Abstract
Accumulation of continuous life stress (chronic stress) often causes gastric symptoms. The development of gastric symptoms may depend on how humans adapt to the stressful events in their daily lives. Although acute stress delays gastric emptying and alters upper gastrointestinal motility in rodents, the effects of chronic stress on gastric motility and its adaptation mechanism remains unclear. Central oxytocin has been shown to have antistress effects. We studied whether central oxytocin is involved in mediating the adaptation mechanism following chronic repeated stress. Mice were loaded with acute and chronic stress (repeated stress for five consecutive days), and solid gastric emptying and postprandial gastric motility were compared between acute and chronic repeated stress. Expression of oxytocin and CRF mRNA in the hypothalamus was studied following acute and chronic repeated stress. Delayed gastric emptying during acute stress (43.1 ± 7.8%; n = 6, P < 0.05) was completely restored to normal levels (72.1 ± 2.4%; n = 6) following chronic repeated stress. Impaired gastric motility induced by acute stress was also restored following chronic repeated stress. Intracerebroventricular injection of oxytocin (0.1 and 0.5 μg) restored the impaired gastric emptying and motility induced by acute stress. The restored gastric emptying and motility following chronic repeated stress were antagonized by intracerebroventricular injection of oxytocin antagonists. Oxytocin mRNA expression in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) of the hypothalamus was significantly increased following chronic repeated stress. In contrast, increased CRF mRNA expression in the SON and PVN in response to acute stress was significantly reduced following chronic repeated stress. Our study suggests the novel finding that the upregulation of central oxytocin expression is involved in mediating the adaptation mechanism following chronic repeated stress in mice.
Keywords: acute stress, chronic stress, corticotropin-releasing factor, hypothalamus-pituitary-adrenal axis
stress is widely believed to play a major role in developing functional gastrointestinal (GI) disorders. Patients with serious stress frequently complain of GI symptoms, and these symptoms are, at least in part, likely due to GI motility disorders. Accumulation of continuous life stress (chronic stress) often causes gastric symptoms. The development of dyspeptic symptoms may also depend on how humans adapt to the stressful events in their daily lives. Although some can adapt to chronic stress, the adaptation mechanism against chronic stress remains unclear.
Animal studies demonstrated that gastric emptying of a solid and liquid meal was delayed by acute stress in dogs (17), rats (31, 45), and mice (16). CRF is known to act in the brain to influence the GI tract. Acute restraint stress inhibits solid gastric emptying via central CRF and peripheral autonomic neural pathways in rats (31, 44). Gastric emptying and acid secretion are attenuated when CRF is exogenously applied to the central nervous system (45). Restraint stress is known to increase CRF mRNA in the amygdala and paraventricular nucleus (PVN), resulting in altered GI motor activities (14).
Many animal studies have been conducted to investigate the effects of acute stress on GI motility. However, relatively few studies have been done on chronic repeated stress. Ochi et al. (36) have recently demonstrated that acute stress delays gastric emptying, while repeated stress for 5 days accelerates gastric emptying in rats. This suggests that homeostatic adaptation may develop in response to repeated stress.
Oxytocin is a cyclic nonapeptide hormone synthesized in the neurosecretory cells that are located in the PVN and supraoptic nucleus (SON) of the hypothalamus. Besides its well-known physiological functions like milk ejection and induction of labor, oxytocin plays an important role in regulating social behavior and positive social interactions in nonhuman mammals (32). In humans, intranasal administration of oxytocin was shown to cause a substantial increase in trusting behavior (20).
Oxytocin is released from the neurohypophysial terminal into the bloodstream and within distinct brain regions in response to stressful or social stimuli. Centrally released oxytocin has anxiolytic effects and antistress effects (3). Anxiolytic and antistress effects of oxytocin are localized within the central amygdala and the PVN both in females and in males. Oxytocin is released in the PVN in response to various stressors, such as shaker stress (34) and forced swimming stress (52) in rats. The SON consists exclusively of magnocellular neurons. In contrast, the PVN consists of both magnocellular and parvocellular neurons. Forced swimming stress increases oxytocin mRNA expression in the magnocellular neurons, but not the parvocellular neurons, of the PVN in rats (53). Restraint stress induces c-fos expression in oxytocinergic magnocellular neurons in the SON and PVN in rats (28). However, it remains unknown whether restraint stress activates parvocellular oxytocinergic neurons of the PVN.
Oxytocin attenuates CRF mRNA expression in the PVN and the activity of hypothalamus-pituitary-adrenal (HPA) axis in response to stress (51). This suggests that anxiolytic and antistress effects of oxytocin are, at least in part, mediated by its inhibitory effect on CRF expression. A recent study demonstrated that water avoidance stress-induced stimulation of colonic motility is attenuated by central administration of oxytocin in rats (25).
The anxiolytic effects and anti-stress effects of central oxytocin are further supported by the study of oxytocin-deficient mice. Oxytocin-deficient mice display more anxiety-related behavior in response to a psychogenic stressor and manifest greater stress-induced hyperthermia, compared with wild-type mice (2). Plasma corticosterone levels after shaker stress are higher in oxytocin knockout mice than those in wild-type mice (24). In oxytocin gene-deficient male mice, enhanced CRF mRNA expression is observed in response to restraint stress in the PVN (35).
