Abstract
BACKGROUND
The levonorgestrel-releasing intrauterine system (LNG-IUS) is a highly effective contraceptive. However, during early months of use unscheduled vaginal bleeding is common, sometimes leading to discontinuation. This study aimed to determine whether intermittent administration of progesterone receptor modulator CDB-2914 would suppress unscheduled bleeding during the first 4 months after insertion of the LNG-IUS.
METHODS
CDB-2914 150 mg, in divided doses, or placebo tablets, were administered over three consecutive days starting on Days 21, 49 and 77 after LNG-IUS insertion, in a double-blind randomized controlled trial of women aged 19–49 years, newly starting use of LNG-IUS. Daily bleeding diaries were completed for 6 months, and summarized across blocks as percentage days bleeding/spotting (BS%).
RESULTS
Of 69 women randomized to receive CDB-2914, and 67 placebo, 61 and 55, respectively, completed the trial. BS% decreased with time in both arms, but showed a much steeper treatment-phase gradient in the placebo arm (P < 0.0001), so that a benefit of CDB-2914 in the 28 days after first treatment (−11% points, 95% CI −19 to −2), converted to a disadvantage by 64 days after the third treatment (+10% points, 95% CI 1–18).
CONCLUSIONS
The effect of CDB-2914 on BS% was initially beneficial but then by third treatment was disadvantageous. Nevertheless, only 3% (4/136) of all women discontinued LNG-IUS. These findings give insight into possible mechanisms and suggest future research directions.
ISRCTN Trial no. ISRCTN58283041; EudraCT no. 2006-006511-72.
Keywords: CDB-2914, LNG-IUS, endometrium, unscheduled bleeding
Introduction
If hormonal methods of contraception are used consistently and correctly they are more than 98% effective in preventing unplanned pregnancy [National Institute for Health and Clinical Excellence (NICE), 2005]. Yet one third of pregnancies worldwide are unintended or mistimed and over 20 million end in induced abortion every year (Guttmacher Institute, 2008). Enthusiasm has therefore grown for long-acting methods which do not depend on compliance for their effectiveness, particularly implants and intrauterine devices/systems which have failure rates of less than 1% (Peterson and Curtis, 2005). An estimated 9.8 million women worldwide use the levonorgestrel-releasing intrauterine system (LNG-IUS). The uptake of LNG-IUS has undoubtedly been further increased because the low dose of LNG released into the uterine cavity leads to endometrial atrophy, so that for many women its use is associated with little or no vaginal bleeding [National Institute for Health and Clinical Excellence (NICE), 2005; Peterson and Curtis, 2005].
However, all hormonal methods of contraception are prone to high discontinuation rates (Trussell, 2007). The commonest reason for premature discontinuation of all methods is unscheduled vaginal bleeding (d'Arcangues et al., 1992). Many women experience persistent vaginal spotting and bleeding during the first 3–6 months of use of the LNG-IUS (Backman et al., 2000; Cox et al., 2002). In a UK study, 10.5% of new users of LNG-IUS had stopped using it by the end of the first year because of bleeding problems, and this accounted for much of the total 5-year cumulative discontinuation rate for bleeding problems (16.7%) (Cox et al., 2002). In a Brazilian study 25% of women complained of vaginal spotting in the first 6 months of use of LNG-IUS and removals due to menstrual bleeding problems were concentrated in this time period (Hidalgo et al., 2002). The cost of LNG-IUS is relatively high, and both insertion and removal use healthcare resource, so premature discontinuation reverses the cost-benefit ratio that applies if used for the full 5 years (Peterson and Curtis, 2005). Although there is a widely held clinical belief that counselling women about unscheduled bleeding with LNG-IUS prevents premature discontinuation, there is no published evidence to support this view (Halpern et al., 2006).
The mechanism underlying irregular/unscheduled bleeding in association with progestogen-only contraceptives (POC) remains to be determined, but may be associated with estrogen withdrawal, especially when the mode of delivery is oral or systemic (Cheng et al., 2000; Gemzell-Danielsson et al., 2002; Massai et al., 2004; Weisberg et al., 2006). With use of LNG-IUS, fewer than half of cycles are ovulatory (Barbosa et al., 1990). In common with other POCs most women continue to have follicle development (Xiao et al., 1990) and thus incomplete suppression of ovarian activity is one mechanism for unscheduled bleeding.
Progesterone receptor modulators (PRMs) have shown a benefit in treating women experiencing unscheduled bleeding with progestogen-releasing contraceptive implants (Cheng et al., 2000; Weisberg et al., 2006). PRMs have also shown a beneficial effect on unscheduled bleeding in prevention studies, administered in conjunction with medroxyprogesterone acetate (Jain et al., 2003), progestogen-only implants (Massai et al., 2004) and the progestogen-only contraceptive pill (Gemzell-Danielsson et al., 2002). That any therapeutic effect of PRMs might be via an ovarian pathway is supported by the finding in the last-mentioned trial, where subjects treated with ORG31710 had better bleeding profiles, that ovulation occurred in 29% of the ORG31710 group compared with 3% or fewer in untreated women (Gemzell-Danielsson et al., 2002). The mechanisms for the effects of PRMs are not known. Following menstruation, a period of unopposed estrogen exposure is required for regeneration of endometrial sex steroid receptors. Administration of a PRM may, via a direct effect on steroid receptor expression, permit simulation of the normal physiological expression of sex steroid receptors post-menses (Cheng et al., 2000).
