Abstract
Acheron (Achn) was originally identified as novel gene that is induced when insect muscles become committed to die at the end of metamorphosis. In separate studies, we have demonstrated that Achn acts upstream of MyoD and is required by mammalian myoblasts to either differentiate or undergo apoptosis following loss of growth factors. In the present study we examined the role of Achn in regulating integrin-extracellular matrix interactions that are required for myogenesis. Both control C2C12 myoblasts and those engineered to express ectopic Achn expressed the fibronectin receptor integrin α5β1 in the presence of growth factors and the laminin receptor α7β1 following growth factor withdrawal. Expression of the laminin receptor was blocked in cells expressing either Achn antisense or an Achn deletion mutant that blocks differentiation. Control cells and those expressing ectopic Achn undergo sequential and transient increases in both substrate adhesion and migration before cell fusion. Blockade of Achn expression reduced these effects on laminin but not on fibronectin. Taken together, these data suggest that Achn may influence differentiation in part via its control of cell adhesion dynamics.
Keywords: C2C12, differentiation, satellite cell, integrin signaling, skeletal muscle
during vertebrate embryogenesis, lineage-restricted myoblasts arise predominantly in the somites. Myogenesis is initiated by a wave of embryonic myoblasts, which migrate out of the somites to sites of muscle development, where they differentiate to form primary muscle fibers (3). This is later followed by a migration of fetal myoblasts, which give rise to secondary muscle fibers. A third population of myoblasts, known as satellite cells, localize between the basal lamina and sarcolemma. Satellite cells are a lineage-restricted stem cell population that is responsible for the continued pre- and postnatal growth of muscle, as well as the repair of damaged fibers (3, 6, 12).
When myoblasts arrive at the appropriate location and have undergone additional rounds of division, they assume one of three distinct fates (7, 16). A subset of cells aligns, upregulates the expression of basic helix-loop-helix (bHLH) transcription factors MyoD and myogenin, and then fuses to form multinucleated myotubes, the precursors of muscle fibers (24, 34). A second population upregulates the bHLH transcription factor Myf5 and arrests as a mitotically quiescent population, which can be subsequently stimulated to proliferate and differentiate (2). A final group of cells fails to upregulate known bHLH or survival proteins and is believed to initiate apoptosis (13). In this way, the population of myoblasts is self-correcting to ensure that needed cells are retained while surplus cells are rapidly removed. This process is recapitulated throughout life when satellite cells become activated following focal muscle damage. A subset of cells repairs muscle, some replicate to replenish the population, and the surplus cells die.
Interactions with both neighboring cells and the extracellular matrix (ECM) provide essential cues for cell fate determination in myoblasts. Cell-ECM interactions are transduced by a family of heterodimeric transmembrane proteins known collectively as integrins (5, 27). A functional integrin is composed of single α- and β-subunits that serve as receptors for specific ECM proteins. Myoblasts express a wide array of integrin subunits that bind a variety of substrates and mediate specific, ECM-dependent intracellular signaling (9, 22, 26). The integrin subunits that have been shown to play significant roles in myoblast differentiation include β1, β3, α4, α5, α6, α7, and αv (reviewed in Ref. 21). The association of β1 with α4, α5, or αv results in a fibronectin receptor, while the association of β1 with either α6 or α7 constitutes a receptor specific for laminin (6, 39). The α4-subunit mediates fusion during secondary myogenesis by recognition of its counterreceptor, VCAM-1, which is expressed on the cell surface of preexisting muscle fibers. During myogenesis, αv associates with β3; however, this pairing forms a nonspecific receptor that binds fibronectin, laminin, and collagen (4). Fibronectin adhesion has been shown to favor myoblast proliferation, while attachment to laminin promotes cell cycle arrest and differentiation (1, 15, 28, 32). During differentiation in vitro, myoblasts upregulate expression of α7 integrin subunits, including the α7A splice variant (11). It has also been shown in vivo that these cells initially express integrin receptors that bind fibronectin, but as myogenesis progresses, there is a shift to laminin receptors, which correlates with the appearance of laminin in the ECM (9). Given the central role of fibronectin and laminin in myogenesis, the current study focuses on the integrins that bind these particular ECM components.
C2C12 cells have proven to be an invaluable tool for elucidating the signaling events that mediate myogenesis (8, 29). C2C12 cells were derived from regenerating adult mouse skeletal muscle and are assumed to represent a satellite cell line that can be maintained indefinitely in vitro without transformation (36). These cells rapidly proliferate when cultured subconfluently in serum-rich growth medium (GM). When cells are switched to a low-serum differentiation medium (DM), they initiate processes that closely parallel in vivo differentiation. Some cells fuse to form myotubes, some arrest as satellite (or reserve cells as they are known in vitro), and the rest undergo apoptosis (13, 23, 29, 38). Careful analysis has demonstrated that the most abundant fibronectin and laminin receptors in C2C12 cells are α5β1 and α7β1, respectively (37).
To better understand how muscle cells make the decision to die during development, we screened the intersegmental muscles from the tobacco hawkmoth Manduca sexta for genes that were induced when the cells became committed to undergo programmed cell death at the end of metamorphosis (30). One of the genes isolated in this screen encoded Acheron (Achn), a novel protein that defines a new subfamily of Lupus antigen (La) RNA binding proteins (31). Achn is phylogenetically conserved, and moth and human Achn proteins share an overall 31% identity and 40% similarity, while within a conserved 227 amino acid region they share 42% identity and 54% similarity. On the basis of its relatedness to La protein, Achn is likely to be an RNA binding protein, although how it regulates differentiation is unknown. It been shown to bind to CASK/lin-2, a signal transduction protein that can shuttle between the membrane and nucleus (35).
Achn is expressed at very low levels in C2C12 cells cultured in GM, but then accumulates when they are transferred to DM (33). Expression of ectopic Achn serves to promote both myotube formation and reserve cell death, while blockade of Achn with either antisense or a putative dominant-negative version (truncated Achn; tAchn) both blocks myotube formation and apoptosis (33). In zebrafish embryos, ectopic Achn promotes muscle formation while injection of an antisense morpholino directed against Achn mRNA blocked myogenesis in vivo (33).
