Abstract
• Background and Aims White lupin is highly adapted to growth in a low-P environment. The objective of the present study was to evaluate whether white lupin grown under P-stress has adaptations in nodulation and N2 fixation that facilitate continued functioning.
• Methods Nodulated plants were grown in silica sand supplied with N-free nutrient solution containing 0 to 0·5 mm P. At 21 and 37 d after inoculation (DAI) growth, nodulation, P and N concentration, N2 fixation (15N2 uptake and H2 evolution), root/nodule net CO2 evolution and CO2 fixation (14CO2 uptake) were measured. Furthermore, at 21 DAI in-vitro activities and transcript abundance of key enzymes of the C and N metabolism in nodules were determined. Moreover, nodulation in cluster root zones was evaluated.
• Key Results Treatment without P led to a lower P concentration in shoots, roots, and nodules. In both treatments, with or without P, the P concentration in nodules was greater than that in the other organs. At 21 DAI nitrogen fixation rates did not differ between treatments and the plants displayed no symptoms of P or N deficiency on their shoots. Although nodule number at 21 DAI increased in response to P-deficiency, total nodule mass remained constant. Increased nodule number in P-deficient plants was associated with cluster root formation. A higher root/nodule CO2 fixation in the treatment without P led to a lower net CO2 release per unit fixed N, although the total CO2 released per unit fixed N was higher in the treatment without P. The higher CO2 fixation was correlated with increased transcript abundance and enzyme activities of phosphoenolpyruvate carboxylase and malate dehydrogenase in nodules. Between 21 and 37 DAI, shoots of plants grown without P developed symptoms of N- and P-deficiency. By 37 DAI the P concentration had decreased in all organs of the plants treated with no P. At 37 DAI, nitrogen fixation in the treatment without P had almost ceased.
• Conclusions Enhanced nodulation in cluster root zones and increased potential for organic acid production in root nodules appear to contribute to white lupin's resilience to P-deficiency.
Keywords: White lupin, Lupinus albus, nitrogen fixation, P deficiency, H2 evolution, 15N2 uptake
INTRODUCTION
White lupin (Lupinus albus) has evolved elegant adaptations for growth under P-deficient soil conditions (Dinkelaker et al., 1995; Keerthisinghe et al., 1998; Watt and Evans, 1999; Neumann and Martinoia, 2002). Rather than forming a mycorrhizal symbiosis, white lupin has a highly synchronous, co-ordinated expression of genes which results in proliferation of cluster roots, root exudation of organic acids and acid phosphatase, as well as the induction of numerous transporters (Gilbert et al., 1999; Neumann and Martinoia, 2002; Vance et al., 2003; Uhde-Stone et al., 2003a). The exudation of organic acids and acid phosphatase solubilize bound forms of P thus increasing the availability of P and micronutrients in cluster root zones (Lamont, 2003). Moreover, the formation of cluster roots results in a striking increase in root surface area, thereby providing enhanced zones for P uptake (Dinkelaker et al., 1989, 1995; Gerke et al., 1994; Neumann et al., 1999; Lamont, 2003).
In agricultural systems, white lupin is readily nodulated by Bradyrhizobium lupini and forms effective nodules. Thus, it is surprising that there are few if any studies of white lupin nodulation and N2 fixation when grown in low P environments. Symbiotic nitrogen fixation has a high P demand because the process consumes large amounts of energy (Schulze et al., 1999), and energy-generating metabolism strongly depends upon the availability of P (Israel, 1987; Plaxton, 2004). Several reports have documented that nodules are a strong P sink and nodule P concentration normally exceeds that of roots and shoots (Sa and Israel, 1991; Drevon and Hartwig, 1997). Legumes frequently respond to mild P-stress through an increase in nodule number but nodules generally are smaller. Mechanistically, the increase in nodule number under low P has been linked to increasing nodule surface area and thereby facilitating oxygen diffusion into the nodule (Ribet and Drevon, 1995). Oxygen diffusion into and availability in the nodule-infected cell zone is regarded as a primary regulatory factor for effective N2 fixation (Layzell et al., 1990) and, as such, is subject to tight regulation (Minchin, 1997; Denison, 1998). Oxygen diffusion into soybean (Ribet and Drevon, 1995), common bean (Vadez et al., 1996) and alfalfa (Schulze and Drevon, 2005) root nodules is facilitated under P-stress conditions. Oxygen diffusion is thought to be facilitated in these species through a change in nodule cortical cell size mediated by osmoregulatory reactions (Drevon et al., 1998). By contrast, regulation of oxygen diffusion in white lupin nodules appears to be brought about by occlusion of nodule cortical-cell free space (De Lorenzo et al., 1993; Iannetta et al., 1993).
It is noteworthy that the plant enzymes phosphoenolpyruvate carboxylase (PEPC; EC 4·1·1·31) and malate dehydrogenase (MDH; EC 1·1·82) play a key role in carbon metabolism of both root nodules and P-deficiency-induced cluster roots. In root nodules, PEPC and MDH are integral enzymes in providing carbon in the form of organic acids for rhizobial bacteroids in infected cells (Rosendahl et al., 1990). Moreover, PEPC and MDH in nodules have been implicated in osmoregulation of the nodule variable-oxygen diffusion barrier (Miller et al., 1998). In P-deficiency-induced cluster roots, expression of PEPC and MDH is increased and a portion of the carbon skeleton of the organic acids exuded from cluster roots is derived from CO2 fixed by PEPC (Johnson et al., 1994, 1996; Uhde-Stone et al., 2003a). Whether root nodules on P-deficient white lupin show any change in expression of PEPC and MDH is not known. Neither is it known whether a relationship exists between cluster root development and root nodule formation in P-stressed white lupin. The objectives of this study were (a) to determine the nodulation profile of white lupin grown under P-stress conditions; (b) to assess if nodule PEPC and MDH were affected by P-stress; and (c) to determine how P-stress affects white lupin nodule physiology.
