Abstract
Disturbances in selective attention represent a core characteristic of schizophrenia, whose neural underpinnings have yet to be fully elucidated. Consequently, we recorded brain activation using functional magnetic resonance imaging (fMRI) while 15 patients with schizophrenia and 15 age-matched controls performed a well-established measure of selective attention- color Stroop negative priming task. We focused on two aspects performance: overriding pre-potent responses (Stroop effect) and inhibition of prior negatively-primed trials (negative priming effect). Behaviorally, controls demonstrated both significant Stroop and negative priming effects, while schizophrenic subjects only showed the Stroop effect. For the Stroop effect, fMRI indicated significantly greater activation in frontal regions – medial frontal gyrus/anterior cingulate gyrus and middle frontal gyrus for controls, but greater activation in medial parietal regions (posterior cingulate gyrus/precuneus) for patients. Negative priming elicited significant activation in right dorsolateral prefrontal cortex for both groups, but also in left dorsolateral prefrontal cortex for patients. These different patterns of fMRI activation may reflect faulty interaction in schizophrenia within networks of brain regions that are vital to selective attention.
Keywords: fMRI, color stroop, negative priming, schizophrenia
1. INTRODUCTION
Founding figures of modern psychiatry, Kraepelin and Bleuler, each emphasized attentional disturbance as the “primary expression of the schizophrenic brain” (Heinrichs, 2005). More contemporary times have seen the study of attention cast within the framework of the burgeoning field of cognitive neuroscience. In this framework attention represents a fundamental adaptation of the healthy brain that has evolved to for the purposes of selecting salient information through both enhancement and suppression of neural activity associated with task-relevant and task-irrelevant representations (Gazzaley et al., 2005). This neural activity is coordinated and organized across networks of brain regions that traverse anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), and medial and lateral parietal sites (Botvinick et al., 2001; Carter and MacDonald, 2001; Fan and Posner, 2004). Schizophrenia may, in theory, compromise the efficiency and integrity of neural circuits underlying attentional selection, and this, in turn, might be expressed in widespread cognitive deficits.
The current study used functional magnetic resonance imaging (fMRI) to examine neural networks of selective attention while patients with schizophrenia and healthy controls performed a Negative Priming (NP) Stroop Color Word test. The Stroop is a well-studied measure of selective attention in which the key stimulus items are color words (e.g., BLUE) that are printed in different color ink (e.g., BLUE in RED ink). The classic Stroop response occurs when the examinee is instructed to identify the color of ink (e.g., RED) of an incongruent color word (e.g., BLUE). These incongruent trials impose heavy demands on selective attention, and elicit slower responses, indicative of greater interference. In addition to these classic interference trials, the NP Stroop Color Word test incorporates another kind of incongruent trials that are negatively-primed. A negatively-primed incongruent trial occurs when the ignored distracter, color name, in the preceding trail becomes the target, color ink, in the subsequent trial. This results in even slower response and greater interference for negatively-primed incongruent trials than for the classic, non-negatively primed incongruent trials (e.g., MacLeod and MacDonald, 2000; MacQueen et al., 2003; van Veen, and Carter, 2005)
The current study aimed principally to examine, within and between patients and healthy controls, behavioral and neural responses to these two kinds of incongruent trials. In imaging studies of healthy subjects, classic Stroop incongruent trials activate a well-specified, widely-distributed network of brain regions of the medial frontal gyrus including the ACC, DLPFC and parietal cortex (Carter et al., 1995; Gruber et al., 2002; Pardo et al., 1990). In addition, findings from healthy subjects have revealed a particularly strong relationship of right DLPFC activation and negative priming (Egner & Hirsch, 2005). For patients, behavioral studies have indicated greater interference on the Stroop, as reflected by longer reaction times for incongruent trials (Barch et al., 2004; Hepp et al., 1996; Salo et al., 2002), which has been linked in fMRI studies to reduced activation within the ACC (Carter et al., 1997; Henik and Salo, 2004).
To our knowledge, no fMRI studies have examined NP Stroop in schizophrenia. However, behavioral studies have pointed to a very interesting pattern of findings in patients for negatively primed, incongruent trials on the Stroop. That is, these data have indicated that whereas healthy controls show the expected negative priming effect of slowest response to these trials in which a previous distracter is now the target, patients with schizophrenia do not: that is, the data indicated significantly reduced negative priming, with the patients failing to show their slowest response for these trials in which a previous distracter is now the target (Laplante et al., 1992; MacQueen et al., 2003; Salo et al., 2002).