In the present study we initially characterized some of the cell-substrate events associated with normal myogenesis, and we then examined the role of Achn in mediating these processes. In control cells, loss of growth factors initiates a transient increase in myoblast adhesion to the substrate. This is followed by a decrease in adhesion and a complimentary increase in cell motility. Motility then declines as cells begin to fuse into myotubes. To determine the possible role of Achn in these processes, we engineered C2C12 cells to stably express ectopic Achn (Achn), antisense Achn (AS-Achn), or an NH2-terminally truncated Achn (tAchn), and then examined their behavior in growth and differentiation media. We observed that Achn regulates integrin subunit expression which parallels changes in cell morphology, adhesion, and motility.
MATERIALS AND METHODS
Cell culture.
C2C12 cells were cultured in GM consisting of Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% (vol/vol) calf serum, 5% fetal bovine serum (Atlanta Biologicals, Norcross, GA), and 100 U/ml of penicillin-streptomycin (Gibco). To induce differentiation, subconfluent cultures were shifted to DM consisting of DMEM supplemented with 2% horse serum (Hyclone) and antibiotics.
LipofectAmine (Gibco) was used to transfect C2C12 cells with a mammalian expression vector (pBABE-puro) encoding full-length human Achn, an NH2-terminally truncated form of Achn lacking the coding region for the first 33 amino acids (tAchn), antisense Achn (AS-Achn), or empty vector according to the manufacturer's protocol. The antibiotic-resistant stable transformants were selected in GM with puromycin (3 μg/ml). About 10 monoclonal lines were randomly chosen from each transfection, and further analyses were performed with representative lines.
Microscopy.
For fluorescence microscopy, cells were fixed for 2 min with 2% paraformaldehyde in PBS at room temperature, permeabilized for 3 min with Karsenti's lysis buffer (0.5% Triton X-100, 80 mM PIPES, 1.0 mM MgSO4, 5.0 mM EGTA, pH 7.0), then fixed for an additional 2 min in 2% paraformaldehyde. Cells were rinsed 3× in PBST (Dulbecco's phosphate-buffered saline, Invitrogen, + 0.05% Tween 20) between each processing step. Actin was labeled with rhodamine-conjugated phalloidin (Molecular Probes) in PBST, while nuclei were labeled with DAPI (Molecular Probes) (1:2,000). Cells were examined under a Nikon TE 2000 inverted microscope using a ×40 oil immersion objective lens. Images were captured and processed with MetaVue software.
RT-PCR.
Expression of integrin subunits was evaluated by semiquantitative (end-point) PCR. RNA was extracted from cells with TRIzol reagent (Invitrogen) according to the manufacturer's instructions and reverse transcribed with qScript (Quanta). PCR reactions were carried out with 32 cycles of amplification at an annealing temperature of 54°C or 59°C using the Platinum Taq system (Invitrogen). The primers used were as follows: β1, 5′-GGCAACAATGAAGCTATCGT-3′ and 5′-CCCTCATACTTCGGATTGAC-3′ (predicted products, A form 282 bp, D form 363 bp); α5, 5′-CATTTCCGAGTCTGGGCCAA-3′ and 5′-TGGAGGCTTGAGCTGAGCTT-3′ (predicted product, 320 bp); α6, 5′-TACCTAGGCTTTTCGCTGGA-3′ and 5′-TAGACGTAAACTGCACCCCC-3′ (predicted product, 293 bp); α7, 5′-TACATGGCCGTGAAATCCCTGGAA-3′ and 5′-TTGGGACAGCAGATGTTAGGCAGT-3′ (predicted products B form 468 bp, A form 581 bp); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GAAGGGCTCATGACCACAGTCCATG-3′, and 5′-TGTTGCTGTAGCCGTATTCATTGTC-3′ (predicted product 454 bp).
Western blot analysis.
Cellular proteins were extracted in Laemmli buffer, fractionated by size via 10% SDS-PAGE, and transferred to Immobilon P membrane (Millipore). Membranes were blocked in 5% nonfat dry milk in PBS-0.05% Tween and reacted overnight at 4°C with primary antisera directed against the following proteins: integrin β1 (1:1,000, Transduction Laboratories, 610467, mouse monoclonal), integrin α5 (1:200, Santa Cruz, sc-10729, rabbit polyclonal), integrin α7A and α7B (both 1:500, generous gifts from Dr. Randy Kramer, University of California, San Francisco), and sarcomeric myosin heavy chain (MHC; 1:100, Developmental Studies Hybridoma Bank, MF-20, mouse monoclonal). Antigens were detected with a horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit antibody (1:2,000, Vector Laboratories) followed by ECL chemiluminescence (NEN) and X-ray film autoradiography (Eastman Kodak).
Cell adhesion.
Ninety-six-well plates were coated with either fibronectin (20 μg/ml) or laminin-1 (40 μg/ml) (BD Biosciences) for 60 min at 37°C and then rinsed with PBS and air-dried. Each of the different Achn-engineered C2C12 cell lines was plated at subconfluent density in 100 μl of adhesion medium (DMEM + 0.1% BSA) and allowed to adhere at 37°C for 60 min (fibronectin) or 90 min. (laminin). The plates were then rinsed twice in PBS to remove unattached cells, and fresh adhesion medium was added to the wells. The cells were lysed with 2 μl of 9% Triton X-100, and adherent cells were quantified with the lactate dehydrogenase activity assay, CytoTox-One (Promega) according to the manufacturer's instructions.
Cell spreading.
Cells were plated on 35-mm dishes coated with fibronectin or laminin-1 in DMEM + 0.1% BSA. Cells were allowed to spread at 37°C, and two fields were imaged from each plate by phase microscopy at ×20 magnification. The same fields were resampled at intervals throughout the assay, as indicated in the figures. Cell were counted and scored from the saved images, and the data are reported as percentage of cells spread relative to total number of cells visible in each field. A “spread cell” is defined as a cell that is nonrefractile and which contains visible cytoplasm extending away from the full circumference of the nucleus.
Migration assays.
Cells were plated on fibronectin or laminin-1-coated 96-well migration inserts (Millipore) in 0.1% BSA DMEM and allowed to migrate for 3–4 h at 37°C. At the end of the assay, the medium was gently aspirated and the unattached cells were removed from the insert. Both sides of the migration chamber were treated with trypsin (0.25%, Invitrogen) for 5 min at 37°C to free adherent cells, which were then transferred to noncoated 96-well plates. A molar equivalent of soybean trypsin inhibitor (Sigma) was added to inactivate the trypsin, and the CellTiter-Glo (Promega), luciferase-based ATP assay was used to determine the ratio of cells that had migrated through the membrane.
Statistical analysis.
