Abstract
• Background Global phosphorus (P) reserves are being depleted, with half-depletion predicted to occur between 2040 and 2060. Most of the P applied in fertilizers may be sorbed by soil, and not be available for plants lacking specific adaptations. On the severely P-impoverished soils of south-western Australia and the Cape region in South Africa, non-mycorrhizal species exhibit highly effective adaptations to acquire P. A wide range of these non-mycorrhizal species, belonging to two monocotyledonous and eight dicotyledonous families, produce root clusters. Non-mycorrhizal species with root clusters appear to be particularly effective at accessing P when its availability is extremely low.
• Scope There is a need to develop crops that are highly effective at acquiring inorganic P (Pi) from P-sorbing soils. Traits such as those found in non-mycorrhizal root-cluster-bearing species in Australia, South Africa and other P-impoverished environments are highly desirable for future crops. Root clusters combine a specialized structure with a specialized metabolism. Native species with such traits could be domesticated or crossed with existing crop species. An alternative approach would be to develop future crops with root clusters based on knowledge of the genes involved in development and functioning of root clusters.
• Conclusions Root clusters offer enormous potential for future research of both a fundamental and a strategic nature. New discoveries of the development and functioning of root clusters in both monocotyledonous and dicotyledonous families are essential to produce new crops with superior P-acquisition traits.
Keywords: Actinorhizal, capillaroid roots, carboxylates, Casuarinaceae, cluster roots, Cyperaceae, dauciform roots, exudation, Fabaceae, Proteaceae, proteoid roots, Restionaceae
INTRODUCTION
Phosphorus (P) is an essential inorganic nutrient for all living organisms. It is required as a structural component in nucleic acids and phospholipids, as an element in intermediates in carbon metabolism, and to allow (in)activation of a wide range of enzymes. After nitrogen (N), P is quantitatively the most important inorganic nutrient for plant growth, and often limits primary productivity in natural systems as well as cropping systems, unless supplied as fertilizer (Vance et al., 2003). P is a non-renewable resource, unlike N, which can be assimilated from N2 into NH3 by free-living and symbiotic N2-fixing micro-organisms, or converted into NH3, 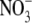 or urea industrially. Moreover, global P reserves are rapidly being depleted; depending on the assumed scenario, current P reserves will be halved (relative to the reserves at the turn of the twentieth century) by 2040 or, more likely, by 2060 (Steen, 1998). Whilst our global P reserves are being depleted, P levels in many agricultural soils are building up, because 80–90 % of P applied as fertilizer is sorbed by soil particles, rendering it unavailable for plants that lack specific adaptation to access sorbed P (Gerke et al., 1994; Jones, 1998a). With decreasing global P reserves, P-fertilizer prices are bound to increase. There is an urgent need to develop crops that are more efficient at acquiring inorganic P (Pi) from soil and/or at using P more efficiently. Equally, it is becoming increasingly important to use crops that reduce the off-site effects of P fertilization, thus reducing the risks of pollution of streams and rivers.
or urea industrially. Moreover, global P reserves are rapidly being depleted; depending on the assumed scenario, current P reserves will be halved (relative to the reserves at the turn of the twentieth century) by 2040 or, more likely, by 2060 (Steen, 1998). Whilst our global P reserves are being depleted, P levels in many agricultural soils are building up, because 80–90 % of P applied as fertilizer is sorbed by soil particles, rendering it unavailable for plants that lack specific adaptation to access sorbed P (Gerke et al., 1994; Jones, 1998a). With decreasing global P reserves, P-fertilizer prices are bound to increase. There is an urgent need to develop crops that are more efficient at acquiring inorganic P (Pi) from soil and/or at using P more efficiently. Equally, it is becoming increasingly important to use crops that reduce the off-site effects of P fertilization, thus reducing the risks of pollution of streams and rivers.
Unlike nitrate, which readily moves in soil towards the roots via both mass flow and diffusion, phosphate (Pi) is highly immobile. Mass flow typically delivers as little as 1–5 % of a plant's P demand, and the amount intercepted by growing roots is only half of that (Lambers et al., 1998). The rest of all required Pi must reach the root surface via diffusion; diffusion coefficients for phosphate in soil are typically very low compared with those for other nutrients: 0·3–3·3 × 10−13 m2 s−1 (Clarkson, 1981). Diffusion is particularly slow in dry soil (e.g. Turner and Gilliam, 1976; Bhadoria et al., 1991). Increasing Pi delivery to roots via mass flow can be achieved by enhanced transpiration rates, but this cannot have a major effect, and would be at the expense of a plant's water-use efficiency. Root interception of Pi can be increased by root proliferation, increased frequency and length of root hairs, a modified root architecture that enhances allocation to shallow soil horizons, and mycorrhizal symbioses. Diffusion of Pi toward the root can be increased by increasing the moisture content of dry soil, or by increasing the Pi concentrations in the soil solution through release of Pi from complexed, sorbed or organic forms of P. This review focuses on structural and functional root traits that enhance Pi acquisition from soil with a low availability of Pi, i.e. soils with a reasonable amount of total P, but where diffusion of Pi towards the root limits plant growth. In particular, it deals with traits of native species naturally occurring on soils with a low Pi availability, to explore the potential of these traits for future crop plants. The focus will be on species native to south-western Australia and the Cape region in South Africa, two of the world's 25 biodiversity hotspots. Both regions were once part of the southern-hemisphere super-continent Gondwanaland, and their soils are ancient and deeply weathered, especially those in Western Australia, some of which are estimated to over be 3 billion years old (White, 1986). As soils weather over thousands to millions of years, both the total P levels and the availability of Pi decline (Walker and Syers, 1976; Crews et al., 1995; Richardson et al., 2004). The decline in availability results from the main initial soil P-containing mineral, calcium apatite, being utilized by organisms to form organic P, and by sorption of P onto the surfaces of other minerals. This mineral-sorbed P is labile and can be desorbed in response to diffusion gradients as a result of Pi uptake by plant roots, or can be chemically displaced by root exudates. Over (geological) time, mineral-sorbed P can also be surrounded (occluded) by Fe and Al, rendering it essentially unavailable to plants. The evolutionary consequence of this decline in P levels and Pi availability is an incredibly diverse array of plant species with present-day root adaptations with remarkable ability to acquire sparingly available soil P, and to use internal P efficiently, and that could be explored for future use in crops.
COMMON ROOT TRAITS TO ENHANCE Pi ACQUISITION
Root architecture
This denotes the spatial configuration of roots of different order and age, with the implication that the overall configuration has some functional significance (Lynch, 1995). In terms of P nutrition, the root architecture of Phaseolus vulgaris when grown at low Pi supply is significant for immobile nutrients such as P because more laterals are produced in shallow soil horizons, where most of the P is located (Lynch and Brown, 2001). Fitter et al. (2002) showed that root architecture had a major effect in Arabidopsis thaliana, both in a pot experiment and in a field experiment conducted under natural conditions, on the fitness of a mutant with a reduced number of lateral roots relative to its isogenic wild-type when P was the limiting nutrient. As expected, when the more mobile nitrate was the limiting nutrient for plant growth, there was no difference in fitness between mutant and wild-type.
Root biomass
Most species allocate more biomass to roots when Pi is limiting for their growth (Brouwer, 1963, 1983). Some of the observed difference in biomass allocation pattern between plants grown with a high vs. a low supply of Pi may be ontogenetic, owing to comparisons of plants at different sizes, rather than a truly plastic response (Kemp and Blair, 1994; Niklas, 1994). However, there is also clear evidence that Pi supply has a direct effect on biomass partitioning, independent of ontogeny (Ryser et al., 1997; De Groot et al., 2001). Interestingly, many Lupinus species, some known to be highly P-efficient, show little change in biomass partitioning to roots as dependent on Pi supply (Keerthisinghe et al., 1998; Pearse et al., 2006; S. J. Pearse, unpubl. data). This low plasticity has been found in both cluster-root-bearing and non-cluster-root-forming Lupinus species (S. J. Pearse, unpubl. data). Effects of Pi supply on biomass partitioning between roots and shoots are thought to involve a decreased production in and export of cytokinins from roots at a low Pi supply, possibly associated with a decreased rate of uptake and metabolism of nitrogen (Kuiper et al., 1989). In the case of Lupinus species, would that mean that they show no change in cytokinin production and export as dependent on their P status? Or might they have a limited response to cytokinins? Do they respond to variation in N supply? Given that we do know that L. albus and L. mutabilis do increase their biomass partitioning to roots (increased root mass ratio) under water stress (Carvalho et al., 2004), a low plasticity in root mass ratio supply is either not typical for all species in this genus or is restricted to effects of P. Is the lack of response to Pi as found for some Lupinus species linked to the capacity of some species in this genus to produce cluster roots? Interestingly, cytokinins play a role in both biomass partitioning (Kuiper et al., 1989) and cluster-root formation, as antagonists of auxins (Neumann et al., 2002). Would that mean that the Lupinus species lacking the capacity to produce root clusters can relatively easily be modified into cluster-forming plants? So far, there are no data in the literature to provide satisfactory answers to many of the questions raised here.
Root length
In field plots of Beta vulgaris, total root-length production over the entire growing season was 3–4 times the size of the living root system at harvest (120 km m−2) in high-P plots, and five times (200 km m−2) in low-P plots (Steingrobe, 2001). The author calculated a 25 % increase in Pi uptake at low P supply as a result of this enhanced root-length production compared with that at the root production of high-P plants. A similarly enhanced root-length production at a low P supply has been observed for Hordeum vulgare (Steingrobe et al., 2001). Increased root production, without a proportional increase in living-root biomass, i.e. enhanced root turnover, allows greater amounts of uptake of immobile soil resources, such as P. Fast root turnover is a very important trait of cluster-root-producing species, as discussed below (Shane and Lambers, 2005a).
Specific root length
In the cases where plants were found to respond to Pi supply with a change in specific root length (SRL), their SRL increased with decreasing Pi supply (Powell, 1974; Christy and Moorby, 1975; Schroeder and Janos, 2005). The increase in SRL is associated with a decrease in root diameter (Powell, 1974), especially for the apical regions of the root system (Mollier and Pellerin, 1999). However, a decrease in root diameter is by no means a universal response to a low Pi supply (e.g. Borch et al., 1999; Schroeder and Janos, 2005).
Root hairs
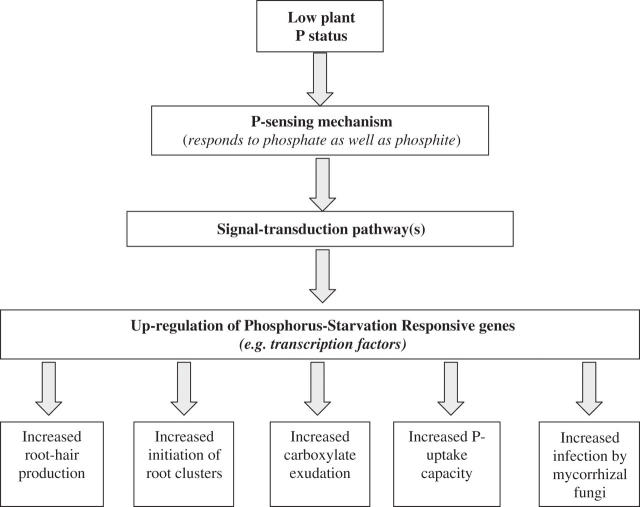
Root hairs are a fairly common root structure, and increased root-hair length and numbers are considered to be an adaptation that enhances Pi acquisition and a plant's competitive advantage when soil Pi is limiting for growth (Bates and Lynch, 2001). Species that develop more and/or longer root hairs, e.g. Lolium perenne, are far more efficient at accessing Pi from soils, and thus show less of a growth response in P-fertilized soils than do species that lack these traits, e.g. Podocarpus totara (Clarkson, 1981). This point was elegantly demonstrated in a comparison of genotypes of Hordeum vulgare; genotypes with the capacity to form longer root hairs (about 1 mm) took up more P, and tended to yield better when Pi was limiting crop growth compared with genotypes having roots hairs half the length (about 0·5 mm) (Gahoonia and Nielsen, 2004). Root-hair abundance and length is enhanced by P deficiency (Schmidt, 2001). The increased growth of root hairs observed for plants grown at low Pi availability can be mimicked in plants grown at high Pi supplies by adding an ethylene precursor to ‘high-P’ roots. Similarly, root-hair growth can be inhibited by adding the ethylene inhibitor 1-amino-cyclopropane-1-carboxylate (ACC) to the medium of ‘low-P’ roots (Zhang et al., 2003). This suggests that ethylene plays a major role in modulating the growth of root hairs in response to plant P nutrition. A recent detailed anatomical analysis in A. thaliana has shown that the effects of low Pi availability and ethylene on root-hair development differ, with low P status leading to a decreased size and increased number of cortical cells, and increased numbers (approximately double) of root-hair-bearing epidermal cell files, whereas ethylene does not (Zhang et al., 2003). Split-root experiments have established that a shoot-derived signal is required for root hairs to increase in length; the signal is translocated to the roots only when the shoot senses a low P status, and root-hair length is even further enhanced by a low P status in the roots (Jungk, 2001). The signal is unknown but given that auxin is also involved in root-hair formation (Schmidt, 2001), the shoot-derived signal might be an auxin. Increased length and abundance of root hairs is one of the typical adaptive P-starvation-induced plant responses (Fig. 1).
Fig. 1.
Plant responses to inorganic phosphorus (Pi) limitation. A low external Pi availability decreases the plant's internal P status. When the plant senses a low P status, P-starvation responses are up-regulated. P-starvation responses, depending on the species, include increased root-hair formation, root-cluster initiation and development, carboxylate exudation, P-uptake capacity, and mycorrhiza formation. Phosphite acts as an analogue of phosphate, and hence suppresses the typical P-starvation responses shown here. Based on Abel et al. (2002) and other references cited in the text.
