Abstract
Collectins are collagenous lectins present in blood, respiratory lining fluid, and other mucosal secretions that play important roles in innate defense against infection. The collectin, surfactant protein D (SP-D), limits infection by viruses and bacteria in the respiratory tract, eye, and female genital tract. Multimeric SP-D has strong antiviral activity and is a potent viral and bacterial agglutinin and opsonin; however, trimers composed of the neck and carbohydrate recognition domain (hSP-D-NCRD) of SP-D lack these activities. We now show that, in contrast, a trimeric neck and CRD construct of bovine serum collectin CL-46 induces aggregation of influenza A virus (IAV) and potently increases IAV uptake by neutrophils. CL-46-NCRD showed calcium-dependent and sugar-sensitive binding to both neutrophils and IAV. Replacement of specific residues of the CRD of human SP-D with those found in bovine serum collectins conferred opsonizing activity. The most effective substitution involved replacement of arginine 343 with valine (hSP-D-NCRD/R343V). hSP-D-NCRD/R343V greatly increased viral uptake by neutrophils and monocytes and also potentiated neutrophil respiratory burst responses. These effects were further increased by cross-linking of hSP-D-NCRD/R343V trimers with MAbs directed against areas of the hSP-D-NCRD not involved in viral binding. Unlike the wild-type human SP-D hSP-D-NCRD, hSP-D-NCRD/R343V also induced viral aggregation. These results indicate that collectins can act as opsonins for IAV even in the absence of the collagen domain or higher order multimerization. This may involve increased affinity of individual CRDs for glycoconjugates displayed on host cells or the viral envelope.
Keywords: influenza virus, neutrophils, CL-46
influenza viruses remain a major cause of morbidity and mortality and have the potential to cause massive mortality if novel pandemic strains emerge through reassortment of human and avian strains or direct adaptation of avian strains to humans (as appears to have occurred in 1918; Refs. 29, 33). Certain groups of subjects are known to be more susceptible to adverse effects of influenza infection, including the very young and old and subjects with a variety of comorbid conditions. It is likely that innate host defense mechanisms play an important role in defense against novel strains associated with antigenic drift or shift. These defense mechanisms may play a greater role in more vulnerable subjects (e.g., those who are immunocompromised in other ways).
We (4, 5, 18, 26) and others (24) have extensively studied the role of collectins in the early defense against influenza A virus (IAV). Mice lacking either surfactant protein A (SP-A) or SP-D experience greater viral replication, inflammation, and illness in the first several days after infection with IAV strains known to be susceptible to inhibition by these host defense lectins in vitro (7, 26, 38). Host defense against IAV can be restored in these mice by instillation or overexpression of wild-type forms of rat SP-D. A trimeric recombinant form of rat SP-D (rSP-Dser15,20, which lacks the ability to form multimers due to replacement of cysteines in the NH2 terminus) can restore antiviral activity in SP-D −/− mice when highly overexpressed, even though this form of SP-D does not improve other aspects of the abnormal phenotype of these mice (38). In vitro, natural human SP-D trimers have substantially reduced activity against IAV (23). These and other results imply that multimerization of SP-D is important for some of its functional activities (10, 11, 13).
The SP-D gene was duplicated in bovidae resulting in at least three additional related collectins, all of which are present in serum, conglutinin, CL-43, and CL-46 (8). These bovine serum collectins are structurally similar to SP-D; however, CL-43 is distinctive in that it only occurs as trimers in vivo and in vitro, whereas the others form dodecameric structures similar to SP-D (9, 12, 28). The bovine collectins also have distinctive monosaccharide binding preferences compared with SP-D. Whereas SP-D has highest affinity for glucose and inositol, bovine conglutinin and CL-46 have higher affinity for N-acetylglucosamine (GlcNAc) and mannose, and CL-43 has higher affinity for mannose, than for glucose. We have demonstrated that conglutinin and CL-43 have strong IAV neutralizing activity and that the intrinsic viral neutralizing activity of the carbohydrate recognition domain (CRD) of conglutinin exceeds that of SP-D (12, 14, 21). The latter conclusion was based on production of a chimeric full-length collectin containing the NH2 terminus and collagen domain of SP-D, coupled to the neck and carbohydrate recognition domain (NCRD) of conglutinin, which was shown to have increased IAV inhibiting activity compared with wild-type SP-D. Furthermore, we have developed NCRD constructs of CL-43 and SP-D (lacking NH2-terminal and collagen domains) and found that the NCRD of CL-43 had viral neutralizing activity, whereas those of human or rat SP-D did not (4). Crystallographic studies of SP-D have shown the calcium ion at the sugar-binding site is flanked by hydrophilic amino acid residues with long side chains, Arg343 or Lys343 and Asp325 or Asn325, depending on the species. Table 1 compares the primary structure of this region of the NCRD of SP-D, CL-46, and other serum collectins. Mutagenesis experiments have shown that ligand binding preferences can be altered via substitutions at one or both of these sites (4, 5). Introduction of a three amino acid (arginine, alanine, and lysine; RAK) insert into the hSP-D-NCRD of SP-D at the same location that this insert is found in CL-43 conferred neutralizing activity. Similar results were obtained with an AAA insert in the same location. The serum collectins mannose-binding lectin (MBL), CL-43, CL-46, and conglutinin all have a hydrophobic amino acid (valine or isoleucine) at position 343 in their NCRD, whereas SP-D and SP-A have an arginine in this location. Recently, we found that introducing such substitutions (hSP-D-NCRD/R343V or hSP-D-NCRD/R343I) in hSP-D-NCRD results in marked increase in viral neutralizing activity. This increase is much more pronounced than that afforded by the RAK or AAA insertions.
Table 1.
Key sequences of the CRDs of human and rat SP-D and bovine serum collectins conglutinin, CL-43, and CL-46
| ↓ | ||
| hSP-D | LVYSNWAPGEPND---DGGS-EDCVEIFTNGKWNDRACGEKRLVVCEF | |
| rSP-D | LVYSNWAPGEPNN---NGGA-ENCVEIFTNGQWNDKACGEQRLVICEF | |
| Conglutinin | LVYSNWAGEPNNSD--EGQPENCVEIFPDGKWNDVPCSKQLLVICEF | |
| CL-43 | LDYSNWAPGEPNNRAKDEG-PENCLEIYSDGNWNDIECREERLVICEF | |
| CL-46 | LVYSNWASGEPNNNN--AGQPENCVQIYREGKWNDVPCSEPLLVICEF | |
| ridge | ridge | |
Amino acids 312–355 are shown. Amino acid 343 is shown in italics. and the location of ridges on either side of the lectin and calcium coordination site are indicated. Conserved residues are shown in bold. The primary calcium coordinating residues are residues 320–322 (EPN). R343 in surfactant protein D (SP-D) is indicated by an arrow. CRDs, carbohydrate recognition domain; hSP-D, trimers composed of human SP-D; rSP-D, trimers composed of rat SP-D.
