Abstract
The Hedgehog (Hh) family of secreted proteins controls many aspects of growth and patterning in animal development. The seven-transmembrane protein Smoothened (Smo) transduces the Hh signal in both vertebrates and invertebrates; however, the mechanism of its action remains unknown. We found that Smo lacking its C-terminal tail (C-tail) is inactive, whereas membrane-tethered Smo C-tail has constitutive albeit low levels of Hh signaling activity. Smo physically interacts with Costal2 (Cos2) and Fused (Fu) through its C-tail. Deletion of the Cos2/Fu-binding domain from Smo abolishes its signaling activity. Moreover, overexpressing Cos2 mutants that fail to bind Fu and Ci but retain Smo-binding activity blocks Hh signaling. Taken together, our results suggest that Smo transduces the Hh signal by physically interacting with the Cos2/Fu protein complex.
Keywords: Smo, Hh, Cos2, Fu, signaling, development
The Hedgehog (Hh) family of secreted proteins governs cell growth and patterning in numerous developmental processes in both vertebrates and invertebrates (Ingham and McMahon 2001). In addition, misregulation of Hh signaling activity has been implicated in many human disorders including cancers (Villavicencio et al. 2000; Taipale and Beachy 2001). In Drosophila wing development, posterior (P) compartment cells express and secrete Hh proteins that diffuse into the anterior (A) compartment and induce neighboring A-compartment cells to express decapentaplegic (dpp), which encodes a member of the TGFβ/BMP family of secreted proteins (Basler and Struhl 1994; Tabata and Kornberg 1994). Dpp then diffuses bidirectionally and functions as a long-range morphogen to control the growth and patterning of cells in the whole wing (Lecuit et al. 1996; Nellen et al. 1996). Although the long-range organizing influence of Hh is mediated by Dpp, Hh functions as a local morphogen to specify patterns near the A/P compartment boundary by activating other genes including patched (ptc) and engrailed (en) in a concentration-dependent manner (Strigini and Cohen 1997).
Hh exerts its biological influence via a conserved, yet not well-defined, signal transduction pathway (Ingham and McMahon 2001). The reception system for the Hh signal consists of two multispan transmembrane proteins Patched (Ptc) and Smoothened (Smo). In the absence of Hh, Ptc inhibits Smo activity through a poorly understood mechanism. In Hh-receiving cells, Hh physically interacts with Ptc and alleviates its inhibition on Smo (Chen and Struhl 1996; Stone et al. 1996), allowing Smo to signal downstream. How Smo activates downstream signaling components is a mystery.
In Drosophila, Hh signal transduction culminates in the activation of Cubitus interruptus (Ci), a member of the Gli family of zinc finger transcription factors (Ingham and McMahon 2001). In imaginal disc development, Ci plays dual roles that are performed by two distinct forms. In the absence of Hh, the full-length Ci (Ci155) undergoes proteolytic processing to generate a truncated form (Ci75) that functions as a repressor to block the expression of Hh-responsive genes such as dpp (Aza-Blanc et al. 1997; Methot and Basler 1999). Hh signaling blocks Ci processing to form Ci75. The accumulated Ci155 acts as an activator to turn on other Hh-responsive genes including ptc and en (Alexandre et al. 1996; Methot and Basler 1999).
Ci processing requires the activities of at least three kinases: the cAMP-dependent protein kinase (PKA), GSK3, and CKI (Jiang and Struhl 1998; Y. Chen et al. 1999; Price and Kalderon 1999, 2002; Wang et al. 1999; Jia et al. 2002; Jiang 2002). These kinases phosphorylate Ci at multiple sites in three clusters in its C-terminal region (Jiang 2002). Hyperphosphorylation of Ci targets it for Slimb/Proteasome-mediated proteolytic processing (Jiang and Struhl 1998; Jiang 2002). Hh appears to induce dephosphorylation of Ci, leading to the blockage of its processing (C.H. Chen et al. 1999).
Hh regulates Ci at multiple levels. In addition to blocking its proteolysis, Hh also induces nuclear translocation of Ci155 and further stimulates its transcriptional activity (Ohlmeyer and Kalderon 1998; C.H. Chen et al. 1999; Wang and Holmgren 1999, 2000; Wang et al. 1999, 2000). In the absence of Hh, Ci155 is retained in the cytoplasm by forming a large protein complex that also includes the kinesin-related protein Costal2 (Cos2), the Ser/Thr kinase Fused (Fu), and the tumor suppressor protein Su(fu) (Methot and Basler 2000; Wang and Holmgren 2000; Wang et al. 2000). The Ci/Cos2/Fu complex binds microtubules, likely through Cos2, in an Hh-regulated manner (Robbins et al. 1997; Sisson et al. 1997; Stegman et al. 2000). The transcriptional activity of Ci155 appears to be further inhibited by PKA phosphorylation and by stoichiometric interaction with Su(fu) (Ohlmeyer and Kalderon 1997; Wang et al. 1999). Su(fu) prevents the maturation of Ci155 into a labile hyperactive form, and Hh alleviates such inhibition through Fu kinase activity (Ohlmeyer and Kalderon 1997). Su(fu) appears to inhibit Ci155 by both impeding its nuclear translocation and inhibiting its transcriptional activity after it enters the nucleus (Methot and Basler 2000; Wang et al. 2000). One possible mechanism for Su(fu) to inhibit the transcriptional activator activity of Ci155 is to recruit transcription corepressors (Cheng and Bishop 2002).
Different layers of negative regulation of Ci appear to be offset by distinct thresholds of Hh signaling activity. Low levels of Hh signaling activity suffice to block Ci processing but do not stimulate the transcriptional activity of Ci155 (Methot and Basler 1999; Wang and Holmgren 1999; Wang et al. 1999). As a consequence, dpp is derepressed, whereas other genes that are activated by Ci155 remain silent. High levels of Hh activate Ci155, at least in part, by alleviating Su(fu)-mediated repression (Ohlmeyer and Kalderon 1998). Cos2 was initially identified as a negative component in the Hh pathway; however, a recent study suggested that it also has a positive role in the pathway, as removal of cos2 function in Hh-receiving cells blocks the transduction of high levels of Hh signaling activity (Wang et al. 2000).
Here we address how Smo relays Hh signal to intracellular signaling components. Although Smo is related to the serpentine family of receptors that transduce signals through trimeric G-proteins (Alcedo et al. 1996; van-den-Heuval and Ingham 1996), no evidence for the involvement of a G-protein in physiological Hh signaling has been obtained (Ingham and McMahon 2001). Here we provide evidence that Smo transduces the Hh signal by physically interacting with the Cos2/Fu complex through its C-terminal tail.
