Abstract
• Background and Aims In dioecious species male and female plants experience different selective pressures and often incur different reproductive costs. An increase in reproductive investment habitually results in a reduction of the resources available to other demands, such as vegetative growth. Tree-ring growth is an integrative measure that tracks vegetative investment through the plant's entire life span. This allows the study of gender-specific vegetative allocation strategies in dioecious tree species thoughout their life stages.
• Methods Standard dendrochronological procedures were used to measure tree-ring width. Analyses of time-series were made by means of General Mixed Models with correction of autocorrelated values by the use of an autoregressive covariance structure of order one. Bootstrapped correlation functions were used to study the relationship between climate and tree-ring width.
• Key Results Male and female trees invest a similar amount of resources to ring growth during the early life stages of Juniperus thurifera. However, after reaching sexual maturity, tree-ring growth is reduced for both sexes. Furthermore, females experience a significantly stronger reduction in growth than males, which indicates a lower vegetative allocation in females. In addition, growth was positively correlated with precipitation from the current winter and spring in male trees but only to current spring precipitation in females.
• Conclusions Once sexual maturity is achieved, tree rings grow proportionally more in males than in females. Differences in tree-ring growth between the genders could be a strategy to respond to different reproductive demands. Therefore, and responding to the questions of when, how and how much asked in the title, it is shown that male trees invest more resources to growth than female trees only after reaching sexual maturity, and they use these resources in a different temporal way.
Keywords: Dendrochronology, sexual maturity, resource allocation, trade-offs, Juniperus
INTRODUCTION
Reproduction is expensive to plants and is often inversely correlated with vegetative growth, indicating that both biological functions compete for the same resources (Harper, 1977; Koenig and Knops, 1998). Plants may compensate for reproductive efforts by improving physiological performance, reducing the number of reproductive events or reducing resources allocated to growth and maintenance (Obeso, 2002). Detection of reproductive costs in the short term might be difficult due to physiological compensation mechanisms (Obeso and Retuerto, 2002); therefore, long-term cumulative variables such as tree-ring width are widely used as a proxy of vegetative investment in woody plants (e.g. Obeso, 1997; Silvertown and Dodd, 1999).
However, little attention has been given to the effect of reproduction on tree-ring growth in dioecious species. Costs of reproduction are age-specific and reproductive investment is variable during plants' lives (Silvertown and Dodd, 1999). Furthermore, plants of different sexes might incur different reproductive costs and thus present different trade-offs between reproduction and growth and develop different resource-use strategies (Obeso, 2002). Thus, little attention has been drawn to the interaction between age and gender in dioecious species and most investigations have not estimated how tree-ring growth varies with size or age at all (Silvertown and Dodd, 1999; but see Bañuelos and Obeso, 2004).
The seasonal scale of resource investment can play a significant role in gender-specific resource allocation. In dioecious species males incur their maximum reproductive effort at the time of flower production. Once pollen is shed, males do not need to invest more resources into current reproduction. In contrast, the main reproductive effort for females starts once the ovules are pollinated because fruit maturation and ripening is a costly task compared with flower production (Verdú and García-Fayos, 1998; Obeso, 2002). Thus, in the short-term, males and females are expected to manage available resources differently. The relationship between tree-ring growth and precipitation from previous months can help us to discern how the different sexes manage resource availability, because precipitation is a proxy of resource availability in semi-arid environments that is positively correlated with tree-ring growth in most species (Cherubini et al., 2003; Austin et al., 2004).
Juniperus thurifera is a dioecious tree inhabiting high mountain, semi-arid areas. Flowering occurs during February, and female cone growth and ripening takes 20 months. Tree-ring growth in this species has been reported to be positively correlated with precipitation from the previous year (Bertaudière et al., 1999). Pavón-García (2005) sampled cores from J. thurifera individuals of different reproductive stages and determined that reproductive maturity in this species started at the age of 31 ± 3·6 years (n = 223). These characteristics make J. thurifera particularly appropriate to study relationships between age and gender-specific reproductive trade-offs and climate in stem growth.
The objectives of the research were to determine the effect of reaching sexual maturity in tree-ring growth of each gender of the dioecious tree J. thurifera, and to detect the resource-use strategies developed to deal with reproductive demands by each gender. More specifically, we wanted to answer the following questions regarding resource investment. (a) When—does sexual maturity affect tree ring growth? (b) How much—do reproductive females differ from males in vegetative investment? (c) How—do climatic relations between precipitation and ring growth reveal gender specific resource allocation strategies?
