Abstract
Sex differences in the incidence of varicose veins have been suggested; however, the venous mechanisms involved are unclear. We hypothesized sex-related differences in venous function and underlying distinctions in intracellular free calcium, [Ca2+]i, signaling and Ca2+-dependent mechanisms of venous contraction. Circular segments of inferior vena cava (IVC) from male and female Sprague-Dawley rats were suspended between two hooks, labeled with fura-2, and placed in a cuvet inside a spectrofluorometer for simultaneous measurement of isometric contraction and the 340/380 fluorescence ratio (indicative of [Ca2+]i). In male IVC, phenylephrine (PHE; 10−5 M) caused significant increase in contraction and [Ca2+]i. In female IVC, PHE-induced contraction was significantly reduced, but [Ca2+]i did not differ significantly from males. Membrane depolarization by KCl (96 mM), which stimulates Ca2+ influx, caused parallel increases in contraction and [Ca2+]i in male IVC, and the KCl-induced contraction was significantly reduced in parallel with [Ca2+]i in female IVC. In male IVC stimulated with 0 Ca2+ KCl solution, the addition of increasing concentrations of extracellular Ca2+ ([Ca2+]e) (0.1, 0.3, 0.6, 1, and 2.5 mM) caused stepwise increases in contraction and [Ca2+]i, and both the KCl-induced [Ca2+]e-contraction curve and the [Ca2+]e-[Ca2+]i curve were reduced in female IVC, suggesting reduced Ca2+ entry via voltage-gated channels. The PHE-induced [Ca2+]e-contraction curve was significantly reduced in females, but the [Ca2+]e-[Ca2+]i curve was similar in female and male IVC, suggesting the involvement of other mechanisms in addition to Ca2+ entry. The [Ca2+]e-contraction and [Ca2+]e-[Ca2+]i curves were used to construct the [Ca2+]i-contraction relationship. The KCl-induced [Ca2+]i-contraction relationship was superimposed in male and female IVC. In contrast, the PHE-induced [Ca2+]i-contraction relationship was reduced and located to the right in female compared with male IVC, suggesting reduced [Ca2+]i sensitivity of the venous contractile myofilaments. The reduced contraction, [Ca2+]i, and [Ca2+]i sensitivity in female veins render them more prone to dilation. These sex-specific reductions in venous function, if they also occur in human veins, may play a role in the greater incidence of varicose veins in females.
Keywords: calcium, sex hormones, vascular smooth muscle, vein
varicose veins is a common disease of the lower extremity characterized by vein valve degeneration and wall dilation and turtuousity (40). In addition to predisposing factors such as diabetes, obesity, smoking, and age (2, 45), sex could influence the incidence of varicose veins. Epidemiology studies have suggested that the incidence of varicose veins is greater in females compared with males (4, 49). The Framingham Heart Study demonstrated that the incidence rate of varicose veins was 2.6% in women compared with 1.9% in men (6). Also, the San Diego Population Study, a cross-sectional study of a multiethnic sample of 2,211 adult men and women, demonstrated varicose veins in 28% of females compared with 15% of males (10, 11). However, the venous mechanisms underlying the sex differences in the incidence of varicose veins are unclear.
Most of our knowledge regarding the sex differences in vascular function comes from studies in arteries. Several studies have demonstrated sex differences in the vascular reactivity of the aorta, coronary, and mesenteric arteries from various species (1, 29, 33, 38, 46, 54, 56). We have also shown that aortic contraction is reduced in female compared with male Wistar-Kyoto and spontaneously hypertensive rats (9, 35). Although numerous studies have demonstrated sex differences in arterial function, little is known regarding the venous function in females compared with males. Also, while some studies have examined the cellular mechanisms of venous smooth muscle contraction (23, 24, 34, 55), whether these mechanisms are influenced by sex is unclear.
The present study was designed to test the hypothesis that the venous tissue reactivity is influenced by sex and that the sex differences in venous function reflect underlying distinctions in intracellular free calcium, [Ca2+]i, signaling and Ca2+-dependent mechanisms of venous contraction.
METHODS
Animals.
Age-matched male and female Sprague-Dawley rats (12 wk, 250–300 g) were purchased from Charles River Laboratories (Wilmington, MA) and maintained in the animal facility on ad libitum standard rat chow and tap water in 12:12-h light-dark cycle. The specific stage of the estrous cycle in female rats was not determined in the present study. Synchronization of adult female rats at a specific stage of the estrous cycle would require the administration of exogenous estrogen and progesterone and abortifacient drugs such as prostaglandin F2α, which could change the vascular reactivity and thus would affect the measurements of the contractile response in the veins. Therefore, female rats were studied using random selection regardless of the stage of the ovarian cycle. Since the ovarian cycle in rats is frequent (every 4 to 5 days) and the estrous stage is short (12 h), the average data from all female rats should cancel out the effects of possible fluctuations in sex hormone levels at specific stages of the ovarian cycle and should, roughly, represent the average changes in vein function during all stages of the ovarian cycle. All procedures followed the National Institutes of Health Guide for the Care of Laboratory Animal Welfare Act and were approved by Harvard's Animal Care and Use Committee.
Tissue preparation.
On the day of the experiment, rats were euthanized by inhalation of CO2. The inferior vena cava (IVC) was rapidly excised, placed in oxygenated Krebs solution, carefully dissected and cleaned of connective tissue under microscopic visualization, and portioned into 3-mm circular segments. The IVC is a very thin and delicate preparation, and extreme care was taken throughout the tissue isolation and dissection procedure to minimize tissue injury.
Isometric contraction.