CDB-2914 is a PRM which binds to the progesterone receptor with high affinity and antagonizes the action of progesterone. CDB-2914 is currently being evaluated for use in several clinical areas including, emergency contraception, and the management of fibroids and endometriosis (Blithe et al., 2003).
LNG-IUS is unique among progestogen-only methods in that troublesome unscheduled bleeding is generally limited to the early months of use (Hidalgo et al., 2002; Baldaszti et al., 2003). The aim of the clinical trial reported here was to determine whether intermittent administration of PRM CDB-2914 (Laboratoire HRA Pharma, Paris, France) would prevent or suppress unscheduled bleeding if taken over the first 3 months after insertion of the LNG-IUS, with the objective of improved LNG-IUS continuation rates.
Materials and Methods
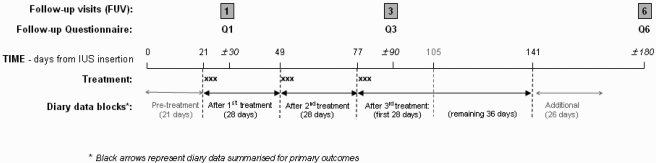
The trial was randomized, double-blind and placebo-controlled; received ethical approval from Lothian research ethics committee; and all participants gave written informed consent. CDB-2914 (50 mg/day) and placebo were packaged to look identical by HRA Pharma, randomized (1:1 in blocks of 20), and numbered sequentially, so that randomization was achieved by dispensing packs in numeric order. ‘Treatment’ comprised taking study medication orally for three consecutive days, with separate treatments starting 21, 49 and 77 days after LNG-IUS insertion (Fig. 1), and study participants were reminded of their treatment dates by email, text messages, telephone calls or letter. Subjects kept a daily record of vaginal bleeding for at least one complete spontaneous menstrual cycle before insertion of the LNG-IUS, and throughout the study. LNG-IUS insertion was not timed to cycle but done at the next available IUS-insertion appointment after completion of screening diaries. At 1, 3 and 6 months after LNG-IUS insertion women completed follow-up questionnaires (the final one asking about continuing LNG-IUS use and overall satisfaction with the method) and returned to the clinic for review. Screening/recruitment commenced January 2005, with randomization (at LNG-IUS insertion) occurring between March 2005 and January 2007, and follow-up ending July 2007. Women eligible to participate were those attending a large family planning clinic and initiating use of an LNG-IUS for contraception, aged 19–49 years with regular menstrual cycles lasting 17–42 days, menstrual periods lasting less than 11 days, and no contraindications to LNG-IUS insertion [WHO Medical Eligibility Criteria Category 3 or 4 conditions (World Health Organization Reproductive Health and Research (WHO), 2008)]. Women breastfeeding, within 3 months of childbirth, or with a chronic medical or psychiatric condition, gynaecological disorders (including fibroids, ovarian cysts or unexplained irregular vaginal bleeding) or on long-term medication were excluded. Adverse events and use of concomitant medication were recorded throughout the study. Premature removal of the LNG-IUS constituted withdrawal from the study. Women requesting this, or otherwise withdrawing from the study, were asked to complete a withdrawal questionnaire collecting data similar to the final questionnaire. A subset of 19 subjects had an endometrial biopsy at insertion and at one or more of the follow-up visits (data on biopsy findings are reported elsewhere).
Figure 1.
Timing of treatments, follow-up visits, questionnaire completion and diary data segments.
Data analyses
Retention of LNG-IUS to 6 months and providing 6 months questionnaire data was deemed study completion, but some who defaulted nevertheless provided information on LNG-IUS continuation. Given the 28 days intervals between treatments, and the ever-changing hormonal background due to the newly inserted LNG-IUS, bleeding diary data was analysed in separate blocks delimited by treatment dates, that is, for the 21 days prior to first treatment, for the 28 days after each of the first two treatments, and for 64 days after third (last) treatment. The primary study outcome was amount of bleeding/spotting after treatment. In order to be able to accommodate occasional non-completed diary days within a block, and for ease of comparison across differing block lengths, bleeding diary data was summarized by follow-up block as percentage days with bleeding/spotting (BS%). However, if the timing of a woman's withdrawal/defaulting meant that half or less of the current diary block was completed, she contributed no BS% value for that block. Secondary outcomes were removal of LNG-IUS within 6 months; the longest run of amenorrhea in the 64 days after third treatment; and five subjective assessments at 6 months of acceptability of bleeding patterns experienced. The final questionnaire also assessed 16 side-effects (tertiary outcomes). Analysis was ‘intention-to-treat’. Mean BS% in the two treatment groups and mean longest run of amenorrhea were compared using the two-sample t-test and proportions in the two treatment groups were compared using Fisher's exact test for binary outcomes and χ2 test for trend for ordinal outcomes. The trend in percentages of days with bleeding/spotting during treatment/follow-up was also analysed using a mixed model with the patient as a random effect and a toeplitz covariance structure to allow for the correlations between the repeated measurements on the same patient, both with and without covariate adjustment for pretreatment bleeding/spotting (first 21 days post LNG-IUS). This approach allows patients to have some missing data, but as a further check for potential distortion of trends, sensitivity analyses were undertaken restricted to women with data for all blocks.
