Abstract
Maintenance of a body weight 10% above or below that “customary” for lean or obese individuals results in respective increases or decreases in the energy expended in low levels of physical activity (nonresting energy expenditure, NREE). These changes are greater than can be accounted for by the altered body weight or composition and are due mainly to altered skeletal muscle work efficiency at low levels of power generation. We performed biochemical analysis of vastus lateralis muscle needle biopsy samples to determine whether maintenance of an altered body weight was associated with changes in skeletal muscle histomorphology. We found that the maintenance of a 10% reduced body weight was associated with significant declines in glycolytic (phosphofructokinase, PFK) enzyme activity and, in particular, in the ratio of glycolytic to oxidative (cytochrome c oxidase, COX) enzyme activity without significant changes in the activities of enzymes relevant to mitochondrial density, respiratory chain activity, or fuel transport; or in skeletal muscle fiber type or glycogen stores. The fractional change in the ratio of PFK/COX activity in subjects following weight loss was significantly correlated with changes in the systemic respiratory exchange ratio (RER) and measures of mechanical efficiency of skeletal muscle at low workloads (pedaling a bicycle to generate 10 or 25 W of power). Thus, predictable changes in systemic skeletal muscle biochemistry accompany the maintenance of an altered body weight and account for a significant portion of the variance in skeletal muscle work efficiency and fuel utilization at reduced body weight.
Keywords: energy metabolism, exercise, obesity, weight gain, weight loss
maintenance of a body weight 10% below “usual” for a lean or obese individual is accompanied by a reduction in systemic energy metabolism (∼300–400 kcal/day less than that predicted solely on the basis of changes in body weight or composition), neuroendocrine function (decreased circulating concentrations of leptin and of bioactive thyroid hormones), autonomic nervous system physiology (decreased sympathetic nervous system tone and increased parasympathetic nervous system tone), and behavior (decreased satiety) that act coordinately to return body weight to its initial level (7, 25, 43, 46, 47, 53, 68). The changes that occur in subjects during maintenance of an elevated body weight following overfeeding involve many of the same systems but are not, in fact, mirror images of the changes following weight loss (7, 25, 43, 46, 47, 53, 63, 64, 68).
Using the same experimental design described in materials and methods, in which in-patient subjects ingesting a liquid formula diet are maintained at their usual weight and following a 10% weight loss or gain on a liquid formula diet, we have found that experimentally reducing or increasing energy stores (mainly adipose tissue mass) lowers energy expenditure per unit of total metabolic mass by ∼15% below or above, respectively, that predicted based on changes in body weight and composition (25). We, and others, have consistently found that the major compartment of energy expenditure that changes following weight loss or gain, is energy expended in physical activity, accounting for the majority of the variance in changes in 24-h energy expenditure at altered body weight (48, 70). Other compartments of energy expenditure, namely resting energy expenditure and the thermic effect of feeding, are also disproportionately decreased and/or increased following weight gain and/or loss, respectively (4, 8, 25, 47). However, these changes account for a smaller fraction of the variance in the changes in 24-h energy expenditure at altered body weight than does energy expended in physical activity. The changes in energy expended in physical activity are not due to changes in time spent in physical activity (25, 48, 71) but are significantly correlated with changes in skeletal muscle work efficiency measured by bicycle ergometry (48).
We hypothesized that a significant fraction of the variance in changes in skeletal muscle fuel utilization and chemomechanical efficiency could be accounted for by changes in skeletal muscle biochemistry and/or molecules known to affect skeletal muscle physiology and to be affected by weight change. We studied the effects of weight loss and weight gain on skeletal muscle fiber type, mitochondrial density, glycogen stores, enzymes involved in transport, and oxidation of glucose and fatty acids, (42, 48), and circulating concentrations of adiponectin, triiodothyronine, glucose, insulin, and leptin (6, 13–16, 24, 42, 43, 59, 75).
MATERIALS AND METHODS
A total of 30 subjects {18 Obese [OB; BMI, (Wt, kg)/(Ht, m)2] ≥30 kg/m2; 8 males and 10 females} and 12 never-obese (NO; BMI < 25 kg/m2; 8 males and 4 females)] subjects were studied at their maximum lifetime weight, which they had maintained within a 2-kg range for at least 6 mo prior to enrollment (34). All subjects were in good health (including prescreening for evidence of diabetes or impaired glucose tolerance), were on no medications, and were aged between 19 and 45 yr. Recruitment procedures and exclusion criteria for these studies, as well as data regarding the effects of weight change on energy expenditure in some of these subjects, have been previously reported (25, 42, 43). All studies were approved by the Institutional Review Board and are consistent with guiding principles for research involving humans (58). Written informed consent was obtained from all subjects prior to enrollment.
Subjects were in-patients in the Clinical Research Center at Rockefeller University Hospital or Columbia Presbyterian Medical Center throughout this study. They were weighed daily at 6:00 AM and were instructed to consume all meals before midnight. As described previously (47), subjects were fed a liquid formula diet [40% of calories as fat (corn oil), 45% as carbohydrate (glucose polymer), and 15% as protein (casein hydrolysate)], plus vitamin and mineral supplements, in quantities sufficient to maintain a stable weight (defined as a mean daily weight variation of less than 10 gm/day for at least 2 wk). This weight plateau is designated as “Wtinitial.”
Following completion of studies at Wtinitial, 25 subjects (18 obese, 7 never-obese; 11 males, 14 females) were provided 800 kcal/day of the same liquid formula diet until they had lost 10% of Wtinitial. The duration of the weight loss phase ranged from 36 to 62 days. Once 10% weight loss had been achieved, caloric intake was adjusted upward until subjects were again weight stable for a minimum of 2 wk as described above. Once weight was stable, all of the studies performed at Wtinitial were repeated. This weight plateau is designated as “Wt−10%.”
Five never-obese subjects (4 males and 1 female) were studied at usual weight and following a 10% weight gain that was achieved by providing them with maximum tolerated intake of self-selected foods (generally 5,000 to 8,000 kcal/day). The period of weight gain averaged 4 to 6 wk. The formula diet was then reinstated and the quantity adjusted downward to maintain constant body weight. This plateau is designated as Wt+10%. When subjects had been weight-stable for at least 14 days at Wt+10%, all of the studies performed at Wtinitial were repeated.
The controlled environment of the clinical research center permits meticulous regulation of subject activity and fitness. Using 24-h Holter monitoring, we have previously reported that the amount of time that subjects spend in physical activity in this environment is not affected by weight loss, weight gain, or duration of participation in this study (25, 47, 48). To prevent changes in physical fitness as a result of the 6–7 mo in-patient stay, and to ensure that subjects did not substantially vary their in-patient physical activity levels during the study, either of which might affect skeletal muscle metabolism/performance, each subject's aerobic fitness was measured by bicycle ergometry upon admission and was then maintained at that level throughout the study. Aerobic fitness was defined as the anaerobic threshold, the point at which pulmonary ventilation increases out of linear proportion to O2 consumption due to the buffering of lactate by endogenous bicarbonate (20). Supervised exercise (treadmill walking or stationary bicycling) was performed three times per week at specified intensities and durations that were adjusted to maintain each subject's anaerobic threshold at its initial level without adjustment for changes in body weight or composition throughout the study (48).
Subjects underwent the following studies while weight was kept stable (by adjustment of formula diet intake) at Wtinitial and Wt−10% or Wt+10%.
Body composition.
Body composition was determined by dual energy X-ray absorptiometry (DXA) (37).
Twenty-four hour energy expenditure.
