Abstract
Expression of the cytokine interleukin-6 (IL-6) by skeletal muscle is hugely increased in response to a single bout of endurance exercise, and this appears to be mediated by increases in intracellular calcium. We examined the effects of endurance exercise on IL-6 mRNA levels and promoter activity in skeletal muscle in vivo, and the role of the calcium-activated calcineurin signaling pathway on muscle IL-6 expression in vivo and in vitro. IL-6 mRNA levels in the mouse tibialis anterior (TA) were increased 2–10-fold by a single bout of treadmill exercise or by 3 days of voluntary wheel running. Moreover, an IL-6 promoter-driven luciferase transgene was activated in TA by both treadmill and wheel-running exercise and by injection with a calcineurin plasmid. Exercise also increased muscle mRNA expression of the calcineurin regulatory gene MCIP1, as did treatment of C2C12 myotubes with the calcium ionophore A23187. Cotransfection of C2C12 myotubes with a constitutively active calcineurin construct significantly increased while cotransfection with the calcineurin inhibitor CAIN inhibited activity of a mouse IL-6 promoter-reporter construct. Cotransfection with a myocyte enhancer-factor-2 (MEF-2) expression construct increased basal IL-6 promoter activity and augmented the effects of calcineurin cotransfection, while cotransfection with the MEF-2 antagonist MITR repressed calcineurin-activated IL-6 promoter activity in vitro. Surprisingly, cotransfection with a dominant-negative form of another calcineurin-activated transcription factor, nuclear factor activator of T cells (NFAT), greatly potentiated both basal and calcineurin-stimulated IL-6 promoter activity in C2C12 myotubes. Mutation of the MEF-2 DNA binding sites attenuated, while mutation of the NFAT DNA binding sites potentiated basal and calcineurin-activated IL-6 promoter activity. Finally, CREB and C/EBP were necessary for basal IL-6 promoter activity and sufficient to increase IL-6 promoter activity but had minimal roles in calcineurin-activated IL-6 promoter activity. Together, these results suggest that IL-6 transcription in skeletal muscle cells can be activated by a calcineurin-MEF-2 axis which is antagonized by NFAT.
Keywords: transcription, cytokine
during a prolonged bout of aerobic exercise, a number of acute physiological changes occur that are designed to meet this increased work demand. The main functions of these changes are to provide increased delivery of oxygen and energy substrate to the contracting muscle to meet the increased energy requirements during the exercise bout and to replenish depleted intramuscular glycogen stores postexercise. During prolonged activation states, glucose and fat stores in the liver and adipose tissue are mobilized for use by contracting muscle (50). Contracting skeletal muscle must, therefore, signal these other tissues so that they can release energy substrate into the vasculature for uptake by the contracting muscle fibers (50).
Recent evidence suggests that IL-6 may be an “exercise factor” released from muscle that signals these other tissues to mobilize their energy stores (50). IL-6 was first identified as a factor secreted by T-cells that is essential for proper differentiation of B-cells (36). A vast amount of subsequent research has demonstrated that IL-6 is a multifunctional cytokine expressed by a wide variety of cell and tissue types. In humans, serum IL-6 levels and muscle IL-6 mRNA levels are increased several-fold during and immediately following a prolonged or intense bout of endurance exercise (14, 19). Moreover, IL-6 infusion induces increased hepatic glycogenolysis (40, 55) and adipose lipolysis in vivo (51). IL-6 is thus released by contracting skeletal muscle and may induce substrate mobilization in target tissues, particularly during prolonged exercise.
However, at present, the molecular signaling pathways governing the exercise-induced increase in muscle IL-6 expression are currently not well defined, though evidence supports a role for calcium-activated signaling pathways, and the calcium-activated phosphatase calcineurin, in particular. Treatment of isolated muscles or muscle cells with a calcium ionophore activates IL-6 expression (26, 27), and pharmacological inhibition of calcineurin attenuates the exercise-induced increase in IL-6 (5, 6, 27). Calcineurin has numerous transcription factor downstream targets, including MEF-2, NFAT, and CREB, among others (8, 9, 15, 28, 35, 47, 57, 67). However, at present, there are no data as to whether calcineurin itself is sufficient to induce IL-6 expression, what its downstream targets are, or whether calcineurin acts through transcriptional or post-transcriptional mechanisms.
The purpose of the present work was to explore the role of calcineurin signaling on IL-6 expression and promoter activity in skeletal muscle in vivo and in vitro. We used quantitative real-time PCR, transgenic mice harboring an IL-6 promoter transgene, and IL-6 promoter-reporter constructs to examine the role of calcineurin on IL-6 expression in vivo and in vitro. Our data demonstrate that exercise is associated with an increase in both IL-6 mRNA levels and in luciferase levels driven by a transgene flanked by the IL-6 promoter and untranslated regions (UTRs) that is accompanied by an increase in expression of MCIP1 mRNA suggestive of increased calcineurin activity. Moreover, plasmid DNA injection of a constitutively active calcineurin construct was sufficient to increase IL-6 promoter activity in mouse hindlimb skeletal muscle in vivo. In addition, we demonstrate that calcineurin activates IL-6 transcription in C2C12 myotubes in vitro and that this activation is dependent upon MEF-2 but is inhibited by NFAT signaling. Together, these data suggest that these pathways may be involved in regulating IL-6 transcription during endurance exercise.
METHODS
Animal studies.
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Colorado, Boulder, and complied with the guidelines of the American Physiological Society on the use of laboratory animals. Male wild-type C57/BL6J mice were obtained from our breeding colony in the Department of Integrative Physiology at the University of Colorado, Boulder. In the treadmill exercise studies, mice were either unexercised (n = 4) or run (n = 4) at 17.3 m/min on a level animal treadmill until exhaustion, which was defined as the point at which animals were unable to get off the shock grid (which was placed at its lowest setting to avoid causing muscle damage or high stress, both of which may induce IL-6 expression) despite low level shock and gentle prodding with a stick, as has been previously used by us (31). Mice were then killed, and the tibialis anterior (TA) was removed, frozen, and stored at −80°C until use. In addition, mice (n = 8 per group) were placed in cages, either lacking or containing a wheel for 3 days. Three days of wheel running was chosen because in many previous studies (1, 20, 21, 29, 32), we have observed that mice can run a highly variable amount the first one or two nights, with some mice hardly running at all initially and others running a great deal; 3 days was chosen because it appears to provide sufficient time for mice to acclimate to the new wheel and start to use it regularly. At the end of the dark cycle on the 3rd day, mice were killed by inhaled anesthesia overdose followed by cervical dislocation, and the TA was isolated and frozen as described.
Transgenic mouse studies.
Mice containing the luciferase coding region flanked by 1,800 base pairs (bp) of the mouse IL-6 promoter and 5′ UTR along with exon 1, intron 1, part of exon 2 of the coding region and 749 bp of the 3′ UTR of the IL-6 gene were created as previously described (46) and kindly provided by Dr. Charles O'Brien of the University of Arkansas. Mice were exercised on the treadmill as described above and then non-exercised (n = 5) or exercised to exhaustion (n = 5); mice were killed and the TA was isolated and homogenized in 1 ml of passive lysis buffer (Promega, Madison, WI), and 10 μl were used to quantify luciferase activity using a firefly luciferase kit (Promega) and a luminometer. In addition, half of the eight mice used above for the wheel-running studies were IL-6-luciferase transgenic mice; one TA was used for the mRNA studies described below and one TA was used for luciferase activity measurement (n = 4).
Quantitative real-time RT-PCR.
RNA was isolated from skeletal muscles and C2C12 myotubes with TRIzol reagent (Invitrogen, Carlsbad, CA) using standard techniques, as described previously (2–4). The reverse transcription (RT) reaction was carried out using 0.5 μg of RNA using the cDNA Archive kit (Applied Biosystems, Foster City), according to the manufacturer's protocol. Primer and probe sets for mouse IL-6, MCIP1/Rcan1, and β-actin were obtained from Applied Biosystems. All real-time PCR procedures were run in triplicate and included a no-RT control, in which purified RNA was run to ensure that no amplification occurred without the RT reaction. In addition, a standard curve ranging from 25 to 0.001 μg of an IL-6 positive control, cDNA created from RNA obtained from C2C12 myotubes treated for 6 h with LPS, was run in duplicate for every assay to produce a standard curve for quantification. All reactions of IL-6 real-time PCRs were run in duplex with β-actin as the internal control, and all IL-6 values were normalized to β-actin levels, which did not change with either exercise treatment (data not shown). In the case of MCIP1, there was interference between MCIP1 and β-actin, and these were therefore run in singleplex and reported separately.
