Abstract
Gap junction channels are formed by two hemichannels in series (one from each neighboring cell), which are in turn connexin hexamers. Under normal conditions, hemichannels at the plasma membrane are mostly closed but can be opened by changes in membrane voltage, extracellular divalent ion concentration, phosphorylation, pH, and redox potential. Recently, interactions between channels have been found to modulate the activity of several ion channels, including gap junction channels. Here, we studied whether connexin46 (Cx46) hemichannels display such behavior. We studied the response of the Cx46 hemichannels expressed in Xenopus laevis oocytes to consecutive depolarization pulses. Hemichannels formed by wild-type Cx46 and a COOH-terminal domain truncation mutant (Cx46ΔCT) were activated by voltage pulses. When the hemichannels were depolarized repeatedly from −60 mV to +80 mV, the amplitude of the outward and tail currents increased progressively with successive pulses. This phenomenon (“current facilitation”) depended on the amplitude of the depolarization, reaching a maximum at approximately +60 mV in oocytes expressing Cx46, and on the interval between pulses, disappearing with intervals longer than about 20 s. The current facilitation was also present in oocytes expressing Cx46ΔCT, ruling out a primary role of this domain in the facilitation. Nominal removal of divalent cations from the extracellular side caused maximal current activation of Cx46 and Cx46ΔCT hemichannels and prevented facilitation. The results suggest that Cx46 hemichannels show a cooperative activation independent of their COOH-terminal domain.
Keywords: connexins, gap junction channels, cataracts, ion channels, lens, cooperativity
gap junctions allow the flow of ions and hydrophilic molecules of up to ∼1,000 Da between neighboring cells. They are formed by aggregates of intercellular channels (gap junction channels), which in turn are formed by the head-to-head docking of two hemichannels (or connexons), each belonging to one of the two adjacent cells (34). Hemichannels are formed by six connexin subunits, each having four transmembrane helices, with the NH2 and COOH termini on the cytoplasmic side (13; see also Ref. 10). The presence of hemichannels at the plasma membrane has been demonstrated in several cell types (24), and their roles in physiological and pathophysiological processes are the subject of intensive research (reviewed in Refs. 24 and 30). Controlled hemichannel opening has important roles in physiological autocrine/paracrine cell signaling, mediates the release of signaling molecules (24), and participates in processes such as cell-volume regulation (18) and glucose uptake (27). In pathological conditions such as ischemia, massive and prolonged hemichannel opening may induce or accelerate cell death (23), probably due to losses of important metabolites such as ATP, amino acids and reduced glutathione, loss of ion gradients, and entry of Ca2+ (4, 28).
The open probability (Po) of hemichannels is modulated by intracellular and extracellular signals, including plasma membrane voltage (5), extracellular divalent ion concentration (32, 35), cytoplasmic pH (15, 17), phosphorylation (1, 2) and redox potential (19–22). Regulation of ion channel gating involving direct interactions between neighboring ion channels (channel cooperativity) has been described (7–9). Cooperative behavior has been observed in P2X2 (8, 9), acetylcholine receptor (12), and hyperpolarization-activated cyclic nucleotide-gated channels (7), as well as gap junction channels formed by connexin30 (Cx30) (34) and hemichannels formed by Cx32 (11). The cooperative behavior of Cx32 hemichannels reconstituted in lipid bilayers is related to the number of open hemichannels and was observed at both positive and negative voltages (11).
In this study, we found that Cx46 hemichannels present cooperative-like behavior (which we refer to as facilitation) when expressed in Xenopus laevis oocytes subjected to repetitive voltage pulses. Hemichannels formed by wild-type Cx46 or a COOH-terminal domain deletion mutant (Cx46ΔCT) display a progressive increase in the amplitude of outward currents and tail currents (i.e., current facilitation) when depolarized repeatedly. This process was dependent on the amplitude of the depolarization, the interval between pulses, and extracellular Ca2+ concentration ([Ca2+]).
