Abstract
We have previously reported that 1) inhibition of cyclooxygenase-2 and PGE2 production reduces hypertrophy after myocardial infarction in mice and 2) PGE2 acting through its EP4 receptor causes hypertrophy of neonatal ventricular myocytes (NVMs) via ERK1/2. It is known that EP4 couples to adenylate cyclase, cAMP, and PKA. The present study was designed to determine interactions between the cAMP-PKA pathway and ERK1/2 and to further characterize events downstream of ERK1/2. We hypothesized that PKA and the small GTPase Rap are upstream of ERK1/2 and that 90-kDa ribosomal S6 kinase (p90RSK) is activated downstream. Treatment of NVMs with PGE2 activated Rap, and this activation was inhibited in part by an EP4 antagonist and PKA inhibition. Transfection of a dominant negative mutant of Rap reduced PGE2 activation of ERK1/2. PGE2 activation of p90RSK was also dependent on EP4, PKA, and Rap. We also tested the involvement of Rap, ERK1/2, and p90RSK in PGE2 regulation of gene expression. PGE2 stimulation of brain natriuretic peptide promoter activity was blocked by either ERK1/2 inhibition or a dominant negative mutation of p90RSK. PGE2 stimulation of c-Fos was dependent on EP4, PKA, ERK1/2, and p90RSK, whereas only the latter two kinases were involved in PGE2 regulation of early growth response-1. Finally, we tested the involvement of EP4-dependent signaling in the NVM growth response and found that the overexpression of EP4 increased NVM cell size. We conclude that EP4-dependent signaling in NVMs in part involves PKA, Rap, ERK1/2, and p90RSK and results in the increased expression of brain natriuretic peptide and c-Fos.
Keywords: prostaglandins, myocytes, prostaglandin E receptor 4
prostaglandin e2 (PGE2) is a 20-carbon proinflammatory prostanoid synthesized from arachidonic acid by cyclooxygenases 1 and 2 (COX1 and COX2) and PGE2 synthases. It has been implicated in pathophysiological conditions including inflammation (18, 24), edema (54), bronchoconstriction (43, 63), fever (3, 60), and tumorigenesis (32, 33, 57). We (44, 45, 56) have previously shown that PGE2 acting through EP4 induces characteristics of hypertrophic growth in neonatal ventricular myocytes (NVMs), including increased cell surface area, protein synthesis, and expression of the marker genes atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). Zamorano and Carmona (76) have shown that PGE2 content increases in the hypertrophied heart in a rat model of chronic pressure overload. In a mouse model of myocardial infarction, both COX2 expression and PGE2 production were increased, and COX2 inhibition reduced hypertrophy and fibrosis as well as improved cardiac function (36). Thus, PGE2 contributes to adverse cardiac remodeling in some pathophysiological models.
The biological actions of PGE2 are mediated through four specific G protein-coupled receptors: EP1, EP2, EP3, and EP4, which differ in their downstream signaling pathways. Activation of EP2 and EP4 couples to Gαs, resulting in increased intracellular cAMP, whereas the EP3 receptor couples to either Gαi or Gαq. EP1 mediates Ca2+ mobilization, although the exact mechanism is not known (50). Our previous studies in NVMs showed that PGE2 stimulated cAMP production, activated the MAPK ERK1/2 (44, 45), and regulated BNP gene expression through EP4 (56). The present study was designed to elucidate 1) the signaling molecules downstream from EP4, including PKA, the small GTPase Rap, and kinases [e.g., p90 ribosomal S6 kinase (p90RSK)]; 2) the effect of EP4 signaling on gene expression [e.g., BNP, early growth response-1 (Egr-1), and c-Fos]; and 3) the effect of EP4 and PKA on myocyte growth as measured by cell size. We found that PGE2 activates Rap1 via both PKA-dependent and PKA-independent pathways but that PKA is not associated with myocyte growth.
METHODS
Supplies and chemicals.
The MEK inhibitor U-0126 was purchased from EMD Chemicals (Gibbstown, NJ), PGE2 was purchased from Cayman Chemical (Ann Arbor, MI), the Epac activator 8CPT-2Me-cAMP (8CPT) was purchased from Tocris Bioscience (Ellisville, MO), and the PKA inhibitor H89 was purchased from BioMol (Plymouth Meeting, PA). The EP4 antagonist ONO-AE3-208 (ONO-208) was a gift from ONO Pharmaceutical (Osaka, Japan). FuGene 6 transfection reagent and phosphatase and proteinase inhibitor cocktail tablets (PhosSTOP and Complete Mini) were obtained from Roche Applied Science (Indianapolis, IN). The Rap1 Activation Assay Kit was purchased from Upstate Biotechnology (Temecula, CA). Primary antibodies against c-Fos, Egr-1, phospho-p90RSK (Thr359/Ser363), RSK1/RSK2/RSK3, GAPDH, phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, and horseradish peroxidase (HRP)-conjugated secondary antibody against rabbit IgG were obtained from Cell Signaling Technology (Boston, MA). β-Actin antibody and HRP-conjugated anti-goat IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody against the hemagglutinin (HA) tag was obtained from Covance (Emeryville, CA). The Coomassie Protein Assay and SuperSignal West Pico Chemiluminescent Substrate kits and Restore Plus Western Blot Stripping Buffer were purchased from Thermo Scientific (Rockford, IL). Precast Tris-glycine polyacrylamide gels and polyvinylidene difluoride (PVDF) membranes were obtained from Invitrogen (San Diego, CA). Fibronectin-coated glass coverslips were purchased from BD Biosciences (San Jose, CA). Routine supplies and chemicals were purchased from Fisher and Sigma.
Expression vectors encoding a dominant negative mutant of Rap [Rap N17 (dnRap)] and Ras [Ras N17 (dnRas)] were obtained from Dr. M. Olah (Univ. of Cincinnati Medical School, Cincinnati, OH) and from Dr. Michael Karin (University of California, San Diego, CA), respectively. The −1818hBNP luciferase (hBNPluc) expression construct has been described previously (39). The HA-tagged EP4 cDNA expression vector and its control vector were obtained from Dr. Barrie Ashby (Temple University). A dominant negative mutation of p90RSK (dnp90RSK) encoded in an adenoviral construct was obtained from Dr. Jun-ichi Abe (Univ. of Rochester). An adenoviral construct encoding the LacZ gene (Ad.CMV.ntLacZ) was obtained from the University of Michigan Viral Vector Core Facility.
