Abstract
• Background and Aims Plant lateral organs such as leaves arise from a group of initial cells within the flanks of the shoot apical meristem (SAM). Alterations in the initiation of lateral organs are often associated with changes in the dimension and arrangement of the SAM as well as with abnormal hormonal homeostasis. A mutation named stem fasciated (stf) that affects various aspects of plant development, including SAM shape and auxin level, was characterized in sunflower (Helianthus annuus).
• Methods F1, F2 and F3 generations were obtained through reciprocal crosses between stf and normal plants. For the genetic analysis, a χ2 test was used. Phenotypic observations were made in field-grown and potted plants. A histological analysis of SAM, hypocotyl, epicotyl, stem and root apical meristem was also conducted. To evaluate the level of endogenous indole-3-acetic acid (IAA), a capillary gas chromatography–mass spectrometry–selected ion monitoring analysis was performed.
• Key Results stf is controlled by a single nuclear recessive gene. stf plants are characterized by a dramatically increased number of leaves and vascular bundles in the stem, as well as by a shortened plastochron and an altered phyllotaxis pattern. By histological analysis, it was demonstrated that the stf phenotype is related to an enlarged vegetative SAM. Microscopy analysis of the mutant's apex also revealed an abnormal enlargement of nuclei in both central and peripheral zones and a disorganized distribution of cells in the L2 layer of the central zone. The stf mutant showed a high endogenous free IAA level, whereas auxin perception appeared normal.
• Conclusions The observed phenotype and the high level of auxin detected in stf plants suggest that the STF gene is necessary for the proper initiation of primordia and for the establishment of a phyllotactic pattern through control of both SAM arrangement and hormonal homeostasis.
Keywords: Helianthus annuus, auxin, fasciated mutant, hormonal homeostasis, phyllotaxis, shoot apical meristem
INTRODUCTION
In higher plants, the zygote divides to produce the embryo, a bipolar structure with one, two or several embryonic leaves (cotyledons), the shoot apical meristem (SAM) and the root apical meristem (RAM). Throughout post-embryonic development new organs are reiteratively differentiated (Sinnott, 1960; Steeves and Sussex, 1989; Carles and Fletcher, 2003). To allow for normal post-embryonic growth, the size of apical meristems has to be accurately regulated. The SAM contributes to the development of the plant via four functions: initiating tissues, initiating organs, communicating with other parts of the plant, and perpetuating itself as a formative region (Steeves and Sussex, 1989; Baurle and Laux, 2003; Castellano and Sablowski, 2005; Bhalla and Singh, 2006). The SAM of dicotyledonous plants can be divided into three zones: (1) a peripheral zone (PZ) of rapidly dividing cells, which produces new lateral organs with regular spacing (phyllotaxis) and regular timing (plastochron); (2) a central zone (CZ) of slowly dividing cells, which has the key role of meristematic cell recovery; and (3) the rib meristem zone, which lies underneath the central zone and gives rise to the pith and vascular structure of the stem (Steeves and Sussex, 1989). Superimposed on these zones is the arrangement of clonally distinct cell layers (Kaplan and Cooke, 1997). In most dicotyledonous plants, the SAM is composed of three layers: L1, or epidermal layer; L2, or sub-epidermal layer; and L3, or corpus. The outermost layers, tunica (L1 and L2), comprise cells that undergo anticlinal division. The L3 is a multilayer group of cells that lie beneath the tunica and divide in all planes, allowing the plant to grow upwards and outwards. Several studies revealed that these zones and layers form separate symplasmic domains (Rinne and Van der Shoot, 1998). For the SAM to function properly, the establishment and maintenance of these zones and layers are essential. When the area of the central pool of stem cells is not closely controlled, two opposite phenotypes can be observed. The increase in SAM size is frequently correlated with loss of typical arrangement of organ primordia, and ribbon-like flattening of normally cylindrical organs (fasciation) frequently arises (White, 1948; Sharma and Fletcher, 2002; Traas and Vernoux, 2002). On the contrary, if the indeterminate fate of the stem cells is not properly maintained, precocious cell differentiation determines the extinction of meristem activity and, consequently, the development of new lateral primordia is prevented (Laux et al., 1996; Long et al., 1996).
The term fasciation has been applied in botany to a wide variety of phenomena even though usually the common trait is a ribbon-like flattening of normally cylindrical plant parts (White, 1948; Gorter, 1965; Binggeli, 1990). All organs of a plant can be fasciated but stem or inflorescence fasciation are the most frequent. The fasciated phenotype has been described in several species as a consequence of pathogen attacks, spontaneous mutations, mutagen treatments, wounding or hormone applications (Gorter, 1965; Gottschalk and Wolff, 1983; Binggeli, 1990; Nadjimov et al., 1999).
Fasciated plants in spontaneous species are often described simply as botanic curiosities but when the phenotype it is not a monstrosity, fasciated mutants can be evaluated in plant breeding programmes in crops or in ornamental species. For example, the significant increase in locule number in tomato cultivars is due to fasciation, while in Celosia cristata the band-like shape of its inflorescence makes it floriculturally attractive (Gorter, 1965). Fasciated plants have also been used to reveal how meristem structure and function are established and maintained in normal plants (Williams and Fletcher, 2005). For example, an extracellular signalling pathway in SAM maintenance depending on the activities of CLAVATA genes has been identified in Arabidopsis thaliana through molecular characterization of fasciated mutants (Clark, 2001). Plants with mutations in any of three loci, CLV1, CLV2 or CLV3, show a progressive increase in meristem size beginning in the embryo and continuing throughout life, indicating a loss of cell division restriction (Clark et al., 1993, 1997). Available evidence indicates that CLV3 encodes a small secreted peptide expressed in outer cell layers (Fletcher et al., 1999) and likely binds to the leucine-rich repeat receptor kinase CLV1 and its putative dimerization partner CLV2, which are expressed in inner cell layers (Clark et al., 1997; Stone et al., 1998; Lenhard and Laux, 2003). Another key element of CLV signalling pathway is the WUSCHEL (WUS) gene product. WUS encodes a novel subtype of the homeodomain transcription factor family that is expressed near the boundary of the CZ and RZ in shoot and floral meristems (Mayer et al., 1998). Both the SAM and floral meristems of wus mutant plants terminate prematurely after the formation of a few organs, indicating that WUS is necessary to promote stem cell activity throughout development (Laux et al., 1996). The WUS expression domain is maintained by FASCIATA1 (FAS1) and FASCIATA2 (FAS2), which encode components of chromatin assembly factor-1 (Kaya et al., 2001). Regular production of leaf primordia that is reflected in stable phyllotaxis and plastochron is another primary function of the SAM. The phyllotaxis is altered in clv and fas A. thaliana mutants as well as in other mutants that show increased SAM size: mgoun1 (mgo1), mgoun2 (mgo2), enhanced response to abscisic acid (era1), pluripetala (plp), abnormal phyllotaxy1 (abph1), corona (cna), jabba-1D, plastochron 1, and shoot organization (sho) (Itoh et al., 1998, 2000; Laufs et al., 1998; Running et al., 1998; Bonetta et al., 2000; Giulini et al., 2004; Green et al., 2005; Williams et al., 2005). Although these genes are supposed to play distinct roles in leaf initiation, it is generally considered that the initiation pattern of leaves is closely associated with the size and shape of the SAM (Fleming, 2005; Reinhardt, 2005). Leaves are not generated in a random fashion, but rather in a consistent pattern over space and time, producing the regular architecture of the plant. Plant hormones have been associated with this process. In particular, auxin appears to be a central player in leaf and flower formation and as a component of phyllotactic patterning (Gälweiler et al., 1998; Reinhardt et al., 2000, 2003; Vernoux et al., 2000; Heisler et al., 2005; Reinhardt, 2005; Jönsson et al., 2006; Smith et al., 2006).
In sunflower (Helianthus annuus), the artificial induction of stem fasciation by auxin treatments has been described as far back as 1940 (Irvine, 1940) and, more recently, two recessive mutations that induce analogous phenotypes were isolated (Shattuck, 1985; Jambhulkar, 2002). Shattuck (1985) evaluated the inheritance of a spontaneous mutant named head and leaf syndrome (hl), while Jambhulkar (2002) has more recently described the morphology and the genetic control of a fasciated mutant induced by gamma rays. Both mutants showed well-documented effects of band-shaped fasciation such as high leaf number, abnormal phyllotaxis and stem enlargement. Fasciation of sunflower inflorescence has also been described by Stoenescu (1974) for the recessive f mutant. Only a preliminary analysis of these mutants' phenotype was reported, without any histological or physiological characterization.
Recently a spontaneous mutant named stem fasciated (stf) was isolated within an inbred line of sunflower, which presents stem and inflorescence fasciation. Here, it is shown that the recessive mutation affects various aspect of plant development, including SAM shape, stem diameter, phyllotaxis and inflorescence development. To learn about the origin of the fasciation in the stf mutant, a detailed morphological characterization of the mutant phenotype throughout development has been performed. Furthermore, to assess the involvement of auxin in the expression of the stf phenotype, endogenous auxin levels and the sensitivity of the mutant to this hormone have been analysed.
MATERIALS AND METHODS
Genetic analysis
The fasciated sunflower mutant arose spontaneously in the inbred line of Helianthus annuus L. ACM 2224 (Department of Crop Plant Biology of the University of Pisa, Italy) in summer 2000. This plant was harvested and its progeny grown in a greenhouse during the winter. No segregation was observed within the progeny. Its phenotype suggested the name stem fasciated (stf). Genetic analysis of the mutation was performed in the experimental field of S. Piero (University of Pisa) with conventional management's practices. Special attention was given to chemical fertilization because the expression of fasciated phenotype was usually influenced by the plant vigour. For the genetic analysis, flowers of stf and normal plants were hand-emasculated. F1, F2 and F3 generations were obtained through reciprocal crosses. Plant growth conditions were as described in Pugliesi et al. (1995). Briefly, parental populations, and F1, F2 and F3 progenies were grown in the field in replicate rows (50-cm inter-row spacing with 25–30 cm between plants, about 8 or 9 plants m−2). At anthesis, plants were classified in all population as either normal or mutant. A χ2 test was used to determine the goodness-of-fit of observed ratios to a theoretical monogenic 3 : 1 ratio.
