Abstract
Gel-forming mucins are the largest complex glycoprotein macromolecules in the body. They form the matrix of gels protecting all the surface epithelia and are secreted as disulfide-bonded polymeric structures. The mechanisms by which they are formed and organized within cells and thereafter released to form mucus gels are not understood. In particular, the initial rate of expansion of the mucins after release from their secretory granules is very rapid (seconds), but no clear mechanism for how it is achieved has emerged. Our major interest is in lung mucins, but most particularly in MUC5B, which is the major gel-forming mucin in mucus, and which provides its major protective matrix. In this study, using OptiPrep density gradient ultracentrifugation, we have isolated a small amount of a stable form of the recently secreted and expanding MUC5B mucin, which accounts for less than 2% of the total mucin present. It has an average mass of ∼150 × 106 Da and size Rg of 150 nm in radius of gyration. In transmission electron microscopy, this compact mucin has maintained a circular structure that is characterized by flexible chains connected around protein-rich nodes as determined by their ability to bind colloidal gold. The appearance indicates that the assembled mucins in a single granular form are organized around a number of nodes, each attached to four to eight subunits. The organization of the mucins in this manner is consistent with efficient packing of a number of large heavily glycosylated monomers while still permitting their rapid unfolding and hydration. For the first time, this provides some insight into how the carbohydrate regions might be organized around the NH2- and COOH-terminal globular protein domains within the granule and also explains how the mucin can expand so rapidly upon its release.
Keywords: mucin granule, secretion, mucus
mucins are large complex glycoprotein macromolecules that provide the basis of gels protecting the surface epithelia in many multicellular organisms. In humans, these macromolecules come in two major kinds, the epithelial or transmembrane mucins characterized by COOH-terminal domains that attach to cell surface membranes (5) and those termed the gel-forming mucins that are secreted as highly assembled polymeric structures (14). There are four such molecules, MUC2, MUC5AC, MUC5B, and MUC6, and they form the largest glycoproteins in the body (10). The functions of these molecules are understood in general terms, i.e., they form the basic infrastructure of the epithelial protective gels, but the mechanisms by which they are made and organized within cells and thereafter released are not understood.
The basis of the polymeric assembly of these large mucin glycoproteins is thought to mimic that of another related glycoprotein called von Willebrand factor (VWF) protein. This molecule provides a vital role in blood coagulation, and genetic defects known to be associated with it relate to a number of diseases (7). Both gel-forming mucins and the VWF assemble into their polymeric structures by first dimerizing in the endoplasmic reticulum via their COOH-terminal domains and subsequently multimerizing through protein domains in the NH2 termini. However, whereas the mucin granules are always spheroidal in shape (2), the VWF granules are more generally cigar shaped (20). A mechanism for the NH2-terminal assembly process in VWF has recently been described (6). This mechanism suggests that the assembling molecules may form extended helical structures around which glycosylated protein regions, linked by the COOH-terminal domains, are organized. Both mucins and the VWF molecules are generally stored in granules that are unpacked upon release from the cell.
However, gel-forming mucins, although similar in some respects to the VWF, are much larger at their individual subunit level, each containing a number of highly glycosylated domains. For instance, the MUC5B subunit (Fig. 1A) has five such regions, all of which have densely arranged, charged O-linked oligosaccharides. These oligosaccharides are so numerous and organized that the glycosylated peptide cannot be proteolytically cleaved even by peptidases as small as trypsin or papain. A MUC5B mucin subunit is ∼400–500 nm in length and arranged into a linear polymeric form. Such a structure is many micrometers in length (Fig. 1, B and C). We have previously employed electron microscopy (EM) to characterize the organization of this structure (9) and in particular used colloidal gold to identify the globular NH2- and COOH-terminal domains (Fig. 1C). These data showed very clearly that the colloidal gold was excluded from the glycosylated regions and only bound to these terminal protein domains.
Fig. 1.