Prior studies have shown that collectins are potent opsonins for viruses and bacteria, strongly increasing uptake of various pathogens by phagocytes (11, 18, 21, 22). In addition, collectins can potentiate activation of phagocytes when bounded to microbial ligands (36). Several lines of evidence support the hypothesis that receptors for collectin NH2 termini and collagen domains may be critical in mediating these effects (2, 6, 37). With respect to IAV, we have shown that dodecamers or multimers of SP-D, MBL, SP-A, or conglutinin potentiate viral uptake and virus-induced respiratory burst responses by neutrophils (1, 13, 15). In the case of SP-D, multimerization of the collectin is important for these effects since full-length trimers or trimers of just the NCRD of SP-D lack opsonizing activity. In contrast, we found that CL-43 trimers and the NCRD of CL-43, or hSP-D-NCRD containing the RAK sequence, increase viral uptake by neutrophils (4, 12). These latter findings were surprising since the wild-type human or rat SP-D-NCRD do not have viral aggregating or opsonizing activity.
For this paper, we sought to determine how NCRD molecules can enhance viral uptake. We first examined the functional activity of bovine CL-46-NCRD and show that, like CL-43-NCRD, it has strong viral opsonizing activity. We then show that the hSP-D-NCRD/R343V and hSP-D-NCRD/R343I mutants of the human hSP-D-NCRD, which resemble the bovine collectins at the 343 position, acquire strong opsonizing activity. Because hSP-D-NCRDs that promote viral uptake by phagocytes all induce viral aggregation to a greater extent than hSP-D-NCRD, it is likely that enhanced aggregating activity accounts for their opsonizing activity.
MATERIALS AND METHODS
Virus preparations.
IAV was grown in the chorioallantoic fluid of 10-day-old chicken eggs and purified on a discontinuous sucrose gradient as previously described (16). The virus was dialyzed against PBS to remove sucrose, aliquoted, and stored at −80°C until needed. Philippines 82/H3N2 (Phil82) strain was kindly provided by Dr. E. Margot Anders (Univ. of Melbourne, Melbourne, Australia). After thawing, the viral stocks contained ∼5 × 108 infectious focus forming units/ml.
Collectin preparations.
hSP-D-NCRD preparations, including the wild-type hSP-D-NCRD (called hSP-D-NCRD hereafter), wild-type rat SP-D-NCRD (rSP-D-NCRD), hSP-D-NCRD/R343V, hSP-D-NCRD/R343I, and hSP-D-NCRD/R343I+RAK mutants, were produced in Escherichia coli as previously described (4, 5). CL-46-NCRD was expressed in Pichia pastoris and purified as previously described (9). Briefly, the α-helical coiled-coil neck region and the CRD of CL-46 were amplified by PCR and ligated into the pPIC9K vector (Invitrogen). The pPIC9K derivatives were transformed into XL-10 E. coli, purified, linearized, and transformed into P. pastoris (GS115). Clones were double-selected by growth on histidine-deficient plates and plates with increasing concentrations of Geneticin. The endotoxin level of all SP-D preparations was 0.1∼0.5 endotoxin units (EU)/ml (Limulus Lysate Assay, Cambrex, Walkersville, MD).
Monoclonal antibodies.
MAbs 246-04 and 246-08 were raised against SP-D by inoculating mice with 10 μg/ml human SP-D as previously described (27). Both of these MAbs bind to the hSP-D-NCRD of human SP-D, although neither blocks binding of SP-D to mannan or IAV (35). Both MAbs are of the IgG2a subtype. The IgG2a isotype control antibody was obtained from eBioscience (http://www.ebioscience.com).
Hemagglutination inhibition assay.
Hemagglutination (HA) inhibition was measured by serially diluting collectins or other host defense protein preparations in round-bottom 96-well plates (Serocluster U-Vinyl plates; Costar, Cambridge, MA) using PBS containing calcium and magnesium as a diluent (18). After adding 25 μl of IAV, giving a final concentration of 40 HA U/ml or 4 HA U/well, the IAV-protein mixture was incubated for 15 min at room temperature, followed by addition of 50 μl of a type O human erythrocyte suspension. The minimum concentration of protein required to fully inhibit the hemagglutinating activity of the viral suspension was determined by noting the highest dilution of protein that still inhibited HA. Inhibition of HA activity in a given well is demonstrated by absence of formation of an erythrocyte pellet. If no inhibition of HA activity was observed at the highest protein concentration used then the value is expressed as > the maximal protein concentration.
Measurement of viral aggregation by collectins.
Viral aggregation was measured by assessing light transmission through stirred suspensions of IAV as previously described (21). In addition, viral aggregates were visualized using electron microscopy (EM) as previously described (31).
Fluorescent focus assay of IAV infectivity.
Madin-Darby canine kidney (MDCK) cell monolayers were prepared in 96-well plates and grown to confluency. These layers were then infected with diluted IAV preparations for 45 min at 37°C in PBS and tested for presence of IAV infected cells after 7 h using a MAb directed against the influenza A viral nucleoprotein [provided by Dr. Nancy Cox, Center for Disease Control (CDC), Atlanta, GA] as previously described (14). IAV was preincubated for 30 min at 37°C with SP-D or control buffer, followed by addition of these viral samples to the MDCK cells. Where indicated, SP-Ds were first incubated with MAbs before adding them to IAV.
Neuraminidase inhibition assay.
Neuraminidase (NA) activity of IAV was measured by an enzyme-linked microplate assay in which Arachis hypogaea (peanut) lectin was used to detect β-d-galactose-GlcNAc sequences exposed after the removal of sialic acid from fetuin (30). Wells of microtiter plate were coated with 50 μl of fetuin (20 μl/ml in PBS; F2379; Sigma) overnight at 4°C and washed with PBS. Dilutions of IAV strains with different concentrations of SP-D were preincubated for 30 min at 37°C, and 50 μl of the mixture was added to fetuin-coated wells and incubated at 37°C for 2 h. After the wells were washed, 50 μl of peroxidase-labeled peanut lectin [20 μl/ml in BSA (0.5%); L6135; Sigma] was added to each well for 30 min at room temperature followed by washing and incubation with 50 μl of TMP-peroxidase (Bio-Rad, Hercules, CA) for 20 min. Finally, 50 μl of 1 N H2SO4 was added to the wells, and the absorbance was measured by ELISA plate reader at 450 nm.