Gastric motility consists of two motor patterns; postprandial contractions and interdigestive contractions (47). The alterations of gastric motor function in response to food intake may play an important role in stress-related functional GI disorders. We have recently demonstrated that acute restraint stress or central injection of CRF completely abolishes the interdigestive gastric contractions in rats (55). Very few studies have been conducted on chronic stress and its long-term effects on GI physiology in mice.
In the current study, we compared the effects of acute and chronic repeated stress on gastric emptying and postprandial gastric motility in conscious mice. Delayed gastric emptying and impaired gastric motility observed during acute stress loading was completely restored following chronic stress in mice. To further investigate the role of oxytocin in stress-induced gastric motor function, oxytocin and oxytocin antagonists were centrally administered. To investigate the changes of central oxytocin and CRF expression following acute stress and chronic stress, oxytocin mRNA expression and CRF mRNA expression in the PVN and SON were measured by real-time PCR. We demonstrated that central oxytocin may play an important role in regulating the adaptation mechanism following chronic repeated restraint stress in mice.
MATERIALS AND METHODS
Animals.
Male Swiss Webster mice weighing 25–30 g were kept in-group cages under conditions of controlled temperature (22–24°C), humidity, and light (12:12-h light-dark cycle starting at 7:00 AM), with free access to laboratory chow and water. All experiments were started at 9:00 AM every day. Protocols describing the use of mice were approved by the Institutional Animal Care and Use Committee of Zablocki VA Medical Center at Milwaukee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals in experiments.
Acute and chronic stress loading.
Restraint stress was induced by placing the mice in a 50-ml falcon tube (2.7 cm in diameter, 7 cm long) with perforated holes for adequate ventilation. The dimensions of the tube effectively restrained the mice, preventing them from turning around and moving forward or backward. Mice were exposed to restraint stress for 90 min. In chronic repeated stress, mice were restrained for 90 min for five consecutive days.
Measurement of solid gastric emptying.
Mice were fasted for 16 h with free access to water. Preweighed pellets (0.2 g) were given, as previously reported (8). The mice that did not consume 0.2 g of food within 10 min were excluded from the study. Immediately after finishing the feeding, the mice were subjected to the restraint stress (both acute and chronic), as described above. After the restraint stress loading for 90 min, mice were killed by pentobarbital (200 mg/kg ip). The stomach was surgically isolated and removed. The gastric content was recovered from the stomach, dried, and then weighed. Solid gastric emptying was calculated according to the following formula, as previously described (31).
Gastric emptying (%) = [1 − (dried weight of food recovered from stomach/weight of food intake)] × 100.
Motility recording.
After an overnight fast, mice were anesthetized with isoflurane (2%). Through a midline laparotomy, the stomach was exposed, and a miniature strain gauge transducer (5 mm × 4 mm) was implanted on the serosal surface of the gastric antrum, as previously described (54). The wires from transducer were exteriorized through abdominal wall, and tunnelled under the skin to the posterior neck. Wires were contained within a protective jacket. After the surgery, mice were housed individually with access to a standard diet and tap water. Mice were allowed to recover for 1 wk before subsequent studies.
Wires from the transducer were connected to a recording system (Power-Lab model 8SP; ADI Instruments, Colorado Springs, CO). Although the animals were wired and connected to the recording system, the animals could freely move during recording, as previously reported (54). Postprandial gastric contractions were measured in conscious, freely moving mice except during the period of restraint stress loading. Gastric motility was monitored before, during, and after the restraint stress. After recording the basal gastric motility for 2 h, mice were exposed to restraint stress for 90 min. After releasing the restraint stress, gastric motility was recorded for two more hours.
Intracerebroventricular injection.
For intracerebroventricular injection, mice were placed in a stereotaxic instrument under the isoflurane anesthesia, 1 wk prior to the gastric emptying and gastric motility studies. A hole was made in each skull using a needle inserted 0.9 mm lateral to the central suture and 0.9 mm posterior to the bregma. A 24-gauge cannula 3 mm in length was implanted into the right lateral ventricle for intracerebroventricular injection. The injector was the same length as the implanted cannula. The cannula was fixed to the skull with dental cement and capped with silicon.
To investigate whether central oxytocin is involved in mediating gastric motility and emptying, oxytocin (0.05, 0.1, and 0.5 μg in 2 μl saline) was injected (icv) 30 min before stress loading. It has been shown that oxytocin (0.1–1.0 μg icv) dose dependently reduced the anxiety responses in mice (42).
To investigate whether endogenous oxytocin is involved in restoring gastric motility and emptying following chronic stress, oxytocin receptor antagonists, tocinoic acid (2 μg) and [d(CH2)51,Tyr(Me)2,Orn8]-oxytocin (100 ng) were injected intracerebroventricularly 30 min prior to stress loading. Saline (2 μl icv)-injected mice served as controls.
Tocinoic acid (20 μg) has been shown to prevent the effect of oxytocin-induced social behavior in male rats (46). [d(CH2)51, Tyr(Me)2,Orn8]-Oxytocin (1–100 ng) has been shown to inhibit the action of oxytocin in sexual physiological stimuli in rats (4, 26).
