Abstract
NF-κB activation in the airway epithelium has been established as a critical pathway in ovalbumin (Ova)-induced airway inflammation in BALB/c mice (Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. J Immunol 173: 7003–7009, 2004). BALB/c mice are susceptible to the development of allergic airway disease, whereas other strains of mice, such as C57BL/6, are considered more resistant. The goal of the present study was to determine the proximal signals required for NF-κB activation in the airway epithelium in allergic airway disease and to unravel whether these signals are strain-dependent. Our previous studies, conducted in the BALB/c mouse background, demonstrated that transgenic mice expressing a dominant-negative version of IκBα in the airway epithelium (CC10-IκBαSR) were protected from Ova-induced inflammation. In contrast to these earlier observations, we demonstrate here that CC10-IκBαSR transgenic mice on the C57BL/6 background were not protected from Ova-induced allergic airway inflammation. Consistent with this finding, Ova-induced nuclear localization of the RelA subunit of NF-κB was not observed in C57BL/6 mice, in contrast to the marked nuclear presence of RelA in BALB/c mice. Evaluation of cytokine profiles in bronchoalveolar lavage demonstrated elevated expression of TNF-α in BALB/c mice compared with C57BL/6 mice after an acute challenge with Ova. Finally, neutralization of TNF-α by a blocking antibody prevented nuclear localization of RelA in BALB/c mice after Ova challenge. These data suggest that the mechanism of response of the airway epithelium of immunized C57BL/6 mice to antigen challenge is fundamentally different from that of immunized BALB/c mice and highlight the potential importance of TNF-α in regulating epithelial NF-κB activation in allergic airway disease.
Keywords: asthma, BALB/c, ovalbumin, tumor necrosis factor-α
asthma is characterized by airway inflammation, elevated levels of Th2 cytokines and antigen-specific IgE, mucus production, and airway hyperresponsiveness (AHR) (11, 53). The transcription factor NF-κB is believed to play a cardinal role in allergic airway disease through its ability to transcriptionally induce the expression of proinflammatory genes (17). In this regard, NF-κB regulates several cytokines and chemokines relevant to asthma and other inflammatory lung diseases, including TNF-α, keratinocyte-derived chemoattractant (KC), macrophage inflammatory proteins, and numerous interleukins (13). NF-κB is activated in the airways of asthmatic patients, suggesting a role in the development of the human disease (16, 45, 49). We previously reported rapid and selective activation of NF-κB within airway epithelium in the ovalbumin (Ova) model of allergic airway disease (37). Transgenic and conditional knockout approaches to prevent activation of the NF-κB pathway specifically within epithelial cells highlighted the functional relevance of NF-κB activation within airway epithelium in the pathogenesis of allergic airway disease (8, 35).
Numerous studies have shown that the phenotype and severity of antigen-induced airway disease in mice are strain-dependent, findings that have been mapped to specific chromosomal regions (6). Ova challenge induces elevated levels of IgE and more pronounced AHR in BALB/c than C57BL/6 mice (7, 47). Conversely, C57BL/6 mice accumulate more eosinophils in the bronchoalveolar lavage (BAL) than BALB/c mice (47, 52), and the localization of these inflammatory cells is histologically different between these two strains. Specifically, in BALB/c mice, eosinophils surround the airways; in C57BL/6 mice, these cells are more diffusely distributed throughout the lung (47). These data suggest that cytokine or chemokine gradients established after Ova sensitization and challenge may be different between these mouse strains. Activation of NF-κB and expression of NF-κB-dependent chemokines were induced in airway epithelium of BALB/c mice subjected to Ova sensitization and challenge, in association with elevated Th2 cytokine levels and AHR (37). Because of the disparate inflammatory cell profiles of C57BL/6 and BALB/c mice in the Ova model, we speculated that these strains may elicit different patterns of NF-κB activation after Ova sensitization and challenge.
The goal of the present study was to compare the functional importance of NF-κB activation within airway epithelium in BALB/c and C57BL/6 mice to unravel whether the extent or locale of NF-κB activation could explain the differences in intensity of allergic airway disease between these strains. We also sought to identify cytokines responsible for the rapid activation of NF-κB in airway epithelium in mice with allergic airway disease by directly comparing the levels of inflammatory cytokines in BAL from C57BL/6 and BALB/c mice. Our findings suggest that differences in levels of TNF-α in BAL from C57BL/6 and BALB/c mice correspond with differences in patterns of NF-κB activation between these strains and highlight the importance of this cytokine in the orchestration of airway NF-κB activation.
METHODS
Animals and reagents.
Female 2- to 4-mo-old BALB/c and C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). CC10-IκBαSR mice were developed as previously reported and backcrossed >10 generations onto the C57BL/6 background (36). All studies using CC10-IκBαSR transgenic animals included wild-type (WT) littermate controls. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Vermont. Antibody to TNF-α and nonspecific IgG were purchased from R & D Systems (Minneapolis, MN).
Model of allergic airway disease.
Allergic airway inflammation was induced as previously reported (35). Briefly, mice were injected intraperitoneally with 40 μg of Ova in the adjuvant aluminum hydroxide (Alum; Pierce Chemical, Rockford, IL) on days 1 and 7 to induce sensitization. Sham-sensitized mice received Alum alone. Mice were then exposed to an aerosolized 1% Ova solution in sterile PBS for 30 min on days 14–16, and lung tissues were harvested on day 18. Alternatively, mice were challenged once for 30 min with 1% Ova, and lung tissues were harvested 2 h later. Airway inflammation and cytokine production were assessed by BAL with 1 ml of PBS.
TNF-α-neutralizing studies.
TNF-α-neutralizing antibody (25 μg in 25 μl) in sterile PBS was delivered intranasally on the day before Ova challenge and 1 h before Ova aerosol challenge. Mice received two doses of TNF-α antibody. Control mice received nonspecific IgG suspended in PBS or PBS vehicle alone.
Respiratory mechanics.