MATERIALS AND METHODS
Growth of plant material
Lupinus albus L. ‘Ultra’ plants were grown in a growth chamber at 20/15 °C with 16-h/8-h day/night cycles. Photosynthetic active radiation was approx. 210 μmol m−2 s−1 at plant height. Seeds were surface sterilized by placing them for 20 min in H2O2. Subsequently, they were rinsed with sterile water and planted in pots (100 × 100 mm) (four plants per pot singled out to two plants per pot after emergence) containing 1 mm diameter silica sand. Plants were watered daily with 200–500 mL of the appropriate nutrient solution. The base nutrient solution consisted of 2 mm MES [2-(N-morpholino) ethane-sulfonic acid] buffer, 1 mm MgSO4, 3 mm K2SO4, 0·95 mm CaCl2, 12 μm Fe (as FeEDTA), 4 μm MnCl2, 22 μm H3BO3, 0·4 μm ZnSO4, 0·05 μm NaMoO4 and 1·6 μm CuSO4. Two treatments were grown, one in which phosphate as Ca(H2PO4)2 was added to the nutrient solution to a concentration of 0·5 mM (+P) and a second without phosphate (−P). The appropriate amount of CaSO4 was added to the −P nutrient solution to ensure equal calcium concentrations. All nutrient solutions were nitrogen free.
Bradyrhizobium lupini (WU 425) was grown in YEM at 28 °C for 1 week. The strain has no uptake hydrogenase (Layzell et al., 1979; Murillo et al., 1989). Plants were inoculated twice at 4 and 6 d after emergence (DAE) with the application of 1 mL of inoculum at the stem of each plant. First nodules appeared 5 d after inoculation. An uninoculated control remained nodule free.
On a separate set of plants grown as described above, nodules were classified into those in or adjacent (10 mm) to proteoid (cluster) root zones and those on normal roots. In addition to the +P and −P treatments, nodules from plants which had been additionally treated with the synthetic auxin naphthalene acetic acid (NAA) or the auxin transport inhibitor 2,3,5-triiodobenzoic acid (TIBA) were counted and classified. The application of NAA and TIBA followed the procedure outlined in Gilbert et al. (2000).
15N2 uptake and H2- and CO2-evolution
At 19–21 d after inoculation (DAI) (subsequently referred to as 21 DAI) and 35–37 DAI (subsequently referred to as 37 DAI), plants were carefully removed from the silica sand. Due to its large grain diameter, the sand could be removed easily without damaging roots. The plants were transferred to 50 mL (21 DAI) or 150 mL (37 DAI) darkened glass containers. The stem base was carefully placed in a hole (diameter 5 mm) through a rubber stopper. The rubber stopper had been sliced to allow the stem to be placed inside the hole. After sealing the cut as well as the stem against the rubber stopper with non-plant toxic silicon rubber, the root/nodule system was placed inside the container and the container was closed. An inlet and outlet tube to the container was placed through the rubber stopper and 25 mL (21 DAI) or 75 mL (37 DAI) of the appropriate nutrient solution were added. An airflow of two times the container volume min−1 was connected to the inlet and outlet for 24 h, sealing the root/nodule container to allow the plants to adapt. The airflow was bubbled through water before entering the root compartment.
For measuring 15N2 uptake the container was filled with nutrient solution and the nutrient solution was then replaced by a 15N2 (99 atom% 15Nexc)/O2 (80/20) mixture. The root/nodule system was exposed to the mixture for 2 h, after which the labelled air was removed by water and the plants were immediately harvested. Nodules were detached, counted, weighed, then macerated in a mortar and pestle and, along with shoots and roots, immediately dried to a constant weight at 70 °C.
For measuring root/nodule H2 and CO2 evolution the container was connected to an airflow (2 vols min−1) from a gas blender (80 % N2/20 % O2). The out-flowing air was analysed for H2 and CO2 with a flow-through H2 analyser and a flow-through CO2 analyser. Apparent nitrogenase activity (ANA) was measured as H2 evolution in an N2/O2 (80/20) mixture while total nitrogenase activity (TNA) was defined as the peak value of H2 evolution after switching to an Ar/O2 (80/20) mixture (Blumenthal et al., 1997). The maximum value was reached about 10–12 min after replacing N2 by Ar. CO2 evolution was measured in an N2/O2 (80/20) mixture.
Calculation of nitrogen fixation and electron allocation from ANA, TNA and 15N2 uptake
The process of nitrogen fixation can be described by the following equations:
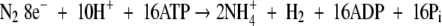 |
(1) |
H+ is reduced concomitantly with N2 on which the measurement of ANA is based. If N2 is replaced by Ar, all electrons will be directed towards H+ reduction and the resulting H2 evolution represents TNA. In a N2/O2 mixture the electron allocation between N2 and H+ can vary. The electron allocation coefficient (EAC) is defined as:
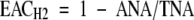 |
(2) |
If the process follows eqns (1) and (2), the EAC would be 0·75 (1−2e−/8e−) and in this case 1 mol N2 would be reduced (fixed) with every mol H2 evolving (see eqns 1 and 2).
To calculate the amount of N2 reduced from an ANA and TNA value, the number of mol H2 evolving along with 1 mol N2 was calculated as follows:
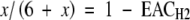 |
(3) |
with x = mol e− flowing onto H+ along with the reduction of 1 mol N2.
Accordingly, the mol H2 evolving (y) along with the reduction of 1 mol N2 would be:
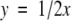 |
(4) |
The ANA value in mol H2 divided by y consequently results in mol N2 reduced (fixed).
The amount of N fixed was calculated from the 15N2 uptake as:
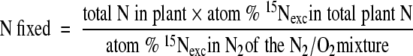 |
(5) |
To calculate the EAC15N from 15N2 uptake and ANA the following equation was used:
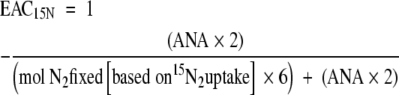 |
(6) |
Root/nodule CO2 fixation
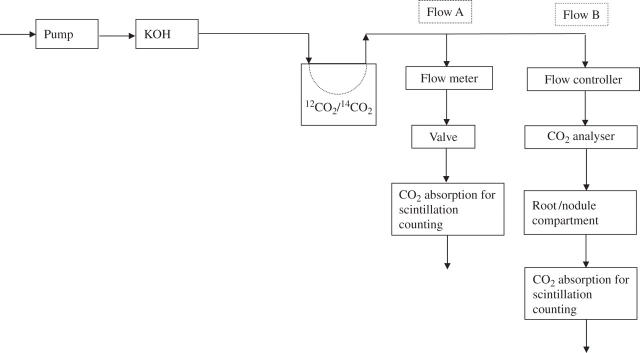
Root/nodule CO2 fixation was measured at 21 DAI on a separate set of plants grown under similar conditions. The experimental set-up is outlined in Fig. 1. A constant, CO2-free airflow was directed through a CO2-permeable silicon rubber tubing located in an airtight mixing chamber. The chamber was filled with a 12CO2/14CO2 mixture. Depending upon the diameter and length of the tubing and the airflow rate, a constant rate of 12CO2/14CO2 mixing was achieved. The airflow emerging from the mixing chamber was split into two streams. The stream designated Flow A was passed directly through a scintillation fluid that absorbs CO2 and counted. The stream designated Flow B was passed sequentially through a CO2 analyser, the compartment containing the nodulated root, and then through scintillation fluid for CO2 capture and counting. The flow control and valve system allowed the airflow to be adjusted so that it contained 400 ppm CO2 (CO2 analyser) and the airflow rate through the root/nodule container was 1 × the container volume min−1.
