the publication by poulsen et al. (19) addresses two issues of general interest and importance, the development of resistance to cisplatin cancer treatment and the possible role of cell volume and/or cell volume regulation in apoptosis. Cell volume regulation is indispensable for life. The constraint of proteins and other large solutes in higher concentration inside than outside the cell must inevitably lead to swelling and cell rupture unless cells are impermeable to solutes and water, express a rigid exoskeleton, or pump out solutes. The need of mammalian cells for metabolically coupled extrusion of solute to maintain volume was early recognized (12, 25), even before the discovery of Na+-K+-activated ATPase by Skou (22). Skou's finding was subsequently and independently incorporated in more concrete models of cell volume regulation in the double-Donnan system of Leaf (11) and the pump-leak hypothesis of Tosteson and Hoffman (24). However, the complexity of the many regulatory volume mechanisms was largely unsuspected before the reports by Kregenow (8) of a regulatory volume decrease (RVD) that likely characterizes almost all mammalian cells and of a regulatory volume increase (RVI) characterizing many cells (7).
RVD refers to the regulatory release of solutes and secondarily water, triggered by acute hyposmotic swelling, tending to restore cell volume to its initial value. RVI refers to the regulatory uptake of solutes and secondarily water, triggered by acute cell shrinkage, again tending to restore the cell volume. The RVI is less robustly demonstrated since some cells do not so respond to simple hyperosmotic shrinkage (4), but the RVI can be commonly elicited by first hypotonically stimulating cells to release solutes and water. The subsequent restoration of an isosmotic extracellular bath commonly triggers a post-RVD RVI (14). Even with this approach, an RVI may not be demonstrable without increasing temperature to physiological levels (15).
A great deal is currently known about the transport mechanisms mediating RVD and RVI (5, 10), and some information is available about their regulation. For example, tonicity-responsive enhancer binding protein (TonEBP) is recognized as an activator of genetic expression of multiple transporters following hypertonic shrinkage (5, 16). However, the osmotic sensors needed to initiate the RVD and RVI remain unclear (5). Despite this limited understanding of cell volume regulation, much information has become available concerning the coupling of regulatory volume mechanisms to a wide spectrum of physiologic and pathophysiologic events (5, 10).
Of particular relevance are the changes in cell volume that may be required both for normal progression through the cell cycle of proliferation and for apoptotic cell death (5, 10). Fundamentally, division of parent cells into daughter cells of similar size requires an increase in cell volume. In contrast, apoptosis usually requires persistent cell shrinkage (5, 9, 17), termed the apoptotic volume decrease (AVD) and reflects, in part, release of cell K+, Cl−, and water (17). Cisplatin and many other drugs kill cancer cells by triggering apoptosis (17) but may become ineffective because of the development of multidrug resistance (MDR). This resistance frequently reflects upregulation of ATP-binding cassette (ABC) transporters, such as P-glycoprotein, that eject the drugs (19). The multidrug resistance has been associated with reduced activity of a volume-regulated anion channel (VRAC) (17), and apoptosis can also be reduced by preventing cellular release of K+ (1). These published data might suggest that 1) resistance to the tumoricidal activity of cisplatin would reflect upregulation of ABC transporters and 2) resistance would be associated with an elevated intracellular K+ concentration. Neither expectation was fulfilled in the study by Poulsen et al. (19).
Poulsen et al. (19) took advantage of wild-type and MDR lines of Ehrlich ascites tumor cells (EATC) to obtain new insights into the mechanisms of cisplatin-triggered apoptosis and MDR. They provide a more complete analysis of the dynamic changes in cell volume and water and ion content during the course of apoptosis than previously measured, defining three stages characterized by an initial volume decrease (AVD1), a transition with partial volume recovery (AVDT), and a secondary volume decrease (AVD2). The changes in AVD1 and AVD2 were blunted and in AVDT exaggerated in an MDR line of EATC that is resistant to daunorubicin-triggered apoptosis and that overexpresses the ABC transporter P-glycoprotein. However, P-glycoprotein does not transport cisplatin, so that the basis of the cisplatin resistance in the MDR EATC line must be mediated by other mechanisms. Interestingly, the intracellular concentrations of K+, Cl−, Na+, and amino acids (measured as ninhydrin-positive substance) were similar in the wild-type and resistant cell lines following incubation with cisplatin. The altered cell volume, alone, is unlikely to account for the resistance to cisplatin-induced apoptosis in the MDR EATC cells since hyperosmotic shrinkage did not enhance the apoptosis in these cells.
