Abstract
Muscle metaboreflex activation during submaximal dynamic exercise in normal subjects elicits a pressor response primarily due to increased cardiac output (CO). However, when the ability to increase CO is limited, such as in heart failure or during maximal exercise, the muscle metaboreflex-induced increases in arterial pressure occur via peripheral vasoconstriction. How the mechanisms of this pressor response are altered is unknown. We tested the hypothesis that this change in metaboreflex function is dependent on the level of CO. The muscle metaboreflex was activated in dogs during mild dynamic exercise (3.2 km/h) via a partial reduction of hindlimb blood flow. Muscle metaboreflex activation increased CO and arterial pressure, whereas vascular conductance of all areas other than the hindlimbs did not change. CO was then reduced to the same level observed during exercise before the muscle metaboreflex activation via partial occlusion of the inferior and superior vena cavae. Arterial pressure dropped rapidly with the reduction in CO but, subsequently, nearly completely recovered. With the removal of the muscle metaboreflex-induced rise in CO, substantial peripheral vasoconstriction occurred that maintained arterial pressure at the same levels as before CO reduction. Therefore, the muscle metaboreflex function is nearly instantaneously shifted from increased CO to increased vasoconstriction when the muscle metaboreflex-induced rise in CO is removed. We conclude that whether vasoconstriction occurs with muscle metaboreflex depends on whether CO rises.
Keywords: integrative cardiovascular regulation, exercise reflexes, peripheral vascular regulation
during dynamic exercise, when oxygen delivery to active skeletal muscle is insufficient to meet the metabolic demands, metabolites (e.g., lactic acid, adenosine, potassium, diprotonated phosphate, H+, arachidonic acid products, and others) accumulate within the active muscle and stimulate group III and IV afferent neurons. These sensory neurons project to the central nervous system, eliciting a reflex increase in sympathetic nerve activity and systemic blood pressure termed the muscle metaboreflex (1, 15, 22, 25, 32, 39). This muscle metaboreflex-mediated pressor response is attributable to increases in cardiac output (CO) and peripheral vasoconstriction (7, 10–12, 15, 20, 21, 26, 33, 37, 39) and partially restores blood flow to the ischemic muscle (27, 29).
During dynamic exercise, if a reduction in blood flow to active skeletal muscle occurs when there is sufficient cardiac reserve (as during mild to moderate exercise in normal subjects), the metaboreflex will increase CO to increase blood supply to the hypoperfused active skeletal muscle (39). Thus the metaboreflex is most often viewed as a flow-sensitive, flow-raising reflex (31, 32, 34). The rise in CO occurs via increases in ventricular contractility, heart rate, and central blood volume mobilization (2, 17, 26, 36, 37).
Augustyniak et al. (6) showed that during near-maximal exercise when CO is at or near-maximal levels, the ability of the metaboreflex to increase blood flow to ischemic active skeletal muscle may become limited and a rise in arterial pressure occurs solely via peripheral vasoconstriction as further increases in CO are not possible. In addition, in subjects with heart failure, the muscle metaboreflex-induced increases in arterial pressure occur solely via peripheral vasoconstriction at all workloads tested, likely due to the impaired ability to improve ventricular contractility (10, 16, 35). Furthermore, Sheriff et al. (37) showed in normal dogs that when CO was fixed (via combined β-blockade and ventricular pacing), the reflex evoked a pressor response due to solely peripheral vasoconstriction (37). Taken together, these previous results suggest that when the ability to increase CO is limited, the mechanisms of the muscle metaboreflex pressor response are shifted from increased CO to increased vasoconstriction. In all of these studies, increases in CO were limited from occurring and with the studies during submaximal exercise, the baseline level of CO during the exercise was well below normal due to the ventricular dysfunction induced by heart failure or acute high dose β-blockade coupled with acute rapid ventricular pacing (6, 15, 37). Therefore, whether the limited ability to increase CO is the trigger of the changes in the mechanisms of the muscle metaboreflex pressor response is uncertain. We tested the hypothesis that this change in metaboreflex function from increased CO to increased vasoconstriction is dependent on the level of CO during the muscle metaboreflex activation. We observed the normal rise in CO with metaboreflex activation and then acutely reduced CO to the same level as observed during the mild exercise immediately before metaboreflex activation. We found that an acute removal of the normal CO response during sustained metaboreflex activation causes an immediate shift to vasoconstriction.