Twenty-four hour energy expenditure (TEE) was measured by empirical determination of weight-maintenance caloric intake as described above (25, 47). The constancy of body composition, as well as weight stability, was confirmed by demonstrating that the respiratory exchange ratio (V̇co2/V̇o2; RER) for subjects at rest in the postabsorptive state was not significantly different from the liquid formula diet “formula quotient” (FQ) of 0.85 (see results) (25). Since body weight and composition are constant, the energy ingested as liquid formula must match the total daily energy expenditure. We have previously shown that TEE measured by such “caloric titration” is highly correlated with TEE directly measured by the “doubly-labeled water” method (R2 = 0.88) (47). Resting energy expenditure (REE) was measured by indirect (hood) calorimetry. Thermic effect of feeding (TEF) was calculated as calories expended above REE following ingestion of liquid formula calories equivalent to 60% of REE measured from 8:00 to 9:00 AM on the day of testing, as previously described (47). Daily energy expenditure above REE and TEF was designated as nonresting energy expenditure (NREE = energy expended above resting in physical activity). NREE was calculated as NREE = TEE − (REE + TEF).
In vivo skeletal muscle fuel utilization.
In vivo skeletal muscle fuel utilization was determined by graded cycle ergometry (51) and magnetic resonance spectroscopy (MRS) (66). After a 10-min period of accommodation, the subjects pedaled at 60 rpm against graded resistance to generate 10 W, 25 W, and 50 W of power in successive 4-min intervals using a Sensormedics 880S bicycle and ergometer with electrical braking (52). Oxygen uptake (V̇o2), carbon dioxide production (V̇co2), and the respiratory exchange ratio (RER) were measured continuously (18) using a Sensormedics VMAX 29 metabolic cart (52). Steady-state values were recorded at 0 W (rest), 10 W, 25 W, and 50 W. Generating 50 W of power is below the anaerobic threshold for even the most sedentary subjects. Below the anaerobic threshold, steady-state V̇o2 and V̇co2 are easily attained within 2–3 min of cycling (69). Skeletal muscle work efficiency was calculated as Work done (kcal/min)/[Energy expended (kcal/min) − Resting energy expenditure (kcal/min)].
This calculation represents the slope of the line relating work performed above energy expenditure at rest (0 work performed) to energy expended during exercise (18, 69).
In vitro studies of skeletal muscle biochemistry and histomorphology.
In vitro studies of skeletal muscle biochemistry and histomorphology were performed by analysis of vastus lateralis muscle needle biopsies (∼200–300 mg) that were obtained under local anesthesia with 1% xylocaine with subjects in the postabsorptive state. Muscle sections (10 μm thick) were stained for myofibrillar adenosine triphosphatase (mATPase) (55) for major fiber type (I, IIA, and IIX) proportion and cross-sectional area. Capillary density around different fiber types was quantified using the amylase-periodic acid stain technique (5). Enzymatic activity levels of creatine kinase, cytochrome c oxidase (COX), hexokinase (HK), glycogen phosphorylase (PHOS), phosphofructokinase (PFK), GAPDH, and 2-hydroxyacyl CoA dehydrogenase (HADH), citrate synthase (CS, an indicator of mitochondrial mass), and carnitine palmitoyl transferase-1 (CPT1) were determined spectrophotometrically (17, 55, 57). The capacity of skeletal muscle to oxidize fatty acids is reflected in the ratio of HADH/CS, and the activity of the mitochondrial respiratory chain is reflected in the ratio of COX/CS (74). The ratio of PFK/COX activity in muscle samples provides an estimate of the relative glycolytic/fatty acid oxidative capacity of muscle in vivo (27, 74), as well as the relative proportions of fast- vs. slow-twitch fiber types (22).
Glycogen is stored in skeletal muscle as both proglycogen (∼4 × 105 Da), which is an acid-insoluble, readily available source of glucose, and macroglycogen, which is an acid-soluble, larger polymer (∼107 Da), less bioavailable source of glucose (3, 21). Muscle glycogen is the predominant source of carbohydrate during exercise, and changes in muscle glycogen availability following weight perturbation could affect skeletal muscle fuel utilization via changes in either the total amount of glycogen stored or the relative size of the metabolically active proglycogen pool (11, 72). Total muscle glycogen, proglycogen, and macroglycogen were assayed in each muscle sample (1). Results are expressed as micromole (glucose equivalents) of glycogen per gram of muscle.
Measurement of circulating concentrations of triiodothyronine (T3) were made by radioimmunoassay(31). Serum glucose was measured by the hexokinase method (Glucose/HK; Roche Molecular Biochemicals, Werk Penzberg, Germany). Plasma insulin was measured by solid-phase 125I-RIA (Coat-a-count; Diagnostics Products, Los Angeles, CA). Total plasma adiponectin and leptin were measured by ELISA (Linco Research, St. Charles, MO). All samples were drawn from subjects in the postabsorptive state at 8:00 AM.
Calculations and statistical analyses.
At each level of power generated during bicycle ergometry studies, the proportion of calories expended that were derived from fatty acids vs. carbohydrate were calculated from the RER (29).
Between-group comparisons (obese vs. never-obese, male vs. female) were made by ANOVA. Within-group comparisons (subjects studied at Wtinitial vs. same subjects studied at Wt+10% or Wt−10%) were made by ANOVA with repeated measures.
Before analyses were performed, normality of data distributions was confirmed by Wilk-Shapiro testing. Statistical significance was prospectively defined as Pα < 0.05. Data were analyzed using Statistica 6.0 software (60).
RESULTS
Stability of body weight and fitness.
The mean ± SD slopes of the regression plots relating body weight to days in the Clinical Research Center during weight stability were 0.12 ± 2.0 g/day (range −5.0 to +7.6 g/day) in subjects at Wtinitial; −0.16 ± 1.9 g/day (range −4.6 to +6.4 g/day) in subjects at Wt+10%; and 0.44 ± 2.1 g/day (range −5.6 to +2.2 g/day) following weight loss. RER at rest was not significantly different from the FQ of 0.85 at any weight plateau.
Stability of fitness was determined by measurement of the anaerobic threshold every 2 wk throughout the study. No significant weight plateau effects on the anaerobic threshold were detected, and values for the anaerobic threshold before and after weight change were significantly correlated (R2 = 0.74, P < 0.001), indicating that there were not significant changes in subject fitness due to weight change or hospitalization (see Table 1). There was a tendency for the ratio of anaerobic threshold to fat-free mass (FFM) to increase following weight loss and to decrease following weight gain (see Table 1), as was predicted since the anaerobic threshold was maintained at initial absolute levels throughout the study.
Table 1.
