Abstract
During platelet-derived growth factor (PDGF)-BB-mediated recruitment to neovascular sprouts, vascular smooth muscle cells (VSMCs) dedifferentiate from a contractile to a migratory phenotype. This involves the downregulation of contractile markers such as smooth muscle (SM) α-actin and the upregulation of promigration genes such as matrix metalloproteinase (MMP)-2. The regulation of MMP-2 in response to PDGF-BB is complex and involves both stimulatory and inhibitory signaling pathways, resulting in a significant delay in upregulation. Here, we provide evidence that the delay in MMP-2 upregulation may be due to the autocrine expression and activation of transforming growth factor (TGF)-β, which is known to promote the contractile phenotype in VSMCs. Whereas PDGF-BB could induce the loss of stress fibers and focal adhesions, TGF-β was able to block or reverse this transition to a noncontractile state. TGF-β did not, however, suppress early signaling events stimulated by PDGF-BB. Over time, though PDGF-BB induced increased TGF-β1 levels, it suppressed TGF-β2 and TGF-β3 expression, leading to a net decrease in the total TGF-β pool, resulting in the upregulation of MMP-2. Together, these findings indicate that MMP-2 expression is suppressed by a threshold level of active TGF-β, which in turn promotes a contractile VSMC phenotype that prevents the upregulation of MMP-2.
Keywords: platelet-derived growth factor-BB, F-actin cytoskeleton, transforming growth factor-β, phenotypic modulation
the recruitment of perivascular cells such as vascular smooth muscle cells (VSMCs) and pericytes is critical for the maturation of nascent vessel sprouts during angiogenesis; failure to do this leads to vessel dysfunction characteristic of diseases such as diabetes (5, 13, 20, 23). This recruitment to angiogenic sprouts is mediated by platelet-derived growth factor (PDGF)-BB expressed by specialized sprout tip endothelial cells (ECs) (1, 5, 11, 24, 29). In addition to serving as a chemoattractant and a mitogen, PDGF-BB induces VSMC dedifferentiation, which is characterized by a program of gene expression changes that includes the downregulation of contractile protein markers (5, 51, 57, 69), and the upregulation of MMP-2 (64). During this process, VSMCs switch from a contractile, quiescent state to a matrix-remodeling, proliferating state (57) and can maintain this dedifferentiated state in the continuous presence of PDGF (58).
Whereas some of the regulatory steps associated with changes in contractile protein marker expression by VSMCs are at least somewhat characterized, little is known about the regulation of markers of VSMC dedifferentiation, particularly MMP-2. MMP-2 is an extracellular matrix-degrading enzyme that plays an important role in vascular remodeling processes. For example, MMP-2 has been linked with VSMC migration/invasion in vitro and in vivo (60, 72, 81) as well as VSMC proliferation (6, 74). MMP-2 is crucial for VSMC migration during atherogenesis (45) but is also important during nonpathological vessel remodeling. The migration of VSMCs toward nascent endothelial tubes is required for vessel maturation (9, 16, 31), and several studies have linked MMP-2 to the angiogenic process. MMP-2 levels peak during the maximal level of angiogenesis in chick chorioallantoic membrane assays (63). Increased MMP-2 levels also correlate with angiogenesis in colorectal carcinoma (42). In a study examining mice with an impaired ability to activate MMP-2, fibroblast growth factor-2-induced angiogenesis was also impaired (83). MMP-2-null mice show decreased angiogenesis in models of experimentally induced neovascularization in the retina (56) and in the cornea (67) and also demonstrate reduced tumor-mediated angiogenesis (38).
We found previously that VSMCs stimulated with PDGF-BB increased MMP-2 expression during the process of phenotypic modulation (64). This MMP-2 upregulation was dependent on the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling axis in response to PDGF-BB but was delayed due to the induction of suppressive signaling pathways that temporarily blocked this process (64). In the present study, we asked what signals or factors are responsible for this delay. We have found that PDGF-BB-induced upregulation of MMP-2 is antagonized by a threshold level of endogenously produced TGF-β that acts by maintaining a contractile phenotype. This threshold level of total, active TGF-β is initially surpassed by PDGF-BB-induced expression of TGF-β1, but, in time, decreases due to the subsequent downregulation of TGF-β2 and TGF-β3. These findings demonstrate the precise balance that exists among signals that support VSMC phenotypic switching and those that antagonize it.
EXPERIMENTAL PROCEDURES
Cell culture and reagents.
The isolation and culture of S31 VSMCs, which were derived from the heart microvasculature of Wistar-Kyoto rats, has been previously described (61, 64). Cultures were maintained in DMEM containing 10% heat-inactivated fetal bovine serum in the absence of antibiotics-antimycotics. For experiments, S31 VSMCs were grown to postconfluence (57) and then serum starved by rinsing the plates once with PBS and placing cells on fresh serum-free medium (DMEM containing 0.02% lactalbumin hydrolysate and 1× antibiotic-antimycotic; Invitrogen, Carlsbad, CA) for 48 h. For all treatments, the following growth factors were diluted in serum-starvation medium at the concentrations indicated in the figure legends: recombinant rat PDGF-BB (R&D Systems, Minneapolis, MN), recombinant human macrophage colony-stimulating factor (M-CSF), and recombinant human TGF-β1 (CHO cell-derived, both from PeproTech, Rocky Hill, NJ). In all experiments, “control” treatment was the addition of fresh serum-free medium for the indicated amount of time. In some cases, cells were also treated for 15 min before growth factor stimulation with the following chemical inhibitors at the concentrations indicated in the figure legends: TGF-β Type I Receptor Kinase Inhibitor III (both from Calbiochem, San Diego, CA) and latrunculin (Lat) B, and LY-294002 (both from Biomol, Plymouth Meeting, PA). S31 VSMCs expressing the chimeric PDGFRβ/CSF-1R were selected and cultured as described previously (64). In most cases, unless noted in the figure legends, all growth factor treatments were continual; that is, additional boluses of the indicated growth factors were added every 24 h. We chose to stimulate cells in this manner due to our observation that PDGF-BB-induced changes in S31 VSMCs begin to revert after 48 h of stimulation if only a single bolus of 25 ng/ml PDGF-BB is used; continual stimulation or a higher dose maintain PDGF-BB-induced changes (Supplemental Fig. 1).
Immunocytochemistry.
Cells were plated onto coverslips at 50% confluence; after 24 h, cells were serum starved for an additional 48 h and then stimulated as indicated before fixation with 4% paraformaldehyde (Polysciences, Warrington, PA) for 10 min. Cells were permeabilized with 0.5% Triton X-100 in PBS before immunostaining. Rhodamine phalloidin (Invitrogen) was used to visualize F-actin stress fibers; focal adhesions were stained with anti-vinculin (Sigma no. V9131, St. Louis, MO) in combination with goat-anti-mouse IgG-conjugated Alexa-488 (Invitrogen). SM α-actin was stained using anti-SM α-actin (Sigma no. A2547) in combination with goat-anti-mouse IgG-conjugated Alexa-594 (Invitrogen). Coverslips were mounted with Vectashield Mounting Medium (Vectashield Laboratories, Burlingame, CA); images were captured on an Olympus AX70 fluorescence microscope (Melville, NY) with QCapture 2.68 software (IBM, Armonk, NY).
RT-PCR, real-time, and semiquantitative PCR.