The structure of MUC5B. A: a typical structural domain representation of the MUC5B subunit. MUC5B has Von Willebrand factor (VWF)-like domains at NH2- and COOH-terminal regions (D, B, C, and CK domains). The central region contains 5 heavily glycosylated mucin domains (TR) and 5 small cysteine-rich (cys) regions. B: a recent electron microscopy image of a conformationally relaxed, linear MUC5B molecule. The MUC5B intact molecule as found in secretions is assembled from multiple large subunits via disulfide bond-mediated interactions between COOH-terminal domains of monomers to form dimers and subsequent interactions between NH2-terminal domains to form higher polymer oligomers. The assignment of the structure as a dimer (dotted bar) is made on the basis of the length (850 nm), which is greater than that obtainable by a single MUC5B subunit molecule. The N↔N terminal region and C↔C terminal region can be delineated by their size. Scale bar, 100 nm. C: an image of the MUC5B molecule as seen in an early surface-based electron microscopy method (9). This surface method entraps and extends the mucin chain, and by using 5 nm of colloidal gold (white dots), the NH2- and COOH-terminal globular protein domains were clearly identified. The molecular distance between the gold is ∼500 nm, which is consistent with the maximal length that a single MUC5B mucin subunit can achieve. Scale bar, 100 nm.
It is difficult to see how such a structure could package around the linear model proposed for the VWF. Indeed, the cigar-like structure that can often be observed for the packaged VWF is never observed for mucin storage granules, which are always close to spherical in shape. The work of Verdugo et al. (17, 18) on the unpacking and secretion of mucins indicates that upon release in exocytosis, these molecules can unfold themselves with great rapidity. This secretion process, which requires the exchange of calcium with sodium, suggests that there must be both ready access to the changing cations and also an ability for the packaged protein not only to change its ionic composition but also expand the whole granule structure uniformly in time rather than stepwise from the granule surface edge. In considering the mucin as a polymer, Verdugo et al. (17) have suggested that mucins in the granule matrix must be folded in a non-random conformation. Polarized microscopy images of partially hydrated mucus granules demonstrate that even at visible wavelengths, it is possible to observe the signature of nematic crystalline structures in mucus, which reveals the presence of large-scale supramolecular organization (19).
However, the formation and organization of these exceptionally large molecules within the cell and the means and rapidity by which they unpack to form mucus gels is still unclear. MUC5B is a major contributor to normal lung mucus, saliva, and cervical mucus and is known to be an important product of the submucosal glands where it can be readily detected in granular form in large clusters of cells. While isolating MUC5B from saliva, we found that a small proportion of it had only partially unfolded from its granules. This has permitted the EM study of the structure of the MUC5B in its unpacking state and allows an approach to the question of how such enormous molecules are packed and how they may unpack into their linear polymeric form. In particular, the images presented here on the early secretion of a MUC5B mucin provide a new model for how mucins are organized in the granule. This topological model would suggest a simple mechanism for fast mucin unfolding and expansion that would permit the observed quick-swelling kinetics of newly exocytosed mucus granules.
MATERIALS AND METHODS
Isolation of the mucin from saliva.
The intact MUC5B mucin used in this study was isolated from saliva by rate zonal centrifugation using the OptiPrep density gradient. Saliva was collected from an individual healthy male donor under University of North Carolina Institutional Review Board-approved protocol. Saliva was stimulated by chewing on Parafilm and collected into a 50-ml falcon tube on ice. Collected saliva was centrifuged at 3,000 g for 20 min at 4°C. A linear OptiPrep gradient (10–30%) in PBS with protease inhibitors (Roche Diagnostics) was created using gradient maker into 12-ml ultraclear SW-40 centrifuge tubes. The saliva supernatant layered onto the gradient. The gradients were centrifuged for 1 h at 210,000 g at 4°C in the SW-40 swinging bucket rotor. One-milliliter fractions were unloaded from the top and subjected to periodic acid-Schiff (PAS) analysis after slot blotting.
Mass and hydrodynamic radii analysis by laser light scattering.