MATERIALS AND METHODS
Experiments were performed on six adult mongrel dogs (weight, ∼20–25 kg) of either sex, selected for their willingness to run on a motor-driven treadmill. The protocols employed in the present study conform with the National Institutes of Health guidelines and were reviewed and approved by the Wayne State University Animal Investigation Committee.
Surgical preparation and procedures.
All animals were accustomed to human handling and trained to run freely on a motor-driven treadmill before they were surgically instrumented in two different procedures (thoracotomy and left-flank abdominal surgery).
Before each surgery, for tranquilization, the animals received an intramuscular injection of acepromazine (0.2 mg/kg). The dogs were anesthetized with thiopental sodium (25 mg/kg iv). Following endotracheal intubation, anesthesia was maintained with isoflurane (2–3%). Before the surgery, the animals received cefazolin (antibiotic, 500 mg iv), carprofen (analgesic, 2.0 mg/kg iv), and buprenorphine (analgesic, 0.1 mg/kg im) and a 72-h transdermal fentanyl patch was applied (analgesic, 125–175 μg/h). In addition, before the left thoracotomy, selective intercostal nerve block was performed with bupivacaine hydrochloride (2.0 mg/kg). Following each surgical procedure, the dogs received a second intravenous dose of cefazolin (500 mg iv) and antibiotics were continued for the length of the experimental protocol at an oral dose of cephalexin (30 mg·kg−1·12 h−1). Moreover, after each surgical procedure, for the following 12 h, buprenorphine and acepromazine were administered (0.05 and 0.5 mg/kg iv, respectively) as needed. Thereafter, carprofen was administered orally (4 mg·kg−1·day−1) for 10 days.
In the first surgical procedure under sterile conditions, a left thoracotomy (fourth intercostal space) was performed. Two hydraulic vascular occluders (In Vivo Metrics) were placed on the superior and inferior vena cavae, respectively. The pericardium was then widely opened, and a blood flow transducer (Transonic Systems) was positioned around the ascending aorta to measure CO. A second blood flow transducer was placed around the left circumflex coronary artery to measure left circumflex coronary blood flow. Two pairs of sonomicrometry crystals (Sonometrics) were implanted in the endocardium of the left ventricle to measure the anterior-to-posterior (short axis) and base-to-apex (long axis) dimensions. A fully implantable telemetered blood pressure transducer (model PAD-70; Data Sciences International) was placed subcutaneously on the left side of the chest. Its catheter was tunneled into the thoracic cavity and located inside the left ventricle for measuring left ventricular pressure. For studies unrelated to the present investigation, three stainless steel ventricular pacing electrodes (O-Flexon, Ethicon) were sutured to the right ventricular free wall. The pericardium was reapproximated loosely, and the chest was closed in layers.
After 10–14 days of recovery, a second surgical session was performed. Through a left abdominal retroperitoneal approach, a blood flow transducer was placed on the terminal aorta to measure blood flow to the hindlimbs. A hydraulic vascular occluder was placed on the terminal aorta just distal to the flow transducer. All arteries branching from the aorta between the iliac arteries and the blood flow transducer were ligated and severed, and a catheter was placed through a lumbar artery proximal to the transducer and occluder to measure arterial blood pressure. Through a 2-cm right neck incision, a catheter was inserted into the jugular vein and advanced to the atrial-caval junction to monitor central venous pressure. All cables, wires, occluder tubing, and the aortic catheter were tunneled subcutaneously and exteriorized between the scapulae. At least 1 wk was allowed for recovery before any experiments.
Experimental procedures.