We used separate mice for each study group. After finishing the experiment, mice were killed, and the implantation site of intracerebroventricular cannula was confirmed by the visualization after injection of a dye via the cannula, as previously reported (7, 19).
Quantitative RT-PCR.
Immediately after finishing acute or chronic stress loading, mice were killed by pentobarbital sodium (200 mg/kg ip). Brain tissues were collected between −0.56 mm and −0.96 mm from bregma, medially 0.8 mm above the ventral tissue edge around the dorsal end of the 3rd ventricle to obtain the PVN, and bilaterally from the optic tract to acquire the SON (10, 39). Total RNA was extracted from the brain tissues using TRIzol (Invitrogen, Carsbad, CA) according to manufacturer's instructions. Trace DNA contamination was removed by DNase digestion (Promega, Madison, WI). cDNA was synthesized from 3 μg total RNA using Superscript III reverse transcriptase (Invitrogen). The following primers were designed to amplify mouse CRF (81 bp; accession no. NM_205769), as previously reported (15). Sense primer: 5′-CCCGGGCAGAGCAGTTAGCT−3′. Antisense primer: 5′-CAAGCGCAACATTTCATTTCC−3′.
The following primers were designed to amplify mouse oxytocin (82 bp; accession no. NM_011025), as previously reported (49). Sense primer: 5′-CCTACAGCGGATCTCAGACTGA−3′. Antisense primer: 5′-TCAGAGCCAGTAAGCCAAGCA−3′. For the internal control, the following primers were designed to amplify a mice β-actin fragment (106 bp; accession no. EF095208). Sense primer: 5′-TGGCACCACACCTTCTACAATGAG−3′. Antisense primer: 5′-GGGTCATCTTTTCACGGTTGG−3′.
Quantitative RT-PCR was performed using SYBR Premix Ex Taq (Takara BIO, Madison, WI), according to manufacturer's instructions. Amplification reactions were performed using a LightCycler 480 (Roche Diagnostics, Mannheim, Germany). Initial template denaturation was performed for 30 s at 95°C. The cycle profiles were programmed as follows: 5 s at 95°C (denaturation), 20 s at 60°C (annealing) and 15 s at 72°C (extension). Forty-five cycles of the profile were run, and the final cooling step was continued for 30 s at 40°C. Quantitative measurement of each mRNA was achieved by establishing a linear amplification curve from serial dilutions of each plasmid containing the amplicon sequence. The relative amount of each mRNA was normalized by the amount of β-actin mRNA. Amplicon size and specificity were confirmed by melting curve analysis and 2% agarose gel electrophoresis.
Statistical analysis.
As previously reported (54), quantification of gastric motility was studied by calculating motility index (MI). MI was equivalent to the area under the curve of the motility recording from baseline. MI was calculated using a computer-assisted system (Power Lab; AD Instruments, AD Instruments, Colorado Springs, CO).
Comparison between group values was performed by one-way ANOVA for gastric emptying and motility studies. Two-way ANOVA was performed for experiments comparing acute and chronic restraint stress with and without drug dosing. The Student's t-test was used to determine the significance among groups. Bonferroni post test was done to compare data replicates in a group. Pearson correlation analysis was performed for oxytocin mRNA expression and gastric emptying rate. P < 0.05 was considered to be statistically significant. Results were shown as means ± SE.
Chemicals.
Oxytocin and tocinoic acid was purchased from (Sigma Aldrich, St. Louis, MO). [d(CH2)51,Tyr(Me)2,Orn8]-Oxytocin was purchased from (Bachem, Torrance, CA).
RESULTS
Effect of acute and chronic repeated restraint stress on gastric emptying.
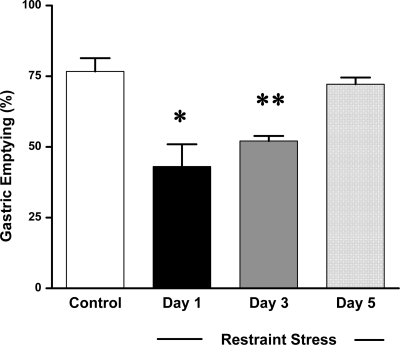
In nonrestraint mice, solid gastric emptying was 76.7 ± 4.6% (n = 6), 90 min after feeding 0.2 g mice chow. Gastric emptying was significantly delayed in mice that received restraint stress at day 1 (43.1 ± 7.8%, n = 6, P < 0.05) and day 3 (52.1 ± 1.7%, n = 6, P < 0.01). Delayed gastric emptying was completely restored to normal levels (72.1 ± 2.4%, n = 6) after five consecutive days of restraint stress (Fig. 1).
Fig. 1.
Effect of acute and chronic restraint stress on solid gastric emptying. Restraint stress significantly delayed the gastric emptying at day 1 and day 3. In contrast, the delayed gastric emptying was completely restored following chronic stress at day 5 (n = 6, *P < 0.05, **P < 0.01).
Effect of acute and chronic repeated-restraint stress on postprandial gastric motility.