Morphological analysis
Phenotypic observations were made on both mutant (stf/stf) and wild-type (STF/STF) plants (ten) taken at random from four replicates grown in field conditions. The number of stem vascular bundles was evaluated on cross-sections of stems of 30-d-old plants (median region of the 4th internode). Other morphological parameters were recorded at the onset of anthesis with the exception of achene yield, which was evaluated at plant maturity. Total leaf area was measured using a DT area meter MK2 (Delta-T Devices, Cambridge, UK). Anthesis of the first tubular flowers was recorded to determine the blooming date. Pollen vitality was evaluated according to Pakòzdi et al. (2002) by performing an overnight stain of pollen grains with acetocarmine (1 % w/v).
When required, seeds of stf and wild type were germinated in Petri dishes on distilled water. Germination took place in a growth chamber in the dark at 23 ± 1 °C. After 3 d, the germinated seeds were transplanted into an alveolar box and then transferred to a growth chamber at 23 ± 1 °C and a 16-h photoperiod. Two weeks later, the seedlings were transplanted into larger pots (3 L) containing a mixture of soil and sand plus an initial dose of complete fertilizer (Osmocote 14–14–14; Sierra, UK). Irradiation was 200 μmol m−2 s−1 provided by mercury vapour lamps (Osram HQI-TS 250 W/NDN; Wembley, UK). The number of expanded leaves was recorded every 5–7 d during 10 weeks of growth. To evaluate the outgrowth of side shoots after decapitation, five potted plants (3 weeks old) from four replicates were deprived of their vegetative SAMs just above the cotyledons.
Histological analysis
Shoot tips, segments of the stem with node and several organs (hypocotyl, epicotyl, stem and root) of stf and wild-type plants were collected from field-grown or potted plants. Shoot tips were collected at different plant ages during both vegetative and reproductive stages (see below for details). Nodal explants, hypocotyls, epicotyls and roots were collected in 10-d-old seedlings while the sampling of stems was obtained from 30-d-old plants (median region of the 4th internode). The materials were fixed for 24 h in FAA (formalin/glacial acetic acid/ethanol/distilled water, 10 : 5 : 50 : 35 v/v) at room temperature before being transferred into 70 % ethanol (Fambrini et al., 2003). Water was removed by graded ethanol series while the dehydrated material was cleared in xylene with five steps according to Ruzin (1999). Paraffin-embedded tissues were sectioned using a rotary microtome (Reichert, Vienna, Austria). Two methods were used to colour the serial 10-μm-thick sections obtained. Median, longitudinal sections of SAM or transverse sections of undeveloped leaves in SAM were stained in Delafield's haematoxylin (BDH Chemicals Ltd, Poole, UK). Sections of hypocotyls, epicotyls, stems and roots were stained in 1 % (w/v) safranin followed by 0·2 % (w/v) fast green (Fluka Chemie GmbH, Germany).
Nucleus size
Serial longitudinal sections of vegetative shoots from 18-d-old seedlings were stained with the Feulgen procedure. In particular, paraffin was removed from slides by immersion in xylene and then, the sections were hydrolysed for 45 min in 5 n HCl at room temperature, and stained in 0·5 % Fuchsin-Schiff's reagent for 1 h (Cavallini et al., 1989). Measurements of nucleus diameter were made on cells of the PZ and the CZ of the SAM using an eyepiece micrometer Periplan 10× MESS (Leitz, Germany) in a Leica DMRB microscope (Leica GmbH, Germany). Data are mean (± s.d.) of three independent experiments with five replications (sections of vegetative shoots).
Indole-3-acetic acid (IAA) analysis
Seedlings deprived of roots (14 d after germination), apical stem segments from 40-d-old plants (median region of the 5th internode) and shoot apices at the floral stage 5 (Marc and Palmer, 1981) of both stf and wild-type plants were collected. Freeze-dried samples (500 mg fresh weight) were extracted in 65 % isopropanol (v/v) with 0·02 m imidazole buffer at pH 7 to which [3H]IAA as radiotracer and [13C6]IAA (JD Cohen, Department of Horticultural Science, Saint Paul, MN, USA), as an internal standard for quantitative mass-spectral analysis, were added. After overnight isotope equilibration, the analysis of free IAA was performed according to Chen et al. (1988). IAA was purified using a Beckman System Gold HPLC with UV detector (Varian UV 50) equipped with a C18 Partisphere column (Whatman, 110 × 5 mm i.d.) and the samples were eluted at 1 mL min−1 20 % acetonitrile/water, 1 % acetic acid. Quantitative IAA analysis was done by a capillary gas–chromatography–mass spectrometry–selected ion monitoring (GC-MS-SIM) using a Hewlett Packard 5890-5970 System equipped with a 12 m Chromopack CPSiI 19 capillary column (i.d. 0·25 mm; film thickness 0·25 μm), carrier gas He (1 mL min−1), injector at 280 °C, oven temperature increased from 50 °C to 110 °C at 30 °C min−1, then 6 °C min−1 to 280 °C, source temperature 270 °C, ionizing voltage 70 eV. Ions monitored were m/z 130 and 136 for the base peak (quinolinium ion) and 189 and 195 for the molecular ion of methyl-IAA and methyl-[13C6]IAA, respectively. The ratios of 130 : 136 and 189 : 195 were used to calculate endogenous levels of IAA. The data are presented as means (± standard deviation) of three independent experiments with three replicates (seedlings, stem segments and shoot apices).
In-vitro treatments
Embryos were surface-sterilized for 1 min in 70 % (v/v) ethanol and for 20 min in 2·8 % (v/v) sodium hypochlorite solution containing 0·01 % Triton X-100 and then rinsed in sterile distilled water. For germination, the embryos were placed on solidified (8 g L−1 Bactoagar, Oxoid Ltd, Basingstoke, UK) MS basal medium (Murashige and Skoog, 1962) without growth regulators. After 1 week, seedlings were transplanted to 20 mL solidified MS medium in 150 mL Erlenmeyer flasks and incubated at 23 ± 1 °C and a 16-h photoperiod in a growth chamber. Irradiation was 30 μmol m−2 s−1 provided by cool-white fluorescent lamps (Philips TLD 36W/33; Philips, Eindhoven, The Netherlands). Hypocotyl and epicotyl explants (0·5–1·0 cm long) were obtained from 3-week-old seedlings and used to induce callus proliferation in Petri dishes on solidified MS basal medium supplemented with 30 g L−1 of sucrose. Cotyledons were obtained from 5-d-old seedlings and cultured as described for hypocotyl and epicotyl explants. A minimum of 40 explants were cultured for each treatment and the experiment was repeated three times. For callus growth measurements, fresh weight of treated explants (30 d) was expressed as a percentage of the initial fresh weight of hypocotyl or epicotyl explants.
IAA treatments
Hormonal treatments were performed according to Lenzi et al. (1995). Briefly, embryos of stf and wild type were placed between two filter paper sheets on a Plexiglas rack partially submerged in distilled water or distilled water supplemented with IAA (0·1, 1 or 10 μm). The treatments were conduced in a growth chamber at 23 ± 1 °C under continuous white light and a photosynthetic photon flux density of 30 μmol m−2 s−1 provided by cool fluorescent lamps. Root length and number of secondary roots were determined after 14 d of culture using 30 seedlings of each genotype for each treatment. The experiment was repeated three times.
Hypocotyl growth in the dark or under light conditions
Embryos of stf and wild type were placed as described for the IAA treatments. The experiments were conducted in a growth chamber at 23 ± 1 °C in the dark or under continuous white light with a photosynthetic photon flux density of 30 μmol m−2 s−1 provided by cool fluorescent lamps. Hypocotyl length was determined after 14 d of culture using 30 seedlings of both genotypes for each treatment. The experiment was repeated three times.
Statistical analysis
Data were treated using analysis of variance procedures, and means were compared by Tukey's test (P = 0·05). Homogeneity of variances was evaluated using Bartlett's test (P = 0·05). Differences between means of Table 1 and Figs 8, 10A and 11 were tested using Student's t-test (P = 0·05 or 0·01). Statistical analyses on percentage data were performed after arcsine transformation.
Table 1.
The effects of the stem fasciated (stf) mutation on some characters of sunflower (Helianthus annuus) plants grown in the field
| Characters | Wild-type | stf |
|---|---|---|
| No. of leaves | 27·8 ± 2·2 | 136·1 ± 19·8** |
| Leaf area per plant (cm2) | 5228·3 ± 253·6 | 8978·8 ± 485·5** |
| Stem height (cm) | 75·2 ± 6·1 | 73·2 ± 6·2n.s. |
| No. of vascular bundles of the stem† | 36·0 ± 1·9 | 63·3 ± 2·1** |
| Onset of anthesis (days) | 63·8 ± 1·4 | 66·7 ± 1·6* |
| No. of head bracts | 29·7 ± 6·3 | 142·0 ± 26·0** |
| No. of ray flowers | 56·0 ± 1·1 | 138·3 ± 7·2** |
| No. of achenes/plant‡ | 605·2 ± 77·9 | 155·0 ± 53·1** |
| Pollen viability (%) | 93·9 ± 0·7 | 65·2 ± 4·1** |
Values are means (± s.d.) from four progenies, with ten replicates each (plants).
P < 0·05; **P < 0·01; n.s., not significant (Student's t-test).
The number of stem vascular bundles was evaluated on cross-sections of stems of 30-d-old plants (medial region of the 4th internode).
The number of achenes was calculated in open-pollinated plants.
RESULTS
Genetic analysis of the stf mutant
Reciprocal crosses between wild-type and the stf mutant resulted in F1 plants only with normal traits and the F2 population segregated into mutant and normal types fitting a monogenic 3 : 1 ratio (490 normal : 181 mutant, χ2 = 1·395, P = 0·20–0·30). The monogenic segregation ratio was confirmed by analysis of F3 progenies. In F3 populations, F2 plants classified as stf only produce mutant progenies. Non-segregating progenies (25) and segregating progenies (61) that fitted the expected 3 : 1 ratio were obtained from F2 normal plants (2336 normal : 719 mutant, χ2 = 3·49, P = 0·05–0·10). These data indicate that stf is controlled by a single nuclear recessive gene as reported for the two previously described sunflower mutants with the fasciated phenotype (Shattuck, 1985; Jambhulkar, 2002). It cannot be excluded that these mutations affect the same locus and crosses among the three genotypes will be performed to test for complementation.
