). Consistent with our previous findings, expression of these molecules was higher in AA compared with DT (Fig. 4), but no difference in the expression of ER stress-related genes was observed between NC animals and HC animals, indicating that the short-term exposure to the HC diet did not further activate the UPR pathway (Fig. 4).
Fig. 4.
Endoplasmic reticulum stress and unfolded protein response pathway gene expression in normal and hypercholesterolemic animals. Transcript expression was normalized to GAPDH in normal and hypercholesterolemic animals in two different regions. HSP, heat shock protein; ATF6α, activating transcription factor 6α; XBP1, X-box binding protein 1. Data are presented as means ± SE. Significance was assessed by Student's t-test. No significance was found between NC and HC samples.
DISCUSSION
Initiation of atherosclerosis occurs at predictable sites despite exposure to systemic risk factors such as elevated low-density lipoprotein (LDL) (8). The focal nature of disease initiation implicates the importance of local changes of endothelial phenotype at the earliest phase of lipid-induced atherogenesis. Here, using an unbiased transcript profiling approach, we distinguish between site-specific endothelial responses predisposing to atherosclerosis and more widespread systemic responses of the endothelium unrelated to regional heterogeneity in vivo. Endothelial upregulation of ABCA1 is an early universal response to a short-term high-fat diet.
Endothelial phenotype heterogeneity in the vascular system is well established (1, 2, 8, 40); however, few studies have addressed site-specific changes in endothelial phenotype in response to atherogenic stimuli (57). Deng et al. (9) demonstrated distinct responses of cultured coronary artery and saphenous vein ECs to oxidized LDL, tumor necrosis factor-α and interleukin-1β. Few genes were commonly induced, but significant differences in oxLDL-induced gene expression were observed based on the cell source. The distinct in vivo spatial response of the endothelium to brief hypercholesterolemia demonstrated in the present study likely plays an important role in modifying the steady-state endothelial gene expression toward disease initiation in atherosusceptible regions.
The accumulation of lipoproteins in the arterial intima is a hallmark of atherosclerosis. Flux of LDL, the most abundant atherogenic plasma lipoprotein, in the arterial wall is controlled by endothelial permeability (56). However, the permeability of the endothelium is not uniform throughout the vascular bed. Our results are in agreement with the observation that the endothelium of atherosusceptible regions in healthy animals is more permeable to LDL than the adjacent atheroresistant endothelium (51).
ABCA1 was the only gene that was upregulated in both susceptible and protected regions in response to hypercholestrolemic diet. Differences in regional expression levels in control animals were also noted. Zhu et al. (60) reported increased expression of ABCA1 transcript in DT of normal C57/BL6 mice compared with AA. Our results are in contrast with their findings and may reflect differences in animal models used.
ABCA1 is a 254-kDa cytoplasmic membrane protein that plays an important role in reverse cholesterol transport by facilitating the release of cellular phospholipids and cholesterol from the plasma membrane to apolipoprotein A-I (ApoA-I) and HDL (24). Plasma levels of HDL, an antiatherogenic lipoprotein, are inversely correlated with coronary artery disease (33). Mutations in ABCA1 gene lead to reductions in plasma HDL and increased risk of cardiovascular pathology as observed in Tangier disease (4, 46, 48) and familial HDL deficiency (6, 30). ABCA1 overexpression in mice has been shown to reduce atherosclerosis, whereas ABCA1 knockout mice have increased atherosclerotic lesions (22). Therefore, in the present study, an increase in ABCA1 expression in ECs may reflect a protective role in maintaining cholesterol homeostasis in the artery wall in response to an atherogenic risk factor.
In vitro studies have demonstrated upregulation of ABCA1 transcript and protein in response to native LDL and cholesterol in cultured ECs; this resulted in cholesterol efflux to ApoA-I (25, 59). However, oxidized LDL has been reported to downregulate ABCA1 transcript and protein expression in cultured ECs by inhibiting the LXR and to inhibit ApoA-I-mediated free cholesterol efflux from the cells (61). It is plausible that lipids detected by Oil red O staining in the atherosusceptible regions after a 2-wk HC diet were native LDL that was not oxidized to a critical level, since endothelial ABCA1 was not downregulated. Additionally, induction of ER stress markers and adhesion molecules, VCAM-1 and E-selectin, which are known to be upregulated with oxidized LDL (13, 50), was not observed. With the use of an endothelial/smooth muscle cell coculture system, ABCA1 has been demonstrated to modulate the cell-mediated oxidative modification of LDL (44). The authors proposed a role for ABCA1 in secretion of ROS by artery wall cells. It is plausible that initial induction of ABCA1 by hypercholesterolemia is important to regulate cellular cholesterol and is protective against atherosclerosis, but its sustained upregulation leads to increased ROS levels in the artery wall that result in LDL oxidation and activation of the endothelium in later stages of atherosclerosis.