MATERIALS AND METHODS
Plasmids, cRNA preparation, and injection into Xenopus laevis oocytes.
In the present studies, we employed wild-type rat Cx46, provided by Dr. Lisa Ebihara (14), and Cx46ΔCT (truncated after Gly240). Details about the engineering and some properties of wild-type and truncated Cx46 have been published (22). Oocyte preparation, manipulation, cRNA production, and injection into the cells were performed as described (22). Oocytes were studied 24–48 h after injection of 12.5 ng of antisense Cx38 oligonucleotide alone, to reduce Cx38 endogenous expression, or in combination with 25 ng of cRNA coding for Cx46 or Cx46ΔCT.
Electrophysiological recordings and calculations.
Hemichannel currents were measured as described (22) in oocytes bathed at room temperature with ND96 solution (in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, and 5 HEPES/NaOH, pH 7.4). Currents were elicited by rectangular pulses ranging from −60 mV to +80 mV, in 10-mV steps, from a holding voltage of −60 mV. Duration and interval between pulses varied and are indicated in the text and/or figures. In the basic pulse protocol, oocytes were held at −60 mV and depolarized for 1.5 s with a +80-mV pulse. At the end of this depolarizing pulse, voltage was returned to −60 mV, for 2.5 s. This cycle was repeated 25 times.
Statistical analysis.
Results are expressed as means ± SE, and n refers to the number of independent experiments. For statistical analyses, each treatment was compared to its respective control, and significance was determined using a one-way ANOVA or paired Student's t-test, as appropriate. Differences were considered significant at P < 0.05.
RESULTS
Wild-type and chimeric Cx46 show progressive outward current increases in response to repetitive depolarizing pulses.
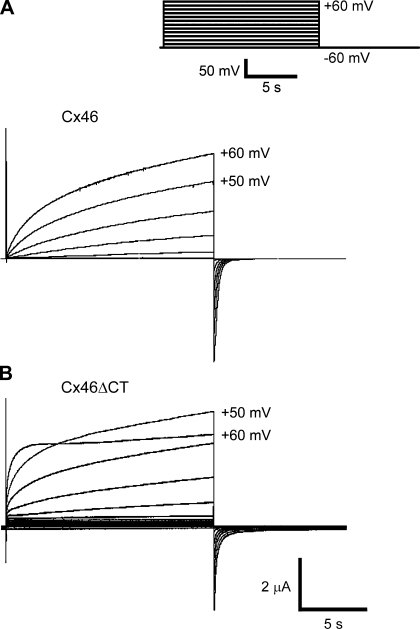
Under control conditions, when Xenopus oocytes expressing Cx46 hemichannels were depolarized from −60 to +60 mV (10 mV steps) for 15 s (Fig. 1A), a slowly activating outward current was observed, followed by a brief tail current upon repolarization to −60 mV (Fig. 1A). In oocytes expressing Cx46ΔCT hemichannels there was a faster initial activation followed by a slower activation at +60 mV (Fig. 1B). At +50 mV, the activation curves of Cx46 and Cx46ΔCT hemichannels were fit by a two-exponential function, yielding the following time constants: for Cx46 (n = 14), 873 ± 54 ms (fast) and 24,536 ± 2,828 ms (slow); for Cx46ΔCT (n = 7), 469 ± 80 ms (fast) and 6,149 ± 913 (slow). Truncation caused statistically significant decreases in the fast and slow time constants. The tail currents were also fit by a two-exponential function, yielding the following time constants: for Cx46 (n = 14), 195 ± 26 ms (fast) and 661 ± 70 ms (slow); for Cx46ΔCT (n = 7), 104 ± 12 ms (fast) and 467 ± 64 (slow). Again, the decreases in the magnitudes of the time constants elicited by the truncation were statistically significant. Therefore, truncation of the COOH-terminal domain accelerates both activation and inactivation of Cx46 hemichannels.
Fig. 1.