Developmental phenotype of the stf mutant
Fasciation is characterized by altered phyllotaxis and stem broadening. These aspects of the mutant phenotype were analysed and the results are presented below. Interestingly, both of these aspects of the stf phenotype became progressively more apparent as the plants developed.
The vigour of stf plants was comparable with respect to the wild type and no differences were observed for plant height (Table 1). By contrast, the number of total leaves and the total leaf area per plant in the mutant were significantly higher than in the wild type (Table 1 and Fig. 1). The number of leaves in stf plants gradually increased from the bottom to the top of the stem, especially in the last section of the stem below the insertion of the inflorescence (Fig. 1A). The mutant showed an increased number of leaves after only 2 weeks after germination (Fig. 1E). This phenotype, easily visible in field-grown plants before floral initiation (Fig. 1B), was maintained during all growing phases (Fig. 1B–D). Both stf leaves and head bracts were always smaller than the ones in wild-type plants (Fig. 2A–J). The shape of the first pair of stf and wild-type leaves was the same. By contrast, the leaves inserted at the basal and median stem nodes in wild-type plants were bigger than those on the mutant and characterized by a well developed petiolar sinus (cordate form) that was not prominent in stf leaves (compare G and H with I and J in Fig. 2).
Fig. 1.

Developmental phenotype of the stem fasciated (stf) mutant of sunflower (Helianthus annuus). (A) Number of visible leaves as a function of the stem length (percentage of total stem length) in wild-type (WT) and stf plants. Data are means (± s.d.) of four independent experiments (progenies) each with ten replicates (plants). Values with the same letter are not significantly different at the P = 0·05 level according to Tukey's test. (B) Forty-day-old stf plant grown in the field (floral stage 5 according to Marc and Palmer, 1981). (C) stf plant 10 d before anthesis. (D) Wild-type (WT) and stf plants at the anthesis. (E) Number of visible leaves as a function of plant development in potted wild-type (WT) and stf plants grown in growth chamber. Data are means (± s.d.) of four independent experiments (progenies) each with ten replicates (plants). Values with the same letter are not significantly different at the P = 0·05 level according to Tukey's test.
Fig. 2.
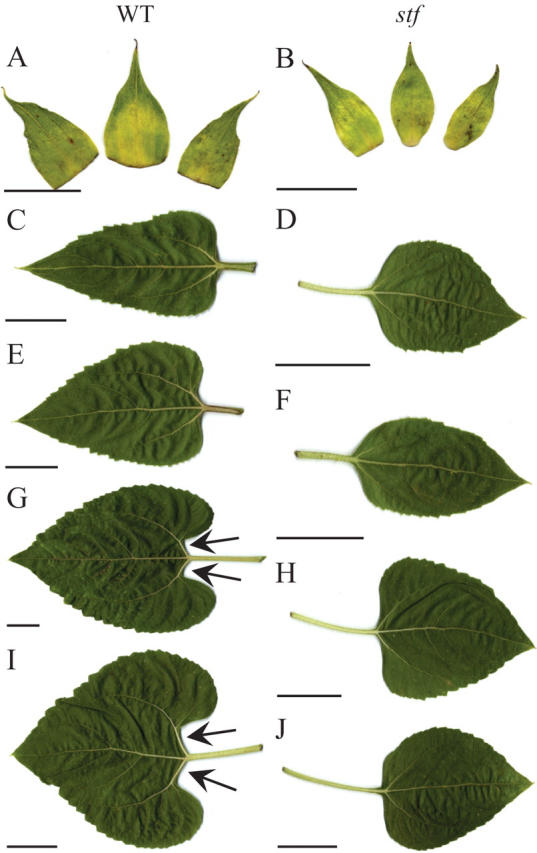
Bracts and leaves of wild-type (WT) and stem fasciated (stf) plants of sunflower (Helianthus annuus). (A, B) Bracts of the inflorescences. (C–J) Leaves from WT and stf plants as a function of the stem length (percentage of the total stem length): (C, D) apical leaves (80–90 % of the total stem length); (E, F) median leaves (60–70 % of the total stem length); (G, H) median leaves (40–50 % of the total stem length); (I, J) basal leaves (10–20 % of the total stem length). Arrows indicate the petiolar sinus visible in WT leaves. Scale bars: A, B = 3·5 cm; C– J = 3 cm.
In the last portion of the stem, just below the inflorescence, the stem diameter of stf plants was significantly larger than in wild-type plants (Fig. 3A, B), while no differences were observed in the basal or median region of the stem. The shape of stf stems was correlated to developmental phases. In the vegetative phase, the stf stem was cylindrical, but after the transition to reproductive phase it gradually broadened at the tip to give a band-shaped fasciation, which was particularly pronounced in the zone just below the inflorescence (Fig. 3B). A central cavity in the pith parenchyma of the stf stem was also present after the transition to reproductive phase (Fig. 3C).
Fig. 3.

Developmental phenotype of the stem fasciated (stf) mutant of sunflower (Helianthus annuus). (A) Stem diameter as a function of the stem length (percentage of the total stem length) in wild-type (WT) and stf plants. Data are means (± s.d.) of four independent experiments (progenies) each with ten replicates (plants). Values with the same letter are not significantly different at the P = 0·05 level according to Tukey's test. (B) Stem of a stf plant deprived of leaves, bracts and ray flowers; arrows indicate the insertion points of the leaves. (C) Hand-made transverse sections at the apical end of wild-type (WT) and stf stems. (D) Stem of an stf plant grown in a growth chamber; arrows indicate the whorled phyllotaxis. (E, F) Transverse sections of shoot tips of 18-d-old wild-type (E) and stf (F) plants, stained in Delafield's haematoxylin. Scale bars: C = 2 cm; E = 2 mm; F = 1·5 mm.
In the wild type, cotyledons and the first pair of leaves are inserted in an opposite arrangement (decussate), which gradually develops into a spiral phyllotaxis of alternate leaves, from the second to the third pair. The diameter of the sunflower SAM increases during development (Marc and Palmer, 1981). This results in a gradual shift from lower to higher phyllotactic numbers. In fact, the phyllotactic pattern undergoes a gradual shift from 2 + 3 to 3 + 5 and to more complex phyllotaxis (e.g. 5 + 8). stf plants showed severe phyllotaxis defects (compare E and F in Fig. 3). Indeed, the first two leaves of stf plants are normally opposite but subsequent leaves are whorled, arranged with a random number of leaves inserted at each node (Fig. 3D), an arrangement that is clearly distinct from the spiral phyllotaxis of normal plants. Although in stf plants the number of leaves at each node is variable, a higher number of leaves are initiated at the top of stf stems with respect to basal ones (Fig. 1A and 3B). Thus, especially at the top of the stf stem, the phytomers were not clearly distinguishable (Fig. 3B).
Sunflower inflorescences are heterogamous with zygomorphic ray flowers located in the outermost whorl of the head and actinomorphic disc flowers arrayed in arcs radiating from the centre of the head (Berti et al., 2005). The fasciated phenotype was also displayed in stf inflorescences, while few minor defects were detected in both ray and disc flowers. Indeed, the stf capitulum developed multiple inflorescences inserted in a single receptacle to generate a profile not as wholly hemispheric as in the control (Fig. 4A–E). The surface of the stf receptacle was wavy with depressed lines from the centre to the circumference which were spaced by areas in relief (Fig. 4B). The spiral phyllotaxis 34 + 55, characteristic of the sunflower inflorescence, was lost (Fig. 4C). stf plants presented a high number of ray flowers and head bracts (Table 1 and Fig. 4B). In deep invaginated and narrow areas, disc flowers were very deformed and/or missing and ray flowers and/or head bracts were differentiated (Fig. 4C). On the contrary, the flowers that developed in portions of stf inflorescences not affected by invagination were very similar to wild-type flowers with respect to shape and organs number (data not shown). In the mutant, the onset of anthesis was delayed in comparison to the wild type (Table 1). In addition, the mutant showed a significantly lower pollen viability than the wild type (Table 1). stf heads were often blemished by a cavity in the central zone (Fig. 4C) and they produced markedly fewer achenes than the wild type (Table 1). stf achenes were also shorter than normal (Fig. 4F). The severity of head malformations varied from plant to plant and depended on general plant vigour. In fact, weak potted stf plants developed a less extreme phenotype than field-grown plants (data not shown).
Fig. 4.
Developmental phenotype of the stem fasciated (stf) mutant of sunflower (Helianthus annuus). (A) Inflorescence of a wild-type plant. (B, C) Inflorescences of stf plants; arrows indicate ray flowers or head bracts differentiated within the stf inflorescence; arrowheads indicate deep invaginated areas within the stf inflorescence. (D, E) Back view of dried wild-type (D) and stf (E) inflorescences; arrows indicate invaginations generating a stf inflorescence with multiple heads. (F) Mature achenes from wild-type (left) and stf (right) plants. Scale bar = 1 cm.
To evaluate side-shoot production, potted plants were decapitated after 3 weeks from germination. In both genotypes, every plant was deprived of the SAM just above the cotyledons. Outgrowth of axillary shoots was always observed in wild-type decapitated plants (20/20), while in the mutant, the rate of side shoot differentiation was strongly reduced (2/20).
Histological analyses of the stf mutant
The developmental phenotype displayed by the mutant suggested that the stf SAM may be enlarged. Indeed, the mutation at the STF locus induces a time-dependent enlargement of the SAM significantly higher than the wild type (Fig. 5A). At a very early stage of seedling growth (3 d old) the stf SAM was similar in size to the one in control plants (Fig. 5B, C). By contrast, after just 10 d of culture, the SAM diameter of the mutant was significantly higher than in wild-type plants (Fig. 5A). An enlarged SAM characterized stf plants also after the transition from vegetative to reproductive stage (Fig. 5A, D, E). By contrast, when floral primordia were initiated, the meristem diameter of stf and wild-type plants was not significantly different (Fig. 5A, F, G). Nevertheless, the SAM of stf mutants showed a different shape with respect to normal plants, often displaying more apical domes along its surface (Fig. 5F, G). Notably, the phenotype of the sporadic side shoots produced by decapitated stf plants retained the fasciated syndrome (Fig. 5H, I). In young stf seedlings, both hypocotyl and epicotyl revealed a normal structure without significant modifications (Fig. 6A–D). On the contrary, stems of 40-d-old stf plants presented more differentiated vascular bundles than normal plants (Table 1 and Fig. 6E, F). There were no structural differences in RAM between stf plants and their normal siblings (Fig. 6G, H), indicating that the effects of the stf mutation were specific to the SAM. However, it was evident that stf seedlings showed a higher number of lateral roots than the wild type (121·5 ± 6·6 vs. 92·2 ± 2·0).
