Abstract
• Background and Aims Evidence from pea rms1, Arabidopsis max4 and petunia dad1 mutant studies suggest an unidentified carotenoid-derived/plastid-produced branching inhibitor which moves acropetally from the roots to the shoots and interacts with auxin in the control of apical dominance. Since the plant hormone, abscisic acid (ABA), known to inhibit some growth processes, is also carotenoid derived/plastid produced, and because there has been indirect evidence for its involvement with branching, a re-examination of the role of ABA in apical dominance is timely. Even though it has been determined that ABA probably is not the second messenger for auxin in apical dominance and is not the above-mentioned unidentified branching inhibitor, the similarity of their derivation suggests possible relationships and/or interactions.
• Methods The classic Thimann–Skoog auxin replacement test for apical dominance with auxin [0·5 % naphthalene acetic acid (NAA)] applied both apically and basally was combined in similar treatments with 1 % ABA in Ipomoea nil (Japanese Morning Glory), Solanum lycopersicum (Better Boy tomato) and Helianthus annuus (Mammoth Grey-striped Sunflower).
• Key Results Auxin, apically applied to the cut stem surface of decapitated shoots, strongly restored apical dominance in all three species, whereas the similar treatment with ABA did not. However, when ABA was applied basally, i.e. below the lateral bud of interest, there was a significant moderate repression of its outgrowth in Ipomoea and Solanum. There was also some additive repression when apical auxin and basal ABA treatments were combined in Ipomoea.
• Conclusion The finding that basally applied ABA is able partially to restore apical dominance via acropetal transport up the shoot suggests possible interactions between ABA, auxin and the unidentified carotenoid-derived branching inhibitor that justify further investigation.
Keywords: Abscisic acid, auxin, branching, apical dominance, branching inhibitor, decapitated shoot, Ipomoea nil, strain violet, Solanum lycopersicum, Helianthus annuus
INTRODUCTION
Apical dominance is the control exerted by the shoot apex over the outgrowth of the lateral buds. This control is thought to be due in part to the indirect effects of apically derived auxin basipetally transported down the shoot. Abscisic acid (ABA), a plastid-produced carotenoid derivative and a known inhibitor of plant growth, was postulated in the late 1970s as a possible auxin-induced second messenger that directly repressed axillary bud outgrowth (Tucker, 1978).
Although subsequent evidence for such a role of ABA in apical dominance has been equivocal, recent data (Sorefan et al., 2003; Schwartz et al., 2004; Foo et al., 2005; Snowden et al., 2005) indicative of an unidentified carotenoid-related and acropetally moving branching signal, most probably an inhibitor, have come from studies with pea rms1, Arabidopsis max4 and petunia dad1 mutants, and justify a closer look at the role of ABA in apical dominance and branching. It should be noted that although the bulk of evidence points to the above-mentioned branching signal as an inhibitor, the possibility that it could also be an appropriately regulated promoter cannot be discounted (Snowden et al., 2005).
RMS1, MAX4 and DAD1 genes encode members of the carotenoid-cleaving dioxygenase (CCD) family that appear to be required for the production of this novel long-distance, root to shoot, presumably xylem-transported, branching inhibitor. Like ABA, it is produced in plastids where the carotenoid biosynthetic pathway occurs, and requires CCD activity (Schwartz et al., 2001; Schwartz et al., 2004; Auldridge et al., 2006). Although there is substantial evidence that this novel branching inhibitor is not ABA (Booker et al., 2004; Ward and Leyser, 2004; Foo et al., 2005), because they are both thought to be carotenoid derivatives (or apocarotenoids) and, hence, indirectly related, it is plausible that they both may share some similar properties and characteristics in their effects on branching. Hence, it is of particular interest at this time for a reappraisal of the inhibitory effects of ABA on lateral bud outgrowth and its interaction with auxin.
During the past three decades, there have been numerous studies carried out to elucidate the role of ABA as a possible inhibitor of axillary bud growth. Early work by Tucker (1972, 1973, 1978), Eliasson (1975) and Zieslin (1978) provided tentative support for the hypothesis that auxin-induced ABA inhibited bud growth. Also, the fact that ABA applied directly to active buds has been found to inhibit their growth (Taylor et al., 1995; Rogan and Smith, 1976; Wareing and Phillips, 1983; Tamas, 1995) was consistent with this hypothesis. Furthermore, there is evidence that ABA-insensitive AB13 confers retardation of growth in vegetative tissue (Rohde et al., 1999) and is ‘… expressed in dormant axillary buds and in accessory buds that were repressed by the growth of the main buds three to four days after decapitation’ (Shimizu-Sato and Mori, 2001). Diminished branching in ABA-hypersensitive era1 mutants (Pei et al., 1998) also has been noted.
However, subsequent studies, including work with ABA-insensitive Arabidopsis mutants (abi1-1 and abi2-1) by Chatfield et al. (2000), have clearly demonstrated the capacity of auxin to inhibit axillary bud outgrowth independently of ABA activity, thus excluding ABA as a second messenger for indoleacetic acid (IAA) in apical dominance in Arabidopsis.