Mycorrhizal associations
The vast majority (82 %) of all higher plant species have the capacity to form a symbiotic association with a mycorrhizal fungus (Brundrett, 2002). It is widely accepted that the ancestral mode of Pi acquisition in higher terrestrial plants was through an association with arbuscular mycorrhizal (AM) fungi; more recent mycorrhizal associations include ectomycorrhizas and orchidaceous mycorrhizas (Brundrett, 2002). At low Pi availability, the mycorrhizal symbiosis often enhances plant Pi uptake and growth, especially when the species has a root system that is relatively coarse with few root hairs, e.g. in Citrus species (Graham and Eissenstat, 1994). However, even when there is no effect on net Pi uptake or growth, there can be a major down-regulation of the roots' high-affinity Pi transporters (Smith et al., 2003), possibly in response to an improved plant P status, owing to rapid Pi uptake by the external mycelium. Further research is required to confirm this contention. For further information on the role of mycorrhizas for plant Pi uptake, the reader is referred to recent reviews (Brundrett, 2002; Söderström, 2002). Present-day non-mycorrhizal species must have lost their ability to form an association with AM fungi. Of the minority of species that do not form a mycorrhizal symbiosis, some have specialized root clusters such as proteoid and dauciform root clusters, and specialized physiology associated with rapid rates of carboxylate exudation, as discussed below. However, it should be noted that these specialized adaptations are not restricted to non-mycorrhizal species. There are, indeed, several mycorrhizal species that also have the capacity to produce root clusters, e.g. Casuarina species (Casuarinaceae), Myrica species (Myricaceae) and Viminaria viminalis (Fabaceae) (Neumann and Martinoia, 2002; Shane and Lambers, 2005a). Mycorrhizal associations can even be formed with the root clusters of Hakea verrucosa, a species naturally occurring on soils containing high levels of nickel (Boulet and Lambers, 2005). Furthermore, some non-mycorrhizal species (e.g. Brassicaceae) lack morphological adaptations like root clusters, but show rapid rates of carboxylate exudation (Hoffland et al., 1989). Finally, some species that lack mycorrhizal associations, e.g. Chenopodiaceae and Urticaceae (Lambers et al., 1998), would appear to have no specialized morphological adaptations, and these species tend to be restricted to relatively nutrient-rich sites and habitats with low competitive pressure (Olsson and Tyler, 2004).
High-affinity Pi transporters
Much remains to be discovered about the expression of high-affinity Pi transporters as dependent on plant P status. Recent discoveries have revealed that sugars are integrally related to P-deficiency-induced expression of one of these transporters in L. albus (Liu et al., 2005). Exogenous sugars stimulate accumulation of transcripts of a high-affinity transporter in dark-grown, P-sufficient seedlings. Conversely, in intact P-deficient plants, expression of this transporter in cluster roots was reduced in girdled plants, and in dark-grown plants in which expression was rapidly restored upon re-exposure to light. Similar results were also obtained for a gene encoding acid phosphatase and a third gene, both being P-deficiency induced. There is obviously cross-talk between phosphorus acquisition and carbon metabolism, similar to that between nitrogen and sulfur uptake and carbon metabolism (Lejay et al., 2003). The promoters of the genes encoding the high-affinity transporter and the acid phosphatase contain a short sequence that is identical to the binding site for a transcription activator for P-deficiency-induced genes in A. thaliana (Rubio et al., 2001).
Enhanced expression of high-affinity, plasma-membrane-bound Pi transporters in roots, and a concomitantly increased P-uptake capacity, is a typical P-starvation response (Burleigh and Harrison, 1999; Dong et al., 1999) (Fig. 1). This response is usually interpreted as an acclimation to a low availability of Pi in soil. However, diffusion of Pi in soil is the key limiting factor for Pi uptake, and changes in kinetic parameters of the roots' P-uptake system, including an increase in Imax (maximum Pi inflow rate), have little effect on a plant's capacity to acquire Pi from soil (Silberbush and Barber, 1983; Raghothama and Karthikeyan, 2005). This is not to say that plants do not need a high-affinity system for Pi uptake; rather, it shows that enhancing the expression of this Pi transport system does not have a proportional effect on Pi uptake, and may have no effect at all. This may explain, partly, why over-expression of a high-affinity phosphate transporter in transgenic Hordeum vulgare had no effect on Pi uptake from soil (Rae et al., 2004). What might be the adaptive significance of differential expression of high-affinity Pi transporters? Species that have a very low capacity to adjust their Pi uptake capacity in response to changes in Pi supply in the root environment show signs of P toxicity at elevated Pi supply (Shane et al., 2004a, b; Shane and Lambers, 2006). Therefore, we suggest that the capacity to down-regulate Pi transporters at high Pi supply is the trait that has adaptive significance, rather than the capacity to up-regulate Pi transporters at a low Pi supply. Although over-expression of high-affinity Pi transporters may not enhance Pi acquisition from soil, we envisage that it might improve internal P utilization. P-starved A. thaliana plants have been found to express high-affinity Pi transporters in roots as well as in developing flowers and fruits (Karthikeyan et al., 2002). If expression of high-affinity Pi transporters can be reduced in reproductive organs of grain crops, accumulation of P in seeds may be decreased, allowing P to be utilized in photosynthetic tissues, which could lead to increased grain production, and greater P return to the soil in organic form.
Effects of phosphite on P-starvation responses
A low plant P status induces the P-starvation responses as discussed above. Many of these responses are suppressed by phosphite, which acts as an analogue of phosphate. Typically suppressed P-starvation responses include increased allocation to root biomass (Varadarajan et al., 2002), enhanced root-hair formation (Ticconi et al., 2001), up-regulation of the high-affinity phosphate transporters and acid phosphatases (Varadarajan et al., 2002), and cluster-root formation (Gilbert et al., 2000). Phosphite inhibits mycorrhiza formation in Zea mays (Seymour et al., 1994), but not in Allium cepa (Sukarno et al., 1998), Eucalyptus marginata, E. globulus or Agonis flexuosa (Howard et al., 2000). The contrasting results for mycorrhization might be due to the fact that phosphite inhibits the expression of high-affinity phosphate transporters (Varadarajan et al., 2002), which would lower the plant P status, and thus indirectly enhance mycorrhization. Phosphite is also used as a fungicide, e.g. to combat the soil-borne plant pathogen Phytophthora cinnamomi in natural ecosystems (Hardy et al., 2001). Considering the effect of phosphite on P-starvation responses, the use of phosphite as a fungicide in pristine ecosystems clearly needs further scrutiny.
Rhizosphere alteration
As discussed above, enhanced root production is an adaptive response to acquire poorly mobile soil resources. An alternative strategy is to enhance the availability of Pi in soil. There are two fundamentally different mechanisms to enhance Pi availability. First, in superficial, dry soil horizons, where most of the P will be located, the mobility of Pi can be enhanced by the release of water into that dry soil. The released water would originate from moister regions in the soil, and be transported inside the root system in a process termed ‘hydraulic redistribution’ (Burgess et al., 1998, 2000). When this process was first described for desert plants that took up water from deep soil layers and released it, at night, into superficial layers, it was termed hydraulic lift. However, it has since been established that water can flow downwards as well as upwards, and it is envisaged that it will also move horizontally, from roots in moist superficial patches via the stem to the roots in drier soil, where the water can be released. Secondly, the concentration of Pi, the only form of P that is taken up by roots, can be enhanced by the release of root exudates, particularly carboxylates and phosphatases (Raghothama and Karthikeyan, 2005). These two strategies to enhance Pi availability are discussed in the next two sections.
ENHANCED Pi UPTAKE ASSOCIATED WITH HYDRAULIC REDISTRIBUTION?
When some roots are in contact with moist soil, while others on the same plant are in dry soil, water may move from moist to dry patches. This was first discovered in desert shrubs, where water can move from moist deep soil layers into shallower dry soil, and was termed ‘hydraulic lift’ (Caldwell and Richards, 1989). The water that is transported from deeper and moist layers into shallower dry soil is available for the species that exhibited hydraulic lift as well as for neighbouring plants (Caldwell and Richards, 1989). Hydraulic lift usually occurs at night, in C3 species, but can also occur during the day in CAM species (Yoder and Novak, 1999). However, water can also move from roots in shallow layers, moistened after rain, to roots deep in the profile (Burgess et al., 1998). Equally, water can move horizontally, via the stem, depending on soil water content (Hultine et al., 2003), and hence the term ‘hydraulic redistribution’ is more appropriate (Burgess et al., 1998, 2000). Hydraulic redistribution is not restricted to woody species, but has also been demonstrated in the crop species Pennisetum americanum (Vetterlein and Marschner, 1993). Water uptake by roots requires the expression of water-channel proteins (aquaporins); these proteins are expressed in a diurnal pattern, with low expression levels at night and increasing expression early in the day (Vandeleur et al., 2005). One would expect that water release also requires expression of water-channel proteins, but this has not yet been investigated.
Because diffusion of Pi in dry soil is very slow (Amijee et al., 1991; Bhadoria et al., 1991), Pi uptake declines with decreasing soil moisture content (Turner and Gilliam, 1976; Vig and Singh, 1983; Mouat and Nes, 1986). Therefore, nutrient uptake from dry shallow patches in soil is expected to increase when the soil is moistened due to hydraulic redistribution (Vetterlein and Marschner, 1993; Horton and Hart, 1998; Huang, 1999), and this could be especially significant for poorly mobile nutrients such as P. Equally, when deeper soil layers contain abundant P reserves, hydraulic redistribution down the profile might enhance the uptake of Pi (McCulley et al., 2004). This interesting concept of enhancing Pi availability by hydraulic redistribution is, however, particularly difficult to approach experimentally, and hence there is little convincing evidence to support it. Valizadeh et al. (2003) found that P banded in dry topsoil was accessed by Triticum aestivum with access to moist subsoil, as a result of to the release of hydraulically lifted water.
In summary, there is a wealth of information on the occurrence of hydraulic redistribution and the use of hydraulically lifted water by neighbouring plants. It is highly likely that hydraulic redistribution enhances Pi acquisition from P-enriched, dry soil patches. However, further research is required to establish the extent to which hydraulic redistribution may favour Pi acquisition.
ENHANCED Pi UPTAKE ASSOCIATED WITH THE RELEASE OF ROOT EXUDATES
In addition to increasing the diffusion coefficient of Pi in soil by hydraulic redistribution as discussed above, root activity can also enhance the concentration of Pi in soil, owing to the release of exudates.
Carboxylates
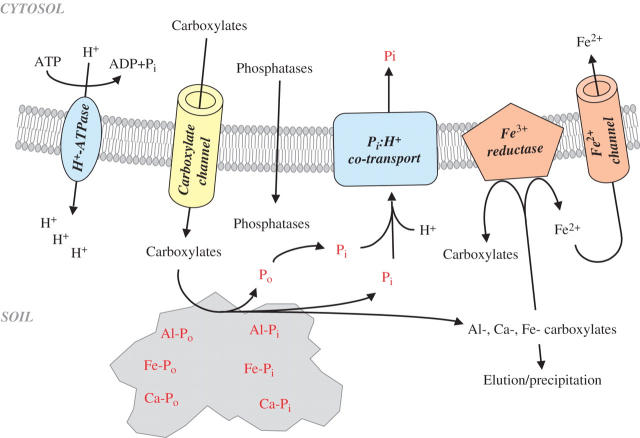
Carboxylates (e.g. citrate, malate) can be major components of exudates released by roots, especially under P deficiency (Gardner et al., 1983; Hoffland et al., 1989; Keerthisinghe et al., 1998). However, some high-exuding plant species, e.g. Cicer arietinum, appear to release carboxylates (mainly malonate) constitutively (Wouterlood et al., 2004). Carboxylates mobilize both inorganic P and organic P (Po), because they complex metal cations that bind phosphate and displace phosphate from the soil matrix by ligand exchange (Fig. 2) (Gerke et al., 2000; Hayes et al., 2000; Jones et al., 2003). The cations excreted together with the carboxylates to maintain charge balance may be protons, leading to rhizosphere acidification (Hinsinger, 2001; Hinsinger et al., 2003). However, other cations, especially K+, are at least as important (Y Zhu et al., 2005), and carboxylate exudation is not invariably associated with acidification (Roelofs et al., 2001). Transport of carboxylates in the anionic form from the cytosol (pH ≈ 7·2–7·5) into a more acidic rhizosphere is likely to result in protonation of the carboxylates in the rhizosphere. This is likely to contribute to scavenging of H+ from the rhizosphere, and hence increase the rhizosphere pH. In fact, unless the soil pH is initially alkaline, acidification does not enhance Pi availability; rather, acidification immobilizes Pi at low pH due to the formation of Fe and Al complexes (Lambers et al., 1998). In addition to P immobilization, acidification can influence the extent of ionization of carboxylates (Jones, 1998b; Hinsinger et al., 2003), which can reduce their chelating ability, potentially rendering them ineffective in acidified soil (Pearse et al., 2006)
Fig. 2.
Effects of carboxylates (and other exudates) on inorganic phosphorus (Pi) and organic P (Po) mobilization in soil. Carboxylates are released via an anion channel. The exact way in which phosphatases are released is not known. Carboxylates mobilize both inorganic and organic phosphorus. Phosphatases hydrolyse organic phosphorus compounds, once these have been mobilized by carboxylates. Carboxylates will also mobilize some of the cations that bind P. Some of these cations (especially Fe) move to the root surface for uptake by the roots. Others move down the soil profile, where they are thought to give rise to the formation of laterite (in the case of Fe) (Pate et al., 2001; Verboom and Pate, 2003) or other precipitates (in the case of Al or Ca) (Verboom and Pate, 2006).
Phenolics and mucilage
Exudation of phenolics may also increase under P deficiency (Neumann and Römheld, 2001; Juszczuk et al., 2004), but this has received less attention in the literature. Similarly, release of mucilage can be enhanced under P deficiency, and this can also enhance Pi availability in soil (Nagarajah et al., 1970; Gaume et al., 2000; Grimal et al., 2001). Phenolics and mucilage act in the same way as carboxylates (Guppy et al., 2005), but tend to be less effective than carboxylates (Neumann and Römheld, 2001). In addition, release of phenolics may serve a fungistatic role (Weisskopf et al., 2006), as further discussed in the section below dealing with root clusters.
