Abstract
Skeletal muscle contraction is reputed not to depend on extracellular Ca2+. Indeed, stricto sensu, excitation-contraction coupling does not necessitate entry of Ca2+. However, we previously observed that, during sustained activity (repeated contractions), entry of Ca2+ is needed to maintain force production. In the present study, we evaluated the possible involvement of the canonical transient receptor potential (TRPC)1 ion channel in this entry of Ca2+ and investigated its possible role in muscle function. Patch-clamp experiments reveal the presence of a small-conductance channel (13 pS) that is completely lost in adult fibers from TRPC1−/− mice. The influx of Ca2+ through TRPC1 channels represents a minor part of the entry of Ca2+ into muscle fibers at rest, and the activity of the channel is not store dependent. The lack of TRPC1 does not affect intracellular Ca2+ concentration ([Ca2+]i) transients reached during a single isometric contraction. However, the involvement of TRPC1-related Ca2+ entry is clearly emphasized in muscle fatigue. Indeed, muscles from TRPC1−/− mice stimulated repeatedly progressively display lower [Ca2+]i transients than those observed in TRPC1+/+ fibers, and they also present an accentuated progressive loss of force. Interestingly, muscles from TRPC1−/− mice display a smaller fiber cross-sectional area, generate less force per cross-sectional area, and contain less myofibrillar proteins than their controls. They do not present other signs of myopathy. In agreement with in vitro experiments, TRPC1−/− mice present an important decrease of endurance of physical activity. We conclude that TRPC1 ion channels modulate the entry of Ca2+ during repeated contractions and help muscles to maintain their force during sustained repeated contractions.
Keywords: calcium, canonical transient receptor potential 1, fatigue
it is well known that adult skeletal muscle fibers do not exchange much Ca2+ with the extracellular medium during contraction. Their sarcoplasmic reticulum (SR) is rich in sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pumps and has a high buffering capacity, mostly due to calsequestrin. Ca2+ extrusion through the plasma membrane is very slow (reviewed in Ref. 54). Therefore, almost all the Ca2+ released from the SR is rapidly restored after stimulation, and for that reason muscle fibers can twitch for hours in the absence of extracellular Ca2+ (8). However, evaluations of Ca2+ influx with the 45Ca2+ uptake technique indicate that each twitch contraction induces a small increase of Ca2+ entry (13).
In a previous study, we showed (24) that a decrease on the order of 30% of the Ca2+ content in the SR store is sufficient to induce an entry of Ca2+ through the so-called store-operated channels. Interestingly, it has been found that a single action potential triggers the release of 0.2–0.3 mM Ca2+ from the SR into the cytoplasm (10), which corresponds to more than a quarter of the SR Ca2+ content (27). In addition, electrically stimulated isotonic contractions of soleus muscle led to a 30-fold increase of 45Ca2+ uptake compared with resting muscle (36). Thus it seems reasonable to assume that store-operated channels are indeed activated during physiological contractions.
In previous work, we have shown that store-operated channels are present in skeletal muscles and that their activity is abnormally increased in Duchenne muscular dystrophy (71), a myopathy due to the lack of dystrophin, a cytoskeletal protein, leading to a perturbation of Ca2+ homeostasis (30–32, 34, 58, 69).
Using a RNA antisense strategy targeted to the transient receptor potential (TRP) box motif [EWKFAR motif found at the COOH terminus, close to the last transmembrane domain in canonical TRP (TRPC) isoforms and modified in melastatin TRP (TRPM) and TRP vanilloid (TRPV) isoforms], we proposed that these channels, abnormally regulated in dystrophic fibers, might be constituted of TRP channel isoforms. In particular, TRPC1 was shown to be localized close to the plasma membrane and to interact with α1-syntrophin that may anchor the store-operated channels to the dystrophin-associated protein complex (70, 71). The role of TRPC1 in Duchenne muscular dystrophy might also involve its interactions with caveolin-3, Src kinase, and Homer proteins (Refs. 35, 67; reviewed in Ref. 1). Several reports propose that TRPC1 channel might constitute a component of store-operated channels (for review, see Refs. 6, 7, 11, 62), possibly associated with another channel called Orai1 (28, 44, 65), and with STIM1, an intrareticular Ca2+ sensor (7, 38, 47, 74, 75). However, this is challenged by different reports showing that store-operated entry of Ca2+ is orchestrated essentially by STIM and Orai1 functioning independently of TRPC channels (Refs. 21, 50; for review, see Ref. 19). In skeletal muscle, this entry seems to occur essentially through the transverse tubule system, a situation that involves molecular agonists distributed throughout junctional membranes (12, 45).
Recently, Lyfenko and Dirksen (50) showed that a Ca2+ entry pathway distinct of the store-operated pathway exists in skeletal muscle (reviewed in Ref. 23). This entry is activated by depolarization. The molecular players have not yet been identified, but this pathway does not involve STIM1 and Orai1.
In this paper, we present new evidence in order to try to evaluate the real importance of TRPC1 channel in the influx of Ca2+ in skeletal muscle and to investigate its role in muscle function. We thus characterized Ca2+ currents and intracellular Ca2+ concentration ([Ca2+]i) transients as well as mechanical function in vitro and in vivo in TRPC1+/+ and TRPC1−/− mice.
MATERIALS AND METHODS
TRPC1+/+ and TRPC1−/− Mice
Generation of TRPC1−/− mice has been described previously (22). TRPC1−/− and TRPC1+/+ were obtained from heterozygous animals. TRPC1−/− were compared with their TRPC1+/+ control sex-matched littermates. TRPC1−/− mice were viable and fertile, with normal litter sizes compared with TRPC1+/+ mice. They appeared to be healthy, had a normal life span, and did not present any signs of major neurological disorder or metabolic disease.
Expression of TRPC Channels
For mRNA quantification, tibialis anterior, extensor digitorum longus (EDL), and soleus muscles were extracted with the Ribopure kit (Ambion) and reverse-transcribed with SuperScript II RNase H (Invitrogen). Gene-specific PCR primers were designed with Primer3 (http://biotools.umassmed.edu/bioapps/primer3_www.cgi). To avoid amplification of genomic DNA, primers were chosen in different exons. All primers (Table 1) were purchased from Eurogentec. The cyclophilin D housekeeping gene and the genes of interest were amplified in parallel. Real-time RT-PCR was performed with 5 μl of cDNA, 12.5 μl of SYBR Green Mix (Bio-Rad), and each primer at 300 nM in a total reaction volume of 25 μl. The reaction was initiated at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 1 min, and extension at 72°C for 10 s.
Table 1.
Sequences of oligonucleotide primers
| Gene | GenBank Accession No. | Primer | 5′–3′ Sequence |
|---|---|---|---|
| TRPC1 | NM_011643 | Fwd | CAGCCTCAGACATTCCAGGT |
| Rev | CTCCACAAGGCTGAGTTCCT | ||
| TRPC2 | NM_011644 | Fwd | ACTTCCTGGACGTGGTCATC |
| Rev | CTGAGCATGCTGGTGACAGT | ||
| TRPC3 | NM_019510 | Fwd | TGGATTGCACCTTGTAGCAG |
| Rev | ACGTGAACTGGGTGGTCTTC | ||
| TRPC4 | NM_016984 | Fwd | AAGCCAAGTGGAGAGAAGCA |
| Rev | ATCCGAGCTGGAGACACACT | ||
| TRPC5 | NM_009428 | Fwd | GCTGAAGGTGGCAATCAAAT |
| Rev | AAGGTTGCTTCTGGGTGAGA | ||
| TRPC6 | NM_013838 | Fwd | CCTCCCTAATGAAACCAGCA |
| Rev | GATTGCCAGCATTCCAAAGT | ||
| TRPC7 | NM_012035 | Fwd | CTGTGAAAACGACCGGAAAT |
| Rev | CCCTTCATTGCTTCATCGTT | ||
| Cyclophilin D | BC019778 | Fwd | CGTCCAGATGAGGAGTCGGA |
| Rev | TAAGCATGATCGGGAGGGTT |
TRPC, canonical transient receptor potential; Fwd, forward; Rev, reverse.