Cloning and mutagenesis.
The mouse and human IL-6 upstream promoter regions were cloned from mouse and human genomic DNA using PCR, as described previously for myostatin (4). Approximately 1,200 bp of the mouse and human upstream promoter regions and the entire 5′ untranslated region to the translation initiation site were amplified using the following primers containing a MluI and an XhoI site at the 5′ and 3′ ends, respectively: 5′TAGTGGACGCGTGGATCCTCCTGCAAGAGACAC-3′ and 5′-TAGTGGCTCGAGAGCTGGGCTCCTGGAGGGGAG-3′ for human and 5′-TAGTGGACGCGTGGATCCTGAGAGTGTGTTTTG-3′ and 5′-TAGTGGCTCGAGAAGCGGTTTCTGGAATTGACTA-3′ for mouse. The resulting PCR product was cut, then ligated into the pGL3basic luciferase expression plasmid (Promega) at these sites, and positive clones were screened by restriction digestion.
Mutagenesis to create IL-6 promoter constructs in which the NFAT, MEF-2, C/EBP, and CREB consensus sites were mutated to eliminate binding of these transcription factors was carried out using PCR and DpnI digestion as previously described (2, 3). For the NF-κB, C/EBP/NI-IL6 and CREB sites, we used mutations previously shown to attenuate IL-6 promoter activity and protein binding ability in other cell types (13, 18). The primers used were as follows (consensus sites in bold, mutations are underlined): for the proximal NFAT (NFAT1): 5′-CATTGCACAATCTTAATAAGGTTCGCAATCAGCCCCACCCGCTCT-3′; middle NFAT (NFAT2): 5′-GGGCTGCGATGGAGTCAGAGCGAACTCAGTTCAGAACATC-3′′; for the proximal MEF-2: 5′-AGTCTCAACCCCCAATAAATAGGGGACTGGAGATGTCTGAGGCTC-3′; for the next-most proximal MEF-2: 5′-TTTCAAAAAACATAGCTTTAGCTCCTTTTTTTTCTCTTTGTAAAACTTCGTGCATGA-3′; for C/EBP/NF-IL6: 5′-CCCCTAGTTGTGTCTTGCCATGCTAAAGGACGTCACAGATATCAATCTTAATAAGGTTTC-3′; for CREB: 5′- CCCCTAGTTGTGTCTTGCGATGCTAAAGCTTGTCACATTGCACAA-3′. After treatment with DpnI, the PCR reaction was used to transform DH5α bacteria, positive colonies were grown up, and plasmid DNA was purified using the Sigma GenElute plasmid miniprep kit, according to the manufacturer's instructions. All mutated promoter constructs were confirmed by sequencing by the Sequencing Core at the University of Colorado, Boulder.
Adenoviral vectors and expression plasmids.
The cytomegalovirus (CMV)-wild type calcineurin adenovirus and the wild-type CMV-MEF-2C, the CMV constitutively active calcineurin (ca-Cn), the CMV constitutively active NFAT3 (NFAT3 Δ317), the CMV-MITR, and the CMV-CAM kinase IV constructs were kindly provided by Drs. Leslie Leinwand of the University of Colorado, Boulder and Eric Olson of the University of Texas Southwestern Medical Center. The CMV-CAIN construct was kindly provided by Dr. Stefano Schiaffino of the University of Padova. The CMV-dominant negative NFAT was kindly provided by Dr. Chi-Wing Chow of Albert Einstein College of Medicine. The CMV-CAM kinase IIdb construct was kindly provided by Dr. Carmen Sucharov of the University of Colorado Health Sciences Center in Denver. The CMV-DCREB, ACREB, and MCREB constructs, which contain a constitutively active form of CREB, a dominant-negative CREB unable to bind DNA, and a dominant-negative CREB unable to be phosphorylated, respectively, were kindly provided by Dr. Peter Watson of the University of Colorado, Denver Health Sciences Center. The CMV-C/EBP-δ construct was kindly provided by Dr. Dov Zipori of The Weizmann Institute, Israel. The 3 CEBP-δ shRNA plasmids were purchased from SA Biosciences.
Plasmid DNA injections.
Plasmid DNA injections were carried out as previously described (2). Briefly, DNA was purified by cesium chloride centrifugation, dialyzed and resuspended in 5 mM Tris solution. Mice (n = 5) were anesthetized with inhaled anesthesia, and 50 μl of plasmid DNA at 2 μg/μl concentration in 0.9% saline was injected into the TA muscle using a Hamilton syringe. Mice were injected on three successive days, and for each mouse, the left TA was injected with CMV green fluorescent protein (GFP) and the right TA was injected with CMV-ca-Cn plasmid. One week after the first injection, mice were killed by cervical dislocation, and each TA was removed and homogenized in passive lysis buffer (Promega). Luciferase readings were taken with 100 μl of firefly luciferase reagent (Promega) and 20 μl of homogenate. One mouse showed extremely low levels of luciferase activity for both muscles and was, therefore, omitted from the study.
Cell culture, infection, and transfection.
C2C12 myoblasts were plated on 0.75% gelatin-coated 6-well plates in proliferation medium consisting of DMEM supplemented with 20% FBS and 1% penicillin/streptomycin (pen/strep). Cells were split after reaching 90% confluence onto 4–6 gelatin-coated 24-well plates and were transfected with Lipofectamine 2000 as previously described (2, 3). Briefly, for each well, 1.5 μl of Lipofectamine 2000 and 1.0 μg of DNA were mixed in 100 μl of FBS-free and pen/strep-free DMEM and allowed to complex for 30 min. For cotransfections, 0.5 μg of promoter construct and 0.5 μg of the expression construct or shRNA plasmid was added. The transfection mix was added to wells containing proliferation medium and allowed to remain on cells for 1–2 days until they reached confluence, at which time the medium was removed and replaced with differentiation medium consisting of DMEM plus 1% horse serum for 2 days to induce differentiation into myotubes, at which time >90% of cells had differentiated into myotubes. All transfection experiments represent 3 replications each with 4–8 wells per replication. For ionophore treatment studies, cells were differentiated for 1.5 days then treated for 6 h with 0.4 μM A23187 or DMSO vehicle alone, as previously described (2). This concentration and duration of ionophore treatment have previously been demonstrated to result in a twofold increase in intracellular calcium levels (30), well within the physiological range. Moreover, we observed minimal myotube apoptosis at 3 or 6 h with this concentration of ionophore (data not shown).
For infections, myotubes were infected with a multiplicity of infection, or MOI, of 100 after 1.5 days of differentiation, then harvested 24 h later for mRNA isolation. Infection of myotubes is more difficult and less efficient than infection of myoblasts due to the down-regulation of the adenoviral receptor with muscle differentiation (42) but was carried out to avoid nonspecific effects of infection of myoblasts on differentiation capability. Myotubes were harvested in passive lysis buffer (Promega) for luciferase reporter studies or with TRIzol to isolate RNA for real-time PCR studies.
Statistical analysis.
Because the same animal was used for both GFP and calcineurin plasmid DNA injection (into the left and right TAs, respectively), a paired t-test was used to evaluated significance with an alpha level of P < 0.05 taken as significant. The effects of exercise on IL-6 mRNA levels and luciferase activity in vivo and of adenovirus infection on IL-6 mRNA levels in vitro, were analyzed using an independent t-test, with P < 0.05 taken as significant. To evaluate the effects of ionophore treatment with and without actinomycin D, infection with different adenoviruses, and luciferase activity of mutated constructs or different plasmid cotransfections in vitro, one-way ANOVA with Fisher's post-hoc test to determine significance at an α value of 0.05.
RESULTS
Exercise parameters.
As shown in Table 1, mice run to exhaustion on the treadmill ran an average of 105.4 ± 10.3 min and 1,823.9 ± 177.8 m before exhaustion. Wheel-running mice ran an average of 7,185.3 ± 322.3 ms per night across the three nights of the study (Table 1).
Table 1.
Distances and times run for treadmill and wheel running mice
| Time, min | Distance, m | |
|---|---|---|
| Treadmill (until exhaustion) | 105.4±10.3 | 1823.9±177.8 |
| Wheel Running, per 24 h | ND | 7185.3±322.3 |
All data are shown as means ± SE. ND, no data.
Exercise and IL-6 mRNA levels.