Results
Smo C-tail is essential for its activity
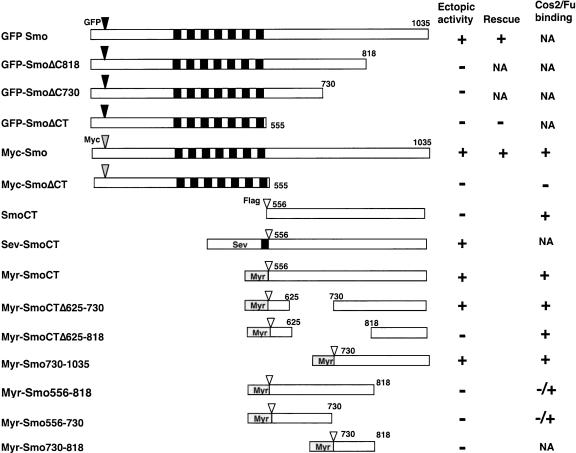
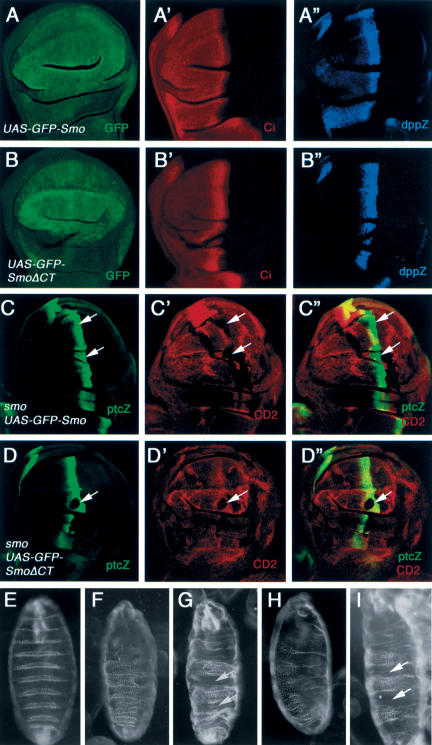
To determine the mechanism by which Smo transduces the Hh signal, we generated epitope-tagged full-length and truncated forms of Smo (summarized in Fig. 1), and assessed their signaling activities in wing imaginal discs using the Gal4/UAS system (Brand and Perrimon 1993). Overexpressing a full-length Smo tagged by GFP at its N terminus (GFP-Smo) by an MS1096 Gal4 driver resulted in ectopic albeit low levels of Hh pathway activation, as evidenced by the accumulation of high levels of Ci155 and ectopic expression of modest levels of dpp-lacZ in A-compartment cells away from the A/P compartment boundary (Fig. 2A-A″). Coexpressing Ptc with GFP-Smo inhibited the ectopic Hh signaling activity caused by overexpressing GFP-Smo alone (Supplementary Fig. 1). These observations suggest that high levels of Smo could at least partially titrate out the inhibitory activity of Ptc, resulting in constitutive Hh pathway activation. In contrast, overexpressing a truncated form of Smo that lacks the C-tail (GFP-SmoΔCT) failed to induce ectopic Hh pathway activation (Fig. 2B-B″), even though its level of expression is comparable to that of full-length Smo (Fig. 2, cf. B and A). Moreover, wing discs expressing multiple copies of UAS-GFP-SmoΔCT by MS1096 did not ectopically activate the Hh pathway (data not shown).
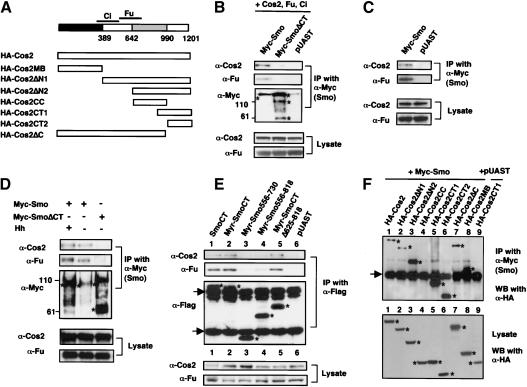
Figure 1.
Tagged Smo and its deletion mutants. Filled and gray boxes indicate the transmembrane domains and myristoylation signal (Myr), respectively. Filled, gray, and open triangles indicate the position where GFP, Myc, or Flag tag was inserted, respectively. The amino acid residues that demarcate each deletion mutant are indicated. Individual constructs were assayed for Hh signaling activity by overexpression using the UAS/Gal4 system in developing wing. Ectopic activity was scored when full-length Ci was stabilized and dpp-lacZ was activated in A-compartment cells away from the A/P compartment boundary. Rescue activity was scored when expressing a given Smo derivative can restore ptc-lacZ in smo mutant clones at the A/P boundary and/or restore naked cuticles in smo zygotic null embryos. Cos2/Fu binding was determined by coimmunoprecipitation. (NA) Not accessed.
Figure 2.
The Smo C-tail is essential for Hh signaling. Wing discs expressing UAS-GFP-Smo (A-A″) or UAS-GFP-SmoΔCT by MS1096 (B-B″) were immunostained to show the expression of GFP (green in A,B), Ci155 (red in A′,B′), and dpp-lacZ (blue in A″,B″). Wing discs containing smo mutant clones and expressing UAS-GFP-Smo (C-C″) or UAS-GFP-SmoΔCT by MS1096 (D-D″) were immunostained to show the expression of ptc-lacZ (green) and CD2 (red). Smo mutant clones are recognized by the lack of CD2 expression (arrows). (E-I) Cuticles were prepared from embryos of the following genotypes growing at 18°C: wild type (E), smo3/smo3 (F), smo3/smo3; prd-Gal4/UAS-GFP-Smo (G), smo3/smo3; prd-Gal4/UAS-GFP-SmoΔCT (H), and smo3/smo3; prd-Gal4/UAS-Myc-Smo (I). The wild-type embryo exhibits alternate naked cuticles and denticles (E), whereas the smo3 homozygote exhibits the typical zygotic null phenotype with a lawn of denticles covering most of the ventral surface (F). Naked cuticles (indicated by arrows) are restored in alternate segments of smo3 homozygotes expressing either UAS-GFPSmo (G) or UAS-Myc-Smo (I), but not in smo3 homozygotes expressing UAS-GFP-SmoΔCT (H).
We also examined if GFP-SmoΔCT possess any Hhinducible activity by expressing UAS-GFP-SmoΔCT in wing discs carrying a smo mutant clone induced by FLP/FRT-mediated mitotic recombination (Xu and Rubin 1993). smo mutant cells expressing GFP-SmoΔCT failed to activate ptc-lacZ expression near the A/P compartment boundary (Fig. 2D-D″), whereas GFP-Smo could functionally substitute the endogenous Smo to activate ptc-lacZ in A-compartment cells near the A/P border (Fig. 2C-C″). In addition, expressing GFP-Smo but not GFP-SmoΔCT in smo zygotic null embryos rescued smo mutant phenotypes (Fig. 2F-H). Similar results were obtained with Myc-tagged full-length (Myc-Smo) and C-terminally truncated Smo (Myc-SmoΔCT; Fig. 2I; data not shown). These results demonstrate that the Smo C-tail is essential for its signaling activity.
Membrane-tethered forms of Smo C-tail possess constitutive Hh signaling activity
To further assess the role of the Smo C-tail in Hh signal transduction, we generated several Flag-tagged Smo deletion mutants that only contain the C-tail portion of Smo (Fig. 1). Sev-SmoCT is a chimeric protein that contains the transmembrane and extracellular domain of Sevenless (Sev) protein fused to SmoCT, whereas Myr-SmoCT contains a myristoylation signal at the N terminus of SmoCT. Surprisingly, A-compartment cells expressing either Sev-SmoCT or Myr-SmoCT accumulate high levels of Ci155 and activate dpp-lacZ in a cell-autonomous manner even when they are distant from the A/P compartment boundary (Fig. 3A-B″), suggesting that both Sev-SmoCT and Myr-SmoCT can block Ci processing and the formation of Ci75. In contrast, cells expressing untethered SmoCT do not ectopically activate the Hh signaling pathway (data not shown), suggesting that membrane targeting of SmoCT is essential for its activity. As Sev-SmoCT and Myr-SmoCT activate the Hh signaling pathway to similar extents, we focused our analyses on Myr-SmoCT.
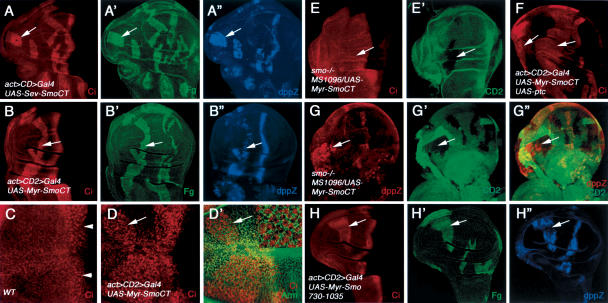
Figure 3.