Human neutrophil and monocyte preparation.
Neutrophils from healthy volunteers were isolated to >95% purity by using dextran precipitation, followed by Ficoll-Paque gradient separation for the separation of mononuclear cells (layering above the Ficoll-Paque) and neutrophils (below the Ficoll-Paque). The neutrophils were purified further by hypotonic lysis to eliminate any contaminating erythrocytes, as previously described (16). Cell viability was determined to be >98% by trypan blue staining. The isolated neutrophils were resuspended at the appropriate concentrations in control buffer (PBS) and used within 2 h. Neutrophil collection was done with informed consent as approved by the Institutional Review Board of Boston University School of Medicine. Peripheral blood mononuclear cells (PBMCs) were taken from the layer above Ficoll-Paque and washed several times in PBS. For fluorescent microscopy, the mononuclear cells were maintained in suspension and hence contained lymphocytes and monocytes. For fluorescent microscopic assays, the mononuclear cell preparations were allowed to adhere to plastic followed by washing off of unattached cells. The adherent cells were predominantly monocytes. RAW cells were obtained from the American Type Culture Collection (Manassas, VA).
Measurement of IAV uptake by neutrophils and monocytes.
FITC-labeled IAV (Phil82 strain) was prepared, and uptake of virus by neutrophils, monocytes, or RAW cells was measured by flow cytometry as previously described (15, 21). In brief, IAV was incubated with neutrophils for 30 min at 37°C in presence of control buffer. Trypan blue (0.2 mg/ml) was added to these samples to quench extracellular fluorescence. Following washing, the neutrophils were fixed with paraformaldehyde, and neutrophil-associated fluorescence was measured using flow cytometry. The mean neutrophil or monocyte fluorescence (>1,000 cells counted per sample) was measured. Monocyte uptake was also assessed by fluorescent microscopy using plastic adherent PBMCs.
Measurement of neutrophil H2O2 production.
H2O2 production was measured by assessing reduction in scopoletin fluorescence as previously described (17). Measurements were made using a POLARstar OPTIMA fluorescent plate reader (BMG Labtech, Durham, NC).
Statistics.
Statistical comparisons were made using Student's paired two-tailed t-test or ANOVA with post hoc test (Tukey). ANOVA was used for multiple comparisons to a single control.
RESULTS
The CL-46-NCRD strongly increases neutrophil uptake of IAV despite lacking NH2-terminal domains.
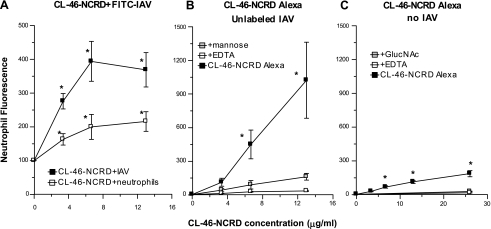
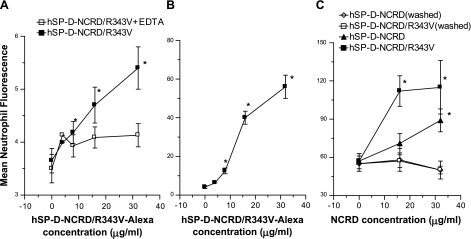
Like full-length CL-43, the CL-43-NCRD, or the RAK insertion mutant of hSP-D-NCRD, CL-46-NCRD increased FITC-labeled IAV uptake by neutrophils (Fig. 1). This effect was most pronounced when the CL-46-NCRD was preincubated with IAV (Fig. 1A, top curve), but significant increase in uptake of the virus also occurred when neutrophils were first incubated with CL-46-NCRD followed by addition of FITC-labeled IAV (Fig. 1A, bottom curve). We also labeled CL-46-NCRD with Alexa Fluor and incubated this with unlabeled IAV (Fig. 1B). This procedure also led to a marked increase in neutrophil fluorescence, which was largely inhibited when the assay was carried out in the presence of mannose or EDTA. Finally, we tested the effect of adding CL-46-NCRD-Alexa alone to neutrophils (no virus included) and showed a small but statistically significant binding to the neutrophils that was inhibited by EDTA or GlcNAc. Overall, these results suggest that CL-46-NCRD forms a complex with IAV leading to increased uptake of the virus and CL-46-NCRD. However, free CL-46-NCRD also bound directly to the neutrophils through its lectin activity, and this was able to increase binding of subsequently added IAV. Note again that wild-type human and rat SP-D-NCRDs do not increase neutrophil uptake of IAV in this manner (Ref. 32 and see below). These results led us to further evaluate the contributions of nonconserved residues near the carbohydrate binding site of serum collectins like CL-43 and CL-46.
Fig. 1.
A trimeric neck and carbohydrate recognition domain (NCRD) construct of bovine serum collectin CL-46 (CL-46-NCRD) increases viral uptake by neutrophils. Preincubation of CL-46-NCRD with influenza A virus (IAV) followed by incubation with human neutrophils caused strong increases in viral uptake (A; CL-46-NCRD+FITC-labeled IAV; P < 0.05 for all doses of CL-46-NCRD compared with binding of virus alone). A shows fluorescence as % of virus only control, and B and C show absolute fluorescence values. Significant increases in viral uptake also occurred when the neutrophils were first incubated with CL-46, followed by washing of the cells and then addition of IAV (A; CL-46-NCRD+neutrophils). Unlabeled IAV was also preincubated with CL-46-NCRD that had been labeled with Alexa Fluor (B). Addition of these complexes to neutrophils resulted in strong increases in neutrophil fluorescence indicating binding of CL-46-NCRD to the cells, presumably in conjunction with IAV. Carrying out these incubations in the presence of mannan or EDTA markedly decreased binding of CL-46-NCRD with neutrophils [significantly less binding by ANOVA compared with binding of CL-46-NCRD-Alexa in control buffer (black rectangles)]. In C, Alexa Fluor-labeled CL-46-NCRD alone was incubated with neutrophils (no virus present). The CL-46-NCRD again bounded to neutrophils in a saccharide- and calcium-dependent manner (C), but the binding was much lower than when CL-46-NCRD had been preincubated with IAV first (B). All results are means ± SE of 4 or more experiments using separate neutrophil donors. *Significantly greater neutrophil fluorescence than control (P < 0.05). GlucNAc, N-acetylglucosamine.
Substitution of valine or isoleucine at position 343 in the human SP-D NCRD confers viral opsonizing activity.