Poulsen et al. (19) have suggested that the resistance to apoptosis is mediated by changes in all three stages of the apoptotic volume decrease. In support, the authors report that the resistant MDR EATC cells display a reduced swelling-activated Cl− current. Furthermore, inhibition of the swelling-activated anion channels with NS3728 reduced the differences in the AVD between the wild-type and MDR lines and also reduced caspase-3 activation in the wild-type cells. As Poulsen et al. (19) note, the implication of this interpretation is that cisplatin resistance might be overcome by incorporating additional channels into the cell membranes. However, the link between changes in membrane transport and stimulation of apoptosis rests obscure, given the similarity of the resulting ionic concentrations in the wild-type and resistant cells.
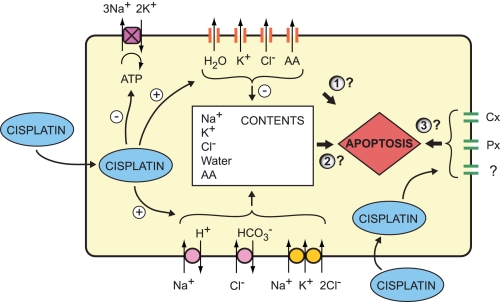
In addition to the possibilities considered by Poulsen et al. (19), a third possible basis for the resistance to apoptosis should be considered (Fig. 1). Increasing evidence suggests that pannexin and connexin hemichannels function as conduits for cell ATP release and in addition as links in signaling cascades (3, 6, 18, 21). The antiapoptotic action of the blocker NS3728 may be mediated by targets other than the VRAC channel. Hemichannels and VRAC channels display substantial cross-inhibition (23, 26).
Fig. 1.
Potential pathways for cisplatin stimulation of caspase activity, based on transport effects on ion, water, and amino acid (AA) transport. Poulsen et al. (19) describe an apoptotic volume decrease (AVD) comprising a sequential reduction, increase and second reduction in cell volume, likely reflecting activation of mechanisms physiologically responsible for both regulatory volume decrease (RVD) and regulatory volume increase (RVI). The signaling events leading to caspase activation may be mediated by 1) altered activity of the regulatory volume transporters themselves, 2) the changes in solute and water content resulting from the activity of the RVD transporters in the early AVD, and/or 3) altered activity of additional membrane transporters, including pannexin (Px) and connexin (Cx) hemichannels and Na+-K+-activated ATPase.
Pelegrin and Surprenant (18) have interpreted their recent data to indicate that the large-bore channel pannexin-1 (Px1) plays a role in P2X7-dependent release of IL-1β from mouse J774 macrophages that is independent of hemichannel activity at the plasma membrane. Px1 also associates with the potassium channel subunit Kvβ3 when heterologously expressed in Neuro2A cells, and is thereby functionally altered (2); Px1 and Kvβ3 are endogenously expressed in the central nervous system (2). Activation of the inflammasome in neurons and astrocytes by elevating external K+ is also thought to be mediated by activation of caspase-1 through an allosteric effect on pannexin-1, which alters its interactions with neighboring proteins (21). These observations suggest that Px1 can form associations with auxiliary subunits in the plasma membrane that are functionally important, apart from releasing ATP and other molecules through its wide-bore conduit. Little is known about the specific interactions of pannexin-1 with its microenvironment in the plasma membrane, but its interaction with Kvβ3 has provided some suggestions (2).
The connexin Cx26 has been found to rescue ouabain-induced disruption of tight-junctional fence and barrier function in Calu-3 cells that is independent of gap-junctional intercellular communication (3, 6). In addition to its functions as a fence and a gate, the multiple protein components of the tight junction provide a platform for trafficking and signaling that regulates the biology of the cell (13, 20).