Cell culture.
This protocol was approved by the Henry Ford Institutional Animal Care and Use Committee in accordance with federal guidelines. Ventricular myocyte-enriched cultures were generated from Sprague-Dawley rat pups (Charles River, Kalamazoo, MI) as previously described (37). Ventricular myocytes (106 cells/well of a 6-well plate) were plated in DMEM (GIBCO-BRL) containing 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 0.1 mM 5′-bromo-2′-deoxyuridine, and 10% FBS (HyClone) for 40 h. Cultures were then maintained under serum-free conditions with DMEM supplemented with 5 mg/l insulin and transferrin and 2.5 mg/l selenium. After 24 h in serum-free medium, the medium was refreshed and cells were treated with 1 μM PGE2 for 5–60 min or 24 h after a 1-h pretreatment with the various inhibitors.
Transfection and luciferase assay.
The transfection of −1818-hBNPLuc by electroporation and luciferase assay were as previously described (39). In other experiments, DNA (e.g., dnRap) was transfected into NVMs with FuGene 6 according to the manufacturer's protocol (1 μg DNA in 5 μl FuGene per 106 cells).
Adenoviral transduction of NVMs.
After NVMs had been plated in serum-free medium, they were transduced with adenovirus (100 plaque-forming units/cell). After 48 h, NVMs were treated with PGE2 and then lysed for Western blot analysis or luciferase assay.
Protein extraction and Western blot analysis.
Protein was isolated from NVMs with a lysis buffer containing 150 mM NaCl, 50 mM Tris·Cl (pH 7.5), 0.5% deoxycholate, 0.1% SDS, 1% Nonidet P-40, PhosSTOP, and Complete Mini. The protein concentration was determined with the Coomassie protein assay kit using BSA as the standard. Aliquots of samples (50 μg protein) were subjected to SDS-PAGE and electrotransferred to a PVDF membrane at 50 V overnight at 4°C. The membrane was incubated in 5% nonfat milk in PBS containing 0.1% Tween 20 (PBS-T) for 1 h at room temperature and then overnight at 4°C in the same buffer containing primary antibody. The membrane was washed with PBS-T and incubated with HRP-conjugated secondary antibody at room temperature for 1.5 h. After being washed, the membrane was developed with SuperSignal West Pico Chemiluminescent reagent at room temperature. The signal was detected through an exposure to Fuji RX film and analyzed by scanning densitometry. The target protein was normalized to the loading control and expressed either as the fold increase versus the control (untreated cells) or as the percentage of PGE2 stimulation.
Rap1 activation assay.
Activated Rap was assayed with a kit from Upstate following its protocol. In brief, 6 × 106 cells from one 10-cm plate were washed with ice-cold PBS, scraped into 1× Rap activation buffer supplemented with Complete Mini, and snap frozen in liquid nitrogen. The cell extract was obtained by centrifugation at 12,000 g for 10 min at 4°C. Cell extract (600 μg) was incubated with 25 μl of immobilized GST-RalGDS-RBD for at least 1 h at 4°C on a rotating platform. The GTP-bound activated Rap was affinity precipitated, washed, and then eluted with 30 μl Laemmli sample buffer by boiling for 5 min. Samples were electrophoresed by SDS-PAGE and transferred to a PVDF membrane to be probed with anti-Rap antibody. To determine total Rap content, 50 μg cell lysate was also analyzed by Rap immunoblot analysis. For various treatment conditions, activated Rap normalized to total Rap was expressed as the fold increase versus the control.
Measurement of cell size.
For the overexpression of HA-EP4 cDNA, 3 × 106 myocytes were transfected by electroporation using Amaxa reagent (Lonza Cologne) with 6 μg of a control plasmid encoding green fluorescent protein (GFP) or HA-EP4 in pcDNA3.1. After transfection, cells were plated onto fibronectin-coated glass coverslips for 40 h, serum deprived for 24 h, and treated for 48 h with vehicle or PGE2. After treatment, cells were washed with PBS and fixed in 4% paraformaldehyde in PBS for at least 15 min. The slides transfected with GFP were immediately mounted in Fluoromount-G (Southern Biotechnology Associates) and stored at 4°C. The slides transfected with HA-EP4 were permeabilized in 0.1% Triton X-100 in PBS for 15 min and blocked for 1 h in 1% BSA with Tris-buffered saline and 0.1% Tween 20 (TBS-T). Cells were incubated with a FITC-conjugated antibody to HA (diluted 1:100 in 1% BSA and TBS-T) for 60 min and then washed with PBS. Slides were then mounted, and immunofluorescence was detected using a fluorescence microscope (Nikon Eclipse E600) attached to a Spot camera (Diagnostic Instruments). Areas of positively transfected cells (at least 60 cells/group) in randomly selected microscopic fields were analyzed using image analysis software (MicroSuite Five) by an observer blinded to the treatment groups.
Statistical analysis.
Data are expressed as means ± SE. Differences in mean values were analyzed by a two-tailed t-test or one-way ANOVA using the Student-Newman-Keuls method for pair-wise multiple comparisons. P values < 0.05 were considered significant.
RESULTS
PGE2 activation of the small GTPase Rap.
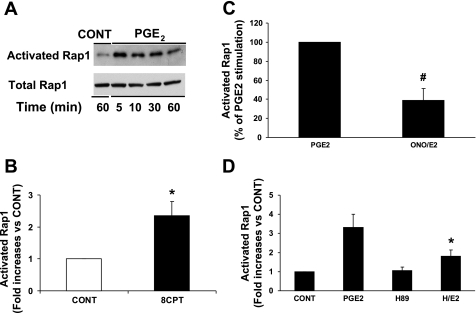
Our previous studies (44, 45) have shown that PGE2 increased intracellular cAMP and activated ERK1/2 in NVMs. cAMP exerts its effects through PKA and/or Epac, an exchange protein directly activated by cAMP, which is usually PKA independent (5, 6). cAMP and Epac can lead to the activation of ERK1/2 through the small GTPase Rap. To investigate whether PGE2 activates Rap, we used a Rap activation assay. In a time-course experiment (Fig. 1A), 1 μM PGE2 activated Rap at 5 min, and the activation persisted for at least 30 min. As a positive control, we treated NVMs with 8CPT (60 μM), which activated Rap by 2.4-fold compared with the control (Fig. 1B). Rap activation by PGE2 was reduced 61% by the EP4 antagonist ONO-208 (10 μM; Fig. 1C) and 46% by H89 (5 μM; Fig. 1D). In addition, we verifed that PGE2 stimulated cAMP in NVMs. PGE2 increased cAMP 5.6-fold (from 2,143 to 12,348 fmol/ml, n = 2), and this was inhibited 54% by ONO-208. In summary, PGE2 stimulated cAMP and induced Rap activation, which was both PKA dependent and independent.