Nevertheless, there is evidence for some interaction between auxin and ABA in the control of lateral bud outgrowth. For most plant systems, there is general agreement that the decapitation of the normally auxin-rich shoot apex that results in the release of apical dominance is also accompanied by a reduction of the lateral bud ABA content (Tamas, 1995; Geuns et al., 2001). Furthermore, it has been widely demonstrated that this bud ABA content reduction can be prevented by IAA application to the cut surface of the stem. However, it is also generally agreed that ABA is not the primary correlative signal of the shoot tip that controls axillary bud outgrowth (Pilate et al., 1989; Tamas, 1995; Guens et al., 2001). In addition, ABA-deficient Arabidopsis mutants exhibit no symptoms of increased branching (H. Klee, University of Florida, pers. comm.).
There is also conflicting evidence as to whether the reduction of lateral bud ABA content, mentioned above, occurs before or after shoot apex decapitation release of the bud outgrowth. Some workers (Van Onckelen et al., 1981; Galoch, 1989; Tamas et al., 1995) report that it occurs before, while others (Knox and Wareing, 1979; Everat-Boueboloux and Charney, 1982; Gocal et al., 1991) report that it occurs after. If the decrease in the level of ABA in the bud does not occur until after the beginning of bud growth, then something other than ABA reduction must be responsible for the initiation of bud outgrowth. In addition, some other workers (Dorffling, 1976; White and Mansfield, 1977; Pilate et al., 1989) have had difficulty determining an unequivocal correlation between bud inhibition and endogenous ABA levels.
Interestingly, the Pearce group (1995) detected high levels of ABA and IAA in inhibited rhizome buds of Elytriga repens (Quackgrass), but also found that the elongating rhizome shoot tips contained as much ABA as did the inhibited lateral buds. Emery et al. (1998) did not find any correlation between endogenous levels of ABA and branch growth in Lupin angustifolius until late growth stages where a significant ABA decrease was noted.
When evaluating and comparing levels of endogenous hormones in various buds and tissues, there is always the difficulty of making valid comparisons of hormone content between whole organs and a specific subset of cells or tissues. This is particularly true because of our lack of knowledge as to the precise sites and mode of hormone action.
Hence, although there is much evidence that is suggestive of some kind of a secondary inhibitory role of ABA in bud growth and branching, fundamental questions remain as to its precise role in apical dominance and the exact nature of its interaction with auxin (Tamas, 1995).
In ten plant types previously tested for auxin restoration of apical dominance in decapitated shoot apices via the classic Thimann and Skoog (1933) experiment (Cline, 1996), it was found that exogenous auxin application to the cut stem surface of the decapitated shoots worked well to repress subsequent lateral bud outgrowth in most cases except for the basal lateral branches of Arabidopsis thaliana (Columbia ecotype). A subsequent study with the axr3-1 gain-of-function mutant which has an increased amplitude in auxin responses did demonstrate an applied auxin restoration of apical dominance (Cline et al., 2001).
Chatfield et al. (2000), in their studies with a unique A. thaliana bioassay system involving analyses of cauline lateral bud outgrowth (not buds of basal branches as in Cline et al., 1996), found not only that apically applied auxin to excised nodes inhibited small lateral bud outgrowth but also that basally applied ABA synergistically repressed axillary bud outgrowth with apically applied auxin.
It was the objective of the present study to re-examine the effects of ABA and ABA–auxin interaction on apical dominance via the Thimann–Skoog test in three herbaceous species (Ipomoea nil, Solanum lycopersicum and Helianthus annuus) and to compare these effects with those found by Chatfield et al. (2000) in A. thaliana and with those of the novel branching inhibitor demonstrated by the pea rms1 (Beveridge et al., 2000) and Arabidopsis max4 (Leyser, 2003) mutants. Analyses were also carried out for the determination of possible acropetal and/or basepetal transport of the branching-inhibitory influence of auxin and ABA with particular attention paid to the effects of basally applied ABA since the transport of the novel carotenoid-derived RMS/MAX4 branching inhibitor is known to be only upwards (acropetal).