Fig. 5.
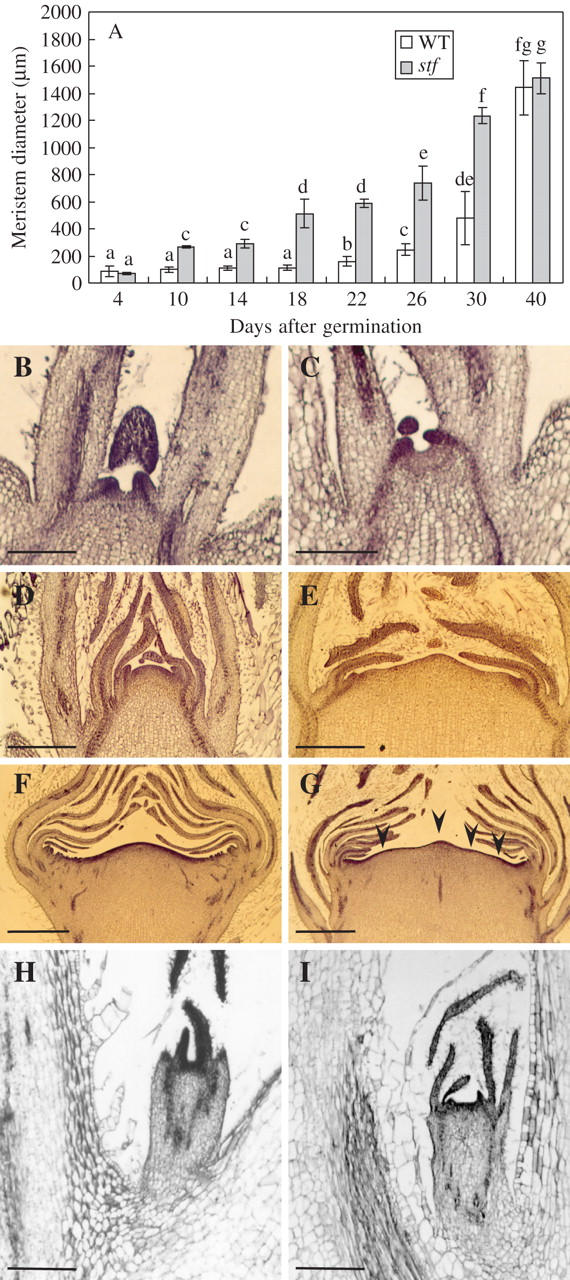
Development of apical and axillary shoot apices in wild-type and in the stem fasciated (stf) mutant of sunflower (Helianthus annuus). (A) Shoot apical meristem (SAM) diameter of stf and wild-type (WT) potted plants. Data are means (± s.d.) of four independent experiments (progenies) each with ten replicates (SAMs). Values with the same letter are not significantly different at the P = 0·05 level according to Tukey's test. (B, C) Median longitudinal section of wild-type (B) and stf (C) shoot apex from 4-d-old seedlings. (D, E) Median longitudinal section of wild-type (D) and stf (E) shoot apex from 30-d-old plants. (F, G) Median longitudinal section of wild-type (F) and stf (G) shoot apex from 40-d-old plants; arrowheads indicate multiple apical domes. (H, I) Median longitudinal section of axillary shoot apex developed from decapitated wild-type (H) and stf (I) plants. Sections were stained in Delafield's haematoxylin. Scale bars: B, C, I = 1 mm; D, E = 500 μm; F, G = 1 mm; H = 400 μm.
Fig. 6.
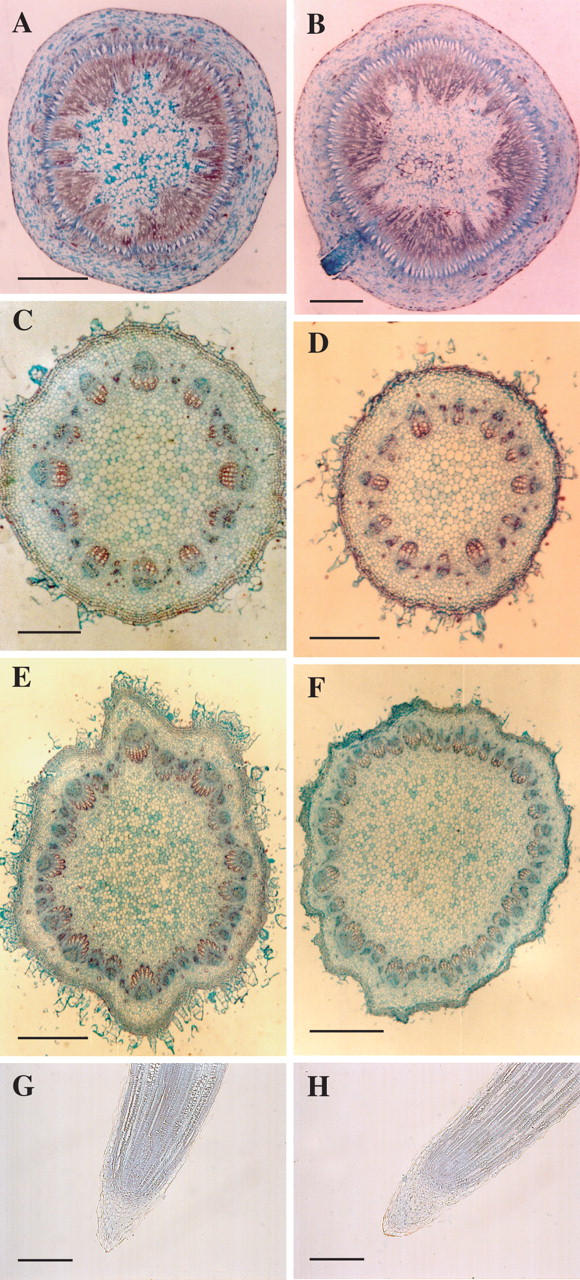
Developmental phenotype of the stem fasciated (stf) mutant of sunflower (Helianthus annuus). (A–F) Transverse section of hypocotyls (A, B), epicotyls (C, D) and stems (E, F) of wild type (A, C, E) and stf (B, D, F). (G, H) Longitudinal section of wild-type (G) and stf (H) roots. Sections were stained in safranin and fast green. Scale bars: A–D = 2 mm; E, F =1 mm; G = 300 μm; H = 400 μm.
Nucleus size in cells of the PZ and CZ of the SAM
To obtain a more precise cytological characterization of the SAM, sections of shoot tips were stained by the Feulgen method. Median longitudinal sections of wild-type sunflower shoot apices reveal a distinctive CZ that includes the central region of the tunica and a portion of the underlying corpus (Fig. 7A). The tunica consists of an overlying epidermal L1 layer and an underlying L2 layer (Fig. 7A, B). Both of these layers are one cell thick, and each remains clonally distinct from the others because the cells within them divide only in an anticlinal orientation, perpendicular to the plane of the meristem. The CZ is recognizable because of the relatively faint staining of its nuclei in contrast to those cells in surrounding region of the apex (PZ) and leaf primordia. In stf SAM the CZ is broader than in the wild type (compare A and C in Fig. 7). The wild type and the PZ of stf SAM showed two distinct layers forming the tunica (Fig. 7A, C, E). By contrast, the L2 layer in the CZ of the stf SAM was no longer visible as in the wild-type apex (compare B and D in Fig. 7). In both wild-type and stf SAM the nuclei of PZ cells were significantly larger than the nuclei of CZ cells (Fig. 7F). Notably, the nucleus size of both PZ and CZ cells of stf was significantly higher than that in cells found in a normal SAM.
Fig. 7.
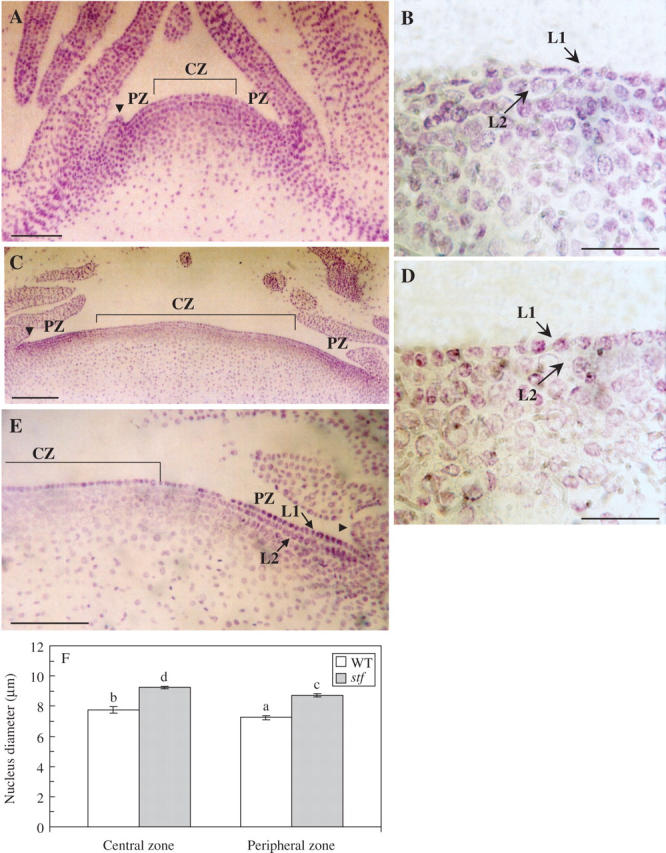
Shoot apical meristem (SAM) of the stem fasciated (stf) mutant of sunflower (Helianthus annuus). (A, C) Median longitudinal section of wild-type (A) and stf (C) shoot apex from 18-d-old seedlings. (B, D) Median longitudinal section of central zone (CZ) of WT (B) and stf (D) SAM. (E) Median longitudinal section of peripheral zone (PZ) of stf SAM. Sections were stained with the Feulgen method. Arrowheads indicate the leaf primordia; arrows indicate the L1 or the L2 layer. (F) Nucleus diameter in cells of the PZ and CZ of wild-type (WT) and stf SAM. Data are means (± s.d.) of three independent experiments (progenies) each with five replicates (SAMs). Values with different letters are significantly different at the P = 0·05 level according to Tukey's test. Scale bars: A, C = 100 μm; B, D = 45 μm; E = 110 μm.