Fig. 1.
Flow scheme of an experimental set-up for measuring short-term root/nodule CO2 fixation. Further explanations are given in the text.
The specific activity of the CO2 (Bq mol−1 CO2) in the airflow could be calculated from the CO2 content (CO2 analyser), the radioactivity collected in scintillation fluid, and the flow rate of Flow A. The root/nodule system was exposed to the 12CO2/14CO2-containing air for 30 min. The plants were immediately frozen in liquid nitrogen, lyophilized, and the 14C determined after oxidization and subsequent liquid scintillation counting. The amount of fixed CO2 (a) was calculated as:
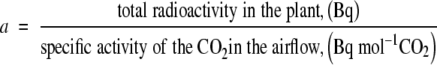 |
(7) |
Enzyme activities
For enzyme assays, nodules were harvested at 21 DAI and kept on ice (10–30 min) until extracted. Approximately 100 mg fresh weight nodules were extracted as previously described (Egli et al., 1989). Total protein in the extract was then determined using Bradford's reagents. PEPC (EC 4·1·1·31), MDH (EC 1·1·1·37), glutamate synthase (GOGAT) (1·4·1·13) and aspartate aminotransferase (AAT) (EC 2·6·1·1) activity was determined by measuring the disappearance of NADH at A340, using protocols previously described (Egli et al., 1989).
RNA gel blot
Nodule RNA extraction, gel separation, blotting and analysis of PEPC and MDH transcript abundance was performed following the procedures outlined in detail in Uhde-Stone et al. (2003b). Equal loading of RNA in each lane was assessed by staining with ethidium bromide.
N and 15N analyses
The dried plant material was weighed and ground to a fine powder. C, N and 15N in the ground material was measured with a combination of a C/N analyser (vario EL Elementaranalysensysteme Hanau) and a 15N emission spectrometer (NOI 7, Fischer Analysen Instrumente, Leipzig).
Statistics
Unless otherwise noted, data were subjected to analyses of variance and the mean values were compared with the t-test, if applicable.
RESULTS
Plant growth, N and P content and nodulation
As can be seen in Table 1, at 21 DAI plants grown under P-deficient conditions did not differ in dry matter (DM) accumulation, % N, total N or root : shoot ratio compared with plants grown under P-sufficient conditions.
Table 1.
Dry matter (DM) accumulation and N- and P-assimilation of white lupin plants grown in silica sand with +P or −P nutrient solution at 21 DAI
| DM (mg) |
% N |
Total N (mg) |
P-concentration (mg P g−1 DM) |
Total P (mg) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| +P | −P | +P | −P | +P | −P | +P | −P | +P | −P | |
| Shoot | 446 | 444 | 3·91 | 3·98 | 17·4 | 17·6 | 8·31 | 3·05* | 3·68 | 1·36* |
| Root | 193 | 206 | 1·94 | 1·92 | 3·7 | 3·9 | 8·72 | 2·58* | 1·68 | 0·54* |
| Nodules | 13·5 | 12·7 | 5·95 | 5·34 | 0·86 | 0·69 | 12·3 | 6·53* | 0·16 | 0·08* |
| Total plant | 652 | 663 | − | − | 21·9 | 22·3 | − | − | 5·51 | 1·98* |
Data are means of six replicates.
Significantly different from the +P treatment, t-test (P ≤ 0·05).
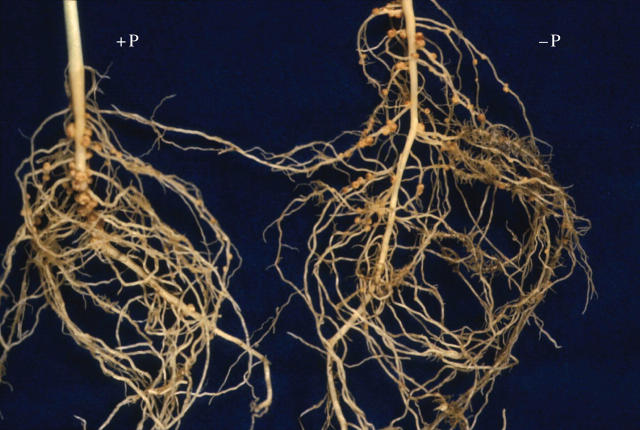
There was, however, a striking difference in P concentration and total plant P with plants grown under P-deficiency conditions having significantly less P than those grown under P-sufficient conditions. Notably, nodules had a higher P concentration than either shoots or roots at 21 DAI. Plants grown under P-deficient conditions at 21 DAI had significantly more nodules than plants grown under P-sufficient conditions, but the nodules on P-deficient plants were smaller than those on P-sufficient plants (Table 2). Nodules in the −P treatment grew predominantly on lateral roots in or in the vicinity of proteoid root clusters (Figs. 2 and 3), while in the +P treatment most nodules were found on the primary root (Fig. 4).
Table 2.
Nodule number and dry matter of white lupin plants grown in silica sand with +P or −P nutrient solution
| 21 DAI |
37 DAI |
|||
|---|---|---|---|---|
| +P | −P | +P | −P | |
| Nodule number | 36 | 55* | 97 | 80* |
| Nodule dry matter (mg) | 13·5 | 12·7 | 80·8 | 47·3* |
| Nodule dry matter/nodule number (mg) | 0·37 | 0·23* | 0·83 | 0·59* |
Data are means of six replicates.
Significantly different from the +P treatment, t-test (P ≤ 0·05).