Protocol sample size was calculated (assuming SD of 8) to give approximately 90% power to detect as significant (at the 5% level) a difference of 4.5 days or more between active and placebo treated groups in the mean number of days of bleeding or spotting in a month of follow-up (equivalent to an absolute difference in BS% of 15%). The calculated 66 patients per group was inflated to a target of 75 women per group, to allow for withdrawals.
Results
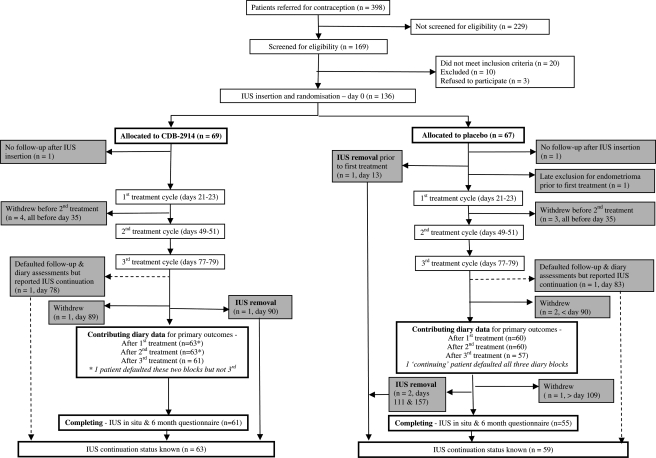
Of 398 women who attended the clinic for advice about using the LNG-IUS, 169 agreed to be screened for eligibility, of whom three subsequently declined to participate, 30 were excluded, and 136 were randomized, 69 to CDB-2914, 67 placebo (Fig. 2). Withdrawals/defaulting in diary completion meant that not all women contributed data to bleeding outcome analyses, with numbers decreasing across follow-up (63 to 61 for women treated with CDB-2914, 60 to 57 for placebo). Rates of study completion were 88% (61/69) and 82% (55/67), respectively (Fig. 2). No serious adverse events were reported and there were minimal differences between groups in compliance with treatment. Considering drug compliance for women who contributed diary or final questionnaire data, 97% (62/64) of the CDB-2914 group took all 45 tablets (or in one case omitted one tablet), and 95% (58/61) of the placebo group took almost all tablets (two women missed five tablets).
Figure 2.
Flow of participants through the trial.
The groups were similar with respect to demographic characteristics, BMI and most menstrual characteristics assessed (Table I), but women randomized to CDB-2914 more often had a history of past discontinuation of a contraceptive method because of weight gain or heavy/irregular bleeding. The timing of insertion of LNG-IUS was similar in both groups, being evenly spread across the range of possible menstrual cycle days, with about half of all women having insertion by 12th to 15th day of cycle, and 21 days later at first treatment about a quarter of each group would have been in the mid-luteal phase.
Table I.
Patient characteristics at recruitment, by randomized group
| CDB-2914 (n = 69) | Placebo (n = 67) | |
|---|---|---|
| Age (years) at LNG-IUS insertion—Mean (SD) | 36.9 (6.5) | 35.8 (7.0) |
| Reported length (days) of most recent menstrual period—Mean (SD) | 5.9 (1.7) | 5.6 (1.5) |
| Length (days) of diary-screened menstrual cycle—Mean (SD) | 28.8 (4.7) | 28.0 (4.1) |
| Days prior to LNG-IUS insertion that most recent menstrual period started—Median (Q1, Q3) | 12 (7, 19) | 15 (10, 21) |
| IUD in situ at insertion visit—N (%) | 5 (7) | 9 (13) |
| Current smoker—N (%) | 13 (19) | 11 (16) |
| Employment—N (%) | ||
| Full-time | 30 (43) | 32 (48) |
| Part-time | 28 (41) | 26 (39) |
| No job | 11 (16) | 8 (12) |
| Number of deliveries—N (%) | ||
| 0 | 14 (20) | 20 (30) |
| 1 | 18 (26) | 13 (19) |
| 2 | 30 (43) | 27 (40) |
| 3 | 6 (9) | 7 (10) |
| 4 | 1 (1) | 0 (0) |
| Previous contraceptive method—N (%) | ||
| Pill | 4 (6) | 3 (4) |
| Cu-IUD | 5 (7) | 9 (13) |
| Barrier—mainly condom* | 53 (77) | 52 (78) |
| Rhythm/withdrawal/ spermicide | 3 (4) | 2 (3) |
| None | 4 (6) | 1 (1) |
| Heaviness of menstrual periods in last 6 months—N (%) | ||
| Light loss | 2 (3) | 0 (0) |
| Moderate loss | 25 (38) | 25 (40) |
| Heavy loss | 26 (40) | 29 (46) |
| Very heavy loss | 12 (18) | 9 (14) |
| Bleeding or spotting in quarter or more of menstrual cycles in past year—N/n (%) | 10/66 (15) | 10/62 (16) |
| Ever stopped using method of contraception due to following reason | ||
| Unacceptable weight gain— N/n (%) | 20/54 (37) | 15/58 (26) |
| Irregular bleeding or spotting—N/n (%) | 16/53 (30) | 12/57 (21) |
| Unacceptably heavy bleeding—N/n (%) | 10/51 (20) | 6/57 (11) |
| Periods stopped altogether— N/n (%) | 1/48 (2) | 1/57 (2) |
*Includes the one woman (in Placebo group) who was using diaphragm.
