Abstract
The purpose of the current study was to determine whether heat shock protein 70 (Hsp70) directly regulates forkhead box O (FOXO) signaling in skeletal muscle. This aim stems from previous work demonstrating that Hsp70 overexpression inhibits disuse-induced FOXO transactivation and prevents muscle fiber atrophy. However, although FOXO is sufficient to cause muscle wasting, no data currently exist on the requirement of FOXO signaling in the progression of physiological muscle wasting, in vivo. In the current study we show that specific inhibition of FOXO, via expression of a dominant-negative FOXO3a, in rat soleus muscle during disuse prevented >40% of muscle fiber atrophy, demonstrating that FOXO signaling is required for disuse muscle atrophy. Subsequent experiments determined whether Hsp70 directly regulates FOXO3a signaling when independently activated in skeletal muscle, via transfection of FOXO3a. We show that Hsp70 inhibits FOXO3a-dependent transcription in a gene-specific manner. Specifically, Hsp70 inhibited FOXO3a-induced promoter activation of atrogin-1, but not MuRF1. Further studies showed that a FOXO3a DNA-binding mutant can activate MuRF1, but not atrogin-1, suggesting that FOXO3a activates these two genes through differential mechanisms. In summary, FOXO signaling is required for physiological muscle atrophy and is directly inhibited by Hsp70.
Keywords: FOXO, atrogin-1, MAFbx, MuRF1, transcriptional regulation
degradation of skeletal muscle protein is, in part, responsible for the skeletal muscle wasting that accompanies systemic diseases such as cancer and diabetes, as well as disuse (1, 8, 20). Although multiple proteolytic systems are involved in muscle protein breakdown, degradation through the ubiquitin proteasome system (UPS) may account for up to 80% of proteolysis during skeletal muscle wasting (20). Before degradation by the UPS, protein substrates must first be polyubiquitinated to confer recognition by the 26S proteasome (11). Involved in this ubiquitination process are the ubiquitin E3 ligases, which tag ubiquitin to specific protein substrates. Two such E3 ligases, the muscle-specific atrogin-1 (MAFbx) and MuRF1, are upregulated in multiple models of skeletal muscle wasting (7), and muscles depleted of these genes demonstrate significant sparing of muscle mass following denervation (3). Because of this requirement of atrogin-1 and MuRF1 in the progression of normal muscle wasting, recent research has trended toward understanding the upstream regulators of these key atrophy genes.
A large body of evidence exists to support the forkhead box subfamily O (FOXO) transcription factors in the regulation of atrogin-1 (14, 15), and, more recently, evidence has emerged to suggest that FOXO factors may also regulate MuRF1 (10, 16, 23). The FOXO transcription factors are the mammalian homologues of the Caenorhabditis elegans DAF-16 protein, and they include FOXO1, FOXO3A, and FOXO4. Each of the DAF-16/FOXO homologues contains a highly conserved forkhead box DNA-binding domain, which recognizes and binds a core DNA sequence found within the DAF-16-binding element and the forkhead-binding element (FBE) in the promoters of target genes. DAF-16/FOXO transcriptional activity is increased in skeletal muscle in response to various catabolic stimuli (15, 16, 23) concomitantly with the increase in atrogin-1 and MuRF1 gene transcription, both of which contain FBEs in their promoter regions. Transfection of FOXO3a alone is sufficient to increase atrogin-1 mRNA, and it can bind to and activate transcription of an atrogin-1 promoter fragment in C2C12 skeletal muscle myotubes, as well as in whole muscle, which is prevented following mutation of the forkhead-binding sites (15). Similar experiments in HepG2 cells demonstrated that both FOXO1 and FOXO3a are sufficient to activate a MuRF1 promoter fragment (23). However, in C2C12 skeletal muscle myotubes, the data are conflicting on whether FOXO1 is sufficient to increase atrogin-1 and/or MuRF1 mRNA expression (10, 18). In addition, out of two FOXO1 transgenic lines created, neither showed an increase in MuRF1 mRNA expression, although one line showed an increase in atrogin-1 (6). Therefore, although FOXO3a is sufficient to increase atrogin-1 transcription, the current data on whether any of the FOXO family members are sufficient to increase MuRF1 transcription in skeletal muscle are more varied.