Constitutive Hh signaling activity associated with the Smo C-tail. Wing discs expressing UAS-Sev-SmoCT (A-A″) or UAS-Myr-SmoCT by act > CD2 > Gal4 (B-B″) were stained for Ci155 (red), Flag (green), and dpp-lacZ (blue). A-compartment cells expressing either Sev-SmoCT or Myr-SmoCT (recognized by Flag staining) accumulate high levels of Ci155 and activate dpp-lacZ in a cell-autonomous fashion. Wild-type wing disc (C) or wing disc expressing Myr-SmoCT by act > CD2 > Gal4 (D-D″) were treated with LMB, followed by immunostaining for Ci155 (red) and Arm (green). In wild-type discs, Ci155 is detected in the nucleus near the A/P border (arrowheads in C). Ci155 translocates into the nucleus in anteriorly situated cells expressing Myr-SmoCT (arrows in D,D′). The inset in D′ shows a high-magnification view of anteriorly situated cells expressing Myr-SmoCT. (E,E′) A wing disc carrying an smo mutant clone and expressing MS1096/UAS-Myr-SmoCT was immunostained to show CD2 expression (green) and the accumulation of Ci155 (red). smo mutant cells (marked by the lack of CD2 expression) expressing Myr-SmoCT accumulate high levels of Ci155. (F) A-compartment cells expressing both UAS-Myr-SmoCT and UAS-ptc by act > CD2 > Gal4 accumulate high levels of Ci155 (arrows). Expression from UAS-ptc was confirmed by staining with an anti-Ptc antibody (data not shown). (G-G″) A wing disc expressing MS1096/UAS-Myr-SmoCT and containing smo3 clones was immunostained to show the expression of dpp-lacZ (red) and CD2 (green). smo3 mutant cells are marked by the lack of CD2 expression. Overexpressing Myr-SmoCT activates dpp-lacZ in smo3 mutant cells (arrows). (H-H″) A wing disc expressing UAS-Myr-Smo730-1035 under the control of act > CD2 > Gal4 was stained for Ci155 (red), Flag (green), and dpp-lacZ (blue). A-compartment cells expressing Myr-Smo730-1035 accumulate high levels of Ci155 and activate dpp-lacZ.
In addition to preventing Ci processing, Hh also promotes nuclear translocation of accumulated Ci155 (C.H. Chen et al. 1999; Wang and Holmgren 2000; Wang et al. 2000). To determine whether Myr-SmoCT stimulates Ci155 nuclear translocation, we treated wing discs expressing Myr-SmoCT with Leptomycin B (LMB), an inhibitor that blocks Ci155 nuclear export (C.H. Chen et al. 1999; Wang and Holmgren 2000; Wang et al. 2000). In wild-type wing discs, A-compartment cells near the A/P boundary accumulate Ci155 in the nucleus, whereas cells situated distantly from the A/P boundary keep Ci155 in the cytoplasm (Fig. 3C; Wang and Holmgren 2000; Wang et al. 2000). In contrast, A-compartment cells expressing Myr-SmoCT accumulate Ci155 in the nucleus regardless of their locations (Fig. 3D,D′), indicating that Myr-SmoCT stimulates Ci155 nuclear translocation in addition to blocking its processing.
Myr-SmoCT activates Hh signaling independent of endogenous Smo
To determine if Myr-SmoCT can elicit Hh signaling activity in the absence of endogenous Smo, we misexpressed Myr-SmoCT in wing discs carrying smo-null mutant clones induced by FLP/FRT-mediated mitotic recombination. As shown in Figure 3, smo- cells expressing Myr-SmoCT accumulate high levels of Ci (Fig. 3E,E′), and activate dpp-lacZ even though they are situated in A-compartment cells distant from the A/P boundary (Fig. 3G-G″), suggesting that Myr-SmoCT possesses constitutive Hh signaling activity independent of endogenous Smo. To determine if Myr-SmoCT is refractory to Ptc inhibition, UAS-Ptc and UAS-Myr-SmoCT were co-overexpressed in wing discs by the act > CD2 > Gal4 driver (Pignoni et al. 1997). As shown in Figure 3F, coexpression of Ptc does not block the ectopic Hh signaling activity caused by overexpressing Myr-SmoCT. Hence, Myr-SmoCT appears to escape the inhibitory regulation by Ptc, which explains why it is constitutively active.
Myr-SmoCT does not fully activate the Hh pathway
Although Myr-SmoCT can stabilize Ci155 and promote its nuclear import, it does not fully stimulate the transcriptional activity of Ci155, as wing discs expressing UAS-Myr-SmoCT by act > CD2 > Gal4 does not activate ptc-lacZ and en in most of the A-compartment cells (Fig. 4B; data not shown). Under this condition, ptc-lacZ is only activated at low levels in anteriormost regions of wing discs (Fig. 4B), where cells are more responsive to low levels of Hh signaling activity (Jia et al. 2002).
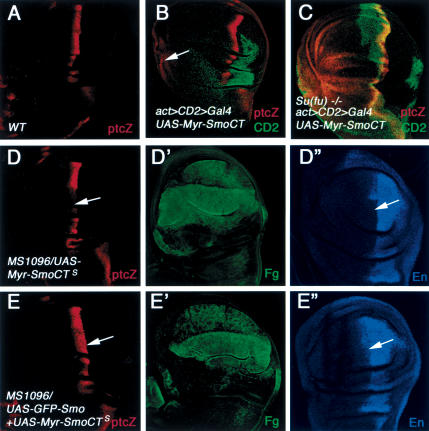
Figure 4.
Myr-SmoCT does not fully activate the Hh pathway and interferes with endogenous Smo. (A) ptc-lacZ expression in a wild-type wing disc. Wild-type (B) or Su(fu)LP homozygous (C) wing discs expressing UAS-Myr-SmoCT by act > CD2 > Gal4 were immunostained to show the expression of CD2 (green) and ptc-lacZ (red). Myr-SmoCT-expressing cells are recognized by the lack of CD2 staining. Myc-SmoCT only activates low levels of ptc-lacZ in anteriormost cells (arrow in B). (C) In contrast, most A-compartment cells expressing Myr-SmoCT activate high levels of ptc-lacZ in Su(fu) homozygous discs. (D-E″) Wing disc expressing a strong line of Myr-SmoCT (UAS-Myr-SmoCTS) alone (D-D″) or in conjunction with GFP-Smo (E-E″) were immunostained to show the expression of ptc-lacZ (red), Flag (green), and En (blue). High levels of Myr-SmoCT inhibit the expression of ptc-lacZ and en in A-compartment cells near the A/P boundary (arrows in D,D″), which is reversed by coexpressing GFP-Smo (arrows in E,E″).
The inability of Myr-SmoCT to fully activate the Hh pathway is not caused by the lack of enough expression. As a matter of fact, the levels of Myr-SmoCT expressed by act > CD2 > Gal4 is much higher than those of endogenous Smo at the A/P compartment boundary, as determined by immunostaining with an antibody against the Smo C-tail (data not shown). In addition, increasing the dose of Myr-SmoCT by expressing multiple copies of UAS-Myr-SmoCT does not further enhance the Hh signaling activity elicited by Myr-SmoCT (data not shown).