AHR was measured using the forced oscillation technique, as previously described (50). Briefly, mice were anesthetized with pentobarbital sodium (90 mg/kg ip). A tracheotomy was then performed using a modified 18-gauge intravenous adapter, and mice were attached to a computer-controlled piston ventilator (flexiVent, SCIREQ, Montreal, QC, Canada). Mice were ventilated with a tidal volume of 0.2 ml and 3-cmH2O positive end-expiratory pressure. Multiple linear regression was used to fit measured pressure and volume in each individual mouse to the model of linear motion of the lung (3, 15). Model fits that resulted in a coefficient of determination <0.85 were excluded.
Determination of AHR.
Dose-response curves to inhaled methacholine (MCh) were determined as follows. MCh was diluted with sterile PBS to concentrations of 3.125, 12.5, and 50 mg/ml and delivered sequentially via a nebulizer (Mystique, Airsep, Buffalo, NY). To expose the animal to aerosol, we diverted the input air line through the nebulizer, increased the tidal volume to 1.0 ml, and slowed the respiratory rate to provide 20 large breaths of aerosol. After each dose, the response was measured by application of a 2-s perturbation every 10 s for a total of 3 min. Airway resistance, tissue resistance, and tissue elastance were measured. The peak response for each variable was determined, and the percent change from baseline was calculated.
Lung histology and immunofluorescence.
Lungs were inflated and fixed with 4% paraformaldehyde to a constant volume of 0.5 ml and then embedded in paraffin. Blocks were cut into 5-μm sections and stained with hematoxylin and eosin for evaluation of histopathology, and mucus production was determined by periodic acid-Schiff staining. For quantification of mucus metaplasia, slides were scored using a scale of 0–3 (0 representing no reactivity and 3 being the highest intensity) for airway periodic acid-Schiff reactivity by two independent, blinded observers. Slides were scored twice by each investigator, and the cumulative score from each mouse was averaged according to treatment group. For immunofluoresence, lungs were inflated with optimal cutting temperature compound in PBS (50% optimal cutting temperature compound by volume) and snap frozen in methyl butane cooled with liquid N2. Immunolocalization for RelA was performed on frozen lung sections with use of an RelA antibody (SC-372, Santa Cruz Biotechnology, Santa Cruz, CA) and Alexa 488-conjugated secondary antibody (Molecular Probes, Eugene, OR) (37). Images were obtained by confocal microscopy (Bio-Rad MRC 1024ES, Carl Zeiss Microimaging, Thornwood, NY).
LPS stimulation.
Escherichia coli 0111:B4 LPS (1 μg; Sigma, St. Louis, MO) in a total volume of 50 μl or an equivalent volume of saline vehicle was administered to C57BL/6 mice via oropharyngeal aspiration. After 2 h, mice were euthanized, and RNeasy columns (Qiagen, Valencia, CA) were used to collect lung tissue for isolation of total RNA.
Cytokine analyses in BAL.
BAL samples from Ova-exposed mice were analyzed using the Bioplex system and a 20-Plex cytokine array (Bio-Rad Laboratories, Hercules, CA).
Quantification of transgene expression in whole lung homogenates.
Standard protocols were used to isolate total RNA from lung tissue. Total RNA was then treated with DNase (Qiagen, Valencia, CA) to remove contaminant DNA carryover. The Maloney murine leukemia virus reverse transcriptase reaction (Promega, Madison, WI) was used to synthesize cDNA from isolated RNA. cDNA samples were analyzed by quantitative and nonquantitative PCR using primers specific for IκBα (36). β-Actin expression was used as a housekeeping gene control. Clca3 and CCL20 expression were determined by TaqMan quantitative PCR using an Assay on Demand for Clca3 or CCL20 and hypoxanthine-guanine phosphoribosyltransferase or GAPDH as housekeeping gene controls (Applied Biosystems, Foster City, CA).
Protein nuclear extraction and EMSA.
For preparation of nuclear extracts from whole lung homogenates (100 mg), the tissue was pulverized in hypotonic buffer and then incubated with 10% NP-40. Nuclei were pelleted and suspended in hypertonic buffer for extraction of nuclear proteins (20). EMSA was then performed with oligonucleotides containing NF-κB DNA-binding motifs (Promega).
Primary tracheal epithelial cell culture.
Mouse tracheal epithelial cell (MTEC) cultures were isolated according to previously published methods (54). Briefly, tracheae were isolated, filled with 0.1% protease type 14 in minimum essential medium, and incubated overnight at 37°C. On the following day, tracheae were flushed, and MTEC were propagated on rat tail collagen I gel (BD Biosciences, San Jose, CA)-coated tissue culture flasks in DMEM-F-12 medium containing 20 ng/ml cholera toxin (List Biologicals Laboratories, Campbell, CA), 4 μg/ml insulin, 5 μg/ml transferrin, 5 μg/ml bovine pituitary extract (Invitrogen, Carlsbad, CA), 10 ng/ml epidermal growth factor (EMD Biosciences, San Diego, CA), 100 nM dexamethasone, 2 mM l-glutamine, and 50 U of penicillin and 50 μg of streptomycin (Pen/Strep, Invitrogen). For experiments, MTEC were plated on Transwell plates (12-mm well diameter, 0.4-μm pore size; Corning, Corning, NY) that were coated with rat tail collagen (50 μg/ml) and grown to confluence before initiation of experiments. TNF-α was then added to the basolateral surface of the MTEC as indicated.
IκB kinase activity assay.
IκB kinase (IKK) activity was assessed by an in vitro kinase assay, as described previously (37). Briefly, anti-IKKγ antibody (Santa Cruz Biotechnology) was used to immunoprecipitate IKK from whole MTEC lysates. Immunoprecipitated IKK was then incubated with GST-IκBα fusion substrate protein in the presence of [32P]ATP for 30 min at 30°C. Phosphorylated GST-IκBα was visualized by SDS-PAGE and autoradiography. A control gel was run in parallel, and equal loading was assessed by Western blotting for β-actin.
Statistical analyses.
Data were subjected to one-way ANOVA followed by Tukey's test for multiple comparisons. Comparisons of two means were conducted by unpaired Student's t-test with the assumption of unequal variance. Analyses with resultant P < 0.05 were determined significant (Excel, Microsoft, Redmond, WA). Each experiment was repeated at least once and showed similar results. Data of individual experiments are presented as means ± SE.