Endogenous levels of IAA
To investigate whether the morphological features of the stf mutant were a consequence of altered levels of endogenous auxin, GC-MS-SIM analyses during different stages of plant development were performed. The level of free IAA in stf seedlings was significantly higher (about 3-fold) than in wild-type seedlings (Fig. 8A). Analogously, IAA concentration in reproductive shoot apices of 40-d-old stf plants, at floral stage 5 (Marc and Palmer, 1981), was higher (about 4·5-fold) than the one present in normal plants (Fig. 8B). By contrast, no significant differences between the two genotypes were observed in the apical portions of stems taken from 40-d-old plants (Fig. 8C).
Fig. 8.
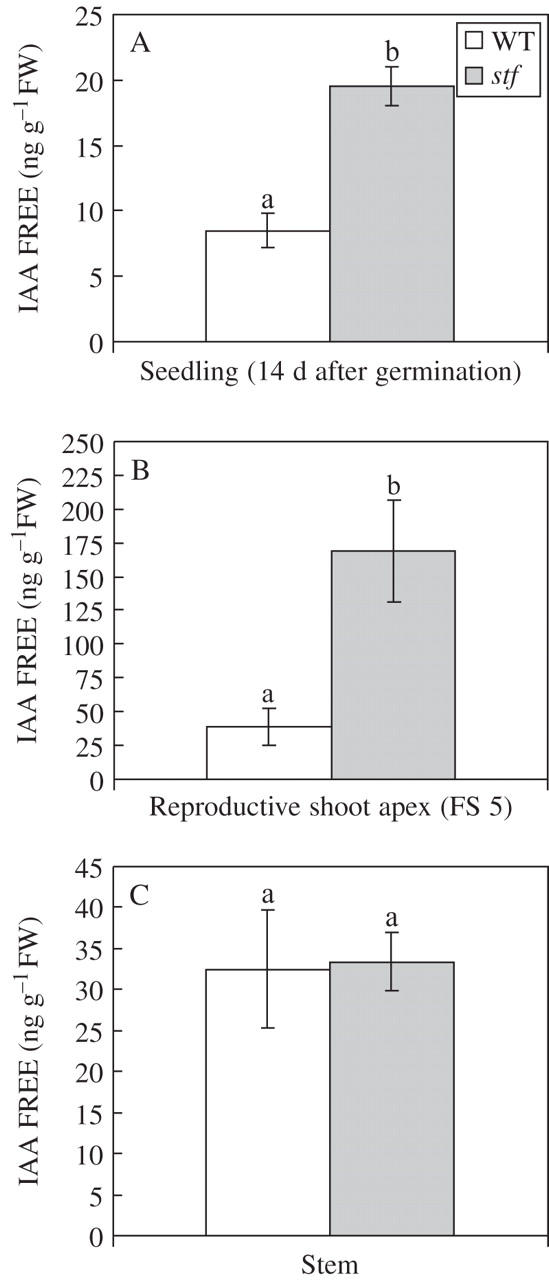
Level of endogenous IAA free in the stem fasciated (stf) mutant of sunflower (Helianthus annuus). (A) Level of endogenous IAA in 14-d-old stf and wild-type (WT) seedlings. (B) Level of endogenous IAA in reproductive shoot apex of 40-d-old plants [floral stage 5 (FS 5) according to Marc and Palmer, 1981]. (C) Level of endogenous IAA in apical stem of 40-d-old plants (median region of the 5th internode). The free IAA concentrations were measured on GC-MS-SIM with stable isotopes as internal standards. Data are means (± s.d.) of three independent experiments each with three replicates. Values with the same letter are not significantly different at the P = 0·01 level according to Student's t-test.
Effect of IAA treatments on root length
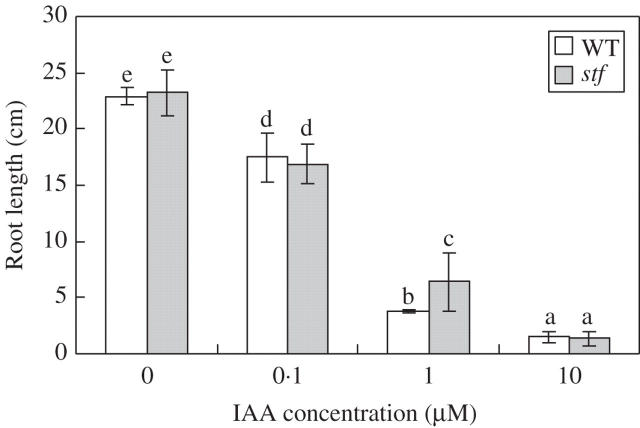
To determine whether the stf mutant is less sensitive to auxin, the response of stf to IAA was examined. After 2 weeks of growth in several concentrations of IAA, a dose-dependent inhibition of the root elongation was observed in both genotypes (Fig. 9). stf roots were only slightly resistant to 1 μm auxin compared with the wild type, suggesting that auxin perception in stf was normal.
Fig. 9.
Effect of indole-3-acetic acid (IAA) treatments on root length of wild-type (WT) and stem fasciated (stf) mutant of sunflower (Helianthus annuus). Data are means (± s.d.) of three independent experiments each with 30 replicates (seedlings). Values with the same letter are not significantly different at the P = 0·05 level according to Tukey's test.
In vitro development of stf seedlings and callus induction from stf organs
Because auxins are required for proliferation of cultured plant cells in vitro, an investigation was carried out to see if stf explants could proliferate in the absence of exogenously applied phytohormones. A significant production of callus from hypocotyl or epicotyl segments of stf seedlings was obtained on MS medium without growth regulators (Fig. 10A–C). By contrast, in wild-type explants, cell division in the cut surface was rapidly lost and the tissues became necrotic (Fig. 10B, C). Notably, cell proliferation in both genotypes is characterized by a basipetal polarity (Fig. 10B, C). Cotyledon explants of the stf mutant were also capable of undergoing phytohormone-free cell proliferation, sometimes producing adventitious roots (Fig. 10D). This experiment demonstrated that all three organs tested (hypocotyl, epicotyl and cotyledon) were able to sustain auxin-autonomous growth.
Fig. 10.
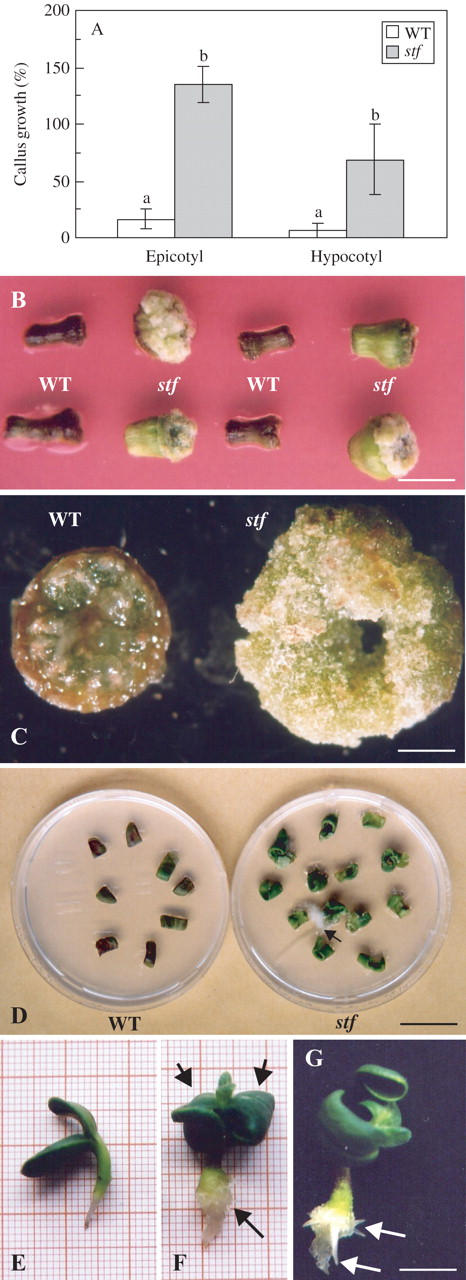
Developmental phenotype of the stem fasciated (stf) mutant cultured in vitro on MS basal medium without growth regulators. (A) Callus growth from hypocotyl and epicotyl explants of wild-type (WT) and stf seedlings. For callus growth measurements, the fresh weight of cultured explants (30 d) was expressed as a percentage of the initial fresh weight of hypocotyl or epicotyl explants. Data are means (± s.d.) of three independent experiments each with five replicates (Petri dishes). Values with different letters are significantly different at the P = 0·01 level according to Student's t-test. Statistical analysis was performed separately for hypocotyl and epicotyl explants. (B) Callus production from hypocotyl explants of wild-type (WT) and stf seedlings. (C) Callus production localized at the basal end of wild-type (WT) and stf hypocotyls. (D) Callus production from cotyledon explants of wild-type (WT) and stf seedlings; arrow indicates an adventitious root. (E, F, G) Seedlings of wild-type (E) and stf (F, G) grown for 1 week on MS basal medium without growth regulators. In (F) arrowheads indicate epinastic cotyledons, while the arrow indicates callus proliferation; in (G) arrows indicate the adventitious roots at the base of the stf hypocotyl. Scale bars: B, G = 1 cm; C = 1·5 mm; D = 2·5 cm.
Wild-type and stf seeds were surface-sterilized and germinated in the light on MS medium deprived of growth regulators (Fig. 10E–G). One week after germination, stf cotyledons were epinastic and a dedifferentiation of the base of hypocotyls was sometimes observed (Fig. 10F). Concomitantly, adventitious root primordia developed in this region (Fig. 10G).