Fig. 1.
PGE2 activates Rap via EP4 and PKA. Neonatal ventricular myocytes (NVMs) were treated with or without 1 μM PGE2 for 0–60 min and then lysed for the Rap1 activation assay as described in methods. A: time course of Rap1 activation. Representative Western blots of activated Rap compared with total Rap protein are shown. Data represent 2 separate experiments. B: Rap activation via Epac. NVMs were treated for 5 min with 60 μM 8CPT-2Me-cAMP (8CPT). Activated Rap is expressed as the fold increase compared with the vehicle-treated control (CONT). *P ≤ 0.05 vs. the control. C: EP4 dependence of Rap activation. NVMs were treated with PGE2 for 5 min after a 1-h pretreatment with or without 10 μM ONO-AE3-208 (ONO). The graph is a summary of data, which are expressed as the percentage of PGE2 stimulation. Bars are means ± SE from 5 separate experiments. #P ≤ 0.01 vs. PGE2. D: PKA dependence of Rap activation. NVMs were treated for 5 min with PGE2 after a 1-h pretreatment with 5 μM H89 (H). Activated Rap is expressed as the fold increase compared with the control, which was treated with DMSO and ethanol vehicle. Data are means ± SE from 5–9 separate experiments. *P ≤ 0.01 vs. PGE2.
PGE2 activation of ERK1/2.
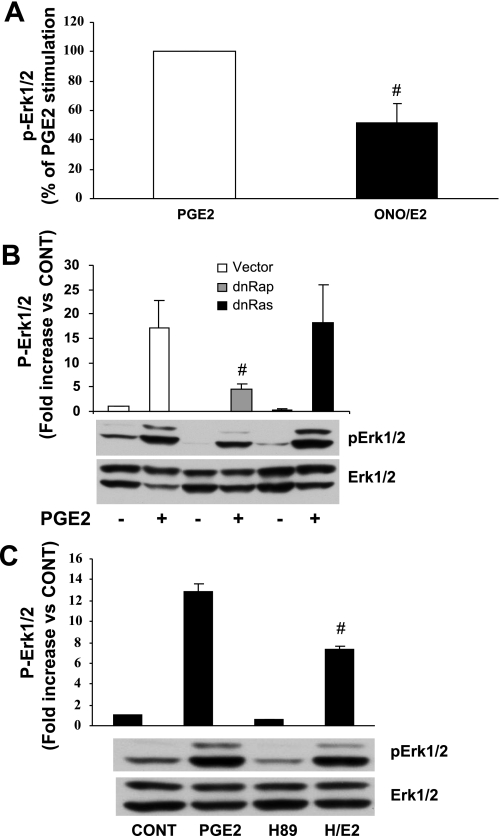
It is known that ERK signaling is initiated by small GTPases such as Rap, which is a member of the Ras family. We (45) have also previously shown that PGE2 activated ERK1/2 via EP4, using the EP4 antagonist L-161982. We first repeated this study using the ONO-208 compound. As shown in Fig. 2A, ERK1/2 activation by PGE2 was inhibited in part by ONO-208. We next tested whether Rap is activated upstream of ERK1/2 in PGE2 signaling. Transfection of NVMs with dnRap significantly decreased PGE2-induced ERK1/2 activation by 75%. dnRas had no effect (Fig. 2B), indicating that the coupling of EP4 to a small GTPase is specific for Rap. In keeping with our results concerning PKA and its role in Rap activation (shown in Fig. 1), PKA inhibition also abrogated PGE2-induced ERK1/2 activation (Fig. 2C). Interestingly, 8CPT had no effect on ERK1/2 phosphorylation (data not shown), suggesting that Epac is not part of the signaling pathway. Thus, the activation of ERK1/2 by PGE2 is partially mediated by EP4, PKA, and Rap1.
Fig. 2.
EP4, PKA, and Rap are involved in PGE2 activation of ERK1/2. NVMs were treated with PGE2 for 5 min after pretreatment with either ONO or H89 for 1 h. Cells were lysed for the Western blot analysis of total and phosphorylated (p-)ERK1/2. A: EP4 dependence of p-ERK1/2 activation. p-ERK1/2 is expressed as a percentage of PGE2 stimulation in n = 7 experiments. #P ≤ 0.01 vs. PGE2. B: Involvement of Rap in the PGE2 activation of ERK1/2. NVMs were transfected with either dominant negative (dn)Rap, dnRas, or a control plasmid (vector) for 24 h. Cells were then treated with PGE2 for 5 min and lysed for protein extraction and Western blot analysis. p-ERK/2 is expressed as the fold increase versus the control vector in NVM without PGE2 treatment. Data are means ± SE from 5 separate experiments. #P ≤ 0.01 vs. vector + PGE2. C: involvement of PKA. p-ERK1/2 activation is expressed as the fold increase versus the control in n = 4 experiments. #P ≤ 0.01 vs. PGE2.
PGE2 activation of p90RSK.
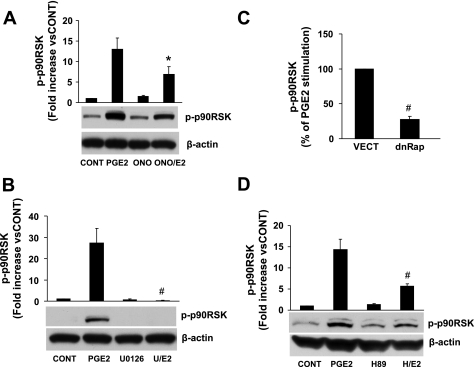
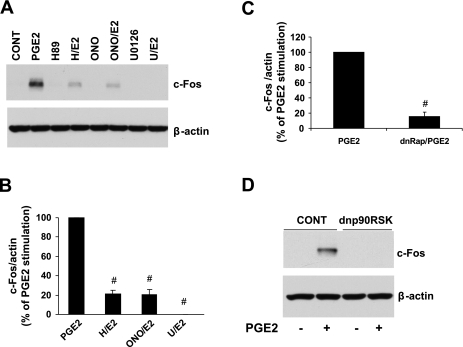
p90RSK is activated by ERK1/2 (13) and has been implicated in cardiac hypertrophy both in vivo and in vitro (4, 34, 71, 74). We found that PGE2 induced a robust activation of p90RSK, which was partially inhibited by the EP4 antagonist ONO-208 (Fig. 3A). As expected, p90RSK activation was totally abrogated by an inhibitor of ERK1/2 activation (U-0126, 10 μM; Fig. 3B). dnRap and PKA inhibition by H89 also significantly inhibited PGE2 activation of p90RSK (Fig. 3, C and D). Thus, p90RSK activation by PGE2 involves EP4 acting through PKA, Rap, and ERK1/2.