RESULTS
NF-κB activation in airway epithelium is not required for antigen-induced inflammation in C57BL/6 mice.
To examine the role of NF-κB activation in airway epithelium in the pathogenesis of allergic airway disease in C57BL/6 mice, we used CC10-IκBαSR mice that had previously been generated in our laboratory; this strain specifically inhibits the NF-κB pathway in airway epithelium. On a BALB/c background, CC10-IκBαSR mice demonstrated substantial attenuation of Ova-induced allergic inflammation, but not AHR (35). These mice were backcrossed onto the C57BL/6 background for >10 generations. In contrast to BALB/c CC10-IκBαSR mice, C57BL/6 CC10-IκBαSR transgenic mice elicited inflammatory responses similar to those elicited by WT transgene-negative littermates in response to sensitization and challenge with Ova. WT and CC10-IκBαSR mice on the C57BL/6 background accumulated similar numbers of inflammatory cells in the BAL fluid, 65.6 ± 12.0 × 104 and 72.9 ± 14.4 × 104 cells/ml, respectively, which reflected similar increases in eosinophils and lymphocytes (Fig. 1A). Evaluation of histopathology also revealed similar cellular infiltrates in C57BL/6 WT and CC10-IκBαSR mice (Fig. 1B). These data suggest that airway inflammation induced by acute challenge with Ova in sensitized C57BL/6 mice occurred independently of NF-κB activation within the bronchiolar epithelium, contrary to our previous findings in BALB/c mice expressing the CC10-IκBαSR transgene (35).
Fig. 1.
NF-κB activation in airway epithelium of C57BL/6 mice is not required for airway inflammation. Wild-type (WT, transgene-negative littermates) and CC10-IκBαSR mice in the C57BL/6 background were immunized with ovalbumin (Ova) or mock-immunized [aluminum hydroxide (Alum)] and challenged with aerosolized Ova for 30 min/day for 3 consecutive days. Lungs were harvested 48 h later, and inflammation was assessed by enumeration of total cells and differentials in bronchoalveolar lavage (BAL) fluid (A) and analysis of histopathology in lung sections stained with hematoxylin and eosin (B). Data are representative of 7 mice in each of the Ova/Ova groups and 5 mice in each of the Alum/Ova groups.
CC10-IκBαSR transgene is expressed in C57BL/6 CC10-IκBαSR mice.
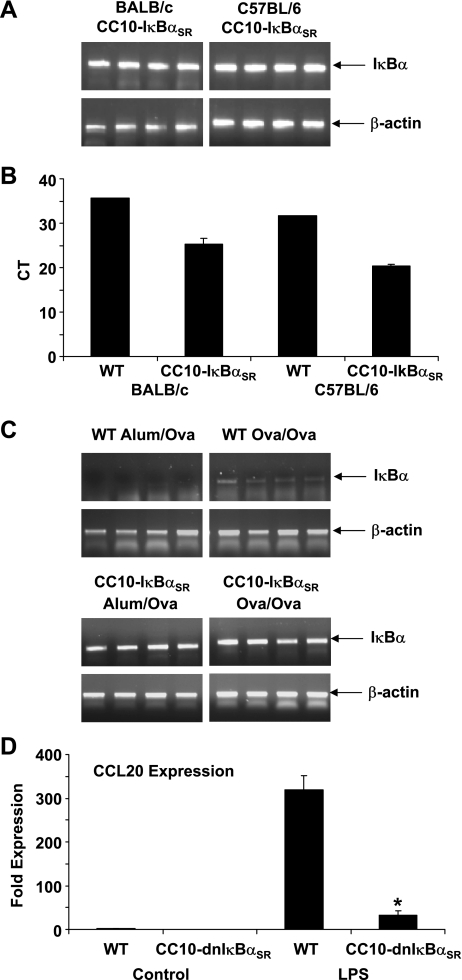
It is possible that the lack of an impact of inflammation on CC10-IκBαSR transgenic mice in the C57BL/6 background may be due to differences in expression of the IκBαSR transgene that could have occurred after backcrossing of the mice onto the C57BL/6 background. We therefore directly compared expression of IκBα in homogenized lung tissues of CC10-IκBαSR transgenic mice in the C57BL/6 and BALB/c backgrounds by traditional and quantitative PCR. We found that expression of total IκBα mRNA in lung homogenates was similar in CC10-IκBαSR mice in C57BL/6 and BALB/c genetic backgrounds (Fig. 2, A and B). Transgenic mice displayed increased levels of total IκBα compared with WT littermates, indicative of enhanced CC10-IκBαSR expression in both background strains. Furthermore, levels of IκBα remained elevated in CC10-IκBαSR mice compared with WT mice subjected to sensitization and challenge with Ova (Fig. 2C). These data strongly suggest that the lack of an impact of the CC10-IκBαSR transgene in the C57BL/6 background on Ova-induced inflammation, in contrast to previous observations in BALB/c mice, is not due to differences in transgene expression levels in the different genetic backgrounds.
Fig. 2.
CC10-IκBαSR mice on the C57BL/6 background express elevated IκBα in lung tissue before and after sensitization and challenge with Ova. A: RT-PCR analysis of IκBα mRNA expression in homogenized lung tissue from CC10-IκBαSR transgenic mice on the C57BL/6 and BALB/c background. B: quantitative PCR measurement of CC10-IκBαSR transgene expression in WT and transgenic BALB/c and C57BL/6 mice. Data are expressed as number of PCR cycles to threshold (CT) for 1 WT mouse compared with 4 transgenic mice of each strain. A decrease in CT indicates more abundant levels of gene expression. CT scale is expressed in log2, indicating that a CT change of 1 is equivalent to a 2-fold change in expression. C: RT-PCR analysis of IκBα expression in homogenized lung tissue from control (Alum/Ova) or Ova-sensitized and challenged (Ova/Ova) WT and CC10-IκBαSR mice on the C57BL/6 background. Samples were run on the same gels, and images were cropped and reassembled for consistency of presentation. Expression of β-actin, a housekeeping gene, was assessed as a control. Data are representative of 7 mice in each of the Ova/Ova groups and 5 mice in each of the Alum/Ova groups. Different lanes represent results from individual mice. D: LPS-induced expression of CCL20 in whole lung homogenates. WT and transgenic mice were instilled with LPS for 2 h, and whole lung RNA was analyzed for CCL20 expression. Data are representative of 3 mice in each of the groups. *P < 0.05 (by ANOVA).