MATERIALS AND METHODS
Study species
Juniperus thurifera L. (Cupressaceae) is a dioecious, long-lived tree with relict distribution from the Tertiary throughout the western Mediterranean basin. It is usually the dominant species in high-elevation, low-density forests with a semi-arid climate. Trees are 5–10 m high and often live for centuries (Bertaudière et al., 1999). Trees reach sexual maturity at the age of 31 ± 3·6 years (n = 223; Pavón-García, 2005). Reproductive output varies strongly from year to year; male and female trees flower at the end of the winter period and following winter pollination females produce reproductive cones over a period of 20 months, after which seed dispersal occurs (Amaral-Franco, 1986; P. García-Fayos, pers. obs.). Gauquelin et al. (2002) found that males grew significantly more than females when comparing the ring-widths of three males and three females from Morocco (t-test, P < 0·01); however in that population females were close to being significantly taller (P = 0·056) than male trees.
Study site
The study was performed at La Puebla de San Miguel, Valencia, Spain. The study site covers approx. 30 ha and is located at 1500 m a.s.l. Vegetation is dominated by J. thurifera trees of different ages, with a tree density of 423 ha−1. Vegetation covers less than 40 % of the rocky soil surface, while the rest of the open spaces have only ephemeral herbaceous vegetation—lichens and sparse Thymus and Genista plants. The mean height (n = 40) of male trees (4·73 ± 0·84 m) was greater than female trees (4·63 ± 0·83 m). However, the difference between the sexes was not significant (F = 0·14; P = 0·71). The climate is Mediterranean, but with harsh winters (the duration of the freezing period is more than 120 d year−1) and warm and dry summers. Mean annual precipitation is 486 mm, with October the wettest month (58·6 mm) and July the driest (26·86 mm). Annual mean temperature is 13 °C, with August the warmest month (mean 22·8 °C) and January the coldest (mean 4·8 °C). Winter precipitation is usually in the form of snow, and accounts for 21 % of the total precipitation. The study area has been used for agriculture, timber and extensive livestock grazing for centuries. Narrow valleys were tilled for rye and barley until land abandonment in 1960 (Rodrigo, 1999). In recent times, reforestation has occurred due to human abandonment of traditional land uses as a consequence of social and economic changes (Lasanta, 1996), although livestock grazing pressure is still noticeable (D. Montesinos, pers. obs.).
Tree-ring and statistical analysis
Logging of the study site in October 2001 provided the opportunity to obtain discs of J. thurifera from 14 male and 14 female trees. The trees were are representative of the variability in age and size of the studied population. Whole discs from the bottom of the trunk were collected and polished for tree-ring growth measurements. Two ring-series were selected on each disc and ring-widths were measured to the nearest 0·01 mm using an incremental measuring table. The tree-ring series were visually cross-dated and synchronized with each other by calculating the ‘Gleichläufigkeit’ coefficient (GLK %; Eckstein and Bauch, 1969) and the t-value after Baillie and Pilcher (1973). Cross-dating was verified statistically using the COFECHA program (Holmes, 1994). Despite difficulties while cross-dating due to the presence of double and missing rings, 11 males and 12 female trees were exactly dated and then used to build chronologies. Two chronologies were made for both the male and female trees: one non-detrended raw-data, and one detrended residual. The non-detrended raw-data chronologies were used to test for differences in growth between male and female trees. The climatic influence on tree growth was studied using the residual version of the chronology, obtained by the program ARSTAN (Holmes, 1994). In this program, the individual raw tree-ring series were standardized in a two-step procedure to remove most of the low-frequency variability that is assumed to be unrelated to climate, such as that due to tree ageing and forest stand development (Cook and Peters, 1981). First, the long-term trend was removed by fitting a negative exponential function or a regression line to each tree-ring series. Second, a more flexible detrending was made by a cubic smoothing spline with a 50 % frequency response of 30 years to reduce further non-climatic variance. After that, autoregressive modelling of the residuals and bi-weight robust estimation of the mean were applied to construct residual chronologies (Cook and Peters, 1981). Residual chronologies are commonly used in dendroclimatic studies because removal of serial autocorrelation is required to analyse the effects of year-to-year climatic variability on tree growth.
Differences between non-detrended ring growth data from male and female trees were tested with SPSS 11·00 with a General Mixed Model with an autoregressive covariance structure of order one. This model permits the study of time-series accounting for temporal autocorrelations among continuous rings from the same tree. Two statistical analyses were made, one testing for differences between sexes among the first 30 years of life of each tree and another testing for differences among the following 120 years, once sexual maturity is achieved (Pavón-García, 2005).