Means ± SE energy expenditure and bicycle ergometry data
| Wtinitial | Wt−10% | Wtinitial | Wt+10% | |
|---|---|---|---|---|
| 24 h energy expenditure (TEE) | ||||
| kcal/24 h | 3100±155 | 2436±113* | 2470±166 | 2985 (186)† |
| kcal/kg FFM | 49.4±1.1 | 41.4±1.3* | 45.3±1.1 | 52.8 (1.6)† |
| Resting energy expenditure (REE) | ||||
| kcal/24 h | 1827±96 | 1663±84* | 1582±84 | 1655 (107) |
| kcal/kg FFM | 29.2±0.9 | 28.3±1.0 | 29.1±1.1 | 29.3 (1.2) |
| Nonresting energy expenditure | ||||
| kcal/24 h | 1175±86 | 691±66* | 813 (84) | 1243 (77)† |
| kcal/kg FFM | 18.6±1.1 | 0.008±0.001* | 14.8 (0.8) | 22.0 (0.05)† |
| Thermic effect of feeding (TEF) | ||||
| kcal/day | 98±8 | 82±13 | 86 (6) | 102 (14) |
| % caloric intake used in TEF | 3.2±0.2 | 3.6±0.4 | 3.5 (0.3) | 3.3 (0.4) |
| Anaerobic Threshold | ||||
| ml O2/min | 1293±55 | 1288±51 | 1492 (66) | 1406 (79) |
| ml O2·kg FFM−1·min−1 | 21.8±1.2 | 24.0±1.4 | 27.1 (2.2) | 24.6 (2.3) |
| Energy expenditure, kcal/min | ||||
| Watts | ||||
| 0 | 1.27±0.07 | 1.15±0.06 | 1.10 (0.06) | 1.15 (0.08) |
| 10 | 2.83±0.23 | 2.36±0.18* | 2.43 (0.18) | 3.18 (0.15)† |
| 25 | 3.65±0.22 | 3.18±0.18* | 3.26 (0.19) | 3.97 (0.13)† |
| 50 | 5.08±0.19 | 4.71±0.25‡ | 4.64 (0.23) | 5.17 (0.15) |
| Energy expenditure, kcal/min-REE | ||||
| Watts | ||||
| 10 | 1.68±0.20 | 1.33±0.16* | 1.33 (0.19) | 2.03 (0.19)† |
| 25 | 2.51±0.17 | 2.16±0.16* | 2.16 (0.21) | 2.92 (0.19) |
| 50 | 3.88±0.16 | 3.68±0.23 | 3.53 (0.24) | 4.03 (0.18) |
| Energy expenditure, kcal·kg−1 FFM·min−1–REE | ||||
| Watts | ||||
| 10 | 0.029±0.002 | 0.026±0.003‡ | 0.026 (0.006) | 0.038 (0.007) |
| 25 | 0.046±0.003 | 0.043±0.003† | 0.041 (0.008) | 0.053 (0.008) |
| 50 | 0.071±0.004 | 0.073±0.005 | 0.067 (0.008) | 0.074 (0.010) |
| Respiratory exchange ratio | ||||
| Watts | ||||
| 0 | 0.85±0.01 | 0.85±0.01 | 0.84 (0.02) | 0.85 (0.03) |
| 10 | 0.83±0.01 | 0.81±0.01‡ | 0.81 (0.02) | 0.83 (0.03)† |
| 25 | 0.85±0.01 | 0.84±0.01 | 0.86 (0.03) | 0.87 (0.03) |
| 50 | 0.88±0.02 | 0.88±0.02 | 0.89 (0.03) | 0.88 (0.03) |
| Efficiency, kcal work/kcal energy expended–REE | ||||
| Watts | ||||
| 10 | 0.114±0.017 | 0.131±0.012* | 0.115 (0.018) | 0.071 (0.006)† |
| 25 | 0.151±0.020 | 0.177±0.013‡ | 0.173 (0.018) | 0.129 (0.008) |
| 50 | 0.189±0.008 | 0.204±0.012 | 0.207 (0.015) | 0.179 (0.007) |
Means ± SE energy expenditure, fitness, fuel utilization, and efficiency. Energy expenditure during exercise is expressed as total calories expended, total calories expended above resting (i.e., total calories expended solely as a result of exercise), and total calories expended above resting per unit of fat-free mass (FFM). Thirty subjects (18 obese, 7 never-obese; 12 males, 13 females) were studied at Wtinitial and Wt−10%. Five subjects (4 never-obese males; 1 never-obese female) were studied at Wtinitial and Wt+10%.
P < 0.001 compared to Wtinitial;
P < 0.05 compared to Wtinitial;
P < 0.01 compared to Wtinitial.
In vivo bicycle ergometry.
As reported previously (48), the ranges of energy expended to generate 10, 25, and 50 W of power approximate those of exercising at ∼20%, 30–50%, and >50% of V̇o2 max, respectively. Our results regarding the effects of maintenance of an altered body weight on in vivo bicycle ergometry studies are similar to those reported previously (48).
Maintenance of a reduced body weight was associated with an approximate 15% increase in skeletal muscle work efficiency during exercise to generate 10 W (P < 0.001) and 25 W of power (P < 0.01), and with a significant decline in RER only at low workloads (P < 0.001 at 10 W, P < 0.01 at 25 W), representing an approximate 12% increase in the fraction of calories derived from the oxidation of fatty acids vs. glucose at 10 W (P < 0.001 compared with zero) and a 7% increase at 25 W (P < 0.01 compared with zero) of power generated (see Table 1, Figs. 1–3). We have previously shown that this effect is still evident even when leg weights were worn by weight-reduced subjects to replace weight lost from Wtinitial from each leg (by DXA), indicating that changes in fuel utilization and efficiency were not simply a function of decreased leg mass (48). No significant effect of maintenance of a reduced body weight on muscle efficiency or fuel utilization at a workload of 50 W was detected.
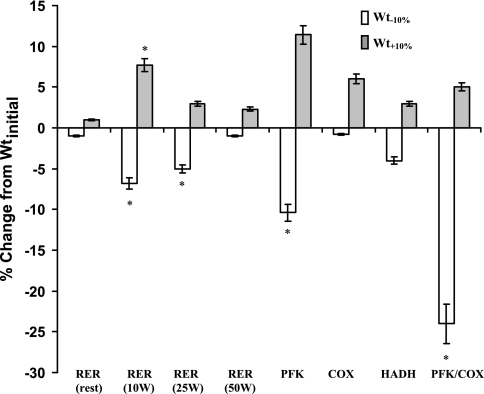
Fig. 1.
Means ± SE percent change in bicycle ergometry, MRS, and in vitro skeletal muscle biopsy data from Wtinitial. Maintenance of a reduced body weight (Wt−10%) is associated with a significant reduction in the respiratory exchange ratio (RER) during low-level exercise (bicycling to generate 10–25W of power) and muscle phosphofructokinase (PFK) and ratio of PFK/cytochrome c oxidase (COX). Maintenance of an elevated body weight (Wt+10%) is associated with a significant increase in RER during low-level exercise (bicycling to generate 10 W of power). HADH, 2-hydroxyacyl CoA dehydrogenase activity. *P < 0.05 compared with zero.
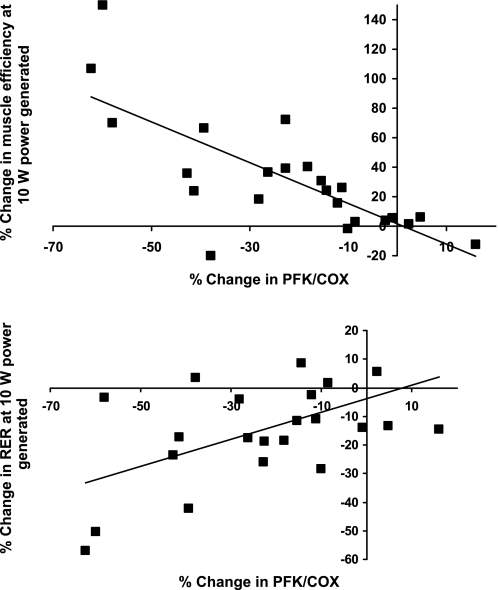
Fig. 3.