Circular segments of IVC were mounted between two home-made cuvet-adaptable stainless-steel hooks; one hook was fixed at the bottom of the cuvet, and the other hook was connected to a Grass force displacement transducer (FT03; Astro-Med, West Warwick, RI). Vein segments were stretched under 0.5 g of basal tension and allowed to equilibrate for 45 min in a water-jacketed cuvet filled with 3 ml of Krebs solution bubbled with 95% O2-5% CO2 at 37°C. We have previously constructed the relationship between basal tension and the contraction to 96 mM KCl in rat IVC, and demonstrated that 0.5 g basal tension produced maximal KCl contraction. Further increases in basal tension did not cause any significant increases in IVC contraction in response to KCl (41). The bathing solution was changed every 10 min. The changes in isometric contraction were recorded using a Grass Polygraph D.C. Driver Amplifier and a chart recorder.
Simultaneous measurement of [Ca2+]i.
The unloaded IVC was mounted in the cuvet and placed in a double-excitation double-emission Fluorolog-3 spectrofluorometer (Instruments S.A., Jobin Yvon-SPEX, Edison, NJ). After measuring the autofluorescence, the IVC segments were incubated in Krebs solution containing the cell-permeable Ca2+ indicator fura-2/AM (5 μM), BSA (3 mg/ml), and the mild detergent cremophor EL (0.25%) for 3 h, as previously described (24, 48). The IVC was washed 3 times in Krebs solution to remove extracellular fura-2/AM, and incubated in normal Krebs for an additional 30 min to allow for deesterification of the trapped intracellular fura-2/AM into the Ca2+-sensitive fura-2. The fura-2-loaded IVC was excited alternately at 340 and 380 nm, and the emitted light was collected at 510 nm every 2 s using DataMax data acquisition and analysis software (Instruments S.A.). The 340/380 ratio was calculated and represented the changes in [Ca2+]i. The signal-to-noise ratio was improved by averaging five consecutive 340/380 fluorescence ratio readings.
Experimental protocols.
IVC segments were first stimulated with 96 mM KCl, and the simultaneous changes in contraction and 340/380 ratio (indicative of [Ca2+]i) were recorded. Once the maximum KCl contraction was reached (within 10 min), the IVC was washed three times in Krebs, 10 min each. The control contraction and [Ca2+]i response to 96 mM KCl was repeated twice prior to further experimentation. IVC segments were then stimulated with phenylephrine (PHE, 10−5 M), and the simultaneous changes in contraction and [Ca2+]i were recorded for 10 min.
To investigate sex differences in the Ca2+ release mechanism from the intracellular stores, IVC segments were incubated in Ca2+-free (2 mM EGTA) Krebs for 5 min, then in nominally 0 Ca2+ Krebs for 5 min, then stimulated with PHE (10−5 M), and the initial contraction and [Ca2+]i were measured. To investigate sex differences in the Ca2+ entry mechanisms, tissues were first stimulated with 0 Ca2+ 96 mM KCl solution or with PHE 10−5 M in 0 Ca2+ Krebs, then increasing extracellular CaCl2 concentrations (0.1, 0.3, 0.6, 1, and 2.5 mM) were added, and the [Ca2+]e-contraction curve and [Ca2+]e-[Ca2+]i curve were constructed. To test for potential sex differences in the [Ca2+]i sensitivity of the contractile proteins, the KCl- and PHE-induced [Ca2+]e contraction and [Ca2+]e-[Ca2+]i curves were used to construct the [Ca2+]i-contraction relationship in IVC of males and females.
Solutions, drugs, and chemicals.
Normal Krebs solution contained (in mM): 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, and 1.2 MgCl2. Krebs solution was bubbled with 95% O2 and 5% CO2 for 30 min, at an adjusted pH 7.4. For nominally 0 Ca2+ Krebs, CaCl2 was omitted. For Ca2+-free Krebs, CaCl2 was omitted and 2 mM EGTA (Sigma, St. Louis, MO) was added. 96 mM KCl was prepared as normal Krebs but with equimolar substitution of NaCl with KCl. Stock solution of PHE (10−1 M, Sigma) was prepared in deionized water. All other chemicals were of reagent grade or better.
Statistical analysis.
Data were analyzed and presented as means ± SE, and data were compared using Student's t-test for unpaired data. Differences were considered statistically significant if P < 0.05.
RESULTS
In male IVC loaded with fura-2 and incubated in normal Krebs solution (2.5 mM Ca2+), the basal [Ca2+]i corresponded to a 340/380 ratio of 1.83 ± 0.09, which was not significantly different from that in female IVC (1.76 ± 0.09).
Effect of PHE.
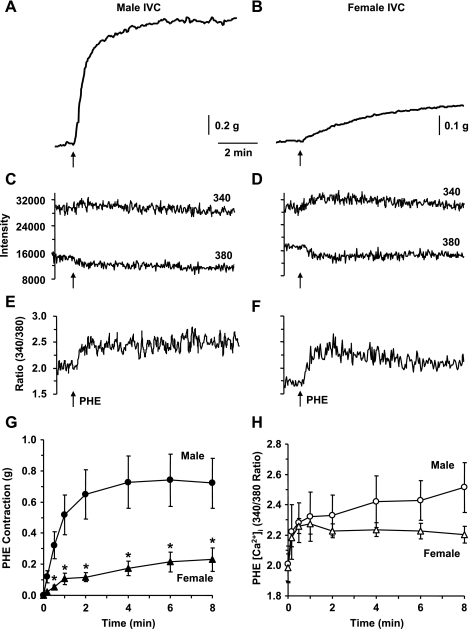
In IVC segments of male rats, the α-adrenergic receptor agonist PHE (10−5 M) caused a significant contraction (Fig. 1A), that reached steady state in 6.27 ± 0.46 min (Table 1) and was maintained for at least 10 min. In female IVC, PHE induced a smaller contraction that reached steady state in 6.50 ± 0.84 min (Table 1 and Fig. 1B). Also, in IVC segments of male and female rats, PHE-induced contraction was associated with simultaneous increase in the fura-2 340-nm fluorescence signal, a decrease in the 380-nm fluorescence signal (Fig. 1, C and D), and an increase in the 340/380 fluorescence ratio (Fig. 1, E and F), indicating simultaneous increase in [Ca2+]i during PHE-induced contraction. In both male and female IVCs the PHE-induced peak [Ca2+]i preceded and reached steady-state before peak and steady-state contraction (Table 1). Cumulative data indicated that the PHE-induced steady-state contraction was significantly reduced in female compared with male IVC (Fig. 1G). In contrast, the PHE-induced steady-state [Ca2+]i was not significantly different between male and female IVC (Fig. 1H).