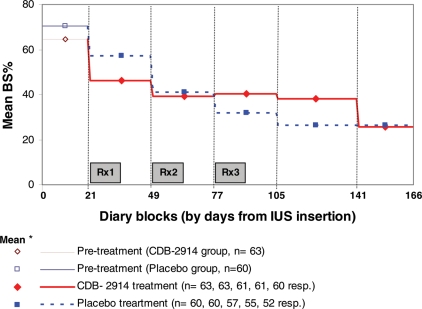
After the first treatment there was a statistically significant difference in BS% in favour of CDB-2914 (Table II), by the equivalent of 3 days [difference −10.6% points, P = 0.011], but for the 64 days following the third treatment women in the CDB-2914 arm reported more days bleeding/spotting, by 6 days [+9.5% points, P = 0.022]. However, in that last block the difference in longest run of days free of bleeding or spotting i.e. days of continuous amenorrhea was not statistically significant (CDB-2914 24 days versus placebo 26 days). Over the treatment and follow-up phase (Days 21–166), both groups showed a decline in BS% (Fig. 3 which for more detailed description splits the third treatment block into the first 28 days and the remainder, and includes data for an additional 26 days, to total 90), with a highly statistically significant time by treatment group interaction (P < 0.0001). This reflects the switch from lower BS% in the CDB-2914 group after first treatment, to greater BS% in the first two diary blocks after third treatment (albeit BS% was very similar across the two groups in the last 26 days). This finding of differing trends persisted regardless of whether: the final 26 days were excluded; an adjustment was made for post-IUS-pretreatment rate of bleeding/spotting; or only women with a complete set of data values were included in the analysis.
Table II.
Percentage days diary-recorded bleeding or spotting (BS%) by study block, and longest consecutive run of days without bleeding/spotting (after third treatment): separately by randomized group, and difference between groups
| Outcome and study interval | Duration of study interval (days) | CDB-2914 |
Placebo |
CDB-2914—Placebo difference |
||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | Difference (95% CI) | P-value | Difference in percentage expressed in days (for the assessment interval) | ||
| Percentage days B/S (BS%) | ||||||||
| Post-LNG-IUS and pretreatment | 21 | 63 | 64.7 (24.7) | 60 | 70.6 (24.1) | −5.9 (−14.8 to 2.8) | # | −1.2 |
| After first treatment | 28 | 63 | 46.7 (20.6) | 60 | 57.3 (24.8) | −10.6 (−18.7 to −2.5) | 0.011 | −3.0 |
| After second treatment | 28 | 63 | 39.6 (19.9) | 60 | 41.2 (24.8) | −1.6 (−9.6 to 6.4) | 0.68 | −0.4 |
| After third treatment | 64 | 61 | 38.2 (23.7) | 57 | 28.7 (20.7) | 9.5 (1.4 to 17.7) | 0.022 | +6.1 |
| Longest consecutive run of days without B/S | ||||||||
| After third treatment | 64 | 61 | 23.7 (14.5) | 57 | 25.6 (13.4) | −2.0 (−7.1 to 3.1) | 0.44 | n.a. |
#Pretreatment therefore not a study outcome and so not formally tested.
Figure 3.
Percentage days bleeding/spotting by diary block, separately by treatment arm. KEY: *mean for group for diary block, plotted at mid-point of block.
There was no statistically significant difference (P = 0.36) in the number of women requesting premature removal of the LNG-IUS, 1/69 (1.6%) in CDB-2914 group and 3/67 (4.5%) in placebo group. The main and secondary reasons given for removal are presented in Table III, together with main reasons given by a further ten women (seven receiving CDB-2914 and three placebo) who indicated in the final questionnaire that they were considering LNG-IUS removal in the next few months.
Table III.