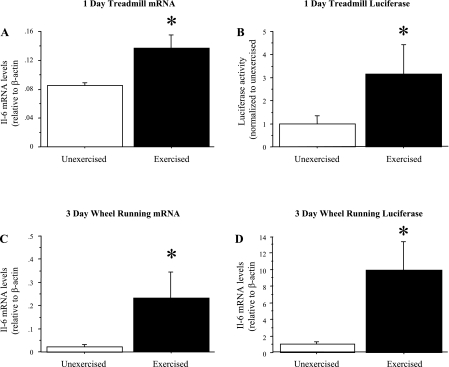
Both acute treadmill exercise and 3 days of voluntary wheel-running exercise resulted in a significant increase in TA IL-6 mRNA levels compared with unexercised controls (Fig. 1). Involuntary treadmill running to exhaustion resulted in a significant increase in IL-6 mRNA levels in the TA (n = 4 mice per group; Fig. 1A), while 3 days of voluntary wheel running resulted in an approximately eight-fold increase in IL-6 mRNA levels in the TA (n = 8 mice per group; Fig. 1C).
Fig. 1.
Skeletal muscle IL-6 mRNA and IL-6 promoter luciferase levels are increased by exercise. IL-6 mRNA levels in the tibialis anterior (TA) from unexercised wild-type C57 mice and from mice run to exhaustion on a treadmill (A), and from unexercised control wild-type C57 mice and mice allowed access to a cage wheel for 3 days. Data represent means ± SE for n = 4 animals for each group. B: IL-6 mRNA levels in the TA in unexercised mice and in mice following 3 days of voluntary wheel running. Data are expressed as means ± SE for n = 8 animals for each group. C: Firefly luciferase levels were quantified from IL-6-luc transgenic mice following a single bout of treadmill exercise Data represent mean ± SE for n = 4 animals for each group. D: both voluntary and treadmill exercise resulted in a significant increase in TA IL-6 mRNA and luciferase levels. Data are expressed as means ± SE for n = 4 animals for each group. *Significantly different from unexercised, P < 0.05.
Exercise and IL-6 transgene activity.
We next attempted to determine whether a transgene containing both upstream and downstream IL-6 regulatory elements flanking a luciferase coding region could recapitulate the responsiveness of the endogenous IL-6 gene to exercise. Luciferase levels in the TA muscle was significantly elevated three-fold from treadmill-exercised compared with unexercised mice (Fig. 1B). In addition, following 3 days of voluntary wheel running, luciferase levels were also significantly elevated in the TA (Fig. 1D).
Exercise and calcineurin activity.
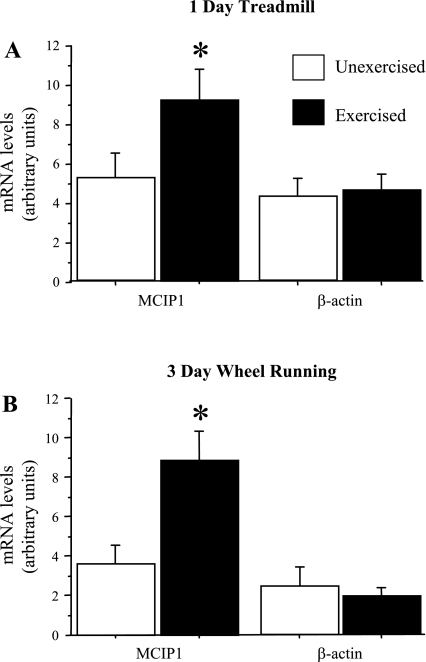
Increases in mRNA levels of the calcineurin regulatory gene MCIP1 have previously been established as a consistent marker of increased calcineurin activity (5, 6). We examined the effects of both treadmill and wheel-running exercise on MCIP1 mRNA expression in mouse hindlimb muscle. As shown in Fig. 2, both acute treadmill and 3-day wheel-running exercise was associated with a significant increase in MCIP1 mRNA levels in the TA (Fig. 2).
Fig. 2.
MCIP1 and β-actin mRNA levels from TA muscles of mice following a single bout of treadmill exercise (A) or following 3 days of voluntary wheel running (B). Data are expressed as means ± SE for n = 4 animals for each group. In both cases, exercise resulted in a significant increase in MCIP1 mRNA levels consistent with calcineurin activation but no change in β-actin mRNA levels. *Significantly different from unexercised, P < 0.05.
IL-6 expression and calcium signaling in C2C12 myotubes in vitro.
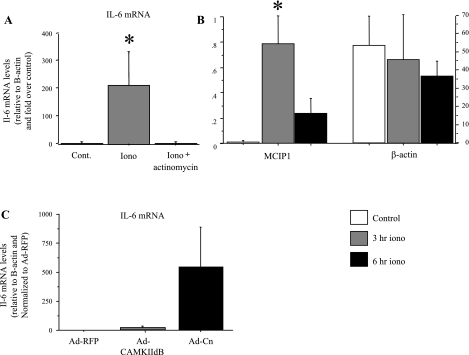
Consistent with previous studies (23), we observed a significant and dramatic increase in IL-6 mRNA levels in C2C12 myotubes in response to calcium ionophore treatment (Fig. 3A). Cotreatment with the transcriptional inhibitor actinomycin D abolished the increase in IL-6 mRNA with calcium ionophore treatment (Fig. 3A), demonstrating that the increase in IL-6 expression in response to calcium ionophore treatment is transcriptional. In addition, treatment with calcium ionophore also increased MCIP1 mRNA levels, while β-actin mRNA levels were not significantly different (Fig. 3B), again consistent with previous research showing an increase in MCIP1 mRNA, indicative of increased calcineurin activity with ionophore treatment (68).
Fig. 3.
IL-6 mRNA and MCIP1 mRNA levels are increased in C2C12 myotubes by calcium ionophore. A: C2C12 myotubes were treated for 6 h with either 1 mM of the calcium ionophore A23187 or with calcium ionophore and actinomycin D. Actinomycin D treatment completely abolished the significant increase in IL-6 mRNA with calcium ionophore treatment. B: MCIP1 and β-actin mRNA levels in untreated C2C12 myotubes and in C2C12 myotubes treated for 3 or 6 h with ionophore. Ionophore treatment resulted in a significant increase in MCIP1 mRNA levels at 3 h and was still elevated at 6 h of ionophore treatment, while β-actin mRNA levels remained unchanged. *Significantly different from untreated, P < 0.05. C: IL-6 mRNA levels in response to infection of C2C12 myotubes with adenoviral constructs containing either a cytomegalovirus (CMV)-RFP control, a CAMKIIdB, or a calcineurin expression vector. Infection with the calcineurin adenovirus increased IL-6 mRNA levels at ∼500-fold, although this was not significant because of high variability in infectivity. Data are expressed as means ± SE for n = 5 independent experiments.
Infection with adenoviruses containing a wild-type calcineurin expression construct showed a trend toward increased IL-6 mRNA levels by ∼500-fold from near-undetectable levels in C2C12 myotubes infected with a control adenovirus (Fig. 3C). This change showed a trend toward significance (P < 0.10) but was not significant due to high variability in infection efficiency. In contrast, infection of myotubes with a CAM kinase IIdb adenoviral construct had no significant effect on IL-6 mRNA levels relative to the CMV red fluorescent protein (RFP) control (Fig. 3C).
Calcineurin and IL-6 expression.
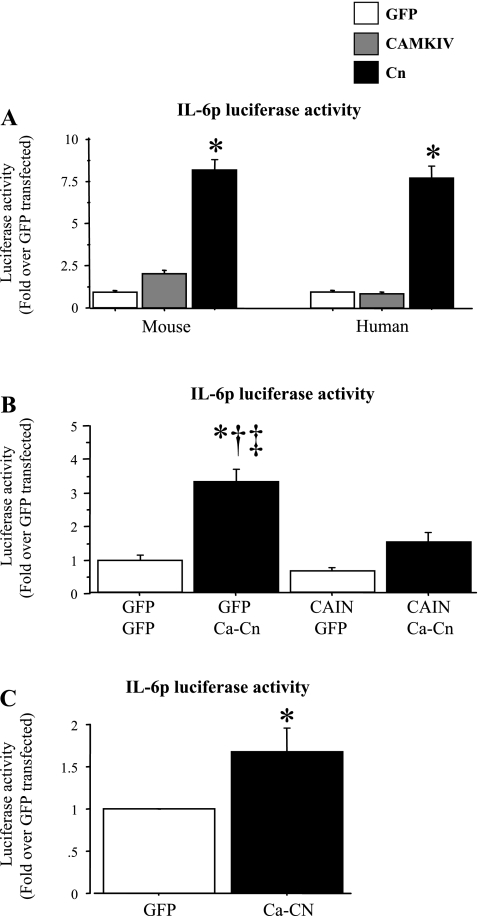
Because the increase in IL-6 mRNA with calcium ionophore treatment appears to be sensitive to actinomycin D treatment and because both exercise and calcium ionophore treatment increased calcineurin activity, as evidenced by increased MCIP1 mRNA levels, we sought to identify possible transcriptional mechanisms underlying IL-6 expression in response to increased calcium/calcineurin activity. Cotransfection with a constitutively active calcineurin construct significantly increased while cotransfection with a CAM kinase IV construct had no significant effect on mouse and human IL-6 promoter activity (Fig. 4A). Specifically, activity of the 1,200-bp mouse and human IL-6 promoter-reporter constructs was significantly increased ∼8-fold in response to constitutively active calcineurin cotransfection (Fig. 4A). Conversely, cotransfection with the calcineurin inhibitor CAIN did not significantly affect basal IL-6 promoter activity but significantly decreased calcineurin-activated IL-6 promoter activity, almost completely abolishing the increase due to calcineurin cotransfection (Fig. 4B). Together, these data confirm that increased calcineurin activity is sufficient to stimulate IL-6 transcription in the absence of increased intracellular calcium levels in C2C12 myotubes in vitro.