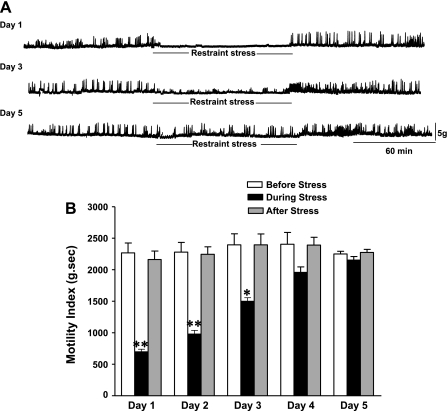
Postprandial gastric motility was measured in mice before, during, and after restraint stress loading. Before stress loading, regular gastric contractions were observed. Immediately after the loading of restraint stress, gastric contractions were significantly attenuated on day 1 (Fig. 2A). Motility index was significantly reduced during the stress loading to 69.0 ± 2.9% of basal (n = 5, P < 0.05). Regular gastric contractions were soon recovered to basal levels after finishing stress loading (Fig. 2A). After three consecutive days of restraint stress, the impaired gastric contractions during stress loading were partially restored. On day 5, restraint stress had no longer an inhibitory effect on gastric contractions (Fig. 2A).
Fig. 2.
Effect of acute and chronic repeated restraint stress on postprandial gastric motility (A) and motility index (B). Acute restraint stress significantly attenuated gastric contractions during the restraint stress loading. The reduced gastric motility in response to acute stress was gradually restored following the repeated stress loading (at day 2 and day 4). On day 5, restraint stress had no more inhibitory effects on gastric contractions (B) (n = 5; *P < 0.05, **P < 0.01).
The reduced MI in response to restraint stress was gradually restored at day 2 and day 4. On day 5, MI was no longer attenuated during stress loading (Fig. 2B).
Effect of oxytocin on gastric emptying during acute stress loading.
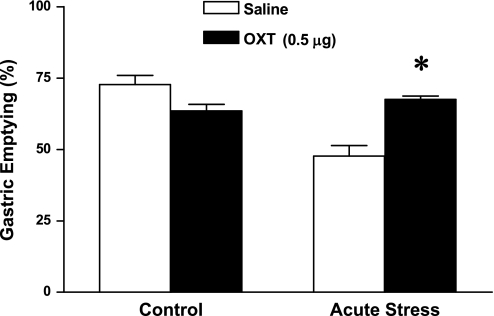
Intracerebroventricular injection of oxytocin (0.05 μg) did not have a significant effect on delayed solid gastric emptying induced by acute stress (47.9 ± 0.7%, n = 6). In contrast, intracerebroventricular injection of oxytocin (0.1 and 0.5 μg) significantly antagonized the delayed gastric emptying induced by acute stress (54.4 ± 1.3% by 0.1 μg and 67.5 ± 1.2% by 0.5 μg; n = 6, P < 0.05), compared with that of saline intracerebroventricularly injected mice (47.6 ± 3.6%; n = 6) (Fig. 3). A two-way ANOVA showed a significant difference in gastric emptying between control and acute stress group (F1,19 = 25.68; P < 0.001).
Fig. 3.
Effect of intracerebroventricular injection of oxytocin (0.5 μg) on gastric emptying during acute restraint stress. Intracerebroventricular injection of oxytocin completely restored delayed gastric emptying induced by acute stress (n = 6; *P < 0.05). A two-way ANOVA showed a significant difference in gastric emptying between control and acute stress group (F1,19 = 25.68; P < 0.001).
Effect of oxytocin on gastric motility during acute stress loading.
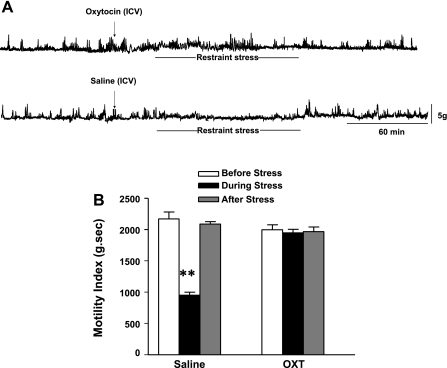
We studied whether intracerebroventricular injection of oxytocin itself affects basal gastric motility before stress loading. MI was slightly yet significantly increased by 7.9 ± 1.8% (n = 5, P < 0.05) of basal contractions by intracerebroventricular injection of oxytocin (0.5 μg). The stimulatory effect of oxytocin was observed immediately after the injection and lasted for 15 to 20 min.
Intracerebroventricular injection of oxytocin (0.5 μg) completely restored impaired gastric contractions induced by acute restraint stress (Fig. 4A). MI was significantly reduced during stress loading to 57.1 ± 2.4% (n = 5, P < 0.05) of basal in saline-injected mice. However, MI was not significantly altered during stress loading of basal in oxytocin-treated mice (Fig. 4B).
Fig. 4.
Effect of intracerebroventricular injection of oxytocin (0.5 μg) on gastric motility (A) and motility index (B) during acute stress loading. Intracerebroventricular injection of oxytocin completely restored the attenuated gastric motility during acute stress loading (A). The calculated motility index showed that the reduction of gastric contractions induced by acute stress was no more observed in mice treated with oxytocin (n = 5; **P < 0.01).
Effect of oxytocin antagonist on gastric emptying in response to acute and chronic repeated restraint stress.