In contrast, there are convincing data demonstrating that FOXO transcription factors are required for atrogin-1 and MuRF1 transcription in response to catabolic stimuli (15, 16, 18). Direct inhibition of FOXOs 1–3 during either fasting or glucocorticoid treatment prevents atrogin-1 transcription (15), while mutation of the FBE is sufficient to block glucocorticoid-induced MuRF1 promoter activity in C2C12 myotubes (23). Furthermore, although constitutively active (c.a.) FOXO1 is not sufficient to activate atrogin-1 and MuRF1 in myotubes, expression of c.a.FOXO1 blocks the ability of IGF-1 to inhibit glucocorticoid-induced atrogin-1 and MuRF1 (18). We recently demonstrated in our lab that expression of dominant-negative (d.n.) FOXO3a blocks both atrogin-1 and MuRF1 transcription during immobilization, providing evidence that FOXO is required for their activation during disuse-induced muscle wasting (16).
In addition, we also recently demonstrated that overexpression of heat shock protein 70 (Hsp70), which is known to be cytoprotective in multiple cell types, is sufficient to inhibit the activation of atrogin-1 and attenuate MuRF1 transcription during disuse muscle atrophy (16). Because Hsp70 overexpression also inhibited the activation of a DAF-16/FOXO reporter plasmid, and both Hsp70 and d.n.FOXO3a inhibit atrogin-1 and MuRF1 transcription, Hsp70 may regulate atrogin-1 and MuRF1 transcription through inhibition of FOXO3a signaling. However, multiple transcription factors, such as glucocorticoids, NF-κB, as well as the other FOXO family members, may regulate atrogin-1 and MuRF1 during muscle wasting (5, 6, 23). Furthermore, the DAF-16/FOXO reporter plasmid responds to each of the skeletal muscle FOXO family members (FOXOs 1, 3, and 4). Consequently, the inhibition of both atrophy gene transcription and the Foxo reporter by Hsp70 may be due to a direct inhibition of any one, or combination of the Foxo proteins, or may instead reflect a downstream affect of inhibiting an alternative pathway.
Therefore, the current study sought to determine whether Hsp70 can specifically and directly regulate FOXO3a signaling and atrophy gene transcription when independently activated in skeletal muscle via transfection of FOXO3a. However, because the physiological significance of inhibiting Foxo signaling directly, in vivo (on muscle fiber cross-sectional area) is not known, we also sought to determine whether FOXO signaling is required for normal muscle wasting during disuse.
MATERIALS AND METHODS
Animals.
All experiments used male Sprague-Dawley rats (250 g) obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in a temperature-controlled facility on a 12-h light-dark cycle, and standard diet and water were provided ad libitum. The University of Florida Institutional Animal Care and Use Committee approved all protocols and animal procedures.
Expression and reporter plasmids.
The d.n.FOXO3a, which consists of only the FOXO3a DNA-binding domain (aa 141–266), hemagglutinin (HA)-tagged FOXO3a wild-type (WT) and FOXO3a triple phosphorylation-mutant expression plasmids were generously donated by Dr. Paul Coffer (University Medical Center, Utrecht, The Netherlands) and have been previously described (4, 17). The enhanced green fluorescent protein (EGFP)-c1 plasmid was purchased from Clontech (Palo Alto, CA) and was cotransfected with the d.n.FOXO3a expression plasmid to visualize fibers expressing plasmid DNA. The Hsp70 expression vector has been described previously (16). The DAF-16 (FOXO) reporter plasmid has also been previously used and described and was obtained from Dr. Alex Toker (Beth Israel Deaconess Medical Center, Boston, MA) (19). The atrogin-1-GL2 promoter reporter was obtained from Dr. William Mitch (Baylor College of Medicine, Houston, TX) while the MuRF1 promoter reporter was a gift from Dr. Steve Shoelson (Joslin Diabetes Center and Harvard Medical School, Boston, MA) and again, have both been previously described (5, 9). The HA-FOXO3a DNA-binding mutant [HA-FOXO3a DBM (H212R)] was obtained through Addgene (Addgene plasmid 8352) and was submitted by Dr. Michael Greenberg and has previously been described (21). Plasmid DNA was amplified and sequentially isolated using an Endotoxin-Free Maxi or Mega Prep Kit (Qiagen, Valencia, CA), ethanol precipitated and resuspended in 1× sterile PBS.
Plasmid injection and electroporation.
In vivo transfection of plasmid DNA into skeletal muscle has been detailed previously (16). For all experiments, 10 μg of the expression or control plasmid(s) and 40 μg of the reporter plasmid were diluted in 50 μl of 1× PBS for injection.
Immobilization and muscle preparation.