Connexin46 (Cx46) hemichannel currents in Xenopus laevis oocytes. A: representative currents obtained in oocytes expressing wild-type Cx46. B: representative currents obtained in oocytes expressing COOH-terminal domain deletion mutant (Cx46ΔCT). Hemichannel currents were measured by whole cell voltage clamp. Inset: pulse protocol for experiments in A and B: holding voltage −60 mV, depolarizing pulses in 10-mV steps, 15-s long, 10-s interval, until +60 mV.
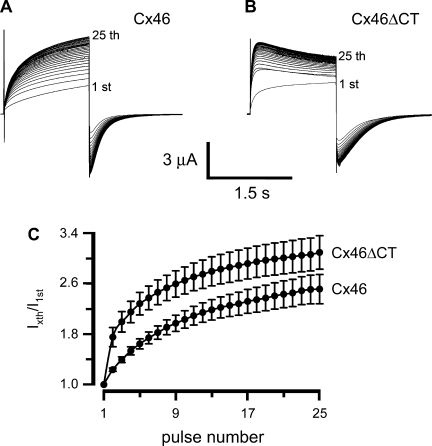
Next, oocytes were depolarized from −60 to +80 mV for 1.5 s, 25 times, with 2.5-s intervals between the pulses (Fig. 2). The consecutive pulses caused a progressive increase in the amplitude of the hemichannel current elicited by depolarization. We refer to this phenomenon as current facilitation. Tail currents also increase in response to consecutive pulses. Oocytes expressing Cx46 hemichannels displayed an outward current with slow activation in response to the first depolarizing pulse (Fig. 2A, 1st). Subsequent pulses elicited progressive increases in the activation rate and amplitude of the currents (Fig. 2A). On the 25th pulse, peak outward current values were 2.5 ± 0.2-fold larger than those in response to the first pulse (Fig. 2C). When the number of pulses increased beyond 25, the current did not increase further (not shown). These data indicate the presence of facilitation induced by successive identical cell membrane depolarizing pulses. The records shown in Fig. 2 and other figures are representative of the number of experiments indicated in the respective legends.
Fig. 2.
Consecutive membrane-depolarizing pulses induce Cx46-hemichannel current facilitation. A: oocytes expressing Cx46 were depolarized 25 consecutive times from −60 to +80 mV (1.5-s-long pulses, 2.5-s intervals). B: same experiment was performed with oocytes expressing Cx46ΔCT. Currents elicited by the 1st and 25th pulses are indicated. C: fractional currents [peak currents after each pulse (Ixth) divided by the current elicited by the first pulse(I1st)] as a function of the number of depolarizing pulses, in oocytes expressing Cx46 (n = 14) and Cx46ΔCT (n = 10). The current amplitudes (Cx46 vs. Cx46ΔCT) were significantly different for pulses 2–15 (P < 0.05). There were no statistical differences for pulses 16–25 (P > 0.05).
In oocytes expressing hemichannels formed by Cx46ΔCT, the response to successive depolarizing pulses was qualitatively similar to that of Cx46 hemichannels (increases in the rate of activation and current amplitude; see Fig. 2B). However, from pulse 4 and on, there was a slow current inactivation following the fast activation (Fig. 2B), as previously observed (27). Figure 2C shows that, relative to the peak currents in response to the first pulse, those following the 25th pulse were 3.1 ± 0.3-fold larger, not different from those elicited in oocytes expressing wild-type Cx46.
Activation and inactivation were fit by two exponentials, which yielded the following time constants: for Cx46 activation, 96 ± 11 ms (fast) and 1,247 ± 170 ms (slow) for the first pulse, and 50 ± 5 ms (fast) and 332 ± 51 ms (slow) for the 25th pulse. First and 25th pulse results were significantly different for both time constants: for Cx46ΔCT activation, 16 ± 3 ms (fast) and 149 ± 4 ms (slow) for the first pulse and 12 ± 2 ms (fast) and 46 ± 4 ms (slow) for the 25th pulse. In this case, only the slow time constant differed significantly.