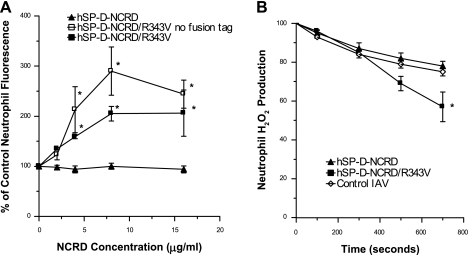
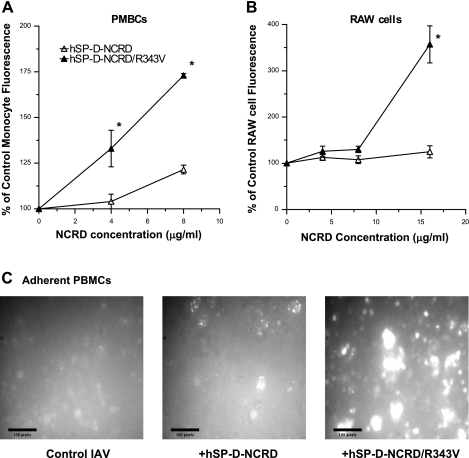
As shown in Table 1, a common feature of the serum collectins is presence of a hydrophobic residue instead of the arginine found at amino acid 343 in SP-D. The R343V substitution in hSP-D-NCRD conferred the capacity to increase viral uptake by neutrophils (Fig. 2A). To rule out a possible contribution of the fusion tag to the opsonizing activity of hSP-D-NCRD/R343V, the protein was also expressed without the tag. The “tagless” hSP-D-NCRD/R343V also caused strong increases in neutrophil uptake of IAV (Fig. 2A). hSP-D-NCRD/R343V also increased neutrophil H2O2 responses to IAV, whereas wild-type hSP-D-NCRD did not (Fig. 2B). hSP-D-NCRD/R343V also increased viral uptake by human PBMCs (Fig. 3A) or RAW 264.7 cells (Fig. 3B) as assessed by flow cytometry using cells in suspension. A similar increase in viral uptake by adherent PBMCs was found using fluorescence microscopy (Fig. 3C).
Fig. 2.
An NCRD construct of human surfactant protein D (SP-D) with an R343V substitution (hSP-D-NCRD/R343V) increases viral uptake and IAV-induced H2O2 production by neutrophils. A shows the effect of preincubating FITC-labeled IAV with hSP-D-NCRD/R343V, hSP-D-NCRD/R343V lacking the fusion tag, or wild-type hSP-D-NCRD on viral uptake by neutrophils. hSP-D-NCRD did not cause any increase in viral uptake, but hSP-D-NCRD/R343V with or without the fusion tag did. At 8 μg/ml hSP-D-NCRD/R343V, the preparation lacking the fusion tag caused a significantly greater increase in uptake than hSP-D-NCRD/R343V with the fusion tag (P < 0.05). B shows neutrophil H2O2 generation as measured by decreases in scopoletin fluorescence. IAV-induced neutrophil H2O2 generation was significantly increased by preincubation of the virus with 16 μg/ml hSP-D-NCRD/R343V (P < 0.05), whereas it was not after preincubation of wild-type hSP-D-NCRD. All results shown are means ± SE of 3 or more experiments. *Significantly greater neutrophil fluorescence than control virus alone (A; P < 0.05) or significantly greater H2O2 generation than was induced by IAV alone or IAV+hSP-D-NCRD (assessed by ANOVA).
Fig. 3.
hSP-D-NCRD/R343V increases IAV uptake by human peripheral blood mononuclear cells (PBMCs) and by RAW 264.7 cells. Experiments shown in A and B were performed as in Fig. 2A, except that human PBMCs and RAW cells were used for flow cytometry. hSP-D-NCRD/R343V significantly increased viral uptake by PBMCs (P < 0.01 at 8 μg/ml) and RAW 264.7 cells (P < 0.01 at 16 μg/ml; n = 4 for each using separate blood donors for the PBMCs). C shows adherent human PBMCs treated with Alexa Fluor-labeled IAV alone (control IAV) or a similar amount of virus pretreated with 8 μg/ml either hSP-D-NCRD or hSP-D-NCRD/R343V. These photos are from 1 representative experiment of 3 that were performed. *Significantly greater cellular fluorescence than control (P < 0.05).
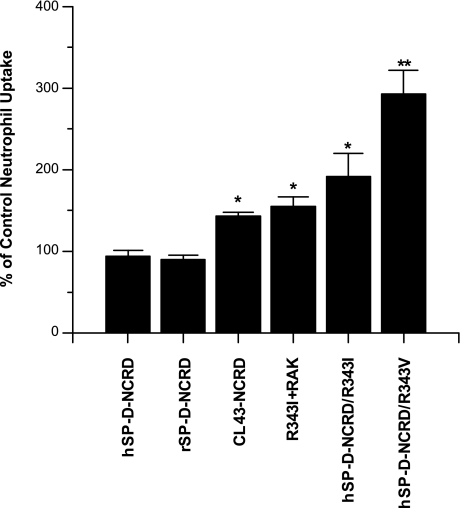
We compared activity of various other wild-type and mutant hSP-D-NCRDs with that of hSP-D-NCRD/R343V in terms of ability to increase neutrophil uptake of IAV (Fig. 4). hSP-D-NCRD/R343I and the combined hSP-D-NCRD/R343I+RAK mutants were prepared. The former construct contains the isoleucine found at residue 343 in CL-43, whereas the latter contains both isoleucine and the RAK insertion present in CL-43. Both mutants increased neutrophil uptake of IAV but not to the same extent as hSP-D-NCRD/R343V (Fig. 4). We (3) have reported that the hSP-D-NCRD/R343V mutant has strong viral neutralizing activity. Hence, it is likely that a major part of the enhanced neutralizing and opsonizing activity seen with CL-46 and CL-43 relates to the presence of the hydrophobic residue at the 343 position. Of interest, the hSP-D-NCRD/R343V mutant had greater opsonizing activity than hSP-D-NCRD/R343I or the combined mutant, hSP-D-NCRD/R343I+RAK. hSP-D-NCRD/R343I+RAK did not have greater activity than the hSP-D-NCRD/R343I single mutant but did have a very similar level of activity to CL-43-NCRD.
Fig. 4.
Comparison of ability of various wild-type or mutant hSP-D-NCRDs to increase neutrophil uptake of IAV. FITC-labeled IAV was preincubated with 16 μg/ml wild-type human or rat (r) SP-D-NCRDs, CL-43-NCRD, or various other mutant versions of hSP-D-NCRD followed by measurement of viral uptake by human neutrophils. Results are expressed as % of control uptake (control = untreated virus). CL-43-NCRD and the mutants, hSP-D-NCRD/R343I, hSP-D-NCRD/R343I+RAK (where RAK indicates arginine, alanine, and lysine insertion; labeled R343V+RAK in figure), and hSP-D-NCRD/R343V, all caused increased uptake (* or ** indicate P < 0.05 compared with virus alone). The increase in uptake caused by hSP-D-NCRD/R343V was significantly greater than that caused by CL-43-NCRD, hSP-D-NCRD/R343I, or hSP-D-NCRD/R343I+RAK as assessed by ANOVA (indicated by **).
hSP-D-NCRD/R343V binds to neutrophils and increases viral uptake.