Fraction 12 from the gradient was chromatographed on a Sephacryl 1000 (15 × 2.5 cm) column and eluted with 0.2 M NaCl at a flow rate of 500 μl/min. The column effluent was passed through an inline Dawn (Wyatt Technologies) enhanced optimal system laser photometer coupled to a Wyatt/Optilab digital signal processing inferometric refractometer to measure light scattering and sample concentration, respectively. Light scattering and refractive index measurements were made continuously, with the light scattering being used only to identify and define the mucin peak and to calculate its molecular weight and radius of gyration. Light scattering measurements were taken continuously at 18 angles between 15° and 151°. The captured data were integrated and analyzed with the Astra software provided with the Dawn.
EM.
The concentration of the mucin pool was adjusted to ∼10 μg/ml and was prepared for EM as described (4). The samples were fixed with 0.6% glutaraldehyde for 4 min at 20°C and mounted on a thin glow-charged, carbon-coated grid for 5 min. To facilitate the mucin adsorption to the supporting film, 2 mM spermidine in TBS (0.15 M NaCl 10 mM Tris, pH 7.5) was added to the final mixture. The samples were then run through washing in water-ethanol as described (4). The samples were shadow cast with tungsten and observed in Tecnai 12 EM at 40 kV. Images were taken at magnifications of 30,000 or 45,000 on Kodak SO163 films and scanned with an Imacon 848 film scanner, and the contrast was optimized and panels were arranged using Adobe Photoshop software.
(Immuno)gold labeling for EM.
Fraction 12 from the gradient, which contains the rapidly moving MUC5B form, was dialyzed against PBS at 4°C for 2 h to remove excess OptiPrep and then incubated overnight at 4°C with a polyclonal rabbit antibody against the NH2-terminal region of MUC5B mucin (13). A replica sample was incubated with rabbit IgG preparation (Vector Laboratories) as a control. The 10-nm gold secondary anti-rabbit antibody (IgG) conjugates were added to the mixtures and incubated for 2 h at 4°C. Glutaraldehyde (0.6%) was added and incubated for 5 min. The mixtures were chromatographed through the Sepharose CL-2B to remove an excess of the primary antibodies and gold secondary antibodies and glutaraldehyde. Fraction 12 was also incubated with 10 nm of colloidal gold for 15 min and then for 5 min with 0.6% glutaraldehyde and subjected to CL-2B chromatography to remove an excess of the gold particles. Mucin-containing fractions, as assessed by light scattering, were analyzed by EM as described above.
RESULTS
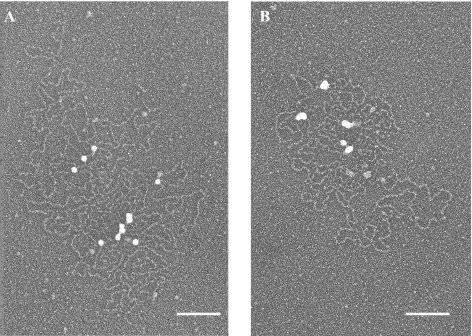
The sedimentation fractionation of the MUC5B is shown in Fig. 2A and where it can be seen as diverse in its mass/size distribution. Our particular focus was a small proportion of very rapidly sedimenting material (fraction 12), and chromatography and physical analysis identifies this material as being between 100 and 200 × 106 g/mol in mass (Fig. 2B). EM suggested it was composed of recently secreted mucins, many of which were still in the process of expansion from their granular form (Figs. 2C and 3A). This may be contrasted with the partially extended form (Figs. 2D and 3B) and the fully linear extended structures of MUC5B (Figs. 2E and 3C) more generally seen in the middle and top fractions, respectively. In their partially expanded form after leaving the granules (Fig. 4A), mucin structures were seen as apparently flexible chains (∼100–200 nm in length) often attached to nodes of ∼10–20 nm in diameter. The number and organization of these nodes suggested that their compaction must account for the granules as seen in goblet cells (mucous cells) (2). Each node is surrounded by a number (4–8 on average) of these flexible chains, some as long as two subunits in length, but most around the length of a single subunit or even less (Fig. 4B). There are 10 or more such nodes in the whole structure. In following the paradigm of the VWF model, it would be anticipated that the flexible chains, clustered around the 10- to 20-nm diameter nodes, are composed of the carbohydrate-rich stretches of the mucins. This view of the situation was tested using colloidal gold particles of 10 nm in size (Fig. 5A). These negatively charged particles have been shown to be repelled from the negatively charged carbohydrate-rich stretches and have in the past been very valuable for distinguishing the presence of the protein-rich as opposed to the highly glycosylated regions in the mucins (9). In Fig. 5A, these gold particles both singly and in tight groups can be observed, attached on the nodes that we take to indicate the presence of clusters of “naked” NH2- and C-OOH terminal domains. A similar experiment employing colloidal gold soaked with appropriate polyclonal anti-antibodies was performed on mucin preparations pretreated with antibodies targeted at the NH2-terminal sequence (Fig. 5B). Again, the colloidal gold is found on the nodes identified at a small number of specific places around the structure.