All experiments were performed after the animals had fully recovered from instrumentation (i.e., active, afebrile, and of good appetite). Before the experimental sessions, each animal was transported to the laboratory, allowed to roam freely for 15–30 min, and then led to the treadmill. The blood flow transducers were connected to the flow meters (Transonic System). Heart rate was computed by a cardiotachometer triggered by the CO signal. The arterial blood pressure and central venous pressure catheters were connected to pressure transducers (Transpac IV; Abbott). The left ventricular pressure implant was turned on and the quality of the signal verified. All crystals were coupled to the sonomicrometer. All data were recorded on analog-to-digital recording systems for subsequent off-line analyses.
After steady-state data were obtained with the animal at rest standing on the treadmill, the treadmill was started and advanced to 3.2 km/h, 0% grade (mild exercise). After all variables had reached steady state (∼5 min), the muscle metaboreflex was activated via partial reduction in hindlimb blood flow through inflation of the vascular occluder placed around the terminal aorta. As reported in previous studies, the activation of muscle metaboreflex during mild exercise induced a significant increase in CO (17, 26, 37, 39). After the collection of steady-state data, CO was acutely reduced and maintained at the same level observed during free-flow exercise (before the muscle metaboreflex activation) via partial occlusion of the inferior and superior vena cavae. The aim of this procedure is to acutely remove the muscle metaboreflex-induced rise in CO. The vena cavae occlusion was maintained until all parameters achieved steady state (∼5 min).
Data analysis.
During the experiments, arterial pressure, central venous pressure, heart rate, left ventricular pressure, CO, hindlimb blood flow, coronary blood flow, and left ventricular short- and long-axis dimensions were collected continuously. Off-line data analyses yielded maximal rates of myocardial isovolmetric relaxation and contraction (dP/dtmin and dP/dtmax), stroke volume, left circumflex coronary vascular conductance, nonischemic vascular conductance [conductance directed everywhere besides the hindlimbs; calculated as (CO − hindlimb blood flow)/(mean arterial pressure − central venous pressure)], and left ventricular volume. Left ventricular volume was calculated as a modified ellipsoid (8). It has been previously demonstrated by Nozawa et al. (24) and other investigators (23) that this method gives a consistent measure of left ventricular volume despite changes in loading conditions, configurations, and heart rate.
Statistical analysis.
Steady-state values at rest, during mild exercise, and during muscle metaboreflex activation and muscle metaboreflex activation plus CO reduction were compared using one-way ANOVA for repeated measures. If a significant interaction term was revealed, then pairwise comparisons of individual means were performed using the test for simple effects. All analyses were performed using Systat software (version 11.0). An α-level of P < 0.05 was set to determine statistical significance. In figures and text, data are expressed as means ± SE.
RESULTS
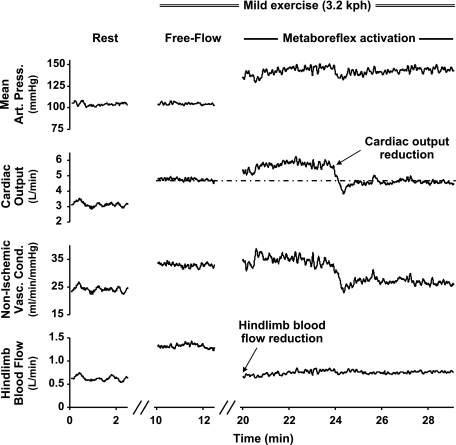
Figure 1 shows an example of changes in mean arterial pressure (MAP), CO, and vascular conductance to all nonischemic areas in response to the imposed changes in hindlimb blood flow from one animal. Figure 2 shows steady-state mean values of MAP, CO, regional conductance, coronary blood flow and conductance, heart rate, stroke volume, and left ventricular end-diastolic volume (LVEDV) at rest, during mild exercise, and during muscle metaboreflex activation and muscle metaboreflex activation during CO reduction. Changes in dP/dtmax and dP/dtmin in each setting are presented in Fig. 3. As expected and in agreement with previous studies (17, 26, 39) from rest to mild exercise, while MAP did not change significantly, we observed increases in heart rate and stroke volume (as a result in CO) and blood flow to the hindlimbs increased. Thus the increase in CO is offset by a rise in total vascular conductance due to the active vasodilation in the exercising skeletal muscles, resulting in little change in MAP. Mild exercise-induced increase in heart rate, stroke volume, and dP/dtmax caused a significant coronary vasodilation that improved blood flow in the left circumflex artery. There were no significant changes in dP/dtmin and LVEDV with mild exercise.