Total RNA was collected using the Purescript RNA Isolation Kit (Gentra Systems, Minneapolis, MN). Total RNA (2 μg) was reverse transcribed with oligo(dT) and either Powerscript Reverse Transcriptase (BD Biosciences, Franklin Lakes, NJ) or Superscript II Reverse Transcriptase (Invitrogen). Real-time PCR was performed as described previously (61). Primers used for real-time PCR of tissue inhibitor of metalloproteinase-2 (TIMP-2) and MMP-2 have been previously described (64). The following real-time PCR primers were used: SM α-actin, upper: 5′-CCCGCTCTGTCTCTAGCAC-3′ and lower: 5′-CACACGAGTAACAAATCAAAGC-3′, calponin, upper: 5′-CTGGATCAGGCAACTATCAGTCTA-3′ and lower: 5′-AGGCAGTACTTGGGATCATACAC-3′, SM22α, upper: 5′-TCTTGAAGGCAGCTGAGGATTATG-3′ and lower: 5′-CACGGTAGTGTCCATCGTTCTTG-3′, TGF-β1, upper: 5′-ACCAACTACTGCTTCAGCTCCA-3′ and lower: 5′-GATCATGTTGGACAACTGCTCC-3′, TGF-β2, upper: 5′-CTGCCTTCGCCCTCTTTACATTGA-3′ and lower: GAGGACTTTAGCTGCATTTACAAG-3′, and TGF-β3, upper: 5′-CGGCAGCATCTAGGCTGGAAATGGGT-3′ and lower: 5′-CTTGTGGGCTCCAGGCTCTAGGGTGA-3′. In cases where P values are shown, unpaired, two-tailed t-tests were performed using Prism 5 Software (GraphPad Software, San Diego, CA). Semiquantitative RT-PCR with primers specific for MMP-2, SM α-actin, TIMP-1, and TIMP-2 was performed as described previously (64), and the sequences of the additional primers used are as follows: c-fos, upper: 5′-ATACGTCTTCCTTTGTCTTCACCT-3′ and lower: 5′-GGAGAAAGAGAAAAGAGACACAGA-3′; c-jun, upper: 5′-TACCAGGCTAGATTGCGGATGAAC-3′ and lower 5′- GCATGCAGGGTGTCTATTCCTACA-3′; and SM22α upper: 5′-CTATAATGGCTTTGGGCAGTTTGG-3′ and lower: 5′- AGGACAGTGGTGGCTCTGGGGTAA-3′. All semiquantitative PCR images are inverted. TIMP-2 was used as a PCR standard in both real-time and semiquantitative PCR, as we have found that its expression is only modestly changed in response to the growth factors analyzed in the relatively long-term experiments presented here.
Western blots and zymography.
SDS lysates were collected and sonicated before bicinchoninic acid assay analysis (BCA, Pierce Biotechnology, Rockford IL), and radioimmune precipitation assay (26) lysates were collected and centrifuged to remove insoluble material before BCA analysis. Western blots were performed as described previously (64) using SDS or RIPA lysates. Samples were analyzed by BCA assay, and equal protein amounts were separated on gels before electrotransfer to nitrocellulose. Antibodies used included anti-SM α-actin, anti-β-tubulin (both from Sigma; A2547, T7816, respectively), anti-phospho-Akt (T308 and S473), anti-Akt, anti-phospho-JNK, anti-phospho-MAPK, anti-MAPK, anti-phospho-PDGFRβ (all from Cell Signaling Technology, Danvers, MA; nos. 9275, 4051, 9272, 9255, 9106, 9102, and 3166, respectively), and anti-PDGFRβ (R&D Systems; no. AF1042). Zymography was performed on conditioned medium (CM) from S31 VSMCs as described previously (64).
Proliferation assays.
Analysis of cell proliferation was performed as described previously (62). Briefly, subconfluent (50% confluent) S31 VSMCs were plated into 96-well plates, serum starved for 48 h, and treated with serum-free DMEM containing 0.2% lactalbumin hydrolysate alone, plus 25 ng/ml PDGF, 10 ng/ml TGF-β1, or the combination of both growth factors. Proliferation was assessed using the CellTiter 96 NonRadioactive Cell Proliferation Assay (Promega, Madison, WI) per manufacturer's instructions.
TGF-β analyses.
ELISA was performed on cultured medium collected from cultures for active TGF-β1 and active TGF-β2 using the Emax ImmunoAssay System (Promega) per manufacturer's instruction. In cases where P values were calculated, unpaired two-tailed t-tests were performed. To measure the relative levels of functional TGF-β in conditioned media, we introduced a TGF-β-responsive SBE4 LUC construct (Addgene, Cambridge, MA) and a β-galactosidase reporter (SV40 β-gal, Promega) into HEK 293T cells using calcium phosphate. After transfection, cells were trypsinized and plated into 48-well plates. S31 VSMCs were treated with 25 ng/ml PDGF-BB, and conditioned medium was collected at the indicated times (Fig. 4). This medium was added to transfected HEK 293T, and cells were incubated for 6 h. Cell lysates were prepared using the Promege Luciferase Assay System with Reporter Lysis Buffer (Promega) and analyzed for luciferase activity, which was normalized to the β-galactosidase activity of the lysates.
Fig. 4.
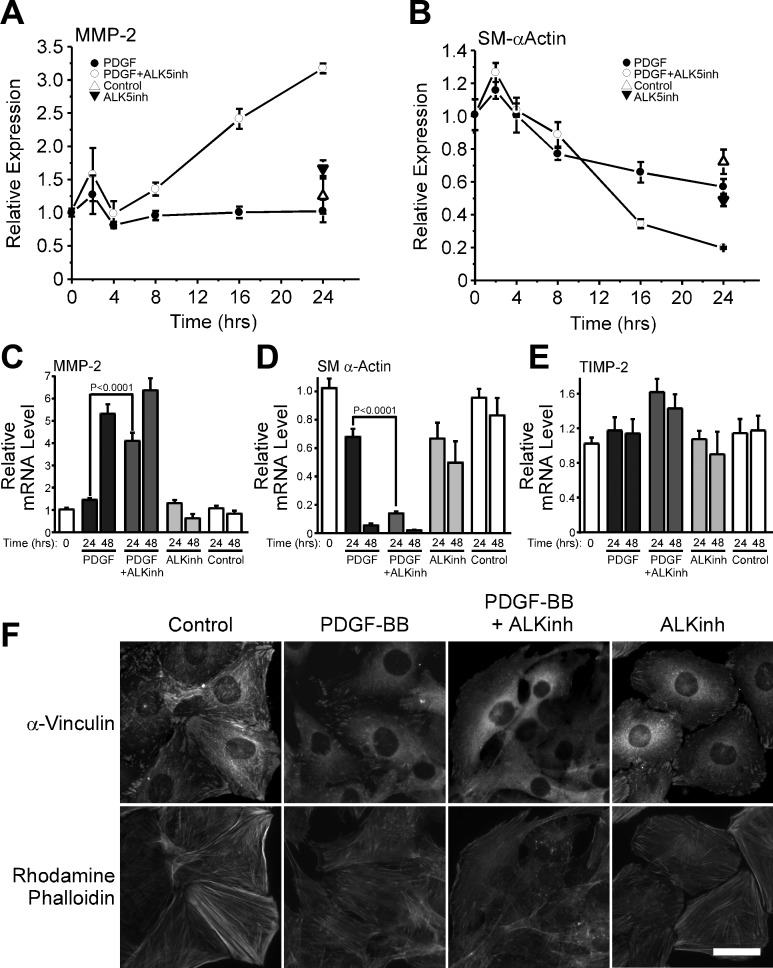
Inhibition of TGF-β-mediated signaling accelerates PDGF-BB-induced upregulation of MMP-2. A–E: S31 VSMC were cultured to postconfluence, serum-starved for 48 h, and then stimulated with 25 ng/ml PDGF-BB with or without 1 μM of an inhibitor of the type 1 receptor ALK5 (ALKinh). Control cells were treated with fresh serum-free medium only. Total RNA was collected at the indicated times and analyzed for the expression of MMP-2 (A, C), SM α-actin (B, D), and TIMP-2 (E). mRNA levels are relative to T = 0, and error bars represent means ± SE. P value was calculated by unpaired, two-tailed t-test. N = 11. F: S31 VSMCs were cultured on glass coverslips and treated as in A–E. Cells were fixed at T = 48 h with paraformaldehyde and stained for F-actin stress fibers with rhodamine phalloidin and focal adhesions with an anti-vinculin antibody. Bar = 30 μM.
RESULTS
Loss of the contractile phenotype precedes PDGF-BB-induced MMP-2 downregulation.