Fig. 2.
Isolation and mass and size analysis of freshly released MUC5B. A: freshly released intact MUC5B was isolated from saliva using a linear OptiPrep gradient (10–30%) in PBS as detailed in materials and methods. One-milliliter fractions were unloaded from the top of the centrifugation tube and subjected to carbohydrate analysis (periodic acid-Schiff). In this study we focused on the most rapidly sedimenting MUC5B form (III, fraction 12), which displays itself as compact granular form. B: dynamic light scattering data from an analysis of the fraction 12 (III, arrow) eluted from the Sephacryl 1000 (15 × 2.5) chromatography. The mucin was recovered in the void. To gain quantitative measurements of the mucin, the flow through the S1000 column was monitored by nine light scattering detectors and then through the refractive index detector. Data for a single angle at one degree (90°) are shown in the diagram. The average molecular weight and radius of gyration were calculated across the whole mucin peak and gave a value of mol wt 150 × 106 and Rg 150 nm. C–E: electron microscopic analysis of different fractions from an OptiPrep density gradient isolation. The MUC5B was isolated using an OptiPrep gradient in which the most rapidly sedimenting material found at fraction 12 (III) is composed of very recently secreted and only partly expanded MUC5B mucin (C), whereas fractions 7–8 (pool II) are mainly dominated by the form that has proceeded further in the process of expansion from their granular organization (D). The linear fully extended form (E) is the major structure seen in fractions 4–5 (pool I). Scale bars, 100 nm. Images are representative of at least 4 different preparations. See Fig. 3 for more images.
Fig. 3.
More electron microscopy images of different fractions from an OptiPrep density gradient isolation. Representative images of pool III (A), pool II (B), and pool I (C) showing recently secreted compact granular, expanding, and fully expanded linear forms of MUC5B, respectively. Scale bars, 100 nm. Histograms show the distribution of the 3 forms of MUC5B over the pools from the gradient (D).
Fig. 4.
The structural form of an unpacking MUC5B. The samples were isolated and prepared for electron microscopy as described in detail in materials and methods. Here at higher resolution, the MUC5B can be observed organized around nodes (arrows) in partially expanded forms (A). In the enlarged image, the interacting mucin structures were seen as apparently flexible loops typically ∼100–200 nm in length, but sometimes longer, and often attached to the dense compact, spherical core structures of ∼15 nm in diameter (B). The number and organization of these structures suggested that they contain the mucin unglycosylated protein domains (NH2- and/or COOH-terminal regions). Scale bars, 100 nm.
Fig. 5.
Distribution of the protein domains of the compact MUC5B form determined by colloidal gold and immunogold labeling. A: an aliquot from fraction 12 of the OptiPrep gradient was incubated with 10 nm of colloidal gold and subjected to CL2B gel filtration chromatography. An aliquot from the void fraction that contains MUC5B-gold complexes was absorbed onto glow-charged carbon grids. The grids were then rotary shadowed with tungsten and visualized by electron microscopy. The gold (white spheres) binds the dense compact, spherical structures and was completely excluded from glycosylated regions. B: an aliquot from fraction 12 of the OptiPrep gradient was incubated with a polyclonal antibody against a region in the NH2 terminus of MUC5B. After incubating with a secondary antibody conjugated with 10 nm of gold, the MUC5B-immunogold complexes were subjected to CL2B gel filtration chromatography. An aliquot from the void fraction that contains MUC5B-immunogold complexes was mounted onto glow-charged carbon grids, shadow cast with tungsten, and visualized by electron microscopy. Typically, 1–3 immunogold particles (white spheres) bind to most of the globular structures, whereas some of the globs remain unlabeled. Scale bars, 100 nm. Images are representative of 5 independent preparations.