For immobilization experiments, disuse muscle atrophy was induced in rat soleus muscles via plaster cast immobilization of both hindlimbs 4 days following plasmid injection and has been detailed previously (16). For muscle fiber atrophy experiments, soleus muscles were collected after 7 days of immobilization or weight-bearing activity and placed in tissue-freezing media on a tongue depressor, at a standardized length, immediately frozen in dry-ice cooled isopentane, and stored at −80°C for subsequent histochemical analysis. For cell signaling studies, muscles were removed following 3 days of immobilization and processed immediately for RNA isolation and cytosolic/nuclear separation, or frozen in liquid nitrogen and stored at −80°C until further biochemical analyses. This 3-day time point was chosen for signaling experiments based on previous work demonstrating that transcriptional changes peak near this earlier time point during multiple conditions of muscle atrophy, including disuse (7, 13), which precede the significant fiber atrophy that is seen after 7 day of disuse muscle wasting (16).
Reporter plasmid analysis.
Luciferase assays were performed on skeletal muscle homogenates according to standard procedures and have been described previously (16). Total muscle luciferase activity was determined using an LMax II microplate luminometer (Molecular Devices, Sunnyvale, CA).
Immunohistochemistry.
Following 7 days of weight-bearing activity or hindlimb immobilization, serial cross sections (10 μm) of soleus muscles transfected with d.n.FOXO3a and EGFP were prepared using a cryostat microtome (Microm HM 550; Microm International, Walldorf, Germany). Sections were fixed in 4% paraformaldehyde and stained with wheat germ agglutinin Texas Red-X conjugate. Fiber images were visualized and captured using an Olympus IX50 camera. From each muscle the area of ∼200 muscle fibers was determined using Image Pro Discovery Software (Media Cybernetics, Bethesda, MD).
RNA isolation, cDNA synthesis, and RT-PCR.
Three days following immobilization or weight-bearing activity, soleus muscles were removed and processed for RNA isolation and cDNA synthesis as previously described (16). cDNA (5 μl) was used as a template for real-time quantitative RT-PCR using primers for FOXO3a (Foxo3a, GenBank accession no. NM_001106395.1) or 18S, (GenBank X03205.1) from Applied Biosystems (Austin, TX) and a 7300 real-time PCR system (Applied Biosystems). TaqMan probe-based chemistry was used to allow detection of PCR products, and quantification of FOXO3a gene expression was performed using the relative standard curve method and normalized to 18S gene expression.
Cytosolic and nuclear separation.
Following 3 days of immobilization or weight-bearing activity, soleus muscles were homogenized in 1 ml of ice-cold lysis buffer (20% glycerol, 0.1% Triton X-100, 10 mM NaCl, 20 mM HEPES, and 1.5 mM MgCl2) containing 1 mM DTT, and phosphatase and protease inhibitory cocktails. Extracts were centrifuged at 880 g for 3 min at 4°C to collect the nuclear pellet. Supernatants were centrifuged three times at 3,500 g for 5 min at 4°C to collect the cytosolic fraction. The nuclear pellet was subsequently washed three times via resuspension in cell lysis buffer and centrifugation at 880 g, followed by 2-h rotation in 5 M NaCl diluted in lysis buffer, all at 4°C. Samples were then centrifuged at 21,900 g for 15 min at 4°C, and the supernatants were saved as the nuclear protein fraction. Confirmation of successful nuclear and cytosolic separation was confirmed via Western blot for histone-H1 and CuZnSOD on each fraction.
Western blot analysis.
Sample preparation and Western blot analysis of muscle homogenates were performed according to standard procedures and have previously been described (16). The following primary antibodies were used according to the manufacturer's directions: anti-HA (HA-FOXO3a) (sc-57592, Santa Cruz Biotechnology, Santa Cruz, CA), anti-SOD-1 (sc-8637, Santa Cruz Biotechnology), anti-histone-H1 (sc-8030, Santa Cruz Biotechnology), anti-FKHRL1 (sc-11351, Santa Cruz Biotechnology), anti-Hsp70 (SPA-810, Stressgen, Ann Arbor, MI), and anti-phospho-FoxO3a (Ser253) (no. 9466S, Cell Signaling Technology).
Statistical analysis.
All data were analyzed using either a two-way ANOVA followed by Bonferroni post hoc comparisons or a Student's t-test (GraphPad Software, San Diego, CA). All data are expressed as means ± SE, and significance was established at P < 0.05 level.