Fig. 1.
Example of changes in mean arterial pressure (Mean Art Press), cardiac output, vascular conductance (Vasc Cond) of all nonischemic areas, and hindlimb blood flow from 1 animal is shown.
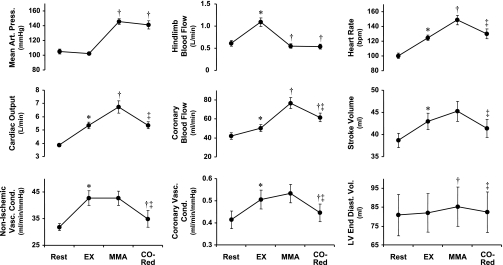
Fig. 2.
Average values of mean arterial pressure, cardiac output, nonischemic vascular conductance, hindlimb and coronary blood flows, coronary vascular conductance, heart rate [in beats/min (bpm)], stroke volume, and left ventricular end-diastolic volume (LV End-Diast Vol) at rest and during mild exercise (EX), muscle metaboreflex activation (MMA), and cardiac output reduction (CO-Red). *P < 0.05, rest vs. EX; †P < 0.05, EX vs. MMA or CO-Red; ‡P < 0.05, MMA vs. CO-Red.
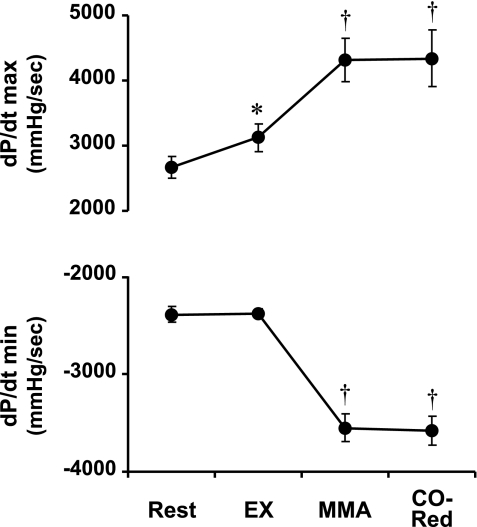
Fig. 3.
Average values of maximal and minimal first derivative of left ventricular pressure (dP/dtmax and dP/dtmin, respectively) at rest and during EX, MMA, and CO-Red. *P < 0.05, rest vs. EX; †P < 0.05, EX vs. MMA or CO-Red.
Muscle metaboreflex generated a significant increase in CO and MAP, whereas no significant regional vasoconstriction/vasodilation occurred. Thus all of the reflex increase in MAP was a result of the rise in CO, which increased via a substantial tachycardia together with a small rise in stroke volume. An increase in LVEDV also occurred, likely reflecting metaboreflex-induced central blood volume mobilization (37). Furthermore, the metaboreflex activation increased dP/dtmax and decreased dP/dtmin, suggesting an important augmentation in left ventricular systolic and diastolic performance (inotropic and dromotropic function). These changes indicate that the rise in CO by the muscle metaboreflex results from the integration of an increase in heart rate and left ventricular systolic and diastolic performance coupled with central blood volume mobilization. Muscle metaboreflex activation increased coronary flow, whereas coronary conductance remained unchanged. Therefore, the reflex-induced rise in left circumflex coronary blood flow was solely due to increased perfusion pressure.