The precise basis for the disease-related reduction in negative priming is still unclear. Behaviorally, many interpretations have emphasized negative priming abnormalities in schizophrenia as evidence of failures in selective attention, specifically in sustained inhibition over time (Laplante et al., 1992; MacQueen et al., 2003; Salo et al., 2002). For patients with schizophrenia, the extent, if any, to which patterns of brain activation might differ as a function of type of incongruent trial may help to understand better the role of failures of inhibition in the disease-related impairment of selective attention. Accordingly, then, in this paper, we combine fMRI and NP Stroop test to examine the dynamics of selective attention in patient and control groups. We hypothesize that each of the incongruent trials will be linked to differential brain activation within prefrontal regions. In particular, we predict that in relation to healthy controls, patients will show reduced ACC activation for the classic Stroop incongruent trials whereas negative priming trails will be related to aberrant DLPFC activation.
2. METHODS
2.1. Subjects
Fifteen male patients diagnosed with chronic schizophrenia, using DSM-IV criteria based on SCID-P interviews and a review of the medical records (mean age 43 (±7), mean age of onset 21.7 (±3.3), mean neuroleptic dose (mg/day in chlorpromazine equivalents 632 (±307)) and 15 male control subjects (mean age 43 (±6)) were matched on gender (all male), handedness, PSES, and age. All subjects gave written informed consent prior to participation in the study, and all were compensated for their time. Criteria for subjects’ inclusion were as follows: right-handedness, ages between 18 and 55 years, no neurological illness, no alcohol or drug dependence in the last 5 years and no abuse in the past year.
2.2. Negative-Priming Color Stroop Paradigm
The Color Stroop/Negative Priming paradigm is a modified, computerized version of single trial/event related classic Color Stroop paradigm, where color names compete and interfere with incongruent ink colors they are written in. In our experiment, stimuli consisted of four color names (red, green, blue, or yellow) presented in one of these four colors (size 44 font) on a black background. Subjects were asked to report the color in which the word was written, by pressing the corresponding response button, ignoring the name of the color itself. Each word (written in 44 Arial font against the black background, presented at the 5 degree angle) appeared centrally on a screen until the subject responded, but not longer than the inter-trial interval (ITI), which was jittered between 3 and 6 seconds (mean 4.4 seconds). A fixation point was present in the center of the screen any time a word was not being shown. In the congruent condition, the target (word color) was the same as the distractor (word text); in the incongruent condition, the target differed from the distractor. In our version of the experiment, in addition to standard stimuli congruency manipulation allowing us to estimate Stroop effect, we manipulated order of the incongruent stimuli, to estimate the negative priming effect. In the negative-priming condition, the target of an incongruent trial became the distractor of an immediately preceding incongruent trial. (Table 1) No color targets or names were ever repeated in consecutive trials. Before the scanning, each session started with detailed instructions where the subjects were asked to identify the color in which the word was printed. Subjects were allowed a practice session, which was immediately followed by the experimental session. Response time and accuracy were recorded. The Stroop Effect was defined as the difference in the response times between incongruent and congruent trials. Negative-priming was defined as the difference in response times for those incongruent primed trials in which the target (ink color of word) had been the distracter (word name) in the preceding trial. The task was presented in four runs, each with 73 events that included 22 congruent trials and 51 incongruent trials (22 manipulated for negative-priming). The inter-trial interval (ITI) was jittered to be between 3 and 6 second (mean 4.4 seconds). Runs were different from each other, and their orders were counterbalanced between subjects. The total length of the experiment was 22 minutes. The task was presented in four runs, each with 73 events that included 22 congruent trials and 51 incongruent trials (22 manipulated for negative-priming). The inter-trial interval (ITI) was jittered to be between 3 and 6 second (mean 4.4 seconds).
Table 1.
Task Design
| Trial # | Stimulus | Distractor (Semantic) | Target (Color) | Type of Trial |
|---|---|---|---|---|
| 1 | BLUE | blue | blue | Congruent |
| 2 | RED | red | yellow | Incongruent |
| 3 | GREEN | green yellow |
red green |
Incongruent with negative priming |
| 4 | YELLOW |
Runs were different from each other, and their orders were counterbalanced between subjects.
