Abstract
We examined whether growth hormone-releasing hormone (GHRH) may promote non-rapid eye movement (NREM) sleep via activation of GABAergic neurons in the preoptic area. Male Sprague-Dawley rats were implanted with EEG, EMG electrodes and a unilateral intracerebroventricular cannula. Groups of rats received injections (3 μl icv) with gonadotropin-releasing hormone (GHRH) (0.1 nmol/100 g body wt) or equal volume of physiological saline at the onset of the dark period and were permitted spontaneous sleep for 90 min. Separate groups of rats were sleep deprived by gentle handling for 90 min, beginning at the time of GHRH or saline injection, at the onset of the dark period. Other groups of rats received intracerebroventricular octreotide (somatostatin analog OCT) injections, intracerebroventricular injection of one of two doses of competitive GHRH antagonist, or intracerebroventricular saline injection at light onset and were then permitted 90 min spontaneous sleep-waking. Rats were killed immediately after the 90-min sleep/wake monitoring period. Brain tissue was processed for immunohistochemistry for c-Fos protein and glutamic acid decarboxylase (GAD). Single c-Fos and dual Fos-GAD cell counts were determined in the median preoptic nucleus (MnPN), and in the core and the extended parts of the ventrolateral preoptic nucleus (cVLPO and exVLPO). Intracerebroventricular GHRH elicited a significant increase in NREM sleep amount. Double-labeled Fos+GAD cell counts were significantly elevated after GHRH injection in the MnPN and VLPO in both undisturbed and sleep-deprived groups. OCT and GHRH antagonist significantly decreased NREM sleep amount compared with control rats. OCT injection increased single c-Fos-labeled cell counts in the MnPN, but not in the VLPO. Double-labeled cell counts were significantly reduced after OCT and the high dose of GHRH antagonist injection in all areas examined. These findings identify GABAergic neurons in the MnPN and VLPO as potential targets of the sleep-regulatory actions of GHRH.
Keywords: preoptic area, growth hormone-releasing hormone, somatostatin, octreotide, median preoptic nucleus, ventrolateral preoptic nucleus
growth hormone-releasing hormone (GHRH) is the main stimulatory neuropeptide that regulates growth hormone (GH) release by the pituitary. Exogenous GHRH stimulates sleep, especially, non-rapid eye movement (NREM) sleep (reviewed in Ref. 39). Intracerebroventricular (icv) administration of GHRH increases NREM sleep and enhances EEG slow-wave activity (SWA) in the neocortical EEG during NREM sleep in rats and rabbits (11, 37, 38). Intravenous administration of GHRH promotes sleep and EEG SWA in humans (25, 33, 54).
In humans, the major daily pulse in GH release occurs early in the nocturnal sleep period, coinciding with the occurrence of Stage 3/4 NREM sleep (58). In male rats, GH release is pulsatile, with peaks in GH release occurring in 3- or 4-h intervals (59). These peaks of GHRH release correlate with episodes of NREM sleep in rats (26, 36). Levels of GHRH mRNA in the rat hypothalamus are maximal early in the light phase, when spontaneous sleep is maximal (5). Collectively, findings in experimental animals and humans indicate that GHRH functions to couple GH release and sleep (53).
Inhibition of endogenous GHRH using either a peptide antagonist (40) or anti-GHRH antibodies (41) suppresses spontaneous sleep in rats, suggesting that GHRH is an endogenous sleep regulatory neuropeptide in this species. The role of endogenous GHRH in the regulation of human sleep is less clear. Somatostatin suppresses GHRH release, and subcutaneous administration of the somatostatin analog, octreotide, has been shown to increase wakefulness and decrease SWA in normal young men (66). Intravenous administration of somatostatin was shown to impair sleep in elderly subjects (14) but had no effect in young men (27, 54). Continuous intravenous infusion of GHRH receptor antagonist in healthy humans has been shown to effectively reduce plasma GH levels without altering sleep-wake amounts (23).
The preoptic area/anterior hypothalamus (POAH) is a candidate brain area for mediating the somnogenic effects of GHRH in rats. Direct microinjection of GHRH into the rat POAH results in increased NREM sleep amounts, while POAH microinjection of a competitive GHRH antagonist results in loss of spontaneous sleep (64). While pointing to the POAH as a potential site of action, studies have not identified the critical POAH nuclei or the specific cell types that might mediate GHRH modulation of sleep.
Two important sleep regulatory nuclei identified in the POAH are the ventrolateral preoptic nucleus (VLPO) and median preoptic nucleus (MnPN). The VLPO and MnPN contains neurons that exhibit elevated discharge rates in NREM and REM sleep compared with waking (55, 56). Both areas contain neurons that express c-Fos protein immunoreactivity (IR) during sleep but not during wakefulness (18, 44, 51). Sleep-related Fos protein IR is colocalized with glutamic acid decarboxylase (GAD) in the rat MnPN and VLPO, indicating that sleep-active neurons are GABAergic (17, 19). The present study was designed to test the hypothesis that the sleep-promoting effects of GHRH involve activation of GABAergic neurons in the VLPO and/or MnPN.
MATERIALS AND METHODS
Animals and Surgery
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Veterans Administration of the Greater Los Angeles Health Care System and were in compliance with National Institutes of Health guidelines.
Male Sprague Dawley rats (n = 65; weight: 300–350 g) were anesthetized with ketamine and xylazine (80 and 10 mg/kg, respectively). Stainless-steel screws for EEG recording were implanted in the skull over the frontal and parietal cortices. Two stainless-steel electrodes were implanted into the dorsal neck muscles to record the electromyogram (EMG).
A stainless-steel single-guide cannula (22 gauge, model C315G; Plastic One, Roanoke, VA) was implanted into the left lateral cerebral ventricle (AP: −0.92 mm, 1.4 mm lateral, and −3 mm in depth to the bregma) (43). The internal cannula (28 gauge, model C315I; Plastic One) inserted into the guide was 0.5 mm longer than the guide cannula. The placement of the cannula and the drainage into the lateral ventricle were verified by means of the drinking response elicited by intracerebroventricular injection of ANG II (Bachem Inc, Torrance, CA) (200 ng in 2 μl) 1 day before the microinjection experiments. ANG II elicits instantaneous drinking via the stimulation of preoptic structures (12). Only those rats exhibiting a positive ANG II tests were used. A dummy cannula was used to seal the bottom tip of the guide cannula. Insulated leads from the screws and EMG electrodes were routed to a miniature plug and attached to the skull with dental cement. Animals were allowed to recover at least 7–10 days after the surgery.