We previously demonstrated that PDGF-BB induces a delayed upregulation of MMP-2 by S31 VSMCs and that this was due to competing signaling pathways (64). When we examined the phenotypic state of S31 VSMC cultures during a time course of PDGF-BB stimulation, we noted that cells stimulated for 8 and 24 h showed only slightly altered morphology compared with nontreated controls and no overt changes in F-actin stress fibers and focal adhesions (Fig. 1A). By 48 h, however, PDGF-BB-stimulated cells contained essentially no actin stress fibers or vinculin-containing focal adhesions (Fig. 1A). This loss of the contractile phenotype was consistent with the progressive reduction of SM α-actin message (Fig. 1B). In contrast, significant changes in MMP-2 mRNA levels did not occur after 24 h of PDGF-BB stimulation but did increase concomitant with the loss of the contractile phenotype (Fig. 1C). TIMP-2, a physiological inhibitor of MMPs, which we have found shows relatively modest changes in expression during the long time courses involved in these studies (64), was used as a control (Fig. 1D). Thus changes in MMP-2 expression occur concomitantly with changes in cell morphology, both of which are delayed in response to PDGF-BB.
Fig. 1.
Platelet-derived growth factor (PDGF)-BB-induced upregulation of matrix metalloproteinase-2 (MMP-2) occurs concomitantly with reorganization of the actin cytoskeleton. A: S31 vascular smooth muscle cells (VSMCs) were cultured on glass coverslips, serum-starved for 48 h, and stimulated with either 25 ng/ml PDGF-BB or no growth factor. At the indicated times, cells were fixed and stained for F-actin stress fibers with rhodamine phalloidin and focal adhesions with anti-vinculin. Bar = 30 μm. B–D: S31 VSMCs were grown to postconfluence, serum-starved for 48 h, and then stimulated every 24 h with either 25 ng/ml PDGF-BB or serum-free medium only. Total RNA was collected at the indicated times and analyzed for SM α-actin (B), MMP-2 (C), and tissue inhibitor of metalloproteinase-2 (TIMP-2) (D) expression by real-time RT-PCR. mRNA levels are relative to T = 0. Error bars represent means ± SE, and P values were calculated by unpaired, two-tailed t-tests. N = 6.
Exogenous TGF-β1 blocks PDGF-BB-induced MMP-2 upregulation.
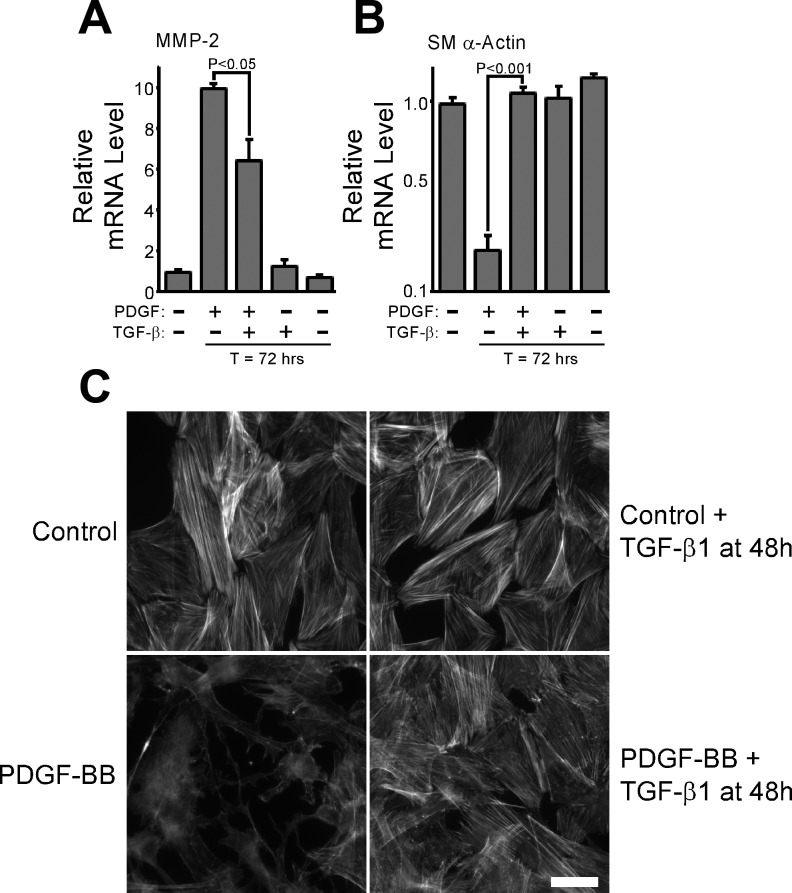
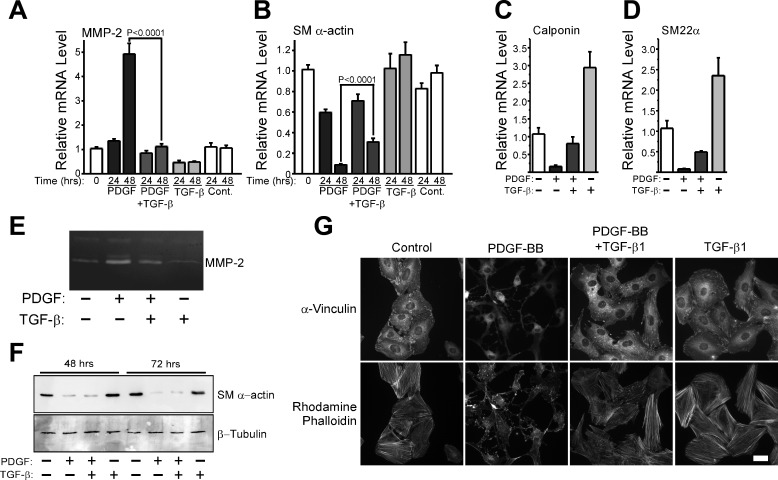
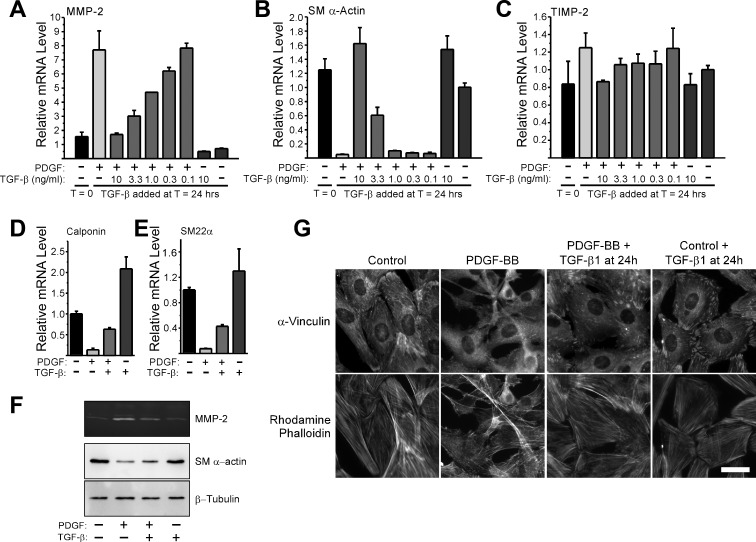
TGF-β is known to be a potent inducer of VSMC differentiation (4, 5, 32, 34), so we hypothesized that endogenous TGF-β may be part of a negative feedback loop established by these cells that limits MMP-2 upregulation in response to PDGF-BB. To evaluate this possibility, we asked if exogenous TGF-β1 could reverse the phenotype of fully dedifferentiated VSMCs. The continuous presence of PDGF-BB can suppress SM α-actin expression (12, 58) and promote increased MMP-2 expression by VSMCs over a prolonged period of time (Supplemental Fig. 1). Therefore, we stimulated S31 VSMCs with PDGF-BB every 24 h for a 48-h period, at which time we restimulated cultures with PDGF-BB plus or minus TGF-β1. At T = 72 h, we analyzed the expression of MMP-2 and SM α-actin and found that TGF-β1, even when added to fully dedifferentiated VSMCs, was able to begin reversing the effects of PDGF-BB-induced upregulation of MMP-2 (Fig. 2A). TGF-β1 also reversed the loss of SM α-actin levels (Fig. 2B) and induced the reformation of actin stress fibers (Fig. 2C). When we continuously stimulated S31 VSMCs with both PDGF-BB and TGF-β1 starting at T = 0, we also found that MMP-2 upregulation was completely inhibited (Fig. 3A). Interestingly, TGF-β1 did not completely block PDGF-BB-induced SM α-actin downregulation when both growth factors were used to simultaneously stimulate cells but did suppress it to a significant extent (Fig. 3B). Two other contractile protein genes showed a differential susceptibility to TGF-β1-mediated inhibition of PDGF-BB-induced downregulation (Fig. 3, C and D). Whereas the mechanism associated with this effect is not known, these results do suggest that the way in which TGF-β1 inhibits the actions of PDGF-BB on contractile protein versus MMP-2 gene expression is different. MMP-2 protein expression by cells stimulated with PDGF-BB was notably higher than by cells costimulated with both PDGF-BB plus TGF-β1, as measured by zymography of cell-conditioned medium (Fig. 3E). PDGF-BB-induced SM α-actin protein downregulation was unaffected by TGF-β1 added concurrently (Fig. 3F), consistent with the message levels. Interestingly, despite an apparent inability to suppress the downregulation of all contractile proteins under the given conditions, TGF-β1 blocked PDGF-BB-induced changes in stress fibers and focal adhesions (Fig. 3G).