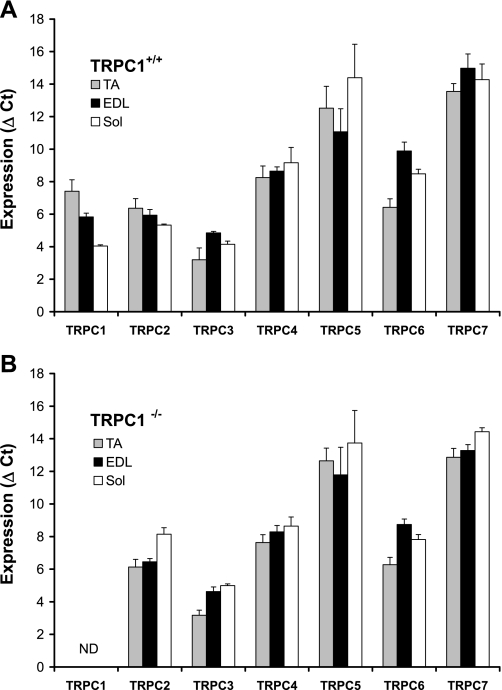
Data were recorded on a MyiQ Real-Time PCR Detection System (Bio-Rad), and cycle threshold (Ct) values for each reaction were determined with analytical software from the same manufacturer. Each cDNA was amplified in duplicate, and Ct values were averaged for each duplicate. The average Ct value for cyclophilin D was subtracted from the average Ct value for the gene of interest (Fig. 1).
Fig. 1.
Quantification of canonical transient receptor potential (TRPC) channels expression in tibialis anterior (TA), extensor digitorum longus (EDL), and soleus (Sol) muscles from TRPC1+/+ and TRPC1−/− mice. A: TRPC1+/+ mice. Each cDNA was amplified in duplicate, and threshold cycle (Ct) values were averaged for each duplicate. The average Ct value for cyclophilin D was subtracted from the average Ct value for the gene of interest, giving ΔCt (n = 5 or 6). Note that gene expression is inversely proportional to ΔCt. B: TRPC1−/− mice. ΔCt values were obtained from TRPC1−/− samples (n = 4–6). ND, not detected.
Muscle Mechanical Measurements
Ten- to sixteen-week old TRPC1+/+ and TRPC1−/− mice were deeply anesthetized by intraperitoneal injection of a solution containing ketamine (10 mg/ml) and xylazine (1 mg/ml) in order to preserve muscle perfusion during dissection of both soleus and EDL muscles. Depth of anesthesia was assessed by the abolition of eyelid and pedal reflexes.
After dissection, the animals were killed by rapid neck dislocation. This protocol has been approved by the Animal Ethics Committee of the Catholic University of Louvain.
Soleus and EDL muscles were bathed in a 1-ml horizontal chamber continuously superfused with HEPES-buffered Krebs solution (100% O2) containing (in mM) 135.5 NaCl, 5.9 KCl, 1 MgCl2, 2.5 CaCl2, 11.6 HEPES sodium, and 11.5 glucose, maintained at a temperature of 20 ± 0.1°C. One end of the muscle was tied to an isometric force transducer and the other end to an electromagnetic motor and length transducer (52). Stimulation was delivered through platinum electrodes running parallel to the muscles. Resting muscle length (L0) was carefully adjusted for maximal isometric force with 100-ms (EDL) or 300-ms (soleus) maximally fused tetani. Force was recorded on a high-speed pen recorder (Sanborn model 320) and digitalized at a sampling rate of 1 KHz with a PCI 6023E i/o card (National Instruments). Normalized stress was expressed relative to cross-sectional area, obtained by multiplying absolute force by the quotient muscle fiber length (mm)/muscle blotted weight (mg) and considering the fiber length equal to L0 for soleus and equal to 0.5 × L0 for EDL (15).
To investigate muscle fatigue, soleus muscles were subjected to 50-Hz stimulation trains of 500-ms duration at 1-s intervals (50% duty cycle) over 2 min.
Adult Muscle Fiber Dissociation
Twelve- to sixteen week-old mice were killed by cervical dislocation. All procedures involving animals were performed in compliance with the Swiss Federal Veterinary Office's guidelines, based on the Swiss Federal Law on Animal Welfare, and were approved by the Cantonal Veterinary Office and by the Animal Ethics Committee of the Catholic University of Louvain. Flexor digitorum brevis (FDB) muscles were removed and incubated for 36 min at 37°C in Krebs solution containing 0.2% collagenase type IV (Sigma). The muscles were removed from this solution, washed twice in Krebs buffer, and suspended in F-12/DMEM supplemented with 2% fetal bovine serum and 1% penicillin-streptomycin (Sigma). Single fibers were mechanically dissociated by passing the muscle repeatedly through fire-polished Pasteur pipettes. Dissociated fibers were plated onto tissue culture dishes coated with extracellular matrix basement membrane (Harbor Bio-products) and allowed to adhere to the bottom of the dish for 2 h.
Muscle Protein Extraction
Muscle protein extraction was done as previously described (64). Briefly, whole EDL muscle was homogenized with Ultraturrax (IKA-Labortechnik, Staufen, Germany) in 500 μl of ice-cold lysis buffer containing 50 mM Tris · HCl (pH 7.5), 1 mM EDTA, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Homogenates were centrifuged 10 min at 10,000 rpm (Sorvall SS-34 rotor) to pellet myofibrillar proteins. These were resuspended in 8 M urea-50 mM Tris · HCl, pH 7.5. The supernatant contained soluble proteins. Myofibrillar and soluble muscle protein contents were determined by using the Bradford protein assay (Bio-Rad, Munich, Germany).
Myosin Heavy Chain Isoform Distribution
Myosin heavy chain (MHC) isoforms were separated as described previously (56) with only slight modifications. Muscles were frozen in liquid nitrogen, ground to powder, and homogenized in SDS solution containing (in mM) 40 dithiothreitol, 5 EDTA, and 100 Tris · HCl (pH 8) with 10% (wt/vol) SDS. Protease inhibitors (Complete, Roche) were added to homogenates. Samples were heated at 100°C for 3 min. For electrophoresis, the separating gel consisted of 30% (vol/vol) glycerol, 8% (wt/vol) acrylamide-N,N′-methylenebisacrylamide (Bis; 99:1), 0.2 M Tris · HCl (pH 8.8), 0.1 M glycine, 0.4% (wt/vol) SDS, 0.1% (wt/vol) ammonium persulfate, and 0.05% (vol/vol) N,N,N′,N′-tetramethylethylenediamine (TEMED). The stacking gel consisted of 30% (vol/vol) glycerol, 4% (wt/vol) acrylamide-Bis (50:1), 70 mM Tris · HCl (pH 6.7), 4 mM EDTA, 0.4% (wt/vol) SDS, 0.1% (wt/vol) ammonium persulfate, and 0.05% (vol/vol) TEMED. The lower running buffer consisted of 0.05 M Tris (base), 75 mM glycine, and 0.05% (wt/vol) SDS. The upper running buffer was at six times the concentration of the lower buffer, but 0.12% (vol/vol) 2-mercaptoethanol was added. Electrophoresis was performed at a constant voltage of 60 V for 22 h, except for the first 40 min (stacking gel penetration), when the current was limited to 10 mA. Bands were scanned and quantified by densitometry.
Histology
Three- and twelve week-old mice were chosen for histological investigations. Both soleus and EDL muscles were dissected, fixed in 10% formalin-PBS on ice for 4 h, embedded in paraffin, and sectioned. Sections were deparaffinated with xylol, rehydrated with alcohol, fixed in 1% acid alcohol (1% acetic acid in ethanol), and air dried. After incubation for 3 min with acidic hemalun, plates were rinsed, treated for 30 s with saturated lithium carbonate, rinsed, and counterstained with 1% eosin. After dehydration with ethanol and xylol, sections were mounted with Entellan (Merck). The size of muscle fiber sections was measured with a homemade planimetry program.
Patch-Clamp Recordings
Cell-attached patch-clamp recordings were performed at room temperature on individual fibers isolated as described above. Fibers were pretreated with 30 μM N-benzyl-p-toluene sulfonamide (BTS) for 10 min to inhibit contractions (18) and immersed in a high-K+ solution composed of (in mM) 10 NaCl, 142 KCl, 2 MgCl2, 0.2 CaCl2, 5 glucose, 0.5 EGTA, and 10 HEPES (pH 7.3). Cells immersed in this solution had resting membrane potentials of 0 ± 2 mV, as verified by measuring membrane potential with sharp electrodes as described previously (n = 6) (40). Patch pipettes contained (in mM) 110 CaCl2, 10 HEPES (pH 7.3) supplemented with 2 mM tetraethylammonium (TEA), 20 μM 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS), and 1 μM nifedipine to inhibit K+, Cl−, and L-type Ca2+ currents. When needed, 100 μM 2-aminoethoxydiphenyl borate (2-APB; Alexis, Lausen, Switzerland) was added to the solution. Pipettes were pulled with a horizontal puller P2000 (Sutter Instrument, Novato, CA) and had a resistance of 3–5 MΩ. Electric currents were acquired and low-pass filtered at 2 kHz with a Multiclamp 700B amplifier and then digitized with a Digidata 1322 digitizer (Molecular Devices, Sunnyvale, CA) at 10 kHz.