Swine spontaneously develop atherosclerotic lesions, and their distribution is very similar to humans, including regions of the first 2 cm of the right and left carotid arteries, arterial curvatures, and proximal regions of the branch orifices (3, 12, 17, 21, 54). Furthermore, hypercholesterolemic diet induces accelerated disease progression similar to humans (5, 20, 45, 47). However, unlike human hypercholesterolemic patients, hypercholesterolemic swine exhibit high plasma HDL and low triglyceride levels (Table S1) (11, 14, 35); therefore, we cannot ignore a possible protective effect of increased plasma HDL in our experiments.
Cholesterol homeostasis in ECs is not well understood. Although hypercholestrolemia significantly increases free cholesterol content in the endothelium in vivo and in vitro, cholesteryl esters prevalent in lipid-laden foam cells do not accumulate in ECs (11, 16, 43). The degree of cholesterol accumulation in ECs is much lower than in smooth muscle cells and macrophages, suggesting more effective control of cholesterol homeostasis in the endothelium (11). Our results implicate an important role for ABCA1 in maintaining vessel wall homeostasis by regulating endothelial cholesterol equilibrium in response to hypercholesterolemia. We recognize that our studies are limited to 2 wk hypercholesterolemia, and ABCA1 protection may be both transient and/or site-specific at a later stage of atherosclerosis development. However, here we report a specific difference in gene responses that separate the general ABCA1 response to hypercholesterolemia from the site-specific responses of atherosusceptible and atheroprotective regions.
Finally, the overall small number of gene expression changes in the endothelium when challenged by hypercholesterolemia suggests that the study is capturing the earliest phenotypic changes to accommodate the hypercholesterolemic environment: ubiquitous protective enhanced reverse cholesterol transport and regional phenotype imbalances that later result in atherosclerosis or resistance to its development.
GRANTS
This work was supported by an American Heart Association predoctoral fellowship (0315286U) and National Institutes of Health Grants HL-062250 and HG-004521.
DISCLOSURES
The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the U.S. Food and Drug Administration, the Department of Health and Human Services or the Public Health Service.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Elisabetta Manduchi for help with the bioinformatics analysis, Tracy Murray and Jennifer Peregoy for help with the animal husbandry, Dr. Daniel Rader for the plasma lipid measurements, and Dr. Yun Fang for helpful discussions.
REFERENCES
- 1.Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ Res 100: 174–190, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bijlenga G, Dahme E, Detweiler DK, Gresham GA, Grunberg W, Howard AN, Kagan AR, Kaplan MM, Van Nie CJ, Rubarth S, Sternby NH, Stunzi H, Uemura K, Whitney JC. Comparative studies of atherosclerosis in swine. Bull World Health Organ 36: 457–465, 1967 [PMC free article] [PubMed] [Google Scholar]
- 4.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 22: 347–351, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bragdon JH, Zeller JH, Stevenson JW. Swine and experimental atherosclerosis. Proc Soc Exp Biol Med 95: 282–284, 1957 [DOI] [PubMed] [Google Scholar]
- 6.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 22: 336–345, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res 105: 453–461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng DX, Tsalenko A, Vailaya A, Ben-Dor A, Kundu R, Estay I, Tabibiazar R, Kincaid R, Yakhini Z, Bruhn L, Quertermous T. Differences in vascular bed disease susceptibility reflect differences in gene expression response to atherogenic stimuli. Circ Res 98: 200–208, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Desrumaux C, Deckert V, Athias A, Masson D, Lizard G, Palleau V, Gambert P, Lagrost L. Plasma phospholipid transfer protein prevents vascular endothelium dysfunction by delivering alpha-tocopherol to endothelial cells. Faseb J 13: 883–892, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Fang Y, Mohler ER, Hsieh E, 3rd , Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circ Res 98: 1064–1071, 2006 [DOI] [PubMed] [Google Scholar]
- 12.French JE, Jennings MA, Poole JCF, Robinson DS, Florey H. Intimal changes in the arteries of ageing swine. Proc Royal Soc Lond 158: 24–42, 1963 [Google Scholar]