Reasons given for having had removal of LNG-IUS before 6 months or for, at Month 6, ‘considering removal in next few months’
| Reason given | IUS removed before 6 months |
Considering IUS removal in next few months |
||||
|---|---|---|---|---|---|---|
| CDB-2914 (n = 1) |
Placebo (n = 3) |
CDB-2914 (n = 7) | Placebo (n = 3) | |||
| Main | 2° | Main | 2° | Main | Main | |
| Bleeding | 1 | 1 | 1 | |||
| Weight gain | 1 | 1 | 1 | 1 | ||
| Depression/Mood change | 1 | 1 | 1 | |||
| Issues with device inside body | 1 | 1 | ||||
| Pain/discomfort/painful breasts | 2 | 1 | 1 | |||
| Bloating/fluid retention | 2 | |||||
| Loss of libido | 1 | |||||
| Skin problems | 1 | |||||
| Possibly to plan pregnancy | 2 | |||||
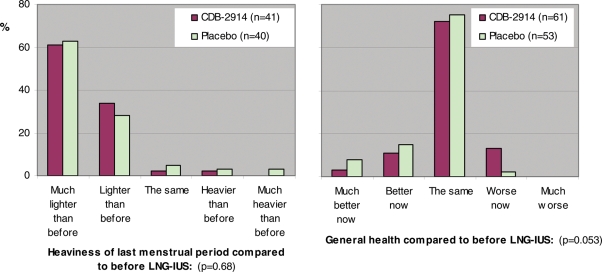
With regard to experience of bleeding as ascertained from final 6 month questionnaire responses, the groups were very similar in reporting menstrual periods having stopped altogether [CDB-2914 24% (14/59) and placebo 23% (12/52), P = 1.00], and having experienced a menstrual period in the last month [72% (43/60) and 70% (38/54), respectively, P = 1.00]. Table IV shows that responses regarding acceptability of vaginal bleeding experience were on the whole favourable, and similar across the two groups—for example, 92% in each group reported lighter bleeding than before insertion (57/60 CDB-2914, 48/52 placebo). However, although a minority of women found vaginal bleeding patterns were ‘more inconvenient than before’, they were predominantly from the CDB-2914 group [39% (22/56) versus 19% (9/48), P = 0.036)]. The groups also showed favourable and similar responses regarding general health and heaviness of most recent menstrual period, compared with before LNG-IUS (Fig. 4). The two groups reported very similar rates over the past 4 weeks of having experienced 13 of the 14 symptoms enquired about in the final in the questionnaire, but women treated with CDB-2914 were more likely to report a ‘tendency to weight gain’ [23% (14/61) versus 4%, (2/55), difference 19%, 95% CI 7% to 32%, P = 0.003).
Table IV.
Comparison between randomized groups in 6 month follow-up questionnaire responses regarding bleeding, both compared with before LNG-IUS, and in absolute terms
| Bleeding since using IUS has been | Lighter than before |
More frequent than before |
More inconvenient than before |
Inconvenient |
Worrying |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| CDB-2914 | Placebo | CDB-2914 | Placebo | CDB-2914 | Placebo | CDB-2914 | Placebo | CDB-2914 | Placebo | |
| Number responding, n | 60 | 52 | 54 | 50 | 56 | 48 | 56 | 49 | 54 | 48 |
| % | % | % | % | % | % | % | % | % | % | |
| Not at all true | 5 | 6 | 44 | 48 | 52 | 67 | 39 | 41 | 70 | 85 |
| Slightly true | 0 | 2 | 17 | 14 | 9 | 15 | 21 | 31 | 17 | 8 |
| Moderately true | 7 | 0 | 4 | 14 | 14 | 4 | 27 | 16 | 9 | 6 |
| Largely true | 13 | 17 | 20 | 10 | 5 | 10 | 5 | 8 | 4 | 0 |
| Very much so | 75 | 75 | 15 | 14 | 20 | 4 | 7 | 4 | 0 | 0 |
| P-value (CDB v placebo) | 1.00 | 0.61 | 0.036 | 0.51 | 0.093 | |||||
Figure 4.
Comparison between randomized groups in 6 month follow-up questionnaire responses regarding heaviness of last menstrual period and general health, as compared with before LNG-IUS and study medication.
Discussion
Against an expectation of diminishing bleeding/spotting across the first months of LNG-IUS use, our comparison of PRM CDB-2914 against placebo showed a short-lived beneficial effect on vaginal bleeding after the first treatment only, which by the last treatment had converted into a disadvantageous effect. Our unexpected findings have high-lighted some important methodological issues.
This is the first study of CDB-2914 in women using LNG-IUS. We therefore selected objective measures of outcome (BS%) and we also undertook questionnaire assessment of acceptability of bleeding experienced. In an exploratory trial such as ours, it is important to have coverage of a range of end-points, and assessment of effect after each of the three intermittent doses. As a check against multiple testing, a single overall analysis was undertaken to compare trends in BS% within groups across treatment blocks, and this confirmed a highly statistically significant group by block interaction (P < 0.001). Nevertheless, to reflect the multiple-testing of outcomes, statistical findings should be interpreted with appropriate caution.