Electrical properties of the channels were studied by measuring activity at series of potentials from −60 to +40 mV in steps of 20 mV, and single-channel currents at these potentials were measured by producing all-point histograms in the regions of TRPC1 activity and performing Gaussian fits to the established peaks. Store-dependent Ca2+ entry was studied by measuring currents at the holding potential of −40 mV; first basal activity was acquired for 5 min, then bath solution was replaced with equivalent high-K+ solution containing 1 μM thapsigargin, and the data acquisition was continued for an additional 20 min. Acquired currents were analyzed with the help of the single-channel search algorithm of the Clampex-10 program with conductance levels set to correspond to typical single-channel currents of TRPC1 or higher-conductance channel observed in patches (typically 0.7 or 2 pA correspondingly at the holding potential of −40 mV), yielding channel activity (NPo) values that were then used to calculate average currents corresponding to a particular mode of Ca2+ entry. Mechanosensitive properties of TRPC1 channels were studied in cell-attached patches held at −40 mV with the suction protocol consisting of steps to suction levels varying from 0 to −100 mmHg in steps of 10 mmHg, applied for 0.5 s, followed by a period of relaxation of 2.5 s, with an HSPC-1 instrument (ALA Scientific Instruments, Westbury, NY) and analyzed similarly to store-dependent currents.
Measurements of [Ca2+]i and Influx of Ca2+
Muscle fibers were loaded for 1 h at room temperature with the membrane-permeant Ca2+ indicator fura-PE3/AM (1 μM) and Pluronic F-127 (0.004%). Before electrical stimulation, they were incubated in the presence of BTS (30 μM) to prevent contraction (18). Cells were illuminated through an inverted Nikon microscope (×40 magnification objective) alternatively at 340 and 380 nm, and the fluorescent light emitted at 510 nm was measured with a Deltascan spectrofluorimeter (Photon Technology International). The ratio R340/380 of the fluorescence intensity emitted at the two excitation wavelengths was calculated, and [Ca2+]i was determined with a previously described calibration (71).
To measure the influx of Ca2+ into muscle fibers, 1 mM MnCl2 was added to the Krebs medium and the influx of Mn2+ through Ca2+ channels was evaluated by the quenching of fura-PE3 fluorescence excited at 360 nm, i.e., at the isosbestic point. The rate of quenching reflected the rate of Ca2+ entry (33, 55). Simultaneously, the ratio of fluorescence obtained with 340-nm and 360-nm excitation wavelengths (R340/360) evaluated [Ca2+]i. Mn2+ entry was first measured at rest and then after Ca2+ was released from the stores with 20 mM caffeine and 1 μM thapsigargin (14).
In Vivo Experiments
Running wheel.
Each mouse was individually housed in a standard mouse cage containing a low-inertia running wheel. Wheel-running distances were measured during the active part of the day (from 6 PM to 6 AM).
Escape test.
For these experiments, the method initially devised by Carlson and Makiejus (16) was applied. Briefly, 4-mo-old mice were placed in front of a tube, and a cuff was wrapped around the tail and connected to a fixed force transducer. In response to gentle pinching of the tail, the mice tried to escape into the tube; this was prevented by the attachment of the tail to the force transducer, and a short peak of force was recorded. The procedure was repeated at short intervals over 2.5 min. The mean of the five highest peaks recorded was calculated for each animal and divided by its body weight (“total body force,” mN/g).
Forced treadmill exercise.
The mice were placed on a homemade treadmill to run with an uphill inclination of 30° at a speed of 5 m/min for 5 min, followed by a progressive increase in speed of 1 m/min every minute up to 17 m/min. The back of the treadmill was equipped with a grid that discharged a mild electrical current, a stimulus aimed at motivating the animal to keep running on the treadmill. The test was stopped when the mouse remained on the shocker plate for 20 s without attempting to reengage the treadmill, and the time to exhaustion was determined.
Wire test protocol.
In this test, animals were suspended by their forelimbs from a 1.5-mm-thick, 60-cm-long metallic wire at 45 cm above soft ground (59). The time until the mouse completely released its grasp and fell down was recorded. Three trials were performed per session, with a 30-s recovery period between trials. The maximum time per trial was set to 180 s. For each mouse, the scores of the three trials were averaged.
Statistics
Data are presented as means ± SE. ANOVA and Student's t-tests and χ2, Pearson's test were used to determine statistical significance.
RESULTS
Expression of TRPC1 and Ca2+ Fluxes in Muscle Fibers
We previously reported (49) that in myoblasts TRPC1 is by far the most widely expressed channel of the TRPC subfamily (at least 100 times more than other isoforms). In adult muscle fibers, we observed that TRPC1, TRPC2, and TRPC3 were the most abundantly expressed TRPC isoforms. It is important to note that in soleus muscle TRPC1 was the most expressed TRPC isoform, whereas this expression was a little bit weaker in EDL and tibialis anterior, where TRPC3 was the predominant isoform. As shown previously, TRPC5 and TRPC7 isoforms are less abundantly expressed (Fig. 1A) (71).
In several investigations, TRPC1 has been reported to be responsible for store-operated Ca2+ entry. In a recent study, we indeed confirmed that repression of TRPC1 in C2C12 myoblasts reduced the store-operated Ca2+ entry (49). Here, we investigated the role of TRPC1 by using TRPC1 knockout mice. The abolition of TRPC1 expression was confirmed by PCR, which showed the absence of TRPC1 mRNA in skeletal muscles of TRPC1 knockout mice. We found that in these muscles TRPC isoforms other than TRPC1 presented an expression pattern that was similar to that observed in muscles from TRPC1+/+ mice (Fig. 1B). To study the role of TRPC1 on Ca2+ fluxes, we evaluated the entry of Ca2+ by microspectrofluorometry and measured Ca2+ currents by the patch-clamp technique in muscle fibers isolated from FDB muscles.
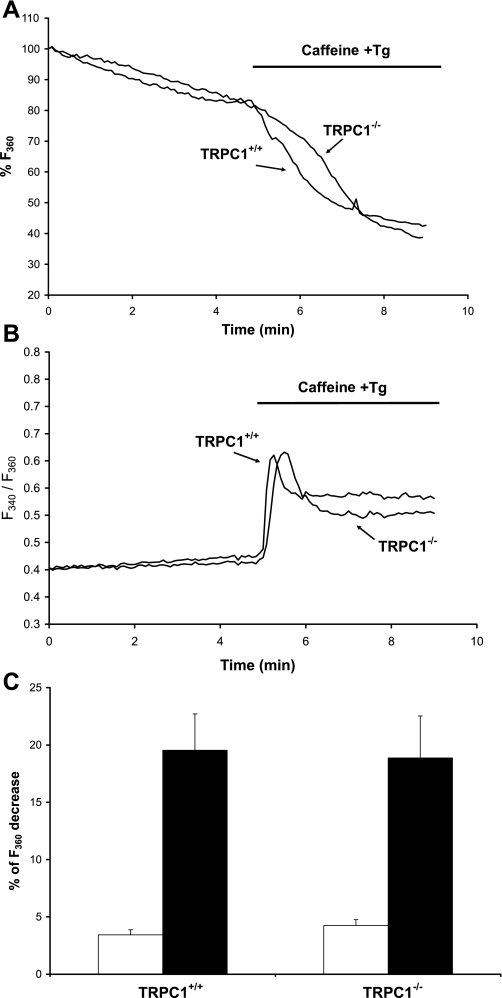
Ca2+ influx was monitored by the Mn2+ quenching of fura-PE3 fluorescence. Mn2+ entry was first measured at rest, and then the release of Ca2+ from the reticulum was triggered by stimulation with 20 mM caffeine and 1 μM thapsigargin (used to activate ryanodine receptors and to inhibit SERCA pumps, respectively) (Fig. 2, A and B). We observed that the rate of quenching of fura-PE3 was similar in muscle fibers from TRPC1+/+ and TRPC1−/− mice at rest. Depletion of the stores by stimulation with thapsigargin significantly increased the quenching rate in both TRPC1+/+ and TRPC1−/− fibers (2-way ANOVA, P < 0.05), but no difference was observed between TRPC1+/+ and TRPC1−/− fibers (Fig. 2C), suggesting that TRPC1 is not indispensable for store-operated entry of Ca2+ and does not play a major role in the basal influx of Ca2+ (at least through Mn2+-permeable Ca2+ channels). In addition, we verified that FDB muscles from TRPC1−/− mice did not change their expression of Stim1 and Orai1, two well-known proteins involved in store-operated influx of Ca2+ (data not shown).
Fig. 2.