Fig. 2.
Transforming growth factor (TGF)-β1 reverses the phenotype of PDGF-BB-induced dedifferentiated VSMCs. A and B: S31 VSMCs were grown to postconfluence, serum-starved for 48 h, and then stimulated (T = 0) with 25 ng/ml PDGF-BB every 24 h. At T = 48, some cultures were stimulated with 10 ng/ml TGF-β1. At T = 0 and T = 72 h, total RNA was collected and analyzed for the expression of MMP-2 (A) and SM α-actin (B). mRNA levels are relative to T = 0, and error bars represent means ± SE. P values were calculated in unpaired, two-tailed t-tests. N = 3. C: S31 VSMCs were cultured on glass coverslips and serum-starved before being treated as in A and B. At T = 72 h, cells were fixed with paraformaldehyde and stained with rhodamine phalloidin to examine F-actin stress fibers.
Fig. 3.
Exogenous TGF-β1 blocks PDGF-BB-induced upregulation of MMP-2 and downregulation of contractile gene expression. A and B: S31 VSMCs were grown to postconfluence, serum starved for 48 h, and then stimulated with 25 ng/ml PDGF-BB, 10 ng/ml TGF-β1, the combination of both growth factors, or serum-free medium only. Cells were restimulated with fresh boluses of each growth factor at T = 24 h. Total RNA was collected at the indicated times, reverse transcribed, and analyzed for the expression of MMP-2 (A) and SM α-actin (B) by real-time PCR. mRNA levels are relative to T = 0. Error bars represent means ± SE, and P values were calculated using an unpaired, two-tailed t-test. N = 17. C: S31 VSMCs were cultured and continually stimulated as in A and B, except that mRNA was analyzed for the expression of calponin (C) and SM22α (D) from 48 h samples only. Error bars represent means ± SE, and N = 3. E: CM from cells treated in A and B was collected at T = 48 h and analyzed for the expression MMP-2 by zymography. F: S31 VSMCs were cultured as in A and B but for 72 h. SDS-lysates were collected at the indicated times and analyzed by Western blot analysis to examine the expression level of SM α-actin and β-tubulin. G: S31 VSMCs were cultured on glass coverslips and serum starved 48 h before stimulation with 25 ng/ml PDGF-BB, 10 ng/ml TGF-β1, the combination of growth factors, or serum-free medium only. Cells were fixed at T = 48 h with paraformaldehyde and stained for F-actin stress fibers with rhodamine phalloidin and focal adhesions with an anti-vinculin antibody. Bar = 30 μm.
TGF-β1 does not block PDGF-BB-induced early signaling events.
Several studies have demonstrated that TGF-β can suppress many of the signaling pathways elicited by PDGF (2, 21, 22, 25, 35). One way TGF-β1 may antagonize PDGF-BB is by altering PDGFRβ levels or receptor dimerization status. However, Western blot analysis of PDGFRβ levels revealed, if anything, increased receptor phosphorylation in cells costimulated with PDGF-BB plus TGF-β1 (Supplemental Fig. 2A). To address the possibility of altered receptor dimerization, we used chimeric receptors that couple the extracellular domain of the CSF-1R with the transmembrane and cytoplasmic domains of the PDGFRβ, as previously described (64). S31 VSMCs do not express significant levels of the CSF-1R and do not respond to M-CSF stimulation. Chimeric PDGFRβ receptors lack the ability to dimerize with PDGFRα. As expected, S31 VSMCs stably expressing the chimeric receptor downregulated SM α-actin and upregulated MMP-2 in response to either M-CSF or PDGF-BB (Supplemental Fig. 2B). In both cases, costimulation with TGF-β1 blocked changes in MMP-2 expression.
Several studies, including our own, have linked the PI3K/Akt signaling axis with MMP-2 upregulation in response to growth factors (7, 46, 48, 64). We thus evaluated Akt activation in S31 VSMCs stimulated with both PDGF-BB and TGF-β1 but noted no differences in the phosphorylation of Akt at threonine-308 or serine-473 between PDGF-BB-stimulated and costimulated cells (Supplemental Fig. 2C). Similarly, there were no marked differences in p44/42-MAPK or JNK activation (Supplemental Fig. 2C). TGF-β1 alone did not appear to stimulate any of these signaling pathways. Consistent with an inability to block early signaling pathways, TGF-β1 did not suppress PDGF-BB-mediated mitogenesis (Supplemental Fig. 3A) nor did it block PDGF-BB-induced upregulation of the immediate-early genes, c-fos and c-jun (Supplemental Fig. 3B), or TIMP-1, another gene upregulated by PDGF-BB (Supplemental Fig. 3C). These data indicate that TGF-β1 suppresses only a subset of the genes and cellular processes regulated by PDGF-BB.
The differential regulation of endogenous TGF-β isoforms by PDGF-BB may govern MMP-2 expression by VSMCs.