RESULTS
FOXO signaling is required for disuse muscle atrophy.
Overexpression of Hsp70 during skeletal muscle disuse was recently demonstrated to inhibit atrophy gene transcription and protect against muscle fiber atrophy (16). In these studies, Hsp70 was also sufficient to block the activation of a FOXO reporter plasmid, which suggests that Hsp70 may regulate muscle mass via inhibition of FOXO signaling. However, although FOXO is linked to the regulation of specific atrophy genes during various catabolic conditions and is required for myotube atrophy in vitro (15), the requirement for FOXO in the progression of fiber atrophy during physiological muscle wasting has not been previously demonstrated. Therefore, in the current study, we first sought to determine whether specific inhibition of FOXO during skeletal muscle disuse prevents muscle fiber atrophy. Rat soleus muscles were injected and electroporated with a d.n.FOXO3a expression plasmid plus EGFP before 7 days of hindlimb immobilization. When coinjecting two plasmids of similar size, cotransduction of a given fiber occurs almost 100% of the time (2, 12). Since EGFP is 4.7 kb and d.n.FOXO3a is 5 kb, the green fluorescent fibers are expressing d.n.FOXO3a (Fig. 1, A and B). Immobilization induced a 40% decrease in the mean cross-sectional area of nontransfected (i.e., black) fibers (weight bearing, 3,084 ± 128 μm2; immobilized, 1,847 ± 88 μm2), which was attenuated by 43% in transfected (i.e., green) fibers expressing d.n.FOXO3a, i.e., those fibers in which FOXO is inhibited (weight bearing, 3,118 ± 108 μm2; immobilized, 2,382 ± 156 μm2) (Fig. 1, C and D). These findings demonstrate that inhibition of FOXO signaling, in a physiological model of muscle wasting, prevents almost half of the muscle fiber atrophy.
Fig. 1.
Forkhead box subfamily O 3A (FOXO3a) signaling is required for disuse muscle fiber atrophy. A and B: representative cross sections taken from the soleus muscle of weight-bearing (A) and 7-day-immobilized (Imm) rats (B), each injected with dominant-negative (d.n.)FOXO3a + enhanced green fluorescent protein (EGFP). The cross-sectional area of green fluorescent fibers (fibers expressing d.n.FOXO3a) was measured and compared with the cross-sectional area of nonfluorescent fibers (fibers not expressing d.n.FOXO3a) within the same muscle. C: muscle fiber cross-sectional area of ∼250 fibers/muscle, from 6 muscles/group. D: frequency distribution of the fiber areas from weight-bearing muscles, immobilized muscles, and immobilized muscles expressing d.n.FOXO3a + EGFP. *P < 0.05 vs. nonexpressing weight-bearing fibers; †P < 0.05 vs. nonexpressing immobilized fibers.
Hsp70 modulates FOXO3a cellular localization during disuse.
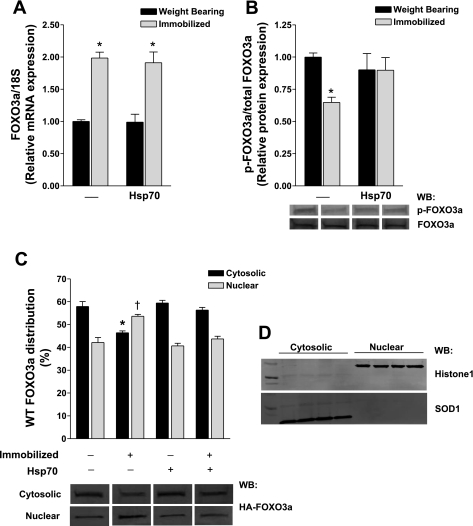
We have previously demonstrated that Hsp70 overexpression can block the activation of a FOXO reporter during skeletal muscle disuse and prevent muscle fiber atrophy (16). Since we now show FOXO signaling can account for ∼40% of disuse muscle fiber atrophy, we sought to determine whether Hsp70 can specifically regulate FOXO3a during disuse. We chose to look specifically at FOXO3a, since its increased expression is sufficient to increase atrogin-1 transcription and cause atrophy in vivo (15). Soleus muscles of rats were injected with either a control or Hsp70 expression plasmid before 3 days of hindlimb immobilization or weight-bearing activity. Following immobilization, FOXO3a mRNA was increased approximately twofold, and it remained elevated in Hsp70-injected muscles (Fig. 2A), demonstrating that Hsp70 does not regulate the transcription of FOXO3a. Despite the increase in FOXO3a mRNA following 3 days of immobilization, total levels of endogenous FOXO3a protein remained unchanged following immobilization at this time point and were not altered by Hsp70. Since Hsp70 does not modulate FOXO3a mRNA or total protein levels at this time point, we determined whether Hsp70 overexpression alters the phosphorylation and nuclear/cytosolic distribution of FOXO3a during weight-bearing conditions or immobilization. Following immobilization, the phosphorylation of FOXO3a (p-FOXO3a/total FOXO3a) decreased by ∼30% compared with weight-bearing conditions (Fig. 2B). Importantly, although Hsp70 did not significantly change the basal levels of p-FOXO3a in weight-bearing muscles, the decrease in phosphorylated FOXO3a in response to immobilization was attenuated by ∼50% in immobilized muscles overexpressing Hsp70. However, since the transfection efficiency of Hsp70 in our hands is ∼60%, this nonsignificant attenuation would likely be much greater, and possibly significant, with a higher transfection efficiency.