2.3. FMRI Study
Imaging was performed using a 3-T whole body MRI Echospeed system (General Electric Medical Systems, Milwaukee, WI). First a sagital anatomical localizer image was acquired, and then the 134 EPI BOLD scans (32 oblique coronal slices; 4 mm thick; TR 2.5 s; TE 30 ms; flip angle 90°) were acquired perpendicular to the AC-PC line. During scanning the subjects performed the Negative-Priming-Color Stroop task. The first 4 scans of each run were discarded, and the rest were subjected to statistical analysis.
Words within the scanner were presented to the subjects with MR compatible visual goggles (Avotec, Inc., Florida, www.avotec.org) using Presentation software (version 0.76, build 11.30.03; Neurobehavioral Systems, www.neurobs.com). Responses were collected using an MRI compatible fiber optic response four-button diamond configuration pad (Current Designs Inc., Philadelphia, PA, www.curdes.com
Data were analyzed using Statistical Parametric Mapping (SPM2). Each subject’s data was co-registered to the first scan of the first session (in order to correct for head movement), normalized to the Montreal Neurology Institute (MNI) template using a nonlinear, 12-parameter affine transformation registration, and smoothed with a 12-mm FWHM Gaussian filter. Activation maps using General Linear Model (SPM2) including contrasts of tested conditions were constructed for each subject (where congruent and incongruent (for Stroop effect), as well as incongruent with and without negative priming (negative priming effect) conditions were first contrasted against each other on the subject level (fixed effect contrast), and then subjected to the second level, random effect analysis on the group level. Another set of random effect analyses compared separately Stroop as well as Negative Priming contrasts between groups. Activation of controls and schizophrenics were subtracted from each other to identify differential activation in both the Stroop (congruent vs. incongruent) and negative-priming (incongruent non-primed vs primed) conditions. Whole brain analysis as well as ROI analysis (WFUPickAtlas SPM2 toolbox) that focused on ACC and DLPFC were performed, and statistical results were reported using cluster level interference method (statistical threshold (p<0.05) corrected for the number of continuous voxels, as implemented in SPM (Friston et al., 1996)).
3. RESULTS
3.1. Behavioral Data
There was no significant difference in overall response time between the two groups (t = −1.162; df = 28; p = 0.260) with a mean response time of 1099.52 ms (±131.29 ms) for normal control subjects and 1210.58 ms (±346.03 ms) for the schizophrenic subjects. The mean difference in response times between congruent and incongruent trials for normal control subjects was 111.39 ms (±47.06ms) and for schizophrenic patients was 122.74 ms (±71.22 ms). The mean difference in response times between the incongruent trials with the negative priming manipulation and those without for the normal control subjects was 30.62 ms (±50.62 ms) and for schizophrenic subjects was 17.87 ms (±49.03 ms).
Statistical tests revealed a strong main effect of Stroop interference (F (1, 34) = 27.35, p < 0.0001), and no group by condition interaction (F (1, 34) = 0.54, p < 0.47). For negative priming, there was a main effect of negative priming (F(1,34)=5.16, p <0.03), and a significant group by NP interaction (F(1,34)=5.65), p <0.023). The significant interaction effect indicated that whereas the control group showed increased slowing for the negative priming trials, the patients did not. To follow-up on the interaction effect, we have conducted paired within-sample t-tests for each group for the NP effect. While normal controls showed a strong NP effect (p < t= −4.709, p < 0.0001), the patient group did not show this effect (t=0.06, p < 0.95).
3.2. Imaging Data
Schizophrenic patients showed less activation associated with Stroop effect for incongruent trials that were not negative primed than normal controls in the medial frontal gyrus/ACC as well as in middle frontal gyrus around the inferior frontal sulcus (Broadman area 9) (Table 2, Figure 1), but showed greater activation in the medial parietal regions, that include posterior cingulate and precuneus (Table 2, Figure 1). For negative priming, schizophrenic patients showed significant activation in both the right and the left DLPFC (Broadman area 6- Table 2, Figure 2) while the healthy subjects only showed significant activation in the right DLPFC (Table 2, Figure 3).
Table 2.