Data were analyzed by single-factor ANOVA, and P < 0.05 was used as the criterion for significance.
RESULTS
Achn affects cell morphology and cytoskeletal organization.
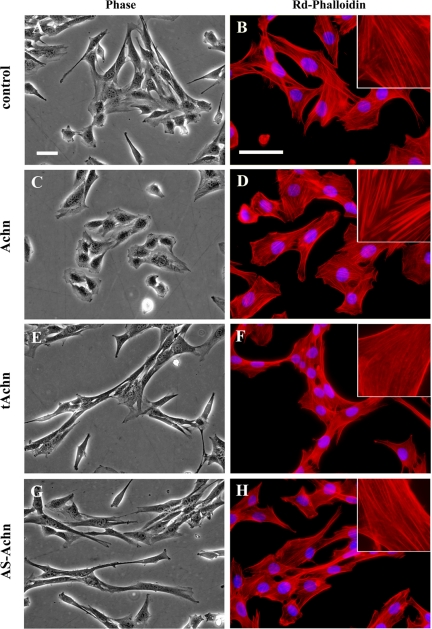
We used phase contrast and fluorescence microscopy to examine control C2C12 cells transfected with an empty retroviral vector (control) or the Achn cDNA (Fig. 1). Phase contrast was used to view living cells while fluorescence microscopy was used to image the actin cytoskeleton of fixed cells that were then stained with rhodamine-conjugated phalloidin. Control C2C12 cells had a fibroblastic appearance and a regular meshwork of actin stress fibers (Fig. 1, A and B). Cells expressing ectopic Achn were more circular, with cytoplasm extending uniformly away from the nucleus and thicker and more prominent actin stress fibers (Fig. 1, C and D). In contrast, cells expressing either truncated Achn (tAchn) (Fig. 1, E and F) or antisense Achn (AS-Achn) (Fig. 1, G and H) were elongated and the cytoplasm appeared as slender extensions from the nucleus. While they were superficially similar (Fig. 1, E and G), the AS-Achn and tAchn cells differed in terms of their cytoskeletal organizations (Fig. 1, F and H). The AS-Achn cells had moderate stress fibers similar to those seen in the vector control cells, while the tAchn-expressing cells had no discernible stress fibers. The diffuse actin staining observed in the tAchn cells is indicative of polymerized but unbundled actin.
Fig. 1.
Effects of Acheron (Achn) on cell morphology. C2C12 myoblasts were engineered to express an empty vector (pBABE-puro) or one of three Achn variants. A and B: empty vector control. C and D: full-length Achn. E and F: truncated Achn (tAchn). G and H: antisense Achn (AS-Achn). A, C, E, and G: live cells imaged by phase contrast microscopy at ×20 magnification. B, D, F, and H: fixed cells with rhodamine (Rd)-phalloidin to label actin and DAPI to label nuclei. Cells were imaged with fluorescence microscopy at ×40 magnification. Scale bars indicate 50 μm. Insets: ×60 magnification of rhodamine-phalloidin-labeled actin.
Achn regulates integrin expression during myoblast differentiation.
Myoblasts undergo a shift in their ability to adhere to ECM components during myogenesis, which in turn regulates both migration and fusion (1, 9, 15, 28). To determine whether Achn alters integrin expression, we used both Western blot analysis and end-point PCR to examine the expression of integrin protein and mRNA, respectively. Given the large number of integrin genes and isoforms expressed in mammalian cells, we chose to restrict our analysis to those integrins that play a role in fibronectin or laminin adhesion and have been previously shown to be expressed in C2C12 cells.
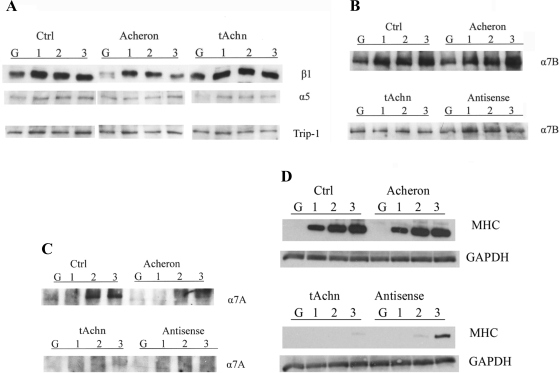
We evaluated integrin expression in each of our different Achn-engineered C2C12 cells lines cultured in either GM or at various times after transfer to DM. Western blot analysis demonstrated that all three of the engineered cell lines displayed detectible levels of integrin β1 in GM, which then accumulated to higher levels following transfer to DM (Fig. 2A). However, at all stages examined the absolute levels of integrin β1 protein were lower in the cells expressing ectopic Achn relative to either the vector controls or the tAchn lines (Fig. 2A). In general, we observed that the expression of β1 integrin protein changed more dramatically during differentiation than its corresponding mRNA (Fig. 3).
Fig. 2.
A–C: Western blot analysis of integrin expression in control (Ctrl) and Achn-engineered C2C12 cells. A: β1 (top) and α5 (middle) integrin expression in control, full-length Achn, and truncated Achn expressing cells in growth medium (G) and after 1, 2, or 3 days after transfer to differentiation medium (DM). The constitutively expressed 26S proteasome subunit Trip-1 served as a loading control (bottom). B: α7B integrin expression in control, full-length Achn, truncated Achn, and antisense Achn-engineered cells. C: α7A integrin expression in C2C12 cells. D: Western blot of muscle-specific myosin heavy chain (MHC) in Achn-engineered C2C12 cell lines in growth medium and after 1, 2, and 3 days in DM. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
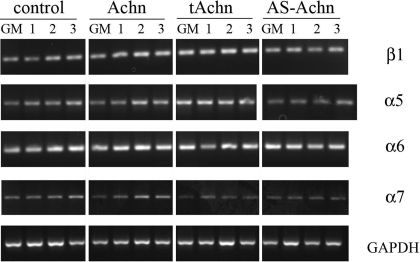
Fig. 3.
RT-PCR analysis of integrin mRNA expression in control and Achn-engineered cells. From top: β1, α5, α6, and α7 in Achn cell lines expressing empty pBABE vector (control), full-length Achn, truncated Achn, and antisense Achn. Cells were analyzed after culture in growth medium (GM) or 1, 2, or 3 days after transfer to DM. GAPDH served as a loading control.
