Abstract
Recent studies report that the menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. The purpose of the present study was to determine whether oral contraceptives (OC) influence sympathetic neural activation during an orthostatic challenge. Based on evidence that sympathetic baroreflex sensitivity (BRS) is increased during the “low hormone” (LH) phase (i.e., placebo pills) in women taking OC, we hypothesized an augmented muscle sympathetic nerve activity (MSNA) response to orthostatic stress during the LH phase. MSNA, mean arterial pressure (MAP), and heart rate (HR) were recorded during progressive lower body negative pressure (LBNP; −5, −10, −15, −20, −30, −40 mmHg; 3 min/stage) in 12 healthy women taking OC (age 22 ± 1 years). Sympathetic BRS was assessed by examining relations between spontaneous fluctuations of diastolic arterial pressure and MSNA. Subjects were examined twice: once during LH phase and once ∼3 wk after LH during the “high hormone” phase (randomized order). Resting MSNA (10 ± 2 vs. 13 ± 2 bursts/min), MAP (85 ± 3 vs. 84 ± 3 mmHg), and HR (62 ± 2 vs. 65 ± 3 beats/min) were not different between phases. MSNA and HR increased during progressive LBNP (P < 0.001), and these increases were similar between phases. Progressive LBNP did not change MAP during either phase. Sympathetic BRS increased during progressive LBNP, but these responses were not different between LH and high hormone phases. In conclusion, our results demonstrate that OCs do not alter cardiovascular and sympathetic neural responses to an orthostatic challenge in young, healthy women.
Keywords: muscle sympathetic nerve activity, arterial blood pressure, lower body negative pressure, baroreflex, menstrual cycle
autonomic adjustments during an orthostatic challenge are essential for the maintenance of arterial blood pressure. Most notably, increases in muscle sympathetic nerve activity (MSNA) are immediate and graded to the level of the orthostatic stress (18). Therefore, altered patterns of sympathoexcitation during orthostatic stress are clinically relevant. Recently, two studies examined MSNA responses to orthostatic stress during the early follicular and midluteal phases of the menstrual cycle in young, eumenorrheic women (2, 4). Despite the use of different methods to induce orthostatic stress [head-up tilt vs. lower body negative pressure (LBNP)], both studies reported an augmented MSNA response to orthostatic stress during the midluteal phase (2, 4). Thus hormonal fluctuations of endogenous estrogen and progesterone associated with a normal menstrual cycle appear to influence sympathoexcitation during orthostatic stress.
In the United States, it is estimated that nearly 20% of women are currently prescribed oral contraceptives; additionally, since their introduction in the 1960s, ∼80% of women have used oral contraceptives at some point in their life (16). The most frequently used oral contraceptive is the combined oral contraceptive pill, which contains a combination of ethinyl estradiol and progestin. These synthetic forms of estrogen and progesterone prevent normal fluctuations of endogenous estrogen and progesterone observed in eumenorrheic women, leading to a suppression of ovulation. To date, only one study has examined the influence of oral contraceptives on MSNA. Minson et al. (11) reported no difference in resting MSNA between low hormone (LH) and high hormone (HH) phases of oral contraception. However, sympathetic baroreflex sensitivity was increased during LH phase compared with HH phase when blood pressure was pharmacologically altered (i.e., modified Oxford technique). Altered sympathetic baroreflex sensitivity could directly influence sympathetic neural responses during an orthostatic challenge, yet the effects of oral contraceptives on MSNA during an orthostatic stress have not been determined.
Therefore, the purpose of the present study was to determine the influence of oral contraceptives on MSNA responses to orthostatic stress in young, healthy women. Progressive LBNP was used to induce the orthostatic stress. Based on previous findings that sympathetic baroreflex sensitivity is augmented during the LH phase of oral contraceptives (11), we hypothesized an augmented MSNA response to lower LBNP during the LH phase.
METHODS
Subjects.
Twelve healthy women (age 22 ± 1 years, height 168 ± 1 cm, weight 65 ± 2 kg) who were taking oral contraceptives participated in the study. Subject exclusion criteria included smoking, diabetes, cardiovascular disease, and autonomic dysfunction. All women were taking combined oral contraception (combined ethinyl estradiol and progestin; described below). All subjects were instructed to abstain from exercise, caffeine, and alcohol for 12 h before the laboratory testing. The Michigan Technological University Institutional Review Board approved the experimental protocols, and all participants signed a written informed consent.
Experimental design.