Results of fMRI analysis
| fMRI results | |||
|---|---|---|---|
| Stroop Effect | |||
| Region | MNI (x,y,z) | Z score | |
| Control > Schizophrenic | |||
| Medial Frontal Gyrus/ACC | (4,44,24) | 2.95 | |
| Right Middle Frontal Lobe | (42,18,32) | 4.18 | |
| Schizophrenic > Control | |||
| Posterior Cingulate/Precuneus | (18,−78,24) | 3.97 | |
| Negative Priming | |||
|---|---|---|---|
| Region | MNI (x,y,z) | Z score | |
| Schizophrenic Activation | |||
| Right DLPFC | (26,0,56) | 3.23 | |
| Left DLPFC | (−26,−8,56) | 2.72 | |
| Control Activation | |||
| Right DLPFC | (30,6,58) | 3.40 | |
Figure 1.
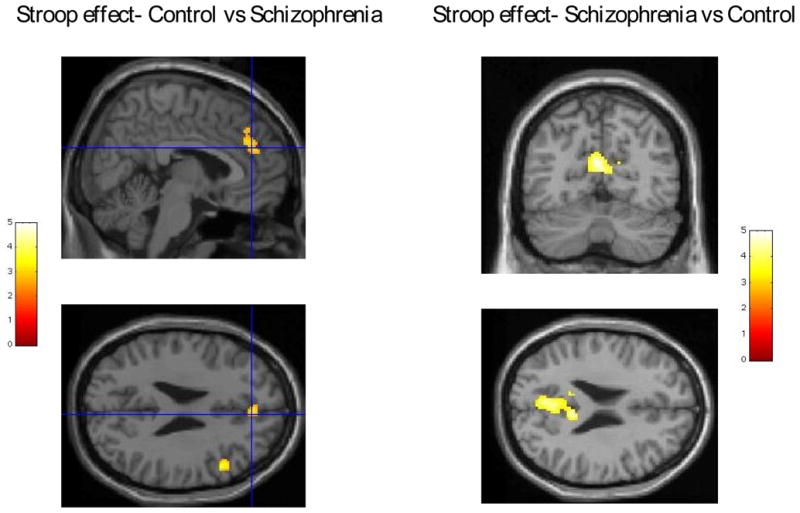
Left panel- activation due to Stroop effect- ACG/medial frontal gyrus and right DLPFC activations in control subjects greater than in schizophrenia. Random effect group analysis (controls vs schizophrenics) superimposed on single subject Talairach brain (provided by SPM2). Right panel-activation due to Stroop effect- posterior cingulate gyrus/precuneus activations in schizophrenia subjects greater than in controls. Random effect group t-test (schizophrenics vs controls) results superimposed on single subject Talairach brain (provided by SPM2)
Figure 2.
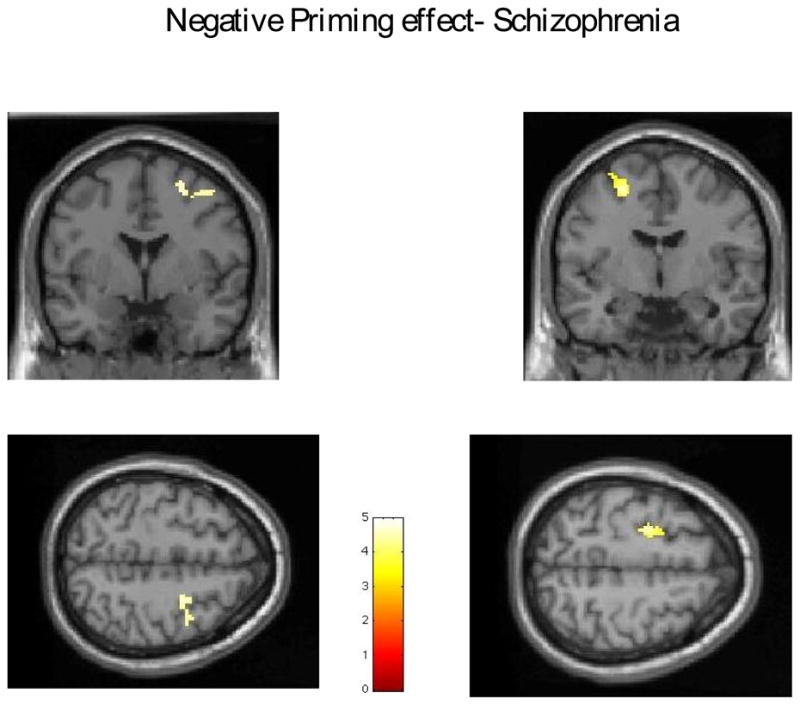
Activation due to Negative priming in schizophrenia- right and left middle frontal gyrus. Group t-test results on single subject Talairach brain (provided by SPM2)
Figure 3.