Fig. 3.
EP4, PKA, Rap, and ERK1/2 are involved in the PGE2 activation of 90-kDa ribosomal S6 kinase (p90RSK). Cells were lysed, and p-p90RSK and the loading control protein β-actin were analyzed by Western blot analysis. A: effect of the EP4 antagonist ONO. p-p90RSK is expressed as the fold increase versus the control. Data are means ± SE from 6 separate experiments. *P ≤ 0.05 vs. PGE2. B: effect of inhibition of ERK1/2 with the MEK inhibitor U-0126 (U; 10 μM). p-p90RSK is expressed as the fold increase versus the control. Data are means ± SE from 3 separate experiments. #P ≤ 0.01 vs. PGE2. C: effect of Rap. p-p90RSK is expressed as the percentage of PGE2 stimulation of NVMs transfected with control vector. Data are means ± SE from 5 separate experiments. #P ≤ 0.01 vs. the control vector. D: effect of PKA inhibition with H89. Data are means ± SE from 5 separate experiments. #P ≤ 0.01 vs. PGE2.
PGE2 regulation of gene transcription.
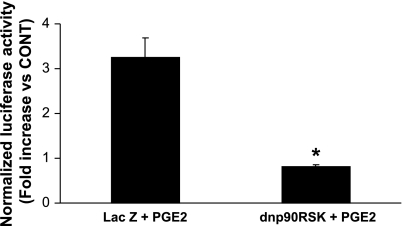
To test the involvement of p90RSK in the regulation of the hBNP promoter, we transfected NVMs with the hBNPluc promoter and then added an adenovirus encoding dnp90RSK. As shown in Fig. 4, PGE2 induced hBNP promoter activity by 3.2-fold compared with the control, and this was abolished by dnp90RSK.
Fig. 4.
p90RSK is involved in the regulation of the brain natriuretic peptide (BNP) promoter. NVMs were transfected with −1818hBNP luciferase and, 3 days later, also infected with an adenovirus encoding either LacZ or dnp90RSK. Cells were treated with PGE2 for the final 24 h of the experiment and then lysed for the assay of luciferase activity. Luciferase activity in the control cells was arbitrarily assigned a value of 1, and activity in PGE2-stimulated cells is expressed as the fold increase versus the control. Data are mean ± SE from 3 separate experiments. *P = 0.025.
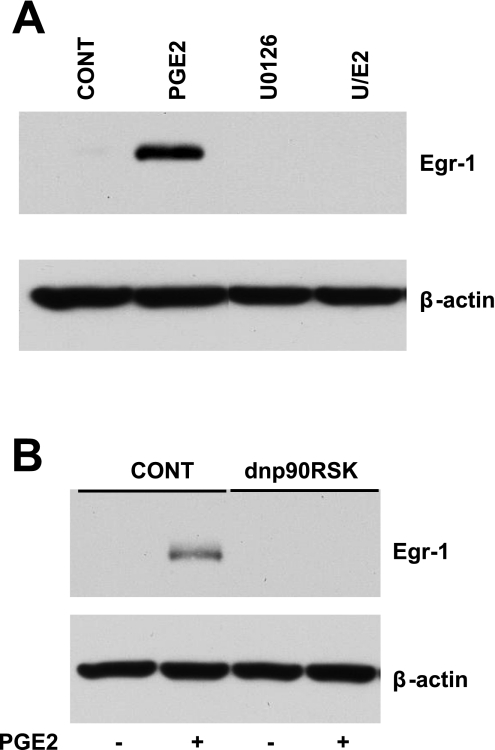
c-Fos and Egr-1, proteins encoded by early response genes, are involved in the transcription of numerous other genes and have been implicated in NVM growth (7, 20, 58, 61). We tested whether PGE2 regulates their expression. Figure 5A shows a representative Western blot demonstrating PGE2 stimulation of c-Fos. Quantitation of multiple Western blots, as shown in Fig. 5B, indicated that this stimulation was dependent on EP4 (80% inhibition with ONO-208), PKA (82% inhibition with H89), and ERK1/2 (100% inhibition with U-0126). The regulation of c-Fos by PGE2 was also dependent on Rap (84% inhibition with dnRap; Fig. 5C) and p90RSK (100% inhibition by dnp90RSK; Fig. 5D). Similar to c-Fos, PGE2 regulation of Egr-1 was completely dependent on ERK1/2 (Fig. 6A) and p90RSK (Fig. 6B). However, the induction of Egr-1 was not affected by the EP4 antagonist, dnRap, and the PKA inhibitor (data not shown).
Fig. 5.
EP4, PKA, Rap, ERK1/2, and p90RSK regulate c-Fos expression. A: representative Western blots showing the effect of ONO, H89, or U-0126 on the PGE2 stimulation of c-Fos. Cells were pretreated for 1 h, treated with PGE2 for 60 min, and then lysed for the detection of c-Fos by Western blot analysis. B: summary graph of data from 4 to 6 separate experiments. c-Fos normalized to β-actin is expressed as a percentage of PGE2 stimulation. #P ≤ 0.01 vs. PGE2. C: Rap involvement. NVMs were transfected with dnRap for 24 h, treated with PGE2 for 60 min, and then lysed for Western blot analysis. The graph is a summary of data from 3 separate experiments, and the data are expressed as a percentage of PGE2 stimulation. #P < 0.01 vs. PGE2. D: involvement of p90RSK in the PGE2 regulation of c-Fos. NVMs were transduced with dnp90RSK adenovirus or LacZ control virus for 48 h, treated with PGE2 for 60 min, and then lysed for Western blot analysis. Blots are representative of 3 separate experiments.