In contrast, the expression of α5 integrin protein did not change appreciably during the 3 days following transfer to DM in any of the cell lines tested (Fig. 2A). By PCR analysis, α5 integrin message level in both control and Achn cell expression was low in GM and then increased slightly during differentiation (Fig. 3). The tAchn cells had higher levels of α5 expression than the control cells, and these levels remained consistent after withdrawal of growth factors. AS-Achn cells were similar to the tAchn line in that α5 expression did not change after transfer to DM. Since the primary fibronectin receptor in myoblasts is α5β1 (37), the relatively stable expression of α5 suggests that myoblasts may not specifically decrease their adhesion to fibronectin during differentiation, a hypothesis that is tested below.
Since myoblasts change their substrate preference from fibronectin to laminin during differentiation (9, 11, 28), we examined the expression of both the A and B isoforms of the laminin-specific integrin subunit, α7. The basal levels of α7B protein and mRNA were higher in control and Achn-expressing cells relative to the tAchn and AS-Achn cells (Figs. 2B and 3). Following transfer to DM, α7B expression also increased in both the control and Achn cells, while in the tAchn and AS-Achn lines, α7B remained essentially unchanged at the level of both protein and mRNA levels (Figs. 2B and 3).
Expression of α7A, the muscle-specific laminin receptor, was undetectable by Western blot in any of the cell lines cultured in GM (Fig. 2C). Following transfer to DM, expression of α7A became discernable in control cells by day 2. In the cells expressing ectopic Achn, there was a faint band on the third day, but it was very close to the limit of detection (Fig. 2C). These changes in integrin expression paralleled the formation of myotubes in the cultures (33 and data not shown) and the induction of muscle-specific myosin heavy chain (MHC) expression (Fig. 2D). The α7A isoform was not detected in the tAchn or AS-Achn cells under any conditions (Fig. 2C). These cells differentiated poorly and had a corresponding reduction in MHC expression (Fig. 2D). Integrin α7A was not detectible by PCR in any of the cells examined.
We also evaluated expression of α6 mRNA, since this subunit also pairs with β1 to form a laminin receptor (Fig. 3). However, this protein was constitutively expressed in all lines at each stage examined.
Achn affects myoblast adhesion to laminin.
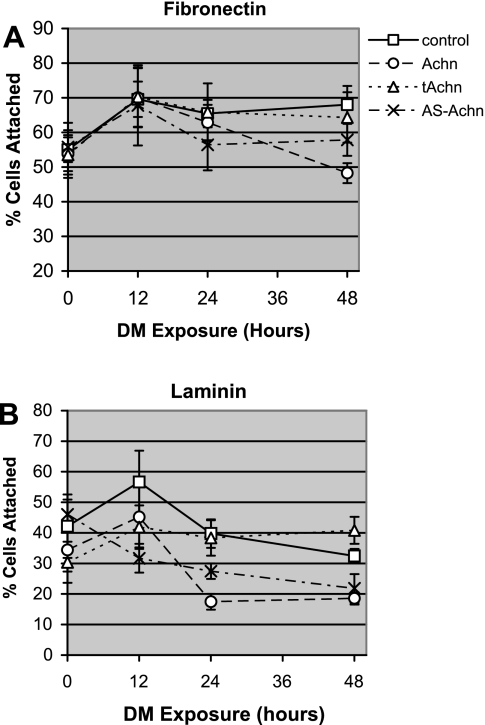
Since Achn regulates the expression of some key integrins required for myogenesis in C2C12 cells, we assayed its effects on myoblast adhesion and migration. About 55% of control cells cultured in GM on a fibronectin substrate were able to adhere to the plate (Fig. 4A). This level of adhesion was comparable to what we observed in the various Achn-engineered lines.
Fig. 4.
Effects of Achn on cell adhesion. Percentage of C2C12 cells attached to fibronectin (A) or laminin-1 (B) after culture in GM (DM exposure = 0) and 12, 24, and 48 h after being switched to DM. Control, empty vector. Values are means ± SE of four independent experiments, each performed in triplicate.
Within 12 h following transfer of control cells to DM, their ability to adhere to fibronectin increased significantly, such that ∼70% of the cells attached to the substrate (Fig. 4A). This level of adherence was maintained during the subsequent 36 h of analysis. All of the Achn-engineered cell lines displayed a comparable increase in fibronectin adhesion during the first 12 h following transfer to DM (P = 0.007, Fig. 4A), although for the Achn and AS-Achn-expressing lines this effect was transient.
The ability of cells to adhere to laminin following transfer to DM was much more varied than we observed for fibronectin under the same conditions. About 40% of the control cells cultured in GM bound to laminin (Fig. 4B). In these cells there was an initial increase in adherence during the first 12 h after transfer to DM, which then fell during the subsequent 36 h. Overall, cells expressing ectopic Achn were less adherent on laminin than the vector control cells. In control, Achn, and tAchn cells, adhesion to laminin at 12 h after transfer to DM was significantly greater than that observed in GM (P = 0.04). Adhesion at this time was also elevated relative to the 24-h time point (P = 0.04), but only in the control and Achn cells. This suggests a discrete peak in adhesion around 12 h after transfer to DM. In contrast, adhesion to laminin in the tAchn cells was not transient and remained elevated throughout the remainder of the test. The AS-Achn cells did not display increased laminin-binding at any time point examined, and, in fact, adhesion consistently declined in these cells after transfer to DM (Fig. 4B).
Achn affects cell spreading on laminin.