Subjects were tested once during the LH phase (3 ± 0 days after start of menstruation) and again during the HH phase (23 ± 0 days after start of menstruation). The initial testing phase (LH vs. HH) was randomized. On the days of testing, the subjects reported for a blood draw to document plasma levels of endogenous estradiol and progesterone. All participants were tested during the same time of day for both trials. After 5 min of supine rest (i.e., baseline), six consecutive stages of progressive LBNP were applied. MSNA, heart rate (HR), and beat-to-beat arterial blood pressure were acquired throughout the study.
LBNP.
All subjects wore a neoprene skirt designed to form an airtight seal between the subject and the LBNP chamber. LBNP was applied below the iliac crest, resulting in a redistribution of blood away from the upper body to the abdomen and lower extremities. Progressive LBNP was applied at −5, −10, −15, −20, −30, and −40 mmHg in 3-min stages after the 5-min baseline period. These levels of LBNP do not typically induce presyncope, but subjects were monitored closely, and the experiment would have been stopped if any presyncopal signs were observed [i.e., symptoms of lightheadedness, nausea, diaphoresis, or a decrease of systolic arterial pressure (SAP) to <80 mmHg]. None of the participants experienced presyncope during the present study.
Oral contraceptives.
All subjects had been taking oral contraceptives for at least 1 year and had been on their current oral contraceptive for a minimum of 2 mo. Only subjects with consistent menstrual cycles were included in the study (26–30 days in length). Nine subjects were taking Ortho-Cyclen or Ortho-Tricyclen (Ortho-McNeil-Janssen Pharmaceuticals), which includes 35 μg of ethinyl estradiol and 0.25 mg of norethindrone during the HH phase. Two subjects were taking Yasmin (Bayer Schering Pharma), which includes 30 μg of ethinyl estradiol and 3.0 mg of drospirenone during the HH phase. One subject was taking Kariva (Barr Laboratories), which includes 20 μg of ethinyl estradiol and 0.15 mg of desogestrel during the HH phase. Thus, all oral contraceptives included consistent doses of ethinyl estradiol (20–35 μg) but varied slightly in the type of progestin (norethindrone, drospirenone, and desogestrel). When the three subjects taking drospirenone and desogestrel were compared with the nine subjects taking norethindrone, results were not different. Thus data are presented as mean values based on all 12 subjects.
Measurements.
Multifiber recordings of MSNA were made by inserting a tungsten microelectrode into the peroneal nerve of a resting leg. A reference electrode was inserted subcutaneously 2–3 cm from the recording electrode. Both electrodes were connected to a differential preamplifier and then to an amplifier (total gain of 80,000) where the nerve signal was band-pass filtered (700–2,000 Hz) and integrated (time constant = 0.1) to obtain a mean voltage display of the nerve activity. Satisfactory recordings of MSNA were defined by spontaneous, pulse synchronous bursts that increased during end-expiratory apnea and did not change during auditory stimulation.
Arterial blood pressure was measured using two techniques. Resting arterial blood pressure was measured three consecutive times (separated by ∼1-min intervals) using an automated sphygmomanometer and reported as a mean value. Beat-to-beat arterial blood pressure was recorded continuously via Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). Although the Finometer accurately determines relative changes in arterial blood pressure, it should not be used to determine absolute values. Thus the Finometer was used to determine precise changes in arterial blood pressure that occurred during LBNP, whereas the sphygmomanometer allowed us to compare baseline arterial blood pressure during the LH and HH phases. Arterial blood pressures are expressed as SAP, diastolic (DAP), and mean (MAP) arterial pressures. We recorded HR using a three-lead electrocardiogram. Microneurography recordings were obtained during both the LH and HH phases in 10 of the 12 subjects. A shift of the neurogram during progressive LBNP prevented repeated-measures analysis of the LBNP data in two subjects; thus we report MSNA responses to LBNP during LH and HH phases in a total of eight subjects.
Data analysis.
Data were imported and analyzed in the WinCPRS software program (Absolute Aliens, Turku, Finland). R-waves were detected and marked in the time series. Bursts of MSNA were automatically detected on the basis of amplitude, with the use of a signal-to-noise ratio of 3:1, within a 0.5-s search window centered on a 1.3-s expected burst peak latency from the previous R-wave. Potential bursts were displayed and edited by one investigator. The average area of the bursts occurring during baseline was normalized to a mean value of 100. MSNA was expressed as bursts per minute and total burst activity (i.e., the sum of the normalized burst areas). HR, blood pressure, and MSNA are reported as the average for the corresponding stage (i.e., 5 min for baseline and 3 min for each LBNP stage).