MATERIALS AND METHODS
Seedlings of I. nil L. Roth, strain violet (syn. Pharbitis nil) (Japanese Morning Glory), S. lycopersicum (Better Boy tomato) and H. annuus L. (Mammoth Grey-striped Sunflower) were propagated in Pro-mix, a general purpose peat–vermiculite growing medium. The seeds of Ipomoea required scarification which was carried out in concentrated sulfuric acid for 35 min with subsequent rinsing in running water. The seedlings were grown under greenhouse conditions (20–30 °C) with supplementary General Electric 400 W mercury vapour lamps (total irradiance: up to 1300 μmol m−2 s−1) for a 16 h photoperiod. For the Thimann–Skoog apical dominance experiments, the main shoot of each plant was decapitated with scissors about 5–15 mm above the lateral bud whose outgrowth was to be analysed. In the case of Ipomoea, this was above the second, third, fourth or fifth node depending upon the particular experiment. For Helianthus it was above the first node, and for Solanum it was above the third or fourth node. Auxin was applied apically as 0·5 % naphthalene acetic acid (NAA) in lanolin to the cut stem surface immediately following decapitation. Lanolin only was applied to decapitated control plants. Abscisic acid (ABA; 1 %) was also applied in lanolin. For the basal hormone treatments, NAA or ABA was applied in a ring around the main shoot about 5–10 mm below the lateral bud to be analysed. In the case of the six Helianthus trials, the NAA or ABA was applied about 10 mm above or below the first node, except for the first trial where is was about 20 mm. Aqueous NAA or ABA (1, 10 or 100 μm, 10–20 μL in 0·05 % Tween-20) was applied directly to the highest single lateral bud of each plant immediately following decapitation of the main shoot and continued daily for 7–10 d. Tween-20 (0·05 %) only was applied to controls. Measurements of lateral bud outgrowth were usually begun within 1 or 2 weeks after the beginning of the treatments. The effects of treatments on the lateral bud outgrowth measurements were tested for three data sets (the Ipomoea data set with 14 experiments, the Solanum data set with four experiments and the Helianthus data set with six experiments) by a mixed model with individual experiments as block, the day as covariate and the plant as random variable. The multiple comparisons were followed to compare differences between the treatments. The marginal means estimates of Figs 2 and 3 are from the unbalanced two-way analysis of variance (ANOVA) models with treatments and individual experiments in the model.
RESULTS
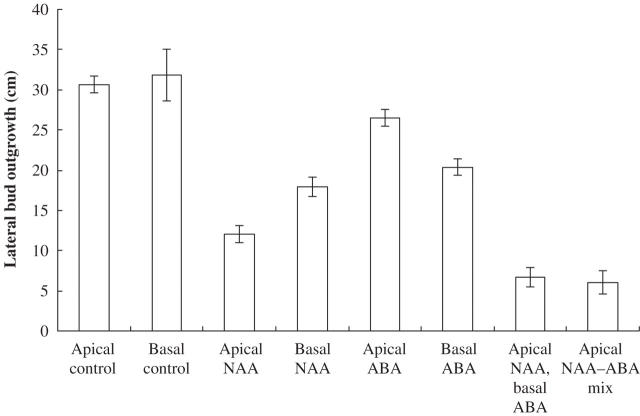
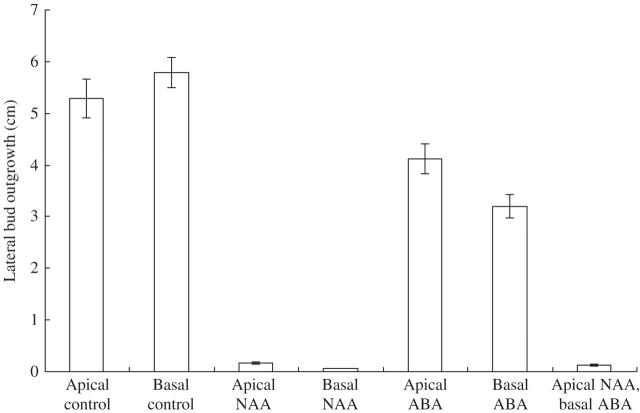
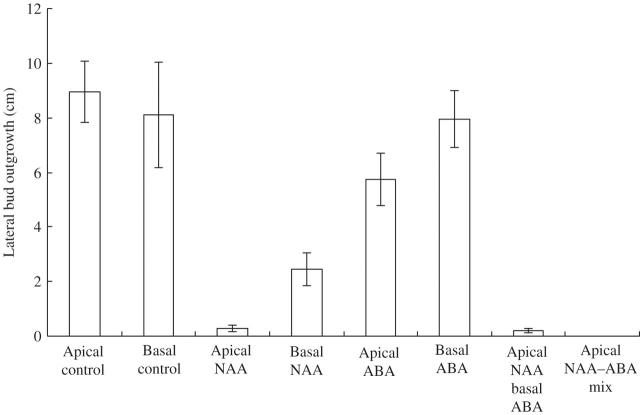
Auxin (0·5 % NAA in lanolin), applied apically to the cut stem surface of a decapitated shoot, 5–15 mm above the highest lateral bud of an I. nil (Fig. 1; Table 1), S. lycopersicum (Fig. 2, Table 2) or H. annuus (Fig. 3, Table 3) shoot in the Thimann–Skoog test, usually vigorously repressed lateral bud outgrowth in all three species, i.e. apical dominance was strongly restored by the apical auxin treatment. When auxin was basally applied in a ring around the stem, 5–10 mm below the highest lateral bud of the decapitated shoot in Solanum, there was complete repression of outgrowth of these buds (Fig. 2). There was also repression in Ipomoea and in Helianthus, although not as strong (Figs 1 and 3).