The time constants for inactivation of the tail currents in oocytes expressing Cx46 were 99 ± 15 (fast) and 330 ± 39 (slow). In oocytes expressing Cx46ΔCT, they were 113 ± 10 (fast) and 267 ± 60 (slow). Both sets of data correspond to the first pulses. The 25th pulse results did not differ significantly.
These results indicate that the facilitation phenomenon is accompanied by a shortening of the fast and slow activation time constants in wild-type Cx46 hemichannels, whereas in Cx46ΔCT hemichannels only the slow time constant is shortened. The inactivation time constants were the same for the 1st and 25th pulses for both Cx46 and Cx46ΔCT hemichannels.
Hemichannel current facilitation depends on the amplitude of the depolarizing voltage.
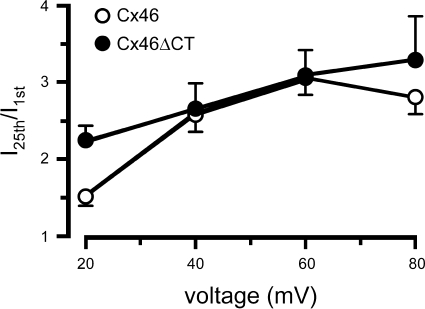
To study the voltage dependence of the progressive hemichannel activation after successive depolarizing pulses, oocytes expressing Cx46 hemichannels were depolarized for 1.5 s, from −60 mV to +20 mV, +40 mV, +60 mV, or +80 mV, 25 consecutive times, with 2.5-s intervals between pulses. Figure 3 shows that depolarizations to +20 mV produced significant increases in the outward currents elicited by consecutive pulses, with a significantly larger effect for Cx46ΔCT (P < 0.01). The average fractional increases in current amplitude elicited by +40-, +60-, and +80-mV pulses were larger than those with 20-mV pulses for both Cx46 and Cx46ΔCT, but the slopes in the range 40–80 mV were not different from zero. These results show a steep voltage dependence of the current facilitation in the 0–20 mV range for Cx46ΔCT and in the 20–40 mV range for Cx46.
Fig. 3.
Hemichannel current facilitation is affected by the amplitude of the depolarizing voltage. Oocytes were depolarized 25 times with 1.5-s pulses at 2.5-s intervals, from a holding voltage of −60 mV to a voltage ranging from +20 to +80 mV. Fractional currents (last pulse/first pulse) are depicted as means ± SE from 10 oocytes expressing Cx46 (○) and 10 oocytes expressing Cx46ΔCT (●). The fractional currents elicited by +20-mV pulses were significantly different (Cx46 vs. Cx46ΔCT, P < 0.005).
Cx46ΔCT hemichannels also displayed voltage dependence of the current facilitation, which was similar to that observed in Cx46 hemichannels. However, at +20 mV the facilitation of Cx46ΔCT was greater than that of Cx46 hemichannels (Fig. 3). These results indicate that although the COOH-terminal domain is not necessary for hemichannel current facilitation, it influences the voltage dependence of the phenomenon at low voltages.
Cx46 hemichannel current facilitation is consistent with a cooperative effect dependent on the number of open hemichannels.