Correlations of the percentage changes in ratios of glycolytic (PFK) to fatty acid oxidative (COX) enzyme activities with skeletal muscle work efficiency (solid circles) and the RER (solid squares) during low-level exercise (bicycling to generate 10 W of power) in subjects studied before (Wtinitial) and after (Wt−10%) weight loss. Regression equations are percentage change in efficiency = −1.38 (PFK/COX) + 1.54, R2 = 0.56, P < 0.0001 and percentage change in RER = 0.48 (PFK/COX) − 3.81, R2 = 0.29; P = 0.008
Maintenance of an elevated body weight was associated with an approximate 25% decrease in skeletal muscle work efficiency during exercise to generate 10 W of power (P < 0.05), and with a significant increase in RER at 10 W of power generated (P < 0.05), representing an approximate 10% decline in the fraction of calories derived from the oxidation of fatty acids vs. glucose (P < 0.05 compared with zero). No significant effect of maintenance of an elevated body weight on muscle efficiency or fuel utilization at workloads of 25 W or 50 W was detected.
Subjects underwent prescribed exercise to maintain their anaerobic threshold throughout the study, irrespective of changes in body composition. We regressed changes in the anaerobic threshold, both absolute and per unit of FFM, against changes in mechanical efficiency of muscle and fuel utilization of muscle at different workloads to examine the possibility that the increased skeletal muscle work efficiency following weight loss was due to an aerobic training effect (56, 65), even though changes in the anaerobic threshold between study periods were not statistically significant. No significant correlations were noted, suggesting that the significant changes observed in chemomechanical efficiency and fuel utilization following weight loss or gain were primarily due to the weight changes rather than any training effects.
When data were analyzed by ANCOVA, in which gender, somatotype (obese vs. non-obese), and weight plateau were entered as dichotomous variables, no significant effects of gender or somatotype on muscle efficiency or fuel preference were noted.
In vitro analysis of muscle biopsy samples.
As shown in Table 2 and Fig. 1, maintenance of a reduced body weight was associated with a significant decline in the activity in skeletal muscle of the glycolytic enzyme, PFK, while there was no significant change in the activity of citrate synthase (CS), an indicator of mitochondrial density. The ratio of glycolytic to fatty acid oxidative enzyme activity, expressed as the ratio of PFK to COX, was also significantly decreased following weight loss. Opposite changes were observed following weight gain (Wt+10%) in 4/5 subjects, but statistical significance was not achieved, probably due to the small number of subjects (n = 5) studied at Wt+10%. No significant effects of altered body weight on fiber type, glycogen stores, or on enzyme activities proportional to mitochondrial density (CS), fatty acid oxidation (COX, HADH), respiratory chain activities (COX/CS ratio), fatty acid transport (CPT1), or glycogen metabolism (PHOS) were noted (see Tables 2 and 3). There was no significant effect of weight perturbation on muscle glycogen content, or the relative proportions of proglycogen or macroglycogen. When data were analyzed by ANCOVA in which sex, somatotype, and weight plateau were each entered as dichotomous variables, no significant effects of sex or somatotype on enzyme activities were noted.
Table 2.
Means ± SE in vitro analyses of muscle biopsies of vastus lateralis muscle
| Wtinitial | Wt−10% | Wtinitial | Wt+10% | |
|---|---|---|---|---|
| Phosphofructokinase, PFK, μM·min−1·g−1 | 56.3±2.9 | 48.7±3.5* | 66.2±5.0 | 73.8±3.2 |
| Creatine kinase, μM·min−1·gm−1 | 407.6±22.1 | 419.6±19.4 | 385.4±22.7 | 413.1±10.3 |
| GAPDH, μM·min−1·g−1 | 441.1±20.2 | 436.7±23.8 | 450.7±30.2 | 496.8±23.2 |
| Citrate synthase, CS, μM·min−1·g−1 | 9.1±0.8 | 9.9 (0.5) | 10.8±0.6 | 11.3±0.9 |
| 2-hydroxyacyl CoA dehydrogenase, HADH, μM·min−1·g−1 | 13.1±0.7 | 13.8±0.5 | 13.9±1.7 | 14.2±3.1 |
| Cytochrome c oxidase, COX; μM·min−1·gm−1 | 5.7±0.5 | 5.8±0.4 | 6.4±0.8 | 7.1±1.0 |
| Hexokinase, μM·min−1·g−1 | 2.4±0.2 | 2.5±0.4 | 2.7±0.2 | 2.9±0.1 |
| Glycogen phosphorylase, μM·min−1·gm−1 | 26.7±1.6 | 28.7±1.9 | 21.6±3.5 | 23.1±3.5 |
| Carnitine palmitoyl tranferase 1, CPT, 1, nM·min−1·g−1 | 0.094±0.005 | 0.089±0.006 | 0.090±0.018 | 0.101±0.004 |
| COX/CS | 0.59±0.02 | 0.59±0.03 | 0.59±0.05 | 0.62±0.04 |
| HADH/CS | 1.70±0.09 | 1.44±0.06 | 1.29±0.05 | 1.25±0.05 |
| PFK/COX | 12.18±1.53 | 9.21±0.70* | 11.0±1.5 | 11.2±1.5 |
Weight loss and weight gain effects on skeletal muscle enzyme activity. Thirty subjects (18 obese, 7 never-obese; 12 males, 13 females) were studied at Wtinitial and Wt−10%. Five subjects (4 never-obese males; 1 never-obese female) were studied at Wtinitial and Wt+10%.
P < 0.05 compared to Wtinitial.
Table 3.
Means ± SE skeletal muscle morphology and glycogen stores
| Wtinitial | Wt−10% | Wtinitial | Wt+10% | |
|---|---|---|---|---|
| % type I fibers | 37.5±2.4 | 33.4±2.5 | 35.2±6.0 | 35.8±4.2 |
| % Type IIA fibers† | 38.5±2.3 | 36.7±3.3 | 38.6±3.9 | 31.3±3.7 |
| % Type IIX fibers† | 20.5±2.8 | 24.1±7.3 | 21.0±7.8 | 24.7±5.0 |
| type I cap. dens. | 4.4±0.1 | 4.3±0.2 | 4.1±0.4 | 4.1±0.3 |
| Type IIA cap. den. | 4.2±0.2 | 4.3±0.2 | 4.0±0.2 | 4.1±0.1 |
| Type IIX cap. den. | 3.5±0.2 | 3.5±0.2 | 3.5±0.2 | 3.5±0.3 |
| Total glycogen, μmol/g | 116.9±5.2 | 115.7±5.0 | 121.2±13.3 | 116.6±16.1 |
| Proglycogen, μmol/g | 80.5±3.8 | 76.1±2.4 | 88.7±10.9 | 81.4±10.6 |
| Macroglycogen, μmol/g | 36.3±2.2 | 41.2±2.3 | 32.2±2.7 | 35.1±6.2 |
| %Proglycogen | 68.9±1.3 | 66.6±1.8 | 72.9±0.9 | 70.1±1.8 |
Means ± SE measures of skeletal muscle morphology and glycogen content. A total of 30 subjects (18 obese, 7 never-obese; 12 males, 13 females) were studied at Wtinitial and Wt−10%. A total of five subjects (4 never-obese males; 1 never-obese female) were studied at Wtinitial and Wt+10%.
Intermediate fiber types (such as IIC and IIAX) were found to represent only a few percent of all fibers, and no differences between groups were found in their proportion, size, or capillary density (data not shown).