As with the CL-46-NCRD, an Alexa Fluor-labeled preparation of hSP-D-NCRD/R343V bounded to neutrophils in a calcium-dependent manner (Fig. 5A). Binding of hSP-D-NCRD/R343V-Alexa was increased ∼10-fold when it was preincubated with unlabeled IAV (Fig. 5B). This indicates that hSP-D-NCRD/R343V forms a complex with IAV that then binds to neutrophils. Preincubation of neutrophils with unlabeled hSP-D-NCRD/R343V also resulted increased binding of FITC-labeled IAV that was added subsequently, and this increase significantly exceeded that of wild-type hSP-D-NCRD (Fig. 5C); however, if the neutrophils were washed free of unbound hSP-D-NCRD/R343V or hSP-D-NCRD before addition of IAV, no increase in viral uptake occurred. The latter result differs from that obtained with the CL-46-NCRD where some increased uptake was observed. These results suggest that direct binding of hSP-D-NCRD/R343V to neutrophils does not play a significant role in it opsonizing activity.
Fig. 5.
Role of binding of hSP-D-NCRD/R343V to neutrophils in its ability to promote viral uptake. A shows that Alexa Fluor-labeled hSP-D-NCRD/R343V bounded to neutrophils in a manner that was significantly reduced in presence of EDTA (P < 0.01 comparing EDTA vs. control buffer at 32 μg/ml; n = 6). B shows effect of preincubating hSP-D-NCRD/R343V-Alexa with unlabeled IAV on binding of hSP-D-NCRD/R343V. Note ∼10-fold increase in neutrophil-associated fluorescence compared with hSP-D-NCRD/R343V-Alexa in absence of IAV. C shows the effect of preincubation of neutrophils with hSP-D-NCRD/R343V or hSP-D-NCRD (30 min at 37°C) on uptake of FITC-IAV added subsequently. Both hSP-D-NCRD and hSP-D-NCRD/R343V caused significant increases in neutrophil uptake of IAV using this method (P < 0.05 for all doses; n = 8); however, the effect of hSP-D-NCRD/R343V was significantly greater at the 16 μg/ml concentration by ANOVA. In contrast, if unbound hSP-D-NCRD/R343V or hSP-D-NCRD were washed off of neutrophils before addition of IAV, no increase in viral uptake was found (n = 4). *Significantly greater neutrophil fluorescence than control (P < 0.05).
hSP-D-NCRD/R343V and CL-46-NCRDs induce viral aggregation.
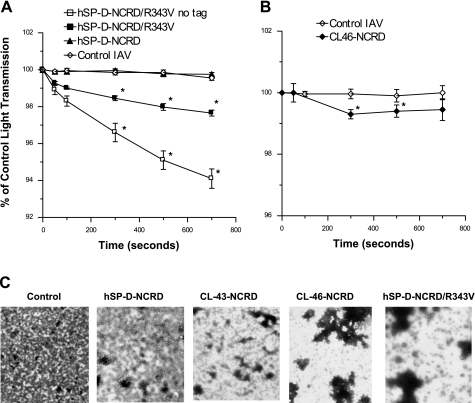
Using light transmission assays, we found that hSP-D-NCRD/R343V caused a subtle viral aggregation response (Fig. 6A). Of interest, the tagless hSP-D-NCRD/R343V caused greater aggregation that hSP-D-NCRD/R343V containing the fusion tag, and both caused significantly greater viral aggregation than hSP-D-NCRD (which did not result in significant aggregation compared with control virus alone). To confirm that the effect was not secondary to aggregation of the protein during storage, an aliquot of purified hSP-D-NCRD/R343V fusion protein was reexamined by gel filtration under the conditions of original isolation; the protein eluted exclusively as a trimer (Ref. 4 and data not shown). Since the gel filtration is carried out in 500 mM NaCl without added calcium, we cannot completely exclude that some protein aggregation occurs after addition of calcium during the incubation with the virus; however, we have also prepared the protein at much higher concentrations in physiological buffer (including calcium) for crystallography and encountered no protein aggregation under those conditions (3). A subtle but statistically significant aggregation response by CL-46-NCRD was also observed using the light transmission assay (Fig. 6B). These aggregation responses were less than we have reported with full-length SP-D dodecamers; hence, we decided to evaluate viral aggregation using EM. As shown in Fig. 6C, viral aggregation caused by hSP-D-NCRD/R343V and CL-46-NCRD was also readily apparent by transmission EM and greatly exceeded the response induces by hSP-D-NCRD. CL-43-NCRD also caused a subtle increase in viral aggregation compared with hSP-D-NCRD. Note that CL-43-NCRD causes greater uptake of IAV, and greater viral aggregation, at 32 μg/ml (data not shown), but the results obtained with 16 μg/ml are shown here for comparison with hSP-D-NCRD/R343V and CL-46-NCRD. It should be noted that the CL-46-NCRD used in these studies was prepared by a different method (i.e., in P. pastoris) from the other NCRDs and thus direct comparisons of activity between CL-46-NCRD and the others NCRDs must be interpreted with caution.
Fig. 6.
hSP-D-NCRD/R343V and CL-46-NCRD cause aggregation of IAV. A shows aggregation of IAV as measured by light transmission through stirred viral suspensions. hSP-D-NCRD/R343V and hSP-D-NCRD/R343V lacking the fusion tag both caused significant viral aggregation (P < 0.01 at points indicated by *; n = 4), whereas wild-type hSP-D-NCRD did not. The hSP-D-NCRD/R343V without fusion tag caused significantly greater aggregation than hSP-D-NCRD/R343V with the tag (labeled hSP-D-NCRD/R343V) as assessed by ANOVA. B shows aggregation caused by CL-46-NCRD (P < 0.05 compared with control virus where indicated by *; n = 3). C shows viral control and aggregation caused by hSP-D-NCRD, CL-43-NCRD, CL-46-NCRD, and hSP-D-NCRD/R343V under electron microscopy (all 16 μg/ml). Results are representative of 3 experiments.
Cross-linking of hSP-D-NCRD/R343V increases its viral aggregating and opsonizing activity and confers NA inhibition activity.