Phosphatases
Organic P typically accounts for 30–80 % of total P in soil (Pederson, 1953; Tarafdar and Claassen, 1988; Adams, 1992). Soil organic P compounds (mainly phosphate mono- and di-esters, Sumann et al., 1998), after having been mobilized by carboxylates, must first be hydrolysed, to release Pi for plant uptake (George et al., 2002) (Fig. 2). Acid phosphatases can hydrolyse a range of organic P compounds (Tarafdar and Claassen, 2001), and both expressed sequence tags for phosphatase (Uhde-Stone et al., 2003) and these enzymes are more abundant in the rhizosphere when plants are P starved (e.g. Li et al., 1997; Gilbert et al., 1999; Yun and Kaeppler 2001; Wasaki et al., 2003). Phytases are required to hydrolyse phytate (= myo-inositol penta- and hexa-phosphates), which is fairly resistant to other phosphatases (Hayes et al., 2000). Phytate can be a major component of the soil organic P pool (Pederson, 1953; McKercher and Anderson, 1968). Phosphatases and phytases in soil may be of microbial origin (Tarafdar and Claassen, 2001), but roots also exude phosphatases (Tarafdar and Claassen, 2001, 2005), and roots of some species also release significant amounts of phytases (Li et al., 1997). Most plants have a very limited capacity to access phytate in the rhizosphere, except in the presence of micro-organisms that can dephosphorylate phytate (Richardson et al., 2001). Transgenic plants of A. thaliana, exhibiting enhanced exudation of extracellular phytase (derived from Medicago truncatula) from their roots, have greater access to phytate than their wild-type (Xiao et al., 2005). Similarly, transgenic plants of Trifolium subterraneum, exhibiting enhanced, constitutive expression and exudation of a phytase derived from Aspergillus niger, had better access to phytate than wild-type plants (George et al., 2004). However, this effect was only pronounced when plants were grown in non-sorbing, sterile laboratory media, and much less so when plants were grown in soil where phytase is rapidly immobilized, limiting its ability to interact with phytate (George et al., 2005). This suggests that phytate can only be dephosphorylated by phytase after it has been mobilized into the soil solution by, for example, carboxylates (Fig. 2).
Exudation as dependent on soil moisture
Phosphate-starvation responses are controlled systemically, via signals originating in the shoot (Abel et al., 2002; Fig. 1). This explains why a low soil moisture content, which reduces the mobility of Pi in soil (e.g. Turner and Gilliam, 1976; Bhadoria et al., 1991), and hence tends to lower the plant's P status, enhances root exudation (Liebersbach et al., 2004). As a consequence, Pi uptake is affected much less by water shortage than expected on the basis of the effect of soil moisture on Pi mobility in soil.
In summary, roots of many species release an array of exudates (e.g. carboxylates, phenolics, protons and other cations, phosphatases, water, mucilage), and thus enhance the availability of Pi in the rhizosphere. The nature and effectiveness of the exudates depends on species as well as environmental conditions. A low plant P status tends to enhance exudation.
SPECIALIZED ROOT STRUCTURES: ROOT CLUSTERS
The specialized roots discussed here, collectively called ‘root clusters’, combine a specialized structure and specialized physiology (see below) to maximize Pi acquisition from soils of low fertility, especially when P is present in ‘sorbed’ or insoluble sources (e.g. rock phosphate and iron phosphate). Proteoid (e.g. Keerthisinghe et al., 1998) and dauciform (Shane et al., 2005; Playsted et al., 2006) root clusters are induced by P deficiency and occasionally by Fe deficiency (reviewed in Shane and Lambers, 2005a). P deficiency induces a wide range of genes in cluster roots of L. albus, including genes involved in carbon metabolism, secondary metabolism, P scavenging and remobilization, plant hormone metabolism, and signal transduction, when compared with P-sufficient and P-deficient non-cluster roots (Uhde-Stone et al., 2003).
There are several ‘types’ of root clusters, occurring in both monocotyledonous and in dicotyledonous species (Fig. 3). The best known examples are the ‘bottlebrush-like’ proteoid roots (Fig. 3A, B) described by Purnell (1960) for woody species of Proteaceae. Proteacean taxa are distributed primarily in Australia and South Africa, but proteoid-like roots have also been described in a range of other species from several families, e.g. in Fabaceae [Lupinus albus (white lupin) and Aspalathus linearis (rooibos)] (Fig. 3E, F) (Dinkelaker et al., 1995; Shane and Lambers, 2005a). Monocotyledonous families containing rushes (Restionaceae from the southern hemisphere) and sedges (Cyperaceae with a worldwide distribution) form root clusters termed ‘dauciform’ roots (in sedges; Fig. 3C, D) and ‘capillaroid’ roots (in rushes; Fig. 3G–I).
Fig. 3.

Root-cluster morphologies induced in Proteaceae, Restionaceae, Cyperaceae and Fabaceae by a low supply of phosphorus. All species are well adapted to soils of extremely low P concentrations and endemic to the South West Botanical Province of Western Australia (WA), or the Cape Floristic Region of South Africa (SA). Plants were raised from seed (Proteaceae and Fabaceae) or cuttings (Restionaceae and Cyperaceae) collected from natural habitats, and then grown in nutrient solutions (glasshouses at the University of Western Australia or University of Cape Town) containing ≤ 1 μm [P] (except for the roots of a plant shown in E, which were collected in soil containing ≤ 10 μg P g−1 in Suid Bokkeveld, SA). (A) Proteoid roots (compound type) of Banksia prionotes; acorn banksia (Proteaceae, WA); scale bar = 13 mm. (B) Proteoid root (simple type) of Hakea prostrata; harsh hakea (Proteaceae, WA); scale bar = 4 mm. (C) Dauciform roots of Lepidosperma squamatum (Cyperaceae, WA); scale bar = 2 mm. (D) Dauciform roots of (Tetraria sp. (Cyperaceae, SA); scale bar = 2 mm. (E) Cluster root of Aspalathus linearis; rooibos (Fabaceae, SA); scale bar = 12 mm. (F) Cluster root of the same species shown in E grown in nutrient solution; scale bar = 4 mm. (G) Capillaroid roots of Thamnochortus fracternus (Restionaceae, SA); scale bar = 4 mm. (H) Capillaroid roots of Mastersiella digitata (Restionaceae, SA); scale bar = 6 mm. (I) Capillaroid roots of Chondropetalum tectorum (Restionaceae, SA); scale bar = 5 mm.
Root cluster morphologies involve formation of compact clusters of (determinate) branch roots (rootlets), or root hairs, in a small soil volume which markedly increases the surface area of the root system (Fig. 3). Moreover, root clusters are ephemeral; even in the woody species that develop them, rootlets remain in the primary state of growth until they senesce, and although we know little about their turnover, it is becoming apparent that these fine roots are physiologically active for little more than a few weeks (Shane et al., 2004c, 2005b; Playsted et al., 2006). The root architecture of field-grown, root-cluster-forming species is patterned toward strongly soil-binding root-cluster development in upper soil layers where levels of nutrients are often enriched. Briefly, the basic root-cluster structures are as follows (the reader is referred to Lamont, 2003, for more details). Proteoid roots formed by species in Proteaceae are either ‘simple’ or ‘compound’, but occasionally both types are found on single root systems (e.g. in South African genera of Leucadendron and Protea; Lamont, 1983). Only a few genera within Proteaceae (e.g. Australian Banksia and Dryandra and South African Orothamnus; Lamont 1982, 1983) produce ‘compound’ proteoid root clusters (e.g. Banksia grandis; Fig. 3A). The ‘compound’ proteoid roots are essentially multiples of the ‘simple’ root clusters, but there are likely to be distinct ecophysiological reasons for the differences between the simple cluster roots and the compound clusters (Fig. 3), which tend to form dense root-mats in natural systems. Many proteacean genera (e.g. Australian Hakea and South African Serruria), and genera of other families [e.g. Fabaceae including the South African Aspalathus (rooibos) and the crop species Lupinus albus] produce ‘simple’ proteoid roots (Fig. 3B, E, F) that include numerous short, determinate rootlets, and each simple proteoid root is separated by unbranched regions (Fig. 3B). The main difference between the simple root clusters of Proteaceae (e.g. Hakea; Fig. 3B) and some species in the Fabaceae (e.g. Aspalathus linearis; Fig. 3F) is most notably the density of rootlets produced per unit root axis, which is far greater in the Proteaceae. Within the genus Lupinus, some species, e.g. L. albus, produce easily recognizable root clusters, whereas others form structures that are distinct from non-cluster roots and have been termed ‘cluster-like’ roots by Hocking and Jeffery (2004), who showed that their physiology (below) is rather similar to that of the root clusters in L. albus.
Dauciform root clusters were first described by Russian plant scientists for Cyperaceae (sedges) (Selivanov and Utemova, 1969, and references cited therein). They were subsequently found in cyperacean species in Great Britain (Davies et al., 1973; Ballard, 2001), continental Europe (Bakker et al., 2005; Güsewell, 2005), and in many parts of Australia (Lamont, 1974; Phillips and Weste, 1984; Shane et al., 2005a; Playsted et al., 2006) and New Zealand (Powell, 1973). Lamont (1974) named them ‘dauciform’ roots, because of the carrot-shape of the dauciform root axis (Fig. 3C, D). Dauciform roots often occur in groupings of 20–30 individuals (Lamont, 1974) and each dauciform root may be as short as 2 mm, e.g. in Carex (cosmopolitan) species, or much longer, e.g. up to 12 mm in Lepidosperma (Western Australian) and Tetraria (South African) species (Fig. 3C, D, respectively) (Lamont, 1974; Shane et al., 2005a; Neumann and Römheld, 2006). Instead of the usual formation of dense clusters of short rootlets, the mature axis of a dauciform root is covered with dense clusters of long (approx. 2 mm; Fig. 3C, D) root hairs. The tips of the dauciform root axis are either indeterminate (and may form additional dauciform roots in sequence along the main axis; see Fig. 3C), or the dauciform root tip is determinate, and the entire dauciform root senesces (cf. figure 1 in Shane et al., 2005b).
The monocotyledonous family of the Restionaceae has a Gondwanan distribution (Pate and Meney, 1999). Approximately 486 species are located mainly in Africa (over 300 species in South Africa) and in mainland Australia and Tasmania (approx. 150 species), and a few species are found in New Zealand, South America (Chile) and South East Asia (Indochina). Approximately half of the Australian taxa develop root clusters, especially species adapted to arid environments, where their development begins only after the onset of seasonal rains (figure 1·3 in Meney and Pate, 1999). These ‘capillaroid’ roots were discovered and named by Lamont (figure 4 in Lamont, 1982), and are characterized by clumps of roots or rootlets, densely covered with exceptionally long root hairs (Fig. 3G–I). Their name (capillaroid) stems from the sponge-like properties on holding soil water (Lamont, 1980, 1982). Little is known about their structure and development in species of Restionaceae and how these specialized roots contribute to plant nutrition and water balance. We have recently found root clusters in South African Restionaceae that are remarkably ‘proteoid-like’ in their morphology (Fig. 3H), and produce distinct (ephemeral) clusters separated by unbranched main root axis. However, most species observed thus far have the morphology typical of that shown in Fig. 3G, I. We hypothesize that the physiology and functioning of capillaroid roots is similar to that of proteoid roots.
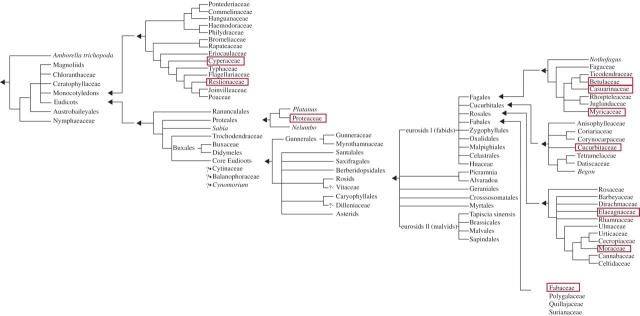
Fig. 4.
Phylogeny of root clusters, based on references cited in the text and on http://tolweb.org/tree?group=angiosperms. Root clusters occur in two monocotyledonous families and eight dicotyledonous families belonging to the Proteales and the Core Eudicots.
Most physiological information about root-cluster functioning has been derived from studies of L. albus, but much has also been discovered about root-cluster functioning in native plants adapted to soils of extremely low Pi concentration. One of the most important aspects is the importance of the influence of the stage of development on root-cluster functioning. The finding that carboxylate (e.g. citrate) release in L. albus (Watt and Evans, 1999) and in proteacean species such as Hakea (H. prostrata, Shane et al., 2004c; H. undulata, Dinkelaker et al., 1997) occurs in an exudative burst strongly supports the view that root development and physiological activity are closely linked as the components of root systems grow and mature (McCully, 1999). Studies of the ‘compound’ (mat-forming) proteoid roots of Banksia integrifolia have also shown that carboxylates, such as citrate, are released into the rhizosphere (Grierson, 1992; Roelofs et al., 2001), but there are no reports on the time course of carboxylate exudation in taxa that produce compound roots. It has now become apparent that dauciform roots in Cyperaceae, although morphologically and anatomically very distinct, also release carboxylates (citrate) in large quantities during a developmentally programmed exudative burst, thus functioning in a way very similar to proteoid roots (Shane et al., 2005b, 2006; Playsted et al., 2006). Another parallel between dauciform and proteoid roots is that both are suppressed when plants have a high P status. Like proteoid roots, dauciform roots release a variety of other compounds, as further discussed below. There is no physiological information on capillaroid roots.
In summary, root clusters differ greatly in their anatomy and morphology, but are rather similar with respect to their physiology. Our knowledge on capillaroid roots is restricted to their anatomy and morphology; their physiology remains to be investigated. The release of carboxylates from root clusters of Proteaceae and Fabaceae, and dauciform roots of Cyperaceae in an exudative burst is bound to be vital for their function, as further discussed in the section ‘Root clusters: combining structure and functioning’.