To test for climatic effects both for male and female trees, bootstrapped correlation functions (Guiot, 1991) were computed using the software DENDROCLIM2002 (Biondi and Waikul, 2004). In each case, the residual chronology was used as an independent variable. Since residual chronologies do not preserve the effects of previous years, the regressors were, separately, seasonal precipitation and temperature from summer and autumn of the previous growing season and winter, spring, summer and autumn of the year in which the ring was formed.
RESULTS
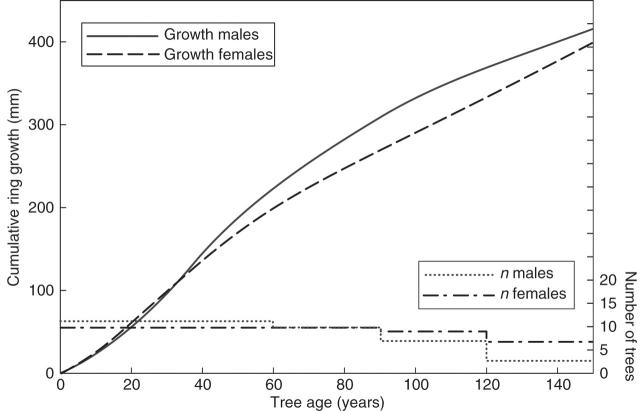
Male and female trees grew increasingly more every year until an approximate age of 30. From that point, ring width decreased gradually in both sexes. However, this reduction was significantly steeper for females, resulting in greater overall ring widths for males compared with female trees (Fig. 1 and Table 1). Mean age of sampled trees was 152 ±74 years (range 47–328).
Fig. 1.
Relationship between cumulative ring growth and tree age. Males and females grow similarly until the age of approx. 30, after which male trees grow significantly more than female trees. Sample sizes are indicated.
Table 1.
Ring growth (mm 10−2; mean ± s.d.) and Lineal Mixed Model values for male and female trees in each age class. Male tree-ring growth is greater than that of females after reaching sexual maturity
| Male trees | Female trees | F | p | |
|---|---|---|---|---|
| Age 1–30 | 321·39 ± 242·77 | 332·78 ± 281·92 | 0·020 | 0·889 |
| Age 31–150 | 293·76 ± 205·61 | 249·44 ± 174·42 | 4·797 | 0·031 |
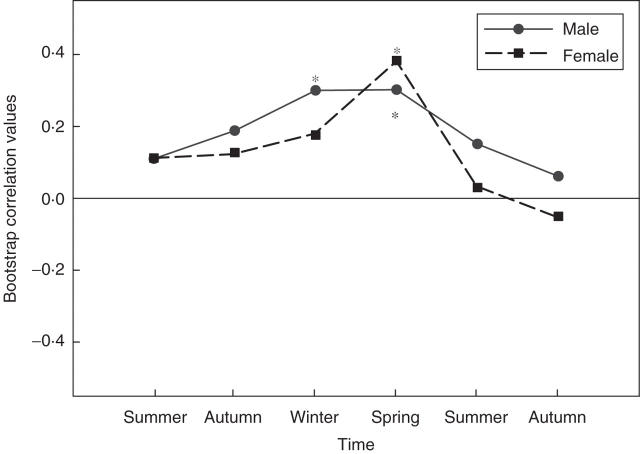
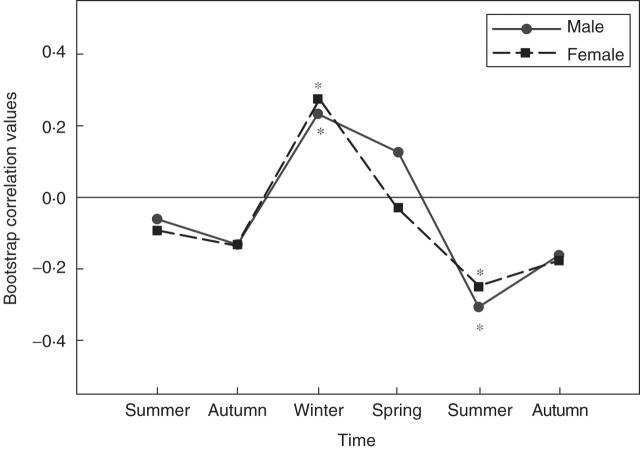
On an annual basis, climate from the current and previous year (precipitation and temperature) accounts for a high proportion of the variance in tree-ring widths for both sexes (r = 0·72 and 0·73 for males and females, respectively). Intra-annually, however, males showed a different growth response than females. Male growth was significantly correlated with current winter and spring precipitation (r = 0·30 and 0·31, respectively) whereas female growth was only significantly correlated to current spring precipitation (r = 0·38; Fig. 2). Temperature from the current year was also correlated with tree-ring width but there was no difference between the sexes. Current winter temperatures correlated positively with tree-ring width for both sexes (r = 0·23 and 0·27 for males and females, respectively; Fig. 3). In contrast, current summer temperatures correlated negatively with tree-ring width for both sexes (r = −0·31 and −0·25 for males and females respectively).