The study was a pragmatic trial aiming to test an intervention which could be used in a routine clinical service setting, so this posed additional challenges. For example, LNG-IUS insertion was not timed to any particular cycle phase, and it was not feasible to classify ovarian function across time, which would have required frequent blood sampling or urine collections. However, the findings are representative of what would transpire in routine clinical practice and the participants are likely to be representative of women in the UK receiving an LNG-IUS for contraception. Over 90% of study participants provided diary data for treatment comparisons, and up to Day 141 after insertion these comparisons had less than 1% of days of recording incomplete. Sensitivity analyses were undertaken to rule out distortions due to patterns of data contribution across time, or chance differences between the groups in tendency to bleed with the LNG-IUS.
A key methodological issue our findings have high-lighted is the disparity in effect sizes between a ‘therapeutic’ study, which recruits into the trial only women already experiencing unscheduled bleeding (Alvarez-Sanchez et al., 1996; Cheng et al., 2000; Glasier et al., 2002; Weisberg et al., 2006), and prevention studies, which recruit from all new users of the contraceptive method. In a prevention study, such as ours, only a subset of participants will have been at risk of unscheduled bleeding and hence able to benefit from the treatment. Even if the treatment is effective, it's apparent ‘effect’, estimated for the entire trial, will be diluted by all the women not susceptible to such bleeding, and hence appear smaller than the effect if estimated in a therapeutic study. There are only two published randomized placebo-controlled double-blind prevention trials using PRMs. Massai et al. (2004) demonstrated, among new users of a levonorgestrel-releasing implant, a reduction by 10% in number of days with bleeding/spotting (by 3 days per month) if treated with mifepristone (100 mg for 2 days every 30 days for 6 months). Among women newly using the oral progestogen-only pill (Cerazette® 75 µg desogestrel daily), a pilot study evaluating the effect of administration of a different PRM, Org 31710 150 mg (Organon, Oss, The Netherlands) once every 28 days for four to seven cycles (Gemzell-Danielsson et al., 2002), found an overall reduction in days bleeding/spotting, per 28 day cycle, of 5% or 1.5 days. Therefore, in prevention trials similar in size to ours, the effect of PRMs on bleeding/spotting has been only modest—reduction in days bleeding/spotting during treatment of 1.5–3 days per month (Gemzell-Danielsson et al., 2002; Massai et al., 2004), and comparable to our finding for the month after first treatment. Where our study differs is in the apparent loss of this treatment efficacy by the third month of LNG-IUS use (negligible effect), and an apparent reversal of effect thereafter.
The disadvantageous bleeding profile for CBD-2914 over the 64 days after last treatment was not much evident in responses to bleeding acceptability items (Table IV). Perhaps this is partly because acceptability was assessed against such a positive change over 6 months, of vaginal bleeding having generally diminished markedly over time, and compared with before LNG-IUS (Table IV, Fig. 4). This raises concern that there is disconnection between objective measures used in research, and acceptability to women of bleeding experienced.
The only side-effect to show a difference between groups was ‘tendency to weight gain’, with an excess in the group administered CDB-2914. There are a number of reasons for caution in interpreting this finding. Since this was one of 14 side-effects assessed, the finding could be chance. Secondly, weight gain has frequently been attributed to LNG-IUS [National Institute for Health and Clinical Excellence (NICE), 2005], and LNG-IUS had been in situ over the entire 6 months, whereas CDB-2914 was administered at three points, the last time 3 months before the questionnaire assessment. Thirdly, despite removals for reported weight gain being higher for LNG-IUS than non-hormonal IUDs, there is no evidence of a difference in actual weight gain between users of the two methods (National Institute for Health and Clinical Excellence (NICE), 2005). Similarly, when we compared actual weight change over the 6 months from LNG-IUS insertion (for the 57 CDB-2914 and 52 placebo-treated women with pre- and 6 months weight measurements), we found no difference between groups (mean gain for placebo minus CDB-2914 = 0.4 kg (95%CI −0.9 to 1.7 kg)). Finally, despite randomization there was a pre-existing (chance) difference between groups in prior discontinuation of contraception due to weight gain, with an excess of women with such a history in the group administered CDB-2914. Furthermore, the average weight gain of women who had responded ‘tendency to weight gain’, was 3.2 kg for the CDB-2914 subgroup (n = 12), and 2 kg for the one placebo group woman. This highlights the difficulty of differentiating effects due to the LNG-IUS that all women received, to the intermittent intervention administered to half the women, and to individual participant characteristics (in terms of susceptibilities to/tolerance of symptoms).