It has been shown that Su(fu) can inhibit Ci155 activity via a mechanism that is independent of Ci nuclear localization (Wang et al. 2000). Hence, one possible reason that Myr-SmoCT fails to activate Ci155 is that it does not alleviate the inhibition of Ci155 by Su(fu). If so, removal of Su(fu) should allow activation of Ci155 accumulated in Myr-SmoCT-expressing cells. We therefore misexpressed Myr-SmoCT in Su(fu) homozygous wing discs. Although Su(fu) homozygous wing discs do not exhibit any discernible phenotypes (Ohlmeyer and Kalderon 1998), overexpressing Myr-SmoCT in Su(fu) wing discs ectopically activated high levels of ptc-lacZ in most A-compartment cells (Fig. 4C).
Deletion analysis of Smo C-tail
To further define the region within the Smo C-tail that mediates Hh pathway activation, we generated a series of deletion mutants that remove various parts of the Smo C-tail and examined their abilities to activate the Hh pathway (Fig. 1). We found that Myr-SmoCTΔ625-730 and Myr-Smo730-1035 can elicit Hh pathway activation, as indicated by the accumulation of Ci155 and ectopic expression of dpp-lacZ (Supplementary Fig. 2; Fig. 3H-H″). In contrast, Myr-Smo556-730, Myr-Smo730-818, Myr-Smo556-818, and Myr-SmoCTΔ625-818 do not possess Hh signaling activity (Supplementary Fig. 2; data not shown). Deleting the C-terminal sequence from full-length Smo (GFP-SmoΔ818 and GFP-SmoΔC730) also abolishes Smo activity as overexpressing these deletion mutants failed to ectopically activate dpp-lacZ (Supplementary Fig. 2; data not shown). These results suggest that the C-terminal half of the Smo C-tail (amino acids 730-1035) is critical for Smo activity.
Smo physically interacts with Cos2/Fu through its C-tail
Interestingly, expressing Myr-SmoCT at high levels inhibits the expression of endogenous ptc and en near the A/P compartment boundary (Fig. 4D-D″), suggesting that Myr-SmoCT can interfere with the ability of endogenous Smo to transduce high levels of Hh signaling activity. The inhibition of ptc and en expression by Myr-SmoCT can be suppressed by coexpressing GFP-Smo (Fig. 4E-E″). One hypothesis that accounts for these observations is that Myr-SmoCT may compete with endogenous Smo for a downstream signaling effector(s).
Several known intracellular Hh signaling components, including Cos2, Fu, and Ci, form large protein complexes (Robbins et al. 1997; Sisson et al. 1997). As no signaling intermediates between Smo and the Cos2 complex have been identified, we sought to determine if Smo transduces Hh signal by physically interacting with the Cos2 complex. To facilitate the detection of complex formation, we transfected S2 cells with DNA constructs expressing Myc-tagged full-length Smo (Myc-Smo) or its C-tail deletion mutant (Myc-SmoΔCT), HA-tagged Cos2, Fu, and Ci. Cell extracts were immunoprecipitated with a mouse αMyc antibody, followed by Western blot analysis with αCos2, αFu, or αCi (2A) antibody. Myc-Smo but not Myc-SmoΔCT pulled down Cos2 and Fu (Fig. 5B), suggesting that Myc-Smo binds the Cos2/Fu complex and the Smo C-tail is essential for this interaction. Ci was not consistently pulled down by Myc-Smo (data not shown). It is possible that Ci/Smo interaction is very dynamic. For example, Ci may dissociate from the Cos2/Fu complex after the complex binds Smo. We also found that Myc-Smo expressed in S2 cells can pull down endogenous Cos2 and Fu (Fig. 5C). As S2 cells do not express Ci, this result indicates that Smo can interact with Cos2 and Fu independently of Ci.
Figure 5.
Smo binds to the Cos2/Fu complex through its C-tail. (A) HA-tagged Cos2 and its deletion mutants. The microtubule-binding (MB) and coiled-coil (CC) domains are indicated by the black and gray boxes, respectively. The Ci- and Fu-binding domains are demarcated by lines above the diagram. (B,C) S2 cells were transfected with Myc-Smo or Myc-SmoΔCT expressing constructs with (B) or without (C) Cos2-, Fu-, and Ci-expressing constructs. The blank expressing vector pUAST was used as a control. Cell extracts were immunoprecipitated (IP) with anti-Myc antibody. Immunoprecipitates and 5% of cell lysates were analyzed by immunoblotting (WB) with indicated antibodies. Myc-Smo but not Myc-SmoΔCT pulled down overexpressed as well as endogenous Cos2 and Fu. Of note, overexpressed Myc-Smo and Myc-SmoΔCT exhibit slow mobility (indicated by asterisks). A similar observation was made with overexpressed vertebrate Smo (Stone et al. 1996). (D) Cell extracts were prepared from 400 wing discs expressing UAS-Myc-Smo, UAS-Myc-SmoΔCT, or UAS-Myc-Smo in conjunction with UAS-Hh under the control of MS1096. Wing disc extracts were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with indicated antibodies. Five percent of lysates were analyzed by direct Western with Cos2 or Fu antibody. Myc-Smo but not Myc-SmoΔCT pulled down endogenous Cos2 and Fu. Ectopic Hh appears to stimulate phosphorylation of Fu bound to Myc-Smo, as indicated by slower mobility. Myc-Smo and Myc-SmoΔCT are indicated by asterisks. (E) S2 cells were transfected with the indicated Flag-tagged Smo constructs. Cell lysates were immunoprecipitated with anti-Flag antibody followed by immunoblotting with anti-Cos2 (top), anti-Fu (middle), or anti-Flag (bottom) antibodies. Asterisks indicate the position of proteins expressed from corresponding Smo constructs. Arrows indicate IgG. (F) S2 cells were transfected with Myc-Smo in conjunction with various HA-tagged Cos2 deletion mutants. Cell extracts were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with anti-HA antibody. Five percent of cell lysates were also subjected to Western blotting with anti-HA antibody (bottom). Asterisks indicate the position of HA-tagged Cos2 proteins expressed from corresponding constructs (bottom) or immunoprecipitated with Myc-Smo (top). Of note, HA-Cos2MB immunoprecipitated with Myc-Smo runs very closely to IgG.
To determine if Smo binds Cos2 and Fu in vivo, we expressed Myc-Smo or Myc-SmoΔCT in wing discs by the MS1096 Gal4 driver. Wing disc extracts were immunoprecipitated with the αMyc antibody, followed by Western blot analysis with αCos2, αFu, and αCi antibodies. As shown in Figure 5D, Myc-Smo but not Myc-SmoΔCT pulled down Cos2 and Fu. Myc-Smo did not pull down detectable levels of Ci (data not shown). We also coexpressed UAS-Hh with UAS-Myc-Smo to determine if the interaction between Myc-Smo and Cos2/Fu is affected. As expected, ectopic expression of Hh stabilizes coexpressed Myc-Smo, as more Myc-Smo was pulled down by the αMyc antibody (Fig. 5D). However, the amounts of Cos2 and Fu pulled down by Myc-Smo only increase modestly (Fig. 5D), suggesting that overexpressing Myc-Smo alone may already saturate Cos2/Fu binding. Interestingly, a significant fraction of Fu bound to Myc-Smo became phosphorylated when Hh was coexpressed, as indicated by its mobility shift (Fig. 5D). Hence, overexpressed Myc-Smo binds Cos2/Fu in vivo, but appears to stimulate Fu phosphorylation only when Hh is coexpressed.