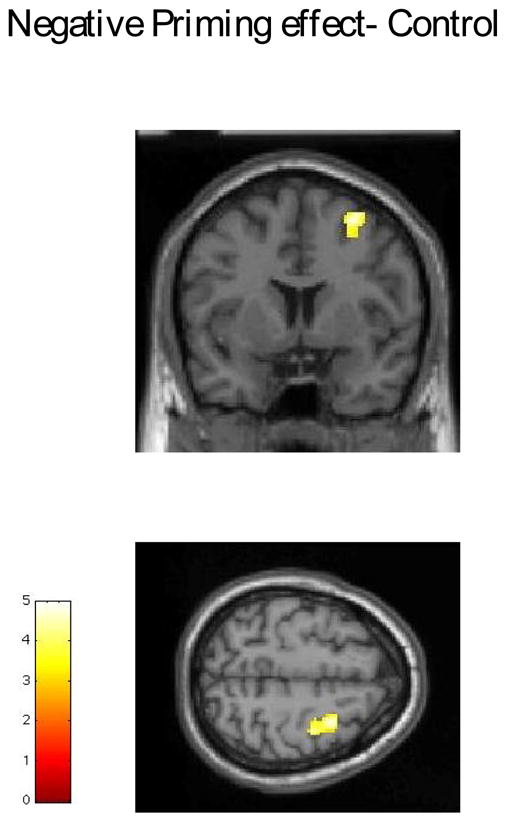
Activation due to Negative priming in control subjects- right middle frontal gyrus. Group t-test results superimposed on single subject Talairach brain (provided by SPM2)
4. DISCUSSION
We recorded patterns of fMRI brain activation while subjects performed a NP Stroop task. Behaviorally, both healthy control and patient groups showed the classic Stroop effect, as reflected by slower reaction time for incongruent trials (e.g., name color font of the word RED printed in BLUE ink). The healthy control group also showed the expected negative priming effect of having their slowest responses to those incongruent trials for which the distracter from the prior trail (e.g., color word RED) became the target (BLUE printed in RED ink) in the current trial. By contrast, the patient group failed to show evidence of negative priming, as they did not have their slowest responses to negatively primed incongruent trials. For the fMRI data, both types of incongruent trials --- classic Stroop and negative primes ---elicited different patterns of brain activation across both groups depending on whether the to-be-identified ink color had been a distracter on the prior trial.
Most important, the current results pointed to group differences in fMRI patterns activated by these different incongruent trials. First, beginning with the classic incongruent trials, in comparison to healthy controls, the patients showed decreased activation in the ACC and medial frontal gyrus, yet increased activation in medial parietal areas, most notably the posterior cingulate gyrus. The decreased ACC activation is consistent with other studies of patients with schizophrenia (Carter et al., 1997; Henik and Salo, 2004), and increased posterior cingulate gyrus/precuneus activation has been also reported before, but with less consistency (Erkwoh et al., 2002; Gur et al., 2007). Together, these three regions are thought to represent key nodes in a prefrontal executive attention network that supports critical cognitive functions of control and performance monitoring that are thought to underlie learning and memory (Botvinick et al., 2001; Nestor et al., 2007).
Second, for negative priming trials, the fMRI findings indicated that schizophrenic patients showed activation in both the right and left DLFPC, while healthy control subjects showed significant activation only in the right DLPFC. To the best of our knowledge, these data represent the first fMRI investigation of Stroop negative priming in schizophrenia, and thus cannot be directly compared to prior literature. However, for the healthy control group, the strong relationship of right DLPFC activation and negative priming replicates recent findings reported by Egner & Hirsch (2005). In addition, and consistent with other behavioral studies (e.g., Salo et al., 2002), the patients in this investigation showed reduced negative priming; that is, they showed less slowing in response times for distracter-turned-target trials. These abnormalities of negative priming may reflect either a hypothesized disease-related reduction in the potency of distracter inhibition (May et al., 1995) or failure in implicit retrieval from a previous instance of a conflicting stimulus (Egner and Hirsch, 2005; Salo et al., 2002; Steel et al., 2001). Neither of these interpretations for the negative priming abnormalities of the patient group can be ruled out by the experimental design of the current study.