Sleep Recording and Sleep Analysis
The animals were housed in individual Plexiglas cages placed in environmental chambers (Fisher Scientific, Pittsburgh, PA). The ambient temperature was regulated at 23 ± 1°C, and a 12:12-h light/dark cycle was maintained throughout the entire experiment (lights on from 0900 to 2100). Food and water were available ad libitum.
Rats were connected to flexible recording cables attached to an electronic swivel (Plastics One) and habituated to the experimental conditions for at least 5 days before recording. Animals were handled daily to reduce the stress during the intracerebroventricular injections of the GHRH, GHRH antagonist, or saline. The cables were connected through commutators to Grass amplifiers (15A94 Quad Neuroamplifiers; Grass Technologies: Astro-Med Industrial Park, West Warwick, RI). The signals were digitized (256-Hz sampling rate) and collected by a computer and stored on compact discs. For sleep-wake scoring, the EEG and EMG were displayed on the computer screen using CED software (CED System; Cambridge Electronic Design, Cambridge, UK; scoring and spectral analysis scripts were provided by R. Nienhuis). The percentage of the time spent in each state of vigilance for the entire 1.5-h period was determined for 10-s epochs. The states of vigilance were determined by a single person without knowledge of the experimental conditions by the usual criteria. Wakefulness was identified by the presence of low amplitude and high-frequency EEG activity with high neck muscle tone. NREM sleep consisted of a high-amplitude slow-wave EEG accompanied by a lower EMG tone compared with the EMG tone of wakefulness. REM sleep was recognized by the presence of a moderate-amplitude EEG with dominant theta-frequency activity along with minimal or no neck muscle tone. EEG spectral power in the delta frequency range (0.5–4.0 Hz; slow-wave activity, or SWA) was calculated by fast Fourier transformation for each artifact-free 10-s epoch of NREM sleep during the 90-min recording period. The average SWA value for all NREM sleep epochs was determined for each animal, and individual group mean SWA values were calculated (Tables 1 and 2). Sleep latency is defined as the time point before the first incident of 40-s continuous NREM sleep episode, measured from the onset of the light (after saline, OCT, low and high dose of GHRH antagonist administration) or dark period (after saline and GHRH administration).
Table 1.
Measured sleep parameters: nonREM sleep, REMS, and EEG SWA
| GHRH | Control D | |
|---|---|---|
| NREMS % | 38.58±2.23** | 11.28±1.61 |
| REMS % | 1.32±0.27 | 0.89±0.32 |
| EEG SWA, μV2 | 3038.58±139.61* | 2628.42±79.27 |
Values are expressed as means ± SE. Values of GHRH treatment are compared to the saline-treated control values during the dark period (Control D). EEG sleep-wake activity (SWA) is defined as EEG delta power (0.5–4.0 Hz) during non-rapid eye movement sleep (NREMS) and calculated for each 10-s epoch during the entire 90-min recording period. REMS, rapid eye movement sleep.
P < 0.05;
P < 0.001.
Table 2.
Measured sleep parameters: NREMS, REMS, and EEG SWA
| OCT | ANTlow | ANThigh | Control L | |
|---|---|---|---|---|
| NREMS % | 4.91±1.08** | 47.53±4.07* | 22.71±2.55** | 61.24±2.17 |
| REMS % | 0* | 1.14±0.28 | 0.36±0.27 | 1.22±0.32 |
| EEG SWA, μV2 | 2578.19±236.85* | 3031.96±190.88* | 2732.57±119.58* | 3497.12±220.17 |
Values are expressed as means ± SE. Values of octreotide (OCT), low dose of GHRH antagonist (ANTlow), and high dose of GHRH antagonist (ANThigh) are compared to the control values (saline treatment) during the light period (Control L). EEG SWA is defined as EEG delta power (0.5–4.0 Hz) during NREM sleep and calculated for each 10-s epoch during the entire 90-min recording period.
P < 0.05;
P < 0.001.
Experimental Protocol
To test for the sleep-promoting activity of GHRH, rats (n = 10) received intracerebroventricular injection of GHRH in 3 μl volume of saline (0.1 nmol/100 g body wt; Bachem, Torrance, CA) or an equal volume of sterile physiological saline (n = 10). GHRH intracerebroventricular injections were given at dark onset (2100). Another group of rats (n = 7) received intracerebroventricular injection of GHRH at 2100 (0.1 nmol/100 g body wt) and were subjected to sleep deprivation for the subsequent 90 min. Rats were aroused by means of gentle stimuli (knocking on the cage, stirring the bedding) when signs of sleep appeared. The records were scored in 10-s epochs. A control group (n = 6) received equal volume of physiological saline at 2100 and was sleep deprived under the same conditions. In all of these groups, EEG/EMG recording continued for 90 min following GHRH or saline intracerebroventricular injection. At the end of this recording period, the animals were killed (see below for details).
Octreotide is a long half-life somatostatin analog that has been documented to suppress GHRH release in the hypothalamus (16). Octreotide (OCT: 0.1 μg/μl; Bachem) (n = 8) or a competitive GHRH antagonist [(N-Ac-Tyrl,D-Arg2) -GRF(1–29) -NH2 low doses 0.5 nmol/kg, (n = 10); high dose: 15 nmol/kg, (n = 8); Bachem] were administered in the volume of 3 μl at the beginning of the light onset. Rats (n = 14) received sterile physiological saline at the same time point in the same volume as the treatment group (3 μl) and served as a control for both the OCT and GHRH antagonist-injected groups. Both treatment and control groups were killed at the same time of day, 90 min after light onset (10:30), when spontaneous sleep amounts in rats are normally high.
Immunostaining Procedure
Animals were anesthetized under deep pentobarbital anesthesia (100 mg/kg), and were transcardially perfused with phosphate buffered (0.01 M) physiological saline (pH: 7.4) followed by 4% paraformaldehyde in phosphate buffer (0.1 M, pH: 7.4). The brains were then quickly removed from the skull, postfixed in the same 4% PFA solution for 1 h, and then transferred consecutively to 20% and 30% sucrose in 0.1 M PB (pH: 7.4) at 4°C until they sank.