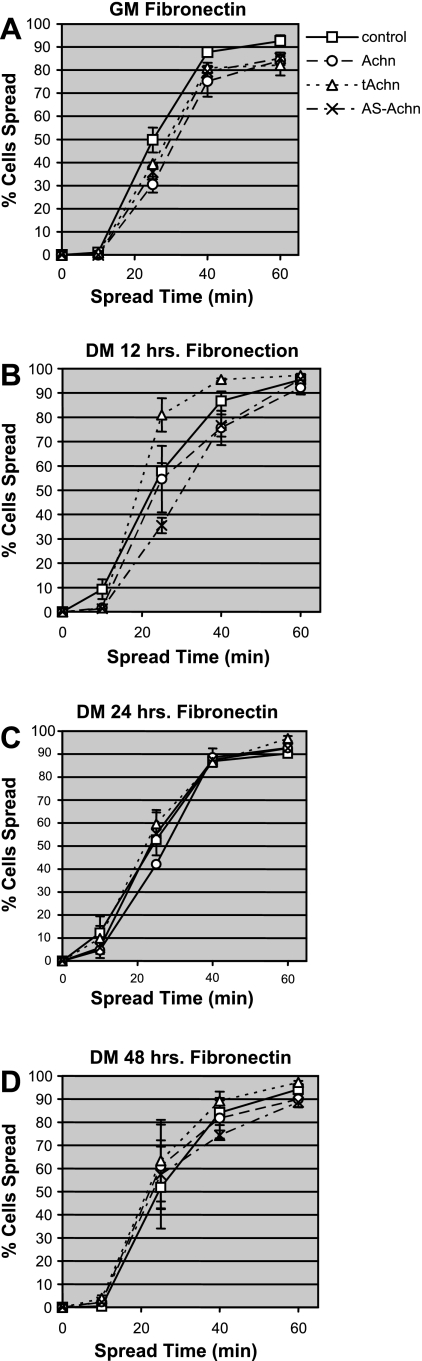
To complement these studies, we cultured all of our engineered cell lines on both laminin and fibronectin and monitored cell spreading during early differentiation. We employed a binary assay designed to measure the rate at which cell populations were able to functionally respond to spreading cues from the ECM. After culture in GM or DM for the indicated time, cells were trypsinized and resuspended in serum-free spreading medium. Cells were allowed to settle onto plastic tissue culture plates coated with either fibronectin or laminin-1, and the rates at which cells adopted a spreading morphology was monitored. Cells were determined to have initiated spreading when they became nonrefractile and their cytoplasm extended visibly around the entire circumference of the nucleus. Control cells maintained in GM spread very quickly on fibronectin (squares, Fig. 5A), with 50% of the cells spread by 25 min. Transfer to DM did not significantly alter the rate at which spreading was initiated in control cells (Fig. 5, B–D).
Fig. 5.
Effects of Achn on the rate of cell spreading on fibronectin. C2C12 cells expressing pBABE-puro vector alone (control), full-length Achn, truncated Achn, or antisense Achn were allowed to spread on fibronectin-coated 35-mm dishes as described. A: cells maintained in GM. B: cells in DM for 12 h. C: cells in DM for 24 h. D: cells in DM for 48 h. Values are means ± SE of three independent experiments, each performed in duplicate.
When cultured in GM, manipulating Achn had only a modest effect on cell spreading on fibronectin (Fig. 5A). However, there were significant differences in the rates at which the tAchn and AS-Achn cells spread after transfer to DM. Under these conditions, the tAchn lines spread 50% faster than the control cells at 12 h, while the AS-Achn line spread ∼50% slower than controls (Fig. 5B). However, these differences were no longer discernable at 24 and 48 h after transfer to DM (Fig. 5, C and D).
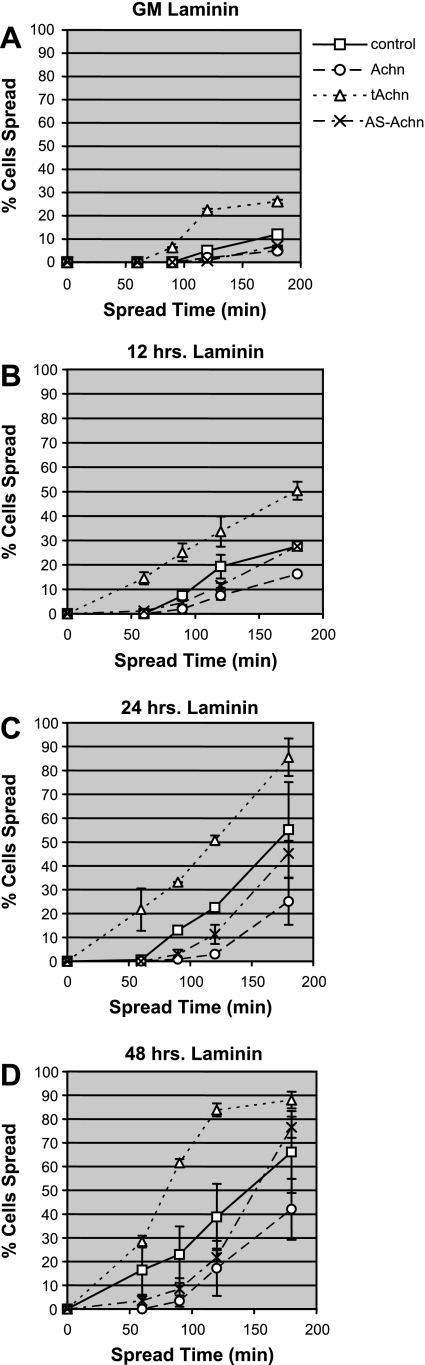
Neither the control cells nor those engineered to express various Achn constructs spread as rapidly on laminin as they did on fibronectin (Fig. 6A). Only ∼12% of control cells cultured on laminin in GM had initiated spreading after 3 h, compared with 50% spreading after 25 min on fibronectin (Fig. 6A vs. Fig. 5A). Spreading improved following transfer to DM (Fig. 6, B–D), such that by 48 h, 65% of the control cells had begun spreading during the 3-h assay period. The Achn and AS-Achn lines behaved comparably to control cells, although their absolute levels of spreading were lower (Fig. 6, A–D). C2C12 cells expressing tAchn displayed a greater ability to spread on laminin than any of the other lines. The response of these cells reached a plateau by 48 h after transfer to DM, something that none of the other lines were able to achieve (Fig. 6D).
Fig. 6.
Effects of Achn on the rate of cell spreading on laminin. C2C12 cells expressing pBABE-puro vector alone (control), full-length Achn, truncated Achn, or antisense Achn were allowed to spread on laminin-coated 35-mm dishes. A: cells maintained in GM. B: cells in DM for 12 h. C: cells in DM for 24 h. D: cells in DM for 48 h. Values are means ± SE of three independent experiments, each performed in duplicate.
Effects of altered Achn expression on motility.