Fig. 4.
Calcineurin is both necessary and sufficient to activate IL-6 promoter activity. Data are expressed as means ± SE for n = 3 independent transfection experiments with 4–8 wells per experiment. A: activity of ∼1,200 bp of the mouse (left) or human (right) IL-6 promoter in C2C12 myotubes in response to cotransfection with either a CMV green fluorescent protein (GFP) control, a CAM kinase IV, or a constitutively active calcineurin plasmid. Cotransfection with the constitutively active calcineurin plasmid was sufficient to significantly activate both the mouse and the human IL-6 promoters, while Cam kinase IV was not. *Significantly different from CMV-GFP control. B: activity of the mouse IL-6 promoter construct in response to calcineurin or CAIN cotransfection. CAIN cotransfection had no effect on basal IL-6 promoter activity but significantly attenuated the increase due to cotransfection with the constitutively active calcineurin plasmid. *Significantly different from CMV-GFP control, P < 0.05. †Significantly different from CAIN alone, P < 0.05. ‡Significantly different from CAIN and Ca-calcineurin cotransfected, P < 0.05. C: injection of IL-6-luc transgenic mouse TA with either a CMV-GFP plasmid or the constitutively active calcineurin construct. Calcineurin plasmid injection significantly increased TA luciferase values relative to GFP injection. *Significantly different from CMV-GFP control.
Moreover, injection of the TA muscle of the IL-6 promoter-reporter transgenic mice with the constitutively active calcineurin plasmid significantly increased luciferase levels compared with injection with a CMV-GFP control plasmid (Fig. 4C). Thus calcineurin is also sufficient to increase IL-6 transcription in vivo as well.
MEF-2 and NFAT and IL-6 promoter activity.
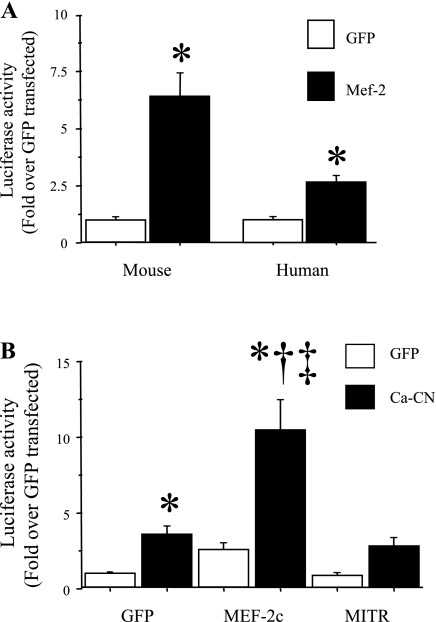
Calcineurin is a phosphatase that frequently acts through dephosphorylation of the MEF-2 and NFAT family of transcription factors to activate transcription (9, 67). We, therefore, examined the role of these transcription factors in mediating both basal and calcineurin-stimulated IL-6 promoter activity in C2C12 myotubes in vitro. Cotransfection with a wild-type MEF-2C expression construct significantly increased both mouse and human IL-6 basal promoter activity in C2C12 myotubes (Fig. 5A). Moreover, cotransfection with the wild-type MEF-2C expression construct also significantly augmented calcineurin-activated IL-6 promoter activity, increasing it 10-fold over the GFP control compared with 3-fold with calcineurin alone (Fig. 5B). In contrast, cotransfection with the MEF-2 antagonist MITR had only minimal and nonsignificant effects on basal IL-6 promoter activity but significantly attenuated calcineurin-activated IL-6 promoter activity, such that it was no longer significantly different from the GFP-GFP cotransfected control (Fig. 5B). MEF-2 thus appears to be both sufficient to induce increased IL-6 promoter activity in the absence of calcium/calcineurin signaling, and necessary for calcineurin-activated IL-6 transcription.
Fig. 5.
MEF-2C is both necessary and sufficient to activate IL-6 promoter activity. Data are expressed as means ± SE for n = 3 independent transfection experiments with 4–8 wells per experiment. A: activity of ∼1,200 bp of the mouse (left) or human (right) IL-6 promoter in C2C12 myotubes in response to cotransfection with either a CMV-GFP control or a wild-type MEF-2C plasmid. Cotransfection with the MEF-2C plasmid was sufficient to significantly activate both the mouse and the human IL-6 promoters. *Significantly different from CMV-GFP control. B: basal (GFP) and calcineurin-activated activity of the mouse IL-6 promoter construct in response to cotransfection with either MEF-2C or the MEF-2 inhibitor MITR. MEF-2C cotransfection significantly increased both basal (GFP) and calcineurin-activated IL-6 promoter activity, while MITR cotransfection had no effect on basal IL-6 promoter activity but significantly attenuated the increase due to cotransfection with the constitutively active calcineurin plasmid. *Significantly different from CMV-GFP control, P < 0.05. †Significantly different from MITR alone, P < 0.05. ‡Significantly different from MITR and Ca-calcineurin cotransfected, P < 0.05.
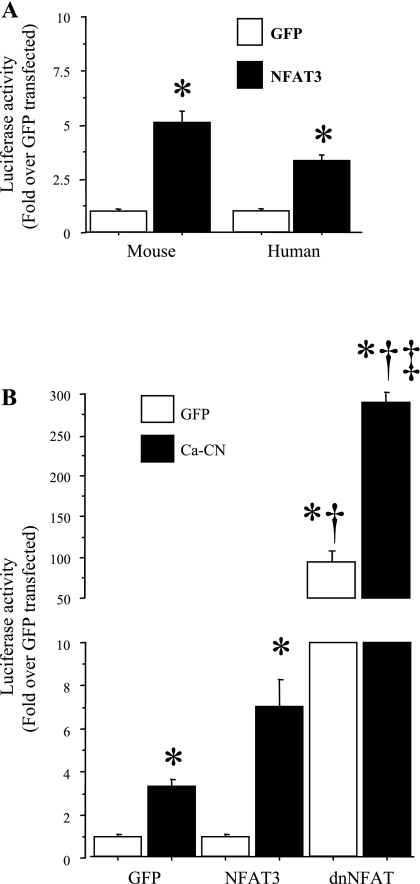
The role of the NFAT family of transcription factors on IL-6 promoter activity was somewhat less clear. Overexpression of a constitutively active form of NFAT3, NFAT3 Δ317, significantly increased basal mouse and human IL-6 promoter activity by three- to five-fold (Fig. 6A). However, the effects of constitutively active and wild-type NFAT cotransfection on both basal and calcineurin-activated activity were dwarfed by the effects of cotransfection with a dominant-negative form of NFAT, which increased basal IL-6 promoter activity nearly 100-fold and increased calcineurin-activated IL-6 promoter activity nearly 300-fold (Fig. 6B). Cotransfection with GFP and ca-CN induced a three-fold increase in IL-6 luciferase activity relative to GFP-GFP transfection, and transfection with ca-CN and dnNFAT induced a similar 3-fold increase in IL-6 promoter-driven luciferase activity relative to transfection with dnNFAT and GFP (Fig. 6B). Thus the relative increase was approximately three-fold in each case with dnNFAT cotransfection, but the magnitude differed with respect to calcineurin status. In summary, while the data support the interpretation that NFAT may act as either an activator or a repressor of IL-6 transcription, given the magnitude of the effects of the dominant-negative NFAT effect, it appears that NFAT acts most potently as a repressor of IL-6 transcription.
Fig. 6.