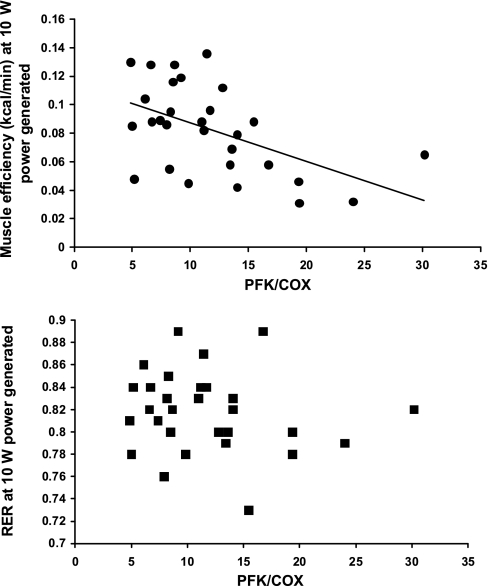
At Wtinitial, skeletal muscle work efficiency, but not RER, during bicycling to generate 10 W of power, was significantly correlated with the glycolytic to oxidative enzyme activity ratio (PFK/COX) in vastus lateralis muscle (see Fig. 2). At Wt−10%, the percentage change from Wtinitial in both skeletal muscle work efficiency and RER during bicycling to generate 10 W of power was significantly correlated with the changes in the glycolytic/oxidative enzyme ratio (see Fig. 3). Even though the maintenance of a reduced body weight was associated with significant declines in RER and increases in skeletal muscle efficiency at both 10 W and 25 W of power generated, the correlations between changes in in vitro studies (RER and efficiency) and in vivo studies (PFK/COX) was significant only at 10 W of power generated. No significant correlation was noted between PFK activity (which was significantly decreased following weight loss) and work efficiency at initial weight [R2 = 0.10, nonsignificant (NS)] or between fractional changes in PFK activity and fractional changes in skeletal muscle work efficiency (R2 = 0.11, NS) or RER (R2 = 0.014, NS) following weight loss.
Fig. 2.
Correlations of the ratios of glycolytic (PFK) to fatty acid oxidative (COX) enzyme activities with skeletal muscle work efficiency (solid circles) and the respiratory exchange ratio (RER, solid squares) during low-level exercise (bicycling to generate 10 W of power) at usual body weight (Wtinitial). Regression equations are efficiency = −0.0026(PFK/COX) + 0.11, R2 = 0.26, P < 0.01. RER = −0.00098 (PFK/COX) + 0.82, R2 = 0.025, not significant.
Glucose, insulin, triiodothyronine, adiponectin, and leptin.
In agreement with our previous studies (43), there were no significant effects of maintenance of an altered body weight on fasting insulin or glucose concentrations. Maintenance of a reduced body weight was associated with significant declines in 8:00 AM circulating concentrations of triiodothyronine (T3) and leptin, and a significant increase in 8:00 AM circulating concentrations of adiponectin (ACRP30). Maintenance of an elevated body weight was associated with a significant increase in 8:00 AM circulating concentrations of leptin and a significant decrease in 8:00 AM circulating concentrations of ACRP30 (see Table 4). Changes in 8:00 AM circulating concentrations of glucose, insulin, T3, ACRP30, or leptin were not significantly correlated with changes in muscle enzyme activity, mechanical efficiency, or fuel utilization by skeletal muscle.
Table 4.
Means ± SE clinical and biochemical data
| Wtinitial | Wt−10% | Wtinitial | Wt+10% | |
|---|---|---|---|---|
| Weight, kg | 109.6±8.9 | 96.4±7.5* | 65.4±3.7 | 71.7±4.2† |
| Height, cm | 170.7±2.0 | 167.1±7.2 | ||
| BMI, kg/m2 | 36.1±2.5 | 31.1±2.2* | 23.6±1.1 | 24.5±0.8† |
| Fat-free mass, kg | 63.5±3.6 | 59.8±3.4* | 54.7±3.8 | 56.9±4.3 |
| Fat mass, kg | 45.4±5.7 | 35.2±4.8* | 10.6±2.2 | 14.8±2.2† |
| Serum glucose, mmol/l | 4.88±0.12 | 4.72±0.13 | 4.55±0.11 | 4.49±0.12 |
| Plasma insulin, pmol/l | 26.8±2.9 | 26.5±3.5 | 23.2±2.7 | 33.2±5.8 |
| Plasma leptin, ng/ml | 34.1±6.8 | 22.4±5.9* | 5.5±3.4 | 7.6±3.4† |
| Serum triiodothyronine, nmol/l | 1.45±0.07 | 1.23±0.04* | 1.51±0.08 | 1.62±0.10 |
| Plasma adiponectin, μg/ml | 5.31±0.52 | 5.77±0.57* | 13.22±1.27 | 11.00±1.16† |
Means ± SE clinical and biochemical characteristics of subjects. Thirty subjects (18 obese, 7 never-obese; 12 males, 13 females) were studied at Wtinitial and Wt−10%. Five subjects (4 never-obese males; 1 never-obese female) were studied at Wtinitial and Wt+10%. BMI, body mass index.
P < 0.001 compared to Wtinitial;
P < 0.05 compared to Wtinitial.
DISCUSSION
This study is an examination of the effects of maintenance of an altered body weight following diet-induced weight change on skeletal muscle physiology and biochemistry in a population of subjects who were isolated as much as possible from other potentially confounding factors as dietary composition, variations in exercise type and intensity, and dynamic weight loss or weight gain. The major findings of this study are 1) maintenance of a reduced body weight is associated with alterations in muscle enzyme activities in vitro (decreased PFK and decreased glycolytic/oxidative enzyme ratio without significant changes in the activity of enzymes related to fatty acid oxidation, mitochondrial density, substrate transport, or substrate availability); and 2) these changes in skeletal muscle biochemistry are significantly correlated with in vivo measures of skeletal muscle fuel utilization and chemomechanical work efficiency at low levels of exercise. In addition, we found that there was a significant negative correlation of skeletal muscle work efficiency and the ratio of PFK/COX in subjects at Wtinitial (see Fig. 2), suggesting that relative glycolytic/oxidative capacity is predictive of muscle efficiency in general (49). This makes sense, since the less efficient myosin heavy chain isoforms preferentially oxidize glucose as fuel and have a higher glycolytic/fatty acid oxidative enzyme ratio (55). The finding that fat oxidation during exercise is increased following weight loss is consistent with other ergometric studies (19, 40) and is most likely reflective of the overall biochemical and molecular changes in skeletal muscle that produce a more chemomechanically efficient organ after weight loss. Overall, however, weight-reduced individuals have lower fat oxidation rates (38, 73). Increasing muscle efficiency during low-level exercise combined with an increased propensity to store ingested calories as fat would function coordinately to favor the regain of lost adipose tissue.
Only five subjects were studied before and after 10% weight gain. We found a significant decrease in skeletal muscle work efficiency at low levels of energy output (see Table 1) and an increase in PFK activity that was not significant (see Table 2) in subjects studied after weight gain compared with Wtinitial. Although these data suggest that maintenance of an elevated body weight may result in reciprocal changes in muscle enzyme activity to those seen following weight loss, the number of subjects studied following weight gain is too small to address this issue. It should be noted in this context that we have previously reported that changes in partitioning of the respective disproportionate decreases and increases in energy expenditure following weight loss and weight gain between REE, NREE, and TEF are not perfectly reciprocal (25, 47).