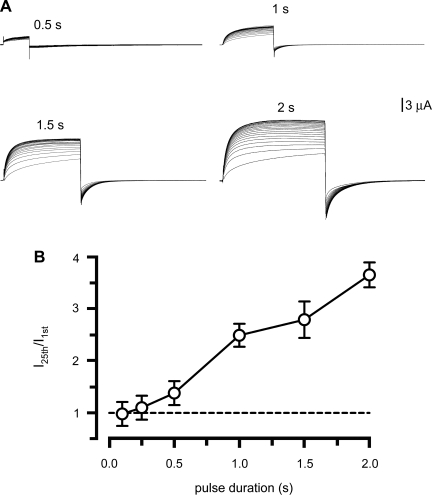
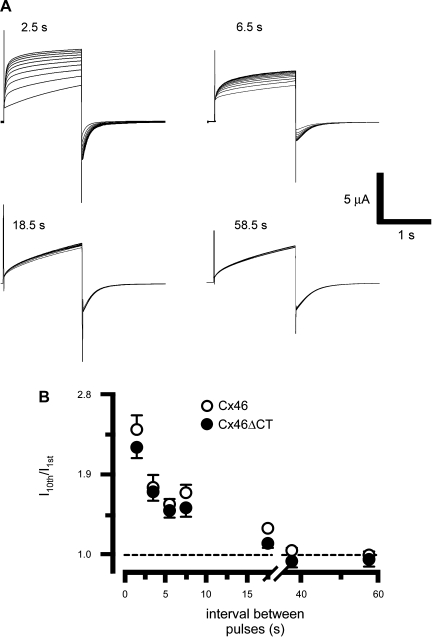
The number of open hemichannels appears to determine the cooperativity of Cx32 hemichannels (11). Since Cx46 hemichannels are slowly activated by positive voltage pulses (Figs. 1 and 2A), during brief pulses many hemichannels will not have enough time to open. To test the hypothesis that Cx46 current facilitation depends on the number of hemichannels open, we studied the effects of +80-mV pulses of varied duration, ranging from 0.1 to 2 s (Fig. 4). As shown in Fig. 2, 1.5-s pulses elicited a clear facilitation, which was enhanced when pulse duration increased, and diminished with shorter pulses (Fig. 4A). These results are summarized in Fig. 4B. There was no significant facilitation with pulses of 0.5 s or less, whereas longer pulses resulted in increasing current facilitation. Repetitive application of 30-s pulses also elicited significant facilitation (not shown). These data support the idea that either the number of activated hemichannels and/or the magnitude of the hemichannel current determine current facilitation. If current facilitation depends on the number of open hemichannels, it is also expected to decrease with the duration of the repolarization to −60 mV between depolarizing pulses. Oocytes expressing Cx46 were depolarized to +80 mV for 1.5 s, for a total of 10 times, and after each depolarizing pulse, they were repolarized to −60 mV for variable times. Fig. 5 shows that when the oocytes were subjected to successive +80-mV pulses for 1.5 s, with 2.5-s interval between pulses, current facilitation was observed (Fig. 5A, 2.5 s). As the interval duration increased, current facilitation decreased (Fig. 5, A and B). Similar results were obtained in oocytes expressing Cx46ΔCT (Fig. 5B). The time constants of the facilitation were 5.6 s for Cx46 and 10.5 s for Cx46ΔCT.
Fig. 4.
The number of activated hemichannels determines the magnitude of the facilitation. A: oocytes expressing Cx46 were depolarized from −60 to +80 mV with pulses of different durations, indicated over the current records. B: fractional currents at different pulse durations (n = 6 each). With pulse durations ≤0.5 s there is no significant facilitation (P > 0.05). At times ≥1 s, current facilitation was observed (P < 0.01 for 0.5 vs. 1 s; P < 0.05 for 1.5 vs. 2 s).
Fig. 5.
The interval between pulses affects hemichannel current facilitation. A: oocytes expressing Cx46 were depolarized 10 times (1.5-s pulses) to +80 mV, from a holding potential of −60 mV, and the time between pulses was changed between 2.5 and 58.5 s. Typical records are shown, with pulse intervals indicated at top. B: Cx46 and Cx46ΔCT hemichannel fractional currents relative to the intervals between pulses (means ± SE, n = 10 for each interval).
Cx46 hemichannel-current facilitation depends on extracellular [Ca2+].