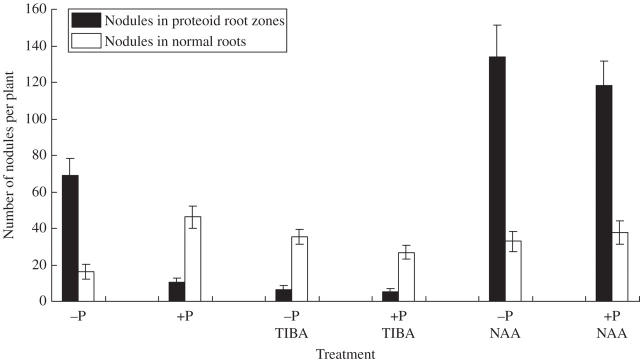
Fig. 2.
Nodulation in cluster root zones of P-deficient white lupin treated with either TIBA or NAA. Black columns show nodules in proteoid (cluster) root zones. White columns show nodules in normal root zones. Data are means of nine replicates. Error bars indicate the s.e.m.
Fig. 3.
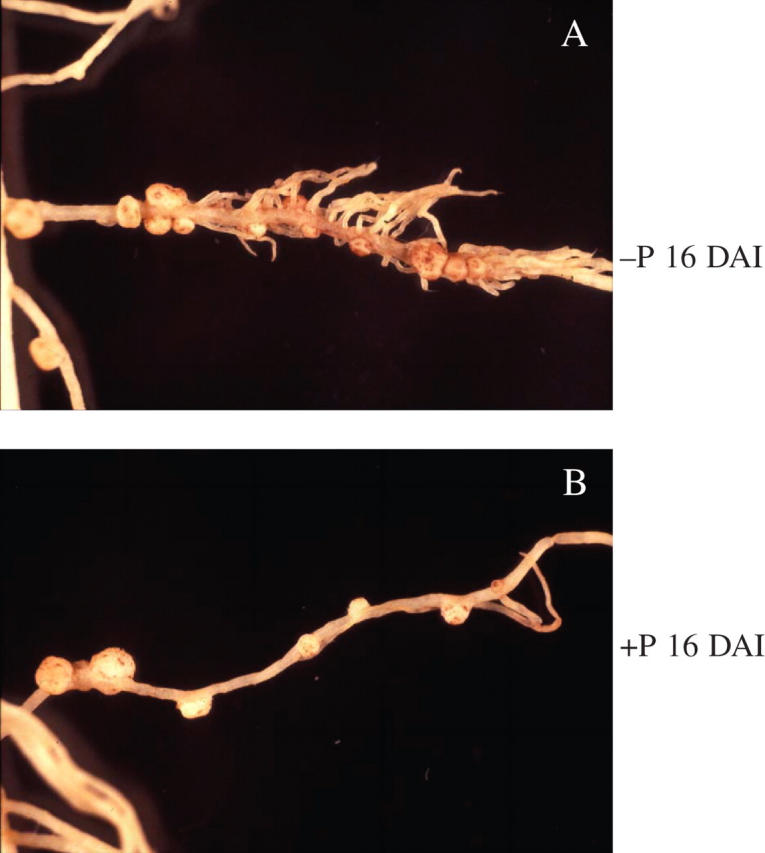
Nodules of white lupin plants in proteoid (cluster) root zones and on normal root segments at 16 d after inoculation (DAI).
Fig. 4.
Nodulated root of white lupin at 21 d after inoculation grown with +P or −P nutrient solution.
By comparison, at 37 DAI plants grown under P-deficient conditions were strikingly reduced in shoot and nodule DM accumulation (Table 3). However, root DM accumulation did not differ resulting in a greater root : shoot ratio for −P plants. The N concentration and total N of −P plants was significantly less than that of +P plants. It was notable again that nodules had a greater N and P concentration than either leaves or roots. Similarly to N, at 37 DAI, P concentration and total P were reduced in −P plants as compared to +P plants. Nodule number and weight were also reduced on −P plants. Total plant P in the −P treatment at both 21 and 37 DAI corresponded to the amount of P that could on average be found in one seed (1·89 ± 0·31 mg P g DM−1, n = 10).
Table 3.
Dry matter (DM) accumulation and N- and P-assimilation of white lupin plants grown in silica sand with +P or −P nutrient solution at 37 DAI
| DM (mg) |
% N |
Total N (mg) |
P-concentration (mg P g−1 DM) |
Total P (mg) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| +P | −P | +P | −P | +P | −P | +P | −P | +P | −P | |
| Shoot | 1884 | 1325* | 3·22 | 2·16* | 60·7 | 28·6* | 8·3 | 1·2* | 15·6 | 1·6* |
| Root | 378 | 376 | 1·53 | 0·56* | 5·8 | 2·1* | 10 | 1·1* | 3·8 | 0·4* |
| Nodules | 80·8 | 47·3* | 5·69 | 4·15* | 4·6 | 1·9* | 13·2 | 3·3* | 1·1 | 0·2* |
| Total plant | 2342 | 1748* | − | − | 71·1 | 32·7* | − | − | 20·4 | 2·1* |
Data are means of six replicates.
Significantly different from the +P treatment, t-test (P ≤ 0·05).
Nitrogen fixation
Nitrogen fixation per unit nodule dry matter as measured by 15N2 uptake did not differ between both treatments at 21 DAI. However, at 37 DAI 15N2 uptake was lower in the +P treatment as compared with +P at 21 DAI, and drastically reduced in the −P treatment (Table 4). This pattern was confirmed by nodule H2 evolution, even though the total amount of N2 fixed calculated on the basis of H2 evolution is lower compared to that based on 15N2 uptake (Table 4). This holds true with the exception of −P at 37 DAI where nitrogen fixation had almost ceased. The lower data for nitrogen fixation calculated from H2 evolution results from a lower EAC measured on the basis of ANA and TNA when compared with the EAC calculated from ANA and 15N2 uptake.
Table 4.
N2 fixation and H2 evolution of white lupin plants grown in silica sand with +P or −P nutrient solution
| 21 DAI |
37 DAI |
|||
|---|---|---|---|---|
| +P | −P | +P | −P | |
| N fixed based on 15N2 uptake [nmol N (mg DM nodule)−1 h−1] | 75·7 | 73·6 | 50·2 | 2·34* |
| N fixed based on H2 evolution [nmol N (mg DM nodule)−1 h−1] | 58·6 | 65·2 | 40·52 | 3·89* |
| H2 evolution [nmol H2 (mg DM nodule)−1 h−1] | ||||
| ANA | 61 | 55 | 44 | 15* |
| TNA | 149 | 153 | 107 | 21* |
| EAC | 0·59 | 0·64* | 0·58 | 0·28* |
| EAC based on 15N2 uptake and H2 evolution (ANA) | 0·65 | 0·67 | 0·63 | 0·19* |
| N fixed (15N2 uptake) nodule−1 [nmol N nodule−1 h−1] | 50·29 | 34·58* | 83·84 | 2·76* |
| N fixed (15N2 uptake) total P−1 [nmol N (mg P in plant)−1 h−1] | 235·9 | 321·1* | 400·4 | 105·34* |
| N fixed (15N2 uptake) nodule P−1 [μmol N (total P in nodules)−1 h−1] | 12·26 | 23·23* | 7·58 | 1·85* |
Data are means of six replicates.