To confirm that the CC10-IκBαSR transgene is functional in the C57BL/6 background, we challenged mice with the known NF-κB activator LPS. LPS rapidly induced expression of the chemokine CCL20 in WT mice (Fig. 2D). CC10-IκBαSR transgenic mice displayed attenuated induction of CCL20 upon LPS stimulation, demonstrating the functional impact of the transgene of NF-κB activation. These data further demonstrate that the CC10-IκBαSR transgene was expressed and was functional in mice on the C57BL/6 background.
Antigen-induced mucus metaplasia and AHR are partially regulated by NF-κB activation in airway epithelium.
Since constitutive NF-κB activity may play a role in epithelial cell biology (38), we examined whether additional components of allergic airway disease were affected after CC10-IκBαSR transgene-mediated inhibition of constitutive NF-κB activity in airway epithelium of C57BL/6 mice. Another hallmark of antigen-induced airway disease in mice is mucus metaplasia. As shown in Fig. 3A, CC10-IκBαSR mice in the C57BL/6 background developed somewhat less mucus metaplasia after Ova challenge than WT mice. Expression of the mucus-associated gene Clca3 (Gob5) was also significantly attenuated in CC10-IκBαSR mice compared with WT mice subjected to sensitization and challenge with Ova (Fig. 3B). These findings are consistent with our previous observations in BALB/c mice expressing the CC10-IκBαSR transgene (35).
Fig. 3.
CC10-IκBαSR expression moderately attenuates mucus metaplasia and enhances airway hyperresponsiveness (AHR) in C57BL/6 mice. A: mucus metaplasia in periodic acid-Schiff-stained airway epithelium from CC10-IκBαSR-expressing and WT mice sensitized and challenged with Ova (Ova/Ova). Alum/Ova represent control (mock-sensitized) groups (Alum/Ova). *P < 0.05 (by ANOVA). B: expression of Clca3 (Gob5) is suppressed in CC10-IκBαSR mice compared with WT controls. RNA from whole lung homogenates of Ova-sensitized and challenged mice (Ova/Ova) or mock-sensitized mice (Alum/Ova) was analyzed by quantitative RT-PCR, and results were normalized to the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase. Data are expressed as fold increases in expression compared with WT Alum/Ova controls. *P < 0.05 (by ANOVA). C: WT or CC10-IκBαSR transgenic mice on the C57BL/6 background were mock-sensitized and challenged with Ova (Alum/Ova) or sensitized and exposed to aerosolized Ova (Ova/Ova). At 48 h after the 3rd Ova challenge, mice were ventilated and challenged with 3.125, 12.5, or 50 mg/ml methacholine (MCh). AHR was assessed by measurement of oscillatory mechanics, and data were fit to the constant-phase model. Airway resistance (a), tissue resistance (b), and tissue elastance (c) are expressed as percent increases from baseline. *P < 0.05 (by ANOVA) vs. WT Ova/Ova groups. Data were obtained from 7 mice in each of the Ova/Ova groups and 5 mice in each of the Alum/Ova groups.
Since AHR in response to antigen is well known to be strain-dependent (47), we next evaluated Ova-induced AHR in CC10-IκBαSR mice in the C57BL/6 background. In WT mice, immunization and challenge with Ova resulted in marginal increases in all parameters of AHR, consistent with the well-known refractory nature of C57BL/6 mice to induce AHR (Fig. 3C). Unexpectedly, central airway resistance and tissue elastance induced by Ova were significantly enhanced in CC10-IκBαSR mice compared with WT mice at two doses of MCh (Fig. 3C), whereas no differences in tissue resistance were observed between WT and CC10-IκBαSR mice on the C57BL/6 background. In contrast, it was previously shown that expression of the CC10-IκBαSR transgene did not affect Ova-induced AHR in BALB/c mice (35). In aggregate, these findings suggest that constitutive activity of NF-κB in airway epithelium of C57BL/6 mice may be partially required to promote mucus metaplasia and attenuate AHR.
NF-κB activation in airway epithelium is not observed in C57BL/6 mice subjected to sensitization and challenge with Ova.
We previously reported that activation of NF-κB occurred rapidly after a single challenge with Ova and was predominantly localized to airway epithelium of sensitized BALB/c mice (37). On the basis of the observation that the localization of inflammatory cells is different between BALB/c and C57BL/6 mice (47) and the finding that inhibition of NF-κB in airway epithelium of C57BL/6 mice did not impact recruitment of inflammatory cells, we compared the patterns of NF-κB activation in response to Ova in C57BL/6 and BALB/c mice. Ova-sensitized BALB/c mice displayed marked nuclear RelA accumulation in airway epithelium 2 h after a single challenge with Ova, in agreement with our previous observations (37). In contrast, no nuclear localization of RelA was apparent in C57BL/6 mice subjected to the same regimen (Fig. 4A), and evaluation of additional time points that extended to 48 h after three challenges also did not reveal evidence of nuclear localization of RelA in bronchiolar epithelium of C56BL/6 mice (data not shown). Interestingly, assessment of activation of NF-κB in homogenized lung tissue by EMSA demonstrated that NF-κB DNA binding increased in response to Ova to a similar extent in both strains of mice (Fig. 4B). These data suggest that C57BL/6 mice, compared with BALB/c mice, are not refractory in terms of NF-κB activation in response to antigen but that the locale of NF-κB activation in response to Ova in the lungs differs between these mouse strains.
Fig. 4.