Fig. 2.
Bootstrap correlation values for the previous six seasons. Male tree-ring growth correlates significantly with winter and spring precipitation, while female tree-ring growth correlates only with spring precipitation. Asterisks indicate statistically significant correlations (P < 0·05).
Fig. 3.
Bootstrap correlation values for the previous six seasons. Male and female tree-ring growth correlate positively with temperature from the previous winter and negatively with temperature from the previous summer. Asterisks indicate statistically significant correlations (P < 0·05).
DISCUSSION
Sexual maturity marks a change-point in J. thurifera tree-ring growth pattern. When reproduction starts, ring growth experiences a strong reduction for individuals of both sexes. Switching of resources between stem growth and reproduction is characteristic of Northern Hemisphere conifers (Koenig and Knops, 1998) and J. thurifera is no exception. Furthermore, the trade-off between the reproductive and the vegetative function is sexually-biased, with females experiencing a stronger reduction in stem growth than males. In the only other work known to us in which the interaction between gender, age of reproduction and stem growth was studied, a similar trade-off in the dioecious, insect-pollinated, Rhamnus alpinus was found (Bañuelos and Obeso, 2004).
In J. thurifera, stem diameter is positively correlated with tree height (data not shown) and thus tree-ring growth is a good proxy for tree height. Males of anemophilous (wind-pollinated) species such as J. thurifera experience a stronger selective pressure than females to increase tree height, since taller males may produce more pollen (Obeso, 2002) and may disperse pollen over longer distances, thereby attaining greater pollination success (Burd and Allen, 1988). In contrast, females do not benefit directly from increased height but do need to accumulate resources during periods of low reproductive investment in order to be able to grow and ripen reproductive cones during the following two years (Obeso, 2002).
Water limitation can limit nutrient availability (Austin et al., 2004) particularly in Mediterranean environments where precipitation and temperature are the main determinants of tree-ring growth (Cherubini et al., 2003). Previous studies have found a positive relationship between current year ring growth and previous year precipitation in J. thurifera (Bertaudière et al., 1999). However, in our study, both sexes were analysed separately. Although yearly growth patterns in relation to climatic conditions are very similar for both sexes, growth patterns differ when seasonal (i.e. within-year) patterns are studied, and as far as we know this is the first work in which gender-specific climatic correlations have been found. In the only other study known to us in which gender-specific climatic correlations were examined (Rovere et al., 2003), correlations between annual tree-ring growth and seasonal climatic variables did not differ between male and female trees of the conifer Austrocedrus chilensis.
In J. thurifera, at the seasonal temporal scale, male trees present a significant positive correlation between tree-ring growth and both current winter and current spring precipitation. This indicates that males are investing a high proportion of their winter- and spring-available resources to growth and not to reproduction, since male cones are fully developed at the end of the winter. In contrast, current female growth correlates only with current spring precipitation. This would suggest that females do not use current winter precipitation for current vegetative growth but rather that they allocate resources for subsequent cone maturation, and only surplus resources from current spring precipitation are used for current growth. Contrary to our findings, correlations between annual growth and seasonal climatic variables did not differ between male and female trees of the conifer Austrocedrus chilensis in the only other work known by us in which gender-specific correlations were studied (Rovere et al., 2003). As far as we know, this is the first work describing a differential ring growth response to climatic variation between male and female plants.
We suggest that male and female trees of J. thurifera have to deal with different selective pressures. Males would benefit from an increase in vegetative investment, since this could increase their reproductive success. On the other hand, females need to store resources to guarantee provisioning for cone maturation. Thus, and responding to the questions asked in the title, it is shown that male trees invest more resources to growth than female trees only after reaching sexual maturity, and in a different temporal way.
ACKNOWLEDGEMENTS
The authors wish to thank the residents of Puebla de San Miguel, Luis María Alcusa, Mayor of the village, and Jesús Monedero, from the Forest Guard, for their help in providing the samples for the present work. Thanks are also given for the help and support of the whole ‘Llavoratori’ team, and especially to Tono Bellido. This work was financed by the Spanish Ministerio de Educación y Ciencia with the project AGL2001-1061. Daniel Montesinos enjoyed a grant (BES-2002-1828) from MEC.