PHYLOGENY OF ROOT-CLUSTER-FORMING SPECIES
Proteoid root clusters were perhaps once considered as a curiosity associated with many proteacean species (Purnell, 1960) and L. albus (Gardner et al., 1983). However, proteoid roots have since been described for a wide range of species in families that are not at all closely related to Proteaceae (Shane and Lambers, 2005a). Although very few species in the genus Lupinus produce the kind of clusters that are found in L. albus (Clements et al., 1993; Skene and James, 2000), many others produce ‘cluster-like roots’, which function in a similar way to the ‘true cluster roots’ (Hocking and Jeffery, 2004). Within the Cyperaceae (sedges), dauciform roots are restricted to two tribes: Cariceae and Rhynchosporeae (Lamont, 1981). Outside the Cyperaceae, dauciform roots have only been reported for Juncus pauciflorus and J. squarrosus (Juncaceae, reeds) (Powell, 1973). Capillaroid roots have been reported exclusively in the Restionaceae (rush) (Lamont, 1982). Viewed in this way, it is obvious that root clusters (a term we use here to refer to all types of clusters: simple and compound proteoid or cluster roots, dauciform roots, capillaroid roots, cluster-like roots; Fig. 3) are actually more widespread in the plant kingdom than considered before.
Root clusters are found in two large monocotyledonous families: Cyperaceae (dauciform roots) and Restionaceae (capillaroid roots) (Fig. 4). Root clusters also occur in several dicotyledonous families. Proteoid roots were first discovered in the Proteaceae (Purnell, 1960), which belong to the Proteales (Eudicots). This accounts for the name ‘proteoid’ roots. There are no records of root clusters in other families within the Proteales; while growing over a year in low-P nutrient solution in the glasshouse, Platanus hybrida (Platanaceae, Proteales) never produced any root clusters (H. M. Stace and H. Lambers, unpubl. data). Apart from the Proteales, within the eudicots, root clusters have been described in Core Eudicots (Rosids) only; that is, they occur in several families that are phylogenetically very distantly related to the Proteaceae (Fig. 4) (Skene, 2000; Shane and Lambers, 2005a). Within the Rosids, root clusters occur in four orders belonging to the eurosids I (fabids): Fagales (Betulaceae, Casuarinaceae, Myricaceae), Cucurbitales (Cucurbitaceae), Rosales (Elaeagnaceae, Moraceae) and Fabales (Fabaceae). Root clusters have obviously evolved several times. What is very striking is that actinorhizal species belonging to different orders and families, namely Betulaceae (Fagales), Casuarinaceae (Fagales), Eleagnaceae (Rosales) and Myricaceae (Fagales), all have the capacity to produce root clusters. Why is there an association between root-cluster-bearing habit and being actinorhizal? Does a plant's capacity to recognize and form an association with Frankia species have something in common with its capacity to develop clusters? Further investigations of the signal-transduction pathways involved in the actinorhizal symbioses and root-cluster formation may lead to fascinating new discoveries. In addition, a careful study of species belonging to the four other actinorhizal families, Rosaceae, Rhamnaceae (both Rosales), Coriariaceae and Datiscaceae (both Cucurbitales) (Swensen, 1996; Vessey et al., 2005), might well reveal more records of cluster-root-bearing species.
Root clusters are no longer the curiosity restricted to plants from ‘down under’, but occur in many distantly related families throughout the plant kingdom. Many species are used as crops, for nuts (Macadamia species), a source of protein (Lupinus species), tea (Aspalathus linearis), timber and pulpwood (Grevillea species) (Shane and Lambers, 2005a). Others may be used in pastures; for example, in eastern Canada and western North America some sedges (Carex sp.) are recognized for their potential for use as forage for grazing (Uresk, 1986; Catling et al., 1994), and Kennedia species for introduction as food (Rivett et al., 1983) or pasture plants (Cocks, 2001). Considering our dwindling P reserves, these cluster-bearing species need to receive greater emphasis in future research.
RELATIONSHIPS BETWEEN P-ACQUISITION STRATEGIES AND SOIL TYPES
Having discussed the structure and functioning of root clusters in different plant families, we now explore where root-cluster-bearing species fit in the landscape. The Western Australian flora offers a unique opportunity to explore that question. Both non-mycorrhizal, cluster-bearing species belonging to the Cyperaceae and Proteaceae, and mycorrhizal species without root clusters are common in south-western Australia, a global biodiversity hotspot (Myers et al., 2000). In addition, there are several species that are both mycorrhizal and cluster-bearing (e.g. Casuarinaceae and Fabaceae). Are the different nutrient acquisition adaptations distributed randomly or linked to certain habitat factors? We can address this question in some detail using McArthur's (1981) detailed descriptions of soil and vegetation for 150 reference sites in south-western Western Australia. Non-mycorrhizal, cluster-bearing Proteaceae predominate on the most P-impoverished soils in the region, whereas mycorrhizal Myrtaceae without root clusters predominate on soils that have somewhat higher P levels (Fig. 5). Whereas Myrtaceae in Western Australia generally dominate forests and tall woodlands, and Proteaceae generally dominate shrublands/heaths and low woodlands, the occurrences of species of the two families are by no means mutually exclusive. Proteaceae understorey species in eucalypt woodlands and in highly diverse mixed heaths of Proteaceae/Myrtaceae are very common. The region offers unique opportunities to study specialization to soil types and coexistence of species with different nutrient acquisition strategies. This flora will allow us to discover the relative advantages of root adaptations as dependent on different soil conditions (Fig. 5), but further work is needed to appreciate the more intricate relationships between soils and roots. Very little information is available for Casuarinaceae, which are mycorrhizal as well as cluster-bearing (Reddell et al., 1997). However, the scarce available data indicate an intermediate position for this group between the cluster-bearing Proteaceae of lower-P soils and the mycorrhizal Myrtaceae of higher-P soils. Solid data for the distribution of cluster-bearing Cyperaceae, Fabaceae and Restionaceae as dependent on soil P levels are lacking, and hence we can only speculate where they fit in Fig. 5.
Fig. 5.
Distribution of species belonging to Proteaceae, Casuarinaceae and Myrtaceae, three major plant families in south-western Australia, as dependent on soil P concentration. The figure is based on information in McArthur (1981) and was prepared as follows. All reference sites where the main species (trees in the case of forests and woodlands, shrubs in the case of shrublands) were either Proteaceae (n = 20 sites), Myrtaceae (n = 82) or Casuarinaceae (n = 5) were selected, and the total P concentration of the A1 horizon was tabulated. Total P was analysed by the Kjeldahl method (Murphy and Riley, 1962). Then, for each family group, the relative frequency of occurrence of P concentration classes was calculated. Total P in the A1 horizon correlated well with total P in the B1 horizon and with bicarbonate-extractable P in the A1 horizon (data not shown).
In summary, the vegetation of Western Australia's global biodiversity hotspot, located on ancient, heavily weathered soils, offers unique opportunities to study the intricate relationships between soils and vegetation, discussed in this review. This flora will allow us to discover the relative advantages of root adaptations as dependent on different soil conditions (Fig. 5), but further work is needed to appreciate the more intricate relationships between soils and roots.
ROOT CLUSTERS: MATCHING STRUCTURE AND FUNCTIONING
Roots of many species release exudates under low-P conditions, but most would not survive in the ancient and severely weathered soils where proteoid roots are so common (Fig. 5). What exactly determines the success of these non-mycorrhizal Proteaceae on the world's most P-impoverished soils? We propose that it is their ‘specialized’ morphology and anatomy (structure), which is matched by their ‘specialized’ physiology and biochemistry (functioning) (Shane and Lambers, 2005a). It should be added, however, that those specializations are merely matters of programming aspects of cellular structures and functions that are not unique to root clusters. Lateral-root formation is a universal process in plants, but only the co-ordinated development of hundreds of lateral roots can give rise to the structures we call cluster roots (Fig. 3). Similarly, production of carboxylates (e.g. citrate and malate) occurs in all plant cells; however, their release in an exudative burst has only been described for root clusters.
In a comparative study of a number of crop species, many of which are known for their release of relatively large quantities of carboxylates, we found that the species that combined carboxylate release with root-cluster formation out-performed other species when grown at severely limiting Pi supply (Fig. 6). An even more convincing case has been made by Paul Reddell (pers. comm.), who compared a number of rainforest proteacean species from north-eastern Australia. Most of these were non-mycorrhizal and cluster-bearing, as expected. However, one of these species was mycorrhizal and did not produce root clusters. Most significantly, this non-cluster-bearing mycorrhizal species was outperformed in terms of biomass production at the lowest soil Pi levels, which confirms our hypothesis on the significance of the cluster-root habit. Bolland et al. (2000) carried out field experiments, using three high-exuding Lupinus species, two with root clusters (L. albus and L. luteus) and another without root clusters (L. angustifolius) (Hocking and Jeffery, 2004). Their results further confirm that root clusters confer a distinct advantage when soil Pi levels are severely limiting for growth in plants that lack these specialized structures.
Fig. 6.
Shoot fresh weights (g) of several crop species grown for 7·5 weeks supplied with basal nutrients and 0 (0-P) or 40 mg P kg−1 dry sand in the form of soluble KH2PO4 (K-P), or sparingly soluble AlPO4 (Al-P), FePO4 (Fe-P), or Ca5OH(PO4)3 (Ca-P) (n = 14). When Pi is supplied in sparingly soluble forms, Lupinus albus and L. cosentinii, which combine high carboxylate release with the formation of cluster roots, performed best.
Carboxylates are a major component of the exudates released by root clusters, and probably the ones most effective at mobilizing phosphorus, but they are not the only component. As briefly discussed above, root clusters often also release phenolics. Although these phenolics may mobilize some phosphorus, it is more likely that their ecophysiological role is to inhibit microbial breakdown of exuded carboxylates (Neumann and Römheld, 2001). Weisskopf et al. (2006) point out three distinctive mechanisms to inhibit microbial breakdown of exudates released from L. albus. First, acidification would slow down breakdown by bacteria. Secondly, excretion of phenolics (mainly isoflavonoids) during the exudative burst slows down fungal metabolism because it leads to fungal sporulation. Finally, release of antifungal cell-wall-degrading enzymes (chitinase and glucanase) prior to the exudative burst would inhibit fungal growth. The fact that exudative bursts result in very high concentrations of carboxylates in the rhizosphere will in itself maximize the effect of the exuded carboxylates, because it allows them to act before microbial populations build up. Additional protection of exuded carboxylates by the mechanisms discovered by Weisskopf et al. (2006) in L. albus further enhances their efficiency. It is therefore highly unlikely that micro-organisms play a significant role in mobilizing phosphorus in the rhizosphere of L. albus cluster roots (Weisskopf et al., 2006). In Proteaceae (e.g. Banksia attenuata; Marschner et al., 2005) and L. albus (Marschner et al., 2002) different bacterial communities are associated with different age classes of proteoid roots, and with proteoid and non-proteoid roots. Furthermore, phosphate-solubilizing bacteria associated with proteoid roots (but not with the non-proteoid roots) of Telopia speciosissima (waratah) increase the solubility of calcium phosphate (Wenzel et al., 1994). However, what remains to be investigated is whether the differences in microbial communities emerged before or after the exudative burst. The bacterial communities may function in P cycling in the ecosystem, but whether they actually enhance the availability of P for the plant remains to be demonstrated. Alternatively, they might increase the rate of decomposition of root clusters after these have depleted most of the P that has been made available by carboxylates and phosphatases.
Some ectomycorrhizas also release carboxylates (e.g. oxalate) and protons, and this will enhance the availability of Pi (e.g. Arvieu et al., 2003). However, as with non-cluster-bearing proteacean and Lupinus species, which are less effective at accessing Pi when soil levels are very low than their cluster-bearing counterparts, ectomycorrhizal species without root clusters are expected to be less effective than non-mycorrhizal species with clusters.
While compound proteoid roots of, for example, Banksia (Grierson, 1992) species appear to function in much the same way as the simple clusters of, for example, Hakea (Shane et al., 2004c) and Lupinus (Watt and Evans, 1999), their morphology is strikingly different (Fig. 3). The greater complexity of compound cluster roots, however, is only due to one extra branching order. Both in the field and in hydroponics, the rootlets of compound clusters develop synchronously, very similar to simple cluster roots. Simple clusters are not necessarily smaller than compound clusters: Lamont (2003) reported simple clusters of Hakea prostrata of 200 mm long and 70 mm wide. Both simple and compound root clusters are concentrated in surface soils (A0 and A1 horizons), but compound clusters tend to form root mats (Lamont, 2003). In ancient, nutrient-impoverished soils, the extremely low concentration of Pi in the soil may result in extremely competitive P recycling. In an ecosystem comprising many species similarly constrained by available P, variation in the strategies for acquisition of Pi as a limiting resource may be important for coexistence. Functionally, mats of surface cluster roots may be similar to root mats found in other nutrient-poor ecosystems with tight nutrient cycles, e.g. tropical rain forests on white sands (Cuevas and Medina, 1988). Such root mats minimize nutrient losses by scavenging of nutrients directly from decomposing litter, or even through contributing to litter decomposition (i.e. release of phosphatases, Grierson and Comerford, 2000; possibly peptidases, Schmidt et al., 2003). Although proteoid roots may form a virtually continuous fabric near the soil surface due to the persistence of senesced cluster roots (Gould, 1998), our field observations suggest that root clusters in their active mature state represent only patches within this mat. The information currently available does not allow us to identify any clear functional difference between simple and compound root clusters. We propose that their functioning as clusters is similar, but that simple and compound clusters have different implications for root-system architecture that may place different constraints or create different opportunities for optimal placement in soil horizons and nutrient-enriched patches. The carbon costs of construction and functioning of cluster roots are high (see below) and dense mats of compound cluster roots are likely to be especially costly. Compound clusters may be more suitable for placement within an existing network of roots (as in a root mat) wherever a favourable (humus-rich) patch appears. Thus, in Banksia woodlands of Western Australia where leaf litter accumulates, proliferation of mats of particularly compound cluster roots just below the leaf litter may facilitate competitive recycling of a resource that is distributed fairly homogeneously (Lamont, 1982). By contrast, in other environments where leaf litter accumulation is less, the resource may be distributed more patchily within the soil and justify the formation of opportunistic simple cluster roots. This is probably more the case in the fire-prone serotinous proteacean ‘fynbos’ of the Cape area of South Africa where leaf litter production is low (78 g m−2) compared with the Australian ‘kwongan’ (194–409 g m−2) (Stock and Allsopp, 1992). In this system fire plays a major role in recycling P. Soil levels of Pi increase significantly after fire and allow early growth of seedlings, following release of seeds from serotinous structures; however, soil Pi rapidly returns to pre-fire levels as the vegetation regrows (Stock and Allsopp, 1992). Simple cluster roots may be more compatible with more explorative root-foraging strategies in which clusters are formed on long axes, which can continue to grow when the clusters senesce. It would be interesting to determine whether compound cluster roots differ from simple cluster roots in their ability to mobilize organic P, and whether the biogeography and ecology of species producing these differing roots are distinct.