Store-operated entry of Ca2+ in muscle fibers from TRPC1+/+ and TRPC1−/− mice. A and B: Ca2+ release from the stores was triggered by stimulation with 20 mM caffeine and 1 μM thapsigargin (Tg) in the presence of 1 mM Mn2+. This induced an increase of intracellular Ca2+ concentration ([Ca2+]i) (reflected by an increase of the fluorescence ratio F340/F360; B) and an increase of Ca2+ entry (reflected by an increase of the quenching rate of fura-PE3 by Mn2+; A). C: comparison of fura-PE3 quenching rates by Mn2+ at rest (gray bars) and after Ca2+ release induced by caffeine and Tg stimulation (black bars representing difference between fura-PE3 quenching rates after and before stimulation) in muscle fibers from TRPC1+/+ (n = 13) and TRPC1−/− mice (n = 14). Results are means ± SE.
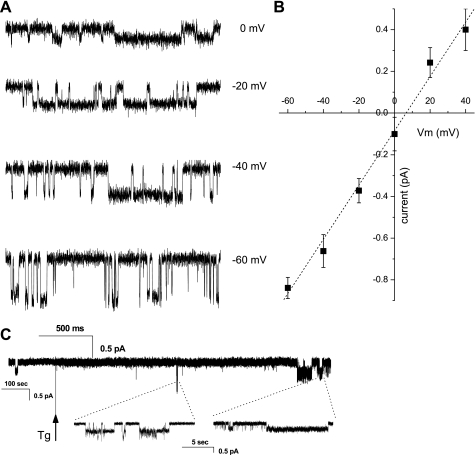
For patch-clamp experiments, muscle fibers were immersed in the relaxing Ca2+-free solution utilized to emphasize currents through store-operated channels (57), and currents were recorded in cell-attached configuration at a series of potentials from −60 to +40 mV in steps of 20 mV (Fig. 3). To investigate store-operated responses, activity was recorded for 5 min in cell-attached patches held at a constant potential of −40 mV. Fibers were then stimulated with 1 μM thapsigargin, and channel activity was recorded over an additional 20 min (Fig. 3C). Typically, patches exhibited Ca2+ currents through essentially two ion channel types, one of small conductance and one of large conductance. Figure 3A shows an example of the small-conductance channel activity recorded at the series of potentials, as indicated on the right. The current-voltage relationship showed a slope conductance of 13 ± 1 pS (n = 7) (Fig. 3B), in agreement with previous reports of TRPC1 activity in overexpression systems (9, 48, 63). Such activity was unambiguously observed in 19% of patches from TRPC1+/+ fibers (11 of 52 patches), an occurrence frequency similar to that observed in wild-type muscle fibers (6 of 31 patches from C57BL/6J mice). However, it was completely absent in TRPC1−/− muscle fibers (0 of 45 patches), allowing us to identify TRPC1 as the channel responsible for this small conductance. As expected (62), it was also significantly inhibited in the presence of 100 μM 2-APB (occurrence falling down to 0%: no observation of TRPC1 activity in 16 patches having a duration of 20 min or longer; P < 0.05, χ2-test). We observed that the channel activity did not significantly increase after thapsigargin stimulation (mean current, in traces where activity was observed, measured at −40 mV over a period of 4 min, of 15 ± 8 fA before and 18 ± 11 fA after thapsigargin stimulation, n = 7; paired Student's t-test comparing values before and after stimulation yields P = 0.85; when normalized per all acquired traces mean currents become 3.2 ± 1.5 fA before and 3.8 ± 2 fA after thapsigargin), clearly showing that TRPC1 activity is not store dependent. In all observed cases, TRPC1 gating was happening in bursts. Unfortunately, the low frequency of observation of such activity (only 3 of 11 patches contained multiple bursts of TRPC1 activity) allowed us to put only a lower boundary estimate on interburst interval at 590 ± 100 s with a mean burst duration of 29.5 ± 10 s and in-burst NPo of 0.48 ± 0.08.
Fig. 3.
Ca2+ currents in cell-attached patches. A: series of traces showing typical single-channel currents in TRPC1+/+ cells in cell-attached configuration at indicated voltages that were not observed in TRPC1−/− cells. B: current-voltage relationship of TRPC1 channel (n = 7). C: sample trace showing Ca2+ current evoked in response to sarcoplasmic reticulum (SR) emptying by thapsigargin (Tg). Patch was held at −40 mV and first basal activity was acquired, followed by the application of thapsigargin-containing solution, as indicated by vertical arrow.
To assess mechanosensitive properties of the TRPC1 channel we performed measurements of Ca2+ currents in cell-attached patches stimulated by application of suction ranging from 0 (no suction) to −100 (stress level often causing patch membrane rupture) mmHg in steps of 10 mmHg. Activity of stretch-activated channels (SAC) could often be evoked with such stimulation (see below); however, application of suction did not cause appearance of additional TRPC1 activity. In 2 cases out of 31 experiments, application of suction occurred when a burst of TRPC1 activity was ongoing. In both cases, the activity stopped with application of suction without reappearing at higher suctions (data not shown). These observations lead us to conclude that TRPC1 channel is not activated by mechanical stimulation.
Membrane patches of TRPC1+/+ fibers also exhibited currents of larger amplitude, corresponding to a conductance of >50 pS and activity increasing on suction application. These currents could thus be associated with the activity of SAC with properties similar to those described in the literature (61, 68). In our experimental conditions (holding potential of −40 mV and 110 mM CaCl2 in the pipette), this conductance was observed in ∼70% of patches (37 of 52 patches) and was responsible for a mean overall current of 23 ± 6 fA (empty traces included). From mean current and occurrence values, we calculated that this conductance therefore seems responsible for ∼88% of the entry of Ca2+ in muscle fibers at rest, the other 12% being due to TRPC1. The activity of this channel increased about twofold after thapsigargin-induced depletion of Ca2+ stores, to a mean current of 40 ± 10 fA (n = 28, P = 0.06). In TRPC1−/− fibers, this conductance was observed with a somewhat smaller occurrence (27 of 45 patches). The related mean current tended to be smaller than that observed in TRPC1+/+ fibers, both at rest (14 ± 6 fA, n = 23) and after thapsigargin stimulation (29 ± 11 fA, n = 23; P = 0.19), although the difference did not reach statistical significance (comparing activity in TRPC1+/+ and TRPC−/− cells before and after thapsigargin application yields P = 0.31 and 0.47, respectively).
Morphology, Phenotype, and Force Production of Muscles of TRPC1−/− Mice
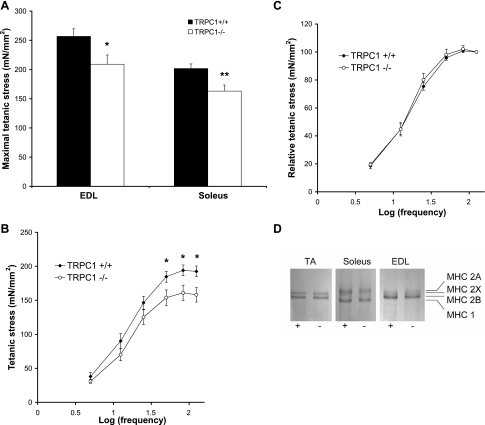
To evaluate muscle function and structure, we carried out mechanical and histological experiments. Isolated soleus and EDL muscles were maximally stimulated to obtain fused tetani. Muscles from TRPC1−/− mice had a strictly normal weight but developed a significantly reduced maximal tetanic force and stress compared with their controls (Fig. 4A and Table 2). Twitch parameters [time to peak (TTP) and half-relaxation time (½RT)] were not different between TRPC1+/+ and TRPC1−/− muscles (Table 2). Investigations of force-frequency relationships in soleus and EDL muscles highlighted the lower stress produced by TRPC1−/− muscles (Fig. 4B), but we could not detect any significant shift in sensitivity to the frequency of stimulation in soleus muscle (Fig. 4C) or in EDL (data not shown), suggesting that the muscles did not change their fast or slow phenotype.
Fig. 4.
Loss of TRPC1 induces a reduction of force production. A: maximal stress (force per cross-sectional area) developed during a 300-ms tetanus stimulated maximally (125 Hz). *P < 0.05 vs. TRPC1+/+; **P < 0.01 vs. TRPC1+/+ (Student's t-test, n = 9). B and C: stress vs. stimulation frequency relationship in soleus muscles. *P < 0.05 vs. TRPC1+/+ (Student's t-test, n = 9). D: SDS-PAGE analysis of myosin heavy chain (MHC) isoforms of TA, soleus, and EDL muscles from TRPC1+/+ (+) and TRPC1−/− (−) mice.
Table 2.