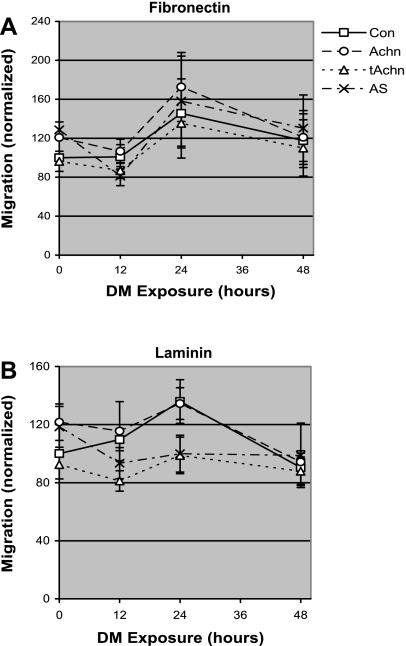
The observation that manipulating Achn could alter integrin expression, cell adhesion, and cell spreading prompted us to determine whether manipulating Achn expression could also affect motility. Control and Achn-engineered cells were cultured in GM or DM, and the percentage of cells that could migrate through an ECM-coated filter was determined. In each experiment, changes in cell motility after transfer to DM were normalized to the motility of control cells cultured in GM (Fig. 7, A and B).
Fig. 7.
Effects of Achn on cell motility during differentiation. C2C12 cells expressing pBABE-puro vector alone (Con), full-length Achn, truncated Achn, or antisense Achn were allowed to attach and migrate through fibronectin-coated (A) or laminin-1-coated (B) 8-μm filters. Cells were assayed in GM (DM exposure = 0) and after 12, 24, and 48 h after transfer to DM. The fraction of cells that migrated through the filter relative to total attached cells was calculated and then normalized to the control values in GM. Values are means ± SE of four independent experiments, each performed in triplicate.
All of the Achn-engineered cell lines cultured on fibronectin displayed a transient increase in motility that peaked at 24 h after transfer to DM (Fig. 7A). This “migration phase” declined over the subsequent 24 h, coincident with the start of myotube formation in the cultures. The increase in cell motility at 24 h constituted a discrete peak that was significantly elevated relative to both 12- and 48-h time points (P = 0.02). As with adhesion and spreading, cell motility was more variable on laminin-1 than on fibronectin (Fig. 7B vs. 7A). Both the control cells and those expressing ectopic Achn displayed transient increases in motility that peaked at 24 h after transfer to DM and were measurably elevated relative to motility observed at 12 or 48 h (P = 0.02). In contrast, the motility of the AS-Achn and tAchn cells dropped within the first 12 h and did not significantly change up to 48 h after transfer to DM. As expected, these lines were significantly less motile at 24 h as compared with control cells (P = 0.01).
DISCUSSION
Extracellular adhesion molecules are potent regulators of myogenic cell fate. Myoblasts adhere to ECM components and migrate long distances during embryogenesis (34). The same behavior is displayed by activated satellite cells engaged in muscle repair (6). In addition to providing a substrate for the migration, components of the ECM also act as signaling molecules that influence cellular decisions to differentiate into myotubes or arrest as satellite/reserve cells. Within the cell, these signals are largely transduced by integrins, which function in both adhesion and intracellular signaling. Cycling myoblasts preferentially express the fibronectin receptor α5β1 but then shift to the laminin receptor α7β1 when they initiate myogenesis (37). This process can be recapitulated in vitro by a number of cell lines including C2C12 myoblasts.
Previous work in our laboratory has demonstrated that Achn is required for myotube formation in both C2C12 cells and zebrafish embryos (33). A variety of cellular and molecular programs have to be coordinated for successful myogenesis, and the current study was initiated in part to help evaluate the role(s) of Achn and integrins in this process.
Alterations in Achn expression had a gross effect on cellular morphology and stress fiber formation, even in the presence of growth factors (Fig. 1). These observations led us to speculate that Achn might modulate cell-substrate adhesion. Cell adhesion to ECM components plays a critical role in myoblast differentiation via integrin-mediated signaling (1, 15, 28). We found that when growth factors were withdrawn, both control cells and those expressing ectopic Achn displayed a rapid increase in the expression of α7 integrins at both mRNA and protein levels (Figs. 2 and 3). The association of α7 integrins with β1 integrin subunits creates an integrin receptor with a binding preference for laminin. It has been established that an upregulation of integrin α7 expression and adhesion to a laminin serve to promote myoblast differentiation (28, 32). Expression of either AS-Achn or tAchn resulted in a significant reduction in the levels of α7 integrin protein and mRNA in both GM and DM (Fig. 2, B and C, and Fig. 3). These cells also failed to demonstrate the anticipated increase in α7 mRNA expression following transfer to DM (Fig. 3). Since the switch to laminin-specific integrins correlates with myoblast differentiation, the failure to induce α7 expression supports the hypothesis that Achn plays a required role in myotube differentiation. This is also supported at the biochemical level by loss of MHC expression in AS-Achn and tAchn-expressing cells (Fig. 2D). At present, it is not known whether the failure to express laminin receptors in Achn-engineered cells induces the block of myogenesis or instead reflects the consequences of blockade at a different level of regulation.
Achn clearly plays a required role in the induction of α7 and may also function to help repress integrin β1 expression (Fig. 2A). This would presumably facilitate differentiation by reducing the inhibitory effects of β1. Sastry et al. (28) observed that β1 integrin subunits induce proliferation and inhibit differentiation, effects that are mediated through α5 signaling. More recently, Lluri and colleagues (18) observed that myotube size is inversely correlated to the level of β1 expression. These data support the hypothesis that Achn promotes differentiation in part through downregulation of β1 integrin expression. The formation of fibronectin receptors may be reduced further by the Achn-dependent upregulation of α7 expression. Newly synthesized α7-subunits can compete with α5 for β1 binding partners, which would help form laminin receptors and thus shift cells toward differentiation and away from proliferation.
Following the trigger to differentiate, myoblasts begin to express muscle-specific integrin isoforms (reviewed in Ref. 21). During this process, integrin β1A is replaced by β1D and the widely expressed α7B isoform is replaced by the muscle-restricted α7A isoform. In our analysis, the levels of α7A and β1D mRNAs were below the level of detection despite repeated efforts to increase signal-to-noise ratio. These transcripts were observed in the ectopic Achn-expressing cells 2 and 3 days after transfer to DM, but only with more than 35 cycles of PCR (data not shown). No specific signal was detectable in the other experimental groups, even at these high cycle numbers.