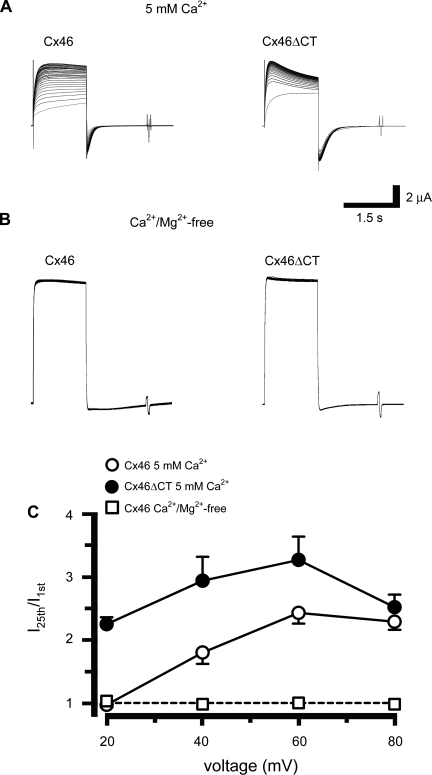
Since extracellular divalent cations block hemichannel currents through decreases in open probability (Po) (35), upon maximal activation by removal of divalent cations, current facilitation by depolarizing pulses will occur only if it involves incorporation of hemichannels to the plasma membrane or activation of hemichannels that cannot be opened by divalent cation removal. Facilitation of Cx46 and Cx46ΔCT hemichannel currents was present at 5 mM extracellular [Ca2+] (Fig. 6A). Cx46 hemichannel currents did not display facilitation at +20 mV, but they did at +40 (P < 0.01 vs. 20 mV) and +60 mV (P < 0.05 vs. 40 mV); with +80 mV there was no additional change (Fig. 6, A and C; compare to Fig. 3). Cx46 facilitation in 1.8 mM [Ca2+] (see Fig. 3) was significantly different (P < 0.001) from that in 5 mM [Ca2+] (Fig. 6C). This indicates that the higher extracellular [Ca2+] displaces the voltage dependence of the facilitation to the right. In contrast, Cx46ΔCT hemichannel currents displayed significant facilitation with +20-mV pulses and there was no additional effect of larger pulses (Fig. 6, B and C). This result indicates that the displacement of the voltage dependence of the facilitation by external Ca requires the presence of the COOH-terminal domain.
Fig. 6.
Cx46 hemichannel current facilitation is affected by extracellular Ca2+ concentration ([Ca2+]). A: oocytes expressing Cx46 (left) or Cx46ΔCT (right) were superfused with ND96 containing 5 mM [Ca2+] and depolarized to +80 mV (examples of n = 5 experiments each). Oocytes were depolarized from −60 to voltages between +20 and +80 mV, 25 times. B: oocytes expressing Cx46 (left) or Cx46ΔCT (right) were depolarized as described in A, but in ND96 nominally free of Ca2+ and Mg2+ (examples of n = 5 experiments each). C: hemichannel fractional currents in oocytes expressing Cx46 (○) or Cx46ΔCT (●) in 5 mM [Ca2+], for all four voltages tested. Exposure of oocytes expressing Cx46 to Ca2+- and Mg2+-free solution abolished hemichannel facilitation (□). A similar result was observed in oocytes expressing Cx46ΔCT (not shown). In 5 mM [Ca2+], the currents observed with Cx46 or Cx46ΔCT differed significantly at +20 mV (P < 0.001) and +40 mV (P < 0.01); there were no differences at +60 or +80 mV (P > 0.05).
At +80-mV depolarization in nominally Ca2+- and Mg2+-free bath solution, Cx46 and Cx46ΔCT hemichannels showed a large outward current with the first pulse, and no facilitation with the following pulses (Fig. 6, B and C). Figure 6C shows that there was no facilitation in the nominal absence of divalent cations, independently of the voltage applied. The simplest explanation for these results is that removal of divalent cations by itself elicits maximal activation of hemichannels at the plasma membrane, thus preventing facilitation. Therefore, facilitation occurs through activation of hemichannels responsive to Ca2+.