When CO was rapidly reduced to the level observed during normal exercise, MAP dropped rapidly but subsequently recovered to a level not significantly different from the value immediately before the acute CO reduction. During the period of CO reduction, blood flow to the hindlimbs was maintained at the same level as before the CO reduction by continuously adjusting the vascular occluder on the terminal aorta to maintain constant the level of muscle metaboreflex activation. With the removal of the metaboreflex-induced rise in CO, profound peripheral vasoconstriction quickly occurred. This even included vasoconstriction in the coronary vasculature. The partial occlusion of the inferior and superior vena cavae reduced venous return and decreased LVEDV and stroke volume to the same levels observed during free-flow exercise although the metaboreflex-induced changes in dP/dtmax and dP/dtmin were sustained. Heart rate also decreased significantly, which is likely due to the Bainbridge reflex (13, 14).
DISCUSSION
The major new finding in the present study is that the muscle metaboreflex function is virtually instantaneously shifted from increased CO to increased vasoconstriction when the muscle metaboreflex-induced rise in CO is acutely removed. These results indicate that the mechanisms involved in the muscle metaboreflex response are continuously dependant on whether a rise in CO occurs.
In both humans and dogs, metaboreflex activation during submaximal dynamic exercise causes substantial increases in CO, which accounts for most, if not all, of the rise in arterial pressure (4, 6, 7, 12, 33, 39). Variable changes in CO occur when the reflex is activated during post-exercise occlusion, which entraps metabolites within skeletal muscle maintaining metaboreceptor activation. However, since this is during the recovery from exercise, the heart rate falls rather than rises, which thereby likely limits any rise in CO (7, 10, 22, 25). When activated during dynamic exercise, the metaboreflex-induced rise in CO acts to partially restore blood flow and oxygen delivery to the hypoperfused muscles (27, 29). However, if a reduction of blood flow to the active skeletal muscles occurs when the ability to increase CO is limited, such as even during mild exercise in heart failure, imposed acute ventricular dysfunction, or maximal exercise when CO is already at maximal levels, the muscle metaboreflex-induced increases in arterial pressure occur solely via peripheral vasoconstriction (4, 15, 37). Given the magnitude of the vasoconstriction observed and the known distribution of CO, a substantial amount of this peripheral vasoconstriction must occur in the active skeletal muscle (18). Whether this vasoconstriction also occurs in the ischemic active skeletal muscle is unknown. Even the coronary circulation is not spared significant vasoconstriction (3, 28). During heavier exercise, blood flow to inactive areas constitutes such a small fraction of total CO that the redistribution of this flow cannot markedly improve oxygen delivery to the active muscle. This could set up a positive-feedback scenario wherein an underperfusion of skeletal muscle triggers reflex vasoconstriction in skeletal muscle. To what extent the ischemic muscle can escape this vasoconstriction is unclear (30).
What causes this immediate shift in metaboreflex mechanisms with the rapid, acute removal of the normal rise in CO is unknown. We do not believe that any substantive changes in the level of activity of the muscle metaboreceptors occurred. In these studies hindlimb blood flow was held constant during the imposed removal of the CO response to maintain the metaboreflex activation steady. We purposely performed these studies during mild exercise so that changes in metaboreceptor activity from other nonischemic active muscles did not occur. A substantial fall in blood flow is required to activate the muscle metaboreflex at this workload (26, 37, 39). With the reflex increase in CO, blood flow to nonischemic active muscle (e.g., the forelimb) actually increases (5, 20). The removal of the metaboreflex-induced increase in CO would only be expected to return blood flow to nonischemic muscles back to the original level, a level wherein no data exist to show tonic metaboreflex activation at this workload even in subjects with heart failure (16, 35).