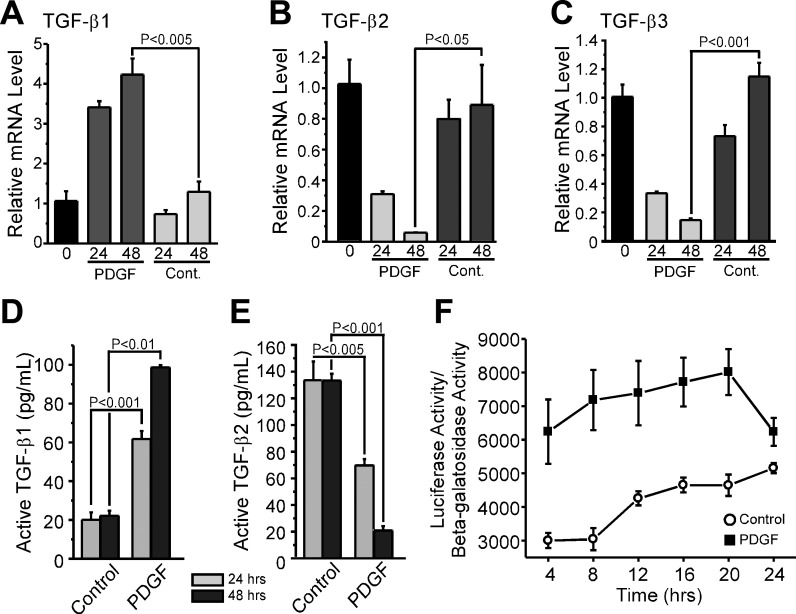
VSMCs are known to express TGF-β isoforms (28), so we sought to determine whether a PDGF-BB-induced increase in endogenous TGF-β expression could transiently suppress MMP-2 upregulation. We costimulated S31 VSMCs with PDGF-BB and an inhibitor of the Type I TGF-β receptor, activin-like kinase (ALK) 5, and found that the inhibitor accelerated MMP-2 upregulation of MMP-2 and SM α-actin downregulation at both early (Fig. 4, A and B, respectively) and later times (Fig. 4, C and D, respectively) post-PDGF-BB stimulation. We also noted that the inhibitor enhanced the ability of PDGF-BB to modify the actin cytoskeleton of S31 VSMCs (Fig. 4F). We next measured the levels of TGF-β isoforms expressed by PDGF-BB-stimulated cells and found that TGF-β1 mRNA was upregulated by 361% by 24 h and 178% by 48 h (Fig. 5A). In contrast, both TGF-β2 and TGF-β3 message levels were significantly downregulated in response to PDGF-BB, with the maximal downregulation of both occurring by 48 h; at this time point, the expression level of TGF-β2 in response to PDGF-BB was only 7% of control values and that of TGF-β3 was 13% of control levels (Fig. 5, B and C). Consistent with message levels, we found that active TGF-β1 protein levels in medium conditioned by S31 VSMCs stimulated with PDGF-BB were increased at 24 and 48 h by 207% and 345%, respectively (Fig. 5D). Again in contrast to TGF-β1, the level of active TGF-β2 decreased in response to PDGF-BB stimulation; by 24 and 48 h poststimulation, TGF-β2 levels were 52% and 16% of control levels, respectively (Fig. 5E). Thus, in nonstimulated cells, the total measurable, activated TGF-β1 and TGF-β2 level was ∼150 pg/ml, whereas PDGF-BB-stimulated cultures had ∼100 pg/ml; TGF-β3 levels were not detectable by ELISA. To confirm that PDGF-BB transiently increases TGF-β levels in the medium of VSMCs, we stimulated S31 VSMCs with PDGF-BB and then used the resultant medium to stimulate human embryonic kidney (HEK)-293T cells transfected with a TGF-β-responsive luciferase reporter construct. We found that luciferase activity could be stimulated by S31 VSMC-conditioned medium from PDGF-BB-stimulated cultures and that this stimulation ultimately approached that induced by nonstimulated cell medium over a 24-h period (Fig. 5F). This indicates that the level of activated TGF-β rapidly increased in response to PDGF-BB, reached a maximum level within 20 h, and then decreased thereafter. Together, these data suggest that, whereas PDGF-BB induces the autocrine expression and activation of TGF-β1, it also induces the simultaneous downregulation of the other TGF-β isoforms. The net result is a decrease in TGF-β over time, allowing PDGF-BB-induced upregulation of MMP-2 expression to proceed.
Fig. 5.
PDGF-BB regulates the expression of TGF-β isoforms in S31 VSMCs. A–C: S31 VSMCs were grown to postconfluence and then serum starved for 48 h before treatment with or without 25 ng/ml PDGF-BB. Total RNA was collected and analyzed by real-time RT-PCR for the expression of TGF-β1 (A), TGF-β2 (B), and TGF-β3 (C). mRNA levels are relative to T = 0. N = 3. D and E: conditioned medium from cells in A–C was collected and analyzed for the levels of active (73) TGF-β1 and (right) TGF-β2 by ELISA at 24 and 48 h. N = 3. F: conditioned medium from S31 VSMCs treated with 25 ng/ml PDGF-BB for the indicated times was added to human embryonic kidney (HEK) 293T cells that had been cotransfected with the TGF-β-responsive reporter SBE4 Luc and a β-galactosidase reporter. HEK 293T cells were lysed after 6 h of treatment with S31 VSMC cultured medium and analyzed by luciferase assay and β-galactosidase assay. N = 3. All error bars represent means ± SE, and all P values were calculated by unpaired, two-tailed t-tests.
TGF-β-mediated promotion of stress fibers is critical to its ability to suppress the upregulation of MMP-2.
Given the known importance of the F-actin-to-G-actin ratio to the regulation of contractile protein gene expression (49, 70), we wanted to determine whether the ability of TGF-β to suppress MMP-2 upregulation was related to its ability to maintain stress fibers and focal adhesions in cells exposed to PDGF-BB. To do this, we stimulated cells with PDGF-BB for 24 h, then added increasing concentrations of TGF-β1 plus additional PDGF-BB, and evaluated the expression of MMP-2 and SM α-actin after an additional 24 h (Fig. 6, A and B). We found that TGF-β1 inhibits MMP-2 upregulation and reverses the loss of SM α-actin expression in a highly dose-dependent manner. We also noted that, at the highest dose of TGF-β1 (10 ng/ml), this late addition of TGF-β1 also reversed the downregulation of the contractile genes calponin and SM22α (Fig. 6, D and E, respectively). At this dose of TGF-β1, we also observed that the effect of blocking MMP-2 upregulation was reflected at the level of protein expression as determined by zymography (Fig. 6F). Adding TGF-β1 24 h after the initial PDGF-BB stimulus resulted in a partial rescue of SM α-actin protein levels (Fig. 6F) and appeared to completely block PDGF-BB-induced reduction of vinculin-staining focal adhesions and F-actin stress fibers (Fig. 6G).
Fig. 6.
Late addition of exogenous TGF-β1 blocks MMP-2 upregulation in response to PDGF-BB. A–C: S31 VSMCs were grown to postconfluence before 48 h of serum starvation. At T = 0, cells were treated with 25 ng/ml PDGF-BB. At T = 24 h, an additional bolus of 25 ng/ml PDGF-BB was added to the cells, with or without the indicated amount of TGF-β1 (ng/ml). At T = 48 h, total RNA was collected, and expression levels of MMP-2, SM α-actin, and TIMP-2 were analyzed by real-time PCR. Error bars represent means ± SE, and N = 3. D and E: S31 VSMCs were treated as in A–C except only the 10 ng/ml concentration of TGF-β1 was added at T = 24 h. At T = 48 h, total RNA was harvested, and calponin and SM22α expression levels were analyzed by real-time PCR. Expression levels are relative to no-addition control. Error bars represent means ± SE and N = 3. F: S31 VSMCs were treated as in D and E, except conditioned medium and SDS lysates were collected at T = 48 h. Conditioned medium was analyzed by zymography for MMP-2 expression (top). SDS lysates were analyzed for SM α-actin (middle) and β-tubulin expression by Western blot (bottom). G: cells were cultured on glass coverslips and serum-starved for 48 h before stimulation with 25 ng/ml PDGF-BB. At T = 24 h, an additional 25 ng/ml bolus of PDGF-BB was added with or without 10 ng/ml TGF-β1. Cells were fixed and T = 48 h and analyzed for F-actin stress fibers with rhodamine phalloidin and focal adhesions with an anti-vinculin antibody. Bar = 30 μm.
This ability of TGF-β1 to block or reverse the effects of PDGF-BB even when added 24 h after an initial PDGF-BB stimulation allowed us to challenge cells with reagents that would normally be cytotoxic during an extended incubation. Using this approach, we stimulated S31 VSMCs with PDGF-BB and rechallenged them 24 h later with fresh PDGF-BB, PDGF-BB plus TGF-β, or TGF-β alone, all in the presence or absence of LatB, a drug that promotes the formation of G-actin. As expected, LatB induced the downregulation of SM α-actin, regardless of other treatments (Fig. 7A). It also blocked the ability of TGF-β to suppress MMP-2 upregulation in response to PDGF-BB (Fig. 7B), and, in all cases, induced a profound loss of stress fibers and focal adhesions (Supplemental Fig. 4).
Fig. 7.
Increasing G-actin levels with latrunculin B blocks TGF-β1-mediated inhibition of PDGF-BB-induced MMP-2 expression. A and B: S31 VSMCs were grown to postconfluence and serum starved for 48 h before stimulation with 25 ng/ml PDGF-BB at T = 0. At T = 24 h, an additional 25 ng/ml bolus of PDGF-BB was added, and as indicated, 10 ng/ml TGF-β1 and/or 1 μM latrunculin B (LatB) were added as well. At T = 48 h, total RNA was collected and analyzed for SM α-actin (A) and MMP-2 (B) expression levels by real-time PCR. Error bars represent means ± SE, and P values were calculated using unpaired, two-tailed t-tests. N = 3.