NFAT has differential effects on IL-6 promoter activity in C2C12 myotubes. Data are expressed as means ± SE for n = 3 independent transfection experiments with 4–8 wells per experiment. A: activity of ∼1,200 bp of the mouse (left) or human (right) IL-6 promoter in C2C12 myotubes in response to cotransfection with either a CMV-GFP control or a constitutively active NFAT3 plasmid. Cotransfection with the constitutively active NFAT3 plasmid was sufficient to significantly activate both the mouse and the human IL-6 promoters. *Significantly different from CMV-GFP control. B: basal (GFP) and calcineurin-activated activity of the mouse IL-6 promoter construct in response to cotransfection with either wild-type NFAT3 or a dominant-negative NFAT (dnNFAT) plasmid. NFAT3 cotransfection had no significant effect on either basal (GFP) or calcineurin-activated IL-6 promoter activity, while cotransfection with the dnNFAT plasmid greatly and significantly increased both basal and calcineurin-activated IL-6 promoter activity. *Significantly different from CMV-GFP control, P < 0.05. †Significantly different from calcineurin transfected alone, P < 0.05. ‡Significantly different from dnNFAT alone, P < 0.05.
Mutagenesis on conserved IL-6 cis-elements.
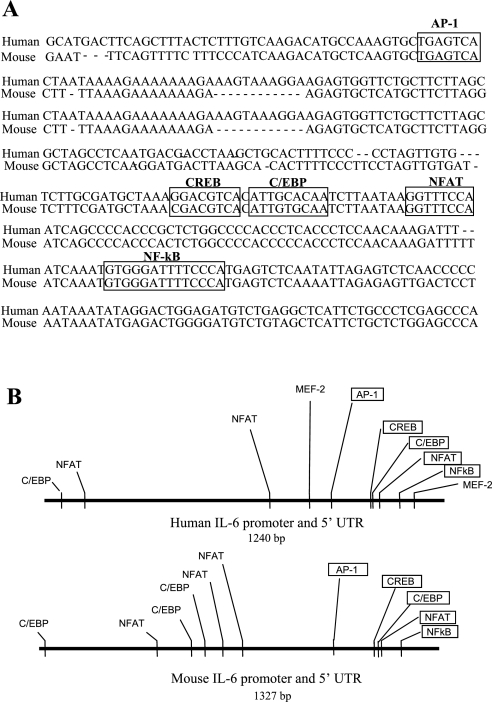
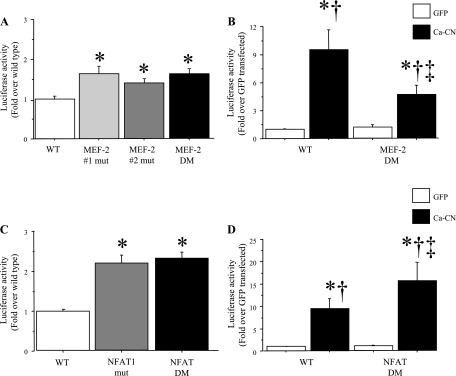
Examination of the mouse and human IL-6 promoter regions revealed several highly conserved elements (Fig. 7), several of which have been described previously (13, 18, 37, 54). Specifically, both the mouse and human IL-6 promoter regions contain conserved AP-1, NF-κB, C/EBP, and CREB consensus sites (Fig. 7). In addition, both the mouse and human IL-6 promoter regions contain several consensus binding sites for MEF-2 and NFAT binding (Fig. 7), but only the most proximal NFAT site is conserved across species. We, therefore, focused on determining whether the NFAT and MEF-2 sites were necessary for basal or calcineurin-activated IL-6 promoter activity by mutating these sites and comparing activity of the mutated IL-6 promoter-reporter constructs to that of the wild-type mouse IL-6 promoter-reporter construct. As shown in Fig. 8, mutation of either or both MEF-2 binding sites in the human IL-6 promoter had a very modest but significant effect on basal IL-6 promoter activity (Fig. 8A), but mutation of both sites significantly attenuated calcineurin-activated IL-6 promoter activity relative to the wild-type human IL-6 construct (Fig. 8B), consistent with the role for MEF-2 demonstrated by the MEF-2C coexpression data above. In contrast, mutation of the either the proximal-most NFAT site, or of the two proximal-most NFAT sites, significantly increased basal IL-6 promoter activity by more than twofold (Fig. 8C) and significantly potentiated calcineurin-activated IL-6 promoter activity relative to the wild-type IL-6 promoter construct (Fig. 8D), consistent with the results from the dominant-negative NFAT cotransfection studies above.
Fig. 7.
A: alignment of ∼400 bp of the human and mouse IL-6 upstream promoter regions. The four sites conserved between both species are boxed and labeled. B: schematic of the upstream promoter regions of the human and mouse 1,200-bp promoter regions showing the consensus sites for MEF-2, NFAT, C/EBP, CREB, Ap1, NF-κB. Conserved sites are boxed.
Fig. 8.
Mutation of the NFAT and MEF-2 binding sites has opposing effects on human IL-6 promoter activity in C2C12 myotubes. Data are expressed as means ± SE for n = 3 independent transfection experiments with 4–8 wells per experiment. A: mutation of either the proximal, the distal, or both MEF-2 binding sites has no significant effect on human IL-6 promoter activity in C2C12 myotubes. B: mutagenesis of both of the MEF-2 binding sites has no effect on basal IL-6 promoter activity but significantly attenuates the increase in IL-6 promoter activity due to calcineurin. *Significantly different from wild-type cotransfected with the CMV-GFP control, P < 0.05; †Significantly different from double mutant cotransfected with the GFP control, P < 0.05. ‡Significantly different from wild-type IL-6 promoter construct cotransfected with the Ca-calcineurin construct, P < 0.05. C: mutation of either the proximal-most, conserved NFAT site, or the proximal-most and next-most-proximal site. Both significantly increase activity of the human IL-6 promoter by approximately twofold in C2C12 myotubes. *Significantly different from wild-type construct, P < 0.05. D: mutagenesis of both proximal-most NFAT sites has no effect on basal Il-6 promoter activity but significantly potentiates the effects of calcineurin activation on the IL-6 promoter. *Significantly different from wild-type IL-6 promoter cotransfected with the CMV-GFP control, P < 0.05. †Significantly different from IL-6 promoter double multant cotransfected with the CMV-GFP control, P < 0.05. ‡Significantly different from wild-type IL-6 promoter cotransfected with the Ca-calcineurin construct, P < 0.05.
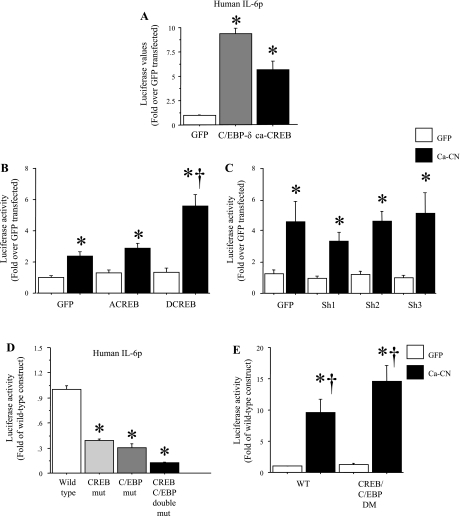
Finally, because CREB and the C/EBP family of transcription factors can also be activated by calcineurin signaling (7, 31, 57, 64) and are known to regulate IL-6 transcription in other cell types (13, 18, 37, 54), we evaluated the effects of these transcription factors on basal and calcineurin-stimulated IL-6 promoter activity. Cotransfection with either a constitutively active CREB or a wild-type C/EBP-δ construct was sufficient to significantly increase IL-6 promoter activity in C2C12 myotubes (Fig. 9A). However, cotransfection with a dominant-negative CREB that is deficient in DNA binding had no effect on basal or calcineurin-activated IL-6 promoter activity, but cotransfection with a dominant-negative CREB that is phosphorylation deficient significantly potentiated calcineurin-activated IL-6 promoter activity (Fig. 9B). Moreover, cotransfection with three different shRNA constructs designed to decrease C/EBP-δ levels had no effect on basal or calcineurin-activated IL-6 promoter activity (Fig. 9C). In addition, mutation of the CREB or C/EBP sites alone or in combination resulted in a significant and dramatic decrease in basal IL-6 promoter activity relative to the wild-type IL-6 promoter construct (Fig. 9D), but mutation of both sites together did not significantly alter calcineurin-activated IL-6 promoter activity relative to the wild-type construct (Fig. 9E). Together, these data suggest that the CREB and C/EBP transcription factors regulate basal but not calcineurin-activated IL-6 transcription in C2C12 myotubes.