Muscle mechanics
| EDL |
Soleus |
|||
|---|---|---|---|---|
| TRPC1+/+ | TRPC1−/− | TRPC1+/+ | TRPC1−/− | |
| Body mass, g | 28.09 ± 1.31 | 29.67 ± 0.99 | 28.64 ± 0.87 | 29.09 ± 1.58 |
| Muscle mass, mg | 8.95 ± 0.18 | 8.93 ± 0.08 | 10.64 ± 0.22 | 10.85 ± 0.39 |
| L0, mm | 12.28 ± 0.12 | 12.04 ± 0.23 | 11.76 ± 0.17 | 11.65 ± 0.16 |
| TTP, ms | 19.11 ± 0.80 | 20.56 ± 0.60 | 35.25 ± 1.73 | 34.38 ± 2.41 |
| 1/2RT, ms | 31.78 ± 3.24 | 32.78 ± 2.97 | 59.17 ± 5.56 | 60.31 ± 5.33 |
| F0, mN | 374.13 ± 16.35 | 310.11 ± 18.21* | 181.28 ± 6.12 | 150.74 ± 8.69* |
| S0, mN/mm2 | 256.77 ± 13.05 | 209.10 ± 13.32* | 201.69 ± 7.82 | 163.17 ± 9.70* |
| n | 14 | 14 | 22 | 18 |
Values are means ± SE. EDL, extensor digitorum longus; L0, resting muscle length; TTP, time to peak; 1/2RT, half-relaxation time; F0, maximal force; S0, maximal stress (force/cross-sectional area).
P < 0.05.
This was further examined by analyzing MHC isoforms in tibialis anterior, soleus, and EDL muscles (Fig. 4D). Proteins corresponding to MHC isoforms were separated by SDS-PAGE in glycerol-containing gels. We did not observe any significant change in isoform distribution between muscles from TRPC1+/+ versus TRPC1−/− mice. Indeed, soleus muscles from TRPC1+/+ and TRPC1−/− mice contained 44 ± 3.7% and 39 ± 3.1%, respectively, of MHC2A, the rest being MHC1 (n = 4 or 5). Tibialis anterior muscles contained 26 ± 5.2% and 33 ± 3.3% of MHC2X, the rest being MHC2B, and EDL muscles contained 12 ± 4.8% and 15 ± 5.5% of MHC2X, the rest being MHC2B (n = 3–5). Trace amounts of MHC2A were detected in tibialis anterior and EDL muscles from both TRPC1+/+ and TRPC1−/− mice, but their levels were too low to be quantified, while no trace of MHC1A was found in EDL muscle.
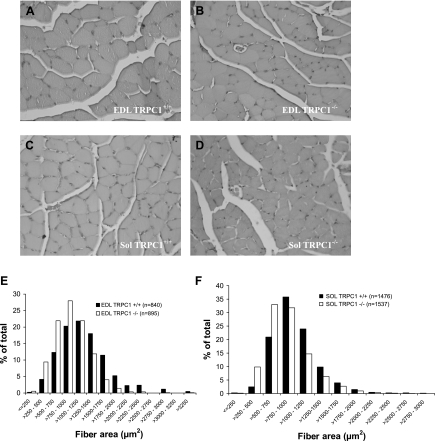
Comparative analysis of histological sections of muscles from 3-mo-old TRPC1+/+ and TRPC1−/− mice did not reveal any major differences (Fig. 5, A–D). In particular, we did not observe any sign of myopathy such as necrosis, central nucleation, or fibrosis. Measurements of the cross-sectional area of the fibers were performed by planimetry. At least 100 fibers were measured in each EDL and 200 fibers in each soleus muscle, in order to determine the mean cross-sectional area of the fibers in a particular muscle. This was repeated on seven different animals and revealed an important decrease of fiber size in both EDL and soleus muscles from TRPC1−/− mice compared with their control littermates [EDL 1,195.7 ± 72 and 939.9 ± 42 μm2 (P < 0.05 n = 7) and soleus 985.7 ± 38 and 855.9 ± 28 μm2 (P < 0.05, n = 7) in TRPC1+/+ and TRPC1−/− muscles, respectively]. Distribution patterns of cross-sectional areas of fibers from different animals were similar for a particular muscle type (Fig. 5B) but clearly showed a shift of the curve of cross-sectional area to smaller values in TRPC1−/− muscles (P < 0.01, χ2, Pearson's test).
Fig. 5.
Histological data. A–D: hematoxylin and eosin-stained cross sections of EDL (A and B) and soleus (C and D) muscles from 3-mo-old TRPC1+/+ (A and C) and TRPC1−/− (B and D) mice. E and F: fiber area distribution in EDL (E) and soleus (F) muscles from TRPC1+/+ and TRPC1−/− mice. The distributions observed in TRPC1+/+ and TRPC1−/− were significantly different (P < 0.01, χ2, Pearson's test).
Finally, we measured the contents of myofibrillar proteins in EDL muscles from TRPC1+/+ and TRPC1−/− mice and observed a significant reduction in TRPC1−/− muscles [95.77 ± 4.28 μg/mg muscle in TRPC1+/+ (n = 9) vs. 72.67 ± 3.01 μg/mg muscle in TRPC1−/− (n = 7); Student's t-test, P < 0.01].
In conclusion, TRPC1−/− muscles produce not only less total force but also less force per cross-sectional area, and their fibers are smaller and contain less myofibrillar proteins.
Muscle Fatigue in TRPC1−/− Mice
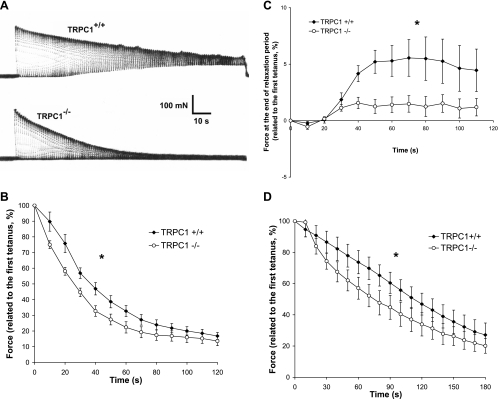
We next investigated the role of TRPC1 in muscle fatigue. Soleus muscles were first chosen because of their dependence on oxidative metabolism, thus limiting the role of anaerobic processes in ionic changes and their contribution to muscle fatigue. In these muscles, TRPC1 was the most abundant TRPC isoform. These muscles were subjected to 50-Hz stimulation trains of 500-ms duration at 1-s intervals (50% duty cycle). Under control conditions, maximal tetanic force progressively declined and after 20–30 s relaxation became incomplete during the 0.5 s separating two successive stimulation periods (Fig. 6A). We previously showed the role of Ca2+ entry in this process. Indeed, in the absence of extracellular Ca2+, the decline of maximal tetanic force was faster and the relaxation during the 0.5-s period separating two successive stimulations was complete (24), suggesting that the latter might be due to cytosolic Ca2+ accumulation (3). Here, using the same protocol, we compared the time course of muscle fatigue in soleus muscles from TRPC1−/− and TRPC1+/+ mice. As shown in Fig. 6, the decline of force was significantly faster in muscles from TRPC1−/− mice than in controls, suggesting that the entry of Ca2+ through TRPC1 channels is necessary for force maintenance during repeated stimulations. Interestingly, in contrast to the situation observed in TRPC1+/+ muscles, the relaxation of TRPC1−/− muscles during the 0.5-s period separating two successive stimulations was complete (Fig. 6C).
Fig. 6.
Involvement of TRPC1 in muscle fatigue: mechanical data. A: representative examples of force records in soleus muscles from TRPC1+/+ and TRPC1−/− mice: tetani of 500 ms every s during 2 min at 50-Hz stimulation frequency. B: quantification of the loss of force during the protocol of fatigue in soleus muscle (force measured every 10th tetanus). Results are expressed relative to the maximal force produced during 1st tetanus. Statistical analysis : *TRPC1+/+ curve (n = 10) different from TRPC1−/− curve (n = 12), P < 0.05, 1-way repeated-measures ANOVA. C: quantification of the force at the end of the relaxation period (0.5 s). Results are expressed relative to the maximal force produced during 1st tetanus. Statistical analysis: *TRPC1+/+ curve (n = 10) different from TRPC1−/− curve (n = 12), P < 0.05, 1-way repeated-measures ANOVA. D: loss of force during the protocol of fatigue in EDL muscle. Statistical analysis: *TRPC1+/+ curve (n = 7) different from TRPC1−/− curve (n = 7), P < 0.05, 1-way repeated-measures ANOVA.