Thirty-micrometer-thick coronal sections were cut on a freezing microtome. Sections were collected between the rostral edge of the optic chiasm and the appearance of the suprachiasmatic nucleus.
Free-floating sections were washed in Tris-buffered saline (TBS; 0.1 M Tris·HCl∕0.9% NaCl; pH: 7.4) and were processed for Fos-protein staining. Sections were incubated overnight in a rabbit anti-c-Fos primary antiserum (Sc52, Santa Cruz Biotechnology, Santa Cruz, CA), at a dilution of 1:15,000 in TBS containing 3% normal goat serum (Vector Laboratories, Burlingame, CA) on a shaking table, at 4°C. Sections were processed with biotinylated goat anti-rabbit IgG (1:600; Jackson ImmunoResearch Laboratories, West Grove, PA) in TBS containing 3% normal goat serum (Vector Laboratories) for 2 h at room temperature followed by reaction with avidin-biotin complex (ABC, Vector Elite kit; 1:200; Vector Laboratories) in TBS, at pH 7.4. The reaction was developed with 3,3′-diaminobenzidine (DAB) and intensified with nickel chloride (Ni-DAB) which produced a black reaction product in cell nuclei. There was no nuclear staining in the absence of the primary antiserum incubation.
To stain for GAD, we used mouse anti-GAD67 monoclonal antibody (MAB5406; Chemicon, Temecula, CA). This antibody, raised against recombinant GAD67 protein, is specific for GAD67 and shows no reactivity to GAD65 by Western blot (manufacturer's technical information). Sections were incubated in the primary antibody (1:1,000) at 4°C for 48 h, then processed with biotinylated anti-mouse IgG (1:600; Jackson ImmunoResearch Laboratories) followed by reaction with avidin-biotin complex (ABC, 1:200; Vector Laboratories) and developed with DAB to produce a brown reaction product. Omission of the GAD primary antiserum resulted in the absence of staining, indicating specificity of the secondary and tertiary treatments. Brains from experimental and control groups were always processed together in pairs for matched immunohistochemical processing.
The sections were mounted on gelatin-coated microscope slides, dehydrated in graded alcohols (50%, 70%, 95%, 100%), cleared in xylene and coverslipped with DPX mounting medium (Electron Microscopy Sciences, Hatfield PA).
Cell Counts
Sections containing the MnPN were taken from ∼0.1 mm rostral to bregma (43) and rostral to the decussation of the anterior commissure through the caudal end of the decussation of the anterior commissure. All sections were examined, and cell counts were determined by a single observer unaware of the experimental conditions or treatment of the animals. The Neurolucida computer-aided plotting system (MicroBrightField, Williston, VT) was used to identify and quantify neurons that were single labeled for Fos-IR or GAD-IR and double-labeled for Fos plus GAD-IR. Section outlines were drawn under 10× magnification. Single Fos-IR neurons and Fos plus GAD-IR double-labeled neurons were mapped in the section outlines under 400× magnifications throughout the entire focal plain. Cell counts were performed directly from Neurolucida plots using a series of counting grids previously used to quantify sleep-related Fos-IR in the rostral and caudal MnPN (18) and the VLPO core (51). The rostral MnPN grid was centered at the tip of the third ventricle rostral to the decussation of anterior commissure in front of bregma, extending 600 μm laterally and 300 μm both dorsally and ventrolaterally (17, 18). The caudal MnPN grid was a counting box 300 μm × 600 μm placed immediately dorsal to the third ventricle at the level of the decussation of anterior commissure (17, 18). Three consecutive sections were collected in both the rostral and the caudal part of the MnPN. Values from the three sections were averaged.
VLPO sections were collected from the beginning of the decussation of the anterior commissure until the first appearance of the suprachiasmatic nucleus. Cell counts were determined bilaterally in six consecutive sections containing the VLPO. The core VLPO grid was 300 μm high and 300 μm wide, and it was placed along the base of the brain just medial to the diagonal band of Broca, as described by Lu et al. (30). The extended VLPO grid was composed by a medial counting box (300 μm high and 400 μm wide) and a dorsal counting box (300 μm high and 200 μm wide). The medial counting box was adjusted to the medial edge of the core VLPO grid, while the dorsal counting box was placed above the core VLPO and medial counting boxes and centered over their common border (30).
Statistical Analysis
All results are reported for each structure in each group as means ± SE. Sleep data were averaged through the entire 90-min recording period. Student's independent t-test was used to assess differences between the treatment and control groups in the measured sleep parameters (NREM sleep; REM sleep; EEG SWA; NREM sleep latency) and in the numbers of the Fos-IR or Fos+GAD-IR cells. An α level of P < 0.05 was considered to be significant for all tests.
RESULTS
Effects of Intracerebroventricular Injection of GHRH
Sleep-wake results.
Intracerebroventricular administration of GHRH significantly increased (P < 0.001) the amount of NREM sleep compared with the control group of rats at the beginning of the active/dark period (Table 1 and Fig. 1). A significant increase (P < 0.001) in the EEG SWA was also observed in the GHRH intracerebroventricularly injected rats compared with the control group. However, REM sleep amount did not change significantly (P = 0.311) following intracerebroventricular injection of GHRH (Table 1). The latency of NREM sleep onset decreased significantly (P < 0.05) after GHRH treatment (22.50 ± 2.68 min) compared with control saline (34.17 ± 4.57 min) treatment group during the dark period (Fig. 2).
Fig. 1.
Examples of the effects of growth hormone-releasing hormone (GHRH) or saline treatment on sleep/wake patterns and EEG slow-wave activity (SWA) (Fig. 1A, GHRH injection; B, saline, control injection) A, top: hypnogram of 90-min continuous recording after intracerebroventricular injection of GHRH. Rat GH06 received intracerebroventricular GHRH injection; we detected 42.22% NREM sleep; 2.22% REM sleep; 55.56% awake during the 90-min recording period. Bottom: EEG SWA values (μV2). B, top: hypnogram of a control rat after intracerebroventricular injection of saline. Rat GH03 had 15.95% NREM sleep; 0.83% REM sleep; 83.21% awake during the 90-min recording time after intracerebroventricular saline injection. Bottom: EEG SWA values (μV2).