Sympathetic baroreflex sensitivity was determined by examining the relations between spontaneous fluctuations in DAP and MSNA (5–8, 12). This analysis has been described in detail previously (2). Briefly, DAPs for each cardiac cycle were grouped into 3 mmHg intervals (bins) during baseline and each stage of LBNP. Burst incidence, total MSNA, and burst strength for each DAP bin were calculated and plotted against the corresponding DAP. The slopes of these relationships were determined using linear regression analysis. All linear regression analyses were weighted for the number of cardiac cycles within each DAP bin, and a minimum r value of 0.5 was used as the criteria for accepting slopes. Thus baseline linear regression slopes were determined from 5 min of data, whereas linear regression slopes for each LBNP stage were determined from 3 min of data.
Statistical analysis.
All data were analyzed statistically using commercial software (SPSS 15.0, SPSS, Chicago, IL). A two-way repeated-measures ANOVA was utilized to determine whether changes in MSNA, SAP, DAP, MAP, HR, and DAP-MSNA slopes occurred during each intervention (baseline, −5, −10, −15, −20, −30, and −40 mmHg) and across trials (LH and HH phases). Resting variables were compared using paired t-tests. Means were considered significantly different at P < 0.05. Results are expressed as means ± SE.
RESULTS
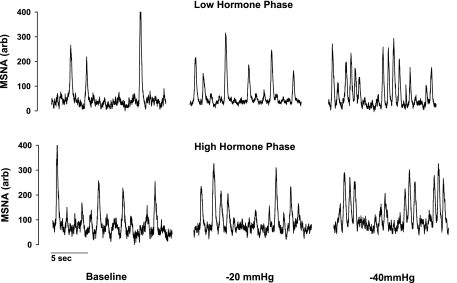
Resting baseline values during the LH and HH phases are presented in Table 1. Plasma estradiol and progesterone were not different between the LH and HH phases. Likewise, resting MSNA, MAP, and HR were similar between the LH and HH phases. Representative neurograms of MSNA during both phases are illustrated in Fig. 1.
Table 1.
Baseline values during low and high hormone phases of oral contraception
| Variable | LH | HH | P Value |
|---|---|---|---|
| Estradiol, pg/ml | 43±7 | 28±4 | 0.08 |
| Progesterone, ng/ml | 1.3±0.1 | 1.5±0.2 | 0.11 |
| MSNA, bursts/min | 10±2 | 13±2 | 0.38 |
| SAP, mmHg | 112±3 | 111±3 | 0.71 |
| DAP, mmHg | 72±3 | 71±3 | 0.60 |
| MAP, mmHg | 85±3 | 84±3 | 0.63 |
| HR, beats/min | 62±2 | 65±3 | 0.12 |
Values are mean ± SE; n = 12 subjects unless noted otherwise. MSNA, muscle sympathetic nerve activity (n = 10); SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial blood pressure; HR, heart rate; LH, low hormone phase; HH, high hormone phase.
Fig. 1.
Representative neurograms obtained from 1 subject at baseline, −20 mmHg, and −40 mmHg of lower body negative pressure (LBNP) during the low and high hormone phases of oral contraception. Oral contraceptives did not alter resting muscle sympathetic nerve activity (MSNA) or MSNA responses to LBNP.
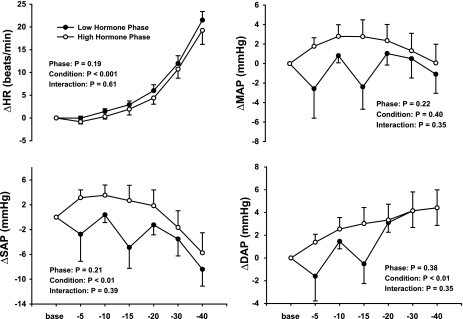
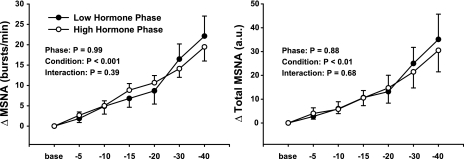
Figure 2 demonstrates that the hemodynamic responses to progressive LBNP were not different between the LH and HH phases of the menstrual cycle. Progressive LBNP increased HR (P < 0.001) and DAP (P < 0.01), decreased SAP (P < 0.01), but did not change MAP (P > 0.40) during the LH and HH phases of the menstrual cycle. Figure 3 demonstrates that progressive LBNP increased MSNA burst frequency (bursts/min; P < 0.001) and total MSNA (arbitrary units; P < 0.01) during both the LH and HH phases and that these increases in MSNA during progressive LBNP were similar between phases (phase × condition interactions; P = 0.39 for burst frequency, P = 0.68 for total MSNA).