DISCUSSION
Junctional currents between paired oocytes expressing Cx32 decreased progressively with successive depolarizing pulses of the same voltage, with recovery over several minutes (16). The kinetics of the effects of repetitive pulses was remarkably similar to that observed by us on Cx46 hemichannels, but the effect of the repetitive voltage pulses was opposite in direction (16). Cx32 hemichannels reconstituted in planar bilayers undergo complex relaxation upon positive-voltage pulses (11). Of the three time constants detected, the slowest was shortened by increases in the number of channels, suggesting a cooperative effect mediated by intermolecular interactions. In studies of Cx30 gap junction channels in HeLa cells it was found that the rate of inactivation is slower in single channels vs. channel assemblies (34), also suggesting a role of intermolecular interactions in the regulation of gap junction channels.
Our data show that Cx46 hemichannel currents display facilitation in response to consecutive depolarizing pulses. This phenomenon was independent of the Cx46 COOH-terminal domain since it was also observed in oocytes expressing hemichannels formed by the COOH-terminal domain truncation mutant Cx46ΔCT. Although the COOH terminus is not necessary for the facilitation, its presence influences the voltage dependence of the phenomenon; i.e., maximum currents after 25 depolarizing pulses are reached at different voltages in oocytes expressing wild-type Cx46 and Cx46ΔCT hemichannels. There were also differences in the kinetics of activation of Cx46 and Cx46ΔCT hemichannels. With Cx46, both fast and slow activation time constants were shortened, whereas in Cx46ΔCT hemichannels, repetitive pulses shortened only the slow time constant. The presence of current facilitation in Cx46ΔCT hemichannels rules out phosphorylation of the COOH-terminal domain or oxidation COOH-terminal domain Cys as essential for the facilitation.
In other studies of gap junction channels or hemichannels, no such facilitation was observed. Gap junction channels in rat Schwann cells gate independently of each other (6). Furthermore, a comparative analysis of single channels and ensembles of Cx50 gap junction channels is consistent with a homogeneous population of noninteracting channels (31). Hence, there are clear instances in which no voltage facilitation of gap junction channels is observed, indicating that this phenomenon is isoform specific. Studies with chimeric channels of isoforms that display and do not display facilitation will be of interest to identify the domain(s) responsible for the phenomenon.
The present studies indicate that a voltage facilitation phenomenon is present in Cx46 hemichannels and that it is modulated by factors known to affect Cx46 hemichannel gating. Our results also support the notion that Cx46 hemichannel current facilitation depends on the number of open hemichannels, as has been suggested for Cx32 hemichannels (11). In response to +20-mV pulses, the number of open Cx46 hemichannels is low and facilitation is absent. At voltage pulses at which the hemichannels are open more frequently, facilitation is clearly apparent. Consistent with the dependence of current facilitation on the number of open hemichannels, it increases with pulse duration and decreases with lengthening of the interval between pulses. A practical consequence of this result is that voltage pulses must be applied at intervals of at least 20 s to study the voltage response of Cx46 hemichannels in the absence of voltage-dependent facilitation. The molecular mechanism underlying the facilitation remains to be determined. A possibility is cooperativity between hemichannels, but another possibility is a signaling mechanism, such as a change in intracellular [Ca2+], a possibility supported by studies of the effects of osmotic swelling of astrocytes on junctional conductance (25).
Opening of Cx46 hemichannels induces or accelerates gap junction formation (34), and hence facilitation could enhance this phenomenon. This may be a protective mechanism from the effect of hemichannel opening on the cells (26). In the case of progressive activation, a number of hemichannels would move to intercellular adhesion regions to form gap junction channels, which are insulated from the extracellular space.
GRANTS
This work was supported by National Institutes of Health Grants GM068586 and GM79629, Texas Advanced Research Program Grant 010674-0046-2007, Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) Grant de Iniciación 11080061, and a Clinical and Basic Science Seed Grant from the Texas Tech University Health Sciences Center School of Medicine.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Catherine F. Hamilton for technical support and Carolina Larraín for comments on the manuscript. We also thank Dr. Lisa Ebihara for providing the wild-type Cx46 DNA.