- 13.Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol 26: 2490–2496, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Geeraert B, De Keyzer D, Davey PC, Crombe F, Benhabiles N, Holvoet P. Oxidized low-density lipoprotein-induced expression of ABCA1 in blood monocytes precedes coronary atherosclerosis and is associated with plaque complexity in hypercholesterolemic pigs. J Thromb Haemost 5: 2529–2536, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Goldblum SE, Ding X, Funk SE, Sage EH. SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sci USA 91: 3448–3452, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA 76: 333–337, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottlieb H, Lalich JJ. The occurrence of arteriosclerosis in the aorta of swine. Am J Pathol 30: 851–855, 1954 [PMC free article] [PubMed] [Google Scholar]
- 18.Grant GR, Liu J, Stoeckert CJ., Jr A practical false discovery rate approach to identifying patterns of differential expression in microarray data. Bioinformatics 21: 2684–2690, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Ifergan I, Assaraf YG. Molecular mechanisms of adaptation to folate deficiency. Vitam Horm 79: 99–143, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ishii A, Vinuela F, Murayama Y, Yuki I, Nien YL, Yeh DT, Vinters HV. Swine model of carotid artery atherosclerosis: experimental induction by surgical partial ligation and dietary hypercholesterolemia. AJNR Am J Neuroradiol 27: 1893–1899, 2006 [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings MA, Florey HW, Stehbens WE, French JE. Intimal changes in the arteries of a pig. J Pathol Bacteriol 81: 49–61, 1961 [DOI] [PubMed] [Google Scholar]
- 22.Joyce CW, Amar MJ, Lambert G, Vaisman BL, Paigen B, Najib-Fruchart J, Hoyt RF, Jr, Neufeld ED, Remaley AT, Fredrickson DS, Brewer HB, Jr, Santamarina-Fojo S. The ATP binding cassette transporter A1 (ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc Natl Acad Sci USA 99: 407–412, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly KA, Allport JR, Yu AM, Sinh S, Sage EH, Gerszten RE, Weissleder R. SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration. J Leukoc Biol 81: 748–756, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest 104: R25–R31, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao H, Langmann T, Schmitz G, Zhu Y. Native LDL upregulation of ATP-binding cassette transporter-1 in human vascular endothelial cells. Arterioscler Thromb Vasc Biol 22: 127–132, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Libby P. Inflammation in atherosclerosis. Nature 420: 868–874, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem 278: 24200–24208, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Luginbuhl H, Jones JE. The morphology and morphogenesis of atherosclerosis in aged swine. Ann NY Acad Sci 127: 763–779, 1965 [DOI] [PubMed] [Google Scholar]
- 29.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18: 716–731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcil M, Brooks-Wilson A, Clee SM, Roomp K, Zhang LH, Yu L, Collins JA, van Dam M, Molhuizen HO, Loubster O, Ouellette BF, Sensen CW, Fichter K, Mott S, Denis M, Boucher B, Pimstone S, Genest J, Jr, Kastelein JJ, Hayden MR. Mutations in the ABC1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet 354: 1341–1346, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Massaro M, Basta G, Lazzerini G, Carluccio MA, Bosetti F, Solaini G, Visioli F, Paolicchi A, De Caterina R. Quenching of intracellular ROS generation as a mechanism for oleate-induced reduction of endothelial activation and early atherogenesis. Thromb Haemost 88: 335–344, 2002 [PubMed] [Google Scholar]
- 32.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci USA 97: 4245–4250, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller NE. Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am Heart J 113: 589–597, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Moens AL, Vrints CJ, Claeys MJ, Timmermans JP, Champion HC, Kass DA. Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease. Am J Physiol Heart Circ Physiol 294: H1971–H1977, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Mohler ER, Sarov-Blat L, 3rd, Shi Y, Hamamdzic D, Zalewski A, Macphee C, Llano R, Pelchovitz D, Mainigi SK, Osman H, Hallman T, Steplewski K, Gertz Z, Lu MM, Wilensky RL. Site-specific atherogenic gene expression correlates with subsequent variable lesion development in coronary and peripheral vasculature. Arterioscler Thromb Vasc Biol 28: 850–855, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Nistor A, Bulla A, Filip DA, Radu A. The hyperlipidemic hamster as a model of experimental atherosclerosis. Atherosclerosis 68: 159–173, 1987 [DOI] [PubMed] [Google Scholar]