Fig. 2.
Changes (Δ) in heart rate (HR) and systolic (SAP), diastolic (DAP), and mean (MAP) arterial pressures during progressive LBNP. Subjects were examined during the low and high hormone phases of oral contraception. Progressive LBNP increased HR and DAP, decreased SAP, and did not change MAP. Hemodynamic responses were similar between phases. Results are means ± SE (n = 12). Base, baseline.
Fig. 3.
Changes in MSNA during progressive LBNP. Subjects were examined during the low and high hormone phases of oral contraception. Progressive LBNP elicited similar increases of MSNA burst frequency and total MSNA during the low and high hormone phases. Results are means ± SE (n = 8).
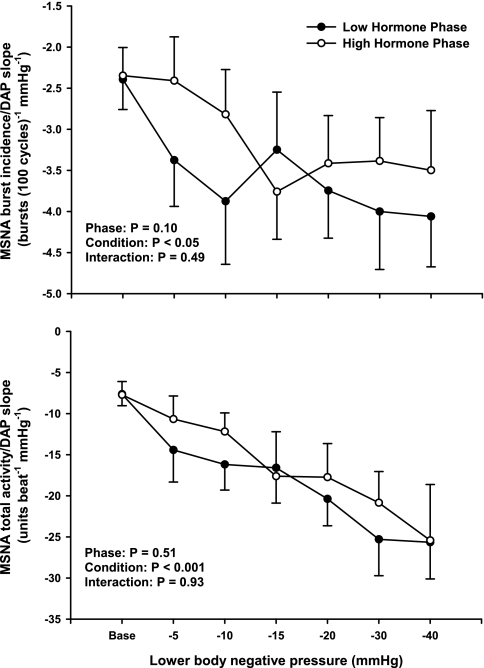
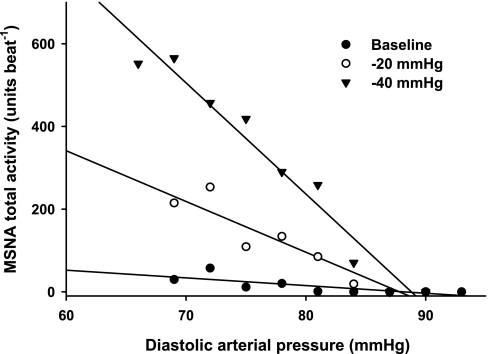
Sympathetic baroreflex sensitivities during LH and HH phases of the menstrual cycle are presented in Fig. 4. The more negative a DAP-MSNA slope becomes, the greater the sympathetic baroreflex sensitivity. A total of 112 linear regression slopes were calculated (8 subjects × 2 phases × 7 conditions), with an average of 7.5 ± 0.2 DAP bins per slope (range 5–14). Progressive LBNP significantly increased sympathetic baroreflex sensitivity during both LH and HH phases (burst incidence condition, P < 0.05; total MSNA condition, P < 0.001). Increases of sympathetic baroreflex sensitivity during LBNP were not different between phases, whether assessed as burst incidence or total MSNA. The correlation coefficients for the burst incidence linear regression slopes ranged from 0.80 ± 0.07 to 0.93 ± 0.03, whereas the correlation coefficients for total MSNA linear regression slopes ranged from 0.74 ± 0.07 to 0.94 ± 0.04 during baseline and all LBNP stages. In contrast, linear regression slopes for “burst strength” did not yield correlation coefficients >0.5; thus linear regression slopes for “burst strength” are not reported. Representative data from one subject is presented in Fig. 5, illustrating that sympathetic baroreflex sensitivities increase (as a function of the MSNA-DAP slope) during progressive LBNP.
Fig. 4.
Linear relations between MSNA and DAP during low and high hormone phases of oral contraception. Progressive LBNP elicited significant reductions in the MSNA-DAP slopes during both low and high hormone phases, indicating sympathetic baroreflex sensitivities increased during orthostatic stress. The increases in sympathetic baroreflex sensitivity were not different between phases. Results are means ± SE (n = 8).
Fig. 5.
Representative linear regression slopes from 1 subject during baseline, −20 mmHg, and −40 mmHg LBNP. Slopes became more negative with increasing levels of LBNP, indicating sympathetic baroreflex sensitivity increased with increasing levels of orthostatic stress. Data are from the low hormone phase, as results were similar between phases.