REFERENCES
- 1.Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem 279: 20058–20066, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bao X, Lee SC, Reuss L, Altenberg GA. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc Natl Acad Sci USA 104: 4919–4924, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beahm DL, Hall JE. Opening hemichannels in nonjunctional membrane stimulates gap junction formation. Biophys J 86: 781–796, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 29: 268–275, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bukauskas FF, Verselis VK. Gap junctions channel gating. Biochim Biophys Acta 1662: 42–60, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanson M, Chandross KJ, Rook MB, Kessler JA, Spray DC. Gating characteristics of a steeply voltage-dependent gap junction channel in rat Schwann cells. J Gen Physiol 102: 925–946, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekker JP, Yellen G. Cooperative gating between single HCN pacemaker channels. J Gen Physiol 128: 561–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding S, Sachs F. Evidence for non-independent gating of P2X2 receptors expressed in Xenopus oocytes. BMC Neurosci 3: 17–28, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujiwara Y, Kubo Y. Density-dependent changes of the pore properties of the P2X2 receptor channels. J Physiol 558: 31–43, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem 65: 475–502, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Ghosh P, Ghosh S. Studies on collective behaviour of gap junction channels. Bioelectrochemistry 68: 150–157, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Keleshian AM, Edson RO, Liu GJ, Madsen BW. Evidence for cooperativity between nicotinic acetylcholine receptors in patch clamp records. Biophys J 78: 1–12, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3,5 Å resolution. Nature 258: 597–602, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115: 1077–1089, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peracchia C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim Biophys Acta 1661: 61–80, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Peracchia C, Salim M, Peracchia LL. Unusual slow gating of gap junction channels in oocytes expressing connexin32 or its COOH-terminus truncated mutant. J Membr Biol 215: 161–168, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Peracchia C, Young KC, Wang XG, Chen JT, Peracchia LL. The voltage gates of connexin channels are sensitive to CO2. Cell Commun Adhes 10: 233–237, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol 148: 1063–1074, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Retamal MA, Cortés CJ, Reuss L, Bennett MV, Sáez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103: 4475–4480, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27: 13781–13792, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Retamal MA, Schalper KA, Shoji KF, Bennett MV, Sáez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci USA 104: 8322–8327, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Retamal MA, Yin SY, Altenberg GA, Reuss L. Modulation of Cx46 hemichannels by nitric oxide. Am J Physiol Cell Physiol 296: C1356–C1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sáez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MVL. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand 179: 9–22, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MVL. Connexin-based gap junction hemichannels: gating mechanism. Biochim Biophys Acta 1711: 215–224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scemes E, Spray DC. Increased intercellular communication in mouse astrocytes exposed to hyposmotic shocks. Glia 24: 74–78, 1998 [PMC free article] [PubMed] [Google Scholar]
- 26.Schalper KA, Orellana JA, Berthoud VM, Sáez JC. Dysfunctions of the diffusional membrane pathways mediated by hemichannels in inherited and acquired human diseases. Curr Vasc Pharmacol 7: 486–505, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Schalper KA, Palacios-Prado N, Orellana JA, Sáez JC. Currently used methods for identification and characterization of hemichannels. Cell Commun Adhes 15: 207–218, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Schalper KA, Palacios-Prado N, Retamal MA, Shoji KF, Martínez AD, Sáez JC. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol Biol Cell 19: 3501–3513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, Jiang JX. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem 283: 26374–26382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia 54: 758–773, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J Physiol 517: 673–689, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277: 10482–10488, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Unwin PN, Zampighi GA. Structure of the junction between communicating cells. Nature 283: 545–549, 1980 [DOI] [PubMed] [Google Scholar]
- 34.Valiunas V, Weingart R. Co-operativity between mouse connexin30 gap junction channels. Pflügers Arch 441: 756–760, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol 132: 315–327, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]