Fig. 1.
Effect of PHE (10−5 M) on contraction and [Ca2+]i in male and female inferior vena cava (IVC). IVC segments from male (A, C, E) and female rats (B, D, F) were loaded with fura-2. The vein segments were stimulated with phenylephrine (PHE) (10−5 M), and the simultaneous changes in contraction (A, B), 340- and 380-nm fluorescence signal (C, D), and 340/380 ratio (E, F) were recorded. Cumulative time-course graphs represent means ± SE of PHE-induced contraction (G) and [Ca2+]i measurements (H) in male and female IVC (n = 5 or 6). *Measurements in female IVC are significantly different compared with corresponding measurements in male IVC (P < 0.05).
Table 1.
Time-to-peak and time-to-steady-state contractions and [Ca2+]i in response to KCl (96 mM) and PHE (10−5 M) in IVC segments of male and female rats
| Male |
Female |
|||
|---|---|---|---|---|
| KCl | PHE | KCl | PHE | |
| Time-to-peak contraction, min | 2.71±0.95 (7) | 3.86±0.57 (5) | 3.23±1.20 (6) | 3.00±0.96 (6) |
| P = 0.737 | P = 0.484 | |||
| Time-to-peak [Ca2+]i, min | 0.93±0.22 (7) | 0.81±0.37 (5) | 0.87±0.23 (6) | 0.67±0.46 (6) |
| P = 0.854 | P = 0.823 | |||
| Time-to-steady-state contraction, min | 5.50±0.65 (7) | 6.27±0.46 (5) | 6.23±0.57 (6) | 6.50±0.84 (6) |
| P = 0.424 | P = 0.826 | |||
| Time-to-steady-state [Ca2+]i, min | 3.31±0.59 (7) | 3.46±0.72 (5) | 3.05±0.41 (6) | 3.30±0.65 (6) |
| P = 0.733 | P = 0.872 | |||
Data are expressed as means ± SE; n = number in parenthesis. P value represents the differences between measurements in female inferior vena cava (IVC) and the corresponding measurements in male IVC. PHE, phenylephrine.
Effect of KCl.
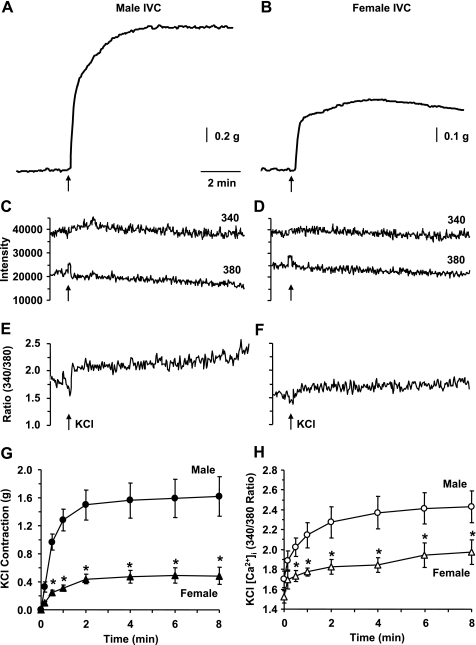
High KCl solution is known to cause membrane depolarization and to induce Ca2+ entry and Ca2+-dependent contraction in vascular smooth muscle (VSM) (26, 35). High KCl (96 mM) caused significant contraction in IVC segments of both male (Fig. 2A) and female rats (Fig. 2B). The KCl-induced contraction was rapid in onset, reached steady state within 5 to 7 min (Table 1), and was maintained for at least 10 min. Also, in IVC segments of both male and female rats KCl caused an increase in the fura-2 340-nm fluorescence signal, a decrease in the 380-nm fluorescence signal (Fig. 2, C and D) and an increase in the 340/380 fluorescence ratio (Fig. 2, E and F), indicating simultaneous increase in [Ca2+]i during KCl-induced contraction. In both male and female IVC, the KCl-induced peak [Ca2+]i preceded and reached steady state before the peak and steady-state contraction (Table 1). Cumulative data indicated that the KCl-induced steady-state contraction (Fig. 2G) and [Ca2+]i (Fig. 2H) were significantly reduced in female compared with male rat IVC, suggesting reduced Ca2+ entry mechanisms of IVC contraction in female rats.
Fig. 2.
Effect of KCl (96 mM) on contraction and [Ca2+]i in male and female rat IVC. IVC segments isolated from male (A, C, E) and female rats (B, D, F) and loaded with fura-2 were stimulated with KCl (96 mM), and the simultaneous changes in contraction (A, B), 340 and 380 nm fluorescence signal (C, D), and 340/380 ratio (E, F) were recorded. Cumulative time-course graphs represent means ± SE of KCl-induced contraction (G) and [Ca2+]i measurements (H) in male and female IVC (n = 6 to 7). *Measurements in female IVC are significantly different compared with corresponding measurements in male IVC (P < 0.05).
PHE-induced [Ca2+]e-contraction and [Ca2+]e-[Ca2+]i curves.