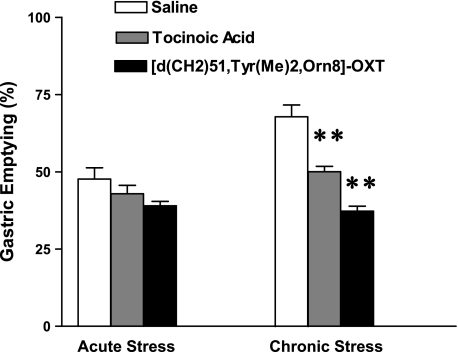
Intracerebroventricular injection of oxytocin antagonists, tocinoic acid (2 μg), and [d(CH2)51,Tyr(Me)2,Orn8]-Oxytocin (100 ng) did not have a significant effect on gastric emptying in nonrestraint mice (Fig. 5). Oxytocin antagonists also did not alter the stress-induced delay in gastric emptying in response to acute stress loading (Fig. 5). A two-way ANOVA indicated a significant difference in gastric emptying between acute and chronic stress group (F2,28 = 8.95; P < 0.01). In chronically stressed mice, intracerebroventricular injection of oxytocin antagonists, tocinoic acid (2 μg), and [d(CH2)51,Tyr(Me)2,Orn8]-Oxytocin (100 ng) significantly delayed solid gastric emptying to 50 ± 1.7% (n = 6, P < 0.01) and 37.2 ± 1.6% (n = 6, P < 0.01), respectively, compared with that of intracerebroventricularly saline-injected (icv) mice (67.8 ± 3.8%, n = 6) (Fig. 5).
Fig. 5.
Effect of intracerebroventricular injection of oxytocin antagonists on gastric emptying during chronic repeated restraint stress loading. In chronically restrained mice, intracerebroventricular injection of oxytocin antagonists, tocinoic acid (2 μg), and [d(CH2)51,Tyr(Me)2,Orn8]-Oxytocin (100 ng) significantly antagonized the restored gastric emptying (n = 6; **P < 0.01), compared with that of the saline-injected mice. In contrast, intracerebroventricular injection of oxytocin antagonists did not alter delayed gastric emptying induced by acute restraint stress. Two-way ANOVA indicated a significant difference in gastric emptying between acute and chronic stress group (F2,28 = 8.95; P < 0.01).
Effect of oxytocin antagonist on gastric motility in response to acute and chronic repeated restraint stress.
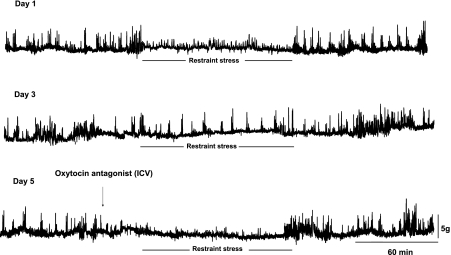
At day 5, MI was no longer attenuated during stress loading. In contrast, MI was significantly reduced to 51.6 ± 1.9% of basal (n = 5, P < 0.05) following the administration of an oxytocin antagonist, [d(CH2)51,Tyr(Me)2,Orn8]-Oxytocin (100 ng) (Fig. 6).
Fig. 6.
Effect of intracerebroventricular injection of an oxytocin antagonist, [d(CH2)51,Tyr(Me)2,Orn8]-oxytocin (100 ng) on gastric motility following chronic repeated restraint stress. At day 1, restraint stress significantly attenuated gastric motility, which was partially restored at day 3 and completely restored at day 5. The administration of an oxytocin antagonist significantly antagonized the restored gastric motility following chronic stress loading. MI was reduced to 51.6 ± 1.9% (n = 5; P < 0.05) of basal by an oxytocin antagonist.
Effects of acute and chronic stress on CRF and oxytocin mRNA expression in the PVN and SON.
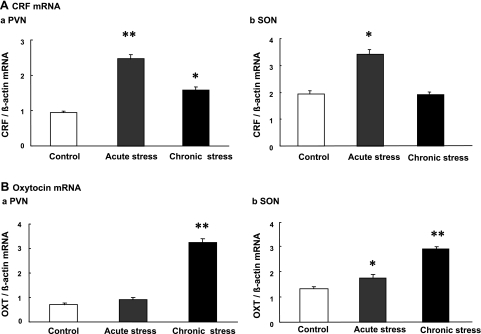
CRF mRNA expression was significantly increased in the PVN and SON in response to acute restraint stress, compared with that of controls. The increased CRF mRNA observed during acute stress was significantly reduced in the PVN following chronic stress. There was no significant increase of CRF mRNA expression observed in the SON following chronic stress loading (Fig. 7A).
Fig. 7.
Effect of acute and chronic repeated restraint stress on CRF mRNA expression (A) and oxytocin mRNA expression (B) in the paraventricular nucleus (PVN) and supraoptic nucleus (SON). CRF mRNA expression showed a significant increase in response to acute stress. The increment of CRF mRNA was much less at the PVN following chronic stress, compared with that of acutely stressed mice. Oxytocin mRNA expression at the PVN and SON showed a more pronounced increase following chronic stress, compared with that of acutely stressed rats. The mRNA expression was standardized with the ratio of internal control, β actin (n = 6; *P < 0.05, **P < 0.01 compared with controls).