Effect of light on hypocotyl length
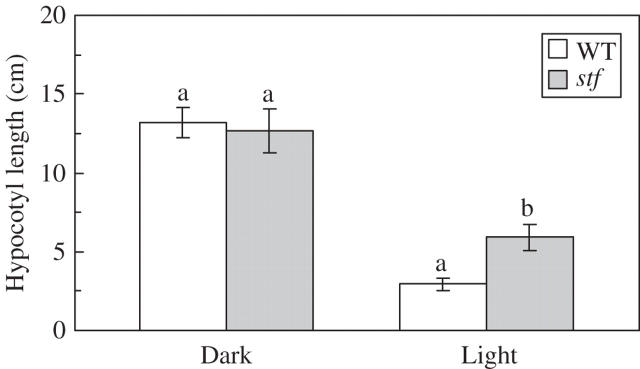
Hypocotyl elongation assay has been performed to compare the sensitivity with continuous light of the stf mutant. After 2 weeks of growth a light-dependent inhibition of hypocotyl elongation was observed in both genotypes (Fig. 11). However, stf mutant has longer hypocotyls than the wild type. By contrast, stf seedlings exhibit normal hypocotyl length in the dark.
Fig. 11.
Effect of light treatment on hypocotyl length of the stem fasciated (stf) mutant of sunflower (Helianthus annuus). Data are means (± s.d.) of three independent experiments each with 30 replicates (seedlings). Values with the same letter are not significantly different at the P = 0·01 level according to Student's t-test. Statistical analysis was performed separately for explants grown under dark and light conditions.
DISCUSSION
Development of the stf SAM in vegetative and reproductive phases
To learn about the control of meristem structure and function in sunflower, the recessive mutation stf, which affects various aspects of plant development, including SAM shape and arrangement, stem diameter, leaf number and shape, phyllotaxis, plastochron, and inflorescence development, have been characterized. This indicates that STF plays multiple developmental roles. In the stf mutant the SAM is enlarged with respect to wild-type plants. Because the SAM increase precedes the manifestation of the other plant defects, it could be the primary cause of the abnormal characteristics proper of the stf mutant. Microscopic analysis of the mutant's apex has also revealed an abnormal enlargement of nuclei in both CZ and PZ and a disorganized distribution of cells in the L2 layer of the CZ. These observations suggest that the STF gene is involved in the control of the pattern of cell differentiation and/or cell proliferation within different regions of the SAM. The particular shape of stf apices after the transition to reproductive phase (i.e. development of multiple apical domes) and the shape of stf inflorescences during anthesis could suggest that the enlarged SAM in the stf mutant can be constituted by a variable number of juxtaposed meristems. In fact, the tri-radiate types of fasciation are common in Compositae (White, 1948), and Jambhulkar (2002) considers juxtaposition of meristems as a potential explanation for the phenotype of another fasciated mutant of sunflower. However, these apical domes were not clearly separated as described for the Arabidopsis mutants mgoun1 (mgo1), mgo2 and mgo3 (Laufs et al., 1998; Guyomarc'h et al., 2004) that, unlike stf plants, also displayed stem bifurcation. In the mgo mutants RAM activity is also affected (Laufs et al., 1998; Guyomarc'h et al., 2004). By contrast, although stf seedlings differentiated a higher number of lateral roots than the wild type, there are no differences in the RAM size and root histology between stf plants and their normal siblings, suggesting that the STF gene product is not required to regulate the RAM structure.
Key regulators of meristem development often affect both shoot and flower meristem function (Clark et al., 1993, 1997; Fletcher, 2002). Indeed, the stf capitulum developed multiple inflorescences where the phyllotactic pattern 34 + 55 was completely disrupted. The extension of the fasciated phenotype to the inflorescence also determines the development of a high number of ray flowers. Nevertheless, no consistent effect of the stf mutation on shape and on flower organ number was observed. This is in contrast to the phenotype of other mutants characterized by enlarged SAM. For example, mutations in the clv1 and clv3 genes increase organ number in all four floral whorls, especially the inner whorls, as well as increasing the total number of whorls (Leyser and Furner, 1992; Clark et al., 1993, 1995). Deformed ray and disc flowers, often with abnormal petal development, were initiated in the boundaries of deep invaginated areas of the stf inflorescence. It is likely that abnormal flowers and bracts were differentiated by the inductive effects of the deep invaginations generated within the stf inflorescences. Wounding the receptacle by puncturing or cutting at an early stage when the receptacle dome was forming (floral stage 3), or later, when the first rows of disc floret primordia were appearing on the rim of the receptacle (floral stage 5), resulted in the initiation of involucral bracts, ray and disc flowers in the wounded area (Palmer and Marc, 1982), reproducing a partial phenocopy of stf inflorescence. The wild-type number of flower organs in the stf mutant could suggest that the STF gene product is not required to regulate the stem cell balance of floret primordia. Analogously, no supernumerary flower organs have been described in other mutants of sunflower with fasciated phenotype (Shattuk, 1985; Jambhulkar, 2002).
The decreased pollen viability displayed by the stf mutant has been also described in other sunflower mutants characterized by a fasciated phenotype (Shattuck, 1985) but, at present, the relationship between the control of SAM size and pollen fertility it is not clear. A pleiotropic effect of the stf mutation on pollen development could be evoked. On the other hand, as pointed out by White (1948), it is not uncommon that anthers of fasciated mutants in several species aborted all or part of their pollen before they mature.
The stf mutation drastically affects stem diameter, leaf morphology and phyllotaxis
In the stf mutant the stem is cylindrical at the base but gradually became more oval just before the insertion of the inflorescence. Therefore, in the upper portion of the shoot, the stf stem show a very abnormal cross-sectional shape. This morphology is not unusual because, especially in annual plants, the fasciated genotypes are often characterized by a broad stem at the tip. Compton's work on Pisum sativum (Compton, 1911) can be cited among the first observations of this phenomenon. Furthermore, the origin of this typical abnormal trait at the anatomical level has been studied in depth by LaMotte et al. (1988) in their characterization of the fasciated mutant f of Glycine max. The division of the stem in two or more sectors has never been observed in the stf mutant. Splitting of stem has been described in the fasciated mutant of Cicer arietinum that shows the development of a broad strap-like appearance from the fifth node (Knights, 1993).
One of the most dramatic phenotypes caused by the stf mutation is the alteration of the phyllotactic pattern. Phyllotaxis is determined by the spatial and temporal regulation of leaf formation at the SAM (Reinhardt, 2005). Leaves are formed in the PZ of SAM which encircles the CZ, the site of the stem cells (Steeves and Sussex, 1989). Inevitably, the field of cells from which primordium formation can occur (i.e. increasing the meristem size) might influence organ formation and phyllotaxis. Indeed, altered meristem size is frequently associated with altered phyllotaxis (Leyser and Furner, 1992; Clark et al., 1993, 1995; Tang and Knap, 1998; Jackson and Hake, 1999; Kaya et al., 2001; Taguchi-Shiobara et al., 2001; Byrne et al., 2003; Giulini et al., 2004; Running et al., 2004).
The most prevalent phyllotactic patterns are distichous (alternate or spiral if one primordium is formed at a time and decussate (opposite) or bijugate if primordia are formed in pairs (Reinhardt, 2005). In sunflower, as well as in many other species, the phyllotactic pattern changes during plant ontogeny. This results from the gradual increase of the diameter of the SAM during development (Marc and Palmer, 1981). Normal sunflower plants exhibit a transition from the initial decussate phyllotaxis to spiral phyllotaxis. In the stf mutant there is irregular leaf initiation rather that a shift to a different phyllotactic pattern. In fact, the whorled phyllotaxis pattern that characterizes the stf mutant is irregular: the number of leaves at each node varies as a function of their node position on the stem. A higher number of leaves are initiated from nodes at the top of stf stems (mature leaves) with respect to basal ones (juvenile leaves). Indeed, at the top of stf stems a clear distinction of phytomers was lost. A similar feature was reported in the sunflower variety Surya, for another fasciated mutant (Jambhulkar, 2002) and in Arabidopsis for the mutants clv1, clv3, fas1 and fas2 (Leyser and Furner, 1992; Clark et al., 1993, 1995).
The stf mutation affects the endogenous auxin levels
Auxin is a key plant hormone that regulates many important plant processes (Berleth et al., 2004). Recently, several studies have suggested that the polar flux and distribution are important cues for lateral organ initiation (Okada et al., 1991; Kuhlemeier and Reinhardt 2001; Benková et al., 2003; Friml, 2003; Reinhardt et al., 2003, 2005; Pfluger and Zambrynski, 2004; Fleming, 2005; Heisler et al., 2005). The stf mutant presented high endogenous auxin levels in young seedlings when the mutant phenotype is not yet visible, whereas auxin perception appears to be normal.
Although there is no clear evidence that auxin endogenous content is important in regulation of phyllotaxis, the disruption of regular phyllotactic pattern in the stf mutant as well as in the f mutant (Tang and Knap, 1998) could suggest the hypothesis that an increased level of IAA might influence the position and number of lateral primordia. The analysis of local distribution of auxin at the shoot meristem level, i.e. using cyto-immunohistological methods, is essential to determine the role of high IAA endogenous content in phyllotactic alteration in these mutants. At the same time, the analysis of expression pattern of key genes required for the induction and the spatial organization of lateral primordia such as PIN-FORMED 1 (PIN1), SHOOTMERISTEMLESS (STM) and CUP-SHAPED COTYLEDON2 (CUC2) (Heisler et al., 2005) could also be informative. On the other hand, SAM enlargement might also alter the position and number of auxin minima and, as a consequence, the setting of fields from which organ primordia arise (Fleming, 2005). In line with this, in clv and fas Arabidopsis mutants, the enlargement of the SAM diameter is coupled with phyllotactic alteration (Leyser and Furner, 1992; Clark et al., 1993, 1997).
By contrast to the high levels of IAA present in the shoot apex, the apical portions of stf stems contain normal IAA levels. Although these contrasting results could suggest an altered basipetal flow of auxin, mutants defective in auxin polar transport (e.g. pin1, aux1 and lop1) exhibit a different phenotype than the one observed in stf (Okada et al., 1991; Bennet et al., 1996; Carland and McHale, 1996). In addition, exogenous treatment with 2,3,5-triiodobenzoic acid does not confer a stf phenotype to wild-type plants (M. Fambrini, unpubl. res.). However, an eventual pleiotropic effect of the stf mutation on the auxin polar transport cannot be excluded on the basis of this indirect evidence. Indeed, if the basipetal auxin flow in the stf mutant is not reduced, a high level of IAA is also expected in the apical portion of the stem. Therefore, analyses of the IAA content on different portions of the stf stem (i.e., basal and median internodes) along with an auxin polar transport assay are both necessary to evaluate the possibility of an effect of the stf mutation on the basipetal flow of auxin.