ANA, Apparent nitrogenase activity; TNA, total nitrogenase activity; EAC, electron allocation coefficient.
Significantly different from the +P treatment, t-test (P ≤ 0·05).
At 21 DAI the −P plants had a significantly higher EACH2 compared with the +P plants, while based on 15N2 uptake, there was no significant difference in EAC15N. Nitrogen fixation per nodule, which was significantly lower in the −P treatment at 21 DAI, was compensated for on a whole-plant basis by the higher nodule number. In terms of P efficiency, the −P treatment fixed the same amount of nitrogen with much less P, both on a plant total-P as well as a nodule-P basis (Table 4).
Root/nodule CO2 evolution and fixation and enzyme activities
As shown in Table 5, total nodule/root CO2 evolution at 21 DAI was fairly comparable in −P and +P plants despite the fact that net nodule/root CO2 evolution was significantly less in −P plants as compared with +P plants. The much greater CO2 fixation rate of −P nodules/roots offset the lower net CO2 evolution of −P plants. Thus, total CO2 evolution per N fixed was fairly equal in +P and −P plants. By 37 DAI net CO2 evolution remained high in +P roots/nodules but decreased in −P roots/nodules. Net CO2 evolution per N fixed at 37 DAI in −P plants was 10-fold that in +P plants, reflecting the continuing respiration of −P roots/nodules but little accompanying N2 fixation.
Table 5.
CO2 evolution and CO2 fixation of nodules and roots of white lupin plants grown in silica sand with +P or −P nutrient solution
| 21 DAI |
37 DAI |
|||
|---|---|---|---|---|
| +P | −P | +P | −P | |
| Net CO2 evolution | ||||
| [nmol CO2 (mg DM nodule + root)−1 h−1] | 187 | 127* | 157 | 75* |
| [μmol CO2 plant−1 h−1] | 38·5 | 27·2* | 71·9 | 31·7* |
| CO2 fixation | ||||
| [nmol CO2 (mg DM nodule + root)−1 h−1] | 45 | 87* | n.d. | n.d. |
| Total CO2 evolution | ||||
| [nmol CO2 (mg DM nodule + root)−1 h−1] | 232 | 214* | n.d. | n.d. |
| [μmol CO2 plant−1 h−1] | 47·8 | 46·8 | n.d. | n.d. |
| Net CO2 evolution/N fixed (15N2 uptake) | ||||
| [μmol CO2 (mol N fixed)−1 plant−1 h−1] | 37·21 | 29·73* | 17·7 | 173·2* |
| Total CO2 evolution/N fixed (15N2 uptake) | ||||
| [μmol CO2 (μmol N fixed)−1 plant−1 h−1] | 46·8 | 50·1 | n.d. | n.d. |
Data are means of six replicates.
Significantly different from the +P treatment, t-test (P ≤ 0·05).
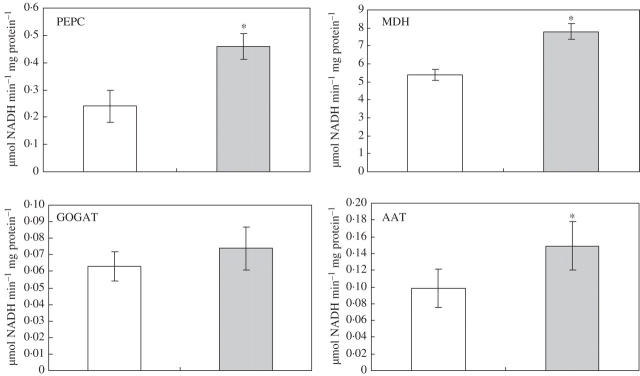
Measurements of MDH and PEPC enzyme activity and gene transcript at 21 DAI reflect the root/nodule in-vivo CO2 fixation. Both enzyme activities and transcripts for PEPC and MDH (Figs. 5 and 6) and AAT enzyme activity (Fig. 5) increased more in −P nodules as compared with +P nodules. By comparison, in vitro enzyme activity for NADH-GOGAT did not increase (Fig. 5). Probing of the same blot with radiolabel showed the increase in PEPC and MDH transcript was not an artefact of RNA loading.
Fig. 5.
In-vitro activity of key enzymes of the C- and N-metabolism in nodules of white lupin grown with +P or −P nutrient solution at 21 d after inoculation. Mean values of six replicates. * Significantly different to the +P treatment, t-test (P ≤ 0·05).
Fig. 6.
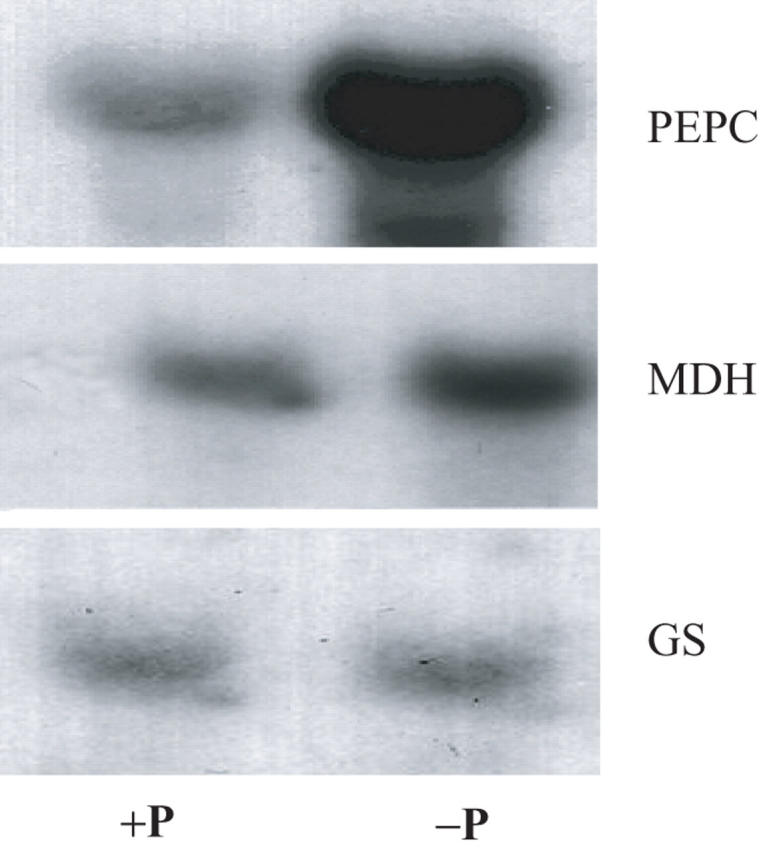
Gel blot analysis of RNA isolated from nodules of +P- and −P-treated white lupin. LaPEPC1 cDNA was used as a probe in the left panel while in the right panel LaMDH1 was used as a probe. Plants grown in presence of sufficient P are designated by (+) and those grown under P stress are designated by (–).
