Abstract
We have previously demonstrated placentas from laboring deliveries at high altitude have lower binding of hypoxia-inducible transcription factor (HIF) to DNA than those from low altitude. It has recently been reported that labor causes oxidative stress in placentas, likely due to ischemic hypoxic insult. We hypothesized that placentas of high-altitude residents acquired resistance, in the course of their development, to oxidative stress during labor. Full-thickness placental tissue biopsies were collected from laboring vaginal and nonlaboring cesarean-section term (37–41 wk) deliveries from healthy pregnancies at sea level and at 3,100 m. After freezing in liquid nitrogen within 5 min of delivery, we quantified hydrophilic and lipid metabolites using 31P and 1H NMR metabolomics. Metabolic markers of oxidative stress, increased glycolysis, and free amino acids were present in placentas following labor at sea level, but not at 3,100 m. In contrast, at 3,100 m, the placentas were characterized by the presence of concentrations of stored energy potential (phosphocreatine), antioxidants, and low free amino acid concentrations. Placentas from pregnancies at sea level subjected to labor display evidence of oxidative stress. However, laboring placentas at 3,100 m have little or no oxidative stress at the time of delivery, suggesting greater resistance to ischemia-reperfusion. We postulate that hypoxic preconditioning might occur in placentas that develop at high altitude.
Keywords: oxidative stress, labor, pregnancy, antioxidants, protein metabolism
it has been postulated that pregnancy at high altitude induces placental hypoxic stress due to the lowered partial pressure of oxygen prevailing. This stress likely contributes to the greater incidence of preeclampsia reported at high altitude (14). Oxidative stress during hypoxia can activate hypoxia-inducible transcription factor (HIF), a heterodimeric transcription factor that increases the transcription of genes encoding, amongst others, glycolytic enzymes. Thereby, it promotes anaerobic glycolysis to maintain ATP synthesis under low oxygen conditions (19, 24). Reports of elevated HIF in placentas from preeclamptic pregnancies at low altitude (16) support the idea that placentas from high-altitude pregnancies may be pushed toward a preeclamptic phenotype.
Surprisingly, we found that HIF activity was lower in normotensive term placentas from high-altitude compared with low-altitude pregnancies (28). However, all of the placentas studied were from laboring deliveries, and recently, it was demonstrated that placentas subjected to labor are oxidatively stressed (4) compared with nonlabored placentas delivered by cesarean-section (C-S). Thus, the acute ischemic/hypoxic stress induced by labor appeared to cause HIF activation at low, but not at high, altitude in our earlier study (28). The lower HIF activity in labored placentas at high altitude suggests that the glycolytic response to labor (acute ischemic hypoxia) is blunted compared with placentas from sea-level pregnancies.
We, therefore, hypothesized that labor at sea level results in high oxidative stress and elevated placental glycolytic activity compared with nonlabored C-S placentas and that the same increase would not be seen in a population residing at 3,100 m. To test this hypothesis, our approach was to collect placental tissue immediately following delivery from laboring and nonlaboring pregnancies at sea level and 3,100 m, and to use quantitative NMR-based metabolomics to distinguish metabolic markers and pathways involved in the placental stress response. Routine metabolomics 1H-NMR analysis will typically detect and quantify all proton-containing smaller molecular weight metabolites (both lipophilic and hydrophilic) <1,000 Da and which are present in concentrations above 10 μM. This means that various major amino acids, carbohydrates, ketone bodies, glycolysis products, osmolytes, antioxidants, as well as various lipids and phospholipids can be simultaneously assessed by 1H-NMR. The addition of 31P-NMR metabolic analysis on the same hydrophilic samples will provide a quantitative information on energy-reach phosphates (ATP, ADP, phosphocreatine), as well as precursors and catabolic products of phospholipids (phosphomonoesters, phosphodiesters) (23, 24). On average, 30–70 endogenous metabolites can be detected (nonselectively) using NMR-based metabolomics (20–23).