DISCUSSION
The structure and organization of the mucin stored in the granule has remained something of a mystery. We do know from the work of Verdugo (16) that the granule upon release from the cell undergoes a very rapid expansion ranging from less than 20 ms in the slug to 3–6 s in the mammalian goblet cell granule. Work in polymer physics on condensation and decondensation of polymers has established the physical principles for such a process and yielded a theory that accounts for the swelling kinetics of the gel (8, 12). In synthetic polymer gels, the relaxation times of volume expansion are inversely correlated with the second power of the linear dimensions of the gel, reflecting the fact that the diffusion of the network is the rate-limiting parameter. For large linear polymers, such a process may take many hours. However, exocytic swelling of mucins and other secretory matrices is much faster (3, 16). It is the mechanisms underpinning the initial rapidity of this process for the mucins that have remained mysterious.
There are two issues that impact on this problem, the first being the structural organization of the mucin polymer itself, and the second, the driving forces that expand the mucin granule. We know in general that the mucins are highly charged polymers enriched in both carboxyl and sulfate groups on their numerous oligosaccharides. Within the cell, the granules are stored at low pH (∼6.0) in an ionic background dominated by calcium. Once the secretory pore is formed, calcium starts the process of exchanging to sodium. Because the negative charges of the cross-linked gel are fixed (i.e., cannot move out of the matrix), the mucin functions as a Donnan system (11) where counterion replacement can only take place by Na ions entering the mucin granule. Exchange of divalent Ca by monovalent counterion Na causes the gel to swell. The swelling is the result of doubling the number of counterions, two Na for each Ca as required by electroneutrality. Increased counterions inside the gel raise the osmotic pressure, and water molecules move into the gel, causing swelling. However, the ion exchange process is highly nonlinear and follows the general law of polymer gel phase transition. The rapid transition from a condensed to solvated phase in mucin release exhibits the characteristic features of a critical phenomena but also suggests that it is complemented by a high degree of cooperativity among the mucin divalent Ca binding sites (16). As found in the heparin matrix of mast cells (3) (a similarly highly negatively charged polysaccharide molecule), a sodium/calcium ion exchange mechanism might trigger the mucus gel phase transition from condensed to solvated phase and drive the postexocytotic swelling of mucus granules (15, 16).
It is also known that a slight increase of extracellular calcium can drastically slow down swelling of newly released goblet cell granules (17), suggesting that Ca/Na exchange could modulate mucin expansion playing an important role in controlling the rheological properties of mucus (1). However, in a randomly entangled granular mucin, the sole existence of a Donnan drive would not necessarily guarantee its rapid isotropic expansion upon granule release, and it is this fact that supports the necessity of the high mucin organization proposed here. Thus, the focus of this paper concerns the topological supramolecular organization of the mucin within the granule and its behavior upon release.
As discussed previously, an organization for assembly of the VWF proposes extended helices constructed by the interacting domains of the NH2 termini. A similar organization for mucins might have been anticipated. However, the very large size and much higher charge and glycosylation number per unit length of the mucin subunit makes this precise model of protein organization improbable, and a somewhat different model for the packaging and expansion of the mucins may be appropriate.
The newly secreted MUC5B granule form is 100–200 × 106 in molecular weight, as assessed by light scattering, and we know from many other studies that the MUC5B mucin subunits are ∼2–2.5 × 106. This suggests the presence of 50–100 subunits or 25–50 dimers per granular form on average. EM of the structure indicates that this number of dimers is to be organized around 10–15 nodes. These nodes are ∼10–20 nm in diameter and have different-length mucin chains emanating from them, many less than a single subunit in length, 100–200 nm) and a few as long as dimers (Figs. 3B and 4, A and B). Consideration of such a macromolecular structural arrangement suggests an efficient mechanism for the initial expansion of the mucin. Thus, it is proposed that on average four to eight dimers may be organized within and around the nodes that are observed. The data coming from previous EM observations (9) indicate that colloidal gold is collected specifically on the NH2- and COOH-terminal protein domains and excluded from the negatively charged carbohydrate-rich regions. Colloidal gold and immuno-EM (Fig. 5, A and B) experiments again show here the nodes are indeed terminal protein domains of the MUC5B, although we cannot rule out the potential contribution of other globular proteins in the organizations of the nodes.