Since neither regional nor global metaboreceptor activity was likely changed with the acute removal of the CO response, this shift in the metaboreflex mechanism must be due to a change in the interaction between the muscle metaboreflex and some other systems. We feel that the baroreflexes are the most likely. The arterial baroreflex buffers the muscle metaboreflex-induced pressor response via an inhibition of peripheral vasoconstriction (18, 19, 38). Muscle metaboreflex activation during mild exercise in baro-denervated dogs causes a twofold greater pressor response that now consists of substantial peripheral vasoconstriction as well as increased CO. Thus the muscle metaboreflex is capable of eliciting profound peripheral vasoconstriction but is prevented from doing so by the rise in arterial pressure that stimulates the arterial baroreflex. In the present study, arterial pressure dropped rapidly during the first few seconds after the CO reduction. This decrease in arterial pressure would unload arterial baroreceptors and thus attenuate arterial baroreflex buffering of the muscle metaboreflex, which would enable metaboreflex-induced peripheral vasoconstriction. However, it is unlikely that arterial baroreflex buffering was markedly changed during the steady state of the imposed reductions in CO since no significant steady-state change in arterial mean, systolic, diastolic, or pulse pressure occurred (albeit our n = 6 may have limited the ability to detect statistically significant small changes). Alternatively, the shift of the muscle metaboreflex function during CO reduction might be associated with the interactions between the muscle metaboreflex and the cardiopulmonary baroreflex (9). Like the arterial baroreflex, the cardiopulmonary baroreflex also acts to buffer the muscle metaboreflex, although the mechanisms mediating this buffering (e.g., attenuated vasoconstriction vs. attenuated rise in CO) have not been investigated (9). The muscle metaboreflex causes substantial central blood volume mobilization (37), as indicated in the present study by the significant rise in LVEDV. This increase in ventricular volume would be expected to load cardiopulmonary baroreceptors that could buffer the sympathoexcitation caused by the metaboreflex activation. With the imposed reduction in CO via partial caval occlusion, preload (i.e., LVEDV) returned to the normal exercise level that could reduce cardiopulmonary baroreceptor buffering and thereby could engender peripheral vasoconstriction.
Limitations of the study.
We found that the level of CO during the muscle metaboreflex activation alters the mechanisms of this reflex pressor response. The response pattern of cardiovascular parameters with muscle metaboreflex activation in heart failure or during severe exercise in normal dogs is remarkably similar to what occurred in the present study. However, this does not prove that the underlying mechanisms of the shift of the muscle metaboreflex function from increased CO to increased vasoconstriction in heart failure or during severe exercise and with CO reduction are the same. Therefore, the data need to be interpreted with caution. In settings wherein a tonic metaboreflex activation persists, as likely occurs during heavier exercise, even transient falls in CO could elicit substantial peripheral vasoconstriction. If the fall is large enough and sustained long enough, both pressure and skeletal muscle blood flow may be compromised and the combined stimulation of two powerful reflexes could elicit massive sympathoactivation.
Conclusions.
During muscle metaboreflex activation, reducing CO to normal exercise levels caused significant vasoconstriction, suggesting that the muscle metaboreflex function is instantaneously shifted from an increased CO to an increased vasoconstriction when the rise in CO is removed. We conclude that whether vasoconstriction occurs with muscle metaboreflex depends on whether CO rises.