The effectivity of both simple and compound clusters is based on a short and intensive ‘extraction’ of soil Pi in a confined volume of soil. This is accomplished by high rootlet density and synchronous exudation of P-mobilizing and antimicrobial compounds (in L. albus), and followed by fast uptake of Pi. Changes of rhizosphere pH and moisture content may assist in P mobilization and uptake, as described above, resulting in divergent root-cluster specializations. The morphological distinction between compound and simple cluster roots is obvious, but there are probably many other, less obvious distinctions in the composition of exudates from cluster roots of species growing in different habitats. For example, although growing in the same area (mere metres apart), Protea obtusifolia and Leucadendron meridianum occur exclusively in shallow pockets of limestone-derived soils, while Protea susannae and Leucadendron coniferum occur exclusively on adjacent, uniformly deep colluvial sands (Mustart et al., 1994). It would be interesting to explore the capacities of the cluster roots of these species to exploit the soils on which they occur. In the Cyperaceae and species from other families, it has been found that calcifuge species exude more acetic acid whereas calcicole species exude more citric and oxalic acid, possibly because of the differential capacity of these carboxylates to solubilize Fe and Pi from the respective soils (Ström et al., 1994). In environments where cluster-root-forming Proteaceae, capillaroid-root-forming Restionaceae and dauciform-root-forming Cyperaceae co-occur, it would be intriguing to establish whether these diverse structures are truly functional analogues, or whether functional distinctions contribute to coexistence. It is likely that these structures all rely on carboxylate exudation for mobilizing inorganic and organic P, as shown for Proteaceae (Shane et al., 2004c) and Cyperaceae (Shane et al., 2005b, 2006; Playsted et al., 2006). However, the composition of those carboxylates (Lambers et al., 2002) and the accompanying enzymes (e.g. Gilbert et al., 1999) and phenolics (Weisskopf et al., 2006) may vary with the nutritional niche that the species occupy.
In summary, current evidence shows that the success of cluster-bearing, non-mycorrhizal species in low-P soils is based on a combination of root structure (root clusters) and root functioning (production and release of carboxylates and other exudates). ‘Root clusters’ as a collective term for cluster roots (e.g. Proteaceae, Fabaceae), capillaroid roots (Restionaceae) and dauciform roots (e.g. Cyperaceae) have diverse anatomical structures, but most probably are all an adaptation to the constraint of low concentration and sparingly soluble P. However, it is possible that there are diverse variations on the ‘root-cluster’ strategy to deal with diverse environments.
EFFECTS OF FAST-EXUDING SPECIES ON NEAREST NEIGHBOUR AND SPECIES IN CROP ROTATIONS
Provided roots of other species are positioned close enough to active root clusters of their neighbours, they are expected to benefit from the activity exhibited by these clusters. Such a beneficial effect of L. albus on the growth and P content of Triticum aestivum was demonstrated by Horst and Waschkies (1987). Cu et al. (2005) subsequently showed that L. albus monocultures preferentially depleted a citric-acid-leachable soil Pi pool, whereas T. aestivum monocultures preferentially depleted the water-leachable soil Pi pool. The mixed cultures depleted both pools. The L. albus monocultures lowered the soil pH by 0·3 pH units, whereas the T. aestivum monocultures raised it by 0·8 pH units; the mixed cultures gave a soil pH intermediate between the two monocultures. Thus, plants of one species may partially offset the effect on soil pH caused by the other. Cluster-root-bearing crop plants such as L. albus as well as high-exuding non-cluster-bearing species would appear to have enormous potential as intercrops (L. Li et al., 2003; Zhang and Li, 2003; S. M. Li et al., 2004; Hauggaard-Nielsen and Jensen, 2005). However, there is also competition between intercropped species, and the effects on growth and yield are therefore not invariably positive (Dessougi et al., 2003). We are unaware of any experimental results for native species in their natural habitats, but envisage that very positive interactions also occur between plants of cluster-root-bearing species and their non-cluster-root-forming neighbours.
Beneficial effects of species with a large capacity to mobilize soil P are not restricted to neighbouring plants, but may extend to the following crop (Kamh et al., 1999). Plants of Zea mays grown after Brassica napus or Beta vulgaris took up more Pi in the presence of a preceding crop's residue (Dessougi et al., 2003). Similar positive effects were observed for both Z. mays (Kamh et al., 2002) and Triticum aestivum (Nuruzzaman et al., 2005) grown after a legume; the legume was grown with sufficient nitrogen, and the effect was ascribed to P mobilization and independent of the N2-fixing potential of the legumes. Little et al. (2004) showed that Olsen-extractable P in plots 8 weeks after sowing potatoes was enhanced after growing L. albus or a combination of L. albus and B. napus as a cover crop relative to that after Avena sativa or B. napus alone. These results provide evidence that cover crops containing L. albus potentially enhance the Pi availability for the following crop. However, it is very unlikely that the carboxylates persist long enough to be of benefit to the next crop; we hypothesize that L. albus roots cause a shift from less available to more available Pi pools.
In summary, high-exuding species, and especially those with root clusters, can have a positive effect on neighbouring crop plants as well as on the following crop. The potential of these P-mobilizing species has been studied in some detail, but much is still to be discovered, especially on native plants in natural systems.
PERSPECTIVES FOR CROP PLANTS IN A WORLD WHERE P RESERVES ARE BEING DEPLETED
As discussed above, many species occurring on severely P-impoverished soils in south-western Australia and the Cape region in South Africa exhibit adaptive root specializations (root clusters) that enhance the availability of Pi in the rhizosphere. Root clusters are a combination of adaptive structures with adaptive physiology. Root clusters are not restricted to species from these Mediterranean regions, but also occur in a large number of species elsewhere in the world (e.g. Cyperaceae) as well as in several crop species. Given the remarkable similarity of form and function among root clusters from distant families, indicating that the structures evolved independently a number of times, the evolution of these traits appears to be a result of intensification of certain common existing elements; therefore, incorporating these traits in new crop species seems to be a reasonable proposal (Skene, 2003). Considering that P reserves are rapidly being depleted, while vast amounts are present in soils that have been fertilized for decades, we should consider options for incorporating root clusters in new crop species. The situations where this strategy would be favourable occur where considerable amounts of Pi can be mobilized that would otherwise remain unavailable. It is important that the soil physicochemical and biological processes that support plant growth remain in place. Because the superior Pi acquisition of high-exuding plant species is based on a localized chemical extraction of soil, certain risks, including decreased pH and excessive Pi depletion, will need to be assessed.
Several advantages of a large capacity to mobilize P in the rhizosphere, especially by root clusters, have been discussed above. Are there also downsides that need to be considered before aiming to introduce root clusters in new crops? In the next paragraphs we discuss possible disadvantages: high carbon costs of root clusters, mobilization of potentially toxic ions in the rhizosphere, and increased risks of P leaching from soil.
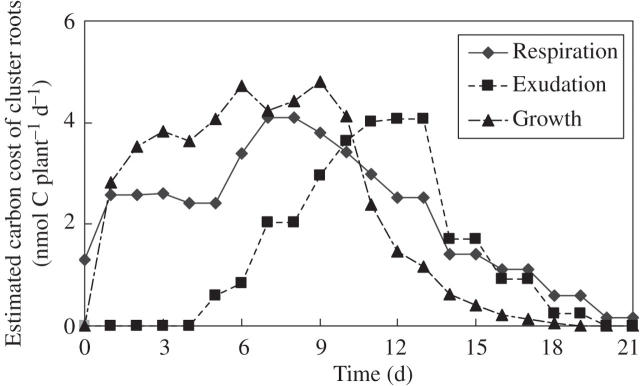
There are obviously costs involved in P mobilization through root-cluster production and functioning. We have used information on leaf photosynthesis, total leaf area and root-cluster production of Hakea prostrata (Shane and Lambers 2005b), combined with data on growth, respiration and carboxylate exudation of the same species (Shane et al., 2004c), to estimate these costs. Our very rough estimates based on these two papers indicate that well over half of all the carbohydrates produced in photosynthesis are required for the growth, respiration and carboxylate exudation of cluster roots in H. prostrata (Fig. 7). This strategy is therefore not one that should be adopted for new crops because it would seriously compromise crop growth; however, this value needs to be qualified. First, cluster roots of H. prostrata, which naturally occurs in a Mediterranean (winter rainfall) environment, are only produced during the wet season (Lamont, 1982) and function only at very low plant P status (Shane et al., 2003). That is, the calculated value should be divided by 4–6 to express the result per annum, as the cluster roots are only active for 2–3 months per year. That brings the estimate much closer to the only other, equally rough, estimate for L. albus: 23 % of whole-plant photosynthate production (Dinkelaker et al., 1989). These estimates are also fairly similar to the estimated cost of 7–20 % to sustain mycorrhizal symbioses (Lambers et al., 1998). Secondly, the root clusters are not only involved in uptake of P, but acquire most other nutrients as well, in particular micronutrients (Shane and Lambers, 2005b) and nitrogen (Schmidt et al., 2003; Hawkins et al., 2005).
Fig. 7.
Estimated carbon costs of cluster roots in Hakea prostrata (total plant fresh mass was approximately 600 g). While the clusters are active, 52–100 % of daily produced photosynthate is utilized in growth, respiration and carboxylate exudation. On an annual basis, these figures should be divided by 4–6, because the clusters of H. prostrata are only active in the wet season (2–3 months). The calculations are based on primary data presented on growth, respiration and carboxylate exudation in Shane et al. (2004c) and on leaf photosynthesis, total leaf area and total root cluster in Shane and Lambers (2005b), assuming that half of the clusters harvested by Shane and Lambers (2005b) were older than 21 d, and hence dead.
Carboxylates not only mobilize nutrients in the rhizosphere needed for growth, but may also enhance the availability of toxic ions, e.g. heavy metals. In a field experiment on an acidic, lateritic ironstone gravel sand, Brennan and Bolland (2003) found greater uptake of cadmium in L. luteus (with root clusters) than in L. angustifolius (without clusters). Similarly, Brennan and Bolland (2005) found greater uptake of cadmium in Brassica napus (high exudation, no root clusters) than in Triticum aestivum (low exudation, no clusters). This is obviously a risk that needs to be considered carefully, especially in view of the fact that many sources for P fertilizer are contaminated with cadmium.
Species that effectively mobilize P in the rhizosphere might do this to the extent that P is leached from superficial soil layers down the profile, and hence contribute to pollution of streams and rivers, contributing to their eutrophication (Djodjic et al., 2004; Fortune et al., 2005). This risk should be managed by measuring both agronomic and environmental soil P saturation, and fertilizing accordingly (Maguire and Sims, 2002). We consider this to be a risk only on soils with a very low capacity to sorb P and that are heavily fertilized; that is, on soils where the ability of crop species to mobilize P would not confer a yield advantage. These are rarely the soils used for agriculture; if they are, (Weaver and Prout, 1993), and if P-mobilizing species are used, then the P-fertilization regime obviously has to be very closely monitored.
Provided the risks of enhanced cadmium uptake and eutrophication are carefully managed, P-acquisition efficient new crops, especially high-exuding, cluster-bearing crops, offer tremendous potential. New crops with improved traits as listed above under ‘Common root adaptations to enhance Pi acquisition’ can be developed using an analysis of quantitative trait loci (QTL) (e.g. Hu et al., 2001; Yan et al., 2004; J. Zhu et al., 2005; Su et al., 2006). Rather than restricting ourselves to crop species that are currently commonly used, we could explore the potential of as yet non-domesticated species with superior P-acquisition traits or use species that are frequently referred to as ‘lost crops’; that is, species that were commonly used several decades ago, but are no longer mainstream species today. It is also likely that breeding for the modern cultivars of high-yielding crops has selected against efficient P-acquisition strategies, which in early selections in a high-P environment might have compromised yield. Crossing modern cultivars with ‘heritage’ crops is an obvious potential strategy. Wide crosses offer possibilities to introduce root clusters into Lupinus species that lack this trait. We have a good understanding of the phylogeny of the genus Lupinus (Ainouche and Bayer, 1999) and of the species within this genus that develop ‘true clusters’ (Clements et al., 1989) or ‘cluster-like roots’ (Hocking and Jeffery, 2004). This should offer perspectives to develop new lupin crops with root clusters. Although root clusters are clearly efficient adaptations for Pi acquistion, in some crops selection for single characters such as longer root hair length may be suffficient to enhance access to P.