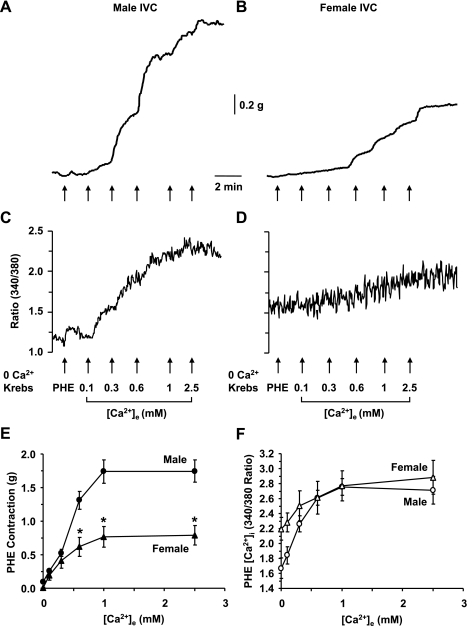
In male IVC incubated in 0 Ca2+ Krebs, stimulation with PHE (10−5 M) caused a measurable contraction (0.094 ± 0.014 g) and little change in [Ca2+]i from 1.65 ± 0.20 to 1.67 ± 0.20 (Fig. 3, A and C). In female IVC, stimulation with PHE caused significantly smaller contraction (0.008 ± 0.008 g, P = 0.002) and insignificant change in [Ca2+]i from 2.06 ± 0.22 to 2.20 ± 0.26 (Fig. 3, B and D). In male IVC, stepwise addition of extracellular Ca2+ caused corresponding increases in PHE-induced contraction (Fig. 3A) and simultaneous increases in [Ca2+]i (Fig. 3C) that reached a maximum at 2.5 mM [Ca2+]e. In female IVC, stepwise addition of [Ca2+]e caused smaller increases in PHE-induced contraction (Fig. 3B), but similar increases in [Ca2+]i (Fig. 3D). The PHE-induced [Ca2+]e-contraction curve was significantly reduced in female compared with male IVC (Fig. 3E). On the other hand, the PHE-induced [Ca2+]e-[Ca2+]i curve was not significantly different between male and female IVC (Fig. 3F).
Fig. 3.
PHE-induced [Ca2+]e-contraction and [Ca2+]e-[Ca2+]i curves in male and female rat IVC. IVC segments from male (A, C) and female rats (B, D) were incubated in Ca2+-free (2 mM EGTA) Krebs for 5 min, then nominally 0 Ca2+ Krebs for 5 min. The tissues were stimulated with PHE (10−5 M), and the initial contraction (A, B) and [Ca2+]i (C, D) were recorded. Increasing concentrations of extracellular CaCl2 (0.1, 0.3, 0.6, 1, 2.5 mM) were added, and the PHE-induced [Ca2+]e-contraction curve (E) and [Ca2+]e-[Ca2+]i curve (F) were constructed and compared in male and female IVC. Data are expressed as means ± SE; n = 4. *Measurements in female IVC are significantly different compared with corresponding measurements in male IVC (P < 0.05).
KCl-induced [Ca2+]e-contraction and [Ca2+]e-[Ca2+]i curves.
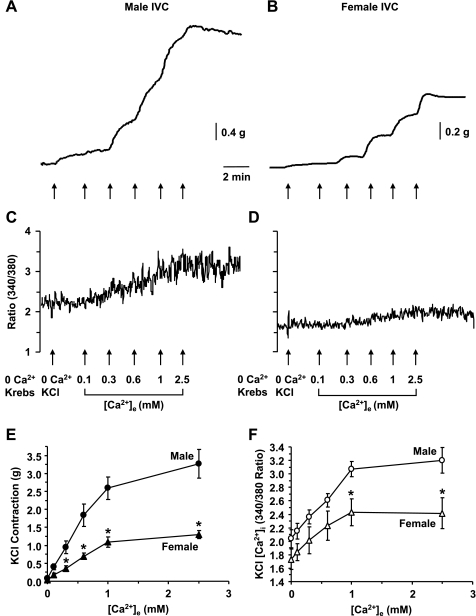
In male IVC incubated in 0 Ca2+ Krebs, stimulation with 0 Ca2+ KCl (96 mM) caused a measurable contraction (0.088 ± 0.022 g) and little change in [Ca2+]i from 1.94 ± 0.17 to 2.04 ± 0.14 (Fig. 4, A and C). In female IVC, stimulation with 0 Ca2+ KCl (96 mM) caused a small contraction (0.026 ± 0.012 g) and little change in [Ca2+]i from 1.62 ± 0.14 to 1.73 ± 0.15 (Fig. 4, B and D). The KCl-induced contraction in 0 Ca2+ medium was significantly greater in male compared with female IVC (P = 0.048), but no significant sex differences in [Ca2+]i were detected under these conditions. In male IVC, stepwise addition of extracellular Ca2+ (0.1, 0.3, 0.6, 1, and 2.5 mM) caused corresponding increases in KCl-induced contraction (Fig. 4A) and simultaneous increases in [Ca2+]i (Fig. 4C) that reached a maximum at 2.5 mM [Ca2+]e. In female IVC, stepwise addition of [Ca2+]e caused smaller increases in KCl-induced contraction (Fig. 4B) and [Ca2+]i (Fig. 4D). The KCl-induced [Ca2+]e-contraction curve (Fig. 4E) and [Ca2+]e-[Ca2+]i curve (Fig. 4F) were significantly reduced in female compared with male IVC, supporting sex differences in the Ca2+ entry mechanisms.
Fig. 4.
KCl-induced [Ca2+]e-contraction and [Ca2+]e-[Ca2+]i curves in male and female rat IVC. IVC segments from male (A, C) and female rats (B, D) were incubated in Ca2+-free (2 mM EGTA) Krebs for 5 min, then nominally 0 Ca2+ Krebs for 5 min. The bathing solution was changed to 0 Ca2+ 96 mM KCl, and the contractile response (A, B) and [Ca2+]i (C, D) were recorded. Increasing concentrations of extracellular CaCl2 (0.1, 0.3, 0.6, 1, 2.5 mM) were added and the KCl-induced [Ca2+]e-contraction curve (E) and [Ca2+]e-[Ca2+]i curve (F) were constructed and compared in male and female IVC. Data are expressed as means ± SE; n = 4 to 5. *Measurements in female IVC are significantly different compared with corresponding measurements in male IVC (P < 0.05).