GRANTS
M. Ichinose and T. K. Ichinose are recipients of research fellowships of the Japan Society for the Promotion of Science for Young Scientists. This research was supported by National Heart, Lung, and Blood Institute Grants HL-55473 and HL-095819.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Jody Helme-Day and Erin Krengel for expert technical assistance and care of the animals.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansorge EJ, Augustyniak RA, Perinot RL, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O'Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ansorge EJ, Shah SH, Augustyniak R, Rossi NF, Collins HL, O'Leary DS. Muscle metaboreflex control of coronary blood flow. Am J Physiol Heart Circ Physiol 283: H526–H532, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Augustyniak RA, Ansorge EJ, Kim JK, Sala-Mercado JA, Hammond RL, Rossi NF, O'Leary DS. Cardiovascular responses to exercise and muscle metaboreflex activation during the recovery from pacing-induced heart failure. J Appl Physiol 101: 14–22, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Augustyniak RA, Ansorge EJ, O'Leary DS. Muscle metaboreflex control of cardiac output and peripheral vasoconstriction exhibit differential latencies. Am J Physiol Heart Circ Physiol 278: H530–H537, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bonde-Petersen F, Rowell LB, Murray RG, Blomqvist GG, White R, Karlsson E, Campbell W, Mitchell JH. Role of cardiac output in the pressor responses to graded muscle ischemia in man. J Appl Physiol 45: 574–580, 1978 [DOI] [PubMed] [Google Scholar]
- 8.Cheng CP, Igarashi Y, Little WC. Mechanism of augmented rate of left ventricular filling during exercise. Circ Res 70: 9–19, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Collins HL, Dicarlo SE. Cardiac afferents attenuate the muscle metaboreflex in the rat. J Appl Physiol 75: 114–120, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Crisafulli A, Salis E, Pittau G, Lorrai L, Tocco F, Melis F, Pagliaro P, Concu A. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am J Physiol Heart Circ Physiol 291: H3035–H3042, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand 131: 339–345, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Hakumäki MO. Vagal and sympathetic efferent discharge in the Bainbridge reflex of dogs. Acta Physiol Scand 85: 414–417, 1972 [DOI] [PubMed] [Google Scholar]
- 14.Hakumäki MO. Seventy years of the Bainbridge reflex. Acta Physiol Scand 130: 177–185, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Ichinose M, Sala-Mercado JA, O'Leary DS, Hammond RL, Coutsos M, Ichinose T, Pallante M, Iellamo F. Spontaneous baroreflex control of cardiac output during dynamic exercise, muscle metaboreflex activation, and heart failure. Am J Physiol Heart Circ Physiol 294: H1310–H1316, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O'Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol 289: H2416–H2423, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O'Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Mittelstadt SW, Bell LB, O'Hagan KP, Clifford PS. Muscle chemoreflex alters vascular conductance in nonischemic exercising skeletal muscle. J Appl Physiol 77: 2761–2766, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Mittelstadt SW, Bell LB, O'Hagan KP, Sulentic JE, Clifford PS. Muscle chemoreflex causes renal vascular constriction. Am J Physiol Heart Circ Physiol 270: H951–H956, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol 77: 2778–2783, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Noda T, Cheng CP, de Tombe PP, Little WC. Curvilinearity of LV end-systolic pressure-volume and dP/dtmax-end-diastolic volume relations. Am J Physiol Heart Circ Physiol 265: H910–H917, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Nozawa T, Cheng CP, Noda T, Little WC. Effect of exercise on left ventricular mechanical efficiency in conscious dogs. Circulation 90: 3047–3054, 1994 [DOI] [PubMed] [Google Scholar]
- 25.O'Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol 74: 1748–1754, 1993 [DOI] [PubMed] [Google Scholar]
- 26.O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998 [DOI] [PubMed] [Google Scholar]
- 27.O'Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999 [DOI] [PubMed] [Google Scholar]
- 28.O'Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol 103: 190–194, 2007 [DOI] [PubMed] [Google Scholar]
- 29.O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962 [DOI] [PubMed] [Google Scholar]
- 31.Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol 24: 117–125, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Rowell LB, O'Leary DS, Kellogg DL., Jr Integration of Cardiovascular Control Systems in Dynamic Exercise New York: Oxford Press, 1996, p. 770–838 [Google Scholar]
- 33.Rowell LB, Savage MV, Chambers J, Blackmon JR. Cardiovascular responses to graded reductions in leg perfusion in exercising humans. Am J Physiol Heart Circ Physiol 261: H1545–H1553, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Rowell LB, Sheriff DD. Are muscle “chemoreflexes” functionally important? News Physiol Sci 3: 250–253, 1988 [Google Scholar]
- 35.Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O'Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Sheriff DD, O'Leary DS, Scher AM, Rowell LB. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am J Physiol Heart Circ Physiol 258: H305–H310, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983 [DOI] [PubMed] [Google Scholar]