To test the hypothesis that response to the ischemic hypoxia stress of labor is blunted in placentas from high-altitude pregnancies, we anticipated changes in metabolites that are involved in responses to hypoxic and or ischemic stress. For example, hypoxia/ischemia places extreme demands on tissue energetics, and 31P-NMR is ideally suited to quantify ATP, ADP, phosphocreatine (PCr) among other phosphor-metabolites. Oxidative stress of hypoxic ischemic insult results in changes in concentrations of endogenous antioxidants and osmolytes such as glutathione, inositol, and taurine (6, 8, 18, 26), and it can affect lipid metabolism (9, 12), which are all detectable in 1H-NMR spectra. Protein catabolism occurs in response to acute hypoxic stress (15), and concentrations of a variety of amino acids (including, alanine, aspartate, arginine, glutamine, and glutamate) can be analyzed by 1H-NMR. Finally, concentrations of placental glucose and lactate are also available from quantitative 1H-NMR on placental extracts, providing important information regarding anaerobic glucose utilization in hypoxic tissues. All of the pathways described above are reflective of changes in mitochondrial activity, which is thought to be the “backbone” of the response to ischemia and/or hypoxia.
METHODS
Subjects.
Informed, written consent was obtained from subjects recruited at St. Vincent's General Hospital in Leadville, CO (3,100 m), Rosie Maternity Hospital, Cambridge, UK (sea level), and University College Hospital, London (sea level), with the approval of the Colorado Multiple Institutional Review Board, the Cambridge Local Research Ethics Committee, and The University College London Hospitals Committee on the Ethics of Human Research. Exclusion criteria included renal disease, cardiac disease, diabetes, chronic hypertension, pregnancy-induced hypertension, or any complication of pregnancy and labor prior to a C-S delivery.
A total of 16 placentas were collected, four per each of four groups: 1) sea level, C-S; 2) sea level, labored; 3) 3,100 m, C-S; and 4) 3,100 m, labored.
Tissue collection.
The placenta was weighed immediately after delivery, and sampled using a systemic random system by which the placenta was divided into five areas. Two full-thickness samples were taken from each area. The samples were immediately (within 5 min of delivery) frozen in liquid nitrogen to minimize hypoxic/ischemic artifacts (20). They were stored at −80°C until processed for nuclear magnetic resonance spectroscopy (MRS) or molecular analyses.
Nuclear Magnetic Resonance Spectroscopy
Sample preparation for 1H- and 31P-NMR spectroscopy.
Placental tissues were extracted using 8% perchloric acid (Sigma-Aldrich, St. Louis, MO), as previously described (22–24). Briefly, ∼0.07–0.15 g of frozen tissues were powdered in a mortar in the presence of liquid nitrogen and added to 4 ml of ice-cold perchloric acid. After centrifugation for 20 min at 1300 g at 4°C, the supernatants were collected, and the pellets were redissolved with 2 ml perchloric acid, vortexed, and centrifuged. Both supernatants, containing the hydrophilic fraction of the extract, were combined, and the mixture was neutralized (pH 7.0) using KOH before centrifuging again to remove potassium perchlorate. Supernatants with their water-soluble metabolites (polar hydrophilic compounds) were then lyophilized overnight to remove water for NMR experiments. The extracted hydrophilic metabolites were dissolved in 0.55 ml of deuterium oxide (D2O) prior to NMR analysis. The pellets from the second centrifugation containing the lipid fraction were dissolved in 4 ml ice-cold water and adjusted to pH 7.0 using KOH, then lyophilized overnight to remove water for MRS experiments. The lipids were dissolved in 1.2 ml of deuterated chloroform/methanol mixture (2:1, vol/vol) prior to 1H-MRS. All deuterated compounds were purchased from Cambridge Isotope (Andover, MA).
Quantitative 1H-NMR and 31P-NMR analysis.