When expressed in S2 cells, both Myr-SmoCT and Myr-Smo730-1035 pulled down endogenous Cos2/Fu (Fig. 5E, lane 2; data not shown), suggesting that SmoCT is sufficient for binding to the Cos2/Fu complex. Consistent with their inability to activate the Hh pathway, both Myr-Smo556-730 and Myr-Smo556-818 pull down diminishing levels of Cos2/Fu (Fig. 5E, lanes 3,4). Intriguingly, Myr-SmoCTΔ625-818, which does not possess Hh signaling activity, appears to bind Cos2/Fu with affinity similar to that of Myr-SmoCT (Fig. 5E, lane 5). In addition, the untethered SmoCT also binds Cos2/Fu (Fig. 5E, lane 1). These observations suggest that binding of Smo to Cos2/Fu per se does not trigger Hh pathway activation. It seems that membrane association and the Smo sequence between amino acids 730 and 818 are also critical for SmoCT to activate the Hh pathway.
To define the Cos2 domain(s) that mediates Smo binding, we generated a series of HA-tagged Cos2 deletion mutants (summarized in Fig. 5A) and examined their ability to bind Myc-Smo in S2 cells by coimmunoprecipitation. It appears that Myc-Smo binds Cos2 through at least two regions of Cos2: the microtubule-binding domain and the C-tail, as HA-Cos2MB, HA-Cos2CT1, and HA-Cos2CT2 were pulled down robustly by Myc-Smo (Fig. 5F, lanes 5,6,8). HA-Cos2ΔN2 also binds strongly to Myc-Smo (Fig. 5F, lane 3). In contrast, HACos2CC was not pulled down by Myc-Smo (Fig. 5F, lane 4). Intriguingly, relatively less HA-Cos2, HA-Cos2ΔN1, and HA-Cos2ΔC were pulled down by Myc-Smo (Fig. 5F, lanes 1,2). One possibility is that Smo-binding domains in these large proteins could be partially “masked.” Alternatively, they might be unstable when bound to Smo in the absence of Fu. Consistent with the latter hypothesis, we found that coexpression of Fu with HA-Cos2 dramatically increases the amount of HA-Cos2 pulled down by Myc-Smo (data not shown).
Blockage of Hh signal transduction by Cos2 deletion mutants
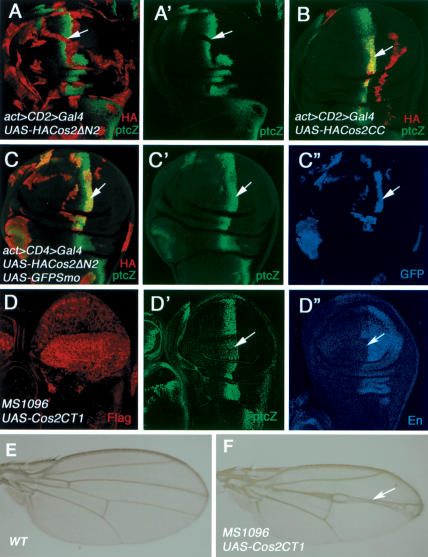
To access the physiological significance of Smo/Cos2 interaction in Hh signal transduction, we expressed Cos2 mutants that lack Ci- and Fu-binding domains but retain Smo-binding activity in wing discs and examined their effects on Hh signaling. We reasoned if Smo transduces Hh signal by recruiting the Cos2/Fu complex, overexpressing such Cos2 deletion mutants should titrate out endogenous Smo and prevent it from interacting with the endogenous Cos2/Fu complex. As a consequence, Hh signal transduction should be blocked. The Ci- and Fu-binding domains have been mapped to the N-terminal half of Cos2 (Fig. 5A; Monnier et al. 2002), and Cos2ΔN2 failed to bind Ci and Fu in yeast (G. Wang and J. Jiang, unpubl.). As shown in Figure 6A, overexpressing Cos2ΔN2 in wing discs blocks Hh signaling, as indicated by the loss of ptc-lacZ. The inhibition of Hh signaling activity by Cos2ΔN2 correlates with Smo binding as Cos2CC, which does not bind Smo, fails to block Hh signaling (Fig. 6B). Moreover, overexpressing the Cos2 C-tail (Cos2CT1) also results in pathway inhibition, as manifested by the reduction of ptc-lacZ and anterior en expression (Fig. 6D-D″) as well as fu-like wing phenotypes (Fig. 6F). If the inhibition of Hh signaling by Cos2 deletion mutants is caused by titrating out endogenous Smo and preventing it from binding to endogenous Cos2/Fu complex, then increasing the amounts of Smo should reverse such a blockage. Indeed, coexpressing GFP-Smo with Cos2ΔN2 restores ptc-lacZ expression (Fig. 6C-C″).
Figure 6.
Blockage of Hh signaling by Cos2 deletion mutants. (A,A′) A wing disc expressing UAS-HA-Cos2ΔN2 by act > CD2 > Gal4 was immunostained for ptc-lacZ (green) and HA (red). Cos2ΔN2 blocks the expression of ptc-lacZ near the A/P border (arrows). (B) A wing disc expressing UAS-HACos2CC by act > CD2 > Gal4. Cos2CC failed to suppress ptc-lacZ expression at the A/P compartment boundary (arrows). (C-C″) A wing disc expressing both UAS-HA-Cos2ΔN2 and UAS-GFP-Smo by act > CD2 > Gal4 was immunostained to show the expression of HA (red), ptc-lacZ (green), and GFP (blue). Coexpression of GFP-Smo with Cos2ΔN2 restores the expression of ptc-lacZ near the A/P border (arrows). (D-D″) A wing disc expressing UAS-Flag-Cos2CT1 by MS1096 was stained to show the expression of Flag (red), ptc-lacZ (green), and En (blue). High levels of Cos2CT1 suppress the expression of ptc-lacZ and en (arrows). A wild-type adult wing (E) or adult wing expressing UAS-Flag-Cos2CT1 by MS1096 (F). Overexpressing Cos2CT1 results in wing phenotypes similar to those caused by the fu mutation.
Discussion
The seven-transmembrane protein Smo plays a central role in transducing Hh signal. In this study, we demonstrated that the Smo C-terminal tail (C-tail) is essential for its function. Surprisingly, we found that the Smo C-tail possesses constitutive albeit low levels of signaling activity when it is tethered to plasma membrane. We provided biochemical evidence that Smo interacts with the Cos2/Fu complex through its C-tail, and Smo/Cos2 interaction is mediated at least in part through the C-terminal region of Cos2. In addition, we provided evidence that Smo/Cos2 interaction is essential for Hh signal transduction. Our results suggest that Smo transduces Hh signal by directly recruiting the Cos2/Fu complex to the plasma membrane rather than by activating a signaling cascade involving a secondary messenger, a mechanism ascribed to most members of the serpentine receptor family.
Smo transduces Hh signal through its C-tail
A previous structure-function study on mammalian Smo using cultured cells suggested that the Smo C-tail is not essential for Smo activity (Murone et al. 1999). Here we showed that Smo deletion mutants lacking the Smo C-tail (SmoΔCT) or part of it (SmoΔC818 and SmoΔC730) are inactive when overexpressed in wing discs. Moreover, SmoΔCT does not substitute endogenous Smo to transduce signal in Hh-receiving cells. Hence, the Smo C-tail is absolutely required for Smo activity. It is not clear what causes the discrepancy. One possibility is that Drosophila Smo may act differently from mammalian Smo. Alternatively, Smo may behave differently in vivo versus in vitro. Consistent with this, we found that mouse Smo without a C-tail fails to induce ventral markers in chick spinal cords, whereas a control full-length Smo does (W. Zhang, R. Lu, and J. Jiang, unpubl.).
Perhaps a more surprising finding of our study is that the Smo C-tail suffices to induce Hh pathway activation. We found that overexpressing the membrane-tethered Smo C-tail (Myr-SmoCT, Sev-SmoCT) blocks Ci processing, induces dpp-lacZ expression, and stimulates nuclear translocation of Ci155. Myr-SmoCT is refractory to Ptc inhibition and activates Hh-pathway independent of endogenous Smo. Membrane tethering appears to be crucial for the Smo C-tail to activate the Hh pathway, as untethered SmoCT has no signaling activity. This is consistent with previous observations that cell surface accumulation of Smo correlates with its activity (Denef et al. 2000; Zhu et al. 2003).