LITERATURE CITED
- Amaral-Franco J. 1986. Juniperus. In: Castroviejo S, Laínz M, López-González G, Montserrat P, Muñoz-Garmendia F, Paiva J, Villar L, eds. Flora Ibérica, Vol. I Lycopodiacieae–Papaveraceae. Madrid: Real Jardín Botánico, CSIC, 181–188.
- Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM. 2004. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141: 221–235. [DOI] [PubMed] [Google Scholar]
- Baillie MGL, Pilcher JR. 1973. A simple crossdating program for tree-ring research. Tree-Ring Bulletin 33: 7–14. [Google Scholar]
- Bañuelos MJ, Obeso JR. 2004. Resource allocation in the dioecious shrub Rhamnus alpinus: the hidden costs of reproduction. Evolutionary Ecology Research 6: 397–413. [Google Scholar]
- Bertaudière V, Montes N, Gauquelin T, Edouard JL. 1999. Dendroecology of thuriferous juniper (Juniperus thurifera L.): example from a French Pyrenean site at Rie mountain. Annals of Forest Science 56: 685–697. [Google Scholar]
- Biondi F, Waikul K. 2004. DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Computers and Geosciences 30: 303–311. [Google Scholar]
- Burd M, Allen TFH. 1988. Sexual allocation strategy in wind-pollinated plants. Evolution 42: 403–407. [DOI] [PubMed] [Google Scholar]
- Cherubini P, Gartner BL, Tognetti R, Braker OU, Schoch W, Innes JL. 2003. Identification, measurement and interpretation of tree rings in woody species from Mediterranean climates. Biological Review 78: 119–148. [DOI] [PubMed] [Google Scholar]
- Cook ER, Peters K. 1981. The smoothing spline: a new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree-Ring Bulletin 41: 53. [Google Scholar]
- Eckstein D, Bauch J. 1969. Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwissenschaftliches Centralblatt 88: 230–250. [Google Scholar]
- Gauquelin T, Bertaudière V, Badri W, Montès N. 2002. Sex ratio and sexual dimorphism in mountain dioecious thuriferous juniper (Juniperus thurifera L., Cupressaceae). Botanical Journal of the Linnean Society 138: 237–244. [Google Scholar]
- Guiot J. 1991. The bootstrapped response function. Tree-Ring Bulletin 51: 39–41. [Google Scholar]
- Harper JL. 1977. The population biology of plants. New York: Academic Press.
- Holmes RL. 1994. Dendrochronology program library user's manual. Tucson, AZ, USA: University of Arizona, Laboratory of Tree Ring Research.
- Koenig WD, Knops JMH. 1998. Scale of mast-seeding and tree-ring growth. Nature 396: 225–226. [Google Scholar]
- Lasanta T. 1996. El proceso de marginación de tierras en España. In: Lasanta T, García-Ruiz JM, eds. Erosión y recuperación de tierras en áreas marginales., Logroño, Spain: Instituto de Estudios Riojanos & Sociedad Española de Geomorfología, 7–32.
- Obeso JR. 1997. Costs of reproduction in Ilex aquifolium: effects at tree, branch and leaf levels. Journal of Ecology 85: 159–166. [Google Scholar]
- Obeso JR. 2002. The costs of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Obeso JR, Retuerto R. 2002. Sexual dimorphism in holly Ilex aquifolium: cost of reproduction, sexual selection or physiological differenciation? Revista Chilena de Historia Natural 75: 67–77. [Google Scholar]
- Pavón-García J. 2005. Biología vegetativa y reproductiva en los primeros estadios de crecimiento de Juniperus thurifera L. Dissertation Thesis. Universidad de Alcalá, Spain.
- Rodrigo C. 1999. Puebla de San Miguel. Valencia: Ayuntamiento de Puebla de San Miguel.
- Rovere AE, Aizen MA, Kitzberger T. 2003. Growth and climatic response of male and female trees of Austrocedrus chilensis, a dioecious conifer from the temperate forests of southern South America. Ecoscience 10: 195–203. [Google Scholar]
- Silvertown J, Dodd M. 1999. The demographic cost of reproduction and its consequences in Balsam Fir (Abies balsamea). The American Naturalist 29: 321–332. [DOI] [PubMed] [Google Scholar]
- Verdú M, García-Fayos P. 1998. Female biased sex ratios in Pistacia lentiscus L. (Anacardiaceae). Plant Ecology 135: 95–101. [Google Scholar]