Fig. 2.
Heat shock protein 70 (Hsp70) regulates FOXO3a posttranscriptionally. A: relative mRNA expression of FOXO3a in weight-bearing and 3-day hindlimb-immobilized muscles injected with a control or Hsp70 expression plasmid, normalized to 18S. B: relative ratio of endogenous phosphorylated (p)-FOXO3a/total FOXO3a in soleus muscles following the same experimental design as in A. Shown are representative Western blots (WB) of p-FOXO3a and total FOXO3a from each group. *Significantly different from weight-bearing control (P < 0.05). C: cellular distribution of wild-type (WT) FOXO3a in the cytosolic and nuclear fractions of soleus muscles injected with WT FOXO3a plus either Hsp70 or the respective control plasmid, and subjected to 3 days of hindlimb immobilization or weight-bearing activity. Included are the representative Western blots of hemagglutinin (HA)-FOXO3a from each fraction. To control for variances in ectopic FOXO3a protein overexpression between muscles, cytosolic and nuclear fractions from a sample were run on the same blot and data are therefore expressed as the percentage of total WT FOXO3a. *Significantly different from weight-bearing, cytosolic fraction (P < 0.05). †Significantly different from weight-bearing, nuclear fraction (P < 0.05). Each bar represents the mean ± SE from at least 6 muscles. D: representative Western blots for Histone1 and SOD1 on nuclear and cytosolic fractions, demonstrating separation of the two fractions.
Because of the low endogenous levels of total FOXO3a in skeletal muscle, accurate quantification of FOXO3a following nuclear and cytosolic fractionation of whole muscle remains difficult. Therefore, to determine whether Hsp70 regulates the subcellular distribution of FOXO3a, we injected an HA-tagged WT FOXO3a expression plasmid into the soleus, plus Hsp70 or the control plasmid before 3 days of weight-bearing activity or immobilization. During weight-bearing conditions, WT FOXO3a was found in both the cytosolic (58%) and the nuclear (42%) fractions and was not significantly altered in weight-bearing muscles coexpressing Hsp70 (Fig. 2C). Three days of immobilization resulted in a 12% increase in WT FOXO3a nuclear localization, which was prevented in immobilized muscles coexpressing Hsp70. Together, these data demonstrate that Hsp70 inhibits FOXO3a nuclear localization during disuse, which may occur through preventing the decrease in phosphorylated FOXO3a.
Hsp70 inhibits FOXO3a-dependent transcription of atrogin-1.