Fig. 6.
ERK1/2 and p90RSK regulate early growth response (Egr)-1 expression. A: representative Western blots. NVMs were treated with PGE2 for 60 min after a 1-h pretreatment with U0126. Cells were lysed for the detection of Egr-1 by Western blot analysis. B: p90RSK is involved in the PGE2 regulation of Egr-1. NVMs were transduced with dnp90RSK adenovirus or LacZ control virus for 48 h, treated with PGE2 for 60 min, and then lysed for Western blot analysis. Blots are representative of 3 separate experiments.
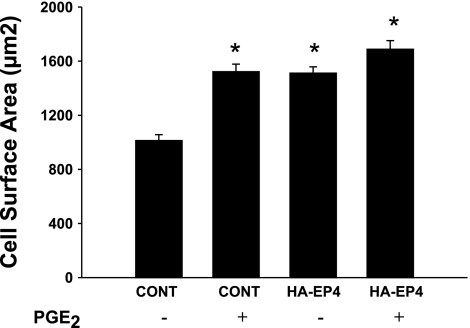
EP4 mediates the increase in myocyte size.
Our previous study (45) showed that PGE2 acting through EP4 increases protein synthesis and the size of NVMs. To directly test the effect of EP4 on NVM growth, we overexpressed EP4 (17) or a control vector expressing GFP and then treated cells with PGE2 for 48 h. Whereas PGE2 increased the cell surface area of control-transfected NVM by 50%, EP4 overexpression alone had the same stimulatory effect (P < 0.001, 70–80 cells counted per treatment; Fig. 7). Although PGE2 treatment of cells overexpressing EP4 resulted in a slightly greater cell surface area than EP4 alone, this did not achieve statistical significance. These data suggest that overexpression of EP4 alone can contribute to NVM growth, probably due to the presence of constitutively synthesized PGE2 (38). Finally, we tested whether PKA inhibition affected PGE2-dependent increases in NVM size, but found no reproducible effect (data not shown).
Fig. 7.
EP4 mediates the PGE2 stimulation of NVM growth. NVMs were transfected with expression vectors as described in methods and then treated with PGE2 for 48 h. Immunofluorescence of either green fluorescent protein-transfected (control) or hemagglutinin (HA)-EP4-transfected cells was detected under a fluorescent microscope, and cell surface area was measured using Microsuite Five. Bars are means ± SE of n = 70–80 cells. *P < 0.001 vs. the untreated control.
DISCUSSION
Our previous studies (45, 56) have shown that PGE2 and EP4 are involved in the regulation of protein synthesis, cell size, and natriuretic peptide expression in NVMs, but these studies did not identify how EP4 coupled to multiple signaling molecules and nuclear targets. The present study demonstrates that the EP4 receptor signals through the small GTPase Rap, ERK1/2, and p90RSK to regulate BNP and c-Fos expression.
EP4 is coupled to adenylate cyclase and the generation of cAMP. In addition to activating PKA, cAMP can bind and activate Epac, resulting in Rap activation (14, 15). The activation of Rap by PKA signaling has also been documented (53, 62, 65). The present study showed that PGE2 activated Rap via both PKA-dependent and -independent mechanisms, given that PKA inhibition did not totally abrogate the effect of PGE2. We have not identified the PKA-independent pathway, but it is not likely Epac, based on the fact that 8CPT does not reproducibly activate either ERK1/2 or p90RSK in NVMs. Consistent with this idea, Morel et al. (49) have shown that Epac activates the hypertrophic program through Rac, calcineurin, and nuclear factor of activated T cells in NVMs. Also, Metrich et al. (46) found that calcineurin but not PKA and Rap played a role in Epac-induced hypertophy in isoproterenol-stimulated adult cardiac myocytes. Thus, the role of Rap may be unique to PGE2/EP4 effects in myocytes.
Rap has been shown to mediate the activation of ERK1/2 (19, 26, 29, 62), and ERK1/2 activates p90RSK (31, 40, 62, 66, 67). ERK1/2 and p90RSK signaling pathways have been implicated in cardiac hypertrophy (8, 21, 34, 48), myocardial ischemia-reperfusion injury (69), and heart failure (70). Our data show that PGE2 via EP4 and Rap activated ERK1/2 and p90RSK, consistent with other studies on the growth of NVM and cardiac dysfunction in vivo.
MAPK signaling cascades regulate transcription by multiple mechanisms, including the phosphorylation and activation of transcription factors, coactivators, corepressors, histones, and the basal transcriptional machinery. Activated ERK1/2 and/or p90RSK have been shown to target c-Fos (2, 11, 22) and Egr-1 (30) as well as participate in the activation of NF-κB (25). Moreover, c-Fos and Egr-1 participate in the transcriptional regulation of many genes associated with cell growth. In our study, PGE2 stimulation of c-Fos and Egr-1 expression was completely dependent on ERK1/2 and p90RSK. In addition, ERK1/2 and p90RSK were involved in the regulation of the BNP promoter. The transcription factors GATA-4 and activator protein (AP)-1 (containing c-Jun and c-Fos family members) have been shown to be regulated by MAPKs (1, 10, 68, 72, 75), and both factors regulate basal and inducible activity of the hBNP promoter (27, 35). Thus, PGE2-dependent activation of ERK1/2 and p90RSK may target GATA-4 and AP-1 family members, enhancing their activity and stimulating BNP promoter activity.
Egr-1 is a transcription factor in the zinc finger family and is referred to as an immediate-early gene, regulating the expression of a number of downstream target genes involved in cardiovascular disease (30). It is regulated by MAPKs, involved in the growth of cardiac myocytes (9, 28, 51, 59, 64), and upregulated in the ischemic myocardium (9, 28, 42, 59, 64). In HEK-293 cells stably overexpressing EP4, PGE2 stimulation resulted in the activation of ERK1/2 and upregulation of Egr-1 (23). In our study in NVMs, we found that PGE2 activated ERK1/2 and upregulated Egr-1 but that this was not dependent on EP4. Thus, unlike c-Fos and the BNP promoter, PGE2 regulation of Egr-1 likely involves a different EP receptor.