Fig. 2.
Horizontal bar graph represents NREM sleep latency values (min) during the light period (top, white background) and the dark period (bottom, gray background). Treatment groups are as follows: octreotide (OCT; 0.1 μg/μl) treatment, low dose of GHRH antagonist treatment (ANT low; 0.5 nmol/kg) treatment, and high dose of GHRH antagonist treatment (ANT high; 15 nmol/kg) compared with the control (light control; saline-treated group) during the light period. GHRH (0.1 nmol)-treated group compared with the control (dark control; saline-treated group) during the dark period. Data are expressed as mean ± SE; *P < 0.05.
Immunohistochemical results.
The numbers of Fos+GAD-IR neurons in the rostral and caudal MnPN of the GHRH injected rats (Fig. 3) was significantly (P < 0.01) elevated compared with saline-treated rats (Fig. 5). Fos-IR single cell counts did not change significantly in the MnPN after GHRH treatment. The numbers of Fos+GAD-IR neurons in both the core VLPO (Fig. 4) and extended VLPO were significantly (P < 0.001) higher following GHRH intracerebroventricular injection compared with the saline-treated control group of rats (Fig. 5). We could not detect significant changes in the number of single Fos-positive cell counts in either part of the VLPO after GHRH treatment compared with control rats.
Fig. 3.
Colocalization of Fos and GAD immunoreactivity in the rostral MnPN. Bright-field photomicrographs of coronal brain section from a GHRH-treated rat are shown on the left column (A, C); images on the right (B, D) show photomicrographs from a control (saline)-treated rat. Low-resolution (top) images (A and B) using 10× objective show the rostral MnPN; higher magnification pictures (bottom C and D; using 40× objective) show areas surrounded by black boxes in A and B, respectively. Black arrows show examples of single Fos-immunoreactive (IR) cells; open arrowheads point to single GAD IR neurons, while open arrows point to double-labeled FOS+GAD IR neurons. 3V, third ventricle. Scale bars in A and B = 100 μm; C and D = 50 μm.
Fig. 5.
Effects of GHRH treatment on the single Fos, single GAD, and Fos+GAD double-labeled IR cell numbers in the examined brain regions: the rostral MnPnN (rMnPN), caudal MnPn (cMnPN), core VLPO, and extended VLPO. Solid bars represent GHRH (0.1 nmol) -treated groups; open bars represent control (saline)-treated groups. Data are represented as means ± SE; *P < 0.05.
Fig. 4.
Examples of colocalization of Fos and GAD immunoreactivity in the core VLPO. A: low magnification (20× objective) bright-field photomicrograph of coronal brain section from a GHRH treated rat. B: high-magnification portion (40× objective) of the same section. C: low-magnification (20× objective), bright-field photomicrograph of coronal brain section form a control (saline)-treated rat. D: high magnification portion (40× objective) of the same section. Black arrows show examples of single Fos-immunoreactive (IR) cells; open arrowheads point to examples of single GAD IR neurons, while open arrows point to double-labeled FOS+GAD IR neurons. OX, optic chiasm. Scale bars on picture A, C = 100 μm; B, D = 50 μm.
Sleep deprivation results.
Vigilance state analysis of EEG and EMG data showed that rats averaged <1 min of sleep during 90 min of sleep deprivation following both intracerebroventricular GHRH and saline administration. Single c-Fos-labeled cell counts did not significantly differ between the two treatment groups. Double-labeled Fos+GAD cell counts were significantly (P < 0.02) elevated after GHRH injection, compared with saline injection, in the MnPN and VLPO (Fig. 6).
Fig. 6.
Effects of sleep deprivation and GHRH treatment on the single Fos, single GAD, and Fos+GAD double-labeled IR cell numbers in the examined brain regions: the rostral MnPn (rMnPN), caudal MnPn (cMnPN), core VLPO, and extended VLPO. Sleep deprivation was initiated at the beginning of the dark period (see materials and methods for details). Solid bars represent GHRH (0.1 nmol) -treated groups; open bars represent control (saline)-treated groups. Data are expressed as means ± SE; *P < 0.05.
Effects of Intracerebroventricular Injection of Somatostatin Analog OCT
Sleep-wake results.
Intracerebroventricular OCT injection elicited arousal and prompt drinking response in the first 1- to 5-min postinjection period. After the cessation of the drinking response, OCT-treated rats displayed long periods of wakefulness compared with the control rats; central OCT treatment significantly decreased (P < 0.001) both NREM sleep time and EEG SWA (Table 2). Following OCT administration, no REM sleep was observable, which resulted in a significant (P = 0.003) decrease from the control value. NREM sleep latency values increased significantly (P < 0.001) after OCT treatment (58.87 ± 8.41) compared with the saline-treated control group of rats (17.88 ± 2.95) during the light period (Fig. 2).
Immunohistochemical results.
The numbers of single Fos-IR cells in the rostral and the caudal MnPN were significantly (P ≤ 0.001) higher in the OCT-treated rats compared with the saline-injected control rats (Fig. 7). However, these differences in the case of the extended and cluster VLPO were not significant. There were significantly (P ≤ 0.001) decreased numbers of Fos+GAD-IR neurons in all four nuclei examined (Fig. 7) after OCT treatment at the beginning of the light period. This decreased Fos activation in the GAD-positive neurons resulted in a drastically decreased percentage of the Fos+GAD double-labeled neurons (Fig. 7).
Fig. 7.
Effects of octreotide (OCT; 0.1 μg/μl) treatment on the single Fos, single GAD, and Fos+GAD double-labeled immunoreactive (IR) cell numbers in the examined brain regions: the rostral MnPn (rMnPN), caudal MnPn (cMnPN), core VLPO, and extended VLPO. Solid bars represent OCT-treated groups; open bars represent control (saline)-treated groups. Data are expressed as means ± SE; *P < 0.05.
Effects of Intracerebroventricular Injection of GHRH Competitive Antagonist
Sleep-wake results.