Other lines of evidence suggest that elevated endogenous auxin levels significantly affect the stf phenotype. In particular, stf plants show a phenotype similar to that exhibited by auxin-over-producing plants. For example, the stf mutant showed epinastic cotyledons, reduced leaf expansion, and long hypocotyls under continuous white light. It is known that auxin influences hypocotyl elongation by light. For example, when compared with wild-type seedlings, auxin-over-producing superroot1 (sur1) and sur2 mutants, as well as 35S::iaaM transgenic seedlings, have longer hypocotyls when grown in light, yet exhibit normal hypocotyl length in the dark (Boerjan et al., 1995; Romano et al., 1995; Delarue et al., 1998). Furthermore, analogously to sur1 and yucca mutants (Boerjan et al., 1995; Zhao et al., 2001), explants of the stf seedlings could be cultured on medium lacking phytohormones, whereas wild-type explants died under these conditions. In this respect, it is noteworthy that in-vitro-cultured explants of fasciated mutants of A. thaliana (Mordhorst et al., 1998) and Mammillaria elongata (Papafotiou et al., 2001) showed a different behaviour than the wild types, suggesting that in these genotypes a genetic mechanism might operate through a hormonal imbalance (Gorter, 1965; Boke and Ross, 1978).
Hypocotyl disintegration concomitant with adventitious root development further supported that stf overproduced IAA. Disintegration and adventitious root formation at the base of hypocotyls could be the result of an increased concentration of, and/or a longer exposure to, IAA in these cells (Boerjan et al., 1995). In fact, if the amount of IAA to be transported from the apical part of the hypocotyl to its base is higher than the capacity to transport IAA, as in auxin overproduction mutants, IAA would accumulate in the lower regions of the hypocotyl, where it would exert its effect (Sánchez-Bravo et al., 1992). The development of a high number of lateral roots expressed by stf seedlings is also consistent with a higher auxin level. Studies have shown that lateral root initiation is dependent on IAA transported from the shoot into the root (Ljung et al., 2001). An excess of adventitious and lateral roots has been detected in well-characterized IAA overproduction mutants of Arabidopsis (e.g. sur1, sur2, rooty and yucca) (Boerjan et al., 1995; King et al., 1995; Delarue et al., 1998; Zhao et al., 2001, 2002; Mikkelsen et al., 2004).
It is noteworthy that in the stf mutant the ability of side shoots' outgrowth from axillary meristems is very low. This result is consistent with the primary role of auxin in the control of apical dominance (Romano et al., 1993; Leyser, 2002, 2003). A major site for auxin synthesis is at the primary shoot apex, particularly in young leaves, from which it is transported down the plant in the polar transport stream (Ljung et al., 2001, 2002). Auxin moving in this fashion can inhibit shoot branching because either blocking polar auxin transport or removing the shoot apex promotes shoot branching, with the branch-promoting effect of removing the apex being negated by the addition of auxin to the decapitated stump (Cline, 1991). In plants overproducing auxin, strong apical dominance is expected. In this respect, it is noteworthy that decapitation of stf plants is also insufficient to remove the auxin inhibition of shoot outgrowth, analogously to the auxin-overproducing mutant f of soybean (Tang and Knap, 1998).
Although genotypes with elevated auxin levels show similar phenotypic changes (e.g. epinastic cotyledons, long hypocotyls in light-grown seedlings, adventitious root formation, increased number of lateral roots, auxin-independent growth in tissue culture), they can be different in regards to other characteristics (Woodward and Bartel, 2005). For example, an enlarged SAM with a concomitantly fasciated phenotype and a high number of leaves was displayed by the soybean mutant f and also by stf plants of sunflower but not by ‘high auxin’ mutants of Arabidopsis (Boerjan et al., 1995; Delarue et al., 1998; Tang and Knap, 1998; Zhao et al., 2001). In addition, although enhanced shoot branching is traditionally correlated with decreased auxin concentration, both the ‘high auxin’ Arabidopsis mutants bushy and supershoot (sps) displayed a bushy habit (Reintanz et al., 2001; Tantikanjana et al., 2001). The reason for these differences is unknown. It is likely that some of these mutations affecting the IAA levels can also affect other aspect of the hormonal homeostasis. For example, the sps mutation of Arabidopsis confers a high endogenous level of free IAA as well as increased levels of zeatin-type cytokinins (Tantikanjana et al., 2001). It has been shown that the SPS gene encodes a cytochrome P450 (CYP79F1), which is thought to catalyse the formation of glucosinolates derived from methionine (Tantikanjana et al., 2001). It is not clear how a lesion in glucosinolate synthesis could affect cytokinin metabolism. The changes in cytokinin level in sps plants may be one of the feedback mechanisms involved in hormone interaction. A quantification of cytokinins in stf plants was not performed and it cannot be excluded that, in addition to elevated level of free IAA, the sensitivity and/or metabolism of other hormones may be affected.
In conclusion, the observed phenotype and the high level of endogenous auxin detected in stf plants suggest that the STF gene may be involved in the proper initiation of primordia and in the establishment of a phyllotactic pattern, probably through control of the SAM arrangement and hormonal homeostasis. However, the dramatically altered phenotype of the stf plants hinders the discrimination between the primary or the secondary effects induced by the stf mutation. Isolation and characterization of the STF gene should clarify its role in shoot development, and also help determine its involvement in auxin biosynthesis.
Acknowledgments
Thanks are due to Dr A. E. Sztein for revising and editing the manuscript.
LITERATURE CITED
- Baurle I, Laux T. 2003. Apical meristems: the plant's fountain of youth. BioEssays 25: 961–970. [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, et al. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, et al. 1996. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950. [DOI] [PubMed] [Google Scholar]
- Berleth T, Krogan NT, Scarpella E. 2004. Auxin signals—turning genes on and turning cells around. Current Opinion in Plant Biology 7: 553–563. [DOI] [PubMed] [Google Scholar]
- Berti F, Fambrini M, Turi M, Bertini D, Pugliesi C. 2005. Mutations of corolla symmetry affect carpel and stamen development in Helianthus annuus. Canadian Journal of Botany 83: 1065–1072. [Google Scholar]
- Bhalla PL, Singh MB. 2006. Molecular control of stem cell maintenance in shoot apical meristem. Plant Cell Reports 25: 249–256. [DOI] [PubMed] [Google Scholar]
- Binggeli P. 1990. Occurrence and causes of fasciation. Cecidology 5: 57–62. [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, et al. 1995. superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. The Plant Cell 7: 1405–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boke NH, Ross RG. 1978. Fasciation and dichotomous branching in Echinocereus (Cactaceae). American Journal of Botany 65: 522–530. [Google Scholar]
- Bonetta D, Bayliss P, Sun S, Sage T, McCourt P. 2000. Farnesylation is involved in meristem organization in Arabidopsis. Planta 211: 182–190. [DOI] [PubMed] [Google Scholar]
- Byrne ME, Kidner CA, Martienssen RA. 2003. Plant stem cells: divergent pathways and common themes in shoots and roots. Current Opinion in Genetics and Development 13: 551–557. [DOI] [PubMed] [Google Scholar]
- Carland FM, McHale NA. 1996. LOP1: a gene involved in auxin transport and vascular patterning in Arabidopsis. Development 122: 1811–1819. [DOI] [PubMed] [Google Scholar]
- Carles CC, Fletcher JC. 2003. Shoot apical meristem maintenance: the art of a dynamic balance. Trends in Plant Science 8: 394–401. [DOI] [PubMed] [Google Scholar]
- Castellano MM, Sablowski R. 2005. Intercellular signalling in the transition from stem cells to organogenesis in meristems. Current Opinion in Plant Biology 8: 26–31. [DOI] [PubMed] [Google Scholar]
- Cavallini A, Zolfino C, Natali L, Cionini G, Cionini PG. 1989. Nuclear DNA changes within Helianthus annuus L.: origin and control mechanism. Theoretical and Applied Genetics 77: 12–16. [DOI] [PubMed] [Google Scholar]
- Chen K-H, Miller AN, Patterson GW, Cohen JD. 1988. A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiology 86: 822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE. 2001. Cell signalling at the shoot meristem. Nature Reviews Molecular Cell Biology 2: 276–284. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. 1993. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119: 397–418. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. 1995. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121: 2057–2067. [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM. 1997. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89: 575–585. [DOI] [PubMed] [Google Scholar]
- Cline MG. 1991. Apical dominance. Botanical Review 57: 318–358. [Google Scholar]
- Compton RH. 1911. The anatomy of mummy pea. New Phytologist 10: 249–255. [Google Scholar]
- Delarue M, Prinsen E, Van Onckelen H, Caboche M, Bellini C. 1998. sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. The Plant Journal 14: 603–611. [DOI] [PubMed] [Google Scholar]
- Fambrini M, Cionini G, Bertini D, Michelotti V, Conti A, Pugliesi C. 2003. MISSING FLOWERS gene controls axillary meristems initiation in sunflower. genesis 36: 25–33. [DOI] [PubMed] [Google Scholar]
- Fleming AJ. 2005. Formation of primordia and phyllotaxy. Current Opinion in Plant Biology 8: 53–58. [DOI] [PubMed] [Google Scholar]
- Fletcher JC. 2002. Shoot and floral meristem maintenance in Arabidopsis. Annual Review of Plant Biology 53: 45–66. [DOI] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. 1999. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911–1914. [DOI] [PubMed] [Google Scholar]
- Friml J. 2003. Auxin transport—shaping the plant. Current Opinion in Plant Biology 6: 7–12. [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, et al. 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230. [DOI] [PubMed] [Google Scholar]
- Giulini A, Wang J, Jackson D. 2004. Control of phyllotaxy by the citokinin-inducible response regulator homologue ABPHYL1. Nature 430: 1031–1034. [DOI] [PubMed] [Google Scholar]
- Gorter CJ. 1965. Origin of fasciation. In: Ruhland W, ed. Handbuch der Pflanzenphysiologie. Vol. 15, Part II. New York, NY: Springer-Verlag, 330–351.
- Gottschalk W, Wolff G. 1983. The alteration of the shoot system by means of mutations. In: Frankel R, Gall GAE, Grossman M, Linskens HF, Riley R, eds. Monographs on theoretical and applied genetics. Induced mutation in plant breeding, Vol. 7. Berlin: Springer-Verlag, 43–64.