Although the Smo C-tail has constitutive Hh signaling activity, it does not possess all the activities associated with full-length Smo. For example, overexpressing Myr-SmoCT in A-compartment cells away from the A/P compartment boundary does not significantly activate ptc and en, which are normally induced by high levels of Hh. In addition, Myr-SmoCT cannot substitute endogenous Smo at the A/P compartment boundary to transduce high levels of Hh signaling activity, as boundary smo mutant cells expressing Myr-SmoCT failed to express ptc in response to Hh (data not shown).
The failure of the Smo C-tail to transduce high Hh signaling activity is due to its inability to antagonize Su(fu). Although Myr-SmoCT blocks Ci processing to generate Ci75, the activity of Ci155 accumulated in Myr-SmoCT-expressing cells is still blocked by Su(fu), as removal of Su(fu) function from Myr-SmoCT-expressing cells allows Ci155 to activate ptc to high levels. As Myr-SmoCT stimulates nuclear translocation of Ci155, the inhibition of Ci155 by Su(fu) in Myr-SmoCT-expressing cells must rely on a mechanism that is independent of impeding Ci nuclear translocation.
Physical interaction between Smo and Cos2/Fu
Several observations prompted us to determine whether Smo could transduce Hh signal by physically interacting with the Cos2/Fu complex. First, although Smo is related to G-protein-coupled receptors, no genetic or pharmacological evidence has been obtained to support the involvement of a G-protein in a physiological Hh signaling process (Ingham and McMahon 2001). Second, Myr-SmoCT can interfere with the ability of endogenous Smo to transduce high levels of Hh signaling activity, which can be offset by increasing the amount of full-length Smo. This implies that Myr-SmoCT may compete with full-length Smo for binding to limiting amounts of downstream signaling components (Fig. 7E,F). Third, extensive genetic screens failed to identify Hh signaling components that may link Smo to the Cos2/Fu complex (Amanai and Jiang 2001; Vegh and Basler 2003).
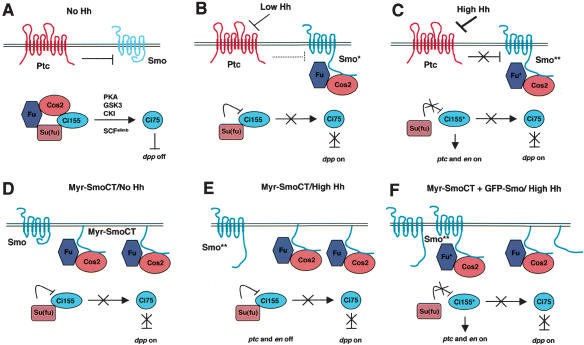
Figure 7.
Signaling by Smo and its C-tail in response to different thresholds of Hh. (A) In the absence of Hh, Ptc prevents cell surface accumulation of Smo. In addition, the Smo C-tail may adopt a “closed” conformation that prevents it from binding to Cos2/Fu. Inside the cell, the full-length Ci (Ci155) forms a complex with Cos2, Fu, and Su(fu). The majority of Ci155 undergoes proteolytic processing to generate the repressor form (Ci75). Ci processing also requires the activity of PKA, GSK3, CKI, and SCFslimb (Jiang 2002). (B) The inhibition of Smo by Ptc is partially alleviated by low levels of Hh (indicated by broken line), leading to an increase of Smo on the cell surface. In addition, the Smo C-tail may adopt an “open” conformation, which allows Smo to bind the Cos2/Fu complex and inhibit its Ci-processing activity. Under this condition, Ci75 is not produced and dpp is derepressed. However, the transcriptional activity of Ci155 is still suppressed by Su(fu). (C) High levels of Hh completely block Ptc, resulting in a further increase in Smo signaling activity. Hyperactive forms of Smo (indicated by two asterisks) stimulate the phosphorylation and activity of bound Fu (indicated by asterisk), which in turn antagonizes Su(fu) to activate Ci155, leading to the expression of ptc and en. (D) Overexpressed membrane-tethered SmoCT binds the Cos2/Fu complex and inhibits Ci processing independently of Hh and endogenous Smo. However, the activity of Ci155 is still blocked by Su(fu). (E) In the presence of Hh, overexpressed Myr-SmoCT competes with activated Smo for binding to Cos2/Fu and prevents it from activating Fu. (F) Increasing the amount of full-length Smo by overexpressing GFP-Smo restores Smo**/Cos2/Fu interaction, allowing Smo** to activate Fu, which in turn stimulates Ci155.
Using a coimmunoprecipitation assay, we demonstrated that Smo interacts with the Cos2/Fu complex both in S2 cells and in wing imaginal discs, and the Smo C-tail appears to be both necessary and sufficient to mediate this interaction. We narrowed down the Cos2/Fu-binding domain to the C-terminal half of the Smo C-tail (between amino acids 818 and 1035). Furthermore, we found that both the microtubule-binding domain (amino acids 1-389) and the C-terminal tail (amino acids 990-1201) of Cos2 interact with Smo. As none of these Cos2 domains binds Fu, this implies that the Cos2/Smo interaction is not mediated through Fu. Ci is also dispensable for Smo/Cos2/Fu interaction, as Smo binds Cos2/Fu in S2 cells in which Ci is not expressed (Aza-Blanc et al. 2000). However, our results did not rule out the possibility that Smo could interact with the Cos2/Fu complex through multiple contacts. For example, Smo could simultaneously contact Cos2 and Fu. Nor did we demonstrate that binding of Cos2 to Smo is direct. Indeed, we failed to detect direct protein-protein interaction between Smo and Cos2 in yeast (data not shown). It is possible that a bridging molecule(s) is required to link Smo to the Cos2/Fu complex. Alternatively, Smo needs to be modified in vivo in order to bind Cos2. It has been shown that Hh stimulates phosphorylation of Smo (Denef et al. 2000); hence, it is possible that phosphorylation of Smo might be essential for recruiting the Cos2/Fu complex.
Several lines of evidence suggest that Smo/Cos2/Fu interaction is important for Hh signal transduction. (1) Deletion of the Cos2-binding domain from Smo, either in the context of full-length Smo or the Smo C-tail, abolishes Smo signaling activity. (2) Overexpressing Cos2 deletion mutants that no longer bind Fu and Ci but retain a Smo-binding domain intercept Hh signal transduction. We previously provided genetic evidence that Cos2 has a positive role in transducing Hh signal in addition to its negative influence on the Hh pathway, as Ci155 is no longer stimulated into labile and hyperactivity forms by high levels of Hh in cos2 mutant cells (Wang et al. 2000). In light of our finding that Smo interacts with Cos2/Fu, the simplest interpretation for a positive role of Cos2 is that it recruits Fu to Smo and allows Fu to be activated by Smo in response to Hh.
Of note, interaction between SmoCT and Cos2/Fu per se is not sufficient for triggering Hh pathway activation. For example, Myr-SmoCTΔ625-818, which binds Cos2/Fu to the same extent as Myr-SmoCT, does not possess Hh signaling activity. The fact that Myr-SmoΔCT625-730 and Myr-Smo730-1035 can activate the Hh pathway suggests that Smo sequence between amino acids 730 and 818 is essential. This domain may recruit factors other than Cos2/Fu to achieve Hh pathway activation. Alternatively, it might target SmoCT to an appropriate signaling environment.