As shown in Fig. 3, the approximate 25% decline in the ratio of glycolytic (PFK) to oxidative (COX) enzyme activities at Wt−10%, is sufficient to account for a significant portion of the increased skeletal muscle work efficiency and utilization of free fatty acids (FFA) as fuel during low level exercise observed in vivo following weight loss. Mechanistically, increased use of FFA vs. glucose as fuel at low levels of power output following weight loss could be due to an increase in the potential of skeletal muscle to oxidize FFA and/or a decrease in the potential for skeletal muscle to oxidize glucose. Our in vitro analyses of enzyme activities in vastus lateralis muscle indicate that the relative increase in fatty acid oxidation at low levels of muscle power output following weight loss is due to a decrease in the capacity of skeletal muscle to oxidize glucose rather than to an increase in muscle capacity to oxidize fatty acids. This finding is consistent with our previous in vivo 31P-NMR spectroscopy analyses of skeletal muscle in similar subjects (48), in which we found no significant change following weight loss in the absolute capacity to oxidize fatty acids per se, but an increase in relative capacity to oxidize FFA vs. glucose, suggesting that the net effect of weight loss is to decrease skeletal muscle capacity to oxidize glucose. The significant correlations of fractional changes in the PFK/COX ratio—but not PFK or COX alone—with fractional changes in mechanical efficiency of muscle and fuel utilization (RER) during low-level exercise suggests that biochemical assessment of the relative capacity of muscle to use glucose or fatty acid as fuel is a better index of the physiological assessment (RER and ergometry) than fractional changes in the activity of either enzyme alone.
We detected these correlations of changes in the biochemical activity of glucose/fatty acid oxidizing enzymes and changes in muscle work efficiency and fuel use following weight change only at low levels of exercise, and the effects of weight alteration on in vivo ergometry studies were more significant at 10 W than at 25 W of power generated. The maximal fatty acid oxidative capacity of muscle is reached at ∼50%-60% of V̇o2 max, depending somewhat upon the subject's level of fitness and muscle fiber type distribution, and is lower than the level at which the maximal rate of glucose oxidation typically occurs (50). Therefore, it is not surprising that correlations of physiological and biochemical assessments of muscle function are most evident at lower levels of exercise characterized by mixed fuel utilization that is not biased by possibly exceeding the maximal fatty acid, but not glycolytic, capacity of muscle. The correlations between skeletal muscle biochemistry and physiology are clearly evident at levels of exercise commensurate with those of daily living, i.e., at levels of exercise that are similar to those at which we detect disproportionate declines in 24-h energy expenditure and energy expended in physical activity following weight loss (25, 48), further supporting the hypothesis that skeletal muscle may represent the major “effector” organ for changes in energy output that favor the regain of lost weight (48).
The decline in utilization of glucose as fuel during low levels of muscle work at Wt−10%, and increase at Wt+10%, could also reflect declines in the availability of glucose for oxidation following weight loss and increases following weight gain. In addition to the lack of changes in circulating glucose concentrations following weight loss, our in vitro studies demonstrate that there is no change in intracellular glycogen. However, no direct measurements of blood flow to or within muscle were made.
Changes in muscle substrate preference and mechanical efficiency at low levels of work, could reflect changes in substrate availability (in particular, the availability of glucose for oxidation), delivery of substrate to muscle, storage or transport of substrate within muscle, or the activity of the mitochondrial respiratory chain. However, these mechanisms are not likely to be significant contributors to weight-change related alterations in skeletal muscle work efficiency or fuel utilization. Weight change did not alter circulating concentrations of glucose, the quantity of intramuscular glycogen in muscle, the activities of enzymes mediating glycogenolysis (PHOS) or fatty acid transport (CPT1), or capillary density, indicating that there are not major effects of weight perturbation on the availability of glucose or fatty acid to skeletal muscle (see Tables 1–3). The stability of COX activity in muscle, which is the rate-limiting step of mitochondrial electron transport chain activity (41, 67, 74), across multiple weight plateaus and normalized to mitochondrial density, as reflected in CS activity, suggests that mitochondrial respiratory chain activity is not affected by weight perturbation (see Table 2).
The changes in the use of glucose as a fuel during low-power output by muscle following weight loss or gain are consistent with the hypothesis that there is an increase in the ratio of predominantly fatty acid oxidative type I to predominantly glycolytic type II skeletal muscle fibers following weight loss, and a decrease in this ratio following weight gain. However, we and others (23) have detected no changes in skeletal muscle fiber type following weight change when fiber types are qualitatively identified by histochemical staining for the predominant isoforms of myofibrillar ATPase. This inference does not eliminate the possibility that the maintenance of an altered body weight is associated with molecular changes within specific muscle fiber types. Possibilities would include shifts in myosin heavy chain (MHC) and sarcoplasmic reticulum Ca2+-ATPases isoform expression toward more or less efficient isoforms (9, 10, 28, 30, 39, 55, 62, 65). Age-related changes in muscle morphology are an example of this disassociation of changes in MHC isoform expression and fiber type. There appear to be little if any changes in the fractional distribution of muscle fiber types with age, even though type II fibers do tend to become smaller (32). However, there is an age-related decline in MHCII protein content and mRNA expression in muscle and an age-related increase in MHCI protein content in muscle (54).
We also examined circulating concentrations of selected molecules that affect skeletal muscle fuel utilization or efficiency and might be altered as a result of weight loss: leptin, adiponectin (ACRP30) insulin, glucose, and triiodothyronine (T3). Low-dose leptin administration reverses the effects of sustained weight reduction on energy expenditure in humans (42), including changes in skeletal muscle work efficiency and fuel utilization of the sort that are reported in this study. These observations support the idea that the increase in skeletal muscle work efficiency following weight loss may be directly or indirectly leptin dependent. Leptin stimulates glucose flux through the Krebs cycle in muscle both directly and indirectly via its effects on thyroid hormones and insulin sensitivity (15, 16, 59) and also promotes fat oxidation both directly and indirectly via the activation of AMP kinase (33). The lack of significant correlations of changes in circulating concentrations of leptin with changes in muscle physiology and biochemistry does not preclude this possibility. We have previously suggested that leptin may act via a “threshold mechanism,” whereby declines in circulating leptin concentrations below a critical level invokes a maximal or near maximal response, i.e., response is not a dose-dependent phenomenon (2, 42, 45).
Insulin directly increases glucose uptake by muscle and could therefore affect the availability of glucose as a substrate for muscle energy. In addition, insulin infusion promotes both FFA transport and oxidation in vastus lateralis, as shown by increased expression of mRNA for CS and COX (neither of which were affected by weight loss in this study) (61). Both weight loss and leptin administration are associated with increased insulin sensitivity (35), potentially resulting in improved delivery of glucose to muscle. Braun et al. (12) compared fuel utilization during treadmill exercise in BMI-matched euglycemic overweight women with and without insulin resistance. The more insulin-sensitive subjects (analogous to weight-reduced subjects) oxidized more carbohydrate during exercise, suggesting, if anything, that weight-reduced subjects should be more predisposed toward carbohydrate oxidation on the basis of greater insulin sensitivity. It should be noted, however, that all subjects in this study are prescreened for evidence of impaired glucose tolerance or type 2 diabetes and that we have, in this selected population, found no significant effects of weight loss on insulin sensitivity (43). Therefore, no conclusions regarding the importance of circulating glucose and insulin concentrations or changes in insulin sensitivity can be drawn from this data set.
Both circulating concentrations of adipocyte complement-related protein (adiponectin, ACRP30), and adipose tissue adiponectin mRNA expression, are low in obese humans and mice, and are increased following weight loss (6, 75). In addition to its promotion of insulin sensitivity, systemic administration of ACRP30 to mice increases fatty acid oxidation by muscle via direct activation of multiple protein kinases and peroxisome proliferator-activated receptor-α (76). We found no correlations of fractional changes in circulating concentration of ACRP30 to skeletal muscle physiology or biochemistry. However, it should also be noted that ACRP30 forms high-, medium-, and low-molecular weight concatemers in the circulation. The circulating concentrations of the high molecular weight isoform, and its ratio to total ACRP30, which were not measured in this study, show the best correlations with many aspects of the biology discussed above (36).