Fig. 9.
Effects of ca-CREB and C/EBP-δ on IL-6 promoter activity in C2C12 myotubes. Data are expressed as means ± SE for n = 3 independent transfection experiments with 4–8 wells per experiment. A: cotransfection with a wild-type CREB or C/EBP-δ plasmid significantly increases mouse IL-6 promoter activity. *Significantly different from GFP-transfected control, P < 0.05. B: cotransfection with a DNA-binding-deficient form of CREB (ACREB) has no effect on calcineurin-activated mouse IL-6 promoter activity but cotransfection with a nonphosphorylatable form of CREB (DCREB) significantly potentiates calcineurin-activated mouse IL-6 promoter activity. *Significantly different from GFP-GFP- transfected control, P < 0.05; †Significantly different from calcineurin cotransfected, P < 0.05. C: cotransfection with three different shRNA constructs against C/EBP-δ has no effect on calcineurin-activated mouse IL-6 promoter activity. *Significantly different from GFP-GFP-transfected control, P < 0.05. D: mutagenesis of the conserved CREB, C/EBP, or both sites significantly attenuated basal activity of the human IL-6 promoter in C2C12 myotubes. *Significantly different from wild-type construct, P < 0.05. E: mutagenesis of the conserved CREB and C/EBP sites does not affect calcineurin-activated human IL-6 promoter activity. *Significantly different from wild-type mice cotransfected with the CMV-GFP control, P < 0.05. †Significantly different from double-mutant mice cotransfected with the GFP control , P < 0.05.
DISCUSSION
Previous studies have strongly suggested that increased intracellular calcium in general (22, 24), and calcineurin activation in particular (5, 6), are major regulators of skeletal muscle IL-6 expression. In the present study, we sought to identify the role of calcineurin and some of its downstream targets on skeletal muscle cell IL-6 expression. Our data suggest that skeletal muscle IL-6 expression can be activated by calcineurin both in vivo and in vitro and that the transcription factor MEF-2 appears to be a downstream effector of this pathway in C2C12 myotubes in vitro.
Previous studies have demonstrated that serum IL-6 protein levels or muscle IL-6 mRNA levels are increased in response to running or cycling in humans (14, 19, 22, 44, 48, 52) or treadmill running in rats (5, 6, 25, 60). Our results are consistent with these previous findings but are in contrast to a previously published report, which reported no significant increase in IL-6 mRNA 60 or 90 min following 1 h of treadmill running or 3 h following a run to exhaustion in mice (12). It is unclear why the present study and the others above observed a significant increase and Colbert et al. (12) did not; however, differences in duration, speed, and other performance variables or other methodological differences may account for some of the difference between this study and the present one.
In addition, we quantified expression of a transgene consisting of the luciferase coding region flanked by 5′ and 3′ regulatory regions from the mouse IL-6 gene in response to treadmill and voluntary wheel-running exercise. Both involuntary treadmill running to exhaustion and 3 days of voluntary wheel running resulted in an increase in luciferase activity in the TA, demonstrating that transgene expression showed the same pattern of expression as the endogenous IL-6 gene. Together, these findings suggest that at least some of the element(s) responsible for inducing increased IL-6 expression in response to treadmill exercise reside within these flanking regulatory regions of the IL-6 gene, although it does not eliminate the possibility that elements upstream or downstream of this may contribute to IL-6 transcription as well.
Given that treadmill exercise significantly increased IL-6 transgene activity, we then sought to identify element(s) within these flanking regions that may be responsive to signaling pathways previously shown to be induced by an acute bout of prolonged exercise. We focused on the upstream promoter region from ∼1,200 bp to the transcription start site, for two reasons. First, previous studies have identified several key element(s) within the proximal promoter sequence responsible for activating IL-6 expression in nonmuscle cells in vitro (i.e., the first 1,000 or so bp; 13), and we sought to better understand the role of these elements in regulating IL-6 transcription in skeletal muscle cells. Secondly, since both mouse and human IL-6 genes appear to respond similarly to exercise, we sought regions showing high-sequence homology between the two species, and homology between the mouse and human IL-6 upstream promoter region is highest within this region and drops off considerably upstream of this (data not shown).
Because exercise is such a complex physiological condition that elicits numerous intracellular and extracellular responses, we shifted our studies to cell culture studies on C2C12 myotubes, where individual stimuli can be evaluated with fewer confounding variables. We sought to focus on one specific signaling pathway, increased calcineurin activation by increased intracellular calcium, for two reasons: 1) previous studies have demonstrated that exercise is associated with an increase in calcineurin activity (5, 6, 9, 15, 67), a finding confirmed in the present study by the increase in MCIP1 mRNA levels, with both treadmill and wheel-running exercise (Fig. 3); 2) inhibition of calcineurin signaling abolishes the increase in IL-6 with treadmill running in rats (5, 6). Given these previous findings, we sought to determine three things: whether increasing calcineurin activity was sufficient to increase IL-6 expression; if so, whether the increase in IL-6 mRNA levels was due to transcriptional regulation via calcineurin; and if so, what the downstream effectors of calcineurin were that were responsible for this effect. Consistent with previous reports, treatment of cultured muscle cells with a calcium ionophore induced a highly significant increase in IL-6 mRNA levels that is transcriptionally driven, since it was abolished by actinomycin D treatment (Fig. 4). These data are consistent with previous studies that have demonstrated that calcium signaling is a critical regulator of IL-6 expression in skeletal muscle cells (27).
As mentioned above, previous studies have suggested that calcineurin signaling is necessary for increased IL-6 expression both in vitro (27) and in vivo (5, 6), but to our knowledge, we are the first to show that overexpression of calcineurin can induce expression of IL-6 in muscle cells, i.e., that calcineurin is sufficient to induce IL-6 expression. One of these previous studies demonstrated that calcium ionophore treatment increases IL-6 expression in primary human myotubes and that this increase is abolished by cotreatment with the calcineurin inhibitor cyclosporin A (27). This is consistent with data from the present study demonstrating that ionophore treatment increases IL-6 promoter activity and that calcineurin activity is necessary for increased IL-6 promoter activity in mouse C2C12 myotubes in vitro. Moreover, the increase in IL-6 mRNA in response to treadmill running exercise was abolished by injection of animals with pharmacological calcineurin inhibitors (5, 6). Together with the plasmid DNA data from the present study, these data establish that calcineurin is both necessary and sufficient to increase skeletal muscle IL-6 expression in vivo.
We, therefore, examined the roles of two key transcription factors downstream of calcineurin signaling, MEF-2 and NFAT. Several lines of evidence from the present study suggest that MEF-2 is relatively unimportant for basal IL-6 transcription but is critical for at least part of the calcineurin-activated increase in IL-6 transcription. First, overexpression of MEF-2C alone is sufficient to significantly increase IL-6 promoter activity and significantly potentiates the effects of calcineurin cotransfection (Fig. 5). Second, cotransfection with the MEF-2 inhibitor MITR, which counteracts the effects of MEF-2 on transcription (33, 61), has minimal effects on basal IL-6 promoter activity but significantly attenuates the effects of calcineurin cotransfection on IL-6 promoter activity (Fig. 5). Finally, mutagenesis of the two MEF-2 consensus sequences in the human IL-6 promoter has minimal effects on basal IL-6 promoter activity but significantly attenuates calcineurin-activated IL-6 promoter activity (Fig. 8). Together, these data strongly suggest that MEF-2 is a key component of calcium-activated, calcineurin-dependent activation of IL-6 transcription in C2C12 myotubes. This is consistent with numerous other studies demonstrating a link between calcineurin and MEF-2 in regulating calcium-dependent gene transcription (9, 67; reviewed in 47).