We have shown that certain MAbs directed against the hSP-D-NCRD do not block activity of SP-D but strongly increase the ability of the wild-type hSP-D-NCRD to bind to and neutralize the virus (32). These MAbs, 246-04 and 246-08, likely bind to the lateral or back surface of the CRD head and cross-link the trimers resulting in greater binding and antiviral activity. As shown in Fig. 7A, MAb 246-08 potentiated the ability of hSP-D-NCRD/R343V to cause viral aggregation. We (30) have previously shown that the ability of SP-D to inhibit viral NA activity is strongly dependent on multimerization. Despite strong activity on various other assays, hSP-D-NCRD/R343V alone did not inhibit viral NA activity; however, it did so when coincubated with MAb 246-04 or 246-08 (Fig. 7B). MAb 246-08 also enabled hSP-D-NCRD/R343V to increase neutrophil uptake of IAV at lower concentrations than when hSP-D-NCRD/R343V was used alone (Fig. 8A). MAb 246-08 also potentiated the ability of hSP-D-NCRD/R343V to increase IAV-induced H2O2 production (Fig. 8B). Of interest, the MAb actually antagonized the ability of higher concentrations (20 μg/ml) of hSP-D-NCRD/R343V to increase neutrophil uptake of IAV. The 246-04 and 246-08 MAbs are of the IgG2a subtype. An IgG2a isotype control MAb alone had no effect on viral uptake and did not significantly alter the effects of 4 μg/ml hSP-D-NCRD or hSP-D-NCRD/R343V on viral uptake (n = 3 experiments using separate neutrophil donors; data not shown). Note also that MAb 246-08 alone did not alter IAV uptake by neutrophils or IAV-induced neutrophil H2O2 responses (Fig. 8B). Hence, it is unlikely that the effects shown in Fig. 8 result from nonspecific binding of MAb 246-08 to neutrophil Fc receptors.
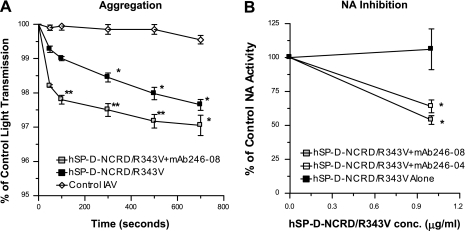
Fig. 7.
Cross-linking of hSP-D-NCRD/R343V by MAbs increases viral aggregating activity and results in neuraminidase (NA) inhibition. The effects of hSP-D-NCRD/R343V and hSP-D-NCRD/R343V combined with the MAb 246-08 on viral aggregation are shown in A. hSP-D-NCRD/R343V caused viral aggregation compared with virus alone (P < 0.05, indicated by *). Aggregation was significantly greater (P < 0.05 where indicated by **; n = 4) with the addition of the MAb. NA inhibition was measured by reduction in binding of peanut lectin to fetuin (B). hSP-D-NCRD/R343V alone did not cause any inhibition of NA activity; however, significant inhibition did occur when hSP-D-NCRD/R343V was combined with the MAbs 246-08 or 246-04 (P < 0.05 compared with control as indicated by *; n = 4). The concentration of MAbs used in these assays was 5 μg/ml.
Fig. 8.
Cross-linking of hSP-D-NCRD/R343V with MAb modifies its ability to increase neutrophil uptake of IAV and increases its ability to potentiate IAV-induced neutrophil H2O2 generation. Neutrophil uptake of IAV (A) and IAV-induced neutrophil H2O2 generation (B) were measured as in Fig. 3. hSP-D-NCRD/R343V alone increased IAV uptake compared with IAV alone (P < 0.05 where indicated by *). At lower concentrations (2 and 4 μg/ml) of hSP-D-NCRD/R343V, addition of MAb 246-08 (labeled MAb in figure) significantly increased the ability of hSP-D-NCRD/R343V to increase neutrophil uptake of IAV (significant difference by ANOVA indicated by **; n = 5 using separate neutrophil donors). The MAb alone (without NCRDs) did not significantly alter neutrophil uptake of IAV (data not shown). In contrast, addition of the MAb significantly reduced neutrophil uptake compared with 16 μg/ml hSP-D-NCRD/R343V alone (# indicates where hSP-D-NCRD/R343V caused significantly greater increase in neutrophil fluorescence than the combination of hSP-D-NCRD/R343V and MAb 246-08 as determined by ANOVA). The MAb alone had no effect on viral uptake (data not shown). hSP-D-NCRD/R343V alone at 4 μg/ml (lower than concentration used in Fig. 3) did not increase IAV-induced neutrophil H2O2 generation, nor did MAb 246-08 alone (B); however, the combination of hSP-D-NCRD/R343V and the MAb caused marked enhancement of neutrophil H2O2 generation (n = 4; P < 0.01 compared with control or hSP-D-NCRD/R343V or MAb alone, indicated by *).
DISCUSSION
We (4, 19) have previously shown that trimers of CL-43 (either full-length or in NCRD form) and hSP-D-NCRD with an insertion of the RAK sequence are able to increase uptake of IAV by neutrophils. By contrast, wild-type human or rat SP-D-NCRDs or the full-length RrSP-Dser15,20 trimers did not have this effect. We now show that CL-46-NCRD also promotes viral uptake by neutrophils. Because the NCRDs do not contain NH2-terminal or collagen domains, these domains are not involved in opsonizing activity.
The key residues that affect binding of SP-D to glycosylated ligands are those present on the ridges flanking the lectin site (e.g., D325 and R343). The RAK insertion lies adjacent to D325 and probably alters or extends the binding surface in that area, allowing a small increase in neutralizing activity and resulting in increases in viral uptake at high concentrations of hSP-D-NCRD; however, the opsonizing activity of CL-46 was much greater than that of RAK, and CL-46 has a distinct insertion in this location (NN). Another common feature of serum collectins that distinguishes them from SP-D is the presence of a hydrophobic residue at 343. We (3) have reported that the hSP-D-NCRD/R343V mutant of hSP-D-NCRD has markedly increased neutralizing activity and now demonstrate that hSP-D-NCRD/R343V also has strong opsonizing activity. hSP-D-NCRD/R343I had increased neutralizing activity compared with hSP-D-NCRD but not as great as hSP-D-NCRD/R343V, and its opsonizing activity was also less. We have recently found that the CL-46-NCRD has increased HA inhibition and neutralizing activity compared with hSP-D-NCRD (K. L. Hartshorn, G. Sorensen, U. Holmskov, and E. C. Crouch, unpublished data). Although CL-43 and CL-46 have insertions near 325, it is not clear whether these insertions contribute to the enhanced opsonizing activity, since the combined RAK+hSP-D-NCRD/R343I mutant had less opsonizing activity than hSP-D-NCRD/R343I alone.