C57BL/6 mice fail to activate NF-κB in airway epithelium early following Ova challenge. A: immunized BALB/c and C57BL/6 mice were challenged with aerosolized Ova once for 30 min, and lungs were harvested 2 h later. Localization of RelA was evaluated via immunofluoresence and confocal microscopy [nuclear SYTOX green (green), RelA (red), and overlap (yellow)]. Data are representative of 4 mice per group and evaluations of multiple images per mouse. Magnification ×200. B: NF-κB DNA-binding activity by EMSA in whole lung homogenates from immunized BALB/c and C57BL/6 mice challenged with aerosolized Ova for 30 min/day for 3 consecutive days and harvested 48 h later. NF-κB-labeled region depicts electrophoretic mobility shift due to RelA and p50 DNA binding. NS indicates nonspecific DNA binding. Each lane represents an independent mouse.
C57BL/6 mice produce suppressed levels of TNF-α compared with BALB/c mice.
The lack of NF-κB activation in airway epithelium of C57BL/6 mice compared with BALB/c mice suggests that levels of proinflammatory cytokines that stimulate NF-κB activation in airways may be different between C57BL/6 and BALB/c mice. To identify potential differences in cytokine levels, we acutely challenged Ova-sensitized C57BL/6 and BALB/c mice with Ova for 30 min and, 2 h later, assessed cytokine levels in BAL fluid. We found significantly higher levels of TNF-α in BAL fluid from BALB/c than C57BL/6 mice (Table 1). In addition, levels of the NF-κB-dependent chemokines keratinocyte-derived chemoattractant and macrophage inflammatory protein-1α were also markedly higher in BAL fluid from BALB/c than C57BL/6 mice. The majority of cytokines measured were similar in C57BL/6 and BALB/c mice, including Th2 cytokines (IL-4 and IL-13) and the eosinophil chemokine eotaxin. These data suggest that variations in TNF-α levels may potentially explain differences in epithelial NF-κB activation between C57BL/6 and BALB/c mice.
Table 1.
Cytokine profiles in BAL fluid of Ova-immunized and challenged C57BL/6 and BALB/c mice
| Mice |
||
|---|---|---|
| Cytokine | BALB/c | C57BL/6 |
| IL-1α | 8,201.5±763.2 | 14,913.0±2,525.5* |
| IL-1β | 3.0±1.9 | 1.6±0.7 |
| IL-2 | 9.9±2.4 | 8.3±0.5 |
| IL-3 | ND | ND |
| IL-4 | 0.9±0.2 | 1.2±0.2 |
| IL-5 | ND | ND |
| IL-6 | 352.5±45.9 | 245.6±30.7 |
| IL-9 | 78.7±7.2 | 76.0±15.0 |
| IL-10 | ND | ND |
| IL-12 p40 | 4.7±0.7 | 26.8±2.4* |
| IL-12 p70 | 5.0±3.2 | 4.3±1.1 |
| IL-13 | 23.4±10.0 | 12.1±7.1 |
| IL-17 | ND | ND |
| TNF-α | 862.8±112.5 | 155.3±76.5* |
| RANTES | 7.3±3.5 | ND |
| MIP-1α | 53.3±11.0 | 7.5±3.9* |
| MIP-1β | 18.1±6.4 | 11.9±3.5 |
| KC | 4,059.8±312.5 | 2,391.6±108.7* |
| G-CSF | 13.5±2.2 | 7.5±2.7 |
| Eotaxin | 931.1±304.7 | 1,070.3±270.7 |
Values (means ± SE of 5 mice/group) are expressed in pg/ml. Ovalbumin (Ova)-sensitized BALB/c and C57BL/6 mice were challenged once with Ova for 30 min and harvested 2 h later for evaluation of bronchoalveolar lavage (BAL) cytokines by Bioplex analysis. RANTES, regulated on activation, normal T cell expressed, and presumably secreted; MIP, macrophage inflammatory protein; KC, keratinocyte-derived chemoattractant; G-CSF, granulocyte colony-stimulating factor; ND, not detected.
P < 0.05 vs. BALB/c (by ANOVA).
Equivalent activation of IKK by TNF-α in MTEC derived from BALB/c or C57BL/6 mice.
To determine whether intrinsic differences exist in the ability of epithelial cells derived from C57BL/6 or BALB/c mice to activate the NF-κB pathway, we isolated MTEC from C57BL/6 and BALB/c mice and used an in vitro kinase assay to analyze the activity of IKK. Since TNF-α is a well-known activator of NF-κB and was shown to be present in different quantities in the BAL fluid from BALB/c and C57BL/6 mice, we stimulated MTEC with TNF-α to evaluate IKK activity. As shown in Fig. 5, MTEC derived from C57BL/6 or BALB/c mice were similarly sensitive to TNF-α with the induction of IKK. These findings suggest that MTEC from C57BL/6 mice are normally responsive to an NF-κB-inducing agonist and that lack of NF-κB activation in airway epithelia from C57BL/6 mice in response to an acute challenge with Ova is likely linked to lower levels of NF-κB-inducing stimuli in airways of BALB/c mice.
Fig. 5.
Primary mouse tracheal epithelial cells (MTEC) derived from BALB/c and C57BL/6 mice activate the NF-κB pathway similarly in vitro. MTEC cultures were established in parallel from BALB/c and C57BL/6 mice. Confluent MTEC on Transwell inserts were treated with TNF-α (50 ng/ml) for 0–60 min. IκB kinase (IKK) activity was determined by an in vitro kinase assay. GST-IκBα indicates phosphorylated IκBα substrate. Western blotting for β-actin was performed on the same cell lysates run on parallel gels as a loading control.
Neutralization of TNF-α attenuated airway epithelial NF-κB activation in BALB/c mice.
On the basis of our findings that levels of TNF-α were high in airways of BALB/c mice (Table 1), we assessed the functional importance of TNF-α in the acute activation of NF-κB induced by Ova in airway epithelium of BALB/c mice. We instilled a TNF-α-neutralizing antibody or control IgG into airways of Ova-sensitized mice before challenge with Ova. We found that sensitization and challenge with Ova led to expected increased nuclear localization of RelA in airway epithelium of mice that received PBS or nonspecific IgG (Fig. 6). In contrast, administration of TNF-α-neutralizing antibody attenuated nuclear localization of RelA to control levels. These data strongly suggest that TNF-α plays a causal role in the rapid activation of NF-κB in airway epithelium of sensitized BALB/c mice subjected to a single challenge with Ova.