In response to low dose (0.5 nmol) of the competitive GHRH antagonist, a significant (P = 0.01) reduction in NREM sleep was observed (Table 2), and the EEG slow wave amplitudes were also significantly decreased (P < 0.01) during the 90-min postinjection period at the beginning of the light onset. A high dose (15 nmol) of the competitive GHRH antagonist resulted in a greater reduction of NREM sleep amount and EEG slow-wave amplitudes (P < 0.01). The REM sleep amount was not significantly altered by either of these treatments (Table 2). Although the latency of NREM sleep onset increased after low dose of the competitive GHRH antagonist (23.034 ± 2.264 min), it did not reach the desired level of significance compared with the control saline-treated rats (17.88 ± 2.95 min) (Fig. 2). A high dose of the competitive GHRH antagonist significantly increased (P < 0.001) NREM sleep latency values (43.16 ± 4.87 min) compared with the control saline-treated group of rats during the light period (Fig. 2).
Immunohistochemical results.
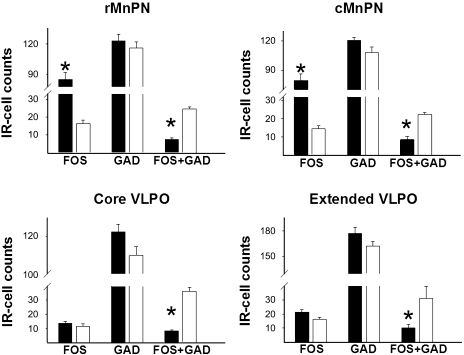
The numbers of single Fos-IR or double Fos+GAD-IR cells did not change significantly in the 0.5 nmol GHRH antagonist-treated group compared with the control group of rats in any of the studied nuclei (Fig. 8). There was only a minor decrease in the numbers of the double-labeled Fos+GAD-positive neurons, which did not reach significance (Fig. 8). However, after high-dose (15 nmol) GHRH antagonist treatment, the number of Fos+GAD-positive neurons was significantly reduced in all examined nuclei (P < 0.001). Other cell counts did not change significantly (Fig. 8).
Fig. 8.
Effects of competitive GHRH antagonist treatment on the single Fos, single GAD, and Fos+GAD double-labeled immunoreactive (IR) cell numbers in the examined brain regions: the rostral MnPn (rMnPN), caudal MnPn (cMnPN), core VLPO, and extended VLPO. Cross-hatched bars represent groups with a low dose of GHRH antagonist treatment (0.5 nmol/kg); solid black bars represent groups with high dose of GHRH antagonist treatment (15 nmol/kg); open bars represent control (saline)-treated groups. Data are expressed as means ± SE; *P < 0.05.
DISCUSSION
Our findings demonstrate that sleep evoked by intracerebroventricular administration of GHRH during the dark phase is accompanied by significant increases in the number of GABAergic neurons in the MnPN and VLPO that express Fos protein IR. Antagonism of GHRH, with either intracerebroventricular administration of OCT or a competitive GHRH receptor antagonist, during the light phase caused suppression of sleep and reduced Fos+GAD immunoreactive cell counts in the MnPN and VLPO. In all of these conditions, increases or decreases in Fos+GAD-immunoreactive cell counts were accompanied by increases or decreases in sleep. Thus, effects of drug administration on MnPN and VLPO neurons may have resulted in changes in sleep-waking amount. However, we found that intracerebroventricular GHRH administration significantly increased Fos expression in VLPO and MnPN GABAergic neurons when animals were not permitted to sleep. Previous work has shown that GABAergic neurons in the MnPN and VLPO are activated during sleep and in response to sleep deprivation (17, 19, 55, 56). Findings support the hypothesis that activation of MnPN and VLPO GABAergic neurons is a mechanism underlying the sleep-promoting effects of GHRH.
Our current results agree with previous reports that central administration of GHRH enhances sleep and EEG slow-wave activity (11, 37, 38) and that GHRH antagonism causes sleep suppression (40, 41). Our findings are also consistent with a previous report that local microinjection of GHRH into the preoptic area augmented sleep (64), and our results identify GABAergic neurons the MnPN and VLPO as possible sites of GHRH action within the preoptic area.
A previous study has shown that microinjections of GHRH directly into the midline preoptic hypothalamus, including the medial preoptic nucleus, effectively promoted sleep (64). The extent of diffusion of GHRH from medial preoptic injection sites was not documented and the population(s) of preoptic area neurons activated during GHRH-induced sleep was not examined in that report. We focused our investigation on GHRH-induced activation of Fos-IR in MnPN and VLPO neurons because these nuclei contain well-characterized populations of GABAergic neurons that are activated during sleep and during recovery sleep following sleep deprivation (17, 19). The prevalence, distribution, and neurotransmitter phenotype of sleep activate neurons in the medial preoptic nucleus are unknown. However, it is possible that sleep-wake effects of intracerebroventricularly administered GHRH and GHRH antagonists involve changes in medial preoptic neuronal activity, as well as actions in the MnPN and VLPO.
There are several lines of evidence indicating that endogenous GHRH plays a role in the regulation of NREM sleep. Studies measuring both the mRNA and protein content of GHRH during normal light-dark cycle or sleep deprivation (4, 15, 63) showed strong correlation between the hypothalamic GHRH synthesis and NREM sleep. These results showed that the mRNA levels of the GHRH were highest at the beginning of the light period, which is followed by an elevated GHRH content in the hypothalamus of sleeping rats. These findings suggest that GHRH is involved in the generation of NREM sleep early in the light phase. In contrast, during the dark or active period (when sleep time occupies only 15–20% of the time), peptide and mRNA content remain low in the rat hypothalamus. Sleep deprivation results in decreased GHRH peptide content and elevation of the GHRH mRNA (15).
The main source of GHRH is in the mediobasal hypothalamus of the rat. GHRH is found in the hypothalamic arcuate nucleus, the surrounding region of the ventromedial nucleus and in the parvocellular part of the paraventricular hypothalamic nucleus (PVN) (5, 8, 29, 35, 49). GHRH neurons in the arcuate nucleus are the major source controlling GH secretion from the pituitary. Although GHRH-containing neurons from outside the arcuate nucleus regulate GH secretion, these neurons may also project to other targets in the brain and could regulate other physiological functions.