Implications for threshold responses to Hh
An important property of Hh family members in development is that they can elicit distinct biological responses via different concentrations. How different thresholds of Hh signal are transduced by Smo to generate distinct transcriptional outputs is not understood. Our results suggest that Smo can function as a molecular sensor that converts quantitatively different Hh signals into qualitatively distinct outputs (Fig. 7).
In the absence of Hh, the cell surface levels of Smo are low (Denef et al. 2000; Zhu et al. 2003). In addition, the Smo C-tail may adopt a “closed” conformation that prevents it from binding to Cos2/Fu (Fig. 7A). Low levels of Hh partially inhibit Ptc, leading to an increase of Smo on the cell surface. In addition, the Smo C-tail may adopt an “open” conformation, which allows Smo to bind the Cos2/Fu complex and inhibit its Ci-processing activity (Fig. 7B). Low levels of Hh signaling activity can be mimicked by overexpression of either full-length Smo or membrane-tethered forms of the Smo C-tail (Fig. 7D). High levels of Hh completely inhibit Ptc, resulting in a further increase in Smo signaling activity. Hyperactive Smo stimulates the phosphorylation and activity of bound Fu, which in turn antagonizes Su(fu) to activate Ci155 (Fig. 7C). Consistent with this, we found that Fu bound to Myc-Smo became phosphorylated in response to ectopic Hh.
The Smo sequence N terminus to SmoCT (SmoN) appears to be essential for conferring high Smo activities. It is not clear how SmoN modulates the activity of SmoCT. SmoN might recruit additional effector(s) or target SmoCT to a microdomain with a more favorable signaling environment. Alternatively, SmoN might function as a dimerization domain that facilitates interaction between two SmoCTs, as in the case of receptor tyrosine kinases (Schlessinger 2000). It is also not clear how Smo/Cos2/Fu interaction inhibits Ci processing. One possibility is that Smo/Cos2 interaction may cause disassembly of the Cos2/Ci complex, which could prevent Ci from being hyperphosphorylated, as Cos2/Ci complex formation might be essential for targeting Ci to its kinases. Consistent with this view, we found that Ci is barely detectable in the Cos2/Fu complex bound to Smo.
Physical association of the receptor complex with a downstream signaling component has also been demonstrated for the canonic Wnt pathway whereby the Wnt coreceptor LRP-5 interacts with Axin, a molecular scaffold in the Wnt pathway (Mao et al. 2001). Hence, Hh and Wnt/Wg pathways appear to use a similar mechanism to transmit signal downstream of their receptor complexes.
Materials and methods
Mutants and transgenes
Su(fu)LP and smo3 are null alleles (Preat 1992; Chen and Struhl 1998). MS1096, act > CD2 > Gal4, prd-Gal4, UAS-ptc, UAS-hh, dpp-lacZ, and ptc-lacZ have been described (Pignoni et al. 1997; Chen and Struhl 1998; Wang et al. 1999). To construct UAS-GFP-Smo, the coding sequence for GFP was amplified by PCR and inserted into the SfiI site (amino acid 35) of smo cDNA. The resulting fusion gene was subcloned into a pUAST vector between the NotI and XhoI sites. GFP-SmoΔCT, GFPSmoΔ730, and GFP-SmoΔ818 were derived from GFP-Smo by introducing a stop codon at amino acids 555, 730, and 818, respectively. Myc-Smo and Myc-SmoΔCT contain six copies of Myc-tag inserted at amino acid 35. To construct UAS-FlagSmoCT, smo cDNA encoding the C-terminal region of Smo from amino acids 556 to 1035 was amplified by PCR and subcloned in the BglII and XhoI sites of a pUAST-Flag vector. To tether SmoCT to the membrane, a myristoylation signal from Src (MGNKCCSKRQ) or the Sevenless transmembrane and extracellular domains (Struhl and Adachi 1998) were inserted at the N terminus of Flag-SmoCT to generate UAS-Myr-SmoCT or Sev-SmoCT. Membrane-tethered deletion mutants of SmoCT were generated by a PCR-based approach using appropriate primers. HA-Cos2 contained two copies of HA tags at the N terminus of Cos2 (Wang et al. 2000). HACos2ΔC was derived from HA-Cos2 by introducing a stop codon after amino acid 993. HA-Cos2ΔN1, HA-Cos2N2, HA-Cos2CT1, and HACos2CT2 contain two copies of HA tag fused N-terminal to amino acids 389, 642, 906, and 991, respectively. HA-Cos2CC was derived from HA-Cos2ΔN2 by introducing a stop codon after amino acid 993. Flag-Cos2CT1 contains one copy of Flag tag N-terminal to amino acid 906. Transformants were generated using standard P-element-mediated transformation. Multiple independent transformant lines were generated and examined for each construct.
Cell culture, transfection, immunoprecipitation, and Western blot analysis
S2 cells were cultured in the Schneider's Drosophila Medium (Invitrogen) with 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. Transfection was carried out using the Calcium Phosphate Transfection Kit (Specialty Media) according to the manufacturer's instructions. Immunoprecipitation and Western blot analyses were performed using standard protocols as previously described (Robbins et al. 1997; C.H. Chen et al. 1999). The antibodies used were mouse αMyc, 9E10 and αHA, F7 (Santa Cruz); mouse αFlag, M2 (Sigma); mouse αCos2 and rabbit αFu (Ascano et al. 2002); and rat αCi, 2A (Motzny and Holmgren 1995).
Immunostaining of imaginal discs
Standard protocols for immunofluorescence staining of imaginal discs were used (Jiang and Struhl 1995). LMB treatment of imaginal disc was done as described (Wang et al. 2000). The antibodies used in this study were rat αCi, 2A (Motzny and Holmgren 1995), rabbit anti-βGal (Cappel), mouse αCD2 (Serotec), rabbit αGFP (Clonetech), and mouse αEn and Arm (DSHB, Iowa University).
Acknowledgments
We thank Bing Wang and Liping Luo for technical assistance, and Gelin Wang and Kazuhito Amanai for initial experiments on Smo/Cos2 interaction. We thank Drs. D. Robbins, M. Scott, S. Cohen, P. Beachy, and G. Struhl for antibodies and fly stocks. This work was supported by grants from the NIH, the Searle Scholar Program from Chicago Trustee, the Leukemia and Lymphoma Society Scholar Program, and the Endowed Scholar Program in Biomedical Science from UT Southwestern Medical Center to J. Jiang.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1136603.