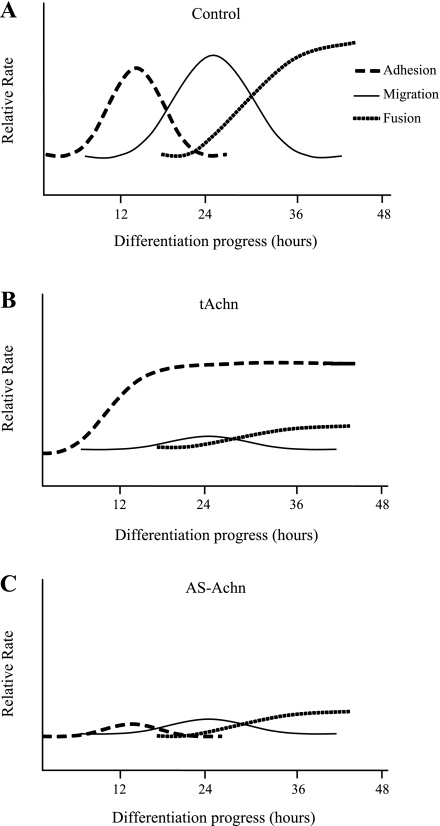
Published data, and our results with control C2C12 cells, suggest the following series of physiological changes in myoblasts when myogenesis is initiated following the loss of growth factors (Fig. 8A). Within hours of transfer to DM, myoblasts increase the expression of both β1 and α7 integrins (Fig. 2). The cells then experience a transient phase of increased adhesion to ECM components like fibronectin and laminin, which resolves by 24 h (Fig. 4). This is followed by a migration phase that peaks at about 24 h after transfer to DM and then returns to baseline by 48 h. This period coincides with the first phase of myotube formation within the cultures and suggests that the majority of motile cells have made contact with either other myoblasts or nascent myotubes. To our knowledge, this is the first description of the temporal relationships of adhesion events in early myogenesis.
Fig. 8.
A: model of adhesion events during early myoblast differentiation. Our data support a model in which cells undergo an adhesion phase that peaks at 12 h following loss of growth factors (dashed line). The resolution of this phase coincides with the initiation of a migratory phase that peaks at 24 h (solid line). Migration in turn declines as cells initiate fusion (dotted line), which results in the conversion of mononucleated myoblasts into multinucleated myotubes. B: expression of truncated Achn alters adhesion dynamics of myoblasts on a laminin substrate. Adhesion increases at 12 h after cells are stimulated to differentiate but remains elevated (dashed line). Both migration (solid line) and fusion (dotted line) are inhibited. C: expression of antisense Achn blocks adhesion (dashed line) as well as migration (solid line) on laminin and also inhibits fusion.
Ectopic Achn appears to have little effect on adhesion and migration on fibronectin when myoblasts are cultured in DM, suggesting that while a reduction of β1 integrin in cells expressing ectopic Achn may affect intracellular signaling, it is not sufficient to affect substrate adhesion. In contrast, ectopic Achn expression had a significant impact on adhesion dynamics on a laminin substrate. We have incorporated these data into our model. Cells expressing tAchn had a modest increase in adhesion at 12 h but did not display either a migration phase on laminin or a subsequent decrease in cell adhesion (Fig. 8B). In contrast, cells expressing AS-Achn displayed neither an adhesion phase nor a migration phase on this substrate (Fig. 8C).
It is well established that when cells initiate migration, they decrease the strength of adhesion to the substrate (20). Migrating cells actively disassemble adhesions in part by deactivation of integrin receptors (reviewed in Refs. 19 and 25). In fact, migration is significantly reduced when integrins are maintained in their high-affinity state (17). Responses of the Achn mutant cells further inform our model and suggest that in order for cells to achieve maximum motility on laminin, they must first upregulate ECM receptors, which results in increased adhesion. We speculate that activation of motility programs leads to the disassembly of adhesion complexes and a decrease the average strength of adhesion. This would explain the correlation between the decline in the adhesion peak and the onset of increased motility. The failure of tAchn cells to become motile is reflected by the persistent maintenance of substrate adhesion. In contrast, the dramatic loss of adhesion in cells expressing ectopic Achn may be due to the combined effects of increased motility and reduced β1 integrin expression. These observations suggest that Achn activity is necessary for cells to become fully motile on laminin, and that the loss of Achn may reduce the ability of myoblasts to contact fusion partners, thus compromising differentiation.
We observed that shortly after switching to differentiation medium, the cells upregulate integrin α7 and displayed enhanced adhesion on laminin. Interestingly, the tAchn cells had the fastest rate of spreading of all the lines tested, while cells expressing ectopic Achn cells had the slowest. These results were unexpected, since cells expressing tAchn contain the lowest levels of α7 integrin, while cells expressing ectopic Achn had α7 integrin levels comparable to that seen in control cells. These data suggest that abundance of specific ECM receptors is only one of the factors influencing cell adhesion dynamics. Other factors may include the organization of the actin cytoskeleton and associated adhesion molecules. We observed that manipulation of Achn expression resulted in alterations in morphology and the structure of the cytoskeleton (Fig. 1). While both AS-Achn and tAchn cells had a similar extended cell shape, the arrangement of their actin was distinct. Expression of tAchn resulted in a cytoskeleton that was less well organized and had few discernible higher-order actin structures. Unbundled actin filaments predominate in this “looser” arrangement, which may facilitate faster and more extensive cell spreading. Expression of ectopic Achn had the opposite effect: the actin was highly bundled into numerous large stress fibers with relatively little unbundled actin. This actin organization may inhibit cell spreading since that process is primarily driven by the polymerization of unbundled actin filaments. We and others have previously observed that bundling of actin filaments inversely correlates with the degree of cell spreading (10, 14). Further investigation will be necessary to elucidate the mechanism(s) and full effects of Achn on the cytoskeleton.
In separate studies we have demonstrated that Achn is a potent regulator of myogenesis (33). The data presented here demonstrate that Achn also regulates integrin expression, which correlates with alterations in cell adhesion, spreading, and migration. These data support the hypothesis that Achn mediates developmental decisions during myogenesis, in part, through its effects on the regulation of cell adhesion dynamics.
GRANTS
This work was supported by grants from the National Institutes of Health, the Collaborative Biomedical Research Program, the Center of Excellence in Apoptosis Research, and the Rays of Hope Foundation.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Randy Kramer (University of California, San Francisco) for providing anti-integrin antisera and Christine Brown and Alex Hirsch for technical assistance.