Fig. 6.
Role of TNF-α in rapid activation of NF-κB in airway epithelium of BALB/c mice after Ova sensitization and challenge. Ova-immunized BALB/c mice were treated with TNF-α-neutralizing antibody (anti-TNF-α), control nonspecific IgG (control IgG), or vehicle control (n = 4 mice/group). Mice were then challenged with aerosolized Ova for 30 min and harvested 2 h later. Frozen lung sections were assessed for nuclear localization of RelA by confocal laser scanning microscopy [nuclear SYTOX green (green), RelA (red), and overlap (yellow)]. Results are representative of 5 images collected, on average, per group. Magnification ×200.
DISCUSSION
NF-κB activation within the airway epithelium has emerged as an important event that orchestrates inflammatory responses in the lungs. Several studies in mice have confirmed that airway epithelial NF-κB activation is required for inflammatory responses to Ova and LPS (8, 35, 36, 44). In the present study, we demonstrate that the requirement for activation of NF-κB within airway epithelium in regulating airway inflammation in the acute Ova model of allergic airway disease is strain-dependent. Contrary to our previous observations in BALB/c mice (35), the CC10-IκBαSR transgene did not dampen Ova-induced inflammation in C57BL/6 mice, consistent with the lack of apparent nuclear translocation of RelA in airway epithelium. However, EMSA of NF-κB in lung homogenates demonstrated comparable increases in DNA-binding activity of NF-κB in response to Ova in both strains, demonstrating that NF-κB activation occurred in C57BL/6 mice but the location of activation in C57BL/6 mice was different from that in BALB/c mice. The differences in the locales of NF-κB activation between C57BL/6 and BALB/c mice may explain the differences in patterns of inflammation in these strains of mice. Although Ova-induced airway inflammation in BALB/c mice is characterized by intense peribronchial inflammation (35, 47), inflammation appears to be more diffuse in C57BL/6 mice (47). These and other previous observations suggest that the epithelium in BALB/c mice secretes NF-κB-dependent inflammatory chemokines (37) and that this response may be attenuated in C57BL/6 mice. These findings are potentially significant, because they correlate with the well-known differences in intensity of allergic airway disease in mice from these different genetic backgrounds.
TNF-α is perhaps the best-studied agonist of the NF-κB pathway and TNF-α signals through binding of its receptors, TNFRI and TNFRII, stimulating IKK, leading to the subsequent phosphorylation and degradation of IκBα, followed by nuclear retention of NF-κB (RelA/p50) and enhanced transcriptional activation of NF-κB-regulated genes (14, 17). The marked differences in the levels of TNF-α in BAL fluid between mouse strains in response to antigen challenge strongly suggest an involvement of TNF-α in the rapid activation of epithelial NF-κB in BALB/c mice. Indeed, neutralization of TNF-α activity attenuated Ova-induced nuclear localization of RelA in airway epithelia of these mice. Our results showing that MTEC from BALB/c and C57BL/6 mice responded similarly to TNF-α in terms of activation of the NF-κB pathway, furthermore, demonstrate that epithelial cells from both strains of mice are equally responsive to TNF-α. These results imply that differences in epithelial NF-κB activation in vivo between C57BL/6 and BALB/c mice may be due to different levels of TNF-α ligand, and not to impaired responsiveness to TNF-α. The importance of TNF-α in regulating epithelial NF-κB activation in the acute Ova model of allergic airway disease in BALB/c mice illuminated in this study was previously unknown.
We did not elucidate the mechanism responsible for rapid increases in TNF-α levels in BAL in Ova-sensitized BALB/c mice after a single challenge with Ova. It is tempting to speculate that rapid degranulation of mast cells was involved because of the timing of increases of TNF-α in BAL, which occurred quite rapidly in relation to new protein synthesis. It is likely that preformed TNF-α was released from mast cells in response to antigen, which is consistent with the rapid increases of TNF-α in BAL. In support of this speculation, a recent study documented a role of mast cell-derived TNF-α in inflammation and AHR in the Ova model of allergic airway disease in mice (31).
In addition to the impact of TNF-α on epithelial NF-κB activation, it is possible that the increased levels of TNF-α in airways of BALB/c mice explain the greater magnitude of AHR in response to Ova than in C57BL/6 mice (47). TNF-α is known to promote smooth muscle sensitization and contractility (24, 30, 34, 42), and elevated levels of TNF-α in BALB/c mice could directly contribute to enhanced AHR in those mice. In support of this notion, TNFRI knockout mice on the C57BL/6 background failed to develop AHR after Ova challenge (23), and transgenic mice on the mixed C57BL/6 129/Sv background that overexpress TNF-α in alveolar epithelial cells showed increases in baseline lung mechanics (tissue resistance) in the absence of antigen sensitization and challenge (29). In contrast, deletion of TNFRII on the C57BL/6 background had no impact on the development of AHR (23), and knockout of both TNFRI and TNFRII on the mixed C57BL/6 129/Sv background resulted in elevated AHR compared with WT mice (41). Another study reported a protective role for TNF-α overexpression on the mixed C57BL/6 129/Sv background in alveolar epithelial cells against Ova-induced AHR (22). The reasons for these apparent discrepancies in the functional importance of TNF-α and TNFR in AHR are unclear but are potentially explained by the different methodologies used to evaluate respiratory mechanics, differences in the effect of location or production of TNF-α on the outcome of TNF-α signaling in a complex cytokine environment, and differences in mouse strains.
Another potential implication of differential production of TNF-α by C57BL/6 and BALB/c mice with regard to AHR would be regulation of regulatory T (Treg) cells. Treg cells are known to inhibit AHR in mice via induction of transforming growth factor-β1 (9, 21), which has also been shown to directly suppress AHR (1). TNF-α has recently been shown to inhibit expression of the Treg cell transcription factor Foxp3 and to attenuate the inflammatory suppressive capacity of Treg cells (46, 51). Furthermore, TNF-α inhibition was shown to enhance expression of Foxp3 in Treg cells (27, 48). In our model, elevated levels of TNF-α in BALB/c mice would perhaps decrease Treg cell function and lead to enhanced AHR compared with C57BL/6 mice. In humans, TNF-α polymorphisms that lead to elevated TNF-α levels have been related to decreased Treg cell numbers (28), and anti-TNF-α therapy was shown to increase Treg cell levels in asthma and Crohn's disease (27, 39). Contrary to this hypothesis, numbers of Treg cells were decreased in the thymus and lymphoid tissue of C57BL/6 mice compared with BALB/c mice (10). However, the potential differences in the number and function of Treg cells in the lungs of various mouse strains have yet to be clarified.