Considerable evidence has accumulated that MEF-2 expression, nuclear translocation, and/or DNA binding are increased by exercise (29, 38, 39,58, 59) and that MEF-2 signaling may play a role in the regulation of another exercise/activity-responsive skeletal muscle gene, that of the GLUT4 glucose uniporter (24, 41, 58, 59). Several studies have demonstrated increased binding of MEF-2 family members to AT-rich sequences in the GLUT4 promoter (38, 39, 58). However, the specific identity of the signals inducing increased MEF-2 expression, nuclear translocation, and/or DNA binding activity is the source of much debate. Both CAM kinase II (CAMKII) and AMP kinase have been implicated, since pharmacological inhibitors of CAMKII attenuate exercise-induced MEF-2 DNA binding and GLUT4 expression (59), while treatment with the AMP kinase activator AICAR increases MEF-2 nuclear translocation and GLUT4 transcription (24). In the present study, cotransfection with a CAMK adenovirus had no effect on IL-6 mRNA levels, nor did cotransfection with a CAMKIV expression construct significantly affect IL-6 promoter activity in C2C12 myotubes, suggesting that this pathway does not play a major role in regulation of IL-6 transcription in these cells under these conditions. In addition, we saw no effect of cotransfection with a constitutively active AMP kinase subunit alpha expression construct on mouse or human IL-6 promoter activity (data not shown), again consistent with the interpretation that AMP kinase signaling is not a major determinant of IL-6 transcription in vitro. The role of calcineurin in exercise-induced MEF-2 activation is also somewhat controversial. Treatment of swimming-exercised rats with the calcineurin inhibitor cyclosporin A did not abolish the increase in MEF2a protein or GLUT4 expression of the triceps or epitrochlearis muscles (17). However, recent data suggest that inhibition of calcineurin does impact expression of GLUT4, and presumably MEF-2 activation, in the fast-twitch TA but not the slow-twitch soleus muscle (41). Taken together with the present data, these data suggest that expression of exercise/activity responsive genes can be regulated by a number of pathways, depending on the gene, but likely influenced by other factors (such as muscle fiber type) as well.
In contrast, our data on the role of NFAT are slightly less clear. Cotransfection with a constitutively active NFAT3 construct significantly increased IL-6 promoter activity. However, cotransfection with a dominant-negative NFAT construct greatly increased basal and calcineurin-activated IL-6 promoter activity, while mutation of two of the NFAT consensus sites in the human IL-6 promoter increased basal and calcineurin-activated IL-6 promoter activity. Thus, the present study provides evidence that NFAT can serve as either a mild activator or as an extremely potent repressor of IL-6 promoter activity. Given that the effects of the dominant-negative NFAT cotransfection dwarfed the effects of constitutively active NFAT cotransfection, it seems likely that NFAT does act as a potent repressor of IL-6 transcription, but the discrepancy between the two pieces of data remains a mystery. One possible explanation of this discrepancy is that there are different isoforms of NFAT, and it may be that NFAT3 acts as an activator, thus explaining why cotransfection of a constitutively active or wild-type form of NFAT3 increased IL-6 promoter activity, but that some other NFAT isoform acts as a potent repressor, thus explaining the effects of cotransfection of the dominant negative NFAT construct, which interferes with binding of all NFAT isoforms (11). While a wealth of published reports has supported the hypothesis that NFAT is activated by calcineurin and typically behaves as an activator of gene transcription, particularly on the expression of activity-dependent genes such as the myoglobin and troponin I slow genes (9, 67; reviewed in 47), there is also a precedent for NFAT also acting as a repressor of transcription of certain genes (10, 45, 53).
Finally, we chose to examine the role of two other transcription factor families, CREB and C/EBP, in basal and calcineurin-activated IL-6 promoter activity for three reasons: 1) binding sites for these transcription factors have been previously identified in the IL-6 promoter region, and they are known to regulate IL-6 promoter activity in other cell types (13, 18, 37, 54); 2) their activity can be altered by calcineurin signaling (7, 28, 31, 56, 64); 3) repression of MEF-2 activity by MITR cotransfection was unable to eliminate all of the calcineurin induction of the IL-6 promoter, suggesting that other pathways contribute to this response. We found that, as has been shown previously for other cell types (13, 18, 37, 54), mutation of the CREB or C/EBP binding sites alone significantly attenuated basal IL-6 promoter activity, and mutation of both together decreased basal IL-6 promoter activity to ∼10% of wild type. In addition, cotransfection with either a constitutively active CREB or a wild-type C/EBP-δ construct significantly increased IL-6 promoter activity, but inhibition of CREB or C/EBP signaling had minimal effects on calcineurin-activated Il-6 promoter activity. Interestingly, calcineurin-activated IL-6 transcription was significantly potentiated by cotransfection with a dominant-negative nonphosphorylatable version of CREB, but cotransfection with a dominant-negative DNA binding-deficient version of CREB was not significantly different from the GFP cotransfected, calcineurin co-transfected control (Fig. 9B). The reason for this is not clear; MCREB, the nonphosphorylatable version, is unable to bind to CREB binding protein (CBP) but is still able to bind to DNA, and thus may partner with some other cofactor that more potently activates transcription in the presence of calcineurin. There is precedent for the DNA binding-deficient and nonphosphorylatable versions of CREB having different physiological effects (56). Thus, our data support the hypothesis that both CREB and C/EBP transcription factors are necessary for basal IL-6 promoter activity and are sufficient to induce increased IL-6 promoter activity but are minimally involved with calcineurin-mediated IL-6 transcription.
The present data support a role for calcineurin/MEF-2 signaling in the activation of IL-6 transcription. However, it should be noted that 1) calcineurin may not be the only calcium-activated signaling pathway that activates IL-6 transcription, particularly during exercise; 2) other calcium-independent pathways, such as those associated with hypoxia/stress and/or energy balance such as HIF-1α or NF-κB (34), may also regulate IL-6 transcription during exercise. Finally, IL-6 transcription can also be stimulated via a cytokine-activated MAP kinase pathway in vitro (34); and 3) post-transcriptional regulation of IL-6 stability (43, 49, 63, 65, 66) may also play a role as well.
Perspectives and Significance
Much progress has been made in identifying the signaling pathways activating the expression of exercise-responsive genes (52, 62; reviewed in 16). IL-6 represents another gene that appears to be activated by a single acute bout of endurance exercise (21, 23), and we have elucidated key aspects of the calcineurin activation of IL-6 gene transcription, and, in particular, have shown that calcineurin is sufficient to increase IL-6 transcription both in vivo and in vitro. Moreover, our data support a central role for MEF-2 in calcineurin-activation of IL-6 transcription. We propose that a calcineurin-MEF-2 pathway may contribute to increased IL-6 mRNA levels with exercise.
GRANTS
D. L. Allen was partly supported during this work by KO1 Grant AR050505-01 from the National Institute for Arthritis, Musculoskeletal and Skin Disease (NIAMS) in the National Institutes of Health. This work was supported by R03 Grant 1R03AR055787-01 from NIAMS.