DISCUSSION
It is generally appreciated that PDGF-BB is a potent stimulator of VSMC dedifferentiation and is critical for the recruitment of these cells to developing vessels (30, 33). We have previously shown that PDGF-BB elicits both stimulatory and inhibitory signals that modulate MMP-2 expression by VSMCs (64). Here, we demonstrate that the primary inhibitory signal may be linked to the autocrine expression and activation of TGF-β1, which promotes the maintenance of actin stress fibers and, through a mechanism not yet known, suppresses MMP-2 upregulation. This suppression is temporary in the VSMC model utilized in this study, and this may be linked to the PDGF-BB-induced downregulation of TGF-β2 and TGF-β3, resulting, over time, in a decreased amount of active TGF-β below a critical threshold level necessary to block PDGF-BB-induced changes in these cells. Indeed, blocking TGF-β1-induced receptor activation accelerated MMP-2 upregulation in response to PDGF-BB. In cases of tissue damage, which involves a rapid increase in PDGF-BB levels, this initial increase in TGF-β1 may limit vessel instability, which, in the absence of increased TGF-β might cause an excessive loss of perivascular cells from vessels. During the stabilization phase of angiogenesis, when nascent vessels become ensheathed with perivascular cells, VSMCs and endothelial cells collaborate to produce active TGF-β (4, 19, 34), which induces VSMC differentiation (58). A disruption in TGF-β-induced signaling leads to an impairment in angiogenesis (14, 18, 47), as characterized by undifferentiated mural cells associated with endothelial cell tubes. This is consistent with our findings that exogenously added TGF-β1 will suppress MMP-2 upregulation in response to PDGF-BB. Importantly, we show that an increase in the available, active pool of total TGF-β will completely reverse or block VSMC dedifferentiation, regardless of phenotypic state.
Whereas the detailed mechanisms remain to be elucidated, our findings suggest that the ability of TGF-β to suppress MMP-2 upregulation is linked to the integrity of the actin cytoskeleton. Forcing the disassembly of actin stress fibers, which is known to result in contractile protein downregulation (58), blocked the ability of TGF-β1 to suppress the upregulation of MMP-2 and accelerated this upregulation in response to PDGF-BB. This suggests that the inhibitory signal that delays PDGF-BB-induced MMP-2 upregulation is dependent on intact actin stress fibers or F-actin. Previous studies have suggested an association between F-actin and the contractile protein markers that characterize a differentiated VSMC phenotype. This regulation was linked mechanistically to the disposition of myocardin-related transcription factor-A (MRTF-A), which associates with G-actin, thus becoming sequestered away from the promoters of contractile protein genes such as SM α-actin (53, 76, 78). Force generation and stress fiber assembly cause the release of MRTF-A, which then acts as a serum response factor cofactor, stimulating the transcriptional activation of contractile protein markers. MMP-2 and the contractile proteins are regulated reciprocally in VSMCs (64), and it appears that the cytoskeleton plays a role in both processes. It is not clear, however, why the concurrent addition of TGF-β and PDGF-BB to VSMCs prevents stress fiber loss, yet fails to prevent the loss of SM α-actin expression. This may be due to PDGF-BB-induced early signals because the addition of TGF-β 24 or 48 h after the initial PDGF-BB stimulation fully restored SM α-actin expression (Figs. 2 and 6). In all cases, TGF-β prevented the upregulation of MMP-2. It has been demonstrated that loss of force generation or stress relaxation will cause the activation of MMP-2 protein (3, 27, 68, 71), and we previously found in fibroblasts that this was accompanied by modest changes in MMP-2 message levels (71). Our present study suggests that the loss of the contractile phenotype is necessary for transcriptional upregulation of MMP-2 in VSMCs, and that TGF-β acts primarily through its ability to promote F-actin to block this upregulation. An examination of the MMP-2 gene shows that there are Smad-binding elements (CAGACA) (41) present in both the proximal promoter and the first intron; however, when we evaluated ∼3500 base pairs of sequence flanking, the 5′ end the rat promoter, and the complete first intron using promoter-reporter assays, we did not observe any suppression by TGF-β, though we did observe PDGF-BB induction (Risinger and Howard, unpublished observation). Future studies will be aimed at determining whether distal elements of the MMP-2 promoter could possibly be involved in regulation elicited by the cytoskeleton or TGF-β signaling.
Whereas PDGF-BB has been previously shown to affect TGF-β isoform expression in SMCs (15, 59, 82) and other cells (75, 77), this is the first demonstration that, in VSMCs, the TGF-β isoforms are reciprocally regulated. It should be noted that the 33% decrease in total active TGF-β levels observed here only reflected the contributions of TGF-β1 and TGF-β2 (Fig. 5). Given that the message level of TGF-β3 decreased much like that of TGF-β2, we assume that a more dramatic change in total active TGF-β levels occur in response to PDGF-BB stimulation. We were unable to measure TGF-β3 protein levels by ELISA, and this could be due to low protein expression or its sequestering to an insoluble pool. Indeed, we were also unable to suppress the ability of endogenous TGF-β to inhibit PDGF-BB-induced MMP-2 upregulation using function-blocking antibodies (Risinger and Howard, unpublished observations), perhaps due to this same sequestration. Previous studies have demonstrated that fibroblasts can sequester TGF-β to the matrix in a form that does not activate receptors but that is nevertheless poised to stimulate TGF-β receptors upon activation (79). In any case, the idea that a net loss of active TGF-β levels facilitates MMP-2 upregulation is supported by the ability of exogenous TGF-β1 to suppress this change in gene expression, regardless of when TGF-β1 was added (Figs. 2, 3, and 6, unpublished observations). It is important to note that our data do not rule out the participation of other TGF-β family members, including the bone morphogenic proteins or activins, in the autocrine suppression of PDGF-BB-induced MMP-2 upregulation, particularly since the ALK inhibitor is known to block ALK5 as well as the activin receptors ALK4 and ALK7 (17). Similarly, the SBE4-Luc construct analyzed may respond to some of these factors as well (37, 44). It is thus possible that TGF-β is one of several factors contributing to the regulation of MMP-2 in these cells.
Finally, our analyses of cellular processes affected by the addition of exogenous TGF-β1 reveal that the combined effects of TGF-β1 and PDGF-BB support vessel stabilization. Depending on context, TGF-β has been found to have an inhibitory (2, 36, 50, 52, 54, 62, 65, 66, 80) or stimulatory (2, 40, 43, 51, 55, 65, 80) effect on PDGF-induced proliferation in SMCs. In contrast, we demonstrated that TGF-β1 had no significant effect on PDGF-BB-induced VSMC proliferation nor did it affect PDGF-BB-induced PDGFRβ receptor activity or immediate downstream signaling. These findings differ from those observed by others using non-SMC cell systems where TGF-β negatively affected PDGF-induced signaling (22, 25, 35). Previous studies have suggested that TGF-β induces PDGF-A expression, which can antagonize PDGF-B-mediated processes in VSMCs (8, 69). We have previously demonstrated that PDGF-AA does not induce S31 VSMCs to proliferate, does not itself alter MMP-2 or SM α-actin expression, and does not affect the ability of PDGF-BB to upregulate MMP-2 or downregulate SM α-actin (64). Given that PDGF-BB can bind PDGFRα and PDGFRβ hetero- and homodimers (10, 29), we needed to determine whether TGF-β1 somehow alters receptor dimerization. Our studies with receptor chimeras suggest that this is not the case. Mural cell proliferation occurs during vessel maturation (31, 39), and, combined with our observations of the effects of TGF-β1 on VSMC phenotypic switching, our findings support the idea that the effects of TGF-β on VSMC phenotype are quite selective and are consistent with those events that would promote vessel stabilization.