Fig. 1.
Lateral bud outgrowth in decapitated shoots of Ipomoea nil. Auxin (0·5 % NAA in lanolin) or 1 % ABA (in lanolin) was applied apically to the cut stem surface above the highest node below the point of decapitation or basally in a ring around the stem below the node. Measurements were begun 1–2 weeks after treatment. Values given are ± s.e. n = 807.
Table 1.
Ipomoea nil treatment interaction
| Treatment | Treatment | Difference (cm) | P-value |
|---|---|---|---|
| Apical control | Basal control | –1·2 ± 3·1 | 0·6915 |
| Apical control | Apical NAA | 18·6 ± 1·0 | <0·0001 |
| Apical control | Basal NAA | 12·7 ± 1·1 | <0·0001 |
| Basal control | Basal NAA | 14·0 ± 3·2 | <0·0001 |
| Apical control | Apical ABA | 4·2 ± 1·1 | 0·0002 |
| Apical control | Basal ABA | 10·3 ± 1·0 | <0·0001 |
| Basal control | Basal ABA | 11·6 ± 3·1 | 0·0003 |
| Apical NAA | Apical ABA | –14·4 ± 1·2 | <0·0001 |
| Apical ABA | Basal ABA | 6·1 ± 1·1 | <0·0001 |
| Basal control | Apical ABA | 5·4 ± 3·2 | 0·0863 |
| Basal NAA | Apical ABA | –8·5 ± 1·2 | <0·0001 |
| Apical NAA | Basal ABA | –8·3 ± 1·1 | <0·0001 |
| Apical NAA | Basal NAA | –5·9 ± 1·2 | <0·0001 |
| Basal NAA | Basal ABA | –2·4 ± 1·1 | 0·0324 |
| Apical control | Apical NAA/ABA mix | 24·6 ± 1·5 | <0·0001 |
| Apical ABA | Apical NAA/ABA mix | 20·4 ± 1·6 | <0·0001 |
| Apical NAA | Apical NAA/ABA mix | 6·0 ± 1·5 | <0·0001 |
| Apical control | Apical NAA/basal ABA | 23·9 ± 1·1 | <0·0001 |
| Apical NAA | Apical NAA/basal ABA | 5·3 ± 1·1 | <0·0001 |
| Basal ABA | Apical NAA/basal ABA | 13·6 ± 1·1 | <0·0001 |
| Basal control | Apical NAA/basal ABA | 25·2 ± 3·2 | <0·0001 |
| Apical NAA/ABA mix | Apical NAA/basal ABA | –0·7 ± 1·6 | 0·6372 |
The ‘Difference’ column is the least square means of one treatment minus the least square means of another treatment. Values given are ± s.e. n=807.
Fig. 2.
Lateral bud outgrowth in decapitated shoots of Solanum lycopersicum. Auxin and ABA were applied as described for Ipomoea. Measured from 11 to 17 d after treatment. Values given are ± s.e. n = 42–48.
Table 2.
Solanum lycopersicum treatment interactiom
| Treatment | Treatment | Difference (cm) | P-value |
|---|---|---|---|
| Apical control | Basal control | –0·3 ± 0·3 | 0·403 |
| Apical control | Apical NAA | 4·9 ± 0·3 | <0·0001 |
| Apical control | Basal NAA | 5·1 ± 0·3 | <0·0001 |
| Basal control | Basal NAA | 5·3 ± 0·3 | <0·0001 |
| Apical control | Apical ABA | 1·0 ± 0·3 | 0·0022 |
| Apical control | Basal ABA | 2·2 ± 0·3 | <0·0001 |
| Basal control | Basal ABA | 2·4 ± 0·3 | <0·0001 |
| Apical NAA | Apical ABA | –4·0 ± 0·3 | <0·0001 |
| Apical ABA | Basal ABA | 1·2 ± 0·3 | <0·0001 |
| Basal control | Apical ABA | 1·2 ± 0·3 | <0·0001 |
| Basal NAA | Apical ABA | –4·1 ± 0·3 | <0·0001 |
| Apical NAA | Basal ABA | –2·8 ± 0·3 | <0·0001 |
| Apical NAA | Basal NAA | 0·1 ± 0·3 | 0·6899 |
| Basal NAA | Basal ABA | –2·9 ± 0·3 | <0·0001 |
| Apical control | Apical NAA/basal ABA | 4·9 ± 0·3 | <0·0001 |
| Apical NAA | Apical NAA/basal ABA | –0·0 ± 0·3 | 0·9199 |
| Basal ABA | Apical NAA/basal ABA | 2·7 ± 0·3 | <0·0001 |
| Basal control | Apical NAA/basal ABA | 5·2 ± 0·3 | <0·0001 |
The ‘Difference’ column is the least square means of one treatment minus the least square means of another treatment. Values given are ±s.e. n = 238.
Fig. 3.