In summary, Poulsen et al. (19) have provided a clear, detailed analysis of the changes in intracellular composition initiated by cisplatin through stimulation of Na+, K+, Cl−, amino acid and water release, and have demonstrated that neither overexpression of P-glycoprotein nor loss of cell K+ is necessarily required for the development of resistance to cisplatin-triggered apoptosis. Precisely how the membrane effects of cisplatin are linked to initiation of apoptosis remains a challenge for further study.
GRANTS
This work was supported by National Institutes of Health Grant EY13624.
DISCLOSURES
No conflicts of interest are declared by the author.
REFERENCES
- 1.Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch Biochem Biophys 462: 176–188, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunse S, Locovei S, Schmidt M, Qiu F, Zoidl G, Dahl G, Dermietzel R. The potassium channel subunit Kvbeta3 interacts with pannexin 1 and attenuates its sensitivity to changes in redox potentials. FEBS J 276: 6258–6270, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Go M, Kojima T, Takano K, Murata M, Koizumi J, Kurose M, Kamekura R, Osanai M, Chiba H, Spray DC, Himi T, Sawada N. Connexin 26 expression prevents down-regulation of barrier and fence functions of tight junctions by Na+/K+-ATPase inhibitor ouabain in human airway epithelial cell line Calu-3. Exp Cell Res 312: 3847–3856, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann EK. Volume regulation in cultured cells. Curr Top Membr Transp 30: 125–180, 1987 [Google Scholar]
- 5.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Kojima T, Murata M, Go M, Spray DC, Sawada N. Connexins induce and maintain tight junctions in epithelial cells. J Membr Biol 217: 13–19, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kregenow FM. The response of duck erythrocytes to hypertonic media. Further evidence for a volume-controlling mechanism. J Gen Physiol 58: 396–412, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kregenow FM. The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol 58: 372–395, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang F, Föller M, Lang K, Lang P, Ritter M, Vereninov A, Szabo I, Huber SM, Gulbins E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol 428: 209–225, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Lang F, Ritter M, Gamper N, Huber S, Fillon S, Tanneur V, Lepple-Wienhues A, Szabo I, Gulbins E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol Biochem 10: 417–428, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Leaf A. Maintenance of concentration gradients and regulation of cell volume. Ann NY Acad Sci 72: 396–404, 1959 [DOI] [PubMed] [Google Scholar]
- 12.Leaf A. On the mechanism of fluid exchange of tissues in vitro. Biochem J 62: 241–248, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DB, Huang E, Ward HJ. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol 290: F20–F34, 2006 [DOI] [PubMed] [Google Scholar]
- 14.McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiol Rev 72: 1037–1061, 1992 [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin CW, Peart D, Purves RD, Carré DA, Peterson-Yantorno K, Mitchell CH, Macknight AD, Civan MM. Timolol may inhibit aqueous humor secretion by cAMP-independent action on ciliary epithelial cells. Am J Physiol Cell Physiol 281: C865–C875, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Miyakawa H, Woo SK, Chen CP, Dahl SC, Handler JS, Kwon HM. Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am J Physiol Renal Physiol 274: F753–F761, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Okada Y, Sato K, Numata T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 587: 2141–2149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem 282: 2386–2394, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Poulsen KA, Andersen EC, Hansen KF, Klausen TK, Hougaard C, Lambert IH, Hoffmann EK. Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am J Physiol Cell Physiol (October21, 2009). doi:10.1152/ajpcell.00654.2008 [DOI] [PubMed] [Google Scholar]
- 20.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286: C1213–C1228, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284: 18143–18151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta 23: 394–401, 1957 [DOI] [PubMed] [Google Scholar]
- 23.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia 54: 758–773, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Tosteson DC, Hoffman JF. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol 44: 169–194, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson TH. Ionic permeability and osmotic swelling of cells. Science 120: 104–105, 1954 [DOI] [PubMed] [Google Scholar]
- 26.Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia 57: 258–269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]