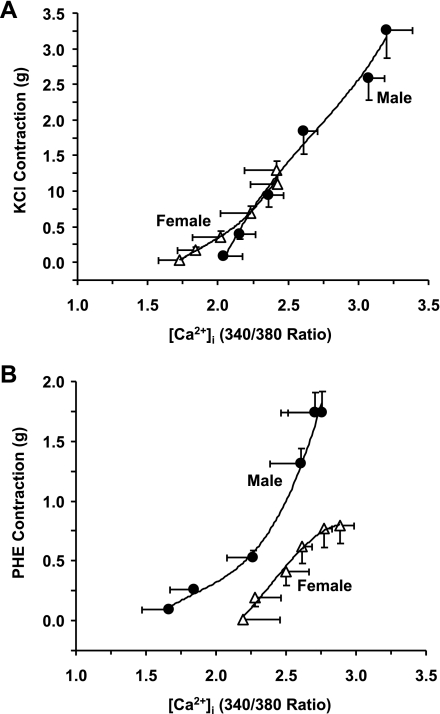
[Ca2+]i-contraction relationship.
The KCl- and PHE-induced [Ca2+]e-contraction and [Ca2+]e-[Ca2+]i curves were used to construct the [Ca2+]i-venocontraction relationship in IVC segments of male and female rats. The KCl [Ca2+]i-contraction relationship in female IVC coincided with that in male rat IVC (Fig. 5A). In contrast, the PHE-induced [Ca2+]i-contraction relationship was reduced and located to the right in female compared with male IVC (Fig. 5B).
Fig. 5.
[Ca2+]i-contraction relationship in IVC segments from male and female rats. The KCl- and PHE-induced [Ca2+]e-contraction and [Ca2+]e-[Ca2+]i curves were used to construct the [Ca2+]i-venocontraction relationship during stimulation by KCl (A) and PHE (B). Data are expressed as means ± SE; n = 4 to 5. To facilitate comparison, data points were best fitted using three-order nonlinear polynomial regression (Microsoft Excel).
DISCUSSION
The present study demonstrates sex differences in venous tissue [Ca2+]i and Ca2+-dependent contraction. Depolarization-induced contraction is reduced in parallel with [Ca2+]i in female compared with male IVC. The α-adrenergic agonist-induced contraction and sensitivity to [Ca2+]i are reduced in female compared with male IVC.
There is a large body of evidence that [Ca2+]i is a major determinant of vascular contraction (20, 25, 26, 44). We found that during stimulation of both male and female rat IVC with KCl or PHE, the time-to-peak and time-to-steady-state [Ca2+]i preceded the time-to-peak and time-to-steady-state contraction (Table 1), suggesting that the increased [Ca2+]i stimulates the IVC contraction.
Membrane depolarization by high KCl is often used to measure Ca2+ influx from the extracellular space through voltage-gated Ca2+ channels (24, 26). Previous studies in rat aortic rings loaded with fura-2 have shown that KCl causes simultaneous increases in contraction and [Ca2+]i (48). KCl also causes simultaneous increases in contraction and [Ca2+]i in rabbit IVC loaded with fura-2 (24). The observation that KCl caused parallel increases in contraction and [Ca2+]i in rat IVC is consistent with previous reports and suggests activation of voltage-gated channels in rat veins. Although the KCl-induced response is generally thought to be mainly due to Ca2+ entry from the extracellular space, we observed a small IVC contraction in response to KCl in 0 Ca2+ medium. This is likely due to some residual Ca2+ that remains bound to the IVC surface membrane despite the IVC incubation in Ca2+-free (2 mM EGTA) Krebs for 5 min and in nominally 0 Ca2+ Krebs for 5 min. The membrane depolarization by 0 Ca2+ KCl would then allow the residual membrane-bound Ca2+ to enter venous smooth muscle, where it may activate Ca2+ release from the intracellular stores via Ca2+-induced Ca2+ release mechanism (20). However, we could not detect any significant increases in IVC [Ca2+]i in response to KCl in 0 Ca2+ medium, suggesting that the contribution of these mechanisms to increases in [Ca2+]i is too small to be measured by our current detection method.
α-Adrenergic agonists have been shown to stimulate contraction and [Ca2+]i in numerous arterial preparations, although the [Ca2+]i profile varies markedly (7, 13, 48). Studies have shown that norepinephrine produces parallel increases in contraction and [Ca2+]i in rat aorta loaded with the fluorescent Ca2+ indicator fura-2 (48). Other studies in ferret aorta loaded with the luminescent Ca2+ indicator aequorin have shown that PHE produces significant contraction with a large transient response followed by a very small maintained increase in [Ca2+]i (13). The difference in the [Ca2+]i profile has been related to differences in the Ca2+ indicator used but could also be related to the thickness of the aortic tissue and difficulties in its loading with Ca2+ indicators. Studies in thinner preparations, such as the ferret portal vein and rabbit IVC have demonstrated a PHE-induced maintained contraction associated with detectable increases in [Ca2+]i (24, 34). The present findings in male rat IVC are consistent with previous reports and demonstrate that PHE is associated with measurable increases in contraction and [Ca2+]i.
In VSM, α-adrenergic agonists are known to activate phospholipase C and to increase the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (5, 31, 32, 36). IP3 diffuses into the cytosol and stimulates Ca2+ release from the intracellular stores. The observed PHE-induced small contraction in 0 Ca2+ Krebs is consistent with activation of Ca2+ release from the intracellular stores. Because the intracellular stores are limited, the maintained PHE contraction is largely due to Ca2+ influx from the extracellular space. Although some studies suggest that α-adrenergic agonists stimulate Ca2+ entry through voltage-gated channels (37), several studies have suggested activation of receptor-operated, ligand-gated, and store-operated channels (3, 20, 27, 28, 57). Assuming that the KCl response is mainly due to Ca2+ entry through voltage-gated channels, the [Ca2+]i profile during IVC stimulation with PHE may involve activation of other Ca2+ entry pathways, such as receptor-operated, ligand-gated, and/or store-operated channels.