Retrograde tracer studies showed direct anatomical connections between the MnPN and the PVN, the MnPN and the arcuate nucleus (48), between the VLPO and PVN and between the VLPO and arcuate nucleus (6). In these studies the neuropeptide phenotype of fibers targeting the MnPN and VLPO from the arcuate nucleus and the PVN was not determined. Thus, the GHRH content of these projections is uncertain. Early GHRH detection studies utilizing detailed mapping technique showed GHRH-containing fibers in the VLPO and MnPN nuclei (49). However, it is not clear whether these projections originate from an intra-arcuate or extra-arcuate source. Severing the lateral projections of the arcuate/medial basal hypothalamus using Halász knife technique, causes reductions in NREM sleep duration but no change in EEG delta power (45). While GHRH-containing neurons in the arcuate nucleus seem to be a functionally important source of projections, other extra-arcuate sources of GHRH (e.g., PVN; 5, 29) may also project to preoptic hypothalamic sleep regulatory nuclei. In situ hybridization studies demonstrate that different pools of GHRH-containing neurons may respond in different ways under various physiological conditions (61). In these experiments, only the extra-arcuate GHRH-containing neurons in the parvocellular portion of the PVN and the periventromedial nucleus responded to sleep deprivation with increased mRNA expression. GHRH neurons in the arcuate nucleus did not exhibit such activation (61).
The presence of GHRH-responsive neurons and GHRH-containing fibers in the POAH (64) raises the question of the localization of GHRH receptors in the VLPO and MnPN area. GHRH receptors have been described (57) by RT-PCR in the periventricular, arcuate, and ventromedial nuclei of the hypothalamus, and also in the anterior hypothalamus. Other studies using in vitro hypothalamic cell culture, prepared from fetal rat brain tissue, combined with an intracellular calcium detection technique (9), found that the GHRH-responding cells were almost all GABAergic neurons. These in vitro cell culture studies additionally support our findings, which demonstrate in vivo activation of MnPN and VLPO GABAergic neurons after intracerebroventricular GHRH administration.
Effect of Competitive GHRH Receptor Antagonist
In the present study we demonstrated that the intracerebroventricular administration of both low and high dose of competitive GHRH receptor antagonist decreases NREM sleep and delta power during NREM sleep. This finding agrees with earlier observations where the effect of GHRH antagonist was dose dependent (40).
The effectiveness of the GHRH antagonist was demonstrated when the peripherally administrated substance blocked GH secretion from the pituitary and decreased its plasma level (7, 31, 32). We cannot exclude the possibility that the decreased NREM sleep depth and amount were due to a decreased GH plasma level after the intracerebroventricular administration of the GHRH antagonist. However, experiments with mutant or transgenic strains of animal indicate that GH is not responsible for changes of NREM sleep and SWA amounts (42, 46). Therefore, alterations in NREM sleep elicited by intracerebroventricular injection of GHRH receptor antagonist are unlikely to be due to suppression of GH release.
Effect of Somatostatin Analog Octreotide
It is well established that sleep is immediately inhibited after both central (e.g., icv) (22) and systemic (47) administration of somatostatin. Our results agree with this and other data (2, 20), showing that somatostatin analog OCT inhibits sleep (both NREM sleep and REM sleep). Somatostatin inhibits the synthesis and release of GHRH (24, 62). It is likely that the sleep-suppressing effect of OCT is the result of a decrease of GHRH release in the hypothalamus (16). This inhibition results in a decreased level of GHRH in the synaptic cleft. GHRH-containing neurons in the arcuate nucleus are innervated by somatostatinergic fibers and express somatostatin receptors (3, 34). The inhibitory effects of OCT are mainly mediated by somatostatin-2 and -5 receptors (28, 50, 65). The main source of the somatostatinergic fiber system, reaching the mediobasal hypothalamus and the median eminence, is located in the anterior part of the periventricular nucleus of the hypothalamus in the rat (1). A limited number of somatostatin-containing perikarya have been localized outside the periventricular nucleus, such as in the arcuate nucleus, ventromedial nucleus and the lateral hypothalamus (10, 13, 52). Another explanation of the decreased sleep effect might be that a portion of the injected OCT may leak out from the cerebral ventricles into the systemic circulation (60) reaching the pituitary and inhibit GH secretion. However, OCT-induced inhibition results in an immediate decrease of total sleep time and SWA in contrast with a slower effect on GH release. Therefore, alterations in NREM sleep elicited by intracerebroventricular injection of OCT can be ascribed to its intrahypothalamic actions.
Central administration of OCT also results in a drinking response, and rats display other behavioral changes (such as feeding, grooming, and exploration), parallel with the reduction of sleep time (2). OCT-induced drinking, vasopressin secretion, and rises in blood pressure are attributed to a stimulation of ANG II release (21). Previous experiments indicate that the arousal response to OCT is not dependent on ANG II-induced drinking response (2) because inhibition of ANG II blocks the effects of OCT on drinking, while it did not interfere with sleep-wake amount (2). Experiments performed with lit/lit mice (containing nonfunctional GHRH-receptors; 42) demonstrate that sleep response to OCT is absent in these mice, whereas OCT-induced drinking seems to be intact. These results suggest that an intact GHRH system is required for the OCT-induced sleep inhibition.
Perspectives and Significance
This experiment is the first to provide functional and anatomical evidence that the GHRH system may regulate NREM sleep via its influence on the sleep-promoting nuclei in the preoptic hypothalamus. Thus, the normal coupling of GH release to sleep may be achieved through the ability of GHRH to both evoke release of GH from the pituitary and to activate sleep-regulatory neurons in the MnPN and VLPO.
GRANTS
This study is supported by The Department of Veterans Affairs and National Institutes of Health Grant MH63323.
DISCLOSURES
No conflicts of interest are delcared by the authors.
ACKNOWLEDGMENTS
The authors thank Mr. Robert Nienhuis for his excellent assistance in data analysis.