References
- Alcedo J., Ayzenzon, M., Von Ohlen, T., Noll, M., and Hooper, J.E. 1996. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the Hedgehog signal. Cell 86: 221-232. [DOI] [PubMed] [Google Scholar]
- Alexandre C., Jacinto, A., and Ingham, P.W. 1996. Transcriptional activation of Hedgehog target genes in Drosophila is mediated directly by the Cubitus interruptus protein, a member of the GLI family of the zinc finger DNA-binding proteins. Genes & Dev. 10: 2003-2013. [DOI] [PubMed] [Google Scholar]
- Amanai K. and Jiang, J. 2001. Distinct roles of Central missing and Dispatched in sending the Hedgehog signal. Development 128: 5119-5127. [DOI] [PubMed] [Google Scholar]
- Ascano M., Nybakken, K.E., Sosinski, J., Stegman, M.A., and Robbins, D.J. 2002. The carboxyl-terminal domain of the protein kinase fused can function as a dominant inhibitor of hedgehog signaling. Mol. Cell. Biol. 22: 1555-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aza-Blanc P., Ramirez-Weber, F., Laget, M., Schwartz, C., and Kornberg, T. 1997. Proteolysis that is inhibited by Hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89: 1043-1053. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P., Lin, H.Y., Ruiz i Altaba, A., and Kornberg, T.B. 2000. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development 127: 4293-4301. [DOI] [PubMed] [Google Scholar]
- Basler K. and Struhl, G. 1994. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368: 208-214. [DOI] [PubMed] [Google Scholar]
- Brand A.H. and Perrimon, N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401-415. [DOI] [PubMed] [Google Scholar]
- Chen Y. and Struhl, G. 1996. Dual roles for patched in sequestering and transducing Hedgehog. Cell 87: 553-563. [DOI] [PubMed] [Google Scholar]
- ____. 1998. In vivo evidence that Patched and Smoothened constitute distinct binding and transducing components of a Hedgehog receptor complex. Development 125: 4943-4948. [DOI] [PubMed] [Google Scholar]
- Chen C.H., von Kessler, D.P., Park, W., Wang, B., Ma, Y., and Beachy, P.A. 1999. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell 98: 305-316. [DOI] [PubMed] [Google Scholar]
- Chen Y., Cardinaux, J.R., Goodman, R.H., and Smolik, S.M. 1999. Mutants of cubitus interruptus that are independent of PKA regulation are independent of hedgehog signaling. Development 126: 3607-3616. [DOI] [PubMed] [Google Scholar]
- Cheng S.Y. and Bishop, J.M. 2002. Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc. Natl. Acad. Sci. 99: 5442-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef N., Neubuser, D., Perez, L., and Cohen, S.M. 2000. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell 102: 521-531. [DOI] [PubMed] [Google Scholar]
- Ingham P.W. and McMahon, A.P. 2001. Hedgehog signaling in animal development: Paradigms and principles. Genes & Dev. 15: 3059-3087. [DOI] [PubMed] [Google Scholar]
- Jia J., Amanai, K., Wang, G., Tang, J., Wang, B., and Jiang, J. 2002. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature 416: 548-552. [DOI] [PubMed] [Google Scholar]
- Jiang J. 2002. Degrading Ci: Who is Cul-pable? Genes & Dev. 16: 2315-2321. [DOI] [PubMed] [Google Scholar]
- Jiang J. and Struhl, G. 1995. Protein kinase A and Hedgehog signalling in Drosophila limb development. Cell 80: 563-572. [DOI] [PubMed] [Google Scholar]
- ____. 1998. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature 391: 493-496. [DOI] [PubMed] [Google Scholar]
- Lecuit T., Brook, W.J., Ng, M., Callega, M., Sun, H., and Cohen, S.M. 1996. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature 381: 387-393. [DOI] [PubMed] [Google Scholar]
- Mao J., Wang, J., Liu, B., Pan, W., Far III, G.H., Flynn, C., Yuan, H., Takada, S., Kimelman, D., Li, L., et al. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7: 801-809. [DOI] [PubMed] [Google Scholar]
- Methot N. and Basler, K. 1999. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96: 819-831. [DOI] [PubMed] [Google Scholar]
- ____. 2000. Suppressor of Fused opposes Hedgehog signal transduction by impeding nuclear accumulation of the activator form of Cubitus interruptus. Development 127: 4001-4010. [DOI] [PubMed] [Google Scholar]
- Monnier V., Ho, K.S., Sanial, M., Scott, M.P., and Plessis, A. 2002. Hedgehog signal transduction proteins: Contacts of the Fused kinase and Ci transcription factor with the Kinesin-related protein Costal2. BMC Dev. Biol. 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzny C.K. and Holmgren, R. 1995. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech. Dev. 52: 137-150. [DOI] [PubMed] [Google Scholar]
- Murone M., Rosenthal, A., and de Sauvage, F.J. 1999. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr. Biol. 9: 76-84. [DOI] [PubMed] [Google Scholar]
- Nellen D., Burke, R., Struhl, G., and Basler, K. 1996. Direct and long-range action of a DPP morphogen gradient. Cell 85: 357-368. [DOI] [PubMed] [Google Scholar]
- Ohlmeyer J.T. and Kalderon, D. 1997. Dual pathways for induction of wingless expression by protein kinase A and Hedgehog in Drosophila embryos. Genes & Dev. 11: 2250-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 1998. Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature 396: 749-753. [DOI] [PubMed] [Google Scholar]
- Pignoni F., Hu, B., Zavitz, K.H., Xiao, J., Garrity, P.A., and Zipursky, S.L. 1997. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 91: 881-891; erratum. 1998. Cell 92: 585. [DOI] [PubMed] [Google Scholar]
- Preat T. 1992. Characterization of Suppressor of fused, a complete suppressor of the fused segment polarity gene of Drosophila melanogaster. Genetics 132: 725-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M.A. and Kalderon, D. 1999. Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development 126: 4331-4339. [DOI] [PubMed] [Google Scholar]
- ____. 2002. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108: 823-835. [DOI] [PubMed] [Google Scholar]
- Robbins D.J., Nybakken, K.E., Kobayashi, R., Sisson, J.C., Bishop, J.M., and Therond, P.P. 1997. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 90: 225-234. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103: 211-225. [DOI] [PubMed] [Google Scholar]
- Sisson J.C., Ho, K.S., Suyama, K., and Scott, M.P. 1997. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell 90: 235-245. [DOI] [PubMed] [Google Scholar]
- Stegman M.A., Vallance, J.E., Elangovan, G., Sosinski, J., Cheng, Y., and Robbins, D.J. 2000. Identification of a tetrameric hedgehog signaling complex. J. Biol. Chem. 275: 21809-21812. [DOI] [PubMed] [Google Scholar]
- Stone D.M., Hynes, M., Armanini, M., Swanson, T.A., Gu, Q., Johnson, R.L., Scott, M.P., Pennica, D., Goddard, A., Phillips, H., et al. 1996. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384: 129-134. [DOI] [PubMed] [Google Scholar]
- Strigini M. and Cohen, S.M. 1997. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development 124: 4697-4705. [DOI] [PubMed] [Google Scholar]
- Struhl G. and Adachi, A. 1998. Nuclear access and action of notch in vivo. Cell 93: 649-660. [DOI] [PubMed] [Google Scholar]
- Tabata T. and Kornberg, T.B. 1994. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 76: 89-102. [DOI] [PubMed] [Google Scholar]
- Taipale J. and Beachy, P.A. 2001. The Hedgehog and Wnt signalling pathways in cancer. Nature 411: 349-354. [DOI] [PubMed] [Google Scholar]
- van-den-Heuval M. and Ingham, P.W. 1996. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature 382: 547-551. [DOI] [PubMed] [Google Scholar]
- Vegh M. and Basler, K. 2003. A genetic screen for hedgehog targets involved in the maintenance of the Drosophila anteroposterior compartment boundary. Genetics 163: 1427-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villavicencio E.H., Walterhouse, D.O., and Iannaccone, P.M. 2000. The Sonic hedgehog-Patched-gli pathway in human development and disease. Am. J. Hum. Genet. 67: 1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.T. and Holmgren, R.A. 1999. The subcellular localization and activity of Drosophila cubitus interruptus are regulated at multiple levels. Development 126: 5097-5106. [DOI] [PubMed] [Google Scholar]
- ____. 2000. Nuclear import of cubitus interruptus is regulated by hedgehog via a mechanism distinct from Ci stabilization and Ci activation. Development 127: 3131-3139. [DOI] [PubMed] [Google Scholar]
- Wang G., Wang, B., and Jiang, J. 1999. Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus. Genes & Dev. 13: 2828-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Amanai, K., Wang, B., and Jiang, J. 2000. Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes & Dev. 14: 2893-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T. and Rubin, G.M. 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223-1237. [DOI] [PubMed] [Google Scholar]
- Zhu A.J., Zheng, L., Suyama, K., and Scott, M.P. 2003. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes & Dev. 17: 1240-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]