Finally, further investigations of the developmental processes involved in cluster-root formation should identify key genes that allow the production of root clusters. Such a molecular approach appears to be most promising for the incorporation of cluster roots in crops that currently lack them, and the tools for such bioengineering are at hand, including the ability to clone root-specific genes with root-tissue-specific promoters that are regulated by nutritional demands (Bucher, 2002). In order to increase P-acquisition efficiency of non-cluster-rooted species, alteration of the architecture of the root system, secretion/exudation of chemical compounds and enzymes into the rhizosphere, and enhanced uptake of Pi would be required. Some progress has been made towards bioengineering enhanced Pi acquisition into plant roots. Selection for longer root hairs may be possible (Rengel and Marschner, 2005). The eto1 mutant plants of A. thaliana, which synthesize more ethylene and have longer root hairs than wild-type plants (Pitts et al., 1998), provide a possible mechanism for altering root architecture. Enhanced uptake of insoluble P (hydroxyapatite) and improved growth has been reported for tobacco constitutively expressing a heterologous citrate synthase (López-Bucio et al., 2000). Over-expression of Pi transporters has been shown to increase Pi uptake by suspension cultures, although there has been no success in increasing whole-plant Pi uptake in barley, probably due to regulation of the homologous Pi transporter utilized (Rae et al., 2004). A. thaliana transformed with heterologous phytase secreted the enzyme only from roots when grown on medium containing low Pi concentrations, enabling growth on phytate as a sole P source (Mudge et al., 2003). However, as discussed above, none of the structures and pathways associated with growth and functioning of root clusters is unique for root clusters. What determines if a cluster will be formed or not is the programming of the structures and processes. Thus, modification of regulatory elements may be a more appropriate mechanism than introduction of heterologous genes. It is envisaged that root-cluster-forming species will soon be used to study species-specific development for which A. thaliana is not a good model (Van Lijsebettens and Van Montagu, 2005).
If we wish to apply information gleaned from native plants for cropping and pasture systems, we should not just be thinking of new crop species and bioengineering. We should also consider new cropping systems, where combinations of species in intercropping systems and ideal rotations are used to maximize the acquistion of Pi from low-P soils. Equally, and although not the focus of this review, there are possibilities to enhance P-use efficiency, in addition to improved P-acquisition efficiency. These approaches should lead to more sustainable cropping systems with less off-site risks of eutrophication of streams and rivers.
CONCLUDING REMARKS
Global P reserves are rapidly being depleted, whilst agricultural soils that have been fertilized for decades contain substantial amounts of P that cannot be accessed by plants lacking specific root adaptations. To acquire soil Pi more efficiently, new crops need to be developed, and there should be a strong focus on species with root clusters, as these represent a combination of form and function that is highly desirable in a world where P will be harder to obtain. There is still much to be learned regarding the role of root clusters in natural systems, and it is envisaged that new knowledge based on investigations of such systems will further enhance our potential to develop new crops and cropping systems, which use P more efficiently.
Acknowledgments
We thank Dr Nick Savidov (University of Alberta, Canada) for translation of Selivanov and Utemova's (1969) publication (in Russian). This work was supported by the Australian Research Council, and a University of Western Australia small grant awarded to Hans Lambers and John Kuo, and conducted as part of the N-P Working Group supported by the ARC-NZ Research Network for Vegetation Function. This review was based, in part, on a plenary lecture presented at the International Plant Nutrition Colloquium, Beijing, China, sponsored by the Annals of Botany. We also acknowledge inspirational discussions with Professor Sally Smith (University of Adelaide, Australia).
LITERATURE CITED
- Abel S, Ticconi CA, Delatorre CA. 2002. Phosphate sensing in higher plants. Physiologia Plantarum 115: 1–8. [DOI] [PubMed] [Google Scholar]
- Adams MA. 1992. Phosphatase activity and phosphorus fractions in karri (Eucalyptus diversicolor F. Muell.) forest soils. Biology and Fertility of Soils 14: 200–204. [Google Scholar]
- Ainouche A-K, Bayer RJ. 1999. Phylogenetic relationships in Lupinus (Fabaceae: Papilionoideae) based on internal transcribed spacer sequences (ITS) of nuclear ribosomal DNA. American Journal of Botany 86: 590–607. [PubMed] [Google Scholar]
- Amijee F, Barraclough PB, Tinker PB. 1981. Modeling phosphorus uptake and utilization by plants. In: Johansen C, Lee KK, Sahrawat KL, eds. Phoshporus nutrition of grain legumes in the semi-arid tropics. Andra Prades: Icrisat, 63–75.
- Arvieu J-C, Leprince F, Plassard C. 2003. Release of oxalate and protons by ectomycorrhizal fungi in response to P-deficiency and calcium carbonate in nutrient solution. Annals of Forest Science 60: 815–821. [Google Scholar]
- Bakker C, Rodenburg J, Van Bodegom PM. 2005. Effects of Ca- and Fe-rich seepage on P availability and plant performance in calcareous dune soils. Plant and Soil 275: 111–122. [Google Scholar]
- Ballard S. 2001. Dauciform roots in sedges: their role in nutrition and response to environmental change. PhD thesis, University of Sheffield, Sheffield, UK.
- Bates TR, Lynch JP. 2001. Root hairs confer a competitive advantage under low phosphorus availability. Plant and Soil 236: 243–250. [Google Scholar]
- Bhadoria PBS, Kaselowsky J, Claassen N, Jungk A. 1991. Phosphate diffusion coefficients in soil as affected by bulk density and water content. Zeitschrift für Pflanzenernährung und Bodenkunde 154: 53–57. [Google Scholar]
- Bolland MDA, Sweetingham MW, Jarvis RJ. 2000. Effects of applied phosphorus on the growth on Lupinus luteus, L. angustifolius and L. albus in acidic soils in the south-west of Western Australia. Australian Journal of Experimental Agriculture 40: 79–92. [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM. 1999. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant, Cell and Environment 22: 425–431. [Google Scholar]
- Boulet FM, Lambers H. 2005. Characterisation of arbuscular mycorrhizal fungi colonisation in cluster roots of Hakea verrucosa F. Muell (Proteaceae), and its effect on growth and nutrient acquisition in ultramafic soil. Plant and Soil 269: 357–367. [Google Scholar]
- Brennan RF, Bolland MDA. 2003. Lupinus luteus cv. Wodjil takes up more phosphorus and cadmium than Lupinus angustifolius cv. Kalya. Plant and Soil 248: 167–185. [Google Scholar]
- Brennan RF, Bolland MDA. 2005. Canola takes up more cadmium and phosphorus from soil than spring wheat. Journal of Plant Nutrition 28: 931–938. [Google Scholar]
- Brouwer R. 1963. Some aspects of the equilibrium between overground and underground plant parts. Mededelingen van het. Instituut voor Biologische en Scheikundig Onderzoek van Landbouwgewassen 213: 31–39. [Google Scholar]
- Brouwer R. 1983. Functional equilibrium: sense or nonsense? Netherlands Journal of Agricultural Science 31: 335–348. [Google Scholar]
- Brundrett MC. 2002. Coevolution of roots and mycorrhizas of land plants. New Phytologist 154: 275–304. [DOI] [PubMed] [Google Scholar]
- Bucher M. 2002. Molecular root bioengineering. In: Waisel Y, Eshel A, Kafkafi U, eds. Plant roots: the hidden half, 3rd edn. New York: Marcel Dekker, 279–294.
- Burgess SSO, Adams MA, Turner NC, Ong CK. 1998. The redistribution of soil water by tree root systems. Oecologia 115: 306–311. [DOI] [PubMed] [Google Scholar]
- Burgess SO, Pate JS, Adams MA, Dawson TE. 2000. Seasonal water acquisition and redistribution in the Australian woody phreatophyte, Banksia prionotes. Annals of Botany 85: 215–224. [Google Scholar]
- Burleigh SH, Harrison MJ. 1999. The down-regulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots. Plant Physiology 119: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MM, Richards J. 1989. Hydraulic lift: water efflux from upper roots improves effectiveness of water uptake by deep roots. Oecologia 79: 1–5. [DOI] [PubMed] [Google Scholar]
- Catling PM, McElroy AR, Spicer KW. 1994. Potential forage value of some eastern Canadian sedges (Cyperaceae: Carex). Journal of Rangeland Management 47: 226–230. [Google Scholar]
- Carvalho IS, Ricardo CP, Chaves M. 2004. Quality and distribution of assimilates within the whole plant of lupines (L. albus and L. mutabilis) influenced by water stress. Journal of Agronomy and Crop Science 190: 205–210. [Google Scholar]
- Christy EK, Moorby J. 1975. Physiological responses of semi-arid grasses I. The influence of phosphorus supply on growth and phosphorus absorption. Australian Journal of Agricultural Research 26: 423–436. [Google Scholar]
- Clarkson DT. 1981. Nutrient interception and transport by root systems. In: Johnson CB, ed. Physiological processes limiting plant productivity. London: Butterworths, 307–314.
- Clements JC, White PF, Buirchell BJ. 1993. The root morphology of Lupinus angustifolius in relation to other Lupinus species. Australian Journal of Agricultural Research 44: 1367–1375. [Google Scholar]
- Cocks PS. 2001. Ecology of herbaceous perennial legumes: a review of characteristics that may provide management. Australian Journal of Agricultural Research 52: 137–151. [Google Scholar]
- Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D, Vitousek PM. 1995. Changes in oil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76: 1407–1424. [Google Scholar]
- Cu STT, Hutson J, Schuller KA. 2005. Mixed culture of wheat (Triticum aestivum L.) with white lupin (Lupinus albus L.) improves the growth and phosphorus nutrition of the wheat. Plant and Soil 272: 143–151. [Google Scholar]
- Cuevas E, Medina E. 1988. Nutrient dynamics within amazonian forests. II Fine root growth, nutrient availability and leaf litter decomposition. Oecologia 76: 222–235. [DOI] [PubMed] [Google Scholar]
- Dakora FD, Phillips DA. 2002. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant and Soil 245: 35–47. [Google Scholar]
- Davies J, Briarty LG, Rieley JO. 1973. Observations on the swollen lateral roots of the Cyperaceae. New Phytologist 72: 167–174. [Google Scholar]
- De Groot C, Marcelis LFM, Van den Boogaard R, Lambers H. 2001. Growth and dry mass partitioning in tomato as affected by phosphorus nutrition and light. Plant, Cell and Environment 24: 1309–1317. [Google Scholar]
- Dessougi HI, zu Dreele A, Claasen N. 2003. Growth and phosphorus uptake of maize cultivated alone, in mixed culture with other crops or after incorporation of their residues. Journal of Plant Nutrition and Soil Science 166: 254–261. [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. 1989. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant, Cell and Environment 12: 285–292. [Google Scholar]
- Dinkelaker B, Hengeler C, Marschner H. 1995. Distribution and function of proteoid roots and other root clusters. Botanica Acta 108: 183–200. [Google Scholar]
- Dinkelaker B, Hengeler G, Neumann G, Eltrop L, Marschner H. 1997. Root exudates and mobilization of nutrients. In: Rennenberg H, Eschrich W, Ziegler H, eds. Trees—contributions to modern tree physiology. Leiden: Backhuys Publishers, 441–452.
- Djodjic F, Borling K, Bergstrom L. 2004. Phosphorus leaching in relation to soil type and soil phosphorus content. Journal of Environmental Quality 33: 678–684. [DOI] [PubMed] [Google Scholar]
- Dong B, Ryan PR, Rengel Z, Delhaize E. 1999. Phosphate uptake in Arabidopsis thaliana: dependence of uptake on the expression of transporter genes and internal phosphate concentrations. Plant, Cell and Environment 22: 1455–1461. [Google Scholar]
- Fitter A, Williamson L, Linkohr B, Leyser O. 2002. Root system architecture determines fitness in an Arabidopsis mutant in competition for immobile phosphate ions but not for nitrate ions. Proceedings of the Royal Society London B 269: 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune S, Lu J, Addiscott TM, Brookes PC. 2005. Assessment of phosphorus leaching losses from arable land. Plant and Soil 269: 99–108. [Google Scholar]
- Gahoonia TS, Nielsen NE. 2004. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant and Soil 262: 55–62. [Google Scholar]
- Gardner WK, Barber DA, Parbery DG. 1983. The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant and Soil 70: 107–124. [Google Scholar]
- Gaume A, Weidler PG, Frossard E. 2000. Effect of maize root mucilage on phosphate adsorption and exchangeability on a synthetic ferrihydrite. Biology and Fertility of Soils 31: 525–532. [Google Scholar]
- George TS, Gregory PJ, Wood M, Read D, Buresh RJ. 2002. Phosphatase activity and organic acids in the rhizosphere of potential agroforestry species and maize. Soil Biology and Biochemistry 34: 1487–1494. [Google Scholar]
- George TS, Richardson AE, Hadobas PA, Simpson RJ. 2004. Characterization of transgenic Trifolium subterraneum L. which expresses phyA and releases extracellular phytase: growth and P nutrition in laboratory media and soil. Plant, Cell and Environment 27: 1351–1361. [Google Scholar]
- George TS, Richardson AE, Simpson RJ. 2005. Behaviour of plant-derived extracellular phytase upon addition to soil. Soil Biology and Biochemistry 37: 977–988. [Google Scholar]
- Gerke J, Römer W, Jungk A. 1994. The excretion of citric and malic acid by proteoid roots of Lupinus albus L.; effects on soil solution concentrations of phosphate, iron, and aluminum in the proteoid rhizosphere in samples of an oxisol and a luvisol. Zeitschrift für Pflanzenernährung und Bodenkunde 157: 289–294. [Google Scholar]
- Gerke J, Beißner L, Römer W. 2000. The quantitative effect of chemical phosphate mobilization by carboxylate anions on P uptake by a single root. I. The basic concept and determination of soil parameters. Journal of Plant Nutrition and Soil Science 163: 207–212. [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL. 1999. Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant, Cell and Environment 22: 801–810. [Google Scholar]
- Gilbert G, Knight JD, Vance CP, Allan DL. 2000. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Annals of Botany 85: 921–928. [Google Scholar]
- Gould SF. 1998. Proteoid root mats bind surface materials in Hawkesbury Sandstone biomantles. Australian Journal of Soil Research 36:1019–1031. [Google Scholar]
- Graham JH, Eissenstat DM. 1994. Host genotype and the formation of VA mycorrhizae. Plant and Soil 159: 179–185. [Google Scholar]
- Grierson PF. 1992. Organic acids in the rhizosphere of Banksia integrifolia L.f. Plant and Soil 144: 259–265. [Google Scholar]
- Grierson PF, Comerford NB. 2000. Non-destructive measurement of acid phosphatase activity in the rhizosphere using nitrocellulose membranes and image analysis. Plant and Soil 218: 49–57. [Google Scholar]
- Grimal JY, Frossard E, Morel JL. 2001. Maize root mucilage decreases adsorption of phosphate on goethite. Biology and Fertility of Soils 33: 226–230. [Google Scholar]
- Güsewell S. 2005. High nitrogen: phosphorus ratios reduce nutrient retention and second-year growth of wetland sedges. New Phytologist 166: 537–550. [DOI] [PubMed] [Google Scholar]
- Guppy CN, Menzies NW, Moody PW, Blamey FPC. 2005. Competitive sorption reactions between phosphorus and organic matter in soil: a review. Australian Journal of Soil Research 43: 180–201. [Google Scholar]
- Hardy GESJ, Barrett S, Shearer BL. 2001. The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australasian Plant Pathology 30: 133–139.