Several studies have shown sex differences in vascular contraction in numerous arterial preparations, and demonstrated that the arterial contraction is reduced in females compared with males (9, 33, 35, 38). On the other hand, little is known regarding the influence of sex on venous tissue function. The present study demonstrates sex differences in PHE- and KCl-induced contraction of rat IVC, with the contraction in females being reduced compared with that in males.
We examined the potential mechanisms underlying the sex differences in IVC contraction. The sex difference may be partly due to differences in the Ca2+-release mechanisms from the intracellular stores because PHE-induced contraction in 0 Ca2+ Krebs was significantly greater in male than female IVC. The sex differences in the Ca2+ release mechanisms in rat IVC is different from previous reports demonstrating the lack of sex differences in the Ca2+ release mechanisms in the rat aorta (9, 35). The sex differences in IVC contraction could also be due to differences in voltage-gated Ca2+ channels because 1) KCl caused parallel increases in contraction and [Ca2+]i, and both responses were reduced in female compared with male IVC; 2) the KCl-induced [Ca2+]e-contraction and [Ca2+]e-[Ca2+]i curves were reduced in female compared with male IVC; and 3) the KCl-induced [Ca2+]i-contraction relationship in female IVC was superimposed or an extension of that in male IVC. The results in rat IVC are consistent with previous reports that the contraction, Ca2+ influx, and [Ca2+]i are reduced in aortic strips and freshly isolated aortic VSM cells of female compared with male rats (9, 35). The reduced Ca2+ entry in female IVC could be due to decreased amount or activity of voltage-gated channels in venous smooth muscle of females and should be examined in future biochemical and electrophysiological studies.
Despite the potential involvement of voltage-gated Ca2+ channels, the sex differences in IVC contraction do not appear to involve differences in ligand-gated channels because 1) PHE contraction was reduced, but [Ca2+]i was similar in female compared with male IVC; 2) the PHE-induced [Ca2+]e-contraction curve was reduced in female compared with male IVC, but the [Ca2+]e-[Ca2+]i curve was similar in both sexes; and 3) the PHE-induced [Ca2+]i-contraction relationship was reduced and located to the right in female compared with male IVC; i.e., for the same increases in [Ca2+]i, PHE produces greater contraction in IVC of males compared with females. The reduction in the PHE-induced [Ca2+]i-contraction relationship in female compared with male IVC suggests sex differences in other venous contraction mechanisms in addition to [Ca2+]i.
The reduced [Ca2+]i-contraction relationship in PHE-stimulated IVC of female compared with male rats suggests that the activity of a [Ca2+]i regulatory pathway that affects the myofilament sensitivity to [Ca2+]i is reduced in female compared with male rat IVC. Several studies have shown that in addition to the role of Ca2+-calmodulin and myosin light chain kinase, Rho-kinase and mitogen-activated protein kinase may contribute to VSM contraction (15–17, 22, 50). Also, PKC has been suggested to play an important role in the regulation of VSM contraction, in part by increasing the [Ca2+]i sensitivity of the contractile proteins (19, 21, 47). We have previously reported sex-related distinctions in PKC activity in rat aortic smooth muscle (18). The present observation that the PHE-induced [Ca2+]i-contraction relationship is reduced in female IVC is consistent with previous reports in arterial smooth muscle and suggests reduced activity of [Ca2+]i sensitization pathways such as PKC or Rho-kinase in venous smooth muscle of female rats.
There are several points that need further explanation and clarification:
It could be argued that the sex differences in vein contraction are caused by differences in the amount of contractile myofilaments. This is less likely because our preliminary Western blot experiments revealed that the amount of actin was similar in protein-matched specimens of male and female IVC (39).
The present study used the fura-2 ratiometric method as an indicator of [Ca2+]i. Although this method provides an indirect measure of [Ca2+]i, it has the advantage of not requiring cell membrane permeabilization, which could affect the receptor-mediated response. Future studies in α-toxin permeabilized IVC would allow manipulation of intracellular Ca2+ and further test for changes in Ca2+ sensitivity of the contractile myofilaments in female compared with male veins.
In the present study 95% O2-5% CO2 was used to oxygenate the vein rings bathed in Krebs solution, which might provide a hyperoxygenated environment, increase uncoupled endothelial nitric oxide synthase (eNOS), and, in turn, produce superoxide instead of nitric oxide (NO) (53) and thereby impair endothelial function in our experimental setup. Studies have shown that estrogen increases eNOS expression and NO production (30) and suppresses the production of reactive oxygen species, such as superoxide in the vasculature (43, 51). We have also shown that the plasma levels of estrogen are greater in female than male rats (18, 35). Also, our preliminary experiments have demonstrated that acetylcholine-induced relaxation is significantly enhanced (P < 0.01) in female IVC (max 80.59 ± 4.06%) compared with male rat IVC (48.02 ± 6.07%) and that estrogen causes significant inhibition of PHE contraction (max 76.53 ± 3.44%) in female IVC (39). Our previous studies on the rat aorta have also shown that estrogen does not affect the Ca2+ release mechanism, but inhibits Ca2+ entry (35). These observations suggest that the sex differences in IVC reactivity could be partly due to an effect of residual estrogen in the female veins on endothelial NO production/biological activity, or other endothelium-derived factors such as prostacyclin or hyperpolarizing factor, or on VSM Ca2+ channel activity. This is less likely because the veins were dissected and washed repeatedly in Krebs solution before starting the experiments. Therefore, the reduction in PHE and KCl contraction in female IVC is more likely due to long-term effect of endogenous estrogen on the mechanisms of contraction of female veins and possible estrogen-mediated downregulation of the Ca2+ channel density. However, a possible role of innate biological differences between male and female veins cannot be ruled out.