REFERENCES
- 1.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 90: 1900– 1908, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Allen LA, Leinwand DL. Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J Biol Chem 277: 45323– 45330, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol 292: C188– C199, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, Madden MC, Mehan RS. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab 294: E918– E927, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Banzet S, Koulmann N, Sanchez H, Serrurier B, Peinnequin A, Alonso A, and Bigard X. Contraction-induced interleukin-6 transcription in rat slow-type muscle is partly dependent on calcineurin activation. J Cell Physiol 210: 596– 601, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Banzet S, Koulmann N, Simler N, Birot O, Sanchez H, Chapot R, Peinnequin A, and Bigard X. Fibre-type specificity of interleukin-6 gene transcription during muscle contraction in rat: association with calcineurin activity. J Physiol 566: 839– 847, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87: 1203– 1214, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Blaeser F, Ho N, Prywes R, Chatila TA. Ca2+-dependent gene expression mediated by MEF2 transcription factors. J Biol Chem 275: 197– 209, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12: 2499– 2509, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choo MK, Yeo H, Zayzafoon M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone 45: 579– 589, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow CW, Rincón M, Davis RJ. Requirement for transcription factor NFAT in interleukin-2 expression. Mol Cell Biol 19: 2300– 2307, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colbert LH, Davis JM, Essig DA, Ghaffar A, and Mayer EP. Tissue expression and plasma concentrations of TNFα, IL-1β, and IL-6 following treadmill exercise in mice. Int J Sports Med 22: 261– 267, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Dendorfer U, Oettgen P, Libermann TA. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol 14: 4443– 4454, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drenth JP, Van Uum SH, Van Deuren M, Pesman GJ, Van der Ven-Jongekrijg J, Van der Meer JW. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-α and IL-1β production. J Appl Physiol 79: 1497– 1503, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Dunn SE, Simard AR, Bassel-Duby R, Williams RS, Michel RN. Nerve activity-dependent modulation of calcineurin signaling in adult fast and slow skeletal muscle fibers. J Biol Chem 276: 45243– 45254, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Fluck M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol 209: 2239– 2248, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Roves PM, Jones TE, Otani K, Han DH, Holloszy JO. Calcineurin does not mediate exercise-induced increase in muscle GLUT4. Diabetes 54: 624– 628, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Grassl C, Luckow B, Schlöndorff D, Dendorfer U. Transcriptional regulation of the interleukin-6 gene in mesangial cells. J Am Soc Nephrol 10: 1466– 1477, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Haahr PM, Pedersen BK, Fomsgaard A, Tvede N, Diamant M, Klarlund K, Halkjaer-Kristensen J, Bendtzen K. Effect of physical exercise on in vitro production of interleukin 1, interleukin 6, tumour necrosis factor-α, interleukin 2 and interferon-γ. Int J Sports Med 12: 223– 227, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol 92: 313– 322, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Haubold KW, Allen DL, Capetanaki Y, Leinwand LA. Loss of desmin leads to impaired voluntary wheel running and treadmill exercise performance. J Appl Physiol 95: 1617– 1622, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J 18: 992– 994, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Holmes AG, Watt MJ, Carey AL, Febbraio MA. Ionomycin, but not physiologic doses of epinephrine, stimulates skeletal muscle interleukin-6 mRNA expression and protein release. Metabolism 53: 1492– 1495, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Holmes BF, Sparling DP, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. Am J Physiol Endocrinol Metab 289: E1071– E1076, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol 528: 157– 163, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juretic N, Garcia-Huidobro P, Iturrieta JA, Jaimovich E, Riveros N. Depolarization-induced slow Ca2+ transients stimulate transcription of IL-6 gene in skeletal muscle cells. Am J Physiol Cell Physiol 290: C1428– C1436, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Keller C, Hellsten Y, Steensberg A, Pedersen BK. Differential regulation of IL-6 and TNF-α via calcineurin in human skeletal muscle cells. Cytokine 36: 141– 147, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kingsbury TJ, Bambrick LL, Roby CD, Krueger BK. Calcineurin activity is required for depolarization-induced, CREB-dependent gene transcription in cortical neurons. J Neurochem 103: 761– 770, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA. Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol 289: H455– H465, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Kubis HP, Hanke N, Scheibe RJ, Meissner JD, Gros G. Ca2+ transients activate calcineurin/NFATc1 and initiate fast-to-slow transformation in a primary skeletal muscle culture. Am J Physiol Cell Physiol 285: C56– C63, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J Biol Chem 280: 26751– 26759, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol 92: 2245– 2255, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol 168: 887– 897, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo G, Hershko DD, Robb BW, Wray CJ, Hasselgren PO. IL-1β stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-κB. Am J Physiol Regul Integr Comp Physiol 284: R1249– R1254, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Mao Z, Wiedmann M. Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J Biol Chem 274: 31102– 31107, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Maza JS, Berek O. Interleukin 6 and cancer treatment. In Vivo 5: 583– 588, 1991 [PubMed] [Google Scholar]
- 37.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, and Akira S. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6, and interleukin 8. Proc Natl Acad Sci USA 90: 10193– 10197, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGee SL, Hargreaves M. Exercise and myocyte enhancer factor 2 regulation in human skeletal muscle. Diabetes 53: 1208– 1214, 2004 [DOI] [PubMed] [Google Scholar]
- 39.McGee SL, Sparling D, Olson AL, Hargreaves M. Exercise increases MEF2- and GEF DNA-binding activity in human skeletal muscle. FASEB J 20: 348– 349, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Metzger S, Goldschmidt N, Barash V, Peretz T, Drize O, Shilyansky J, Shiloni E, Chajek-Shaul T. Interleukin-6 secretion in mice is associated with reduced glucose-6-phosphatase and liver glycogen levels. Am J Physiol Endocrinol Metab 273: E262– E267, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Murgia M, Jensen TE, Cusinato M, Garcia M, Richter EA, Schiaffino S. Multiple signalling pathways redundantly control glucose transporter GLUT4 gene transcription in skeletal muscle. J Physiol 587: 4319– 4327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nalbantoglu J, Pari G, Karpati G, Holland PC. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther 10: 1009– 1019, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk HD, Holtmann H, Kollias G, and Gaestel M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem 277: 3065– 3068, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Nieman DC, Davis JM, Henson DA, Gross SJ, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, McAnulty SR, McAnulty LS, Triplett NT. Muscle cytokine mRNA changes after 2.5 h of cycling: influence of carbohydrate. Med Sci Sports Exerc 37: 1283– 1290, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Nguyen T, Lindner R, Tedeschi A, Forsberg K, Green A, Wuttke A, Gaub P, Di Giovanni S. NFAT-3 is a transcriptional repressor of the growth-associated protein 43 during neuronal maturation. J Biol Chem 284: 18816– 18823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Brien CA, Jilka RL, Fu Q, Stewart S, Weinstein RS, Manolagas SC. IL-6 is not required for parathyroid hormone stimulation of RANKL expression, osteoclast formation, and bone loss in mice. Am J Physiol Endocrinol Metab 289: E784– E793, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Olson EN, Williams RS. Remodeling muscles with calcineurin. Bioessays 22: 510– 519, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508: 949– 953, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paschoud S, Dogar AM, Kuntz C, Grisoni-Neupert B, Richman L, Kuhn LC. Destabilization of interleukin-6 mRNA requires a putative RNA stem-loop structure, an AU-rich element, and the RNA-binding protein AUF1. Mol Cell Biol 26: 8228– 8241, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Wolsk-Petersen E, Febbraio M. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proc Nutr Soc 63: 263– 267, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA, and Pedersen BK. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am J Physiol Endocrinol Metab 288: E155– E162, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B, Neufer PD. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol 541: 261– 271, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rana ZA, Gundersen K, Buonanno A. Activity-dependent repression of muscle genes by NFAT. Proc Natl Acad Sci USA 105: 5921– 5926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray A, Sassone-Corsi P, Sehgal PB. A multiple cytokine- and second messenger-responsive element in the enhancer of the human interleukin-6 gene: similarities with c-fos gene regulation. Mol Cell Biol 9: 5537– 5547, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ritchie DG. Interleukin 6 stimulates hepatic glucose release from prelabeled glycogen pools. Am J Physiol Endocrinol Metab 258: E57– E64, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Sarkar SA, Gunter J, Bouchard R, Reusch JE, Wiseman A, Gill RG, Hutton JC, Pugazhenthi S. Dominant-negative mutant forms of the cAMP response element binding protein induce apoptosis and decrease the anti-apoptotic action of growth factors in human islets. Diabetologia 50: 1649– 1659, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Schwaninger M, Blume R, Oetjen E, Lux G, Knepel W. Inhibition of cAMP-responsive element-mediated gene transcription by cyclosporin A and FK506sss after membrane depolarization. J Biol Chem 268: 23111– 23115, 1993 [PubMed] [Google Scholar]
- 58.Smith JA, Collins M, Grobler LA, Magee CJ, Ojuka EO. Exercise and CaMK activation both increase the binding of MEF2A to the Glut4 promoter in skeletal muscle in vivo. Am J Physiol Endocrinol Metab 292: E413– E420, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Smith JA, Kohn TA, Chetty AK, Ojuka EO. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab 295: E698– E704, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Spangenburg EE, Brown DA, Johnson MS, Moore RL. Exercise increases SOCS-3 expression in rat skeletal muscle: potential relationship to IL-6 expression. J Physiol 572: 839– 848, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sparrow DB, Miska EA, Langley E, Reynaud-Deonauth S, Kotecha S, Towers N, Spohr G, Kouzarides T, Mohun TJ. MEF-2 function is modified by a novel co-repressor, MITR. EMBO J 18: 5085– 5098, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsunoda N, Maruyama K, Cooke DW, Lane DM, Ezaki O. Localization of exercise- and denervation-responsive elements in the mouse GLUT4 gene. Biochem Biophys Res Commun 267: 744– 571, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Wang SW, Pawlowski J, Wathen ST, Kinney SD, Lichenstein HS, Manthey CL. Cytokine mRNA decay is accelerated by an inhibitor of p38-mitogen-activated protein kinase. Inflamm Res 48: 533– 538, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Waters V, Sokol S, Reddy B, Soong G, Chun J, Prince A. The effect of cyclosporin A on airway cell proinflammatory signaling and pneumonia. Am J Respir Cell Mol Biol 33: 138– 144, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weigert C, Dufer M, Simon P, Debre E, Runge H, Brodbeck K, Haring HU, Schleicher ED. Upregulation of IL-6 mRNA by IL-6 in skeletal muscle cells: role of IL-6 mRNA stabilization and Ca2+-dependent mechanisms. Am J Physiol Cell Physiol 293: C1139– C1147, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J 18: 4969– 4980, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J 20: 6414– 6423, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res 87: E61– E68, 2000 [DOI] [PubMed] [Google Scholar]