All NMR analyses were performed by the magnetic resonance scientist (N. J. Serkova), who was blinded to the group assignment of the samples. For NMR analysis, the dissolved hydrophilic and lipophilic extracts were transferred into 5-mm NMR glass tubes (Wilmad LabGlass, Buena, NJ). The hydrophilic extracts were analyzed by high-resolution 1H-NMR using a 500-MHz, high-resolution Bruker DRX system equipped with Bruker TopSpin software (Bruker Biospin, Fremont, CA) (22). An inverse TXI 5-mm probe was used for all 1H-NMR experiments. To suppress water residue in the extracts, a standard Bruker water presaturation sequence was used (“zgpr”). An external reference, trimethylsilyl propionic-2,2,3,3,-d4 acid (TMSP, 0.5 mmol/l for hydrophilic and 1.2 mmol/l for lipid extracts), was used for metabolite quantification of fully relaxed 1H-MRS spectra and as a 1H chemical shift reference (0 ppm). For metabolite identification in water-soluble and lipid extracts, a two-dimensional (2D)-1H, 13C-HSQC (heteronuclear single quantum correlation) NMR sequence was used. The 1H-NMR peaks for single metabolites were identified and referred to a metabolite chemical shift library. After performing Fourier transformation and making phase and baseline corrections, each 1H peak was integrated using 1D WINNMR software (Bruker Biospin). The absolute concentrations of single metabolites were then referred to the TMSP integral and calculated according to the equation:
where Cx is the metabolite concentration, Ix is the integral of metabolite 1H peak, Nx is the number of protons in metabolite 1H peak (from CH, CH2, CH3, etc.), C is the TMSP concentration, I is the integral of TMSP 1H peak at 0 ppm (:9 since TMSP has 9 protons), V is the volume of the extract, and M is the weight of placental tissue sample. The final metabolite concentrations were expressed as micromoles per gram of placental tissue.
The water-soluble (hydrophilic) placental extracts were additionally analyzed by 31P-NMR spectroscopy immediately after 1H-NMR and the addition of 100 mmol/l EDTA to chelate divalent ions bound to ATP (22). Phosphorous spectra were obtained on a Bruker 300-MHz Advance spectrometer (31P-NMR frequency: 121.5 MHz) equipped with a 5-mm QNP 31P/13C/19F/1H probe using a composite pulse-decoupling (CPD) program. An external standard in a thin capillary, methyl diphosphoric acid (MDP, 2.3 mmol/l D2O, Sigma-Aldrich), was placed into the NMR tube to serve as a reference for both chemical shift (18.6 ppm) and phosphor metabolite quantification.
Statistics.
A prospective power test indicated that to detect a 50% difference between means in four groups, at 80% power (α ≤ 0.05, STD = 0.5), a total n = 16 or n = 4 per group was needed.
Differences between group means (altitude and/or labor) were determined by one-way ANOVA with Sheffe's post hoc analyses. Significance was set at P < 0.05 for all statistical analyses.
RESULTS
Women from sea level and 3,100 m were of similar age and body size and had similar gravidity and parity, and there were no significant differences in gestational age, birth weight, blood pressure, or length of labor (Table 1). All neonates had Apgar scores between 7 and 9.
Table 1.
Characteristics of pregnancies
| Sea Level |
3100 m |
|||
|---|---|---|---|---|
| C-S Normotensive | Labor Normotensive | C-S Normotensive | Labor Normotensive | |
| Birth weight, kg | 3.44 (0.537) | 3.55 (0.218) | 3.48 (0.403) | 2.97 (0.353) |
| Maternal age, yr | 30 (6) | 34 (3) | 31 (5) | 27 (9) |
| BMI, kg/m2 | 23 (1.9) | 23 (3.7) | 26 (4.9) | 22 (6.6) |
| Blood pressure, mmHg | ||||
| Systolic | 118 (8) | 126 (11) | 116 (12) | 117 (6) |
| Diastolic | 75 (4) | 76 (6) | 74 (6) | 73 (6) |
| Length of Labor, h | NA | 15 (5) | NA | 9 (6) |
| Gravidity/parity | 2.3/2.3 | 2.0/2.0 | 2.5/1.8 | 4.3/3.0 |
| Gestational age, wk | 38 (0.7) | 39 (3.0) | 40 (0.5) | 39 (0.9) |
Data are presented as mean ± SD, BMI, basal metabolic index; NA, not applicable.
Energetic State
There were no differences in ATP or ADP with altitude, although the ATP/ADP ratio was lower at high altitude for C-S (Fig. 1). Glucose and lactate concentrations were similar between nonlabored placentas at the two altitudinal levels, indicating no change in the balance of aerobic and anaerobic metabolism prior to delivery (Fig. 2). Phosphocreatine (PCr) trended higher with increased altitude.
Fig. 1.