We demonstrate that the opsonizing activity of hSP-D-NCRD/R343V is not confined to neutrophils but is also present for PBMCs and RAW cells. It is particularly notable that hSP-D-NCRD/R343V and other mutant hSP-D-NCRDs are able to act as opsonins because these preparations fully lack NH2-terminal and collagen domains and do not form multimers. A variety of studies support the concept that NH2-terminal and collagen domains are important for binding to macrophage surface receptors and that these receptors play important roles in promotion of phagocytosis and cell activation in response to complexes of collectins and microbial or apoptotic cell ligands (6, 25). Our results convincingly show, however, that trimeric collectin hSP-D-NCRDs can also act as opsonins to enhance neutrophil or monocyte/macrophage uptake of IAV. We have recently shown that small dodecamers of SP-D lacking the collagen domain but retaining the NH2 terminus and hSP-D-NCRD trimers cross-linked with MAbs also promote neutrophil uptake of IAV (32, 34). Hence, using several methods, we have shown that the collagen domain per se in not required for antiviral or opsonizing activity. The current results further demonstrate that these activities can be reproduced even in the absence of higher levels of multimerization or cross-linking of trimers.
Both hSP-D-NCRD/R343V and CL-46-NCRD showed detectable calcium-dependent binding to neutrophils; however, this does not appear to be a major factor in their ability to increase viral uptake. Preincubation of neutrophils with CL-46-NCRD or hSP-D-NCRD/R343V followed by later addition of IAV increased viral uptake. In the case of hSP-D-NCRD/R343V, this effect was abolished by thoroughly washing off unbound hSP-D-NCRD/R343V, and washing off unbound CL-46-NCRD greatly decreased its opsonizing activity. The more likely explanation for their opsonizing activity is their shared ability to cause viral aggregation. This mechanism is similar to that we (31) recently proposed for some defensins. As for defensins, the aggregating activity of CL-46-NCRD or hSP-D-NCRD/R343V was much more evident by EM than with light transmission assays (31). The mechanism through which a trimer can induce aggregation is still unclear since we did not find any evidence of self-aggregation in the hSP-D-NCRD/R343V preparation. It is likely that an hSP-D-NCRD trimer can cross-link IAV particles when a sufficient amount of the hSP-D-NCRD is present in relation to the viral concentration. This implies that individual lectin sites on a trimer can bind to separate viral particles if the affinity for viral saccharides is high enough. Alternatively, hSP-D-NCRD/R343V or CL-46-NCRD could self-aggregate after binding to the viral ligand, leading to aggregation.
Of additional interest, the antiviral activities of hSP-D-NCRD/R343V and other mutant hSP-D-NCRDs were further increased by cross-linking of the hSP-D-NCRDs with MAbs. We have previously shown that these MAbs bind to the hSP-D-NCRD but not to the neck or the area adjacent to the lectin site (23, 32). These antibodies are able to cross-link hSP-D-NCRD such that it can mediate neutralizing and other antiviral activities (32). We now show that these MAbs also increase the ability of hSP-D-NCRD/R343V to cause viral aggregation and potentiate neutrophil uptake of IAV and neutrophil respiratory burst responses. At suboptimal concentrations of hSP-D-NCRD/R343V for promotion of viral uptake or respiratory burst responses of neutrophils, the MAbs potentiated opsonizing activities. Of interest, however, MAb interfered with enhancement of uptake by optimal doses of hSP-D-NCRD/R343V. It is possible that the combination of higher concentrations of hSP-D-NCRD/R343V and the MAb results in aggregates that are too large to be ingested by neutrophils. However, further study will be needed to clarify this issue.
Although hSP-D-NCRD/R343V did not inhibit viral NA activity on its own, it did so when the MAbs were added. Our current hypothesis is that NA inhibition by collectins relies on binding to the HA and steric hindrance of the NA such that it cannot reach surface-bound sialic acid ligands (30). It is possible that the hSP-D-NCRD/R343V trimer is not as effective as full-length collectins at inhibiting NA activity due to its smaller size and that cross-linking by the MAbs allows it to mediate greater steric hindrance of NA activity.
Summary.
NCRD trimers of collectins can mediate increased uptake of virus by neutrophils and monocytes. This activity can be modulated by introduction of specific mutations around the lectin site of hSP-D-NCRD that increase the apparent affinity of the hSP-D-NCRD for IAV and neutralizing activity. It is likely that enhanced uptake relates to viral aggregating activity of the hSP-D-NCRD in a manner similar to that described for the full-length molecules. We infer that binding activity of individual CRDs is sufficiently great to allow bridging interactions by trimers. Thus far, all of the hSP-D-NCRDs that can serve as opsonins for IAV also have increased viral binding and antiviral activity. Given the phenotype of hSP-D-NCRD/R343V and related mutants, this is likely dependent on the presence of a hydrophobic residue at position 343, with further contributions of other residues on the CRD. Aggregating and opsonizing activity of mutant hSP-D-NCRDs can be further enhanced by cross-linking with MAbs that bind to hSP-D-NCRD without inhibiting its lectin activity. Hence, these activities of hSP-D-NCRD can be increased both by modification of key CRD residues to improve viral binding and by cross-linking of hSP-D-NCRD trimers. The latter effect can be accomplished with MAbs or through modification of NH2-terminal and collagen domains to optimize multimeric assembly.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-06931 (K. L. Hartshorn) and HL-29594 (E. C. Crouch).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Brown-Augsburger P, Hartshorn K, Chang D, Rust K, Fliszar C, Welgus H, Crouch E. Site directed mutagenesis of Cys15 and Cys20 of pulmonary surfactant protein D: expression of a trimeric protein with altered anti-viral properties. J Biol Chem 271: 13724–13730, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Chroneos Z, Abdolrasulnia R, Whitsett J, Rice W, Shepherd V. Purification of a cell-surface receptor for surfactant protein A. J Biol Chem 271: 16375–16383, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Crouch E, Hartshorn K, Horlacher T, McDonald B, Smith K, Cafarella T, Seaton B, Seeberger P, Head J. Recognition of mannosylated ligands and influenza A virus by human SP-D: contributions of an extended site and residue 343. Biochemistry 48: 3335–3345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crouch E, Tu Y, Briner D, McDonald B, Smith K, Holmskov U, Hartshorn K. Ligand specificity of human surfactant protein D: expression of a mutant trimeric collectin that shows enhanced interactions with influenza A virus. J Biol Chem 280: 17046–17056, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Crouch EC, Smith K, McDonald B, Briner D, Linders B, McDonald J, Holmskov U, Head J, Hartshorn K. Species differences in the carbohydrate binding preferences of surfactant protein D. Am J Respir Cell Mol Biol 35: 84–94, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardai S, Xiao Y, Dickinson M, Nick J, Voelker D, Green K, Henson P. By binding SIRP or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115: 13–23, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Giannoni E, Sawa T, Allen L, Wiener-Kronish J, Hawgood S. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 34: 704–710, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gjerstorff M, Hansen S, Jensen B, Dueholm B, Horn P, Bendixen C, Holmskov U. The genes encoding bovine SP-A, SP-D, MBL-A, conglutinin, CL-43 and CL-46 form a distinct collectin locus on Bos taurus chromosome 28 (BTA28) at position q.1.8–1.9. Anim Genet 35: 333–337, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Hansen S, Holm D, Moeller V, Vitved L, Bendixen C, Reid KB, Skjoedt K, Holmskov U. CL-46, a novel collectin highly expressed in bovine thymus and liver. J Immunol 169: 5726–5734, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hartshorn K, Chang D, Rust K, White M, Heuser J, Crouch E. Interactions of recombinant human pulmonary surfactant protein D and SPD multimers with influenza A. Am J Physiol Lung Cell Mol Physiol 271: L753–L762, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Hartshorn K, Crouch E, White M, Colamussi M, Kakkanatt A, Tauber B, Shepherd V, Sastry K. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am J Physiol Lung Cell Mol Physiol 274: L958–L969, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Hartshorn K, Holmskov U, White M, Meschi J, Crouch E. Distinctive antiviral properties of collectin-43 (CL-43). Biochem J 366: 87–96, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartshorn K, Reid K, White M, Morris S, Jensenius J, Crouch E. Neutrophil deactivation by influenza A viruses: mechanisms of protection after viral opsonization with collectins and hemagglutination-inhibiting antibodies. Blood 87: 3450–3461, 1996 [PubMed] [Google Scholar]
- 14.Hartshorn K, Sastry K, Chang D, White M, Crouch E. Enhanced antinfluenza activity of a recombinant pulmonary surfactant protein D and serum conglutinin fusion protein. Am J Physiol Lung Cell Mol Physiol 278: L90–L98, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Hartshorn K, White M, Shepherd V, Reid K, Jensenius J, Crouch E. Mechanisms of anti-influenza activity of pulmonary surfactant proteins A and D: comparison with other collectins. Am J Physiol Lung Cell Mol Physiol 273: L1156–L1166, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Hartshorn KL, Collamer M, Auerbach M, Myers JB, Pavlotsky N, Tauber AI. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol 141: 1295–1301, 1988 [PubMed] [Google Scholar]
- 17.Hartshorn KL, Collamer M, White MR, Schwartz JH, Tauber AI. Characterization of influenza A virus activation of the human neutrophil. Blood 75: 218–226, 1990 [PubMed] [Google Scholar]
- 18.Hartshorn KL, Crouch EC, White MR, Eggleton P, Tauber AI, Chang D, Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest 94: 311–319, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartshorn KL, Holmskov U, Hansen S, Zhang P, Meschi J, Mogues T, White MR, Crouch EC. Distinctive anti-influenza properties of recombinant collectin 43. Biochem J 366: 87–96, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartshorn KL, Sastry K, Brown D, White MR, Okarma TB, Lee YM, Tauber AI. Conglutinin acts as an opsonin for influenza A viruses. J Immunol 151: 6265–6273, 1993 [PubMed] [Google Scholar]
- 22.Hartshorn KL, Sastry K, White MR, Anders EM, Super M, Ezekowitz RA, Tauber AI. Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest 91: 1414–1420, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartshorn KL, White MR, Tecle T, Tornoe I, Sorensen GL, Crouch EC, Holmskov U. Reduced influenza viral neutralizing activity of natural human trimers of surfactant protein D. Respir Res 8: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawgood S, Brown C, Edmondson J, Stumbaugh A, Allen L, Goerke J, Clark H, Poulain F. Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J Virol 78: 8565–8572, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol 9: 1871–1879, 2007 [DOI] [PubMed] [Google Scholar]
- 26.LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 167: 5868–5873, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Madsen J, Kliem A, Tornoe I, Skjolt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol 164: 5866–5870, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Rothmann A, Mortensen H, Holmskov U, Hojrup P. Structural characterization of bovine collectin-43. Eur J Biochem 243: 630–635, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Stevens J, Corper AL, Basler CF, Taubenberger JK, Palese P, Wilson IA. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science 303: 1866–1870, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Tecle T, White MR, Crouch EC, Hartshorn KL. Inhibition of influenza viral neuraminidase activity by collectins. Arch Virol 152: 1731–1742, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Tecle T, White MR, Gantz D, Crouch EC, Hartshorn KL. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol 178: 8046–8052, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Tecle T, White MR, Sorensen G, Gantz D, Kacak N, Holmskov U, Smith K, Crouch EC, Hartshorn KL. Critical role for cross-linking of trimeric lectin domains of surfactant protein D in antiviral activity against influenza A virus. Biochem J 412: 323–329, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Tumpey TM, Maines TR, Van Hoeven N, Glaser L, Solorzano A, Pappas C, Cox NJ, Swayne DE, Palese P, Katz JM, Garcia-Sastre A. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315: 655–659, 2007 [DOI] [PubMed] [Google Scholar]
- 34.White M, Kingma P, Tecle T, Kacak N, Linders B, Heuser J, Crouch E, Hartshorn K. Multimerization of surfactant protein D, but not its collagen domain, is required for antiviral and opsonic activities related to influenza virus. J Immunol 181: 7936–7943, 2008 [DOI] [PubMed] [Google Scholar]
- 35.White MR, Crouch E, van Eijk M, Hartshorn M, Pemberton L, Tornoe I, Holmskov U, Hartshorn KL. Cooperative anti-influenza activities of respiratory innate immune proteins and neuraminidase inhibitor. Am J Physiol Lung Cell Mol Physiol 288: L831–L840, 2005 [DOI] [PubMed] [Google Scholar]
- 36.White MR, Crouch E, Vesona J, Tacken PJ, Batenburg JJ, Leth-Larsen R, Holmskov U, Hartshorn KL. Respiratory innate immune proteins differentially modulate the neutrophil respiratory burst response to influenza A virus. Am J Physiol Lung Cell Mol Physiol 289: L606–L616, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5: 58–68, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Hartshorn K, Crouch E, Ikegami M, Whitsett J. Complementation of pulmonary abnormalities in SP-D (-/-) mice with an SP-D/conglutinin fusion protein. J Biol Chem 277: 22453–22459, 2002 [DOI] [PubMed] [Google Scholar]