- 37.O'Connell BJ, Denis M, Genest J. Cellular physiology of cholesterol efflux in vascular endothelial cells. Circulation 110: 2881–2888, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Okuda M, Inoue N, Azumi H, Seno T, Sumi Y, Hirata K, Kawashima S, Hayashi Y, Itoh H, Yodoi J, Yokoyama M. Expression of glutaredoxin in human coronary arteries: its potential role in antioxidant protection against atherosclerosis. Arterioscler Thromb Vasc Biol 21: 1483–1487, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Osterwalder T, Cinelli P, Baici A, Pennella A, Krueger SR, Schrimpf SP, Meins M, Sonderegger P. The axonally secreted serine proteinase inhibitor, neuroserpin, inhibits plasminogen activators and plasmin but not thrombin. J Biol Chem 273: 2312–2321, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA 101: 2482–2487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passerini AG, Shi C, Francesco NM, Chuan P, Manduchi E, Grant GR, Stoeckert CJ, Jr, Karanian JW, Wray-Cahen D, Pritchard WF, Davies PF. Regional determinants of arterial endothelial phenotype dominate the impact of gender or short-term exposure to a high-fat diet. Biochem Biophys Res Commun 332: 142–148, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Polacek DC, Passerini AG, Shi C, Francesco NM, Manduchi E, Grant GR, Powell S, Bischof H, Winkler H, Stoeckert CJ, Jr, Davies PF. Fidelity and enhanced sensitivity of differential transcription profiles following linear amplification of nanogram amounts of endothelial mRNA. Physiol Genomics 13: 147–156, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Reckless JP, Weinstein DB, Steinberg D. Lipoprotein and cholesterol metabolism in rabbit arterial endothelial cells in culture. Biochim Biophys Acta 529: 475–487, 1978 [DOI] [PubMed] [Google Scholar]
- 44.Reddy ST, Hama S, Ng C, Grijalva V, Navab M, Fogelman AM. ATP-binding cassette transporter 1 participates in LDL oxidation by artery wall cells. Arterioscler Thromb Vasc Biol 22: 1877–1883, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Reiser R, Sorrels MF, Williams MC. Influence of high levels of dietary fats and cholesterol on atherosclerosis and lipid distribution in swine. Circ Res 7: 833–846, 1959 [DOI] [PubMed] [Google Scholar]
- 46.Remaley AT, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, Peterson KM, Koch C, Arnould I, Prades C, Duverger N, Funke H, Assman G, Dinger M, Dean M, Chimini G, Santamarina-Fojo S, Fredrickson DS, Denefle P, Brewer HB., Jr Human ATP-binding cassette transporter 1 (ABC1): genomic organization and identification of the genetic defect in the original Tangier disease kindred. Proc Natl Acad Sci USA 96: 12685–12690, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowsell HC, Downie HG, Mustard JF. The experimental production of atherosclerosis in swine following the feeding of butter and margarine. Can Med Assoc J 79: 647–654, 1958 [PMC free article] [PubMed] [Google Scholar]
- 48.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 22: 352–355, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol 165: 2156–2164, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Sanson M, Auge N, Vindis C, Muller C, Bando Y, Thiers JC, Marachet MA, Zarkovic K, Sawa Y, Salvayre R, Negre-Salvayre A. Oxidized low-density lipoprotein triggers endoplasmic reticulum stress in vascular cells. Prevention by oxygen-regulated protein 150 expression. Circ Res 104: 328–336, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. .I Focal increases in arterial LDL concentration precede development of fatty streak lesions. Arteriosclerosis 9: 895–907, 1989 [DOI] [PubMed] [Google Scholar]
- 52.Simionescu M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler Thromb Vasc Biol 27: 266–274, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Simionescu N, Vasile E, Lupu F, Popescu G, Simionescu M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am J Pathol 123: 109–125, 1986 [PMC free article] [PubMed] [Google Scholar]
- 54.Skold BH, Getty R, Ramsey FK. Spontaneous atherosclerosis in the arterial system of aging swine. Am J Vet Res 27: 257–273, 1966 [PubMed] [Google Scholar]
- 55.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem 272: 20963–20966, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Tarbell JM. Mass transport in arteries and the localization of atherosclerosis. Annu Rev Biomed Eng 5: 79–118, 2003 [DOI] [PubMed] [Google Scholar]
- 57.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 24: 12–22, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest 116: 607–614, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng L, Liao H, Liu Y, Lee TS, Zhu M, Wang X, Stemerman MB, Zhu Y, Shyy JY. Sterol-responsive element-binding protein (SREBP) 2 down-regulates ATP-binding cassette transporter A1 in vascular endothelial cells: a novel role of SREBP in regulating cholesterol metabolism. J Biol Chem 279: 48801–48807, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Zhu M, Fu Y, Hou Y, Wang N, Guan Y, Tang C, Shyy JY, Zhu Y. Laminar shear stress regulates liver X receptor in vascular endothelial cells. Arterioscler Thromb Vasc Biol 28: 527–533, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y, Liao H, Xie X, Yuan Y, Lee TS, Wang N, Wang X, Shyy JY, Stemerman MB. Oxidized LDL downregulates ATP-binding cassette transporter-1 in human vascular endothelial cells via inhibiting liver X receptor (LXR). Cardiovasc Res 68: 425–432, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.