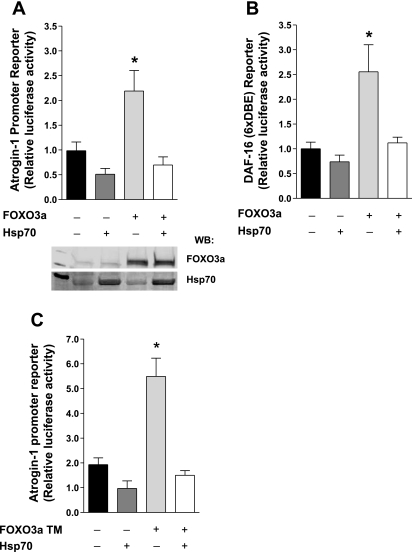
We have previously shown that Hsp70 blocks the disuse-induced transcription of atrogin-1, a known FOXO3a target gene (16). Since FOXO3a is alone sufficient to increase atrogin-1 transcription (14, 15) and is required for atrogin-1 promoter activation during disuse (16), we next sought to determine whether Hsp70 can specifically and directly block FOXO3a-dependent transcription from the atrogin-1 promoter. Although transfection of FOXO3a was sufficient to induce atrogin-1 mRNA expression by ∼30% (P = 0.08), this is likely an underestimate due to the transfection efficiency. We therefore utilized an atrogin-1 promoter reporter construct since coinjection of two or more plasmids results in cotransfection almost 100% of the time. Therefore the atrogin-1 promoter is only reporting from those fibers overexpressing FOXO3a and/or Hsp70. In these experiments, soleus muscles were injected with an atrogin-1 promoter-reporter construct plus WT FOXO3a and either Hsp70 or a control plasmid. WT FOXO3a caused an approximately twofold induction of the atrogin-1 promoter, which was abolished in muscles coexpressing Hsp70, demonstrating that Hsp70 can directly inhibit FOXO3a-induced transcription of atrogin-1 (Fig. 3A). Further evidence that Hsp70 suppresses FOXO3a-dependent transcription was obtained from similar experiments using a Foxo-responsive reporter, which contains six concatemerized DAF-16 binding sites (Fig. 3B).
Fig. 3.
Hsp70 represses FOXO3a-dependent transcription of atrogin-1. A and B: relative luciferase activity from the soleus muscle of rats 4 days following injection with either an atrogin-1 promoter-reporter (A) or a DAF-16 reporter (B), plus WT FOXO3a and Hsp70 or the respective control plasmids. A representative Western blot showing FOXO3a and Hsp70 overexpression is depicted in A. *Significantly different from control-injected group (P < 0.05). †Significantly different from WT FOXO3a-injected group (P < 0.05). DBE, DAF-16-binding element. C: relative luciferase activity from the soleus muscles of 3-day-immobilized rats injected with an atrogin-1 promoter-reporter plus FOXO3a triple mutant (TM) and Hsp70 or the respective control plasmids, normalized to their respective weight-bearing group. *Significantly different from weight-bearing control-injected group (P < 0.05). Each bar represents the mean ± SE from at least 6 muscles.
Because Hsp70 prevented the immobilization-induced decrease in phosphorylated FOXO3a in earlier experiments, we next sought to determine whether Hsp70 could similarly inhibit a FOXO3a triple mutant (TM), which is mutated in the three Akt phosphorylation sites, from activating atrogin-1 transcription during disuse. Soleus muscles were injected with an atrogin-1 promoter-reporter construct, plus FOXO3a TM, Hsp70, or the respective control plasmids before 3 days of weight-bearing activity or immobilization. Immobilization-induced an approximately twofold increase in atrogin-1 transcription in control-injected muscles which was further increased to more than fivefold in immobilized muscles expressing FOXO3a TM (Fig. 3C). Importantly, expression of Hsp70 was sufficient to block both immobilization-induced and FOXO3a TM-induced atrogin-1 transcription, demonstrating that Hsp70 inhibits FOXO3a signaling, not only through preventing the decrease in phospho-FOXO3a and nuclear translocation, but also through an additional mechanism that does not require FOXO3a phosphorylation by Akt.
FOXO3a is sufficient to activate MuRF1 through a DNA-binding-independent mechanism.
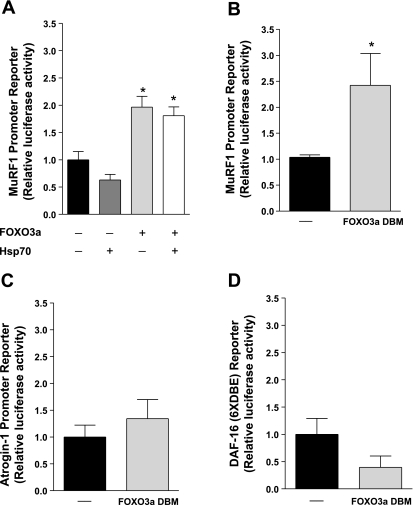
As stated previously, overexpression of Hsp70 during skeletal muscle disuse attenuates the transcription of another FOXO target gene, MuRF1, in addition to atrogin-1 (16). To determine whether Hsp70 can similarly prevent FOXO3a-induced transcription from a MuRF1 promoter, soleus muscles were injected with a MuRF1 promoter-reporter construct plus WT FOXO3a and Hsp70 or the respective control plasmids. WT FOXO3a induced the MuRF1 promoter approximately twofold over control-injected muscles. However, although Hsp70 inhibited the atrogin-1 promoter-reporter, cotransfection of Hsp70 did not prevent WT-FOXO3a from activating the MuRF1 promoter (Fig. 4A). Because Hsp70 inhibited FOXO3a from activating atrogin-1 as well as the DAF-16 reporter, which both require FOXO DNA-binding to activate transcription (15), these data suggest that FOXO3a may activate MuRF1 in a DNA-binding-independent manner.