Oxytocin mRNA expression was not significantly increased in the PVN in response to acute stress. In the SON, a small, but significant, increase of oxytocin mRNA expression was observed in response to acute stress. Chronic stress significantly increased oxytocin mRNA expression in the PVN, as well as SON, compared with that of controls and acutely stressed mice (Fig. 7B).
Correlation between oxytocin mRNA expression and gastric emptying.
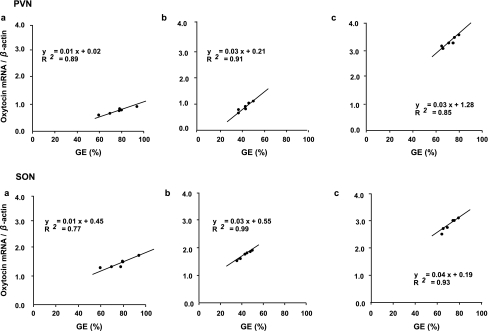
There was a significant positive correlation observed between oxytocin mRNA expression and gastric emptying in the PVN and SON in control (Fig. 8a ), acute stress (Fig. 8b) and chronic repeated stress groups (Fig. 8c).
Fig. 8.
Correlation between oxytocin mRNA expression and gastric emptying (GE) in the PVN and SON in control (A), acute stress (B), and chronic repeated stress (C) in mice. There was a significant positive correlation observed between oxytocin mRNA expression and gastric emptying (n = 6; P < 0.05).
DISCUSSION
We have previously shown that acute stress delays solid gastric emptying via central CRF and peripheral sympathetic pathways in rats (31). Others have suggested that the inhibitory effect of acute stress on gastric emptying is mediated via the impaired activity of parasympathetic pathway in rats (44).
In contrast to acute stress, a recent study suggests that chronic stress seems to have an inverse effect on gastric emptying in rats (36). Our recent study demonstrated that delayed gastric emptying induced by acute restraint stress is no longer observed following a 5-day consecutively repeated stress in rats (55). Our current study also demonstrates that delayed gastric emptying observed in acute stress was fully restored following stress loading for five consecutive days in mice.
Oxytocin and its receptors are also found all throughout the GI tract in humans (29) and rats (48). Oxytocin exerts its activity on the GI tract through both central and peripheral mechanisms. In healthy fasting humans, intravenous infusion of oxytocin (0.33 U/min) increases gastric emptying of semi-solid meals (40). In contrast, intraperitoneal administration of oxytocin (0.8 mg/kg) inhibits gastric emptying of a nonnutrient liquid meal in rats (22). Others have shown that administration of oxytocin (4 pmol) into the dorsal motor nucleus of the vagus inhibits gastric motility in anesthetized rats (43). Intravenous injection of oxytocin (0.5 to 2.0 U/kg) abolishes the peristaltic contractions of the GI tract in anesthetized dogs (27). On the other hand, intravenous injection of oxytocin (0.1–0.8 μg/kg) was shown to increase the gastric motility in rabbits (21). Oxytocin (10−9−10−6 M) dose dependently increased the contraction of the muscle strips of gastric body and antrum in rats. Tetrodotoxin and atropine do not influence the effect of oxytocin, suggesting the direct action on smooth muscle cells (41).
It remains unclear whether central administration of oxytocin affects solid gastric emptying and postprandial gastric contractions in rodents. Flanagan et al. (13) have previously shown that centrally administered oxytocin (10 μg icv) inhibits gastric motility under normal conditions in conscious rats (13). Our current study shows that oxytocin (0.5 μg icv) fails to alter gastric emptying in nonstressed mice, while gastric motility was slightly increased by intracerebroventricular injection of oxytocin. Enhanced gastric motility induced by central administration of oxytocin may not influence solid gastric emptying in conscious mice under normal conditions. Thus, it is likely that the effects of oxytocin on the GI tract may vary among species under normal conditions.
Next, we studied the central effects of oxytocin on gastric emptying and gastric motility in response to acute and chronic repeated stress in mice. We demonstrated that central administration of oxytocin antagonized the inhibitory effect of acute restraint stress on gastric emptying and motility. As the central administration of oxytocin did not cause any effects on gastric emptying in nonstressed conditions, the normalization of gastric emptying caused by intracerebroventricular oxytocin observed in acute stress loading may be associated with the stress responses.
To further investigate the role of oxytocin in the adaptation mechanism following chronic repeated stress, two different oxytocin antagonists, tocinoic acid and [d(CH2)51,Tyr(Me)2,Orn8]-Oxytocin were administered. Either of the oxytocin antagonists failed to evoke any significant effects on gastric emptying in nonrestrained mice. In contrast, oxytocin antagonists reversed the restored gastric emptying and gastric motility following chronic restraint stress in mice. This suggests that endogenous oxytocin may play an important role in the adaptation mechanism following chronic stress.
Previous studies have shown that central administration of oxytocin antagonists into the PVN resulted in a significant increase in basal release of ACTH in male and female rats (33). This suggests that endogenous oxytocin in the PVN mediates its antistress responses through the inhibition of HPA axis.