Other PGE2 receptors may mediate PGE2-induced ERK1/2 activation and the downstream transcriptional regulation of genes. Nicola et al. (52) and Chuang et al. (12) have shown that EP3 activated ERK1/2 in trophoblast cells and fibroblasts, respectively, whereas Meyer-Kirchrath et al. (47) have shown that EP3 overexpression in the mouse heart resulted in the activation of a pro-hypertrophic signaling pathway. Unpublished data from our laboratory have indicated that the EP3 agonist sulprostone activates ERK1/2, and an EP3 antagonist inhibits this in part, with complete inhibition occurring with the combination of EP3 and EP4 antagonists (Q. He and M. C. LaPointe, unpublished observations). Luttrell et al. (41) demonstrated that the Gβγ subunit of Gαi is involved in Src-dependent activation of ERK1/2. Since the EP3 receptor couples to Gαi, it is possible that a similar mechanism is involved in its activation of ERK1/2. It is also possible that the EP3 receptor is responsible for the upregulation of Egr-1, as its regulation by PGE2 was not inhibited by the EP4 antagonist.
EP4 is expressed in the adult heart (73) and seems to be the major EP mediating the actions of PGE2 in the heart and cultured NVMs (21, 48, 73). Using a mouse model of myocardial infarction, we have shown that either inhibition of COX2 to decrease PGE2 production (36) or cardiac-specific deletion of EP4 (55) reduced cardiac hypertrophy, as measured by myocyte cross-sectional area. In addition, Degousee et al. (16) have shown that mice with global knockout of inducible PGE2 synthase (microsomal PGE synthase-1) had reduced cardiac PGE2 levels, myocyte cross-sectional area, and surface area compared with wild-type mice after myocardial infarction, although knockout hearts were more dilated and had impaired fractional shortening. Using a global EP4 knockout mouse, Xiao et al. (73) found that infarct size in response to ischemia-reperfusion injury was larger in knockout mice than in wild-type mice, suggesting a cardioprotective role for EP4 and PGE2. This implies that PGE2 and EP4 may contribute to the adaptive compensatory growth and/or survival of myocytes, which is necessary for maintaining function.
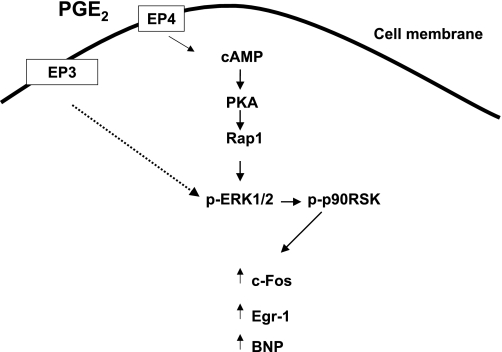
In summary, our previous data have indicated that PGE2 signaling through EP4 and ERK1/2 contribute to NVM growth. However, our present study shows that the PKA-dependent signaling pathway defined in the present study does not seem to result in NVM growth. As shown in Fig. 8, the clearly defined mediators of EP4 include cAMP, PKA, the small GTPase Rap, ERK1/2, and p90RSK. Downstream of these kinases, EP4 mediates the regulation of c-Fos and BNP gene expression. Another early response gene, Egr-1, is upregulated by PGE2 but does not seem to be regulated by EP4. A limitation of this study is that we have not determined whether PGE2 acting through EP4 activates the transcription factors c-Fos and Egr-1 as well as increases their expression. If they are activated, then they may have the potential to act as additional growth-promoting factors in PGE2-stimulated NVMs. Nonetheless, detailed understanding of EP4 signaling in NVM in vitro provides insight into the potential physiological and pathological impact of PGE2 in the heart.
Fig. 8.
EP4-dependent PGE2 signaling in NVMs. This schematic summarizes our data related to PGE2 and EP4 signaling in NVMs. EP4 acting through cAMP and PKA activates the small GTPase Rap. Downstream of Rap is ERK1/2 and p90RSK. p90RSK acts to stimulate the expression of c-Fos, Egr-1, and BNP. Other EPs (in particular, EP3) may act at the level of activation of ERK1/2.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant P01-HL-28982 (Dr. LaPointe, project III Principal Investigator).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Zizheng Hou and David Taube for the excellent technical assistance. The authors also thank Dr. Mariela Mendez for the assistance with the EP4 overexpression experiments.
REFERENCES
- 1.Ai J, Wang Y, Tan K, Deng Y, Luo N, Yuan W, Wang Z, Li Y, Wang Y, Mo X, Zhu C, Yin Z, Liu M, Wu X. A human homolog of mouse Lbh gene, hLBH, expresses in heart and activates SRE and AP-1 mediated MAPK signaling pathway. Mol Biol Rep 35: 179–187, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bjorbaek C, Zhao Y, Moller DE. Divergent functional roles for p90rsk kinase domains. J Biol Chem 270: 18848–18852, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Blatteis CM, Li S, Li Z, Feleder C, Perlik V. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostaglandins Other Lipid Mediat 76: 1–18, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bogoyevitch MA, Sugden PH. The role of protein kinases in adaptational growth of the heart. Int J Biochem Cell Biol 28: 1–12, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4: 733–738, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bruneau BG, Piazza LA, de Bold AJ. α1-Adrenergic stimulation of isolated rat atria results in discoordinate increases in natriuretic peptide secretion and gene expression and enhances Egr-1 and c-Myc expression. Endocrinology 137: 137–143, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19: 6341–6350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buitrago M, Lorenz K, Maass AH, Oberdorf-Maass S, Keller U, Schmitteckert EM, Ivashchenko Y, Lohse MJ, Engelhardt S. The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nat Med 11: 837–844, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Charron F, Tsimiklis G, Arcand M, Robitaille L, Liang Q, Molkentin JD, Meloche S, Nemer M. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev 15: 2702–2719, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen RH, Chung J, Blenis J. Regulation of pp90rsk phosphorylation and S6 phosphotransferase activity in Swiss 3T3 cells by growth factor-, phorbol ester-, and cyclic AMP-mediated signal transduction. Mol Cell Biol 11: 1861–1867, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang PC, Sun HS, Chen TM, Tsai SJ. Prostaglandin E2 induces fibroblast growth factor 9 via EP3-dependent protein kinase Cδ and Elk-1 signaling. Mol Cell Biol 26: 8281–8292, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem 273: 1496–1505, 1998 [DOI] [PubMed] [Google Scholar]
- 14.de Rooij J, Rehmann H, van TM, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 275: 20829–20836, 2000 [DOI] [PubMed] [Google Scholar]