Both incongruent trials induce response competition, pitting the dominant and natural response of reading a color word against the decidedly weaker and unnatural response of identifying the color of its font. Both trials require resolving competing and conflicting task demands for which the ACC of the prefrontal executive attention network is thought to play a particularly critical role. In addition, the fMRI data suggested medial and dorsolateral prefrontal sites may mediate negative priming. The fMRI findings that these two types of incongruent trials elicited differential brain activation may have important functional significance. For example, simulation studies have suggested a similar division of labor of ACC-mediated monitoring and conflict resolution on one hand, and lateral prefrontal-mediated control and inhibition on the other hand (see Botivinick et al., 2001). These simulations have revealed a prefrontal executive attention network that enables the ultimate selection of the weaker yet correct response of font color over the dominant but incorrect response of color word via coordinated activity of anterior cingulate and prefrontal sites: as simulated, the anterior cingulate monitors performance, and then provides feedback for prefrontal sites for on-line adjustments in control and inhibition (Botvinick et al., 2001).
Thus, for patients with schizophrenia, their abnormal patterns of fMRI activation for these incongruent trials may signify disease-related disturbance in a prefrontal executive attention network. Moreover, these imaging results conform well with recent structural evidence of abnormalities in the cingulum bundle, a key white matter tract connecting the anterior cingulate to other frontal sites of the executive attention network (Kubicki et al., 2003b). Consistent with the current findings, these structural abnormalities have in turn correlated with poorer performance on neuropsychological measures of attention in patients with chronic schizophrenia (Nestor et al., 2004; Nestor et al., 2007). In addition, for the patient group, incongruent trials elicited relative increase in activity in the medial parietal cortex (posterior cingulate/precuneus) that was not observed in healthy controls. These regions have been theorized to be involved in conscious awareness (Cavanna and Trimble, 2006; Vogt and Laureys, 2005), and as part of the “default network” have shown metabolic activation during rest, and relative decrease of activation during goal-directed action in healthy controls (Greicius et al., 2003; Gusnard et al., 2001). A relative increase in the activity within this region observed in our experiment in schizophrenia patients may reflect insufficient “suspension” of its baseline activity, either due to the impaired recruitment of brain systems required for target detection (Gur et al., 2007), or altered connectivity between anterior and posterior medial regions of the brain (Garrity et al., 2007).
It is worth acknowledging that our study has multiple limitations- our population is chronic, medicated, and is limited to males only, thus results might not be easily generalized onto entire schizophrenia population, and medication as well as age could be the additional confounds of the study results. Sample size is also relatively small (15 controls and 15 schizophrenics) although comparable with other published fMRI clinical studies.
In summary, the study combined fMRI and the NP Stroop in order to examine neural circuitry underlying selective attention in patients with schizophrenia. The results revealed a distinct pattern of behavioral response and brain activation for the patients in comparison to healthy controls, which suggested evidence of a specific impairment in selective attention in schizophrenia. We cannot however rule out the contribution of group differences in task strategy as contributing to the current results. Other limitations of the current study include the relatively small group sizes as well as that all patients were males with long histories of anti-psychotic medication prescribed for the treatment of their chronic schizophrenia. Future studies are needed to examine how the robustness of these behavioral and brain abnormalities in selective attention and how they might be related to the core cognitive problem of the disease.
Acknowledgments
The authors would like to acknowledge the National Institute of Health (R03 MH068464-01 to MK, R03 MH 078036 to MN, K05 MH070047, R01 MH 40799 to MN and MK and P50MH080272 (CIDAR Award, CW, MN and MK), and the VA Schizophrenia Center Grant (CW, MN). This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149 (MK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barch DM, Carter CS, Cohen JD. Factors influencing Stroop performance in schizophrenia. Neuropsychology. 2004;18(3):477–84. doi: 10.1037/0894-4105.18.3.477. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biological Psychiatry. 2005;58(1):47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychology Reviews. 2001;108(3):624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. American Journal of Psychiatry. 2001;158(9):1423–8. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage. 1995;2(4):264–72. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. American Journal of Psychiatry. 1997;154(12):1670–5. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Where memory meets attention: neural substrates of negative priming. J Cognitive Neuroscience. 2005;17(11):1774–84. doi: 10.1162/089892905774589226. [DOI] [PubMed] [Google Scholar]