Energy metabolites in term placental tissue from pregnancies delivered by nonlaboring C-section (C-S) and laboring vaginal procedures at sea level and 3,100 m. When letters above the columns differ, columns are significantly different (P < 0.05). Data are expressed as means ± SD.
Fig. 2.
Antioxidant reserves and metabolic markers of hypoxia in term placental tissue from pregnancies delivered by C-S and laboring vaginal procedures at sea level and 3,100 m. When letters above the columns differ, columns are significantly different (P < 0.05). Data are expressed as means ± SD.
Labor at sea level was associated with 2.5-fold higher placental concentrations of ATP, ADP, glucose, lactate, and PCr compared with the C-S controls (P < 0.0001, and = 0.014, 0.0007, 0.015, and 0.022, respectively), but there was no increase in the ATP/ADP ratio (Fig. 1). In contrast, glucose was only slightly elevated following labor at high altitude compared with the C-S controls, as was the ATP/ADP ratio (Fig. 1; P < 0.0001, and P = 0.015, and P = 0.004, respectively). There were, however, no changes in ADP, lactate, or PCr.
Despite variable changes in ATP and ADP concentrations, the adenylate energy charge remained equivalent between all groups.
Oxidative Stress Markers
The concentrations of the placental osmolytes and antioxidants taurine and inositol were greater in nonlabored placentas at 3,100 m vs. sea level (Fig. 2; P = 0.023), but there was no difference in glutathione. The concentration of monounsaturated fatty acids (MUFA) trended lower at 3100 m, while concentrations of polyunsaturated fatty acids (PUFA) and cholesterol were greater. Accordingly, the PUFA/MUFA ratio was greater at 3,100 m compared with sea level.
Labor was associated with a 2- to 3-fold greater level of glutathione at sea level and higher cholesterol, with no change in placentas at high altitude (Fig. 2). At 3,100 m, the concentrations of taurine and inositol were lower following labor, while the level of PUFA was greater (P = 0.023, P = 0.014, P < 0.0001, and P < 0.0001, respectively).
Amino acids.
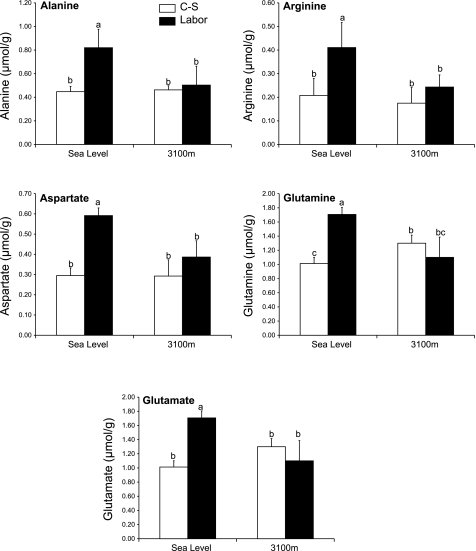
The concentrations of amino acids were similar at the two-altitudinal levels, with the exception of glutamine, which was greater at high altitude (Fig. 3, P = 0.002).
Fig. 3.
Amino acid concentrations in term placental tissue from pregnancies delivered by C-S and laboring vaginal procedures at sea level and 3,100 m. When letters above the columns differ, columns are significantly different (P < 0.05). Data are expressed as means ± SD. PUFA, polyunsaturated fatty acids; MUFA, monounsaturated fatty acids.
Placentas laboring at sea level had greater concentrations of alanine, arginine, glutamate, glutamine, and aspartate than C-S placentas at sea level (Fig. 3; P = 0.002, P = 0.007, P = 0.0003, P = 0.0002, and P = 0.0005, respectively). However, at 3,100 m, there were no differences in amino acid concentrations between labored and nonlabored placentas. Consequently, labored placentas at sea level had consistently greater amino acid concentrations than the labored placentas from pregnancies at 3,100 m.
DISCUSSION
Our results demonstrate that placentas from 3,100 m do not show metabolic evidence of hypoxia-induced stress, irrespective of whether they are labored or delivered by C-S. In contrast, placentas from sea level had significant evidence of glycolysis, oxidative stress, and stress-induced protein metabolism following labor. In their review, Hochachka and Lutz (11) concluded that metabolic adaptation to chronic hypoxia is characterized by cells and tissues altering their metabolism to reduce production of, and demand for, ATP. In the current study, term placentas from all pregnancies at 3,100 m had metabolic profiles that differed from those at sea level, suggesting that adaptation to chronic hypoxia had occurred. Further, when challenged with acute ischemic hypoxia (labor), the metabolic profiles resembled that of tissue preconditioned to hypoxia (11).