A role of TNF-α/NF-κB signaling in the pathogenesis of human asthma is emerging. Patients with refractory asthma were reported to have increased expression of membrane-bound TNF-α, TNFRI, and TNF-α-converting enzyme by peripheral-blood monocytes, whereas treatment with the soluble TNF-α receptor etanercept was reported to have a beneficial effect on airflow limitations and quality of life (4). In a separate study, neutralization of TNF-α with the monoclonal antibody drug infliximab in patients with moderate asthma resulted in a decrease in the number of exacerbations. This improvement in clinical outcome was correlated to a significant decrease in TNF-α in treated patients (12). It also appears that anti-TNF-α therapy is most effective in patients with more severe disease, which is consistent with the present study, in which we demonstrated that different levels of TNF-α in airways between C57BL/6 and BALB/c mice directly correlated with the intensity of AHR in these strains of mice (47). Furthermore, polymorphisms in the TNF-α gene have been associated with asthma severity and risk (2, 18, 26), and TNF-α variants have been shown to lead to altered levels of TNF-α protein in patients and were associated with asthma incidence (5, 43). These collective findings, along with our present observations, suggest that TNF-α may be an important target in the treatment of asthma (19).
It is important to note that expression of the CC10-IκBαSR transgene in C57BL/6 mice resulted in decreased mucus metaplasia and increased AHR. These results are puzzling, because they occurred in the absence of detectable increases in the activation of NF-κB in airway epithelium. However, it is possible that the basal NF-κB activity that is constitutively present may regulate airway epithelial biology and that its inhibition may have consequences for subsequent responses to antigen by regulating the activation of other pathways. In this regard, we previously demonstrated that constitutive inhibition of NF-κB in a line of lung epithelial cells enhanced baseline activation of JNK and facilitated TNF-α-stimulated activation of JNK (32). It is therefore possible that expression of the CC10-IκBαSR transgene in C57BL/6 mice altered the activity of other signaling pathways, which could explain the attenuation of mucus metaplasia and enhanced AHR in response to antigen, although this scenario remains to be further tested.
The differences in TNF-α/NF-κB pathway activation between mouse strains highlighted in this study may be informative toward our understanding of differences in asthma severity and management of patients, inasmuch as our present results show that genetic variations control distinct signaling events triggered in response to inhaled antigen, which in turn dictate the phenotypic outcome. The NF-κB pathway has been identified as a promising target for drug intervention in a variety of lung diseases, including asthma (25, 33, 40). Therefore, a further understanding of the molecular regulation of TNF-α and NF-κB pathways in patients with allergic airway disease could be paramount toward developing approaches to optimize targeting of anti-inflammatory therapeutics.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-60014 and R01 HL-079331 (Y. M. W. Janssen-Heininger), R01 HL-074295 (A. van der Vliet), and F32 HL-082121 (J. F. Alcorn) and National Center for Research Resources Center for Biomedical Research Excellence Grant P20 RL-15557 (C. G. Irvin).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Douglas Taatjes (Cell Imaging Facility, University of Vermont) for expert advice with confocal laser scanning microscopy.
REFERENCES
- 1.Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JH, Janssen-Heininger YM, Irvin CG. Transforming growth factor-β1 suppresses airway hyperresponsiveness in allergic airway disease. Am J Respir Crit Care Med 176: 974–982, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki T, Hirota T, Tamari M, Ichikawa K, Takeda K, Arinami T, Shibasaki M, Noguchi E. An association between asthma and TNF-308G/A polymorphism: meta-analysis. J Hum Genet 51: 677–685, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol 94: 1297–1306, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor-α in refractory asthma. N Engl J Med 354: 697–708, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bilolikar H, Nam AR, Rosenthal M, Davies JC, Henderson DC, Balfour-Lynn IM. Tumour necrosis factor gene polymorphisms and childhood wheezing. Eur Respir J 26: 637–646, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Boyce JA, Austen KF. No audible wheezing: nuggets and conundrums from mouse asthma models. J Exp Med 201: 1869–1873, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med 160: 1150–1156, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by IκB kinase-β-dependent genes in airway epithelium. Proc Natl Acad Sci USA 102: 17723–17728, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol 296: L307–L391, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Oppenheim JJ, Howard OM. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD25− responder T cells than C57BL/6 mice. J Leukoc Biol 78: 114–121, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 22: 789–815, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, Zacharasiewicz AS, Turner J, Barnathan ES, Kon OM, Barnes PJ, Hansel TT. The effects of a monoclonal antibody directed against tumor necrosis factor-α in asthma. Am J Respir Crit Care Med 174: 753–762, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol 281: L1037–L1050, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE 2006: re13, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Hart L, Lim S, Adcock I, Barnes PJ, Chung KF. Effects of inhaled corticosteroid therapy on expression and DNA-binding activity of nuclear factor κB in asthma. Am J Respir Crit Care Med 161: 224–231, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Hayden MS, West AP, Ghosh S. NF-κB and the immune response. Oncogene 25: 6758–6780, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hong SJ, Kim HB, Kang MJ, Lee SY, Kim JH, Kim BS, Jang SO, Shin HD, Park CS. TNF-α (−308 G/A) and CD14 (−159T/C) polymorphisms in the bronchial responsiveness of Korean children with asthma. J Allergy Clin Immunol 119: 398–404, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, Beckett P, Al Ali M, Chauhan A, Wilson SJ, Reynolds A, Davies DE, Holgate ST. Tumour necrosis factor (TNFα) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax 60: 1012–1018, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen YM, Driscoll KE, Howard B, Quinlan TR, Treadwell M, Barchowsky A, Mossman BT. Asbestos causes translocation of p65 protein and increases NF-κB DNA binding activity in rat lung epithelial and pleural mesothelial cells. Am J Pathol 151: 389–401, 1997 [PMC free article] [PubMed] [Google Scholar]