GRANTS
This work was supported by National Institutes of Health Grant GM-63638, the Presbyterian Health Foundation, and the Oklahoma Center for the Advancement of Science and Technology to E. W. Howard and National Institutes of Health Grant GM-60651 to J. J. Tomasek.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENT
We greatly appreciate the technical assistance of Zenoba Hines.
REFERENCES
- 1. Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest 112: 1142–1151, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agrotis A, Saltis J, Bobik A. Effect of transforming growth factor-beta 1 on platelet-derived growth factor receptor binding and gene expression in vascular smooth muscle cells from SHR and WKY rats. Clin Exp Pharmacol Physiol 21: 145–148, 1994. [DOI] [PubMed] [Google Scholar]
- 3. Ailenberg M, Silverman M. Trichostatin A-histone deacetylase inhibitor with clinical therapeutic potential-is also a selective and potent inhibitor of gelatinase A expression. Biochem Biophys Res Commun 298: 110–115, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Antonelli-Orlidge A, Saunders KB, Smith SR, D'Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci USA 86: 4544–4548, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Auge N, Maupas-Schwalm F, Elbaz M, Thiers JC, Waysbort A, Itohara S, Krell HW, Salvayre R, Negre-Salvayre A. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation 110: 571–578, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Bae IH, Park MJ, Yoon SH, Kang SW, Lee SS, Choi KM, Um HD. Bcl-w promotes gastric cancer cell invasion by inducing matrix metalloproteinase-2 expression via phosphoinositide 3-kinase, Akt, and Sp1. Cancer Res 66: 4991–4995, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 63: 515–524, 1990. [DOI] [PubMed] [Google Scholar]
- 9. Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103: 159–165, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Betsholtz C. Role of platelet-derived growth factors in mouse development. Int J Dev Biol 39: 817–825, 1995. [PubMed] [Google Scholar]
- 11. Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 131: 1847–1857, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Blank RS, Owens GK. Platelet-derived growth factor regulates actin isoform expression and growth state in cultured rat aortic smooth muscle cells. J Cell Physiol 142: 635–642, 1990. [DOI] [PubMed] [Google Scholar]
- 13. Carmeliet P. Angiogenesis in health and disease. Nat Med 9: 653–660, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Carvalho RL, Jonker L, Goumans MJ, Larsson J, Bouwman P, Karlsson S, Dijke PT, Arthur HM, Mummery CL. Defective paracrine signalling by TGFbeta in yolk sac vasculature of endoglin mutant mice: a paradigm for hereditary haemorrhagic telangiectasia. Development 131: 6237–6247, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Chen XL, Chen ZS, Ding Z, Dong C, Guo H, Gong NQ. Antisense extracellular signal-regulated kinase-2 gene therapy inhibits platelet-derived growth factor-induced proliferation, migration and transforming growth factor-beta(1) expression in vascular smooth muscle cells and attenuates transplant vasculopathy. Transpl Int 21: 30–38, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Crocker DJ, Murad TM, Geer JC. Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol 13: 51–65, 1970. [DOI] [PubMed] [Google Scholar]
- 17. DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol 65: 744–752, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 121: 1845–1854, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Ding R, Darland DC, Parmacek MS, D'Amore PA. Endothelial-mesenchymal interactions in vitro reveal molecular mechanisms of smooth muscle/pericyte differentiation. Stem Cells Dev 13: 509–520, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J 21: 4307–4316, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engel L, Ryan U. TGF-beta 1 reverses PDGF-stimulated migration of human aortic smooth muscle cells in vitro. In Vitro Cell Dev Biol Anim 33: 443–451, 1997. [DOI] [PubMed] [Google Scholar]
- 22. Fontenay M, Bryckaert M, Tobelem G. Transforming growth factor-beta 1 inhibitory effect of platelet-derived growth factor-induced signal transduction on human bone marrow fibroblasts: possible involvement of protein phosphatases. J Cell Physiol 152: 507–519, 1992. [DOI] [PubMed] [Google Scholar]
- 23. Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature 438: 960–966, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314: 15–23, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Ghosh Choudhury G, Kim YS, Simon M, Wozney J, Harris S, Ghosh-Choudhury N, Abboud HE. Bone morphogenetic protein 2 inhibits platelet-derived growth factor-induced c-fos gene transcription and DNA synthesis in mesangial cells. Involvement of mitogen-activated protein kinase. J Biol Chem 274: 10897–10902, 1999. [DOI] [PubMed] [Google Scholar]
- 26. Gines P, Li X, Zamarripa JL, Brown SE, Wieder ED, Nakamura T, Guzelian PS, Schrier RW, Heasley LE, Nemenoff RA. Tyrosine kinase growth factor receptors but not seven-membrane-spanning receptors or phorbol esters activate mitogen-activated protein kinase in rat hepatocytes. Hepatology 22: 1296–1303, 1995. [PubMed] [Google Scholar]
- 27. Gingras D, Page M, Annabi B, Beliveau R. Rapid activation of matrix metalloproteinase-2 by glioma cells occurs through a posttranslational MT1-MMP-dependent mechanism. Biochim Biophys Acta 1497: 341–350, 2000. [DOI] [PubMed] [Google Scholar]
- 28. Gohongi T, Fukumura D, Boucher Y, Yun CO, Soff GA, Compton C, Todoroki T, Jain RK. Tumor-host interactions in the gallbladder suppress distal angiogenesis and tumor growth: involvement of transforming growth factor beta1. Nat Med 5: 1203–1208, 1999. [DOI] [PubMed] [Google Scholar]
- 29. Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1378: F79–113, 1998. [DOI] [PubMed] [Google Scholar]
- 30. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79: 1283–1316, 1999. [DOI] [PubMed] [Google Scholar]
- 31. Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153: 543–553, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999. [DOI] [PubMed] [Google Scholar]
- 33. Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D'Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res 84: 298–305, 1999. [DOI] [PubMed] [Google Scholar]
- 34. Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141: 805–814, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hung LM, Tsai CH, Chen JK. TGF-beta1 selectively suppresses PDGF receptor signaling pathways in MG-63 human osteosarcoma cell. Life Sci 61: 685–693, 1997. [DOI] [PubMed] [Google Scholar]
- 36. Hwang DL, Latus LJ, Lev-Ran A. Effects of platelet-contained growth factors (PDGF, EGF, IGF-I, and TGF-beta) on DNA synthesis in porcine aortic smooth muscle cells in culture. Exp Cell Res 200: 358–360, 1992. [DOI] [PubMed] [Google Scholar]
- 37. Itoh F, Asao H, Sugamura K, Heldin CH, ten Dijke P, Itoh S. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J 20: 4132–4142, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 58: 1048–1051, 1998. [PubMed] [Google Scholar]
- 39. Jain RK. Molecular regulation of vessel maturation. Nat Med 9: 685–693, 2003. [DOI] [PubMed] [Google Scholar]
- 40. Janat MF, Liau G. Transforming growth factor beta 1 is a powerful modulator of platelet-derived growth factor action in vascular smooth muscle cells. J Cell Physiol 150: 232–242, 1992. [DOI] [PubMed] [Google Scholar]
- 41. Jonk LJ, Itoh S, Heldin CH, ten Dijke P, Kruijer W. Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-beta, activin, and bone morphogenetic protein-inducible enhancer. J Biol Chem 273: 21145–21152, 1998. [DOI] [PubMed] [Google Scholar]