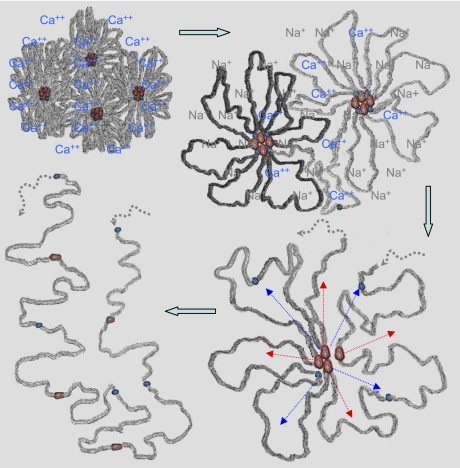
Our data indicate that a granule might contain around 25–50 dimers, and thus we would expect to see a similar number of colloidal gold particles. We do not; it is more like 5–12, i.e., the same as the number of nodes, and thus we conclude that the nodes are largely accounted for by clusters of interacting NH2- and COOH-terminal domains around which carbohydrate-rich protein regions are collected. A diagrammatic representation of this has been given in Fig. 6.
Fig. 6.
Our model of MUC5B organization after granular release. MUC5B is organized around the nodes that are mainly composed of their NH2- (red) and COOH- (blue) terminal protein domains. These nodes are ∼10–20 nm in diameter and have different-length mucin chains (gray) emanating from them. In the granule, mucins are stored as highly compacted by the presence of Ca ions (top left). After the granular release, exchange of divalent Ca2+ by monovalent counterion Na+ causes the mucin chains to expand. The expansion is the result of doubling the number of counterions, 2 Na for each Ca as required by electroneutrality. Increased counterions inside the gel raise the osmotic pressure, and water molecules move into the gel, causing it to expand (top right). This initial change in volume happens rapidly and appears to offer access to the globular NH2- and COOH-terminal protein domains in the nodes, which may be modified and become dissociated, perhaps by a proteolytic process (bottom right), to bring them to their linear conformation (bottom left).
An important question arises from these results: if the mucin expansion is rapid (in seconds), then why are the compact mucin forms still observed? The full answer to this is not trivial and still under investigation. It would appear that the maturation of the mucin has two parts. The first involves a release and rapid expansion of the granule form that requires the exchange of Ca for Na (16). Recent data on the VWF D-domains indicate that the binding of calcium is necessary for their interactions, which are strong and pH dependent (6). This is consistent with the notion that the matrix of secretory granules, including goblet and mast cell granules, undergo phase transition from condensed to solvated phase upon release from the cell. The process is triggered and driven by Na/Ca exchange (3, 11, 16). The second part, the unfolding of the expanded form and the establishment of the linear structure, is not so well understood. It is not clear if this second unfolding step results from an active enzymatic processing or from the dynamics of low energy interactions present in mucus. In our own work, we have discovered that the small amount of the expanded granule form we isolate is stable and appears to have escaped from some active process. We say this because the purified expanded granule form we show here is stable even in 4 M GuHCl (unpublished observation). This is consistent with the possibility that additional bindings other than Ca bonds might be present in the mucin network. There are other factors that must be considered. The linear charge density of individual mucins, involving the modification of the oligosaccharides, may be variable, i.e., the organization and number of the carboxyl and sulfate groups is not clear. Variation in these factors could have a profound effect both on the rate of Ca to Na exchange and subsequent granule expansion. It is thus possible that in our experiments we have identified a subgroup of granular forms that mature in size and structure more slowly. The kinetics and control of these complex potential modifications have not been identified and are under investigation.