REFERENCES
- 1.Alpert LC, Brawer JR, Patel YC, Reichlin S. Somatostatinergic neurons in anterior hypothalamus: immunohistochemical localization. Endocrinology 98: 255– 258, 1976 [DOI] [PubMed] [Google Scholar]
- 2.Beranek L, Hajdu I, Gardi J, Taishi P, Obál F, Jr., Krueger JM. Central administration of the somatostatin analog octreotide induces captopril-insensitive sleep responses. Am J Physiol Regul Integr Comp Physiol 277: R1297– R1304, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bertherat J, Dournaud P, Bérod A, Normand E, Bloch B, Rostene W, Kordon C, Epelbaum J. Growth hormone-releasing hormone-synthesizing neurons are a subpopulation of somatostatin receptor-labeled cells in the rat arcuate nucleus: a combined in situ hybridization and receptor light-microscopic radioautographic study. Neuroendocrinology 56: 25– 31, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Bredow S, Taishi P, Obal F, Jr, Guha-Thakurta N, Krueger JM. Hypothalamic growth hormone-releasing hormone mRNA varies across the day in rats. NeuroReport 7: 2501– 2505, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Bruhn TO, Anthony EL, Wu P, Jackson IM. GRF immunoreactive neurons in the paraventricular nucleus of the rat: an immunohistochemical study with monoclonal and polyclonal antibodies. Brain Res 424: 290– 298, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci 22: 977– 990, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coy DH, Murphy WA, Sueiras-Diaz J, Coy EJ, Lance VA. Sructure-activity studies on the N-terminal region of growth hormone releasing factor. J Med Chem 28: 181– 185, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Daikoku S, Kawano H, Noguchi M, Nakanishi J, Tokuzen M, Chihara K, Nagatsu I. GRF neurons in the rat hypothalamus. Brain Res 399: 250– 261, 1986 [DOI] [PubMed] [Google Scholar]
- 9.De A, Churchill L, Obal F, Jr, Simasko SM, Krueger JM. GHRH and IL1β increase cytoplasmic Ca2+ levels in cultured hypothalamic GABAergic neurons. Brain Res 949: 209– 212, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Dierickx K, Vandesande F. Immunocytochemical localization of somatostatin-containing neurons in the rat hypothalamus. Cell Tissue Res 201: 349– 359, 1979 [DOI] [PubMed] [Google Scholar]
- 11.Ehlers C, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology 42: 467– 474, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the brain of the rat. J Physiol (Lond) 210: 457– 474, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finley JC, Maderdrut JL, Roger LJ, Petrusz P. The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience 6: 2173– 2192, 1981 [DOI] [PubMed] [Google Scholar]
- 14.Frieboes RM, Murck H, Schier T, Holsboer F, Steiger A. Somatostatin impairs sleep in elderly human subjects. Neuropsychopharmacology 16: 339– 345, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Gardi J, Obal F, Jr, Fang J, Zhang J, Krueger JM. Diurnal variations and sleep deprivation-induced changes in rat hypothalamic GHRH and somatostatin contents. Am J Physiol Regul Integr Comp Physiol 277: R1339– R1344, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Gardi J, Szentirmai E, Hajdu I, Obal F, Jr., Krueger JM. The somatostatin analog, octreotide, causes accumulation of growth hormone-releasing hormone and depletion of angiotensin in the rat hypothalamus. Neurosci Lett 315: 37– 40, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol 556: 935– 946, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effect of ambient warming. Am J Physiol Regul Integr Comp Physiol 279: R2079– R2088, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci 26: 9426– 9433, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajdu I, Obal F, Jr, Fang J, Krueger JM, Rollo CD. Sleep of transgenic mice producing excess rat growth hormone. Am J Physiol Regul Integr Comp Physiol 282: R70– R76, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hajdu I, Obal F, Jr, Gardi J, Laczi F, Krueger JM. Octreotide-induced drinking, vasopressin and pressure responses: role of central angiotensin and acetylcholine. Am J Physiol Regul Integr Comp Physiol 279: R271– R277, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Havlicek V, Rezek M, Friesen H. Somatostatin and thyrotropin releasing hormone: central effects on sleep and motor system. Pharmacol Biochem Behav 4: 455– 459, 1976 [DOI] [PubMed] [Google Scholar]
- 23.Jessup SK, Malow BA, Symons KV, Barkan AL. Blockade of endogenous growth hormone-releasing hormone receptors dissociates nocturnal growth hormone secretion and slow-wave sleep. Eur J Endocrinol 151: 561– 566, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Katakami H, Downs TR, Frohman LA. Inhibitory effects of hypothalamic medial preoptic area somatostatin on growth hormone-releasing factor in the rat. Endocrinology 123: 1103– 1109, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Kerkhofs M, Van CE, Van OA, Caufriez A, Thorner MO, Copinschi G. Sleep-promoting effects of growth hormone-releasing hormone in normal men. Am J Physiol Endocrinol Metab 264: E594– E598, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Kimura F, Tsai CW. Ultradian rhythm of growth hormone secretion and sleep in the adult male rat. J Physiol (Lond) 353: 305– 315, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupfer DJ, Jarrett DB, Ehlers CL. The effect of SRIF on the EEG sleep of normal men. Psychoneuroendocrinology 17: 37– 43, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Lanneau C, Peineau S, Petit F, Epelbaum J. Somatostatin modulation of excitatory synaptic transmission between periventricular and arcuate hypothalamic nuclei in votro. J Neurophysiol 84: 1464– 1474, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Liposits Z. Ultrastructure of hypothalamic paraventricular neurons. Crit Rev Neurobiol 7: 89– 162, 1993 [PubMed] [Google Scholar]
- 30.Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci 22: 4568– 4576, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumpkin MD, McDonald JK. Blockade of growth hormone-releasing factor (GRF) activity in the pituitary and hypothalamus of the conscious rat with a peptidic GFR antagonist. Endocrinology 124: 1522– 1531, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Lumpkin MD, Mulroney SE, Haramati A. Inhibition of pulsatile growth hormone (GH) secretion and somatic growth in immature rats with a synthetic GH-releasing factor antagonist. Endocrinology 124: 1154– 1159, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Marshall L, Molle M, Boschen G, Steiger A, Fehm HL, Born J. Greater efficacy of episodic than continuous growth hormone-releasing hormone (GHRH) administration in promoting slow-wave sleep (SWS). J Clin Endocrinol Metab 81: 1009– 1013, 1996 [DOI] [PubMed] [Google Scholar]