The present study was performed on endothelium-intact IVC segments, and the sex-related reduction in PHE and KCl contraction in female IVC can be due to effects of endogenous estrogen on both endothelium-dependent and endothelium-independent mechanisms. Although the rat IVC is a very thin and delicate tissue, we were able to carefully preserve the endothelium during the dissection procedure and to measure significant acetylcholine-induced relaxation in the rat IVC (39). In preliminary experiments, we attempted to remove the endothelium using traditional methods, such as rubbing the vein interior around the tip of forceps or with filter paper. Unfortunately, these harsh methods appeared to cause significant injury to the VSM layer, and the response to either PHE or KCl was almost undetectable in these veins. Another approach to minimize the role of the endothelium is to block not only the endothelium-derived NO, but also prostacyclin and hyperpolarizing factor using a cocktail of nitro-l-arginine methyl ester, indomethacin, and K+ channel blockers, respectively. Future studies should examine whether such a multifaceted intervention would eliminate the sex differences in PHE and KCl contractions in rat veins (suggesting that the sex differences involve endothelium-dependent mechanisms) or the differences would still be observed in veins treated with blockers of endothelium-derived vasodilators (suggesting that the sex differences involve endothelium-independent mechanisms).
Previous studies in rat aorta have demonstrated sex-related difference in contraction of the aorta of intact female compared with intact male rats (35, 52). Studies have also shown that both androgens and estrogens influence vascular function (8, 12, 14). Interestingly, we found that aortic VSM [Ca2+]i was not different in castrated male compared with intact male rats, suggesting that the sex differences in arterial function may not be related to endogenous androgens (35). On the other hand, aortic VSM [Ca2+]i was greater in ovariectomized (OVX) female rats compared with intact female rats, and the difference was eliminated in estrogen-replaced OVX females, suggesting a role of endogenous estrogen in the control of the [Ca2+]i mechanisms of arterial contraction (35). Whether endogenous estrogen compared with androgens plays a role in the observed sex differences in venous [Ca2+]i is unclear and should be examined in future studies.
Finally, Although the rats used in the present study were postpubescent, they were still relatively young. Whether the observed sex differences in vein function are maintained during longer periods of exposure to sex hormones in more mature rats should be examined. More importantly, it would be interesting to test whether the sex differences in vein function are maintained, or abolished, with old age and in the postmenopausal period.
In conclusion, the present study provides evidence of sex differences in venous tissue contraction and demonstrates that the [Ca2+]i, Ca2+-dependent contraction, and the myofilament contraction sensitivity to [Ca2+]i are reduced in female compared with male rat IVC. The role of endogenous sex hormones in the reduced mechanisms of contraction in female veins should be further investigated in future studies.
Perspectives and Significance
Reduction in the contraction, [Ca2+]i, and [Ca2+]i sensitivity in female veins should render them more prone to dilation. We should caution that the present study was conducted on rat IVC. Although the rat is a four-legged animal, the rat is a consistent breed, and studies on rat veins can avoid the variability related to age, body weight, and other confounding factors that are encountered in studies on human veins. Also, our preliminary studies on the rat IVC have produced consistent contractile response (41, 42), making it a reliable model to perform mechanistic studies on venous tissue and to test the mechanisms underlying the sex differences in venous function. To enhance the relevance of the studies to lower extremity veins, future mechanistic studies need to be performed on rat iliac and femoral vein. Also, to enhance the relevance of the studies to human varices, future mechanistic studies need to be performed on human greater saphenous vein from males and females. Although the present results were demonstrated in the rat IVC, the observed sex-specific reductions in venous function, if they also occur in human veins, may play a role in the greater incidence of varicose veins in females. Future studies on more relevant peripheral rat veins such as the femoral or iliac veins, as well as in human saphenous vein, should help to further define the causes of the sex differences in venous function and the incidence of varicose veins.
GRANTS
This work was supported by grants from The National Heart, Lung, and Blood Institute (HL-65998 and HL-70659), and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD-60702). Dr. Y. Xia was a visiting scholar from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, P. R. China, and a recipient of a scholarship from the China Scholarship Council.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Barber DA, Miller VM. Gender differences in endothelium-dependent relaxations do not involve NO in porcine coronary arteries. Am J Physiol Heart Circ Physiol 273: H2325– H2332, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol 15: 175– 184, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature 328: 275– 278, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med 355: 488– 498, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312: 315– 321, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med 4: 96– 101, 1988 [PubMed] [Google Scholar]
- 7.Chen W, Khalil RA. Differential [Ca2+]i signaling of vasoconstriction in mesenteric microvessels of normal and reduced uterine perfusion pregnant rats. Am J Physiol Regul Integr Comp Physiol 295: R1962– R1972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crews JK, Khalil RA. Antagonistic effects of 17β-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol 19: 1034– 1040, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Crews JK, Murphy JG, Khalil RA. Gender differences in Ca2+ entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 34: 931– 936, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Criqui MH, Denenberg JO, Bergan J, Langer RD, Fronek A. Risk factors for chronic venous disease: the San Diego Population Study. J Vasc Surg 46: 331– 337, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Criqui MH, Jamosmos M, Fronek A, Denenberg JO, Langer RD, Bergan J, Golomb BA. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol 158: 448– 456, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidge ST, McLaughlin MK. Endogenous modulation of the blunted adrenergic response in resistance-sized mesenteric arteries from the pregnant rat. Am J Obstet Gynecol 167: 1691– 1698, 1992 [DOI] [PubMed] [Google Scholar]