REFERENCES
- 1.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development 117: 1183–1198, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151: 1221–1234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biressi S, Molinaro M, Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev Biol 308: 281–293, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Blaschuk KL, Guerin C, Holland PC. Myoblast alpha v beta3 integrin levels are controlled by transcriptional regulation of expression of the beta3 subunit and down-regulation of beta3 subunit expression is required for skeletal muscle cell differentiation. Dev Biol 184: 266–277, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Boettiger D, Enomoto-Iwamoto M, Yoon HY, Hofer U, Menko AS, Chiquet-Ehrismann R. Regulation of integrin alpha 5 beta 1 affinity during myogenic differentiation. Dev Biol 169: 261–272, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev 16: 525–532, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F. The formation of skeletal muscle: from somite to limb. J Anat 202: 59–68, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burattini S, Ferri P, Battistelli M, Curci R, Luchetti F, Falcieri E. C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem 48: 223–233, 2004 [PubMed] [Google Scholar]
- 9.Cachaco AS, Pereira CS, Pardal RG, Bajanca F, Thorsteinsdottir S. Integrin repertoire on myogenic cells changes during the course of primary myogenesis in the mouse. Dev Dyn 232: 1069–1078, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Coghill ID, Brown S, Cottle DL, McGrath MJ, Robinson PA, Nandurkar HH, Dyson JM, Mitchell CA. FHL3 is an actin-binding protein that regulates alpha-actinin-mediated actin bundling: FHL3 localizes to actin stress fibers and enhances cell spreading and stress fiber disassembly. J Biol Chem 278: 24139–24152, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Collo G, Starr L, Quaranta V. A new isoform of the laminin receptor integrin alpha 7 beta 1 is developmentally regulated in skeletal muscle. J Biol Chem 268: 19019–19024, 1993 [PubMed] [Google Scholar]
- 12.Cossu G, De AL, Borello U, Berarducci B, Buffa V, Sonnino C, Coletta M, Vivarelli E, Bouche M, Lattanzi L, Tosoni D, Di DS, Berghella L, Salvatori G, Murphy P, Cusella-De Angelis MG, Molinaro M. Determination, diversification and multipotency of mammalian myogenic cells. Int J Dev Biol 44: 699–706, 2000 [PubMed] [Google Scholar]
- 13.Dee K, Freer M, Mei Y, Weyman CM. Apoptosis coincident with the differentiation of skeletal myoblasts is delayed by caspase 3 inhibition and abrogated by MEK-independent constitutive Ras signaling. Cell Death Differ 9: 209–218, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Glenn HL, Jacobson BS. Arachidonic acid signaling to the cytoskeleton: the role of cyclooxygenase and cyclic AMP-dependent protein kinase in actin bundling. Cell Motil Cytoskeleton 53: 239–250, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gullberg D, Ekblom P. Extracellular matrix and its receptors during development. Int J Dev Biol 39: 845–854, 1995 [PubMed] [Google Scholar]
- 16.Hollway GE, Currie Myotome meanderings PD. Cellular morphogenesis and the making of muscle. EMBO Rep 4: 855–860, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol 134: 1551–1562, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lluri G, Langlois GD, Soloway PD, Jaworski DM. Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates myogenesis and beta1 integrin expression in vitro. Exp Cell Res 314: 11–24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lock JG, Wehrle-Haller B, Stromblad S. Cell-matrix adhesion complexes: master control machinery of cell migration. Semin Cancer Biol 18: 65–76, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Lynch L, Vodyanik PI, Boettiger D, Guvakova MA. Insulin-like growth factor I controls adhesion strength mediated by alpha5beta1 integrins in motile carcinoma cells. Mol Biol Cell 16: 51–63, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem 278: 14587–14590, 2003 [DOI] [PubMed] [Google Scholar]
- 22.McDonald KA, Horwitz AF, Knudsen KA. Adhesion molecules and skeletal myogenesis. Semin Cancer Biol 6: 105–116, 1995 [Google Scholar]
- 23.Montarras D, Lindon C, Pinset C, Domeyne P. Cultured myf5 null and myoD null muscle precursor cells display distinct growth defects. Biol Cell 92: 565–572, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Parker MH, Seale P, Rudnicki MA. Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat Rev Genet 4: 497–507, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Robinson PA, Brown S, McGrath MJ, Coghill ID, Gurung R, Mitchell CA. Skeletal muscle LIM protein 1 regulates integrin-mediated myoblast adhesion, spreading, and migration. Am J Physiol Cell Physiol 284: C681–C695, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Samson T, Will C, Knoblauch A, Sharek L, von der Mark K, Burridge K, Wixler V. Def-6, a guanine nucleotide exchange factor for Rac1, interacts with the skeletal muscle integrin chain alpha7A and influences myoblast differentiation. J Biol Chem 282: 15730–15742, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sastry SK, Lakonishok M, Wu S, Truong TQ, Huttenlocher A, Turner CE, Horwitz AF. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J Cell Biol 144: 1295–1309, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz LM, Gao Z, Brown C, Parelkar SS, Glenn H. Cell death in myoblasts and muscles. Methods Mol Biol 559: 313–32, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz LM, Kosz L, Kay BK. Gene activation is required for developmentally programmed cell death. Proc Natl Acad Sci USA 87: 6594–6598, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valavanis C, Wang Z, Sun D, Vaine M, Schwartz LM. Acheron, a novel member of the Lupus Antigen family, is induced during the programmed cell death of skeletal muscles in the moth Manduca sexta. Gene 393: 101–109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von der Mark K, Ocalan M. Antagonistic effects of laminin and fibronectin on the expression of the myogenic phenotype. Differentiation 40: 150–157, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Glenn H, Brown C, Valavanis C, Liu JX, Seth A, Thomas JE, Karlstrom RO, Schwartz LM. Regulation of muscle differentiation and survival by Acheron. Mech Dev 126: 700–709, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Wei Q, Paterson BM. Regulation of MyoD function in the dividing myoblast. FEBS Lett 490: 171–178, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Weng H, Kim C, Valavanis C, Wang Z, Schwartz LM. Acheron, an novel LA antigen family member, binds to CASK and forms a complex with Id transcription factors. Cell Mol Biol Lett 14: 273–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation 7: 159–166, 1977 [DOI] [PubMed] [Google Scholar]
- 37.Yao CC, Ziober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the alpha 7 integrin receptor. J Cell Sci 109: 3139–3150, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Yoshida N, Yoshida S, Koishi K, Masuda K, Nabeshima Y. Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J Cell Sci 111: 769–779, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z, Gruszczynska-Biegala J, Zolkiewska A. ADP-ribosylation of integrin alpha7 modulates the binding of integrin alpha7beta1 to laminin. Biochem J 385: 309–317, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]