Fig. 4.
FOXO3a induces MuRF1 through a DNA-binding independent mechanism. A: relative luciferase activity from soleus muscles 4 days following injection with a MuRF1 promoter-reporter plus WT FOXO3a and Hsp70 or the respective control plasmids. B–D: relative luciferase activity from soleus muscles 4 days following injection with a MuRF1 promoter-reporter (B), an atrogin-1 promoter-reporter (C), or a DAF-16 reporter (D), plus a FOXO3a DNA-binding mutant (FOXO3a DBM) or the respective control plasmid. Each bar represents the mean ± SE from at least 8 muscles. *Significantly different from control-injected group (P < 0.05).
To test this, soleus muscles were injected with a control or FOXO3a DNA-binding mutant (FOXO3a DBM) plus the MuRF1 promoter. Similar experiments were repeated with FOXO3a DBM using the atrogin-1 promoter-reporter and the DAF-16 reporter. Expression of the FOXO3a DBM significantly activated the MuRF1 promoter (Fig. 4B), but not the atrogin-1 promoter or the DAF-16 reporter (Fig. 4, C and D). These data demonstrate that in contrast to atrogin-1, FOXO3a can activate MuRF1 through a DNA-binding-independent mechanism.
DISCUSSION
The present studies substantiate the role of FOXO in the progression of physiological muscle wasting by demonstrating that FOXO-dependent transcription can account for >40% of disuse muscle fiber atrophy. Although this finding is significant and novel, it is not surprising. Transfection of d.n.FOXO3a prevents the immobilization-induced promoter activation of atrogin-1 and MuRF1 (16), both of which, when specifically inhibited, attenuate muscle wasting due to denervation (3). Furthermore, our findings are consistent with data from Sandri et al. (15), which demonstrate that transfection of d.n.FOXO3a in vitro prevents dexamethasone-induced atrogin-1 mRNA expression and myotube atrophy. Moreover, although it was previously demonstrated that small interfering RNA-mediated knockdown of FOXOs 1–3 prevents the activation of an atrogin-1 promoter reporter following 7 days of fasting (15), these studies did not determine, in vivo, whether this also prevents muscle fiber atrophy. Therefore, to our knowledge, this study is the first to report that inhibition of FOXO signaling, during a physiological model of muscle wasting, prevents almost half of muscle fiber atrophy. It is important to mention, however, that expression of d.n.FOXO3a (which lacks its transactivation domain) likely reduces the function of FOXO1 and FOXO4 in addition to FOXO3a (15), due to the homology between the FOXO DNA-binding domains. Thus, the inhibition of atrogin-1 and MuRF1 in previous studies, and muscle fiber atrophy in the current study through expression of d.n.FOXO3a, likely reflects the inhibition of total FOXO signaling.
Since FOXO signaling can account for almost half of disuse muscle atrophy, our previous finding that Hsp70 overexpression inhibits DAF-16 (FOXO) reporter activation and muscle fiber atrophy during disuse suggests a link between Hsp70 and FOXO in the regulation of muscle mass. However, since there is no published evidence that Hsp70 regulates FOXO signaling in any cell type, it is not known whether Hsp70 directly regulates FOXO signaling or whether inhibition of FOXO transactivation by Hsp70 is a downstream consequence of Hsp70 regulating an alternative pathway. Data presented here provide evidence that Hsp70 directly represses FOXO3a-induced transcription of atrogin-1, via both Akt-dependent and -independent mechanisms. During normal physiological conditions, the IGF-1/phosphatidylinositol 3-kinase/Akt axis inhibits FOXO transcription factors through Akt-phosphorylation of FOXO on specific threonine and serine residues that result in the retention of FOXO in the cytosol (4). During conditions of muscle wasting, decreases in FOXO phosphorylation by Akt allow FOXO to localize to the nucleus (15). We show here that Hsp70 inhibits the disuse-induced decrease in phosphorylated FOXO3a and prevents FOXO3a nuclear localization, which likely explains, in part, Hsp70's inhibition of FOXO3a-induced transcription. However, because Hsp70 also inhibited a FOXO3a TM (which cannot be phosphorylated by Akt) from activating gene promoter transcription, these data suggest that Hsp70 inhibits FOXO3a-induced transcription through an additional mechanism. In other cell types, FOXO-induced transcription has been shown to be regulated by multiple factors in addition to Akt-phosphorylation, including other posttranslational modifications and interactions with cofactor proteins. Determining whether similar mechanisms exist in skeletal muscle to regulate FOXO3a, and whether Hsp70 can mediate these, although beyond the scope of the current study, certainly warrants further investigation.