- 13.DeFeo TT, Morgan KG. Calcium-force relationships as detected with aequorin in two different vascular smooth muscles of the ferret. J Physiol 369: 269– 282, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geary GG, Krause DN, Duckles SP. Gonadal hormones affect diameter of male rat cerebral arteries through endothelium-dependent mechanisms. Am J Physiol Heart Circ Physiol 279: H610– H618, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Hilgers RH, Todd J, Jr, Webb RC. Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens 25: 1687– 1697, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hilgers RH, Webb RC. Molecular aspects of arterial smooth muscle contraction: focus on Rho. Exp Biol Med (Maywood) 230: 829– 835, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev 76: 967– 1003, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol 280: C34– C45, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kanashiro CA, Khalil RA. Signal transduction by protein kinase C in mammalian cells. Clin Exp Pharmacol Physiol 25: 974– 985, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Khalil R, Lodge N, Saida K, van Breemen C. Mechanism of calcium activation in vascular smooth muscle. J Hypertens Suppl 5: S5– S15, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Khalil RA, Lajoie C, Resnick MS, Morgan KG. Ca2+-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am J Physiol Cell Physiol 263: C714– C719, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Khalil RA, Menice CB, Wang CL, Morgan KG. Phosphotyrosine-dependent targeting of mitogen-activated protein kinase in differentiated contractile vascular cells. Circ Res 76: 1101– 1108, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Khalil RA, Morgan KG. Phenylephrine-induced translocation of protein kinase C and shortening of two types of vascular cells of the ferret. J Physiol 455: 585– 599, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalil RA, van Breemen C. Intracellular free calcium concentration/force relationship in rabbit inferior vena cava activated by norepinephrine and high K+. Pflügers Arch 416: 727– 734, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Khalil RA, van Breemen C. Mechanisms of calcium mobilization and homeostasis in vascular smooth muscle and their relevance to hypertension. In: Hypertension: Pathophysiology, Diagnosis, and Management, edited by Laragh JH, Brenner BM. New York: Raven, 1995, p. 523– 540 [Google Scholar]
- 26.Khalil RA, van Breemen C. Sustained contraction of vascular smooth muscle: calcium influx or C-kinase activation? J Pharmacol Exp Ther 244: 537– 542, 1988 [PubMed] [Google Scholar]
- 27.Large WA. Receptor-operated Ca2+-permeable nonselective cation channels in vascular smooth muscle: a physiologic perspective. J Cardiovasc Electrophysiol 13: 493– 501, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Leung FP, Yung LM, Yao X, Laher I, Huang Y. Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol 153: 846– 857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto T, Kakami M, Kobayashi T, Kamata K. Gender differences in vascular reactivity to endothelin-1 (1–31) in mesenteric arteries from diabetic mice. Peptides 29: 1338– 1346, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol 90: 3F– 6F, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Michell RH. Inositol lipid breakdown as a step in alpha-adrenergic stimulus-response coupling. Clin Sci Lond 68Suppl 10: 43s– 46s, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Michell RH. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta 415: 81– 47, 1975 [DOI] [PubMed] [Google Scholar]
- 33.Miller VM, Barber DA, Fenton AM, Wang X, Sieck GC. Gender differences in response to endothelin-1 in coronary arteries: transcription, receptors and calcium regulation. Clin Exp Pharmacol Physiol 23: 256– 259, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Morgan JP, Morgan KG. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol 351: 155– 167, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca2+]i in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol 278: C834– C844, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Nånberg E, Putney J., Jr Alpha 1-adrenergic activation of brown adipocytes leads to an increased formation of inositol polyphosphates. FEBS Lett 195: 319– 322, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Nelson MT, Standen NB, Brayden JE, Worley JF., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature 336: 382– 385, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233– R249, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Raffetto JD, Khalil RA. Estrogen receptor-specific endothelium-dependent and -independent pathways of venous relaxation in female rat. Implications in sex-related differences in varicose veins. In Proceedings of the American Venous Forum 21st Annual Meeting Salem, MA: American Venons Forum, 2009 [Google Scholar]
- 40.Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology 23: 85– 98, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J Vasc Surg 48: 447– 456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg 45: 373– 380, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, Procaccio V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J Pharmacol Exp Ther 325: 782– 790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rembold CM, Murphy RA. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res 63: 593– 603, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Robertson L, Evans C, Fowkes FG. Epidemiology of chronic venous disease. Phlebology 23: 103– 111, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest 99: 2429– 2437, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol 70: 1537– 1547, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato K, Ozaki H, Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther 246: 294– 300, 1988 [PubMed] [Google Scholar]
- 49.Sisto T, Reunanen A, Laurikka J, Impivaara O, Heliövaara M, Knekt P, Aromaa A. Prevalence and risk factors of varicose veins in lower extremities: Mini-Finland Health Survey. Eur J Surg 161: 405– 414, 1995 [PubMed] [Google Scholar]
- 50.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325– 1358, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Song JY, Kim MJ, Jo HH, Hwang SJ, Chae B, Chung JE, Kwon DJ, Lew YO, Lim YT, Kim JH, Kim JH, Kim MR. Antioxidant effect of estrogen on bovine aortic endothelial cells. J Steroid Biochem Mol Biol 117: 74– 80, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Stallone JN, Crofton JT, Share L. Sexual dimorphism in vasopressin-induced contraction of rat aorta. Am J Physiol Heart Circ Physiol 260: H453– H458, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Sullivan JC, Pollock JS. Coupled and uncoupled NOS: separate but equal? Uncoupled NOS in endothelial cells is a critical pathway for intracellular signaling. Circ Res 98: 717– 719, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Tostes RC, David FL, Carvalho MH, Nigro D, Scivoletto R, Fortes ZB. Gender differences in vascular reactivity to endothelin-1 in deoxycorticosterone-salt hypertensive rats. J Cardiovasc Pharmacol 36Suppl 1: S99– S101, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Tykocki NR, Gariepy CE, Watts SW. Endothelin ET(B) receptors in arteries and veins: multiple actions in the vein. J Pharmacol Exp Ther 329: 875– 881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varbiro S, Matrai M, Szekeres M, Nadasy GL, Szaky E, Mericli M, Banhidy F, Monos E, Szekacs B. Intramural coronary artery constrictor reactivity to thromboxane is higher in male than in female rats. Gynecol Endocrinol 22: 44– 47, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res 88: 84– 87, 2001 [DOI] [PubMed] [Google Scholar]