- Hauggaard-Nielsen H, Jensen ES. 2005. Facilitative root interactions in intercrops. Plant and Soil 274: 237–250. [Google Scholar]
- Hawkins H-J, Wolf G, Stock WD. 2005. Cluster roots of Leucadendron laureolum (Proteaceae) and Lupinus albus (Fabaceae) take up glycine intact: an adaptive strategy to low mineral nitrogen in soil? Annals of Botany 96: 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Simpson RJ, Richardson AE. 2000. The growth and phosphorus utilisation of plants in sterile media when supplied with inositol hexaphosphate, glucose 1-phosphate or inorganic phosphate. Plant and Soil 220: 165–174. [Google Scholar]
- Hinsinger P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil 237: 173–195. [Google Scholar]
- Hinsinger P, Plassard C, Tang C, Jaillard B. 2003. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant and Soil 248: 43–59. [Google Scholar]
- Hocking P, Jeffery S. 2004. Cluster root production and organic anion exudation in a group of old world lupins and a new world lupin. Plant and Soil 258: 135–150. [Google Scholar]
- Hoffland E, Findenegg GR, Nelemans JA. 1989. Solubilization of rock phosphate by rape II. Local root exudation of organic acids as a response to P-starvation. Plant and Soil 113: 161–165. [Google Scholar]
- Horst WJ, Waschkies C. 1987. Phosphatversorgerung von Sommerweizen (Triticum aestivum L.) in Mischkultur mit Weiszer Lupine (Lupinus albus L.). Zeitschrift für Pflanzenernährung und Bodenkunde 150: 1–8. [Google Scholar]
- Horton JL, Hart SC. 1998. Hydraulic lift: a potentially important ecosystem process. Trends in Ecology and Evolution 13: 232–235. [DOI] [PubMed] [Google Scholar]
- Howard K, Dell B, Hardy GE. 2000. Phosphite and mycorrhizal formation in seedlings of three Australian Myrtaceae. Australian Journal of Botany 48: 725–729. [Google Scholar]
- Hu B, Wu P, Liao CY, Zhang WP, Ni JJ. 2001. QTLs and epistasis underlying activity of acid phosphatase under phosphorus sufficient and deficient condition in rice (Oryza sativa L.). Plant and Soil 230:99–105. [Google Scholar]
- Huang B. 1999. Water relations and root activities of Buchloe dactyloides and Zoysia japonica in response to localized soil drying. Plant and Soil 208: 179–186. [Google Scholar]
- Hultine KR, Williams DG, Burgess SSO, Keefer TO. 2003. Contrasting patterns of hydraulic redistribution in three desert phreatophytes. Oecologia 135: 167–175. [DOI] [PubMed] [Google Scholar]
- Jones DL. 1998a. Organic acids in the rhizosphere—a critical review. Plant and Soil 205: 25–44. [Google Scholar]
- Jones DL. 1998b. Sorption of organic acids in acid soils and its implications in the rhizosphere. European Journal of Soil Science 49: 447–455. [Google Scholar]
- Jones DL, Dennis PG, Owen AG, Van Hees PAW. 2003. Organic acid behavior in soils—misconceptions and knowledge gaps. Plant and Soil 248: 31–41. [Google Scholar]
- Jungk A. 2001. Root hairs and the acquisition of plant nutrients from soil. Journal of Plant Nutrition and Soil Science 164: 121–129. [Google Scholar]
- Juszczuk IM, Wiktorowska A, Malusá E, Rychter AM. 2004. Changes in the concentration of phenolic compounds and exudation induced by phosphate deficiency in bean plants (Phaseolus vulgaris L.). Plant and Soil 267: 41–49. [Google Scholar]
- Kamh M, Horst WJ, Amer F, Mostafa H, Maier P. 1999. Mobilization of soil and fertilizer phosphate by cover crops. Plant and Soil 211:19–27. [Google Scholar]
- Kamh M, Abdou M, Chude V, Wiesler F, Horst WJ. 2002. Mobilization of phosphorus contributes to positive rotational effects of leguminous cover crops on maize grown on soils from northern Nigeria. Journal of Plant Nutrition and Soil Science 165: 566–572. [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG. 2002. Regulated expression of Arabidopsis phosphate transporters. Plant Physiology 130: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisinghe G, Hocking P, Ryan PR, Delhaize E. 1998. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant, Cell and Environment 21: 467–478. [Google Scholar]
- Kemp PD, Blair GJ. 1994. Phosphorus efficiency in pasture species. VIII. Ontogeny, growth, P acquisition and P utilization of Italian ryegrass and phalaris under P deficient and P sufficient conditions. Australian Journal of Agricultural Research 45: 669–688. [Google Scholar]
- Kuiper D, Kuiper PJC, Lambers H, Schuit JT, Staal M. 1989. Cytokinin contents in relation to mineral nutrition and benzyladenine addition in Plantago major ssp. pleiosperma. Physiologia Plantarum 75:511–517. [Google Scholar]
- Lambers H, Chapin FS III, Pons TL. 1998. Plant physiological ecology. New York: Springer.
- Lambers H, Juniper D, Cawthray GR, Veneklaas EJ, Martinez-Ferri E. 2002. The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant and Soil 238: 111–122. [Google Scholar]
- Lambers H, Cramer MD, Shane MW, Wouterlood M, Poot P, Veneklaas EJ. 2003. Structure and functioning of cluster roots and plant responses to phosphate deficiency. Plant and Soil 248: ix–xix. [Google Scholar]
- Lamont B. 1974. The biology of dauciform roots in the sedge Cyathochaete avenacea. New Phytologist 73: 985–996. [Google Scholar]
- Lamont B. 1981. Specialized roots of non-symbiotic origin in heathlands. In: Specht RL, ed. Ecosystems of the world, Vol 9B, Heathlands and related shrublands. Analytical studies. Amsterdam: Elsevier Scientific, 183–195.
- Lamont B. 1982. Mechanisms for enhancing nutrient uptake in plants, with particular reference to Mediterranean South Africa and Western Australia. Botanical Review 48: 597–689. [Google Scholar]
- Lamont BB. 1984. Specialised modes of nutrition. In: Pate JS, Beard JS, ed. Kwongan—plant life of the sandplain. Nedlands: University of Western Australia Press, 227–252.
- Lejay L, Gansel X, Cerezo M, Tillard P, Muller C, Krapp A, et al. 2003. Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase. Plant Cell 15: 2218–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Tang C, Rengel Z, Zhang FS. 2003. Chickpea facilitates phosphorus uptake by intercropping wheat from an organic phosphorus source. Plant and Soil 248: 305–312. [Google Scholar]
- Li M, Osaki M, Rao IM, Tadano T. 1997. Secretion of phytase from the roots of several plant species under phosphorus-deficient conditions. Plant and Soil 195: 161–169. [Google Scholar]
- Li SM, Li L, Zhang FS, Tang C. 2004. Acid phosphatase role in chickpea/maize intercropping. Annals of Botany 94: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebersbach H, Steingrobe B, Claassen N. 2004. Roots regulate ion transport in the rhizosphere to counteract reduced mobility in dry soil. Plant and Soil 260: 79–88. [Google Scholar]
- Little SA, Hocking PJ, Greene RSB. 2004. A preliminary study on the role of cover crops in improving soil fertility and yield for potato production. Communications in Soil Science and Plant Analysis 35: 471–494. [Google Scholar]
- Liu J, Samac DA, Bucciarelli B, Allan DL, Vance CP. 2005. Signaling of phosphorus deficiency induced gene expression in white lupin requires sugars and phloem transport. Plant Journal 41: 257–288. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Martínez de la Vega O, Guevara-García A, Herrera-Estrella L. 2000. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nature Biotechnology 18: 450–453. [DOI] [PubMed] [Google Scholar]
- Lynch JP. 1995. Root architecture and plant productivity. Plant Physiology 109: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. 2001. Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant and Soil 237: 225–237. [Google Scholar]
- Maguire RO, Sims JT. 2002. Measuring agronomic and environmental soil phosphorus saturation and predicting phosphorus leaching with Mehlich 3. Soil Science Society of America Journal 66: 2033–2039.
- Marschner P, Neumann G, Kania A, Weiskopf L, Lieberei R. 2002. Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant and Soil 246: 167–174. [Google Scholar]
- Marschner P, Grierson PF, Rengel Z. 2005. Microbial community composition and functioning in the rhizosphere of three Banksia species in native woodland in Western Australia. Applied Soil Ecology 28: 191–201. [Google Scholar]
- McArthur WM. 1991. Reference soils of south-western Australia. Perth: Department of Agriculture.
- McCulley RL, Jobbágy EG, Pockman WT, Jackson RB. 2004. Nutrient uptake as a contributing explanation for deep rooting in arid and semi-arid ecosystems. Oecologia 141: 620–628. [DOI] [PubMed] [Google Scholar]
- McKercher RB, Anderson G. 1968. Characterisation of the inositol penta- and hexaphosphate fractions of a number of Canadian and Scottish soils. Journal of Soil Science 19: 302–310. [Google Scholar]
- Mollier A, Pellerin S. 1999. Maize root system growth and development as influenced by phosphorus deficiency. Journal of Experimental Botany 50: 487–497. [Google Scholar]
- Mouat MCH, Nes P. 1986. Influence of soil water content on the supply of phosphate to plants. Australian Journal of Soil Research 24:435–440. [Google Scholar]
- Mudge SR, Smith FW, Richardson AE. 2003. Root-specific and phosphate-regulated expression of phytase under the control of a phosphate transporter enables Arabidopsis to grow on phytate as a sole P source. Plant Science 165: 871–878. [Google Scholar]
- Murphy J, Riley JP, 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36. [Google Scholar]
- Mustart PJ, Cowling RM, Dunne TT. 1994. Reproductive traits of two closely related species-pairs on adjacent, different soil types in South African Fynbos. Plant Ecology 111: 161–171. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Dafonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Nagarajah S, Posner AM, Quirk JP. 1970. Competitive adsorption of phosphate with polygalacturonate and other organic anions on kaolinite and oxide surfaces. Nature 228: 83–85. [DOI] [PubMed] [Google Scholar]
- Neumann G, Martinoia E. 2002. Cluster roots—an underground adaptation for survival in extreme environments. Trends in Plant Science 7: 162–167. [DOI] [PubMed] [Google Scholar]
- Neumann G, Römheld V. 2001. The release of root exudates as affected by the plant physiological status. In: Pinto R, Varanini Z, Nannipieri Z, eds. The rhizosphere: biochemistry and organic substances at the soil–plant interface. New York: Marcel Dekker Inc., 41–93.
- Neumann G, Römheld V. 2006. The release of root exudates as affected by the plant physiological status. In: Pinto R, Varanini Z, Nannipieri Z, eds. The rhizosphere: biochemistry and organic substances at the soil–plant interface. 2nd edn. Boca Raton, FL: CRC Press, in press.
- Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E. 2000. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Annals of Botany 85: 909–919. [Google Scholar]
- Niklas KJ. 1994. Plant allometry: the scaling of form and process. Chicago: University of Chicago Press.
- Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ. 2005. Phosphorus uptake by grain legumes and subsequently grown wheat at different levels of residual phosphorus fertiliser. Australian Journal of Agricultural Research 56: 1041–1047. [Google Scholar]
- Olsson PA, Tyler G. 2004. Occurrence of non-mycorrhizal plant species in south Swedish rocky habitats is related to exchangeable soil phosphate. Journal of Ecology 92: 808–815. [Google Scholar]
- Pate JS, Verboom WH, Galloway PD. 2001. Co-occurrence of Proteaceae, laterite and related oligotrophic soils: coincidental associations or causative inter-relationships? Australian Journal of Botany 49: 529–560. [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H. 2006. Triticum aestivum shows a greater biomass response to a supply of aluminium phosphate than Lupinus albus, despite releasing fewer carboxylates into the rhizosphere. New Phytologist 169: 515–524. [DOI] [PubMed] [Google Scholar]
- Pederson NEJ. 1953. On phytin phosphorus in the soil. Plant and Soil 4: 252–266. [Google Scholar]
- Phillips D, Weste G. 1984. Field resistance in three native monocotyledon species that colonize indigenous sclerophyll forest after invasion by Phytophthora cinnamomi. Australian Journal of Botany 32: 339–353. [Google Scholar]
- Pitts RJ, Cernac A, Estelle M. 1998. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant Journal 16: 553–560. [DOI] [PubMed] [Google Scholar]
- Playsted CWS, Johnston ME, Ramage CM, Edwards DG, Lambers H. 2006. The functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytologist 170: 491–500. [DOI] [PubMed]
- Powell CL. 1973. Effect of P fertiliser on root morphology and P uptake of Carex coriacea. Plant and Soil 41: 661–667. [Google Scholar]
- Powell CL. 1974. Mycorrhizal status of rushes and sedges in New Zealand. PhD thesis, University of Otago. Otago, New Zealand.