Thus, in conclusion, we envisage the initial expansion of the carbohydrate-rich regions around the nodes as being enforced by the exchange of calcium with sodium ions. This is a rapid expansion that changes the dimensions of the granule from ∼350 nm in diameter to 1,000 nm. This initial change in volume happens rapidly and appears to offer access to the terminal globular protein domains in the nodes, which may be modified (perhaps by a proteolytic process), to bring them to their linear conformation (Fig. 6). The end result of such a process is the modification of most of the mucins into predominantly linear sequences of different sizes. This model implies that the rate-limiting parameter for swelling is not the slow reptational diffusivity of the large mucin polymers such as takes place in synthetic polymer gels (15), but rather the rapid access of sodium ions into the carbohydrate-rich network. This rapid exposure of the dominant, highly charged carbohydrate-rich regions from Ca to Na ions dramatically increases the Donnan drive and steers mucin hydration. Thus notwithstanding the ion exchange imparted to the driving force, it is the ordering of the subunits that enables the rapidity of mucin hydration and the concurrent mucin expansion that make mucus a transportable gel.
GRANTS
This work was supported by a gift from an anonymous donor for research targeted to proteomics of cystic fibrosis lung disease (J. K. Sheehan), Cystic Fibrosis Foundation/National Institutes of Health (NIH) Bridge Grant SHEEHA05PO, and NIH Grants HL-084934 (J. K. Sheehan), ES-13773 (J. D. Griffith), GM-31819 (J. D. Griffith), and CA-16086 (J. D. Griffith).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Chin WC, Quesada I, Steed J, Verdugo P. Modeling polyanion-Ca crosslinking in secretory networks: assessment of charge density and bond affinity in polyanionic secretory networks. Macromol Symp 227: 89–96, 2006 [Google Scholar]
- 2.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 70: 487–512, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Fernandez JM, Villalon M, Verdugo P. Reversible condensation of mast cell secretory products in vitro. Biophys J 59: 1022–1027, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith JD, Christiansen G. Electron microscope visualization of chromatin and other DNA-protein complexes. Annu Rev Biophys Bioeng 7: 19–35, 1978 [DOI] [PubMed] [Google Scholar]
- 5.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol 70: 431–457, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Huang RH, Wang Y, Roth R, Yu X, Purvis AR, Heuser JE, Egelman EH, Sadler JE. Assembly of Weibel-Palade body-like tubules from N-terminal domains of von Willebrand factor. Proc Natl Acad Sci USA 105: 482–487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem 67: 395–424, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Schott H. Kinetics of swelling of polymers and their gels. J Pharm Sci 81: 467–470, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Sheehan JK, Carlstedt I. Electron microscopy of cervical-mucus glycoproteins and fragments therefrom. The use of colloidal gold to make visible ‘naked’ protein regions. Biochem J 265: 169–177, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheehan JK, Thornton DJ. Heterogeneity and size distribution of gel-forming mucins. Methods Mol Biol 125: 87–96, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Tam PY, Verdugo P. Control of mucus hydration as a Donnan equilibrium process. Nature 292: 340–342, 1981 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Fillmore DJ. Kinetic of swelling of gel. J Chem Phys 70: 1214–1218, 1979 [Google Scholar]
- 13.Thornton DJ, Gray T, Nettesheim P, Howard M, Koo JS, Sheehan JK. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L1118–L1128, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 70: 459–486, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Verdugo P. Dynamics of biopolymer networks. Polymer Bull 58: 139–143, 2007 [Google Scholar]
- 16.Verdugo P. Goblet cells secretion and mucogenesis. Annu Rev Physiol 52: 157–176, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Verdugo P, Aitken M, Langley L, Villalon MJ. Molecular mechanism of product storage and release in mucin secretion. II. The role of extracellular Ca2+. Biorheology 24: 625–633, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Verdugo P, Deyrup-Olsen I, Aitken M, Villalon M, Johnson D. Molecular mechanism of mucin secretion: I. The role of intragranular charge shielding. J Dent Res 66: 506–508, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Viney C, Huber AE, Verdugo P. Liquid crystalline order in mucus. Macromolecules 26: 852–855, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol 23: 101–112, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]