We next studied muscle fatigue of EDL, a fast glycolytic muscle. The protocol was adapted to avoid the possibility of metabolic changes masking the possible role of Ca2+ entry in the process. Muscles were subjected to 125-Hz stimulation trains of 100-ms duration at 2-s intervals. We observed that maximal force declined faster in muscles from TRPC1−/− than TRPC1+/+, similarly as described for soleus muscles (Fig. 6D).
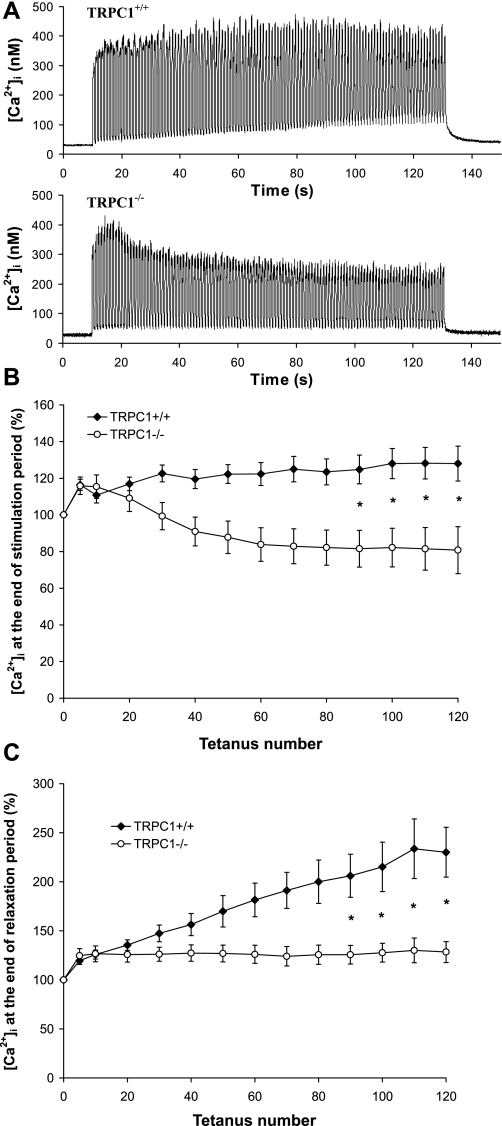
To further investigate the role of Ca2+ entry and Ca2+ accumulation in the evolution of force during the protocol of fatigue, we measured [Ca2+]i transients during fatigue experiments. For technical reasons we performed these measurements on isolated fibers from FDB muscles. FDB muscle contains IIA and IIX fibers and therefore presents an intermediate metabolism (oxidative and glycolytic); we previously showed (60) that its contraction is much slower than that of EDL. We therefore used the same protocol of stimulation as described for soleus. As shown in Fig. 7, A and B, the amplitude of [Ca2+]i transients induced by repetitive stimulations was maintained throughout the fatigue protocol in TRPC1+/+ muscle fibers, indicating that the important loss of force observed under control conditions (Fig. 6) is not due to a decrease of Ca2+ release from the SR. However, in TRPC1−/− muscle fibers, we observed a small and progressive decrease of the amplitude of [Ca2+]i transients, which could explain the accentuated decline of force observed in TRPC1−/− muscles. We also observed that Ca2+ progressively accumulated in fibers after repetitive stimulation (Fig. 7C). Indeed, [Ca2+]i measured at the end of the relaxation period (limited to 500 ms) was almost 2.5 times higher at the end of the fatigue protocol than at the beginning. Interestingly, this was not observed in muscle fibers from TRPC1−/− mice, in which [Ca2+]i at the end of the relaxation period returned to baseline levels throughout the protocol. This fits with the complete relaxation observed in TRPC1−/− muscles (Fig. 6C) and suggests that the incomplete relaxation observed in TRPC1+/+ muscles is due to an accumulation of [Ca2+]i in the cytoplasm.
Fig. 7.
Involvement of TRPC1 in muscle fatigue: [Ca2+]i transients. A: examples of [Ca2+]i transients measured in flexor digitorum brevis (FDB) fibers from TRPC1+/+ (top) and TRPC1−/− mice (bottom) submitted to the fatigue protocol described in Fig. 6. B: quantification of maximal tetanic [Ca2+]i during the protocol of fatigue ([Ca2+]i measured at 1st, 5th, and then every 10th tetanus). Results are expressed relative to maximal [Ca2+]i obtained during 1st tetanus. C: quantification of the [Ca2+]i obtained at the end of each relaxation period during the protocol of fatigue. ([Ca2+]i measured at 1st, 5th, and then every 10th tetanus). Results are expressed relative to maximal [Ca2+]i obtained during 1st tetanus. Statistical analysis: *TRPC1+/+ (n = 13) different from TRPC1−/− (n = 22), P < 0.05, 1-way repeated-measures ANOVA followed by Bonferroni multiple-comparison test.
TRPC1 Knockout Mice Present In Vivo Signs of Fatigue
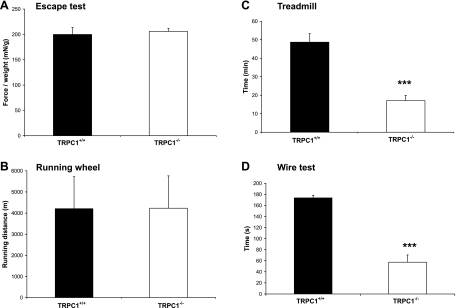
To study the role of TRPC1 in skeletal muscle functions, we used four functional tests designed to investigate in vivo the ability of the animal to perform voluntary exercise (running wheel test and escape test) or endurance and forced exercise (treadmill test and wire test). The running wheel test was used to evaluate spontaneous voluntary exercise during the active part of the day. We did not observe any difference in the running distance between TRPC1+/+ and TRPC1−/− mice (Fig. 8B). Likewise, the escape test used to evaluate the whole body force developed by a mouse to escape in response to a stimulus (pinching of the tail) showed similar performances in TRPC1+/+ and TRPC1−/− mice (∼200 mN/g) (Fig. 8A).
Fig. 8.
In vivo testing. A: escape test: whole body force developed in response to a stimulus (gentle pinching of the tail). Procedure was repeated at short intervals for 2.5 min. Results are presented as means of the 5 highest peaks of force recorded, relative to body weight (mN/g) (n = 10 for each strain). B: running wheel: spontaneous running activity measured during the active part of the day (n = 11 for each strain). C: forced treadmill exercise: duration of exercise before exhaustion. ***P < 0.001 vs. TRPC1+/+ (Student's t-test, n = 10) D: wire test: mice were suspended from a horizontal metallic wire. Time until mice completely released their grasp and fell down is shown; scores of the 3 trials were averaged. ***P < 0.001 vs. TRPC1+/+ (Student's t-test, n = 10).
In contrast, TRPC1+/+ and TRPC1−/− mice performed very differently in the two endurance tests. These two tests were designed to evaluate the resistance of the animal to endurance exercise. As described in materials and methods, treadmill exercise was performed in uphill inclination (30°). During the first 5 min mice were forced to run at 5 m/min, and then the speed was increased by 1 m/min up to 17 m/min. TRPC1+/+ mice ran easily during the progressive increase of treadmill speed and resisted beyond 45 min of the test. Interestingly, TRPC1−/− mice presented difficulties when speed was progressively increased and ran only <20 min (n = 10). The performance of the TRPC1−/− mice in the treadmill test was two to three times lower than that observed in a control population (P < 0.001, Fig. 8C), suggesting a predisposition to muscle fatigue. A similar result was obtained with the wire test, which also evaluates muscle force and resistance to fatigue. In this test, a mouse is suspended from a horizontal wire and the time until the mouse releases its grasp and falls down is recorded (test stopped after 180 s, 3 successive trials). We observed that TRPC1+/+ mice rarely fell down before the end of each trial. However, TRPC1−/− mice were unable to resist so long [57 ± 13 s (n = 11) in TRPC1−/− vs. 174 ± 4s (n = 10) in TRPC1+/+ mice; Fig. 8D].
DISCUSSION
In this paper, we show that the influx of Ca2+ through TRPC1 channels represents a minor part of the entry of Ca2+ into muscle fibers at rest (∼12% of the total influx of Ca2+). We also clearly show that the activity of TRPC1 channels is not store dependent. However, TRPC1-related Ca2+ entry is detectable in muscle fatigue experiments. Indeed, TRPC1 channels modulate the entry of Ca2+ during repeated muscle contractions and allow muscle to maintain force production during sustained stimulations. Finally, we show that muscles from TRPC1−/− mice display a smaller fiber cross-sectional area and generate less force than their controls and express less myofibrillar proteins.