Lateral bud outgrowth in decapitated shoots of Helianthus annuus. Auxin and ABA were applied as described for Ipomoea. Measured 14–23 days after treatment. Values given are ± s.e. n = 8–61.
Table 3.
Helianthus annuus treatment interaction
| Treatment | Treatment | Difference (cm) | P-value |
|---|---|---|---|
| Apical control | Basal control | −1·0 ± 2·1 | 0·6331 |
| Apical control | Apical NAA | 8·8 ± 1·0 | <0·0001 |
| Apical control | Basal NAA | 6·6 ± 1·0 | <0·0001 |
| Basal control | Basal NAA | 7·6 ± 2·1 | 0·0003 |
| Apical control | Apical ABA | 3·4 ± 1·0 | 0·0006 |
| Apical control | Basal ABA | 1·1 ± 1·0 | 0·2573 |
| Basal control | Basal ABA | 2·1 ± 2·0 | 0·3146 |
| Apical NAA | Apical ABA | −5·5 ± 1·0 | <0·0001 |
| Apical ABA | Basal ABA | −2·3 ± 1·0 | 0·0172 |
| Basal control | Apical ABA | 4·4 ± 2·1 | 0·0367 |
| Basal NAA | Apical ABA | −3·2 ± 1·0 | 0·0009 |
| Apical NAA | Basal ABA | −7·7 ± 1·0 | <0·0001 |
| Apical NAA | Basal NAA | −2·2 ± 1·0 | 0·0211 |
| Basal NAA | Basal ABA | −5·5 ± 1·0 | <0·0001 |
| Apical control | Apical NAA/ABA mix | −10·3 ± 1·2 | <0·0001 |
| Apical ABA | Apical NAA/ABA mix | 6·9 ± 1·2 | <0·0001 |
| Apical NAA | Apical NAA/ABA mix | 1·4 ± 1·2 | 0·2338 |
| Apical control | Apical NAA/basal ABA | 9·0 ± 1·0 | < 0·0001 |
| Apical NAA | Apical NAA/basal ABA | 0·1 ± 1·0 | 0·9081 |
| Basal ABA | Apical NAA/basal ABA | 7·9 ± 1·0 | <0·0001 |
| Basal control | Apical NAA/basal ABA | 9·9 ± 2·1 | <0·0001 |
| Apical NAA/ABA mix | Apical NAA/basal ABA | −0·3 ± 1·2 | 0·2719 |
The ‘Difference’ column is the least square means of one treatment minus the least square means of another treatment. Values given are ±s.e. n = 389.
In preliminary trials, there was some repression detected on bud outgrowth when aqueous auxin (1, 10 and 100 μm) was applied directly to the sensitive lateral buds of decapitated Ipomoea shoots (Table 4). However, the fact that there was no consistency in the dose–response correlation suggested a lack of direct auxin effect.
Table 4.
Effect of 10–20 μL of aqueous auxin (NAA) or ABA in 0·05 % Tween-20 on bud outgrowth when applied directly to the highest lateral bud of decapitated Ipomea nil
| 0 μm | 1 μm | 10 μm | 100 μm | |
|---|---|---|---|---|
| Control | 17·1 ± 3·2 | – | – | – |
| NAA | – | 8·3 ± 1·4 | 10·6 ± 1·7 | 8·7 ± 1·5 |
| ABA | – | 9·1 ± 1·7 | 7·2 ± 1·2 | 2·2 ± 0·3 |
Measured after 1 week. Data lengths are in cm, ±s.e. n = 21–26.
ABA (1 % in lanolin) applied apically to the cut stem surfaces of a decapitated shoot in the Thimann–Skoog experiment had a slight repressive effect on bud outgrowth in Ipomoea, Solanum and Helianthus (Figs 1–3). However, when ABA was basally applied, a significantly stronger, but moderate, inhibition of the outgrowth of the highest lateral buds was exhibited both in Ipomoea and Solanum (Figs 1 and 2, Tables 1 and 2). Although no testing was done, the lack of inhibition in Helianthus (Fig. 3 and Table 3) may have been due to insufficient penetration of the basally applied ABA (in lanolin) in the rings around the stems in this somewhat slow-growing herbaceous species.
When aqueous ABA (1, 10 and 100 μm) was applied directly to axillary buds of decapitated Ipomoea seedlings, there was a significant repressive effect (directly proportional to the concentration) on their outgrowth (Table 4). To determine whether the inhibitory effect of ABA might have been due to a toxic effect rather than to a physiological one, single buds/plant at the second node were given daily treatments of aqueous 100 μm ABA for 1 week. The ABA treatments then were discontinued and the adjacent main shoot was immediately decapitated to release apical dominance. After another week, the outgrowth of the previous ABA-treated lateral buds was compared with that of water-treated controls. The previous ABA-treated buds exhibited vigorous subsequent outgrowth with little or no apparent residual toxic effects (data not shown).
When apical auxin and basal ABA treatments were combined on the same plants, there was a small but significant additive repressive effect on lateral bud outgrowth in Ipomoea (Fig. 1, Table 1). This effect was difficult to detect in the other two species possibly due to the very strong inhibitory effect of the apical auxin treatment alone.