Placental energetics.
In comparing C-S and laboring placentas at sea level, it is apparent that the labor process produced greater ATP and ADP concentrations and elevated glycolytic activity. This probably reflects a stress-induced activation of anaerobic glycolysis to compensate for high-energy demand. Indeed, ATP/ADP ratio and adenylate energy charge were maintained. These findings support the premise that labored placentas have experienced a hypoxic ischemic insult, consistent with the findings in a recent report from Cindrova-Davies et al. (4).
Placentas from nonlabored high altitude pregnancies had lower ATP/ADP ratios than those at sea level. However, labor at 3,100 m generated greater ATP and ATP/ADP ratios, without large changes in glucose or lactate. The trend toward higher concentrations of PCr at 3,100 m suggests that placentas were preconditioned to store energy for use during ischemic/hypoxic stress. Analogous to our findings in high-altitude placentas, chronic hypoxia results in increased PCr concentrations in rat hippocampal slices (17). PCr, used as an energetic reserve to maintain ATP levels, can inhibit ADP-stimulated mitochondrial respiratory activity (29). Thus, the greater PCr concentration at 3,100 m most likely plays a role in lowering glycolytic activity, and reducing ATP production both prior to and during labor.
Lactate paradox.
Following acclimatization to high altitude, a phenomenon, known as the lactate paradox, describes a lower circulating lactate concentration in individuals following exercise at a given work load than that seen in the same individual when acutely hypoxic and unacclimatized (30). In the current study, we observed a similar pattern in acclimatized placentas from pregnancies at 3,100 m, which, when stressed by labor, did not yield the elevated lactate concentrations seen in stressed sea-level placentas. This is possibly explained by the notion that acclimatization to hypoxia leads to tighter coupling of ATP demand and production, possibly via mitochondrial adaptation, leading to a decreased reliance on anaerobic glycolysis (10). In the current study, higher ADP at sea level may have stimulated mitochondrial respiration and thus ATP production (29). Conversely, at 3,100 m, the lower ADP concentration may have played a role in blunting the stimulation of mitochondrial respiration, as evidenced by decreased glycolysis and ATP production in response to labor stress.
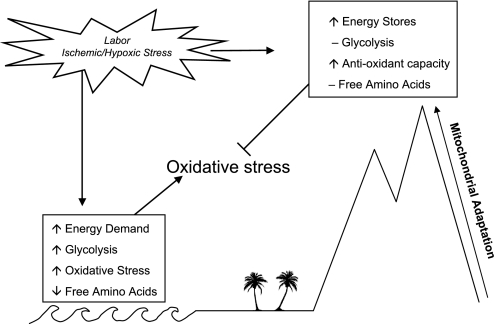
Overall, it appears that labor resulted in a standard metabolic response to ischemia and/or hypoxia characterized by glycolysis and increased mitochondrial respiration at sea level. In contrast, the metabolic stress response was blunted in placentas from pregnancies at 3,100 m, yet ATP/ADP ratios were maintained and even elevated (Fig. 4).
Fig. 4.
Potential mechanism of placental adaptation to pregnancy at 3,100 m, as well as the metabolic responses to labor stress based on the results of the current study.
Oxidative stress.
Nonstressed placentas from high altitude vs. sea-level pregnancies had greater, or trended toward greater, concentrations of taurine and inositol, suggesting greater potential antioxidant capacity and volume regulation. Labor at sea level produced greater total glutathione (Unfortunately, NMR only detects total glutathione and the ratio of GSH/GSSG is unknown), suggesting the need for antioxidants during ischemic hypoxic insult. Interestingly, cholesterol elevated with labor at sea level, but not at 3,100 m. Cholesterol is elevated following renal ischemic reperfusion injury in association with mitochondrial damage and apoptosis (13, 31, 32). Reports suggest that increasing cholesterol in response to mitochondrial stress may be a protective mechanism during ischemic reperfusion injury (13, 31). Our data indicating greater cholesterol at altitude may reflect another preconditioning effect in response to gestation at 3,100 m.