- Erkwoh R, Sabri O, Schreckenberger M, Setani K, Assfalg S, Sturz L, Fehler S, Plessmann S. Cerebral correlates of selective attention in schizophrenic patients with formal thought disorder: a controlled H2 15O-PET study. Psychiatry Research. 2002;115(3):137–53. doi: 10.1016/s0925-4927(02)00045-8. [DOI] [PubMed] [Google Scholar]
- Fan J, Posner M. Human attentional networks. Psychiatry Praxis. 2004;31(Suppl 2):S210–4. doi: 10.1055/s-2004-828484. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. I. Encoding. Brain. 1998a;121(Pt 7):1239–48. doi: 10.1093/brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain. 1998b;121(Pt 7):1249–56. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164(3):450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. Journal of Cognitive Neuroscience. 2005;17(3):507–17. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Holcomb P, Soraci S, Yurgelun-Todd D. Stroop performance in normal control subjects: an fMRI study. Neuroimage. 2002;16(2):349–60. doi: 10.1006/nimg.2002.1089. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Loughead J, Snyder W, Kohler C, Elliott M, Pratiwadi R, Ragland JD, Bilker WB, Siegel SJ, Kanes SJ, Arnold SE, Gur RC. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. American Journal of Psychiatry. 2007;164(3):442–9. doi: 10.1176/ajp.2007.164.3.442. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. National Reviews Neuroscience. 2001;2(10):685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. The primacy of cognition in schizophrenia. American Psychology. 2005;60(3):229–42. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- Henik A, Salo R. Schizophrenia and the stroop effect. Behavavioral and Cognitive Neuroscience Reviews. 2004;3(1):42–59. doi: 10.1177/1534582304263252. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999;122(Pt 7):1367–81. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Hepp HH, Maier S, Hermle L, Spitzer M. The Stroop effect in schizophrenic patients. Schizophrenia Research. 1996;22(3):187–95. doi: 10.1016/s0920-9964(96)00080-1. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fizbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Nestor PG, Huh T, Kikinis R, Shenton ME, Wible CG. An fMRI study of semantic processing in men with schizophrenia. Neuroimage. 2003a;20(4):1923–33. doi: 10.1016/s1053-8119(03)00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biological Psychiatry. 2003b;54(11):1171–80. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante L, Everett J, Thomas J. Inhibition through negative priming with Stroop stimuli in schizophrenia. British Journal of Clinical Psychology. 1992;31(Pt 3):307–26. doi: 10.1111/j.2044-8260.1992.tb00998.x. [DOI] [PubMed] [Google Scholar]
- MacDonald PA, Joordens S, Seergobin KN. Negative priming effects that are bigger than a breadbox: attention to distractors does not eliminate negative priming, it enhances it. Memory and Cognition. 1999;27(2):197–207. doi: 10.3758/bf03211405. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends in Cognitive Sciences. 2000;4(10):383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Galway T, Goldberg JO, Tipper SP. Impaired distractor inhibition in patients with schizophrenia on a negative priming task. Psychological Medicine. 2003;33(1):121–9. doi: 10.1017/s0033291702006918. [DOI] [PubMed] [Google Scholar]
- May CP, Kane MJ, Hasher L. Determinants of negative priming. Psychology Bulletin. 1995;118(1):35–54. doi: 10.1037/0033-2909.118.1.35. [DOI] [PubMed] [Google Scholar]
- Neill DB, Kaufman JL. Deficits in behavioral responding to regulatory challenges after lesions of ventrobasal thalamus in rats. Physiology and Behavavior. 1977;19(1):47–52. doi: 10.1016/0031-9384(77)90157-3. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, Shenton ME. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18(4):629–37. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophrenia Research. 2007;90(1–3):308–15. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Scinces. 1990;87(1):256–9. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Henik A, Nordahl TE, Robertson LC. Immediate versus sustained processing in schizophrenia. Journal of the International Neuropsychological Society. 2002;8(6):794–803. doi: 10.1017/s1355617702860076. [DOI] [PubMed] [Google Scholar]
- Steel C, Haworth EJ, Peters E, Hemsley DR, Sharma T, Gray JA, Pickering A, Gregory L, Simmons A, Bullmore ET, Williams SC. Neuroimaging correlates of negative priming. Neuroreport. 2001;12(16):3619–24. doi: 10.1097/00001756-200111160-00049. [DOI] [PubMed] [Google Scholar]
- Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. American Journal of Psychiatry. 2005;162(10):1849–58. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- Tipper SP. The negative priming effect: inhibitory priming by ignored objects. Quarterly Journal of Experimental Psychology A. 1985;37(4):571–90. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. Neuroimage. 2005;27(3):497–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in Brain Research. 2005;150:205–17. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