- Purnell HM. 1960. Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Australian Journal of Botany 8: 38–50. [Google Scholar]
- Rae AL, Jarmey JM, Mudge SR, Smith FW. 2004. Over-expression of a high-affinity phosphate transporter in transgenic barley plants does not enhance phosphate uptake rates. Functional Plant Biology 31: 141–148. [DOI] [PubMed] [Google Scholar]
- Raghothama KG, Karthikeyan AS. 2005. Phosphate acquisition. Plant and Soil 274: 37–49. [Google Scholar]
- Reddell P, Yun Y, Shipton WA. 1997. Cluster roots and mycorrhizae in Casuarina cunninghamiana: their occurrence and formation in relation to phosphorus supply. Australian Journal of Botany 45: 41–51. [Google Scholar]
- Rengel Z, Marschner P. 2005. Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytologist 168: 305–312. [DOI] [PubMed] [Google Scholar]
- Richardson AE, Hadobas PA, Hayes JE, O'Hare CPO, Simpson RJ. 2001. Utilization of phosphorus by pasture plants supplied with myo-inositol hexaphosphates is enhanced by the presence of soil micro-organisms. Plant and Soil 229: 47–56. [Google Scholar]
- Richardson SJ, Peltzer DA, Allen RB, McGlone MS, Parfitt RL. 2004. Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia 139: 267–276. [DOI] [PubMed] [Google Scholar]
- Rivett DE, Tucker DJ, Jones GP. 1983. The chemical composition of seeds from some Australian plants. Australian Journal of Agriculture 34: 427–432. [Google Scholar]
- Roelofs RFR, Rengel Z, Cawthray GR, Dixon KW, Lambers H. 2001. Exudation of carboxylates in Australian Proteaceae: chemical composition. Plant, Cell and Environment 24: 891–904. [Google Scholar]
- Rubio V, Linhares F, Solano F, Martin A, Iglesias J, Leyva A, Paz-Ares J. 2001. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes and Development 15: 2122–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser P, Verduyn B, Lambers H. 1997. Phosphorus allocation and utilization in three grass species with contrasting response to N and P supply. New Phytologist 137: 293–302. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Mason M, Sangtiean T, Stewart GR. 2003. Do cluster roots of Hakea actities (Proteaceae) acquire complex organic nitrogen? Plant and Soil 248: 157–165. [Google Scholar]
- Schmidt W. 2001. From faith to fate: ethylene signalling in morphogenetic responses to P and Fe deficiency. Journal of Plant Nutrition and Soil Science 164: 147–154. [Google Scholar]
- Schroeder MS, Janos DP. 2005. Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 15: 203–216. [DOI] [PubMed] [Google Scholar]
- Selivanov IA, Utemova LD. 1969. Root anatomy of sedges in relation to their mycotrophy. Transactions of Perm State Pedagogical Institute 68: 45–55. (in Russian). [Google Scholar]
- Seymour NP, Thompson JP, Fiske ML. 1994. Phytotoxicity of fosetyl-Al and phosphonic acid to maize during production of vescicular arbuscular mycorrhizal inoculum. Plant Disease 78: 441–446. [Google Scholar]
- Shane MW, Lambers H. 2005a. Cluster roots: a curiosity in context. Plant and Soil 274: 99–123. [Google Scholar]
- Shane MW, Lambers H. 2005b. Manganese accumulation in leaves of Hakea prostrata R. Br. (Proteaceae) and the significance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiologia Plantarum 274: 441–450. [Google Scholar]
- Shane MW, Lambers H. 2006. Systemic suppression of cluster-root formation and net P-uptake rates in Grevillea crithmifolia at elevated P supply: a proteacean with resistance for developing symptoms of ‘P toxicity’. Journal of Experimental Botany 57: 413–423. [DOI] [PubMed] [Google Scholar]
- Shane MW, De Vos M, De Roock S, Cawthray GR, Lambers H. 2003. Effects of external phosphorus supply on internal phosphorus concentration and the initiation, growth and exudation of cluster roots in Hakea prostrata R.Br. Plant and Soil 248: 209–219. [Google Scholar]
- Shane MW, McCully ME, Lambers H. 2004a. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). Journal of Experimental Botany 55: 1033–1044. [DOI] [PubMed] [Google Scholar]
- Shane MW, Szota C, Lambers H. 2004b. A root trait accounting for the extreme phosphorus sensitivity of Hakea prostrata (Proteaceae). Plant, Cell and Environment 27: 991–1004. [Google Scholar]
- Shane MW, Cramer MD, Funayama-Noguchi S, Millar AH, Day DA, Lambers H. 2004c. Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiology 135: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, Dixon KW, Lambers H. 2005a. The occurrence of dauciform roots amongst Western Australian reeds, rushes and sedges, and the impact of phosphorus supply on dauciform-root development in Schoenus unispiculatus (Cyperaceae). New Phytologist 165: 887–898. [DOI] [PubMed] [Google Scholar]
- Shane MW, Cramer MD, Cawthray GR, Kuo J, Lambers H. 2005b. Specialised ‘dauciform’ roots of Cyperaceae exhibit convergent physiology with ‘proteoid’ roots of Proteaceae to challenge phosphate-deficient environments. In: Li CJ, Zhang FS, Dobermann A, et al., eds. Plant nutrition for food security, human health and environmental protection. Beijing: Tsinghua University Press, 522–523.
- Shane MW, Cawthray GR, Cramer MD, Kuo J, Lambers H. 2006. Specialised ‘dauciform’ roots of Cyperaceae are structurally distinct, but functionally analogous with ‘cluster’ roots. Plant, Cell and Environment, in press. [DOI] [PubMed]
- Silberbush M, Barber SA. 1983. Sensitivity of simulated phosphorus uptake to parameters used by a mechanistic-mathematical model. Plant and Soil 74: 93–100. [Google Scholar]
- Skene KR. 2000. Pattern formation in cluster roots: Some developmental and evolutionary considerations. Annals of Botany 85: 901–908. [Google Scholar]
- Skene KR. 2003. The evolution of physiology and development in the cluster root: teaching an old dog new tricks? Plant and Soil 248: 21–30. [Google Scholar]
- Skene KR, James WM. 2000. A comparison of the effects of auxin on cluster root initiation and development in Grevillea robusta Cunn. Ex R. Br. (Proteaceae) and in the genus Lupinus (Leguminosae). Plant and Soil 219: 221–229. [Google Scholar]
- Smith SE, Smith FA, Jakobsen I. 2003. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiology 133: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström B. 2002. Challenges for mycorrhizal research into the new millennium. Plant and Soil 244: 1–7. [Google Scholar]
- Steen I. 1998. Phosphorus availability in the 21st century: management of a non-renewable resource. Phosphorus and Potassium 217: 25–31. [Google Scholar]
- Steingrobe B. 2001. Root renewal of sugar beet as a mechanism of P uptake efficiency. Journal of Plant Nutrition and Soil Science 164: 533–539. [Google Scholar]
- Steingrobe B, Schmid H, Claassen N. 2001. Root production and root mortality of winter barley and its implication with regard to phosphate acquisition. Plant and Soil 237: 239–248. [Google Scholar]
- Stock WD, Allsopp N. 1992. Functional perspective of ecosystems. In: Cowling RM, ed. The ecology of fynbos: nutrients, fire and diversity. Cape Town: Oxford University Press, 241–259.
- Ström L, Olsson T, Tyler G. 1994. Differences between calcifuge and acidifuge plants in root exudation of low molecular weight organic acids. Plant and Soil 167: 239–245. [Google Scholar]
- Su J, Xiao Y, Li M, Liu Q, Li B, Tong Y, et al. 2006. Mapping QTLs for phosphorus-deficiency tolerance in wheat (Triticum aestivum L.). Plant and Soil 281: 25–36.
- Sukarno N, Smith FA, Scott ES, Jones GP, Smith SE. 1998. The effect of fungicides on vesicular-arbuscular mycorrhizal symbiosis. III. The influence of VA mycorrhiza on phytotoxic effects following application of fosetyl-Al and phosphonate. New Phytologist 139: 321–330.
- Sumann M, Amelung W, Haumaier L, Zech W. 1998. Climatic effects on soil organic phosphorus in the North American great plains identified by phosphorus-31 nuclear magnetic resonance. Soil Science Society of America Journal 62: 1580–1586. [Google Scholar]
- Swensen SM. 1996. The evolution of actinorhizal symbioses: evidence for multiple origins of the symbiotic association. American Journal of Botany 83: 1503–1512. [Google Scholar]
- Tarafdar JC, Claassen N. 1988. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biology and Fertility of Soils 5: 308–312. [Google Scholar]
- Tarafdar JC, Claassen N. 2001. Comparative efficiency of acid phosphatase originated from plant and fungal sources. Journal of Plant Nutrition and Soil Science 164: 279–282. [Google Scholar]
- Tarafdar JC, Claassen N. 2005. Preferential utilization of organic and inorganic sources of phosphorus by wheat plant. Plant and Soil 27: 285–293. [Google Scholar]
- Ticconi CA, Delatorre CA, Abel S. 2001. Attenuation of phosphate starvation responses by phosphite in Arabidopsis. Plant Physiology 127: 963–972. [PMC free article] [PubMed] [Google Scholar]
- Turner FT, Gilliam JW. 1976. Increased P diffusion as an explanation of increased P availability in flooded rice soil. Plant and Soil 45: 365–377. [Google Scholar]
- Uhde-Stone C, Zinn KE, Ramirez M, Li A, Vance CP, Allan DL. 2003. Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant Physiology 131: 1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uresk DW. 1986. Food habits of cattle on mixed-grass prairie on the Northern Great Plains. Prairie Naturalist 18: 211–218. [Google Scholar]
- Valizadeh GR, Rengel Z, Rate AW. 2003. Response of wheat genotypes efficient in P utilisation and genotypes responsive to P fertilisation to different P banding depths and watering regimes. Australian Journal of Agricultural Research 54: 59–65. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423–447. [DOI] [PubMed] [Google Scholar]
- Vandeleur R, Niemietz C, Tilbrook J, Tyerman SD. 2005. Roles of aquaporins in root responses to irrigation. Plant and Soil 274: 141–161. [Google Scholar]
- Van Lijsebettens M, Van Montagu M. 2005. Historical perspectives on plant developmental biology. International Journal of Developmental Biology 49: 453–465. [DOI] [PubMed] [Google Scholar]
- Varadarajan DK, Karthikeyan AS, Matilda PD, Raghothama KG. 2002. Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiology 129: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboom WH, Pate JS. 2003. Relationships between cluster root-bearing taxa and laterite across landscapes in southwest Western Australia: an approach using airborne radiometric and digital elevation models. Plant and Soil 248: 321–333. [Google Scholar]
- Verboom WH, Pate JS. 2006. Bioengineering of soil profiles in semiarid ecosystems: the ‘phytotarium’ concept. A review. Plant and Soil, in press.
- Vessey JK, Pawlowski K, Bergman B. 2005. Root-based N2-fixing symbioses: legumes, actinorhizal plants, Parasponia sp. and cycads. Plant and Soil 274: 51–78. [Google Scholar]
- Vetterlein D, Marschner H. 1993. Use of a microtensiometer technique to study hydraulic lift in a sandy soil planted with pearl millet (Pennisetum americanum [L.] Leeke). Plant and Soil 149: 275–282. [Google Scholar]
- Vig AC, Singh NT. 1983. Yield and P uptake by wheat as affected by P fertilization and soil moisture regime. Nutrient Cycling in Agroecosystems 4: 21–29. [Google Scholar]
- Walker TC, Syers JK. 1976. The fate of phosphorus during pedogenesis. Geoderma 15: 1–19. [Google Scholar]
- Wasaki J, Yamamura T, Shinano T, Osaki M. 2003. Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant and Soil 248: 129–136. [Google Scholar]
- Watt M, Evans JR. 1999. Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiology 120: 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DM, Prout AL. 1993. Changing farm practice to meet environmental objectives of nutrient loss to Oyster Harbour. Nutrient Cycling in Agroecosystems 36: 177–184. [Google Scholar]
- Weisskopf L, Fromin N, Tomasi N, Aragno M, Martinoia E. 2005. Secretion activity of white lupin's cluster roots influences bacterial abundance, function and community structure. Plant and Soil 268: 181–194. [Google Scholar]
- Weisskopf L, Abou-Mansour E, Fromin N, Tomasi N, Santelia D, Edelkott I, et al. 2006. White lupin has developed a complex strategy to limit microbial degradation of the secreted citrate required for phosphate nutrition. Plant, Cell and Environment 29: 919–927. [DOI] [PubMed]
- Wenzel CL, Ashford AE, Summerell BA. 1994. Phosphate-solubilising bacteria associated with proteoid roots of warath [Telopia speciosissima (Sm.) R.Br.]. New Phytologist 128: 487–496. [DOI] [PubMed] [Google Scholar]
- White ME. 1986. The greening of Gondwana. The 400 million year story of Australian plants. Frenchs Forest: Reed Books.
- Wouterlood M, Cawthray GR, Scanlon TT, Lambers H, Veneklaas EJ. 2004. Carboxylate concentrations in the rhizosphere of root tips of chickpea increase during plant development, but are not correlated with P supply. New Phytologist 162: 745–753. [DOI] [PubMed] [Google Scholar]
- Xiao K, Harrison MJ, Wang Z-Y. 2005. Transgenic expression of a novel M. truncatula phytase gene results in improved acquisition of organic phosphorus by Arabidopsis. Planta 222: 27–36. [DOI] [PubMed] [Google Scholar]
- Yan X, Liao H, Beebe SE, Blair MW, Lynch JP. 2004. QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant and Soil 265: 17–29. [Google Scholar]
- Yoder CK, Novak RS. 1999. Hydraulic lift among native plant species in the Mojave Desert. Plant and Soil 215: 93–102. [Google Scholar]
- Yun SJ, Kaeppler SM. 2001. Induction of maize acid phosphatase activities under phosphorus starvation. Plant and Soil 237:109–115. [Google Scholar]
- Zhang FS, Li L. 2003. Using competitive and facilitative interactions in intercropping systems enhances crop productivity and nutrient-use efficiency. Plant and Soil 248: 305–312. [Google Scholar]
- Zhang Y-L, Lynch JP, Brown KM. 2003. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. Journal of Experimental Botany 54: 2351–2361. [DOI] [PubMed] [Google Scholar]
- Zhu J, Kaeppler SM, Lynch JP. 2005. Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant and Soil 270: 299–310. [Google Scholar]
- Zhu Y, Yan F, Zörb C, Schubert S. 2005. A link between citrate and proton release by proteoid roots of white lupin (Lupinus albus L.) grown under phosphorus-deficient conditions? Plant and Cell Physiology 46: 892–901. [DOI] [PubMed] [Google Scholar]