- 42. Kim TS, Kim YB. Correlation between expression of matrix metalloproteinase-2 (MMP-2), and matrix metalloproteinase-9 (MMP-9) and angiogenesis in colorectal adenocarcinoma. J Korean Med Sci 14: 263–270, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ko FN, Yang YC, Huang SC, Ou JT. Coagulation factor Xa stimulates platelet-derived growth factor release and mitogenesis in cultured vascular smooth muscle cells of rat. J Clin Invest 98: 1493–1501, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem 277: 4883–4891, 2002. [DOI] [PubMed] [Google Scholar]
- 45. Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol 26: 1120–1125, 2006. [DOI] [PubMed] [Google Scholar]
- 46. Lee SJ, Bae SS, Kim KH, Lee WS, Rhim BY, Hong KW, Kim CD. High glucose enhances MMP-2 production in adventitial fibroblasts via Akt1-dependent NF-kappaB pathway. FEBS Lett 581: 4189–4194, 2007. [DOI] [PubMed] [Google Scholar]
- 47. Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science 284: 1534–1537, 1999. [DOI] [PubMed] [Google Scholar]
- 48. Liao J, Wolfman JC, Wolfman A. K-ras regulates the steady-state expression of matrix metalloproteinase 2 in fibroblasts. J Biol Chem 278: 31871–31878, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem 276: 341–347, 2001. [DOI] [PubMed] [Google Scholar]
- 50. Majack RA. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J Cell Biol 105: 465–471, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Majack RA, Majesky MW, Goodman LV. Role of PDGF-A expression in the control of vascular smooth muscle cell growth by transforming growth factor-beta. J Cell Biol 111: 239–247, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mii S, Ware JA, Kent KC. Transforming growth factor-beta inhibits human vascular smooth muscle cell growth and migration. Surgery 114: 464–470, 1993. [PubMed] [Google Scholar]
- 53. Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113: 329–342, 2003. [DOI] [PubMed] [Google Scholar]
- 54. Morisaki N, Kawano M, Koyama N, Koshikawa T, Umemiya K, Saito Y, Yoshida S. Effects of transforming growth factor-beta 1 on growth of aortic smooth muscle cells. Influences of interaction with growth factors, cell state, cell phenotype, and cell cycle. Atherosclerosis 88: 227–234, 1991. [DOI] [PubMed] [Google Scholar]
- 55. Nishio E, Watanabe Y. Transforming growth factor beta is a modulator of platelet-derived growth factor action in vascular smooth muscle cells: a possible role for catalase activity and glutathione peroxidase activity. Biochem Biophys Res Commun 232: 1–4, 1997. [DOI] [PubMed] [Google Scholar]
- 56. Ohno-Matsui K, Uetama T, Yoshida T, Hayano M, Itoh T, Morita I, Mochizuki M. Reduced retinal angiogenesis in MMP-2-deficient mice. Invest Ophthalmol Vis Sci 44: 5370–5375, 2003. [DOI] [PubMed] [Google Scholar]
- 57. Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995. [DOI] [PubMed] [Google Scholar]
- 58. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. [DOI] [PubMed] [Google Scholar]
- 59. Pan D, Yang J, Lu F, Xu D, Zhou L, Shi A, Cao K. Platelet-derived growth factor BB modulates PCNA protein synthesis partially through the transforming growth factor beta signalling pathway in vascular smooth muscle cells. Biochem Cell Biol 85: 606–615, 2007. [DOI] [PubMed] [Google Scholar]
- 60. Pauly RR, Passaniti A, Bilato C, Monticone R, Cheng L, Papadopoulos N, Gluzband YA, Smith L, Weinstein C, Lakatta EG, et al. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res 75: 41–54, 1994. [DOI] [PubMed] [Google Scholar]
- 61. Phelps ED, Updike DL, Bullen EC, Grammas P, Howard EW. Transcriptional and posttranscriptional regulation of angiopoietin-2 expression mediated by IGF and PDGF in vascular smooth muscle cells. Am J Physiol Cell Physiol 290: C352–C361, 2006. [DOI] [PubMed] [Google Scholar]
- 62. Quarck R, Berrou E, Magnier C, Bobe R, Bredoux R, Tobelem G, Enouf J, Bryckaert M. Differential up-regulation of Rap1a and Rap1b proteins during smooth muscle cell cycle. Eur J Cell Biol 70: 269–277, 1996. [PubMed] [Google Scholar]
- 63. Ribatti D, Nico B, Vacca A, Iurlaro M, Roncali L. Temporal expression of the matrix metalloproteinase MMP-2 correlates with fibronectin immunoreactivity during the development of the vascular system in the chick embryo chorioallantoic membrane. J Anat 195: 39–44, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Risinger GM, Jr, Hunt TS, Updike DL, Bullen EC, Howard EW. Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J Cell Biol 281: 25915–25925, 2006. [DOI] [PubMed] [Google Scholar]
- 65. Saltis J, Agrotis A, Bobik A. Differential regulation by transforming growth factor-beta 1 of platelet-derived growth factor-stimulated proliferation of vascular smooth muscle cells from SHR and WKY rats. Clin Exp Pharmacol Physiol 19: 396–399, 1992. [DOI] [PubMed] [Google Scholar]
- 66. Saltis J, Bobik A. Developmental regulation of transforming growth factor-beta 1 responses and vascular smooth muscle growth in spontaneously hypertensive rats. J Hypertens 13: 1441–1448, 1995. [PubMed] [Google Scholar]
- 67. Samolov B, Steen B, Seregard S, van der Ploeg I, Montan P, Kvanta A. Delayed inflammation-associated corneal neovascularization in MMP-2-deficient mice. Exp Eye Res 80: 159–166, 2005. [DOI] [PubMed] [Google Scholar]
- 68. Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 48: 2105–2114, 2007. [DOI] [PubMed] [Google Scholar]
- 69. Seifert RA, Coats SA, Raines EW, Ross R, Bowen-Pope DF. Platelet-derived growth factor (PDGF) receptor alpha-subunit mutant and reconstituted cell lines demonstrate that transforming growth factor-beta can be mitogenic through PDGF A-chain-dependent and -independent pathways. J Cell Biol 269: 13951–13955, 1994. [PubMed] [Google Scholar]
- 70. Staus DP, Blaker AL, Taylor JM, Mack CP. Diaphanous 1 and 2 regulate smooth muscle cell differentiation by activating the myocardin-related transcription factors. Arterioscler Thromb Vasc Biol 27: 478–486, 2007. [DOI] [PubMed] [Google Scholar]
- 71. Tomasek JJ, Halliday NL, Updike DL, Ahern-Moore JS, Vu TK, Liu RW, Howard EW. Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. J Cell Biol 272: 7482–7487, 1997. [DOI] [PubMed] [Google Scholar]
- 72. Turner NA, Hall KT, Ball SG, Porter KE. Selective gene silencing of either MMP-2 or MMP-9 inhibits invasion of human saphenous vein smooth muscle cells. Atherosclerosis 193: 36–43, 2007. [DOI] [PubMed] [Google Scholar]
- 73. Tziakas DN, Lazarides MK, Tentes IK, Georgiadis GS, Eleftheriadou E, Chalikias GK, Kortsaris A, Hatseras DI. Gelatinases [matrix metalloproteinase-2 (MMP-2) and MMP-9] induce carotid plaque instability but their systemic levels are not predictive of local events. Ann Vasc Surg 19: 529–533, 2005. [DOI] [PubMed] [Google Scholar]
- 74. Uzui H, Lee JD, Shimizu H, Tsutani H, Ueda T. The role of protein-tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis 149: 51–59, 2000. [DOI] [PubMed] [Google Scholar]
- 75. Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Cell Biol 263: 7741–7746, 1988. [PubMed] [Google Scholar]
- 76. Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316: 1749–1752, 2007. [DOI] [PubMed] [Google Scholar]
- 77. Villiger PM, Lotz M. Differential expression of TGF beta isoforms by human articular chondrocytes in response to growth factors. J Cell Physiol 151: 318–325, 1992. [DOI] [PubMed] [Google Scholar]
- 78. Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev 14: 558–566, 2004. [DOI] [PubMed] [Google Scholar]
- 79. Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wrenn RW, Raeuber CL, Herman LE, Walton WJ, Rosenquist TH. Transforming growth factor-beta: signal transduction via protein kinase C in cultured embryonic vascular smooth muscle cells. In Vitro Cell Dev Biol 29A: 73–78, 1993. [DOI] [PubMed] [Google Scholar]
- 81. Zempo N, Kenagy RD, Au YP, Bendeck M, Clowes MM, Reidy MA, Clowes AW. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg 20: 209–217, 1994. [DOI] [PubMed] [Google Scholar]
- 82. Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, Iwao H. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol 23: 795–801, 2003. [DOI] [PubMed] [Google Scholar]
- 83. Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA 97: 4052–4057, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.