A systematic review of interventions for unscheduled bleeding occurring with POC included no trials involving the LNG-IUS (Abdel-Aleem et al., 2007). Therefore a key question is whether adjuvant administration of PRM CDB-2914 would have a beneficial effect on unscheduled bleeding with LNG-IUS. The beneficial effect found during the second month of LNG-IUS use (after first treatment) may be consistent with an effect on ovarian function. However, with the LNG-IUS, high-dose local delivery of LNG results in local changes in endometrial steroid response and structural integrity of endometrial blood vessels (Fraser and Hickey, 2000; Guttinger and Critchley, 2007). Within a few months this profound endometrial atrophy may mask the endocrine effects of ovarian disruption, and if so, would also mask any benefit of a treatment that reduced bleeding due to ovarian disruption. Another consequence of treatment to be considered is that it might itself precipitate bleeding. In a prevention study with a PRM administered in new users of oral progestogen-only contraception, the active treatment group had relatively more bleeding/spotting episodes commencing in the 7 days following treatment (Gemzell-Danielsson et al., 2002). A similar withdrawal bleed effect is apparent in our study also, with a marked difference between groups in timing of onset of next bleeding/spotting episode after the third ‘treatment’. Of the women administered CDB-2914, 53% (32/62) began a new bleeding/spotting episode within 4 days of starting the treatment, compared with 15% (9/58) of placebo-treated women, and the episodes were twice as likely to exceed 8 days. Therefore, preventative administration of PRM has the regrettable property that it might induce bleeding in a woman who would otherwise have had (virtually) none. This might explain the excess BS% in the CDB-2914 group for the 28 days following third treatment (by 9% points, or 2.5 days). However, induced withdrawal bleeds cannot explain the excess bleeding/spotting persisting over the following 36-day diary block (Fig. 3). This might have been a chance finding, or CDB-2914 might exacerbate whatever mechanism accounts for unscheduled bleeding persisting in the fourth/fifth months of LNG-IUS use.
In summary, our study is the first report of an intervention in women using a LNG-IUS. There appears to be some beneficial preventative effect of CDB-2914 on bleeding/spotting, but only in the very earliest months of use. In our study the first treatment started on Day 21 after insertion, yet before this there were high average rates of bleeding/spotting reported (67% of days). We do not know if CDB-2914 would have provided timelier benefit if administered earlier. Given the unpredictability as to which LNG-IUS users will in the first 3 months experience unscheduled bleeding, and our observations that PRMs appear to induce withdrawal bleeding, these compounds have little promise for prevention. However, in the absence of anything better, and given the safety profile of PRMs, they have potential for therapeutic use, among women experiencing excessive bleeding in the first few weeks of LNG-IUS use. An exploratory therapeutic trial is needed, with not only drug (CDB-2914 and placebo) but also stopping point (10 or 14 weeks) randomized.
Effective interventions to ameliorate unacceptable bleeding for women using long-acting progestogen contraception remain elusive. Discontinuation of LNG-IUS is only the tip of the iceberg. If an intervention could be found, improved bleeding profiles would enhance quality of life worldwide for the many women wishing to continue with a LNG-IUS for contraception.
Authors’ Roles
R.M.B., H.O.D.C., A.F.G. and P.W. contributed to conception and design; A.G., S.N., A.F.G., P.W. and H.O.D.C. contributed to acquisition of data; P.W., R.J.L., A.F.G. and H.O.D.C. contributed to analysis; P.W., R.J.L., A.F.G., H.O.D.C., R.M.B. and A.G. contributed to interpretation of findings; all contributed to drafting of article and approved final version.
Funding
This work was supported by the National Institute of Child Health and Human Development (NICHD; R01 HD43209-01).
Acknowledgements
We acknowledge the following for invaluable contributions to the research: Professor David Baird re design of study and analysis/interpretation of results; Dr Jennifer Guise re assistance in design of questionnaires and recruitment/interviews; Ms Joan Kerr for recruitment and study co-ordination/record-keeping; Ms Lyn Chalmers for design of data-entry database; Ms Dorothy Thom and Richard Warner for accurate data entry, in particular of diary data; and for general support the staff of Dean Terrace Family Planning Clinic, Edinburgh. We also thank the study participants for completion of diaries and questionnaires, and attending for follow-up visits. The authors wish to thank Dr André Ulmann and Dr Erin E Gainer of HRA Pharma (Paris, France) for providing CDB-2914.