Overall, MUFA was greater at sea level, while PUFA was greater at 3,100 m, resulting in higher PUFA/MUFA ratios at 3,100 m. High [PUFA/MUFA] ratios represent a saturation index, which inversely correlates with lipid peroxidation (low LPO). Greater concentrations of PUFA suggest a preconditioning protective adaptation against LPO. In chronically hypoxic tissue high PUFA concentrations protect membrane integrity (12). In response to an acute hypoxic ischemic insult, such as in kidney transplant tissue, damage from LPO lowers PUFA concentrations (21).
Protein metabolism.
Concentrations of placental amino acids, with the exception of glutamine, were the same at sea level and 3,100 m. Placentas stressed by labor at sea level had consistently greater amino acid concentrations than nonstressed placentas, suggesting protein catabolism was utilized as an energy source, as generally occurs during acute hypoxic stress (15). However, at 3,100 m, amino acid concentrations were not different between laboring and nonlaboring placentas. These data suggest that proteins are not used as a significant fuel source during acute ischemic hypoxic stress in placentas adapted to 3,100 m.
Energetic, oxidative stress, and amino acid metabolic pathways are complex and interrelated. Examining the data from the current study in these simplified metabolic systems provides insight into specific pathways that may be important to placental adaptation to chronic hypoxia. The current data provide a snapshot in time of the metabolic processes occurring, and because birth is a time of great physiological change, these metabolites are likely in continual flux. Across the differing conditions of altitude and labor, three primary metabolic pathways (energy, oxidative stress, and protein metabolism) are consistently affected and appear to play a role in placental adaptation to chronic hypoxia.
In general, placentas supporting healthy fetuses at altitude develop mechanisms that efficiently handle acute ischemic/hypoxic stress. Our data suggest that metabolic adaptations include developing energy reserves, elevating antioxidant capacity, and reducing the stress-induced inhibition of protein synthesis. Mitochondrial activity is integral to all of these processes (1, 2, 5, 25, 27), critical to cellular response to hypoxia (3, 7), and is likely a large contributor to the preconditioning in placentas at high altitude observed in this study.
Conclusion
Placentas from high-altitude normotensive pregnancies are exposed to a lower maternal arterial Po2 and oxygen saturation throughout development yet support healthy fetuses. The lack of oxidative stress in response to ischemia/hypoxia (labor) suggests that adaptation blunts the stress response of placental tissues to acute hypoxia. It seems unlikely that a placenta could support a healthy fetus for 9 mo, while in a state of chronic hypoxic stress that would induce strong glycolytic metabolism. Thus, our data suggest that by term, the placenta at high altitude has moderated its response to low oxygen delivery to sustain a healthy fetus.
GRANTS
Professors G. J. Burton and E. Jauniaux gratefully acknowledge financial support for their research from the Wellcome Trust (069027/Z/02/Z). Dr. A. J. Murray thanks the Research Councils UK for his research fellowship. The Metabolomics NMR Core is supported in part by the University of Colorado–Denver Clinical Translational Science Award (UL1RR025780).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Stephen Charnock Jones for providing laboratory space for placental collection at the Rosie Maternity Hospital, Cambridge, UK and Ms. Andrea Merz for sample preparation for MRS analyses. We offer special thanks to the obstetrical nurses of St. Vincent's General Hospital, Leadville, CO; University Hospital, Denver, CO; the Rosie Maternity Hospital, Cambridge, UK; and University College Hospital, London, UK for their cooperation in attaining these samples.