When animals are subjected to acute stress, CRF is secreted from the hypothalamus, resulting in the secretion of corticosterone from the adrenal cortex to guard against stress disorders. Central administration of CRF significantly elevates oxytocin secretion both in male and female rats (9). On the other hand, central oxytocin attenuates CRF mRNA expression in the PVN in response to restraint stress (50). This suggests that oxytocin may have a direct or indirect influence on CRF production and/or secretion from the PVN through a negative feedback mechanism (37).
We studied the changes of CRF and oxytocin mRNA expression in the PVN and SON following acute and chronic stress in mice. CRF mRNA expression in PVN and SON was significantly increased following acute restraint stress, which was reduced to control levels at day 5 of chronic repeated stress. In contrast, oxytocin mRNA expression was significantly increased following chronic repeated stress. Further analysis showed that there was a significant positive correlation between oxytocin mRNA expression and gastric emptying. To our knowledge, this is the first demonstration of upregulated oxytocin expression in the hypothalamus following chronic repeated stress in mice.
Plasma corticosterone levels are significantly reduced following chronic repeated stress in mice (38) and rats (55), suggesting the attenuation of HPA axis following chronic stress. Oxytocin attenuates stress-induced activity of HPA axis and thus modulates the regulation of the stress response (50); it is very likely that chronic repeated stress may upregulate oxytocin expression at the PVN and SON, which, in turn, attenuates CRF expression and HPA activity. Further studies are needed to elucidate the CRF-oxytocin interactions in the adaptation mechanism following chronic repeated stress.
The mechanism of upregulation of oxytocin expression following chronic repeated stress remains to be determined. Ghrelin plays an important role in mediating gastric emptying (6) and interdigestive gastric contractions (5, 54) in rodents. It has been shown that the expression of gastric ghrelin is upregulated following chronic stress in rats (36, 55) and mice (23). Plasma ghrelin levels are increased by β-adrenergic agonists (18) and sympathetic nerve stimulation (30) in rats. We have previously shown that restraint stress stimulates the sympathetic pathway in rats (31). Thus, the upregulation of ghrelin expression might be explained by activated sympathetic nerves following chronic stress (23). Ghrelin released from the stomach has been shown to activate various neuropeptides and neuronal circuits at the central nervous system (1, 11). We cannot exclude the possibility that increased gastric ghrelin expression may directly or indirectly stimulate oxytocin expression during the adaptation process following chronic stress.
Another possibility for the mechanism of upregulated oxytocin expression following chronic repeated stress is the lack of a feedback system. Glucocorticoids secreted in response to stress activate the HPA axis and cause negative feedback onto the hypothalamus, suppressing oxytocin secretion (12). Reduced plasma cortisol levels following chronic repeated stress may result in upregulation of oxytocin expression.
Although mice can adapt to a single, repeated stressful event, it is necessary to establish an animal model of chronic loading of different types of stress (chronic complicated stress) and to study whether adaptation develops following chronic complicated stress loading. A better understanding of the mechanism of stress-induced GI tract dysfunction and how adaptation to stress takes place may lead to better treatments for these disorders in humans.
Perspectives and Significance
Functional GI disorders are frequently associated with continuous life stress (chronic stress). In our modern society, most individuals encounter both mental and social stress on a daily basis. GI symptoms may develop as a result of accumulation of daily stress in some individuals; however, others will adapt to the stressful environment without developing GI symptoms. Our current study reveals that delayed gastric emptying observed in acute stress loading was completely restored following repeated chronic stress in mice. The adaptation mechanism involves upregulation of oxytocin expression and downregulation of CRF expression. Our study contributes to the better understanding of the mechanism of stress-induced functional GI disorders.
GRANTS
This study was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (RO1 DK62768) to T. Takahashi.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116: 3229– 3239, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol 16: 319– 324, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Amico JA, Miedlar JA, Cai HM, Vollmer RR. Oxytocin knockout mice: a model for studying stress-related and ingestive behaviours. Prog Brain Res 170: 53– 64, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Argiolas A, Melis MR, Vargiu L, Gessa GL. d(CH2)5Tyr(Me)-[Orn8]vasotocin, a potent oxytocin antagonist, antagonizes penile erection and yawning induced by oxytocin and apomorphine, but not by ACTH-(1–24). Eur J Pharmacol 134: 221– 224, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Ariga H, Imai K, Chen C, Mantyh C, Pappas TN, Takahashi T. Fixed feeding potentiates interdigestive gastric motor activity in rats: importance of eating habits for maintaining interdigestive MMC. Am J Physiol Gastrointest Liver Physiol 294: G655– G659, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Ariga H, Nakade Y, Tsukamoto K, Imai K, Chen C, Mantyh C, Pappas TN, Takahashi T. Ghrelin accelerates gastric emptying via early manifestation of antro-pyloric coordination in conscious rats. Regul Pept 146: 112– 116, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 52: 947– 952, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology 116: 1287– 1292, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Bruhn TO, Sutton SW, Plotsky PM, Vale WW. Central administration of corticotropin-releasing factor modulates oxytocin secretion in the rat. Endocrinology 119: 1558– 1563, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Bunck M, Czibere L, Horvath C, Graf C, Frank E, Kessler MS, Murgatroyd C, Muller-Myhsok B, Gonik M, Weber P, Putz B, Muigg P, Panhuysen M, Singewald N, Bettecken T, Deussing JM, Holsboer F, Spengler D, Landgraf R. A hypomorphic vasopressin allele prevents anxiety-related behavior. PLoS ONE 4: e5129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab 4: 323– 331, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Di S, Tasker JG. Rapid synapse-specific regulation of hypothalamic magnocellular neurons by glucocorticoids. Prog Brain Res 170: 379– 388, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res 578: 256– 260, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Gomez F, Manalo S, Dallman MF. Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology 145: 59– 70, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gourcerol G, Gallas S, Mounien L, Leblanc I, Bizet P, Boutelet I, Leroi AM, Ducrotte P, Vaudry H, Jegou S. Gastric electrical stimulation modulates hypothalamic corticotropin-releasing factor-producing neurons during post-operative ileus in rat. Neuroscience 148: 775– 781, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Gue M, Fioramonti J, Bueno L. Comparative influences of acoustic and cold stress on gastrointestinal transit in mice. Am J Physiol Gastrointest Liver Physiol 253: G124– G128, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Gue M, Peeters T, Depoortere I, Vantrappen G, Bueno L. Stress-induced changes in gastric emptying, postprandial motility, and plasma gut hormone levels in dogs. Gastroenterology 97: 1101– 1107, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Hosoda H, Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regul Pept 146: 12– 18, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Ishiguchi T, Nakajima M, Sone H, Tada H, Kumagai A, Takahashi T. Gastric distension-induced pyloric relaxation: central nervous system regulation and effects of acute hyperglycaemia in the rat. J Physiol 533: 801– 813, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 435: 673– 676, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Li L, Kong X, Liu H, Liu C. Systemic oxytocin and vasopressin excite gastrointestinal motility through oxytocin receptor in rabbits. Neurogastroenterol Motil 19: 839– 844, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Liu CY, Chen LB, Liu PY, Xie DP, Wang PS. Effects of progesterone on gastric emptying and intestinal transit in male rats. World J Gastroenterol 8: 338– 341, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11: 752– 753, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am J Physiol Regul Integr Comp Physiol 287: R1494– R1504, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga M, Konagaya T, Nogimori T, Yoneda M, Kasugai K, Ohira H, Kaneko H. Inhibitory effect of oxytocin on accelerated colonic motility induced by water-avoidance stress in rats. Neurogastroenterol Motil 21: 856– e59, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Melis MR, Spano MS, Succu S, Argiolas A. The oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin reduces non-contact penile erections in male rats. Neurosci Lett 265: 171– 174, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Milenov K, Kasakov L. Effect of synthetic oxytocin on the motor and bioelectrical activity of the stomach and small intestines (in vivo). Acta Physiol Pharmacol Bulg 3–4: 31– 40, 1975 [PubMed] [Google Scholar]
- 28.Miyata S, Itoh T, Lin SH, Ishiyama M, Nakashima T, Kiyohara T. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull 37: 391– 395, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Monstein HJ, Grahn N, Truedsson M, Ohlsson B. Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study. Regul Pept 119: 39– 44, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Mundinger TO, Cummings DE, Taborsky GJ., Jr Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology 147: 2893– 2901, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol 288: R427– R432, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol 20: 858– 865, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol 12: 235– 243, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res 781: 56– 60, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced up-regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventricular nucleus of oxytocin gene-deficient male mice. J Neuroendocrinol 15: 1054– 1061, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K, Arakawa T. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci 82: 862– 868, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Patel H, Chowdrey HS, Lightman SL. Lactation abolishes corticotropin-releasing factor-induced oxytocin secretion in the conscious rat. Endocrinology 128: 725– 727, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 145: 5431– 5438, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Paxinos GFK. The Mouse Brain in Stereotaxic Coordinates ( 2nd ed.), San Diego, CA: Academic, 2001 [Google Scholar]
- 40.Petring OU. The effect of oxytocin on basal and pethidine-induced delayed gastric emptying. Br J Clin Pharmacol 28: 329– 332, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin J, Feng M, Wang C, Ye Y, Wang P, Liu C. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neurogastroenterol Motil 21: 430– 438, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology 185: 218– 225, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides 8: 505– 513, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 117: 33– 40, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tache Y, Maeda-Hagiwara M, Turkelson CM. Central nervous system action of corticotropin-releasing factor to inhibit gastric emptying in rats. Am J Physiol Gastrointest Liver Physiol 253: G241– G245, 1987 [DOI] [PubMed] [Google Scholar]
- 46.Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience 146: 509– 514, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest 59: 1158– 1166, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J Comp Neurol 512: 256– 270, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wesolowski SR, Allan MF, Nielsen MK, Pomp D. Evaluation of hypothalamic gene expression in mice divergently selected for heat loss. Physiol Genomics 13: 129– 137, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci 24: 2974– 2982, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 138: 2829– 2834, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience 85: 1209– 1222, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Wotjak CT, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci 13: 2273– 2281, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Zheng J, Ariga H, Taniguchi H, Ludwig K, Takahashi T. Ghrelin regulates gastric phase III-like contractions in freely moving conscious mice. Neurogastroenterol Motil 21: 78– 84, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol 296: R1358– R1365, 2009 [DOI] [PubMed] [Google Scholar]