- 21.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakhama A, Gelfand EW. Naturally occurring lung CD4+CD25+ T cell regulation of airway allergic responses depends on IL-10 induction of TGF-β. J Immunol 178: 1433–1442, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kanehiro A, Lahn M, Makela MJ, Dakhama A, Fujita M, Joetham A, Mason RJ, Born W, Gelfand EW. Tumor necrosis factor-α negatively regulates airway hyperresponsiveness through γδ T cells. Am J Respir Crit Care Med 164: 2229–2238, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kanehiro A, Lahn M, Makela MJ, Dakhama A, Joetham A, Rha YH, Born W, Gelfand EW. Requirement for the p75 TNF-α receptor 2 in the regulation of airway hyperresponsiveness by γδ T cells. J Immunol 169: 4190–4197, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kang BN, Tirumurugaan KG, Deshpande DA, Amrani Y, Panettieri RA, Walseth TF, Kannan MS. Transcriptional regulation of CD38 expression by tumor necrosis factor-α in human airway smooth muscle cells: role of NF-κB and sensitivity to glucocorticoids. FASEB J 20: 1000–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Remick DG. Tumor necrosis factor inhibitors for the treatment of asthma. Curr Allergy Asthma Rep 7: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Ye YM, Lee SK, Choi JH, Holloway JW, Park CS, Park HS. Association of TNF-α genetic polymorphism with HLA DPB1*0301. Clin Exp Allergy 36: 1247–1253, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Lin YL, Shieh CC, Wang JY. The functional insufficiency of human CD4+CD25 high T-regulatory cells in allergic asthma is subjected to TNF-α modulation. Allergy 63: 67–74, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Lopez P, Gomez J, Prado C, Gutierrez C, Suarez A. Influence of functional interleukin 10/tumor necrosis factor-α polymorphisms on interferon-α, IL-10, and regulatory T cell population in patients with systemic lupus erythematosus receiving antimalarial treatment. J Rheumatol 35: 1559–1566, 2008 [PubMed] [Google Scholar]
- 29.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-α overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 171: 1363–1370, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore PE, Lahiri T, Laporte JD, Church T, Panettieri RA, Jr, Shore SA. Selected contribution: synergism between TNF-α and IL-1β in airway smooth muscle cells: implications for β-adrenergic responsiveness. J Appl Physiol 91: 1467–1474, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and T(H)2 cytokine production in an asthma model in mice. J Allergy Clin Immunol 120: 48–55, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Pantano C, Shrivastava P, McElhinney B, Janssen-Heininger Y. Hydrogen peroxide signaling through tumor necrosis factor receptor 1 leads to selective activation of c-Jun N-terminal kinase. J Biol Chem 278: 44091–44096, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Park GY, Christman JW. Nuclear factor-κB is a promising therapeutic target in inflammatory lung disease. Curr Drug Targets 7: 661–668, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Parris JR, Cobban HJ, Littlejohn AF, MacEwan DJ, Nixon GF. Tumour necrosis factor-α activates a calcium sensitization pathway in guinea-pig bronchial smooth muscle. J Physiol 518: 561–569, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-κB activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 173: 7003–7009, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-κB activation in lipopolysaccharide-induced airway inflammation. J Immunol 170: 6257–6265, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-κB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol 160: 1325–1334, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramis I, Bioque G, Lorente J, Jares P, Quesada P, Rosello-Catafau J, Gelpi E, Bulbena O. Constitutive nuclear factor-κB activity in human upper airway tissues and nasal epithelial cells. Eur Respir J 15: 582–589, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Ricciardelli I, Lindley KJ, Londei M, Quaratino S. Anti-tumour necrosis-α therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn's disease. Immunology 125: 178–183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth M, Black JL. Transcription factors in asthma: are transcription factors a new target for asthma therapy? Curr Drug Targets 7: 589–595, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Rudmann DG, Moore MW, Tepper JS, Aldrich MC, Pfeiffer JW, Hogenesch H, Tumas DB. Modulation of allergic inflammation in mice deficient in TNF receptors. Am J Physiol Lung Cell Mol Physiol 279: L1047–L1057, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Sakai H, Otogoto S, Chiba Y, Abe K, Misawa M. Involvement of p42/44 MAPK and RhoA protein in augmentation of ACh-induced bronchial smooth muscle contraction by TNF-α in rats. J Appl Physiol 97: 2154–2159, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, Sharma A, Kumar S, Sharma SK, Ghosh B. Association of TNF haplotypes with asthma, serum IgE levels, and correlation with serum TNF-α levels. Am J Respir Cell Mol Biol 35: 488–495, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol 287: L143–L152, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S. The allergen Der p1 induces NF-κB activation through interference with IκBα function in asthmatic bronchial epithelial cells. Biochem Biophys Res Commun 236: 522–526, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Stoop JN, Woltman AM, Biesta PJ, Kusters JG, Kuipers EJ, Janssen HL, van der Molen RG. Tumor necrosis factor-α inhibits the suppressive effect of regulatory T cells on the hepatitis B virus-specific immune response. Hepatology 46: 699–705, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 281: L394–L402, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108: 253–261, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vignola AM, Chiappara G, Siena L, Bruno A, Gagliardo R, Merendino AM, Polla BS, Arrigo AP, Bonsignore G, Bousquet J, Chanez P. Proliferation and activation of bronchial epithelial cells in corticosteroid-dependent asthma. J Allergy Clin Immunol 108: 738–746, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 114: 104–111, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, van Dongen H, Scherer HU, Huizinga TW, Toes RE. Suppressor activity among CD4+,CD25++ T cells is discriminated by membrane-bound tumor necrosis factor-α. Arthritis Rheum 58: 1609–1618, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol 285: L32–L42, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 17: 255–281, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 18: 800–812, 1982 [DOI] [PubMed] [Google Scholar]