- 15.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Degousee N, Fazel S, Angoulvant D, Stefanski E, Pawelzik SC, Korotkova M, Arab S, Liu P, Lindsay TF, Zhuo S, Butany J, Li RK, Audoly L, Schmidt R, Angioni C, Geisslinger G, Jakobsson PJ, Rubin BB. Microsomal prostaglandin E2 synthase-1 deletion leads to adverse left ventricular remodeling after myocardial infarction. Circulation 117: 1701–1710, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Desai S, April H, Nwaneshiudu C, Ashby B. Comparison of agonist-induced internalization of the human EP2 and EP4 prostaglandin receptors: role of the carboxyl terminus in EP4 receptor sequestration. Mol Pharmacol 58: 1279–1286, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Downey GP, Gumbay RS, Doherty DE, LaBrecque JF, Henson JE, Henson PM, Worthen GS. Enhancement of pulmonary inflammation by PGE2: evidence for a vasodilator effect. J Appl Physiol 64: 728–741, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Ehses JA, Pelech SL, Pederson RA, McIntosh CH. Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J Biol Chem 277: 37088–37097, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Freire G, Ocampo C, Ilbawi N, Griffin AJ, Gupta M. Overt expression of AP-1 reduces alpha myosin heavy chain expression and contributes to heart failure from chronic volume overload. J Mol Cell Cardiol 43: 465–478, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Frias MA, Rebsamen MC, Gerber-Wicht C, Lang U. Prostaglandin E2 activates Stat3 in neonatal rat ventricular cardiomyocytes: a role in cardiac hypertrophy. Cardiovasc Res 73: 57–65, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol 151: 65–77, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem 278: 12151–12156, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Gauvreau GM, Watson RM, O'Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med 159: 31–36, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Ghoda L, Lin X, Greene WC. The 90-kDa ribosomal S6 kinase (pp90rsk) phosphorylates the N-terminal regulatory domain of IκBα and stimulates its degradation in vitro. J Biol Chem 272: 21281–21288, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Guo FF, Kumahara E, Saffen D. A CalDAG-GEFI/Rap1/B-Raf cassette couples M1 muscarinic acetylcholine receptors to the activation of ERK1/2. J Biol Chem 276: 25568–25581, 2001 [DOI] [PubMed] [Google Scholar]
- 27.He Q, Mendez M, LaPointe MC. Regulation of the human brain natriuretic peptide gene by GATA-4. Am J Physiol Endocrinol Metab 283: E50–E57, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Iwaki K, Sukhatme VP, Shubeita HE, Chien KR. α- and β-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. Fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an α1-mediated response. J Biol Chem 265: 13809–13817, 1990 [PubMed] [Google Scholar]
- 29.Karliner JS, Simpson PC. β-Adrenoceptor and adenylate cyclase regulation in cardiac myocyte growth. Basic Res Cardiol 83: 655–663, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Khachigian LM. Early growth response-1 in cardiovascular pathobiology. Circ Res 98: 186–191, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Yu HS, Park HG, Jeon WJ, Song JY, Kang UG, Ahn YM, Lee YH, Kim YS. Dose-dependent effect of intracerebroventricular injection of ouabain on the phosphorylation of the MEK1/2-ERK1/2-p90RSK pathway in the rat brain related to locomotor activity. Prog Neuropsychopharmacol Biol Psychiatry 32: 1637–1642, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Kitamura T, Itoh M, Noda T, Tani K, Kobayashi M, Maruyama T, Kobayashi K, Ohuchida S, Sugimura T, Wakabayashi K. Combined effects of prostaglandin E receptor subtype EP1 and subtype EP4 antagonists on intestinal tumorigenesis in adenomatous polyposis coli gene knockout mice. Cancer Sci 94: 618–621, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klimberg VS, Kornbluth J, Cao Y, Dang A, Blossom S, Schaeffer RF. Glutamine suppresses PGE2 synthesis and breast cancer growth. J Surg Res 63: 293–297, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Kuster GM, Pimentel DR, Adachi T, Ido Y, Brenner DA, Cohen RA, Liao R, Siwik DA, Colucci WS. α-Adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1-sensitive oxidative modification of thiols on Ras. Circulation 111: 1192–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 35.LaPointe MC. Molecular regulation of the brain natriuretic peptide gene. Peptides 26: 944–956, 2005 [DOI] [PubMed] [Google Scholar]
- 36.LaPointe MC, Mendez M, Leung A, Tao Z, Yang XP. Inhibition of cyclooxygenase-2 improves cardiac function after myocardial infarction in the mouse. Am J Physiol Heart Circ Physiol 286: H1416–H1424, 2004 [DOI] [PubMed] [Google Scholar]
- 37.LaPointe MC, Sitkins JR. Phorbol ester stimulates the synthesis and secretion of brain natriuretic peptide from neonatal rat ventricular cardiocytes: a comparison with the regulation of atrial natriuretic factor. Mol Endocrinol 7: 1284–1296, 1993 [DOI] [PubMed] [Google Scholar]
- 38.LaPointe MC, Sitkins JR. Phospholipase A2 metabolites regulate inducible nitric oxide synthase in myocytes. Hypertension 31: 218–224, 1998 [DOI] [PubMed] [Google Scholar]
- 39.LaPointe MC, Wu G, Garami M, Yang XP, Gardner DG. Tissue-specific expression of the human brain natriuretic peptide gene in cardiac myocytes. Hypertension 27: 715–722, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Luo J, Kintner DB, Shull GE, Sun D. ERK1/2-p90RSK-mediated phosphorylation of Na+/H+ exchanger isoform 1. A role in ischemic neuronal death. J Biol Chem 282: 28274–28284, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem 271: 19443–19450, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Lyn D, Liu X, Bennett NA, Emmett NL. Gene expression profile in mouse myocardium after ischemia. Physiol Genomics 2: 93–100, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Melillo E, Woolley KL, Manning PJ, Watson RM, O'Byrne PM. Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am J Respir Crit Care Med 149: 1138–1141, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Mendez M, LaPointe MC. Trophic effects of the cyclooxygenase-2 product prostaglandin E2 in cardiac myocytes. Hypertension 39: 382–388, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Mendez M, LaPointe MC. PGE2-induced hypertrophy of cardiac myocytes involves EP4 receptor-dependent activation of p42/44 MAPK and EGFR transactivation. Am J Physiol Heart Circ Physiol 288: H2111–H2117, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc'h F. Epac mediates β-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res 102: 959–965, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Meyer-Kirchrath J, Martin M, Schooss C, Jacoby C, Flogel U, Marzoll A, Fischer JW, Schrader J, Schror K, Hohlfeld T. Overexpression of prostaglandin EP3 receptors activates calcineurin and promotes hypertrophy in the murine heart. Cardiovasc Res 81: 310–318, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Miyatake S, Manabe-Kawaguchi H, Watanabe K, Hori S, Aikawa N, Fukuda K. Prostaglandin E2 induces hypertrophic changes and suppresses alpha-skeletal actin gene expression in rat cardiomyocytes. J Cardiovasc Pharmacol 50: 548–554, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, Lompre AM, Vandecasteele G, Lezoualc'h F. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res 97: 1296–1304, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 79: 1193–1226, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Neyses L, Nouskas J, Luyken J, Fronhoffs S, Oberdorf S, Pfeifer U, Williams RS, Sukhatme VP, Vetter H. Induction of immediate-early genes by angiotensin II and endothelin-1 in adult rat cardiomyocytes. J Hypertens 11: 927–934, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Nicola C, Chirpac A, Lala PK, Chakraborty C. Roles of Rho guanosine 5′-triphosphatase A, Rho kinases, and extracellular signal regulated kinase (1/2) in prostaglandin E2-mediated migration of first-trimester human extravillous trophoblast. Endocrinology 149: 1243–1251, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Obara Y, Labudda K, Dillon TJ, Stork PJ. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci 117: 6085–6094, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Peana AT, Bennardini F, Buttu L, Pippia P, Meloni MA, Stuffler RG, Maccarrone M. Effect of simulated microgravity on PGE2-induced edema and hyperalgesia in rat paws: pharmacological data and biochemical correlates. J Gravit Physiol 11: 41–42, 2004 [PubMed] [Google Scholar]
- 55.Qian JY, Harding P, Liu Y, Shesely E, Yang XP, LaPointe MC. Reduced cardiac remodeling and function in cardiac-specific EP4 receptor knockout mice with myocardial infarction. Hypertension 51: 560–566, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qian JY, Leung A, Harding P, LaPointe MC. PGE2 stimulates human brain natriuretic peptide expression via EP4 and p42/44 MAPK. Am J Physiol Heart Circ Physiol 290: H1740–H1746, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Rask K, Zhu Y, Wang W, Hedin L, Sundfeldt K. Ovarian epithelial cancer: a role for PGE2-synthesis and signalling in malignant transformation and progression. Mol Cancer 5: 62, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saadane N, Alpert L, Chalifour LE. Altered molecular response to adrenoreceptor-induced cardiac hypertrophy in Egr-1-deficient mice. Am J Physiol Heart Circ Physiol 278: H796–H805, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Sadoshima J, Takahashi T, Jahn L, Izumo S. Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc Natl Acad Sci USA 89: 9905–9909, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saigusa T, Iriki M. Regional differentiation of sympathetic nerve activity during fever caused by intracerebroventricular injection of PGE2. Pflügers Arch 411: 121–125, 1988 [DOI] [PubMed] [Google Scholar]
- 61.Sakai H, Urasawa K, Oyama N, Kaneta S, Saito T, Kitabatake A, Tsutsui H. Induction of c-fos mRNA expression by pure pressure overload in cultured cardiac myocytes. Int Heart J 48: 359–367, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Schmitt JM, Stork PJ. PKA phosphorylation of Src mediates cAMP's inhibition of cell growth via Rap1. Mol Cell 9: 85–94, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, Vaghi A, Folco GC, Bianco S, Robuschi M. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am J Respir Crit Care Med 153: 572–575, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Shamim A, Pelzer T, Grohe C, Neyses L. Induction of Egr-1 mRNA and protein by endothelin 1, angiotensin II and norepinephrine in neonatal cardiac myocytes. Mol Cell Biochem 195: 11–17, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Simpson PC, Long CS, Waspe LE, Henrich CJ, Ordahl CP. Transcription of early developmental isogenes in cardiac myocyte hypertrophy. J Mol Cell Cardiol 21, Suppl 5: 79–89, 1989 [DOI] [PubMed] [Google Scholar]
- 66.Simpson PJ, Lucchesi BR. Free radicals and myocardial ischemia and reperfusion injury. J Lab Clin Med 110: 13–30, 1987 [PubMed] [Google Scholar]
- 67.Starksen NF, Simpson PC, Bishopric N, Coughlin SR, Lee WM, Escobedo JA, Williams LT. Cardiac myocyte hypertrophy is associated with c-myc protooncogene expression. Proc Natl Acad Sci USA 83: 8348–8350, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugden PH. An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol 35: 871–886, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Takeishi Y, Abe J, Lee JD, Kawakatsu H, Walsh RA, Berk BC. Differential regulation of p90 ribosomal S6 kinase and big mitogen-activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circ Res 85: 1164–1172, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Takeishi Y, Huang Q, Abe J, Che W, Lee JD, Kawakatsu H, Hoit BD, Berk BC, Walsh RA. Activation of mitogen-activated protein kinases and p90 ribosomal S6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc Res 53: 131–137, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Takeishi Y, Huang Q, Abe J, Glassman M, Che W, Lee JD, Kawakatsu H, Lawrence EG, Hoit BD, Berk BC, Walsh RA. Src and multiple MAP kinase activation in cardiac hypertrophy and congestive heart failure under chronic pressure-overload: comparison with acute mechanical stretch. J Mol Cell Cardiol 33: 1637–1648, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Tu VC, Bahl JJ, Chen QM. Distinct roles of p42/p44(ERK) and p38 MAPK in oxidant-induced AP-1 activation and cardiomyocyte hypertrophy. Cardiovasc Toxicol 3: 119–133, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation 109: 2462–2468, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Yamazaki T, Komuro I, Yazaki Y. Molecular aspects of mechanical stress-induced cardiac hypertrophy. Mol Cell Biochem 163–164: 197–201, 1996 [DOI] [PubMed] [Google Scholar]
- 75.Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene 320: 3–21, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Zamorano B, Carmona MT. Prostaglandin-E2 and cyclic adenosine 3′-5′ monophosphate levels in the hypertrophied rat heart. Biol Res 25: 85–89, 1992 [PubMed] [Google Scholar]