REFERENCES
- 1.Alaynick WA. Nuclear receptors, mitochondria and lipid metabolism. [see comment] Mitochondrion 8: 329– 337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand MD, Kesseler A. Control analysis of energy metabolism in mitochondria. Biochem Soc Trans 23: 371– 376, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Chavez A, Miranda LF, Pichiule P, Chavez JC. Mitochondria and hypoxia-induced gene expression mediated by hypoxia-inducible factors. Ann NY Acad Sci 1147: 312– 320, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones D. S. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol 171: 1168– 1179, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Lisa F, Ziegler M. Pathophysiological relevance of mitochondria in NAD(+) metabolism. FEBS Lett 492: 4– 8, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Engelman DT, Watanabe M, Engelman RM, Rousou JA, Kisin E, Kagan VE, Maulik N, Das DK. Hypoxic preconditioning preserves antioxidant reserve in the working rat heart. Cardiovasc Res 29: 133– 140, 1995 [PubMed] [Google Scholar]
- 7.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401– 408, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1α and NF-κB redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem 275: 21130– 21139, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Hlavackova M, Neckar J, Jezkova J, Balkova P, Stankova B, Novakova O, Kolar F, Novak F. Dietary polyunsaturated fatty acids alter myocardial protein kinase C expression and affect cardioprotection induced by chronic hypoxia. Exp Biol Med (Maywood) 232: 823– 832, 2007 [PubMed] [Google Scholar]
- 10.Hochachka PW, Beatty CL, Burelle Y, Trump ME, McKenzie DC, Matheson GO. The lactate paradox in human high-altitude physiological performance. News Physiol Sci 17: 122– 126, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Hochachka PW, Lutz PL. Mechanism, origin, and evolution of anoxia tolerance in animals. Comp Biochem Physiol C Toxicol Pharmacol 130: 435– 459, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Jezkova J, Novakova O, Kolar F, Tvrzicka E, Neckar J, Novak F. Chronic hypoxia alters fatty acid composition of phospholipids in right and left ventricular myocardium. Mol Cell Biochem 232: 49– 56, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Naito M, Bomsztyk K, Zager RA. Renal ischemia-induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. Am J Pathol 174: 54– 62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer SK, Moore LG, Young DZ, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 180: 1161– 1168, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Pettersen EO, Juul NO, Ronning OW. Regulation of protein metabolism of human cells during and after acute hypoxia. Cancer Res 46: 4346– 4351, 1986 [PubMed] [Google Scholar]
- 16.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25: 763– 769, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Brain Res Dev Brain Res 156: 202– 209, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Sasaki H, Ray PS, Zhu L, Otani H, Asahara T, Maulik N. Hypoxia/reoxygenation promotes myocardial angiogenesis via an NF-κB-dependent mechanism in a rat model of chronic myocardial infarction. J Mol Cell Cardiol 33: 283– 294, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269: 23757– 23763, 1994 [PubMed] [Google Scholar]
- 20.Serkova N, Bendrick-Peart J, Alexander B, Tissot van Patot MC. Metabolite concentrations in human placentae and their changes due to the collection time after delivery. Placenta 24: 227– 235, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Serkova N, Fuller TF, Klawitter J, Freise CE, Niemann CU. H-NMR-based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney Int 67: 1142– 1151, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Serkova N, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics 31: 15– 24, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Serkova NJ, Niemann CU. Pattern recognition and biomarker validation using quantitative 1H-NMR-based metabolomics. Expert Rev Molec Diagn 6: 717– 731, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Serkova NJ, Reisdorph NA, Tissot van Patot MC. Metabolic markers of hypoxia: systems biology application in biomedicine. 18: 81– 95, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann NY Acad Sci 1147: 37– 52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storey KB. Gene hunting in hypoxia and exercise. Adv Exp Med Biol 588: 293– 309, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Takeo S, Nasa Y. Role of energy metabolism in the preconditioned heart–a possible contribution of mitochondria. Cardiovasc Res 43: 32– 43, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Tissot van Patot MC, Bendrick-Peart J, Beckey VE, Serkova N, Zwerdlinger L. Greater vascularity, lowered HIF-1/DNA binding and elevated GSH as markers of adaptation to in vivo chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L525– L532, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 537: 971– 978, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West JB. Point: the lactate paradox does/does not occur during exercise at high altitude. J Appl Physiol 102: 2398– 2399, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Zager RA, Burkhart KM, Johnson AC, Sacks BM. Increased proximal tubular cholesterol content: implications for cell injury and “acquired cytoresistance.” Kidney Int 56: 1788– 1797, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Zager RA, Kalhorn TF. Changes in free and esterified cholesterol: hallmarks of acute renal tubular injury and acquired cytoresistance. Am J Pathol 157: 1007– 1016, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]