Importantly, our finding that FOXO3a differentially activates the atrogin-1 and MuRF1 promoters, further supports the notion that FOXO-induced transcription is complex and should not be surprising given the complexities of promoter transactivation. In fact, recent data collected in C2C12 myotubes demonstrated that although atrogin-1 is a FOXO1 target gene that is DNA binding dependent, MuRF1 and Bnip3 are DNA-binding-independent FOXO1-induced target genes (10). In agreement with these findings we report here, for the first time, that FOXO3a can activate the MuRF1 promoter in vivo, through a DNA-binding-independent mechanism. In this scenario, FOXO3a may act as a coregulatory factor for a second transcription factor (e.g., via protein-protein interactions) to activate MuRF1 transcription. In a recent review, van der Vos and Coffer (22) highlight multiple FOXO-binding partners which associate with FOXO to regulate target gene transcription, and thus may play a role in FOXO3a-induced transcription from the MuRF1 promoter. To our knowledge, the only indication of a protein-protein interaction with FOXO in activating the MuRF1 promoter comes from a recent study by Waddell et al. (23). Their work demonstrated that FOXO1, but not FOXO3a, synergized with the glucocorticoid receptor to activate the MuRF1 promoter in response to dexamethasone treatment in HepG2 cells (23). Although this synergistic activation was prevented following mutation of either the FBE or the glucocorticoid response element (GRE), it is unknown whether the FBE and/or GRE are necessary for FOXO1 to activate the MuRF1 promoter when expressed alone. Although we did not determine in the current study whether FOXO3a interacts with the glucocorticoid receptor in skeletal muscle to activate the MuRF1 promoter, this certainly warrants further investigation.
In conclusion, this study provides direct evidence that Hsp70 can counteract FOXO3a-dependent transcription of atrogin-1 which has important clinical implications in the muscle wasting field. Given our finding that FOXO-dependent transcription can account for >40% of disuse muscle fiber atrophy, the direct inhibition of FOXO3a signaling by Hsp70 likely explains, in part, our previous finding that Hsp70 prevents disuse muscle atrophy. Furthermore, because FOXO misregulation is evident in multiple pathological conditions, our finding that Hsp70 can regulate FOXO3a may have important ramifications in health and disease that extend beyond skeletal muscle. In addition, our finding that FOXO3a can activate MuRF1 transcription independently of FOXO3a DNA binding in vivo is novel, and it identifies an important distinction between FOXO3a-induced transcription of atrogin-1 and MuRF1. Further investigation is necessary to determine the coactivator proteins and/or transcription factors that are required for FOXO3a to activate the MuRF1 promoter.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R03AR056418 (to A. R. Judge). S. M. Senf is supported by a T32 from the National Institute of Child Health and Human Development Grant T32-HD-043730.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Joseph McClung for critical reading and editorial comments.
REFERENCES
- 1.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest 114: 370–378, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzghoul MB, Gerrard D, Watkins BA, Hannon K. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J 18: 221–223, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279: 41114–41123, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18: 39–51, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129: 227S–237S, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 15: 1537–1545, 2004 [DOI] [PubMed] [Google Scholar]
- 10.McLoughlin TJ, Smith SM, DeLong AD, Wang H, Unterman TG, Esser KA. FoxO1 induces apoptosis in skeletal myotubes in a DNA binding-dependent manner. Am J Physiol Cell Physiol 297: C548–C555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Rana ZA, Ekmark M, Gundersen K. Coexpression after electroporation of plasmid mixtures into muscle in vivo. Acta Physiol Scand 181: 233–238, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21: 140–155, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 22: 3836–3845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol 168: 5024–5031, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol 25: 8520–8530, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawa NE, Jr, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest 100: 197–203, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr, DiStefano PS, Chiang LW, Greenberg ME. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296: 530–534, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene 27: 2289–2299, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab 295: E785–E797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]