DISCUSSION
The understanding of P-stress on nodulation and N2-fixation has been extended by showing (a) white lupin nodulation and continuing N2-fixation is extremely tolerant of insufficient P for a comparatively extended time in its growth cycle (21–27 DAE); (b) individual nodule dry mass accumulation is more sensitive to P-deficiency than either nodule number or total nodule mass per plant; (c) the P-concentration threshold for nodule function is much higher than that for leaves or roots; and (d) P stress induces nodule enzyme activities of PEPC and MDH, two key enzymes involved in carbon cycling and energy substrates for N2 fixation.
Remarkably, nodulated white lupin shoots did not show any symptoms of nutrient stress when grown in the presence of N- and P-free medium for a period as long as 27 DAE. Although seeds provided some P, the −P treatment led to markedly low P concentrations by 21 DAI but N concentration and total N remained unaffected. Because −P plants were grown under symbiotic conditions, nodulated by Bradyrhizobium and fixing N2, these results clearly show that white lupin is well adapted for growth under P-deficiency stress conditions. In fact, at 21 DAI, N2-fixation measured as both 15N2 uptake and H2 evolution clearly demonstrate that total N2 fixation in +P and −P plants is equivalent. This occurs even though N2 fixed per nodule is less in −P plants because nodule number at 21 DAI is much greater in −P plants. Thus, total nodule mass is equivalent in −P and +P plants. At 37 DAI, this picture changes. As P deficiency becomes more pronounced and P deficiency symptoms are more evident N2 fixation had almost ceased, as confirmed by 15N2 uptake and H2 evolution. It is quite apparent that a certain internal threshold of P concentration is required for lupin plants to continue optimal N2 fixation and physiological function. This threshold lies between 3·05 and 1·12 mg P g DM−1 for shoots and 6·53−3·33 mg P g DM−1 for nodules. The existence of such thresholds is supported by observations relating legume dry matter accumulation to P content (Bell et al., 1990). Moreover, the shoot P threshold reported for clover, Medicago and soybean is approx. 2·7 mg P g DM−1 (Israel, 1987; Tang et al., 2001; Hoch-Jensen et al., 2002). By contrast, a shoot P concentration of 2·5 mg P g DM−1 resulted in a 50 % dry matter reduction in common bean (Olivera et al., 2004).
Similar to legume nodules in other studies (Jakobson, 1985; Israel, 1987; Hoch-Jensen et al., 2002), lupin nodules contained a greater P concentration than other tissues. Whether the difference is due to selective partitioning of P to nodules or P uptake by nodules is not clear. A particularly high demand of the bacterial part of the nodule might also play a role. It is very apparent that the nodule P concentration of 6·5 mg P g DM−1 found at 21 DAI in plants grown without P supported N2 fixation rates equal to that of plants receiving P fertilization and having a nodule P concentration of 12·3 mg P g DM−1. However, by 37 DAI the P concentration of nodules from plants receiving no P had decreased to 3·3 mg P g DM−1 accompanied by a severe impact on nodule N2 fixation. In Medicago truncatula (Tang et al., 2001) nodule functioning was fairly efficient when nodule P concentration was 4–5 mg P g DM−1. Similar nodule P concentrations support adequate N2 fixation in pea and soybean.
Phosphorus stress had a greater impact on shoot growth as compared with that of root growth. In fact, root dry matter accumulation was comparable in P-stressed and P-sufficient plants. Shoot dry matter accumulation increased 3-fold in P-stressed plants between 21 and 37 DAI as compared with the 4·2-fold increase in P-sufficient plants. Thus, roots seem to have a more pliable response to P deficiency than shoots.
The present observations with white lupins appear to support the view that phosphorus deficiency affects nitrogen fixation through secondary effects rather than direct involvement of P in nitrogenase functioning (Jakobson, 1985; Israel, 1987; Ribet and Drevon, 1995). However, this is at most an indirect indication and the question can only be adequately addressed when P deficiency is experimentally achieved through continuous low supply, which would more readily reflect the conditions in a soil with low P availability. Nevertheless, up to 21–27 DAI the plants showed clear adaptative features, comprising both morphological and physiological phenomena.
Consistent with previous reports for other legumes, it was found that mild P stress in white lupin leads to the formation of more and smaller nodules (Ribet and Drevon, 1995), i.e. individual nodule mass is initially more affected by emerging P-deficiency than nodule number or nodule dry matter per plant. Additionally, in white lupin, these nodules seem to be located in the vicinity of cluster (proteoid) root zones where P uptake is presumably highest, especially at low soil P. In separate experiments, nodule number and cluster root formation were evaluated in response to P-stress and auxin treatment (Fig. 2). Consistent with earlier experiments (Tables 1 and 2) nodule number increased in response to P-deficiency and the greatest number of nodules was associated with cluster root zones. When nodule number was calculated on the basis of cluster root weight, P-deficient plants had 3-fold more nodules per gram of cluster root than P-sufficient plants (54 nodules g−1 cluster root vs. 17 nodules g−1 cluster root). In addition, an auxin analogue NAA stimulated cluster root formation and nodule development in cluster root zones. In comparison, TIBA an auxin transport inhibitor, inhibited cluster root formation and nodule development in cluster root zones. The effects of NAA and TIBA on cluster roots were independent of P, similar to the findings of Gilbert et al. (2000). These results show that cluster root zones appear to be more susceptible to nodulation than normal roots. Whether an increased susceptibility to nodulation in cluster root zones is due to exudation of nodulation signals or the prolific emergence of root and root hairs combined with enhanced organic acid synthesis is worthy of study. The altered location of nodules under P stress indicates that tissue P concentration might be involved in regulation of nodule initiation (Almeida et al., 2000), although the underlying mechanisms are unclear. Recent studies (Liu et al., 2005) have shown that the expression of P stress-induced genes are activated in nodules of plants grown with adequate P, suggesting that nodules may frequently be P-limited even under adequate fertilizer.