Circulating triiodothyronine is decreased following weight loss and increased following weight gain (43) and increases the proportion of the more glycolytic, less chemomechanically efficient MHCII isoforms in skeletal muscle (13, 14) via an inhibitory response element in the promoter region of MHC1. Changes in circulating concentrations of T3 may constitute a mechanism by which the relative proportion of MHC1 vs. MHC2 isoforms in muscle is increased following weight loss and decreased following weight gain. We found no correlation of fractional changes in circulating concentrations of T3 to skeletal muscle physiology or biochemistry, but as with possible threshold effects of leptin described above, this lack of correlation does not necessarily preclude a prominent role for T3 in mediating changes in skeletal muscle that occur following weight loss.
We and others have previously reported that maintenance of a reduced weight is not associated with a decline in time spent in physical activity in in-patients (25, 48) and may, in fact, be associated with an increase in time spent in physical activity in out-patients (44, 71). The observation that increased skeletal muscle efficiency is evident only at low levels of physical activity suggests other potential mechanisms for the decline in NREE in following weight loss (25, 48). The weight-reduced individual may preferentially spend more time ambulating at workloads that maximize their skeletal muscle efficiency, i.e., that levels of activity performed in non-exercise activity thermogenesis are subtly but significantly altered to conserve energy following weight loss (26). From a therapeutic standpoint, exercise post-weight reduction might be more effective at higher workloads, i.e., that the weight-reduced individual might “escape” this increased efficiency by altering the intensity of exercise even without necessarily increasing the work performed.
Perspectives and Significance
These studies demonstrate that maintenance of an altered body weight is associated with concordant in vivo and in vitro changes in skeletal muscle fuel utilization and work efficiency that favor the return to preperturbation levels of energy stores. These concordant physiological and biochemical changes are most evident at low levels of muscle work, i.e., at levels of activity similar to those of daily activities. These data suggest possible molecular mechanisms for the specific changes in skeletal muscle work efficiency that are present in individuals maintaining an altered body weight (42, 48). Manipulation of these responses by exercise, pharmacotherapy, or other means might be useful in the treatment of obesity, particularly in improving maintenance of reduced body weight after otherwise successful weight reduction.
GRANTS
These studies were supported in part by National Institutes of Health Grants DK30583, DK26687, DK37948, DK64773, RR00102, RR00645, and RR024156.
DISCLOSURES
None of the authors have any conflicts of interest or anything to disclose relevant to this manuscript.
ACKNOWLEDGMENTS
We would like to thank Drs. Ellen Murphy and Sanobar Parkar for their work as research coordinators during some aspects of these studies, Taylor Lloyd for supervising many of the exercise studies, and Josée St-Onge for expert technical assistance in muscle sample analyses. The late Dr. Jean-Aimé Simoneau was instrumental in the design of these studies and provided invaluable instruction in many of the techniques utilized. We are indebted to our research subjects and to the members of the nursing and nutrition staffs of the Rockefeller University Hospital and Irving Center for Clinical Research at Columbia Presbyterian Medical Center for dedicated help with their care.
REFERENCES
- 1.Adamo K, Graham T. Comparison of traditional measurements with macroglycogen and proglycogen analysis of muscle glycogen. J Appl Physiol 84: 908– 913, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Ahima R, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratosflier E, Flier J. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250– 252, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Alonso M, Lomako J, Lomako W, Whelan W. A new look at the biogenesis of glycogen. FASEB J 9: 1126– 1137, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Amati F, Dube J, Shay C, Goodpaster B. Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J Appl Physiol 105: 825– 831, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281– R283, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand 95: 203– 205, 1975 [DOI] [PubMed] [Google Scholar]
- 6.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79– 83, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Aronne L, Mackintosh R, Rosenbaum M, Leibel R, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol 38: R222– R225, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Astrup A, Gotzsche P, van den Werken K, Ranneries C, Toubro S, Raben A, Buemann B. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr 69: 1117– 1122, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Barclay C, Constable J, Gibbs C. Energetics of fast- and slow-twitch muscle fibers of the mouse. J Physiol 472: 61– 80, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blei M, Conley K, Kushmerick M. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol (Lond) 465: 203– 222, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomstrand E, Saltin B. Effect of muscle glycogen on glucose, lactate, and amino acid metabolism during exercise and recovery in human subjects. J Physiol 514: 293– 302, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun B, Sharoff C, Chipkin S, Beaudoin F. Effects of insulin resistance on substrate utilization during exercise in overweight women. J Appl Physiol 97: 991– 997, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Caiozzo V, Baker M, Baldwin K. Novel transitions in MHC isoforms: separate and combined effects of thyroid hormone and mechanical unloading. J Appl Physiol 85: 2237– 2248, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Canepari M, Cappelli V, Pellegrino M, Zanardi M, Reggiani C. Thyroid hormone regulation of MHC isoform composition and myofibrillar ATPase activity in rat skeletal muscles. Arch Physiol Biochem 106: 308– 315, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Ceddia R, WIlliam W, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes 23: 75– 82, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Ceddia R, William W, Curi R. The response of skeletal muscle to leptin. Front Biosci 1: D90– D97, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Colberg S, Simoneau J, Thaete F, Kelley D. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest 95: 1846– 1853, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.de Meis L, Arruda A, Carvalho D. Role of sarco/endoplasmic seticulum Ca2+-ATPase in thermogenesis. Biosci Rep 25: 181– 190, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol 38: 1132– 1139, 1975 [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster G, Katsiaris A, Kelley D. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52: 2191– 2197, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Horton ES, Terjung RL. Exercise, Nutrition, and Energy Metabolism. New York: Macmillan, 1988 [Google Scholar]
- 21.Huang M, Chin-fang L, Lin R, Chen R. The exchange between proglycogen and the metabolic role of the protein-rich glycogen in rat skeletal muscle. J Clin Invest 93: 501– 505, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaworski A, Porter M, Holmback A, Downham D, Lexell J. Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand 176: 215– 225, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kempen K, Saris S, Kuipers H, Glatz J, and Van der Vusse G. Skeletal muscle metabolic characteristics before and after energy restriction in human obesity: fibre type, enzymatic beta-oxidative capacity and fatty acid-binding protein content. Eur J Clin Invest 28: 1030– 1037, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Leibel R, Chua S, Rosenbaum M. Obesity. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver C, Beaudet A, Sly W, Valle D. New York: McGraw-Hill, 2001, chapt. 157, p. 3965– 4028 [Google Scholar]
- 25.Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621– 628, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Levine J. Non-exercise activity thermogenesis (NEAT). Nutr Rev 62: S82– S87, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Linossier M, Dormois D, Perrier C, Frey J, Geyssant A, Denis C. Enzyme adaptations of human skeletal muscle during bicycle short-sprint training and detraining. Acta Physiol Scand 161: 439– 445, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Loukianov E, Ji Y, Baker D, Reed T, Babu J, Loukianova T, Greene A, Shull G, Periasamy M. Sarco(endo)plasmic reticulum Ca2+ ATPase isoforms and their role in muscle physiology and pathology. Ann NY Acad Sci 853: 281– 289, 1998 [DOI] [PubMed] [Google Scholar]