When a mix of 0·5 % NAA and 1 % ABA in lanolin was applied apically to the cut stem surface of decapitated Ipomoea plants via the Thimann–Skoog experiment, the repression of lateral bud outgrowth was somewhat stronger than when either hormone treatment was given alone (Fig. 1, Table 1).
DISCUSSION
That auxin applied apically to the decapitated shoot via the Thimann–Skoog procedure restores apical dominance was definitively demonstrated in the present study with three herbaceous species. Lateral bud outgrowth was strongly repressed by the apically applied 0·5 % NAA. This result confirms those of various workers (Phillips, 1975; Tamas, 1995; Cline, 1996) and is consistent with the hypothesis that the apically applied auxin moves down the shoot basipetally. The indication here that basally applied auxin may be somewhat less effective than apically applied auxin in restoring apical dominance in Ipomoea and Helianthus is consistent with the hypothesis that acropetal auxin transport is less efficient than basepetal auxin transport. The slightly stronger inhibition by basally applied auxin in the case of Solanum may have been due to the close proximity of the application (5–10 mm from the bud) site which led to significant effects of diffusible auxin.
However, the lack of persuasive evidence for strong growth inhibition by direct application of auxin to lateral buds of decapitated shoots (also confirmed by other workers, Phillips, 1975) suggests that repression of axillary bud outgrowth by NAA application to decapitated shoots is probably indirect. The suggestion of Morris et al. (2005) that pre-auxin acting processes may promote the initiation of decapitation-induced bud elongation deserves consideration. Booker et al. (2003) have suggested that the mechanism of auxin repression of bud outgrowth involves apically derived auxin moving down the polar transport stream and operating in the xylem and interfascicular schlerenchyma.
The Thimann–Skoog experiment did not work effectively with ABA, and the results were generally the reverse of those obtained with auxin. In contrast to the strong growth-repressive effects of direct ABA application to buds, ABA applied apically to the decapitated shoot had only a slight effect in restoring apical dominance, i.e. lateral bud outgrowth was not inhibited nearly as much as with the auxin treatment. There was relatively little evidence for substantial ABA movement down the shoot. Chatfield et al. (2000) also found no inhibitory response with apically applied ABA in their Arabidopsis assay system with the Columbia ecotype.
In contrast to this lack of convincing evidence for strong basipetal ABA transport in the shoots, we did find in at least two (Ipomoea and Solanum) of the three herbaceous species tested that basally applied ABA partially inhibited lateral bud outgrowth. Hence, these results are consistent with the hypothesis that ABA transport in the shoot is stronger in the acropetal than in the basipetal direction (or at least moderately so in our study) which is also similar to that of the novel unidentified carotenoid-related and acropetally moving branching inhibitor suggested by the research with pea rms1 (Beveridge, 2000), Arabidopsis max4 (Leyser, 2003) and petunia dad1 (Napoli et al., 1999) mutants.
If there is validity to the hypothesis of Snowden et al. (2005) that the unidentified mobile xylem branching signal may be a promoter rather than an inhibitor, then it is possible that ABA might be repressing lateral bud outgrowth by the reduction of the acropetal delivery of the branching promoter via the inhibition of transpiration. Consistent with this, the supply of xylem-carried solutes, including cytokinins, to axillary buds is immediately enhanced after decapitation (Turnbull et al., 1997). Thomas and Hay (2003) have suggested the control of branching by some other ‘root-supplied’ factor. It is unlikely that root-derived cytokinins alone control shoot branching because overproduction of cytokinins in roots does not lead to shoot branching (Faiss et al., 1997). Alternatively, Bennett et al. (2006) have proposed that the unidentified, carotenoid-related, Arabidopsis MAX-dependent hormone controls branching by regulating shoot auxin transport capacity. Somewhat similarly, Lazer and Goodman (2006) envisage an apocarotenoid flavenoid control of auxin transport. However, the fact that ABA and the novel long-distance hormone controlled by rms, max and dad genes may be derived from a common precursor may mean that changes in ABA content may relate indirectly to changes in content of the novel signal.
Chatfield et al. (2000) reported strong inhibition of lateral bud growth with basally applied ABA in Landsberg erecta but not in the Columbia ecotype of A. thaliana. However, they did find (as did we in Ipomoea) suggestive evidence for an additive repression with apically applied NAA and basally applied ABA in both types. They also observed some ABA counteractive effects on NAA repression of bud growth in the Columbia ecotype when both ABA and NAA were apically applied. They suggested that these effects might be due to ABA inhibition of auxin transport. However, no such reduction in apical NAA repression of lateral bud outgrowth by apical ABA could be detected in the present study, perhaps due to a lack of sufficient sensitivity in our system.