P-deficiency stress is accompanied by increased respiration per fixed N2 in nodules (Schulze and Drevon, 2005) which may result in a carbon shortage in nodules of −P plants. This could become more severe as photosynthesis is impaired due to reduced phosphorylation processes in shoots (Foyer and Spencer, 1986). With respect to nitrogen fixation white lupin plants reacted to any potential carbon shortage in the −P treatment in two ways. First, the EAC was slightly increased, leading to a more (carbon) efficient nitrogen fixation in the first half of the growth cycle. Secondly, PEPC and MDH expression and activity were strongly increased, with the result of a nearly doubled root/nodule CO2 fixation. Although the measurement of CO2 fixation, based on applied 14CO2, will most probably underestimate the actual fixation, in the present case that would even increase the difference between the treatments. Nodule CO2 fixation has been shown to be closely connected to nitrogen fixation activity (Schulze et al., 1998) and nodule organic acid production is central for functioning nitrogen fixation (Vance, 1998). In addition, CO2 fixation by cluster roots contributes a significant amount of carbon for organic acid synthesis (Johnson et al., 1994, 1996). Due to the strong CO2 uptake in the −P treatment, a lower net CO2 release was observed, although the CO2 release per unit fixed N was higher (Table 5). Thus, in fact, a shift towards the PEPC-MDH pathway could result in greater carbon efficiency of N assimilation when the recovered carbon is used for asparagine formation and N-transport. AAT activity was increased, possibly leading to a higher portion of C derived from CO2-fixation in the N-exporting compounds and maybe a increased portion of asparagine versus glutamine as the N export compound resulting in a lower C : N ratio of the N-transporting compounds. Furthermore, if additional malate was produced, it could be used to feed the bacteroid respiration (Schulze et al., 2002). These combined effects may contribute to more efficient carbon fixation and assimilation of nitrogen in the −P treatment.
Finally, a further notable aspect of the present results is that in the system described here the TNA value based on H2 evolution after a switch to Ar/O2 seems to underestimate the actual total nitrogenase activity by about 20 % when compared with 15N2 uptake. The reasons are not immediately apparent, yet could be various. The mechanism that brings about the argon-induced decline in nitrogenase activity (Witty et al., 1984) might have an immediate effect before the onset of the time-dependent decline, which is probably due to oxygen diffusion restriction (Schulze, 2004). Moreover, a certain unavoidable delay of every open-flow gas-exchange measurement system might somewhat moderate the H2 evolution peak after switching to an Ar/O2 mixture.
In conclusion, it has been shown that white lupin can maintain sufficient nitrogen fixation rates solely on the basis of seed P for as long as 3 weeks after nodulation. Adaptation is achieved by a shift to a more carbon efficient N assimilation and increased nodulation in cluster root zones. These characteristics add to the high adaptability of this species to a low P environment.
Acknowledgments
This work was supported by a fellowship of the ‘Deutsche Akademie der Naturforscher—Leopoldina’ (FKZ BMBF-LPD 9801-19) to Joachim Schulze. The programme of the academy is made possible through funding by the ‘Bundesministerium für Bildung und Forschung’.
LITERATURE CITED
- Almeida JPF, Hartwig UA, Frehner M, Nösberger J, Lüscher A. 2000. Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.). Journal of Experimental Botany 51: 1289–1297. [PubMed] [Google Scholar]
- Blumenthal JM, Russelle MP, Vance CP. 1997. Nitrogenase activity is affected by reduced partial pressure of N2 and NO3−. Plant Physiology 114: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RW, Edwards DG, Asher CJ. 1990. Growth and nodulation of tropical food legumes in dilute solution culture. Plant and Soil 122: 249–258. [Google Scholar]
- De Lorenzo C, Iannetta PPM, Fernandez-Pascual M, James EK, Lucas MM, Sprent JI, et al. 1993. Oxygen diffusion in lupin nodules. II. Mechanisms of diffusion barrier operation. Journal of Experimental Botany 44: 1469–1474. [Google Scholar]
- Denison FR. 1998. Decreased oxygen permeability: a universal stress response in legume nodules. Botanica Acta 111: 191–192. [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. 1989. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant, Cell and Environment 12: 285–292. [Google Scholar]
- Dinkelaker B, Hengeler C, Marschner H. 1995. Distribution and function of proteoid roots. Botanica Acta 108: 183–200. [Google Scholar]
- Drevon JJ, Hartwig UA. 1997. Phosphorus deficiency increases the argon-induced decline of nodule nitrogenase activity in soybean and alfalfa. Planta 200: 463–469. [Google Scholar]
- Drevon JJ, Frangne N, Fleurat-Lessard P, Payre H, Ribet J, Vadez V, et al. 1998. Is nitrogenase-linked respiration regulated by osmocontractile cells in legume nodules? In: Elmerich C, Kondorosi, A, Newton W, eds. Biological nitrogen fixation for the 21st century. Dordrecht: Kluwer, 465–466.