- 29.McArdle W, Katch F, Katch V. Exercise Physiology. Baltimore, MD: Williams & Wilkins, 1996, p. 145– 147 [Google Scholar]
- 31.Miles L. Handbook of Radioimmunoassay. New York: Marcel Dekker, 1977, chapt. 4. [Google Scholar]
- 32.Moumard J, Weidner M, Gavigan K, Tyndall G, Hickey M, Alshami A. Fiber type and citrate synthase acitivty in the human gastrocnemius and vastus lateralis with againg. J Appl Physiol 85: 1337– 1341, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Muoio D, Dohm G, Fiedorek F, Tapscott E, Coleman R. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 46: 1360– 1363, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Najjar M, Rowland M. Anthropometric Reference Data and Prevalence of Overweight. National Center for Health Statistics Series 11 No 238, DHHS publication (PHS) 87– 1688, Washington, DC: Public Health Service, 1987 [PubMed] [Google Scholar]
- 35.Naslund E, Andersson I, Degerblad M, Kogner P, Kral J, Rossner S, Hellstrom P. Associations of leptin, insulin resistance and thyroid function with long-term weight loss in dieting obese men. J Intern Med 248: 299– 308, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Pajvani U, Du X, Combs T, Berg A, Rajala M, Schultness T, Engel J, Brownlee M, Scherer P. Structure-function studies of adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 278: 9073– 9085, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Pietrobelli A, Formica C, Wang Z, Heymsfield S. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol Endocrinol Metab 271: E941– E951, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Poynten A, Markovic T, Maclean E, Furler S, Freund J, Chisholm D, Campbell L. Fat oxidation, body composition and insulin sensitivity in diabetic and normoglycaemic obese adults 5 years after weight loss. Int J Obes 27: 1212– 1218, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Rall JA. Energetic aspects of skeletal muscle contraction: implications of fiber types. Exerc Sport Sci Rev 13: 33– 74, 1985 [PubMed] [Google Scholar]
- 40.Ranneries C, Bulow J, Buemann B, Christensen N, Madsen J, Astrup A. Fat metabolism in formerly-obese women. Am J Physiol Endocrinol Metab 274: E155– E161, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen U, Rasmussen H. Human skeletal muscle mitochondrial capacity. Acta Physiol Scand 168: 473– 480, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel R. Low dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579– 3586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbaum M, Hirsch J, Murphy E, Leibel R. The effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 71: 1421– 1432, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum M, Leibel R. Reply to Weinsier et al. Am J Clin Nutr 73: 657– 658, 2001 [Google Scholar]
- 45.Rosenbaum M, Leibel R. The role of leptin in human physiology. N Engl J Med 341: 913– 915, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Rosenbaum M, Leibel R, Hirsch J. Medical Progress: Obesity. N Engl J Med 337: 396– 407, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Rosenbaum M, Ravussin E, Matthews D, Gilker C, Ferraro R, Heymsfield S, Hirsch J, Leibel R. A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol Regul Integr Comp Physiol 270: R496– R504, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau J, Heymsfield S, Joanisse D, Hirsch J, Murphy E, Matthews D, Segal K, Leibel R. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183– R192, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Saez L, Leinwand L. Characterization of diverse forms of myosin heavy chain expressed in adult human skeletal muscle. Nucleic Acids Res 14: 2951– 2969, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahlin K, Sallstedy EK, Bishop D, Tonkonogi M. Turning down lipid oxidation during heavy exercise—what is the mechanism? J Physiol Pharmacol 59: 19– 30, 2008 [PubMed] [Google Scholar]
- 51.Segal K, Presta E, Gutin B. Thermic effect of food during graded exercise in normal weight and obese men. Am J Clin Nutr 40: 995– 1000, 1984 [DOI] [PubMed] [Google Scholar]
- 52.Sensormedics VMax Reference Manual. Yorba Linda, CA: Sensormedics, 1998 [Google Scholar]
- 53.Shetty P. Adaptation to low energy intakes: the responses and limits to intakes in infants, children, and adults. Eur J Clin Nutr 53Suppl1: S14– S33, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Short K, Vittone J, Bigelow M, Proctor D, Coenen-Schimke J, Rys P, Nair K. Changes in myosin heavy chain mrRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol 99: 95– 102, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 257: E567– E572, 1989 [DOI] [PubMed] [Google Scholar]
- 56.Simoneau J. Adaptation of human skeletal muscle to exercise-training. Int J Obes 4: S9– S13, 1995 [PubMed] [Google Scholar]
- 57.Simoneau J, Colberg S, Thaete F, Kelley D. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J 9: 273– 278, 1985 [PubMed] [Google Scholar]
- 59.Solinas G, Summermatter S, Mainieri D, Gubler M, Pirola L, Wymann M, Rusconi S, Montani J, Seydoux J, Dulloo A. The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett 577: 539– 544, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Statsoft Statistica, Release 5. Tulsa, OK: Statsoft, 1997 [Google Scholar]
- 61.Stump C, Short K, Bigelow M, Schimke J, Nair K. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100: 7996– 8001, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Theriault R, Theriault G, Simoneau JA. Human skeletal muscle adaption in response to chronic low-frequency electrical stimulation. J Appl Physiol 77: 1885– 1889, 1994 [DOI] [PubMed] [Google Scholar]
- 63.Tremblay A, Despres J, Theriault G, Fournier G, Bouchard C. Overfeeding and energy expenditure in humans. Am J Clin Nutr 56: 857– 862, 1992 [DOI] [PubMed] [Google Scholar]
- 64.Tremblay A, Poehlman ET, Nadeau A, Dessault J, Bouchard C. Heredity and overfeeding-induced changes in submaximal exercise V̇o2. J Appl Physiol 62: 539– 544, 1987 [DOI] [PubMed] [Google Scholar]
- 65.Tremblay A, Simoneau J, Bouchard C. Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism 43: 814– 818, 1994 [DOI] [PubMed] [Google Scholar]
- 66.Vandenborne K, Walter G, Ploutz-Snyder L, Staron R, Fry A, DeMeirler K, Dudley G, Leigh J. Energy-rich phosphates in slow and fast human skeletal muscle. Am J Physiol Cell Physiol 268: C869– C876, 1995 [DOI] [PubMed] [Google Scholar]
- 67.Villani G, Greco M, Papa S, Attardi M. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J Biol Chem 273: 31829– 31836, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Wadden T. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med 229: 688– 693, 1993 [DOI] [PubMed] [Google Scholar]
- 69.Wasserman K, Hansen J, Sue D, Casaburi R, Whipp B. Principles of exercise testing and interpretation. Baltimore, MD: Lippincott Williams & Wilkins, 1999, p. 36 [Google Scholar]
- 70.Weigle D, Sande K, Iverius P, Monsen E, Brunzell J. Weight loss leads to a marked decrease in nonresting energy expenditure in ambulatory human subjects. Metabolism 37: 930– 936, 1988 [DOI] [PubMed] [Google Scholar]
- 71.Weinsier R, Hunter G, Zuckerman P, Redden D, Darnell B, Larson D, Newcomer B, Goran M. Energy expenditure and free-living physical activity in black and white women: comparison and after weight loss. Am J Clin Nutr 71: 1138– 1146, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Weltan S, Bosch A, Dennis S, Noakes T. Influence of muscle glycogen on metabolic regulation. Am J Physiol Endocrinol Metab 274: E72– E82, 1998 [DOI] [PubMed] [Google Scholar]
- 73.Weyer C, Pratley R, Salbe A, Bogardus C, Ravus E, Tataranni P. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab 85: 1087– 1094, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Wiedermann F, Vielhaber S, Schroder R, Elger C, Kunz W. Evaluation of methods for the determination of mitochondrial respiratory chain enzyme activities in human skeletal muscle. Analyt Biochem 279: 55– 60, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Yang W, Lee W, Funahashi T, Tanaka S, Matuszawa Y, Chao C, Chen C, Tai T, Chuang L. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 86: 3815– 3819, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Yoon M, Lee G, Chung J, Ahn Y, Hong S, Kim J. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55: 2562– 2570, 2006 [DOI] [PubMed] [Google Scholar]