- Green KA, Prigge MJ, Katzman RB, Clark SE. 2005. CORONA, a member of the class III homeodomain leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. The Plant Cell 17: 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyomarc'h S, Vernoux T, Traas J, Zhou D-X, Delarue M. 2004. MGOUN3, an Arabidopsis gene with TetratricoPeptide-Repeat-related motifs, regulates meristem cellular organization. Journal of Experimental Botany 55: 673–684. [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, et al. 2005. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Current Biology 15: 1899–1911. [DOI] [PubMed] [Google Scholar]
- Irvine VC. 1940. X-radiation and growth substances as affecting growth primordial tissues. Proceedings of the Society of Experimental Biology and Medicine 43: 453–455. [Google Scholar]
- Itoh J-I, Hasegawa A, Kitano H, Nagato Y. 1998. A recessive heterochronic mutation, plastochron 1, shortens the plastochron and elongates the vegetative phase in rice. The Plant Cell 10: 1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh J-I, Kitano H, Matsuoka M, Nagato Y. 2000. SHOOT ORGANIZATION genes regulate shoot apical meristem organization and the pattern of leaf primordium initiation in rice. The Plant Cell 12: 2161–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Hake S. 1999. Control of phyllotaxy in maize by the abphyl1 gene. Development 126: 315–323. [DOI] [PubMed] [Google Scholar]
- Jambhulkar SJ. 2002. Growth and morphology and inheritance of fasciation mutation in sunflower. Journal of Genetics and Breeding 56: 327–330. [Google Scholar]
- Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E. 2006. An auxin-driven polarized transport model for phyllotaxis. Proceedings of the National Academy of Sciences of the USA 103: 1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Cooke TJ. 1997. Fundamental concepts in the embryogenesis of dicotyledons: a morphological interpretation of embryo mutants. The Plant Cell 9: 1903–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya H, Shibahara K, Taoka K, Iwabuchi M, Stillman B, Araki T. 2001. FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104: 131–142. [DOI] [PubMed] [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB. 1995. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. The Plant Cell 7: 2023–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights EJ. 1993. Fasciation in chickpea: genetics and evaluation. Euphytica 69: 163–166. [Google Scholar]
- Kuhlemeier C, Reinhardt D. 2001. Auxin and phyllotaxis. Trends in Plant Science 6: 187–189. [DOI] [PubMed] [Google Scholar]
- LaMotte CE, Curry TM, Palmer RG, Albertsen MC. 1988. Developmental anatomy and morphology of fasciation in the soybean (Glycine max). Botanical Gazette 149: 398–407. [Google Scholar]
- Laufs P, Dockx J, Kronenberger J, Traas J. 1998. MGOUN1 and MGOUN2: two genes required for primordium initiation at the shoot apical and floral meristem in Arabidopsis thaliana. Development 125: 1253–1260. [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KFX, Berger J, Jürgens G. 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96. [DOI] [PubMed] [Google Scholar]
- Lenhard M, Laux T. 2003. Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development 130: 3163–3173. [DOI] [PubMed] [Google Scholar]
- Lenzi A, Fambrini M, Barotti S, Pugliesi C, Vernieri P. 1995. Seed germination and seedling growth in a wilty mutant of sunflower (Helianthus annuus L.): effect of abscisic acid and osmotic potential. Environmental and Experimental Botany 35: 427–434. [Google Scholar]
- Leyser O. 2002. Molecular genetics of auxin signalling. Annual Review of Plant Biology 53: 377–398. [DOI] [PubMed] [Google Scholar]
- Leyser O. 2003. Regulation of shoot branching by auxin. Trends in Plant Science 8: 541–545. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Furner IJ. 1992. Characterisation of three shoot apical meristem mutants of Arabidopsis thaliana. Development 116: 397–403. [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. 2001. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. The Plant Journal 28: 465–474. [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, et al. 2002. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Molecular Biology 50: 309–332. [DOI] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JL, Barton MK. 1996. A member of the KNOTTED class of homeodomain proteins encoded by SHOOTMERISTEMLESS gene of Arabidopsis. Nature 379: 66–69. [DOI] [PubMed] [Google Scholar]
- Marc J, Palmer JH. 1981. Photoperiodic sensitivity of inflorescence initiation and development in sunflower. Field Crops Research 4: 155–165. [Google Scholar]
- Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. [DOI] [PubMed] [Google Scholar]
- Mikkelsen MD, Naur P, Halkier BA. 2004. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. The Plant Journal 37: 770–777. [DOI] [PubMed] [Google Scholar]
- Mordhorst AP, Voerman KJ, Hartog MV, Meijer EA, Van Went J, Koornneef M, et al. 1998. Somatic embryogenesis in Arabidopsis thaliana is facilitated by mutations in genes repressing meristematic cell divisions. Genetics 149: 549–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Nadjimov UK, Scott IM, Fatkhullaeva GN, Mirakhmedov MS, Nasirullaev BU, Musaev DA. 1999. Conditioning of fasciation by gibberellin and genotype in cotton (Gossypium hirsutum L.). Journal of Plant Growth Regulation 18: 45–48. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. 1991. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. The Plant Cell 3: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakòzdi K, Taller J, Alföldi Z, Hirata Y. 2002. Pepper (Capsicum annuum L.) cytoplasmic male sterility. Journal of Central European Agriculture 3: 150–158. [Google Scholar]
- Palmer JH, Marc J. 1982. Wound-induced initiation of involucral bracts and florets in the developing sunflower inflorescence. Plant and Cell Physiology 23: 1401–1409. [Google Scholar]
- Papafotiou M, Balotis GN, Louka PT, Chronopoulos J. 2001. In vitro plant regeneration of Mammillaria elongata normal and cristate forms. Plant Cell, Tissue and Organ Culture 65: 163–167. [Google Scholar]
- Pfluger J, Zambrynski P. 2004. The role of SEUSS in auxin response and floral patterning. Development 131: 4697–4707. [DOI] [PubMed] [Google Scholar]
- Pugliesi C, Fambrini M, Barotti S, Lenzi A, Baroncelli S. 1995. Inheritance of the ‘Basilicum Leaf’ mutation in sunflower (Helianthus annuus L.). Journal of Heredity 86: 76–78. [Google Scholar]
- Reinhardt D. 2005. Regulation of phyllotaxis. International Journal of Developmental Biology 49: 539–546. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C. 2000. Auxin regulates the initiation and radial position of plant lateral organs. The Plant Cell 12: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennet M, et al. 2003. Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Frenz M, Mandel T, Kuhlemeier C. 2005. Microsurgical and laser ablation analysis of leaf positioning and dorsoventral patterning in tomato. Development 132: 15–26. [DOI] [PubMed] [Google Scholar]
- Reintanz B, Lehnen M, Reichelt M, Gershenzon J, Kowalczyk M, Sandberg G, et al. 2001. bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. The Plant Cell 13: 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne PLH, Van der Schoot C. 1998. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 125: 1477–1485. [DOI] [PubMed] [Google Scholar]
- Romano CP, Cooper ML, Klee HJ. 1993. Uncoupling auxin and ethylene effects in transgenic tobacco and Arabidopsis plants. The Plant Cell 5: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano CP, Robson PRH, Smith H, Estelle M, Klee H. 1995. Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Molecular Biology 27: 1071–1083. [DOI] [PubMed] [Google Scholar]
- Running MP, Fletcher JC, Meyerowitz EM. 1998. The WIGGUM gene is required for proper regulation of floral meristem size in Arabidopsis. Development 125: 2545–2553. [DOI] [PubMed] [Google Scholar]
- Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, et al. 2004. Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferase. Proceedings of the National Academy of Sciences of the USA 101: 7815–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzin SE. 1999. Plant microtechnique and microscopy. New York, NY: Oxford University Press.
- Sánchez-Bravo J, Ortuño A, Botía JM, Acosta M, Sabater F. 1992. The decrease in auxin polar transport down the lupin hypocotyl could produce the indole-3-acetic acid distribution responsible for the elongation growth pattern. Plant Physiology 99: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Fletcher JC. 2002. Maintenance of shoot and floral meristem cell proliferation and fate. Plant Physiology 129: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck V. 1985. Inheritance of a morphological mutant in sunflower. Genetica Agraria 39: 187–192. [Google Scholar]
- Sinnott EW. 1960. Meristems. In: Plant morphogenesis. New York, NY: McGraw-Hill Book Company, 55–91.
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. 2006. A plausible model of phyllotaxis. Proceedings of the National Academy of Sciences of the USA 103: 1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM. 1989. Patterns in plant development, 2nd edn. Cambridge: Cambridge University Press.
- Stoenescu F. 1974. Genetics. In: Vranceanu AV, ed. Floarea-soarelui. Bucarest: Editura Academiei Republicii Socialiste, Romania, 92–125.
- Stone JM, Trotochaud AE, Walker JC, Clark SE. 1998. Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiology 117: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi-Shiobara F, Yuan Z, Hake S, Jackson D. 2001. The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes and Development 15: 2755–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Knap HT. 1998. Fasciation mutation enhances meristematic activity and alters pattern formation in soybean. International Journal of Plant Science 159: 249–260. [Google Scholar]
- Tantikanjana T, Yong JWH, Letham DS, Griffith M, Hussain M, Ljung K, et al. 2001. Control of axillary bud initiation and shoot architecture in Arabidopsis through the SUPERSHOOT gene. Genes and Development 15: 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas J, Vernoux T. 2002. The shoot apical meristem: the dynamics of a stable structure. Philosophical Transactions of the Royal Society London, Series B 357: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J. 2000. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127: 5157–5165. [DOI] [PubMed] [Google Scholar]
- White OE. 1948. Fasciation. Botanical Review 14: 319–358. [Google Scholar]
- Williams L, Fletcher JC. 2005. Stem regulation in the Arabidopsis shoot apical meristem. Current Opinion in Plant Biology 8: 582–586. [DOI] [PubMed] [Google Scholar]
- Williams L, Grigg SP, Xie M, Christensen S, Fletcher JC. 2005. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 132: 3657–3668. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. 2005. Auxin regulation, action, and interaction. Annals of Botany 95: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. 2001. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, et al. 2002. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes and Development 16: 3100–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]