Patch-clamp experiments performed on adult fibers from TRPC1+/+ mice revealed the presence of two ion channel types, one of large conductance exhibiting mechanosensitive properties and characteristics similar to those described in the literature (24, 61, 68) and one of small conductance (13 pS). The small-conductance activity presented the characteristics of TRPC1 measured in overexpression systems (9, 48, 63), was inhibited by 2-APB, and was completely absent in TRPC1−/− fibers, strongly suggesting that this current is carried by TRPC1 channels. We clearly show that in adult skeletal muscle fibers TRPC1 is not store dependent. Although we cannot rule out an effect of the lack of TRPC1 on the presence or the activity of other (possibly unknown) channels, we show by fura-PE3 quenching experiments that, even if TRPC1 channels were present in the T tubules (where they cannot be patched), they do not play an indispensable role in store-operated entry of Ca2+. These results support previous observations performed in smooth muscle (22) but appear contradictory to the situation observed in myoblasts. Indeed, we previously showed (49) that in myoblasts a partial repression of TRPC1 [using a short hairpin RNA (shRNA) strategy] decreased the store-operated entry of Ca2+. Two observations can be proposed to explain this discrepancy. First, we showed that TRPC1 repression slowed down myoblast migration and differentiation. It has been shown that store-operated Ca2+ entry increases during differentiation (67). It is therefore possible that the difference observed in the store-operated entry of Ca2+ reflects the difference in myoblast-myotube maturation. Alternatively, this difference between myoblasts and adult muscle fibers could be due to the interaction of TRPC1 with myogenic factors. Indeed, TRPC1 has been shown to interact with the a-isoform of the inhibitor of myogenic family (I-mfa) (17, 51). I-mfa inhibits store-operated currents through the TRPC1 channel. However, the interaction between TRPC1 and I-mfa can be relieved by binding of myogenic factors such as myogenin to I-mfa. I-mfa has therefore been proposed to function as a molecular switch to suppress the store dependence of TRPC1. The binding of I-mfa to myogenic factors might explain our observations that TRPC1 seems to be store dependent in myoblasts (49) but not in adult fibers.
To investigate the role of TRPC1 in skeletal muscle function, we first compared the force produced by EDL and soleus muscles of TRPC1−/− and TRPC1+/+ mice. We observed a significant decrease of force per cross-sectional area developed by muscles from TRPC1−/− mice, without any shift in the frequency-response curve. The decrease of force did not seem to be related to the maximal [Ca2+]i reached during contraction. Indeed, we did not observe a significant decrease of Ca2+ release in adult FDB fibers submitted to electrical stimulation (see Fig. 7A, 1st tetanus of experiment). Interestingly, similar results were obtained in Homer 1−/− mice, in which the activity of TRPC1 channel seemed to be increased (67). In agreement with force measurements, we observed a decrease of 24% in the contents of myofibrillar proteins. We did not observe any major typical sign of myopathy such as necrosis, regeneration, central nuclei, or fibrosis. We did, however, observe a decrease of the mean cross-sectional area of fibers without any change in MHC isoform distribution (in particular, we did not observe any significant increase of type I fibers, which are smaller). This particular phenotype (smaller fibers with decreased content of myofibrillar proteins) might reflect a slower development that could be due, as mentioned above, to the interactions between TRPC1 and myogenic factors.
To unravel the possible role of TRPC1-related entry of Ca2+ in muscle function, we studied muscle fatigue in TRPC1+/+ and TRPC1−/− mice. Muscle fatigue consists of a progressive decrease in relaxation speed and force production when muscles are stimulated maximally and repeatedly. It has been classically attributed to the accumulation of intracellular lactic acid (39) resulting in acidosis, but it is now clear that this effect contributes little to muscle fatigue (2) and that intracellular ionic changes play a major role in this process (2, 66).
In particular, a partial failure of the SR to release Ca2+ during tetanus has been proposed as a possible cause of fatigue (2, 5). This seems to be due to the accumulation of Ca2+ phosphate in the SR (25, 43). We hypothesized that the entry of Ca2+ might also modulate the amount of releasable Ca2+ from the SR. For example, fast twitch muscles intermittently stimulated at 40 Hz for a total of 30 s show a net influx equivalent to 5–10% of the Ca2+ already present in the SR (3). Similarly, we previously showed (24) that in the absence of external Ca2+ a faster decline of force is observed. Here, we observed that the decline of force was faster in TRPC1−/− than in TRPC1+/+ muscles. We therefore compared Ca2+ transients during all contractions of the fatigue protocol. In normal muscle, we did not observe any decrease of the [Ca2+]i reached during successive tetani. This is probably due to the fact that, for technical reasons, all [Ca2+]i measurements were performed in the presence of BTS to avoid movement artifacts, a situation that minimizes ATP consumption and avoids Pi accumulation (and thus accumulation of Ca2+ phosphate in the SR). In contrast, the release of Ca2+ progressively decreased in muscle fibers from TRPC1−/− mice, emphasizing the need for Ca2+ entry through TRPC1-related channel to allow maintenance of a normal level of [Ca2+] in the SR and a normal production of force during repeated stimulations.
Soleus muscle fatigue was accompanied by a slowing of relaxation evidenced by an incomplete loss of force at the end of the 0.5-s period of relaxation between two contractions (Fig. 6). The mechanisms inducing this slowing of relaxation are still not completely understood (3). Some investigators have shown that relaxation slowing might be due to a decrease of SERCA pump activity. However, this effect might be partially counteracted by a progressive decrease of myofibrillar Ca2+ sensitivity (72, 73). Other studies have proposed a progressive alteration of cross-bridge function (42). Finally, metabolic changes related to fatigue such as the progressive acidosis and the accumulation of ADP and Pi exert their effect on myosin and on SERCA pump and may therefore also contribute to slowing of relaxation (4, 20, 26). Our experiments clearly show that slowing of relaxation is related to an accumulation of cytosolic Ca2+. Indeed, in the absence of TRPC1 channel, the release of Ca2+ from the SR decreases progressively and the accumulation of cytosolic Ca2+ at the end of the relaxation period observed in normal conditions disappears. The consequence is a complete relaxation during the whole experimental protocol of fatigue.
Finally, we investigated whether this susceptibility to fatigue had some repercussions in vivo. Interestingly, we observed that TRPC1−/− mice performed much worse than TRPC1+/+ mice in the wire test and in the forced treadmill test, two tests designed to evaluate the resistance of the animal to endurance exercise. In contrast, these mice performed totally normally in the running wheel test and in the escape test, two voluntary exercises designed to grossly evaluate spontaneous activity and the force of the animal. We therefore propose that the loss of endurance observed in TRPC1−/− mice is due, at least partially, to the diminution of force production observed in vitro as well as to the accentuation of muscle fatigue. Obviously, this does not exclude possible additional effects not related to muscle fatigue.
In conclusion, our results emphasize the importance of TRPC1-related entry of Ca2+ in maintaining force production during sustained trains of contractions and the importance of TRPC1 in the acquisition of adult muscle phenotype.
The gating mechanisms of TRPC1 are, however, still unclear. It might be constitutively active, and because it is responsible only for a small part of the total entry of Ca2+ at rest, its repression could be undetectable in FURA-PE3 quenching studies but emphasized under conditions where Ca2+ accumulates in the cytosol, as in fatigue experiments. Alternatively, TRPC1 channel might also be activated during contraction. However, we show that it is not store dependent. Launikonis and colleagues (46) recently described an excitation-induced Ca2+ current from the transverse tubular membrane; this current activates following single action potentials, but its biophysical properties plead against TRPC1. Finally, the TRPC1 isoform has been proposed to form a stretch-activated cation channel when artificially reconstituted in CHO cells (53). However, this issue is still controversial because, under physiological conditions, mechanosensitive gating has not been reported for this channel (22, 37). Here, we show that in the native environment of skeletal muscle fibers, the TRPC1 channel does not exhibit mechanosensitive properties.
In a previous study, we reported (71) that some TRP channel isoforms had an abnormally increased activity in dystrophin-deficient muscle fibers. The channel involved seemed to be store dependent and mechanosensitive but was insensitive to 2-APB (24, 29). The results presented here therefore suggest that the TRP channel detected in these dystrophic fibers was not TRPC1. In addition, another isoform (TRPV2) was recently implicated in the physiopathology of the disease (41, 76).