That exogenous ABA can directly inhibit bud growth was also confirmed in a preliminary study with the demonstration of repression by direct ABA treatments of lateral buds of decapitated shoots. Hence, although there is substantial evidence for the general growth-inhibitory properties of ABA (Emery et al., 1998), it appears that it is auxin that plays the role as the primary, albeit indirect, correlative signal of the shoot apex that controls apical dominance, whereas ABA functions as a secondary inhibitor of bud growth, independently in some respects of auxin activity. Although ABA is not the second messenger for auxin, at least in Arabidopsis (Chatfield et al., 2000), the presence of the auxin-rich shoot apex or the presence of exogenous auxin applied in the place of the removed shoot apex is required to maintain endogenous ABA levels.
The suggestive evidence in the present study for acropetal transport of ABA as a branching inhibitor in two of three widely divergent herbaceous species provides support for a possible connection with the unidentified apocarotenoid and acropetally moving branching inhibitor suggested by pea rms1, Arabidopsis max4 and petunia dad1 mutant research. As efforts (Beveridge, 2000; Sorefan et al., 2003; Turnbull et al., 2004; Foo et al., 2005; Snowden et al., 2005) accelerate to elucidate and to identify this latter branching inhibitor and its interaction with auxin, our understanding of the various pathways and interacting genetic factors involved will also undoubtedly expand, as will the importance of elucidating the interaction with ABA, albeit in a probable secondary role with respect to branching control.
Acknowledgments
Appreciation is expressed to Dr Christine Beveridge, ARC Centre of Excellence in Integrative Legume Research, The University of Queensland, and to Dr Steven P. Chatfield, Plant Agriculture Department, University of Guelph, for their helpful suggestions on the manuscript. Thanks is also expressed for support by the Philip Brown fund.
LITERATURE CITED
- Auldridge M, Block A, Vogel J, Dabney-Smith C, Mila I, Bouzayan M, et al. 2006. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant Journal 45: 982–993. [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. 2006. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology 16: 553–563. [DOI] [PubMed] [Google Scholar]
- Beveridge C. 2000. Long-distance signaling and a mutational analysis of branching in pea. Plant Growth Regulation 32: 193–203. [Google Scholar]
- Booker J, Chatfield S, Leyser O. 2003. Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. 2004. MAX3CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Current Biology 14: 1–20. [DOI] [PubMed] [Google Scholar]
- Chatfield S, Stirnberg P, Forde B, Leyser O. 2000. The hormonal regulation of axillary bud growth in Arabidopsis. Plant Journal 24: 159–169. [DOI] [PubMed] [Google Scholar]
- Cline M. 1996. Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Annals of Botany 78: 255–266. [Google Scholar]
- Cline M, Chatfield S, Leyser O. 2001. NAA restores apical dominance in the axr3-1 mutant of Arabidopsis thaliana. Annals of Botany 87: 61–65. [Google Scholar]
- Dorffling K. 1976. Correlative bud inhibition and abscisic acid in Acer pseudoplatanus and Syringa vulgaris. Physiologia Plantarum 38: 319–322. [Google Scholar]
- Eliasson L. 1975. Effect of indoleacetic acid on the abscisic acid level in stem tissue. Physiologia Plantarum 34: 117–120. [Google Scholar]
- Emery R, Longnecker N, Atkins C. 1998. Branch development in Lupin angustifolius L. II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. Journal of Experimental Botany 49: 555–562. [Google Scholar]
- Everat-Bourbouloux A, Charney D. 1982. Endogenous abscisic acid levels in stems and axillary buds of intact or decapitated broadbean plant (Vicia faba L.). Physiologia Plantarum 54: 440–445. [Google Scholar]
- Faiss M, Zalubilova J, Strnad M, Schmulling T. 1997. Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant Journal 12: 401–415. [DOI] [PubMed] [Google Scholar]
- Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge C. 2005. The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17: 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galoch E, Zielinska M, Burkacka-Laukajtys E. 1989. The effect of decapitation on the levels of IAA and ABA in the lateral buds of Betula pendula Roth. Acta Physiologiae Plantarum 20: 399–403. [Google Scholar]
- Geuns J, Smets R, Struyf T, Prinsen E, Valcke R, Van Onckelen H. 2001. Apical dominance in Pssu-ipt-transformed tobacco. Phytochemistry 58: 911–921. [DOI] [PubMed] [Google Scholar]
- Gocal F, Pharis R, Yeung E, Pearce D. 1991. Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiology 95: 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J, Wareing P. 1979. Apical dominance in Phaseolus vulgaris L. The possible roles of ABA and IAA. Journal of Experimental Botany 35: 239–244. [Google Scholar]
- Lazar G, Goodman H. 2006. MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis. Proceedings of the National Academy of Sciences of the USA 103: 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. 2003. Regulation of shoot branching by auxin. Trends in Plant Science 8: 541–546. [DOI] [PubMed] [Google Scholar]