References
- Abdel-Aleem H, d'Arcangues C, Vogelsong KK, Gülmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives. Cochrane Database of Systematic Reviews. 2007 Issue 2. Art. No.: CD003449. DOI: 10.1002/14651858.CD003449.pub3. [Google Scholar]
- Alvarez-Sanchez F, Brache V, Thevenin F, Cochon L, Faundes A. Hormonal treatment for bleeding irregularities in Norplant implant users. Am J Obstet Gynecol. 1996;174:919–922. doi: 10.1016/s0002-9378(96)70326-5. [DOI] [PubMed] [Google Scholar]
- Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 users. BJOG. 2000;107:335–339. doi: 10.1111/j.1471-0528.2000.tb13228.x. [DOI] [PubMed] [Google Scholar]
- Baldaszti E, Wimmer-Puchinger B, Loschke K. Acceptability of the long-term contraceptive levonorgestrel-releasing intrauterine system (Mirena): a 3-year follow-up study. Contraception. 2003;67:87–91. doi: 10.1016/s0010-7824(02)00482-1. [DOI] [PubMed] [Google Scholar]
- Barbosa I, Bakos O, Olsson SE, Odlind V, Johansson ED. Ovarian function during use of a levonorgestrel-releasing IUD. Contraception. 1990;42:51–66. doi: 10.1016/0010-7824(90)90092-a. [DOI] [PubMed] [Google Scholar]
- Blithe DL, Nieman LK, Blye RP, Stratton P, Passaro M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68:1013–1017. doi: 10.1016/s0039-128x(03)00118-1. [DOI] [PubMed] [Google Scholar]
- Cheng L, Zhu H, Wang A, Ren F, Chen J, Glasier A. Once a month administration of mifepristone improves bleeding patterns in women using subdermal contraceptive implants releasing levonorgestrel. Hum Reprod. 2000;15:1969–1972. doi: 10.1093/humrep/15.9.1969. [DOI] [PubMed] [Google Scholar]
- Cox M, Tripp J, Blacksell S. Clinical performance of the levonorgestrel intrauterine system in routine use by the UK Family Planning and Reproductive Health Research Network: 5-year report. J Fam Plann Reprod Health Care. 2002;28:73–77. doi: 10.1783/147118902101196225. [DOI] [PubMed] [Google Scholar]
- d'Arcangues C, Odlind V, Fraser IS. Dysfunctional uterine bleeding induced by exogenous hormones. In: Alexander NJ, d'Arcangues C, editors. Steroid Hormones and Uterine Bleeding. Washington: AAAS Press; 1992. pp. 81–105. [Google Scholar]
- Fraser IS, Hickey M. Endometrial vascular changes and bleeding disturbances with long-acting progestins. Steroids. 2000;65:665–670. doi: 10.1016/s0039-128x(00)00188-4. [DOI] [PubMed] [Google Scholar]
- Gemzell-Danielsson K, van Heusden AM, Killick SR, Croxatto HB, Bouchard P, Cameron S, Bygdeman M. Improving cycle control in progestogen-only contraceptive pill users by intermittent treatment with a new anti-progestogen. Hum Reprod. 2002;17:2588–2593. doi: 10.1093/humrep/17.10.2588. [DOI] [PubMed] [Google Scholar]
- Glasier AF, Wang H, Davie JE, Kelly RW, Critchley HO. Administration of an antiprogesterone up-regulates estrogen receptors in the endometrium of women using Norplant: a pilot study. Fertil Steril. 2002;77:366–372. doi: 10.1016/s0015-0282(01)02997-1. [DOI] [PubMed] [Google Scholar]
- Guttinger A, Critchley HO. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007;75:S93–S98. doi: 10.1016/j.contraception.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Guttmacher Institute. Facts on Induced Abortion Worldwide. 2008. http://www.guttmacher.org/pubs/fb_IAW.html .
- Halpern V, Grimes DA, Lopez LM, Gallo MF. Strategies to improve adherence and acceptability of hormonal methods of contraception. Cochrane Database of Systematic Reviews. 2006 doi: 10.1002/14651858.CD004317.pub2. Issue 1. Art. No.: CD004317. DOI:10.1002/14651858.CD004317.pub2. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception. 2002;65:129–132. doi: 10.1016/s0010-7824(01)00302-x. [DOI] [PubMed] [Google Scholar]
- Jain JK, Nicosia AF, Nucatola DL, Lu JJ, Kuo J, Felix JC. Mifepristone for the prevention of breakthrough bleeding in new starters of depo-medroxyprogesterone acetate. Steroids. 2003;68:1115–1119. doi: 10.1016/s0039-128x(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Massai MR, Pavez M, Fuentealba B, Croxatto HB, d'Arcangues C. Effect of intermittent treatment with mifepristone on bleeding patterns in Norplant implant users. Contraception. 2004;70:47–54. doi: 10.1016/j.contraception.2004.02.009. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) Long-acting reversible contraception. 2005. http://guidance.nice.org.uk/CG30 . [PubMed]
- Peterson HB, Curtis KM. Clinical practice. Long-acting methods of contraception. N Engl J Med. 2005;353:2169–2175. doi: 10.1056/NEJMcp044148. [DOI] [PubMed] [Google Scholar]
- Trussell J. Contraceptive Efficacy. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart FH, Kowal D, editors. Contraceptive Technology: Nineteenth Revised Edition. New York NY: Ardent Media; 2007. [Google Scholar]
- Weisberg E, Hickey M, Palmer D, O'Connor V, Salamonsen LA, Findlay JK, Fraser IS. A pilot study to assess the effect of three short-term treatments on frequent and/or prolonged bleeding compared to placebo in women using Implanon. Hum Reprod. 2006;21:295–302. doi: 10.1093/humrep/dei273. [DOI] [PubMed] [Google Scholar]
- World Health Organization Reproductive Health and Research (WHO) Geneva: WHO; 2008. Quality Care in Family Planning, Medical Eligibility Criteria for Contraceptive Use. http://www.who.int/reproductive-health/publications/mec/index.htm . [Google Scholar]
- Xiao BL, Zhou LY, Zhang XL, Jia MC, Luukkainen T, Allonen H. Pharmacokinetic and pharmacodynamic studies of levonorgestrel-releasing intrauterine device. Contraception. 1990;41:353–362. doi: 10.1016/0010-7824(90)90035-t. [DOI] [PubMed] [Google Scholar]