GRANTS
This work was supported by the “Association française contre les myopathies” (AFM), the “Association belge contre les maladies neuro-musculaires” (ABMM), by grant ARC 05/10-328 from the General Direction of Scientific Research of the French Community of Belgium, the “Programme d'excellence Marshall” (DIANE convention) from the “Region Walonne,” by grants of the Swiss National Science Foundation (SNSF), the Swiss Foundation for Research on Muscular Diseases, and in part by the Intramural Research Program of the National Institutes of Health (project Z01-ES-101684 to L. Birnbaumer).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 23: 297–328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DG. Skeletal muscle function: role of ionic changes in fatigue, damage and disease. Clin Exp Pharmacol Physiol 31: 485–493, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Allen DG, Lannergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol 80: 497–527, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol 536: 657–665, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambudkar IS. TRPC1: a core component of store-operated calcium channels. Biochem Soc Trans 35: 96–100, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium 42: 213–223, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(beta-aminoethyl ether)-N,N′-tetracetic acid. Biochim Biophys Acta 267: 605–608, 1972 [DOI] [PubMed] [Google Scholar]
- 9.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9: 472–479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baylor SM, Chandler WK, Marshall MW. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol 344: 625–666, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium 33: 433–440, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Berbey C, Allard B. Electrically silent divalent cation entries in resting and active voltage-controlled muscle fibers. Biophys J 96: 2648–2657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi CP, Shanes AM. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol 42: 803–815, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boittin FX, Gribi F, Serir K, Beny JL. Ca2+-independent PLA2 controls endothelial store-operated Ca2+ entry and vascular tone in intact aorta. Am J Physiol Heart Circ Physiol 295: H2466–H2474, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson CG, Makiejus RV. A noninvasive procedure to detect muscle weakness in the mdx mouse. Muscle Nerve 13: 480–484, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Chen CM, Kraut N, Groudine M, Weintraub H. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell 86: 731–741, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol 4: 83–88, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Clapham DE. A STIMulus package puts orai calcium channels to work. Cell 136: 814–816, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Dawson MJ, Gadian DG, Wilkie DR. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol 299: 465–484, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW., Jr TRPC channels function independently of STIM1 and Orai1. J Physiol 587: 2275–2298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietrich A, Kalwa H, Storch U, Mederos YSM, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch 455: 465–477, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Dirksen RT. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol 587: 3149–3151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducret T, Vandebrouck C, Cao ML, Lebacq J, Gailly P. Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J Physiol 575: 913–924, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutka TL, Cole L, Lamb GD. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol 289: C1502–C1512, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Edwards RH, Hill DK, Jones DA. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol 251: 287–301, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev 57: 71–108, 1977 [DOI] [PubMed] [Google Scholar]
- 28.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Franco-Obregon A, Jr, Lansman JB. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J Physiol 481: 299–309, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gailly P. New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim Biophys Acta 1600: 38–44, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Gailly P, Boland B, Himpens B, Casteels R, Gillis JM. Critical evaluation of cytosolic calcium determination in resting muscle fibres from normal and dystrophic (mdx) mice. Cell Calcium 14: 473–483, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Gailly P, De Backer F, Van Schoor M, Gillis JM. In situ measurements of calpain activity in isolated muscle fibres from normal and dystrophin-lacking mdx mice. J Physiol 582: 1261–1275, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gailly P, Hermans E, Gillis JM. Role of [Ca2+]i in “Ca2+ stores depletion-Ca2+ entry coupling” in fibroblasts expressing the rat neurotensin receptor. J Physiol 491: 635–646, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gailly P, Hermans E, Octave JN, Gillis JM. Specific increase of genetic expression of parvalbumin in fast skeletal muscles of mdx mice. FEBS Lett 326: 272–274, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG. TRPC1 binds to caveolin-3 and is regulated by Src kinase—role in Duchenne muscular dystrophy. J Cell Sci 121: 2246–2255, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Gissel H, Clausen T. Excitation-induced Ca2+ uptake in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 276: R331–R339, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch 455: 1097–1103, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 42: 173–182, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Hill A, Kupalov P. Anaerobic and aerobic activity in isolated muscle. Proc R Soc Lond B 105: 313–322, 1929 [Google Scholar]
- 40.Hirn C, Shapovalov G, Petermann O, Roulet E, Ruegg UT. Nav1.4 deregulation in dystrophic skeletal muscle leads to Na+ overload and enhanced cell death. J Gen Physiol 132: 199–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet 18: 824–834, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Jones DA, de Ruiter CJ, de Haan A. Change in contractile properties of human muscle in relationship to the loss of power and slowing of relaxation seen with fatigue. J Physiol 576: 913–922, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabbara AA, Allen DG. The role of calcium stores in fatigue of isolated single muscle fibres from the cane toad. J Physiol 519: 169–176, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem 284: 9733–9741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Launikonis BS, Rios E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol 583: 81–97, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Launikonis BS, Stephenson DG, Friedrich O. Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J Physiol 587: 2299–2312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA 105: 2895–2900, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Singh BB, Ambudkar IS. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5-S6 region. J Biol Chem 278: 11337–11343, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Louis M, Zanou N, Van Schoor M, Gailly P. TRPC1 regulates skeletal myoblast migration and differentiation. J Cell Sci 121: 3951–3959, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol 586: 4815–4824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma R, Rundle D, Jacks J, Koch M, Downs T, Tsiokas L. Inhibitor of myogenic family, a novel suppressor of store-operated currents through an interaction with TRPC1. J Biol Chem 278: 52763–52772, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Marechal G, Beckers-Bleukx G. Force-velocity relation and isomyosins in soleus muscles from two strains of mice (C57 and NMRI). Pflügers Arch 424: 478–487, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7: 179–185, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Martonosi AN, Pikula S. The network of calcium regulation in muscle. Acta Biochim Pol 50: 1–30, 2003 [PubMed] [Google Scholar]
- 55.Merritt JE, Jacob R, Hallam TJ. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem 264: 1522–1527, 1989 [PubMed] [Google Scholar]
- 56.Mizunoya W, Wakamatsu J, Tatsumi R, Ikeuchi Y. Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal Biochem 377: 111–113, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol 38: 744–749, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulido SM, Passaquin AC, Leijendekker WJ, Challet C, Wallimann T, Ruegg UT. Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Lett 439: 357–362, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Raymackers JM, Debaix H, Colson-Van Schoor M, De Backer F, Tajeddine N, Schwaller B, Gailly P, Gillis JM. Consequence of parvalbumin deficiency in the mdx mouse: histological, biochemical and mechanical phenotype of a new double mutant. Neuromuscul Disord 13: 376–387, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Raymackers JM, Gailly P, Schoor MC, Pette D, Schwaller B, Hunziker W, Celio MR, Gillis JM. Tetanus relaxation of fast skeletal muscles of the mouse made parvalbumin deficient by gene inactivation. J Physiol 527: 355–364, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rolland JF, De Luca A, Burdi R, Andreetta F, Confalonieri P, Conte Camerino D. Overactivity of exercise-sensitive cation channels and their impaired modulation by IGF-1 in mdx native muscle fibers: beneficial effect of pentoxifylline. Neurobiol Dis 24: 466–474, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Rychkov G, Barritt GJ. TRPC1 Ca2+-permeable channels in animal cells. Handb Exp Pharmacol: 23–52, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol 577: 479–495, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schakman O, Gilson H, de Coninck V, Lause P, Verniers J, Havaux X, Ketelslegers JM, Thissen JP. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology 146: 1789–1797, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Soboloff J, Spassova MA, Dziadek MA, Gill DL. Calcium signals mediated by STIM and Orai proteins—a new paradigm in inter-organelle communication. Biochim Biophys Acta 1763: 1161–1168, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Stephenson DG, Lamb GD, Stephenson GM. Events of the excitation-contraction-relaxation (E-C-R) cycle in fast- and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiol Scand 162: 229–245, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Stiber JA, Zhang ZS, Burch J, Eu JP, Zhang S, Truskey GA, Seth M, Yamaguchi N, Meissner G, Shah R, Worley PF, Williams RS, Rosenberg PB. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol 28: 2637–2647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suchyna TM, Sachs F. Mechanosensitive channel properties and membrane mechanics in mouse dystrophic myotubes. J Physiol 581: 369–387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turner PR, Westwood T, Regen CM, Steinhardt RA. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335: 735–738, 1988 [DOI] [PubMed] [Google Scholar]
- 70.Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B. Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. FASEB J 21: 608–617, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol 158: 1089–1096, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Westerblad H, Allen DG. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol 468: 729–740, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Westerblad H, Lannergren J, Allen DG. Slowed relaxation in fatigued skeletal muscle fibers of Xenopus and mouse. Contribution of [Ca2+]i and cross-bridges. J Gen Physiol 109: 385–399, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium 42: 205–211, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanou N, Iwata Y, Schakman O, Lebacq J, Wakabayashi S, Gailly P. Essential role of TRPV2 ion channel in the sensitivity of dystrophic muscle to eccentric contractions. FEBS Lett (October 16, 2009). doi:10.1016/j.febslet.2009.10.033 [DOI] [PubMed] [Google Scholar]