- 34.McCarthy GF, Beaudet A, Tannenbaum GS. Colocalization of somatostatin receptors and growth hormone releasing factor immunoreactivity in neurons of the rat arcuate nucleus. Neuroendocrinology 56: 18– 24, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Merchenthaler I, Thomas CR, Akimura A. Immunocytochemical localization of growth hormone-releasing factor (GHRF)-containing structures in the rat brain using anti-rat GHRF serum. Peptides 5: 1071– 1075, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Mitsugi N, Kimura F. Simultaneous determination of blood levels of corticosterone and growth hormone in the male rat: relation to sleep-wakefulness cycle. Neuroendocrinology 41: 125– 130, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Nistico G, DeSarro GB, Bagetta G, Müller EE. Behavioural and electrocortical spectrum power effects of growth hormone releasing factor in rats. Neuropharmacology 26: 75– 78, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Obal F, Jr, Alfoldi P, Cady AB, Johannsen L, Sary G, Krueger JM. Growth hormone-releasing factor enhances sleep in rats and rabbits. Am J Physiol Regul Integr Comp Physiol 255: R310– R316, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Obal F, Jr, Krueger JM. GHRH and sleep. Sleep Med Rev 8: 367– 377, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Obál F, Jr, Payne L, Kapás L, Opp M, Krueger JM. Inhibition of growth hormone-releasing factor suppresses both sleep and growth hormone secretion in the rat. Brain Res 557: 149– 153, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Obál F, Jr, Payne L, Opp M, Alföldi P, Kapás L, Krueger JM. Growth hormone-releasing hormone antibodies suppress sleep and prevent enhancement of sleep after sleep deprivation. Am J Physiol Regul Integr Comp Physiol 263: R1078– R1085, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Obal F, Jr, Alt J, Taishi P, Gardi J, Krueger JM. Sleep in mice with nonfunctional growth hormone-releasing hormone receptors. Am J Physiol Regul Integr Comp Physiol 284: R131– R139, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The Rat Brain in Stereotxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- 44.Peterfi Z, Churchill L, Hajdu I, Obal F, Jr, Krueger JM, Parducz A. Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience 124: 695– 707, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Peterfi Z, Makara GB, Obál F, Jr., Krueger JM. The anterolateral projections of the medial basal hypothalamus affect sleep. Am J Physiol Regul Integr Comp Physiol 296: R1228– R1238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterfi Z, Obal F, Jr, Taishi P, Gardi J, Kacsoh B, Unterman T, Krueger JM. Sleep in spontaneous dwarf rats. Brain Res 1108: 133– 146, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Rezek M, Havlicek V, Hughes KR, Friesen H. Cortical administration of somatostatin (SRIF): effect on sleep and motor behavior. Pharmacol Biochem Behav 5: 73– 77, 1976 [DOI] [PubMed] [Google Scholar]
- 48.Saper CB, Levishon D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res 288: 21– 31, 1983 [DOI] [PubMed] [Google Scholar]
- 49.Sawchenko PE, Swanson LW, Rivier J, Vale WW. The distribution of growth-hormone-releasing factor (GRF) immunoreactivity in the central nervous system of the rat: an immunohistochemical study using antisera directed against rat hypothalamic GRF. J Comp Neurol 237: 100– 115, 1985 [DOI] [PubMed] [Google Scholar]
- 50.Schindler M, Humphrey PPA, Emson PCR. Somatostatin receptors in the central nervous system. Prog Neurobiol 50: 9– 47, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science 271: 216– 219, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Shiosaka S, Takatsuki K, Sakanaka M, Inagaki S, Takagi H, Senba E, Kawai Y, Iida H, Minagawa H, Hara Y, Matsuzaki T, Tohyama M. Ontogeny of somatostatin-containing neuron system of the rat: immunohistochemical analysis. II. Forebrain and diencephalon. J Comp Neurol 204: 211– 224, 1982 [DOI] [PubMed] [Google Scholar]
- 53.Steiger A. Sleep and endocrine regulation. Front Biosci 8: s358– s376, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Steiger A, Guldner J, Hemmeter U, Rothe B, Wiedemann K, Holsboer F. Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls. Neuroendocrinology 56: 566– 573, 1992 [DOI] [PubMed] [Google Scholar]
- 55.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol 543: 665– 677, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res 803: 178– 188, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Takahashi T, Okimura Y, Yoshimura K, Shigeyoshi Y, Kaji H, Abe H, Chihara K. Regional distribution of growth hormone-releasing hormone (GHRH) receptor mRNA in the rat brain. Endocrinology 136: 4721– 4724, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J Clin Invest 47: 2079– 2090, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tannenbaum GS, Martin JB. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98: 562– 570, 1976 [DOI] [PubMed] [Google Scholar]
- 60.Tannenbaum GS, Patel YC. On the fate of centrally administered somatostatin in the rat: massive hypersomatostatinemia resulting from leakage into the peripheral circulation has effects on growth hormone secretion and glucoregulation. Endocrinology 118: 2137– 2143, 1986 [DOI] [PubMed] [Google Scholar]
- 61.Toppila J, Alanko L, Asikainen M, Tobler I, Stenberg D, Porkka-Heiskanen T. Sleep deprivation increases somatostatin and growth hormone-releasing hormone messenger RNA in the rat hypothalamus. J Sleep Res 6: 171– 178, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Yamauchi N, Shibasaki T, Ling N, Demura H. In vitro relese of growth hormone-releasing factor (GRF) from the hypothalamus: somatostatin inhibits GRF release. Regul Pept 33: 71– 78, 1991 [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Chen Z, Taishi P, Obál F, Jr, Fang J, Krueger JM. Sleep deprivation increases rat hypothalamic growth hormone-releasing hormone mRNA. Am J Physiol Regul Integr Comp Physiol 275: R1755– R1761, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, Obal F, Jr, Zheng T, Fang J, Taishi P, Krueger JM. Intrapreoptic microinjection of GHRH or its antagonist alters sleep in rats. J Neurosci 19: 2187– 2194, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng H, Bailey A, Jiang MH, Honda K, Chen HY, Trumbauer ME, Van der Ploeg LHT, Schaeffer JM, Leng G, Smith RG. Somatostatin receptor subtype 2 knockout mice are refractory to growth hormone-negative feedback on arcuate neurons. Mol Endocrinol 11: 1709– 1717, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Ziegenbein M, Held K, Künzel H, Murck H, Antonijevic IA, Steiger A. The somatostatin analogue octreotide impairs sleep and decreases EEG sigma power in young male subjects. Neuropsychopharmacology 29: 146– 151, 2004 [DOI] [PubMed] [Google Scholar]