- Morris S, Cox M, Ross J, Krisantini S, Beveridge C. 2005. Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiology 138: 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Beveridge C, Snowden K. 1999. Reevaluating concepts of apical dominance and the control of axillary bud outgrowth. Current Topics in Developmental Biology 44: 127–169. [DOI] [PubMed] [Google Scholar]
- Pearce D, Taylor J, Robertson J, Harker K, Daly E. 1995. Changes in abscisic acid and indole-3-acetic acid in axillary buds of Elytrigia repens released from apical dominance. Physiologia Plantarum 94: 110–116. [Google Scholar]
- Pei Z, Ghassemian M, Kwak C, McCourt P, Schroeder J. 1998. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282: 287–290. [DOI] [PubMed] [Google Scholar]
- Phillips I. 1975. Apical dominance. Annual Review of Plant Physiology 26: 342–367. [Google Scholar]
- Pilate G, Sossountzov L, Miginiac E. 1989. Hormone levels and apical dominance in the aquatic fern Marsilea drummondii A. Br. Plant Physiology 90: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan P, Smith D. 1976. Experimental control of bud inhibition in rhizomes of Agropyron repens (L.) Beauv. Zeitschrift für Plflanzenphysiologie 78: 113–121. [Google Scholar]
- Rohde A, Van Montagn M, Boerjan W. 1999. The ABSCISIC ACID-INSENSITIVE 3 (AB13) gene is expressed during vegetative quiescence processes in Arabidopsis. Plant, Cell and Environment 22: 261–270. [Google Scholar]
- Schwartz S, Xiaoqiong Q, Zeevaart J. 2001. Characterization of a novel carotenoid cleavage dioxygenase from plants. Journal of Biological Chemistry 276: 25208–25211. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Xiaoqiong Q, Loewen M. 2004. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. Journal of Biological Chemistry 279: 46940–46945. [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato S, Mori H. 2001. Control of outgrowth and dormancy in axillary buds. Plant Physiology 127: 1405–1413. [PMC free article] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogne K, Goussot M, Bainbridge K, Foo E, et al. 2003. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes and Development 17: 1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden K, Simkin A, Janssen B, Templeton K, Loucas H, Simons J, et al. 2005. The decreased apical dominance/petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth and flower development. Plant Cell 17: 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas I. 1995. Hormonal regulation of apical dominance. In: Dordrecht D, ed. Plant hormones: physiology, biochemistry and molecular biology, 2nd edn. The Netherlands: Kluwer Academic Publishers, 340–353.
- Taylor J, Robertson J, Harker K, Bhalla M, Daly E, Pearce D. 1995. Apical dominance in rhizomes of quackgrass, Elytrigia repens: the effect of auxin, cytokinin and ABA. Canadian Journal of Botany 73: 307–314. [Google Scholar]
- Thimann K, Skoog F. 1933. Studies on the growth hormones of plants III. The inhibition of growth substance on bud development. Proceedings of the National Academy of Science of the USA 19: 714–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Hay M. 2003. Relationships among shoot sinks for resources exported from nodal roots regulate branch development of distal non-rooted portions of Trifolium repens L. Journal of Experimental Botany 54: 2091–2104. [DOI] [PubMed] [Google Scholar]
- Tucker D. 1978. Apical dominance in the tomato: the possible roles of auxin and abscisic acid. Plant Science Letters 12: 273–278. [Google Scholar]
- Tucker D, Mansfield T. 1972. Effects of light quality on apical dominance in Xanthium strumarium and the associated changes in endogenous levels of abscisic acid and cytokinins. Planta 102: 140–151. [DOI] [PubMed] [Google Scholar]
- Tucker D, Mansfield T. 1973. Apical dominance in Xanthium strumarium. Journal of Experimental Botany 24: 731–740. [Google Scholar]
- Turnbull C, Raymond M, Dodd I, Morris S. 1997. Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum) during release of apical dominance. Planta 202: 271–276. [Google Scholar]
- Turnbull C, Zhang B, Young N. 2004. Metabolomic approaches to discovering novel regulators of shoot branching. IPGSA Conference 2004. Canberra, Australia, 69.
- Van Onckelen H, Horemans S, De Greef J. 1981. Functional aspects of abscisic acid metabolism in cotyledons of Phaseolus vulgaris L. seedlings. Plant and Cell Physiology 22: 507–515. [Google Scholar]
- Ward S, Leyser O. 2004. Shoot branching. Current Opinion in Plant Biology 7: 73–78. [DOI] [PubMed] [Google Scholar]
- Wareing P, Phillips I. 1983. Abscisic acid in bud dormancy and apical dominance. In: Addicott F, ed. Abscisic acid. New York: Pradger Publishers, 303–329.
- White J, Mansfield T. 1977. Correlative inhibition of lateral bud growth in Pisum sativum L. Studies of the role of abscisic acid. Annals of Botany 41: 1163–1170. [Google Scholar]
- Zieslin N, Spiegelstein H, Halevy A. 1978. Components of axillary bud inhibition in rose plants. IV. Inhibitory activity of plant extracts. Botanical Gazette 139: 64–68. [Google Scholar]