- Egli MA, Griffith SM, Miller SS, Anderson MP, Vance CP. 1989. Nitrogen assimilating enzyme activities and enzyme protein during development and senescence of effective and plant gene-controlled ineffective alfalfa nodules. Plant Physiology 91: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, Spencer C. 1986. The relationship between phosphate status and photosynthesis in leaves. Planta 167: 369–375. [DOI] [PubMed] [Google Scholar]
- Gerke J, Römer W, Jungk A. 1994. The excretion of citric and malic acid by proteoid roots of Lupinus albus: effects on soil solution concentrations of phosphate, iron, and aluminium in the proteoid rhizosphere in samples of an oxisol and a luvisol. Journal of Plant Nutrition and Soil Science 157: 289–294. [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL. 1999. Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant, Cell and Environment 22: 801–810. [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL. 2000. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Annals of Botany 85: 921–928. [Google Scholar]
- Hoch-Jensen H, Schjoerring JK, Soussana JF. 2002. The influence of phosphorus deficiency on growth and nitrogen fixation of white clover plants. Annals of Botany 90: 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetta PPM, De Lorenzo C, James EK, Fernandez-Pascual M, Sprent JI, Lucas MM, et al. 1993. Oxygen diffusion in lupin nodules. I. Visualization of diffusion barrier operation. Journal of Experimental Botany 44: 1461–1467. [Google Scholar]
- Israel DW. 1987. Investigations of the role of phosphorus in symbiotic dinitrogen fixation. Plant Physiology 84: 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson I. 1985. The role of phosphorus in nitrogen fixation by young pea plants (Pisum sativum). Physiologia Plantarum 88: 190–196. [Google Scholar]
- Johnson JF, Allan DL, Vance CP. 1994. Phosphorus stress-induced proteoid roots show altered metabolism in Lupinus albus. Plant Physiology 104: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JF, Vance CP, Allan DL. 1996. Phosphorus deficient Lupinus albus: altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase. Plant Physiology 112: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisinghe G, Hocking PJ, Ryan PR, Delhaize E. 1998. Effect of phosphorus supply on the formation and function of poteoid roots of white lupin (Lupinus albus L.). Plant, Cell and Environment 21: 467–478. [Google Scholar]
- Lamont BB. 2003. Structure, ecology and physiology of root clusters—a review. Plant and Soil 248: 1–9. [Google Scholar]
- Layzell DB, Rainbird RM, Atkins CA, Pate JS. 1979. Economy of photosynthate use in nitrogen-fixing legume nodules: observations on two contrasting symbioses. Plant Physiology 64: 888–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell DB, Hunt S, Palmer GR. 1990. Mechanism of nitrogenase inhibition in soybean nodules: pulse-modulated spectroscopy indicates that nitrogenase activity is limited by O2. Plant Physiology 92: 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Samac DA, Bucciarelli B, Allan DA, Vance CP. 2005. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. The Plant Journal 41: 257–268. [DOI] [PubMed] [Google Scholar]
- Miller SS, Driscoll BT, Gregerson RG, Gantt JS, Vance CP. 1998. Alfalfa malate dehydrogenase (MDH): molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. The Plant Journal 15: 173–184. [DOI] [PubMed] [Google Scholar]
- Minchin FR. 1997. Regulation of oxygen diffusion in legume nodules. Soil Biology and Biochemistry 29: 881–888. [Google Scholar]
- Murillo J, Villa A, Chamber M, Ruiz-Argueso T. 1989. Occurrence of H2-uptake hydrogenase in Bradyrhizobium sp. (Lupinus) and their expression in nodules of Lupinus spp. and Ornithopus compressus. Plant Physiology 89: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G, Martinoia E. 2002. Cluster roots—an underground adaptation for survival in extreme environments. Trends in Plant Science 7: 162–167. [DOI] [PubMed] [Google Scholar]
- Neumann G, Massonneau A, Martinoia E, Römheld V. 1999. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208: 378–382. [Google Scholar]
- Olivera M, Tejera N, Iribarne C, Ocana A, Lluch C. 2004. Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): effect of phosphorus. Physiologia Plantarum 121: 498–505. [Google Scholar]
- Plaxton WC. 2004. Plant response to stress: biochemical adaptations to phosphate deficiency. In: Goodman R, ed. Encyclopedia of plant and crop science. New York, NY: Marcel Dekker, 976–980.
- Ribet J, Drevon JJ. 1995. Increase in permeability to oxygen and in oxygen uptake of soybean nodules under limiting phosphorus nutrition. Physiologia Plantarum 94: 298–304. [Google Scholar]
- Rosendahl L, Vance CP, Pedersen WB. 1990. Products of dark CO2 fixation in pea root nodules support bacteroid metabolism. Plant Physiology 93: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa T-M, Israel DW. 1991. Energy status and functioning of phosphorus-deficient soybean nodules. Plant Physiology 97: 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze J. 2004. How are nitrogen fixation rates regulated in legumes? Journal of Plant Nutrition and Soil Science 167: 125–137. [Google Scholar]
- Schulze J, Drevon JJ. 2005. P-deficiency increases the O2 uptake per N2 reduced in alfalfa. Journal of Experimental Botany 56: 1779–1784. [DOI] [PubMed] [Google Scholar]
- Schulze J, Shi L, Blumenthal J, Samac DA, Gantt JS, Vance CP. 1998. Inhibition of alfalfa root nodule phosphoenolpyruvate carboxylase through an antisense strategy impacts nitrogen fixation and plant growth. Phytochemistry 49: 341–346. [Google Scholar]
- Schulze J, Adgo E, Merbach W. 1999. Carbon costs associated with N2 fixation in Vicia faba L. and Pisum sativum L. over a 14-day period. Plant Biology 1: 625–631. [Google Scholar]
- Schulze J, Tesfaye M, Litjens R, Bucciarelli B, Trepp G, Miller S, et al. 2002. Malate plays a central role in plant nutrition. Plant and Soil 247: 133–139. [Google Scholar]
- Tang C, Hinsinger P, Drevon JJ, Jaillard B. 2001. Phosphorus deficiency impairs early nodule functioning and enhances proton release in roots of Medicago truncatula L. Annals of Botany 88: 131–138. [Google Scholar]
- Uhde-Stone C, Zinn KE, Yanez MR, Li A, Vance CP, Allan DL. 2003a. Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant Physiology 131: 1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde-Stone C, Gilbert G, Johnson JM, Litjens R, Zinn KE, Temple SJ, Vance CP, Allan DL. 2003b. Acclimation of white lupin to phosphorus deficiency involves enhanced expression of genes related to organic acid metabolism. Plant and Soil 248: 99–116. [Google Scholar]
- Vadez V, Rodier F, Payre H, Drevon, JJ. 1996. Nodule permeability to O2 and nitrogenase-linked respiration in bean genotypes varying in the tolerance of N2 fixation to P deficiency. Plant Physiology and Biochemistry 34: 871–878. [Google Scholar]
- Vance CP. 1998. Nodule carbon metabolism: organic acids for N2 fixation. In: Elmerich C, Kondorosi A, Newton W, eds. Biological nitrogen fixation for the 21st century. Dordrecht: Kluwer, 443–448.
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 432–449. [DOI] [PubMed] [Google Scholar]
- Watt M, Evans JR. 1999. Proteoid roots: physiology and development. Plant Physiology 121: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JF, Minchin FR, Sheehy JE, Minguez I. 1984. Acetylene- induced changes in the oxygen diffusion resistance and nitrogenase activity of legume root-nodules. Annals of Botany 53: 21–27. [Google Scholar]