DISCUSSION
The present study examined the influence of oral contraceptives on MSNA at rest and during graded LBNP. Our results reveal three novel findings. First, MSNA, blood pressure, and HR responses to orthostatic stress are similar during LH and HH phases of oral contraception. Although this was contrary to our original hypothesis, we believe that these findings can be explained when one considers the recent studies demonstrating an augmentation of MSNA responses to orthostatic stress during the midluteal phase in eumenorrheic women (2, 4). Second, oral contraceptives did not alter sympathetic baroreflex sensitivity. This finding challenges previous data that suggested an increased sympathetic baroreflex sensitivity during the LH phase (11). Third, oral contraceptives did not alter resting MSNA. This finding supports previously presented data (11). Overall, this study advances our understanding of how oral contraceptives influence sympathetic neural activity at rest and during an orthostatic challenge. Moreover, when taken together with recent studies demonstrating augmented MSNA responses to orthostatic stress during the high endogenous hormone phase (i.e., midluteal) in eumenorrheic women (2, 4), these findings support the concept that fluctuations of endogenous estradiol and progesterone help modulate patterns of sympathoexcitation during an orthostatic stress.
It is important to consider the present data in context with two recent studies that examined the influence of menstrual phase on MSNA responses to othostatic stress in young, eumenorrheic women (2, 4). These studies reported that total MSNA, but not burst frequency, responses to head-up tilt (4) and LBNP (2) are significantly augmented during the midluteal phase of the menstrual cycle when compared with the early follicular phase. Thus it appears that the menstrual cycle is associated with altered MSNA patterns of sympathoexcitation during an orthostatic stress and that there is an augmentation of MSNA when levels of estradiol and progesterone are elevated.
In contrast to these previous findings in eumenorrheic women, the present study demonstrated no difference in either MSNA burst frequency or total MSNA responses to LBNP during the LH and HH phases of oral contraception. Therefore, unlike their eumenorrheic counterparts, women taking oral contraceptives do not experience altered patterns of sympathetic neural activation during a monthly menstrual cycle. In fact, the MSNA responses to LBNP were remarkably similar during the LH and HH phases (Fig. 3). The oral contraceptives in the present study used a combination of ethinyl estradiol and synthetic progestin to suppress ovulation, resulting in similar levels of endogenous estradiol and progesterone during the LH and HH phases (Table 1). When taken in conjunction with the findings of Carter et al. (2) and Fu et al. (4), our findings suggest the fluctuations of endogenous estradiol and progesterone in young, healthy women play an important role in sympathoexcitaton during orthostatic stress. The physiological significance of this relationship remains unclear, but it may have implications for the control of arterial blood pressure during orthostasis.
Sympathetic baroreflex sensitivity was determined in the present study by examining the relations between spontaneous fluctuations of DAP and MSNA (5–8, 12). Our data demonstrate no differences in sympathetic baroreflex sensitivity during LH and HH phases of oral contraception at rest or during progressive LBNP. However, much like the eumenorrheic women (2, 4), sympathetic baroreflex sensitivity increased as the level of LBNP became more negative. Thus, taken together with previous studies of eumenorrheic women (2, 4), it appears that fluctuations of endogenous or synthetic forms of estrogen and progesterone do not alter sympathetic baroreflex sensitivity in young, healthy women.
To our knowledge, only one other study has assessed sympathetic baroreflex sensitivity in women taking oral contraceptives, and this study reported augmented sympathetic baroreflex sensitivity during the LH phase compared with the HH phase in women who were taking oral contraceptives (11). The discrepancies between our present study and Minson et al. (11) may be due to differences in the technique used to assess sympathetic baroreflex sensitivity. The present study used the spontaneous DAP-MSNA slope analysis, whereas Minson et al. (11) used the modified-Oxford technique, a pharmacological method that induces arterial blood pressure changes through intravenous bolus injections of sodium nitroprusside and phenylephrine. Both techniques offer various strengths and weaknesses. In particular, the modified-Oxford offers a time-honored technique that elicits changes in arterial blood pressure outside the normal “physiological” range, but these ranges can only be reached during pharmacological manipulation. In contrast, the spontaneous DAP-MSNA slope analysis determines sympathetic baroreflex sensitivity during nonpharmacological conditions but is limited to a smaller range of blood pressure (i.e., the spontaneous fluctuations of blood pressure, typically ∼20 mmHg range). Therefore, differences between the present study and Minson et al. (11) may be related to the different arterial pressure ranges used to assess sympathetic baroreflex sensitivity. Nevertheless, the results of the present study do not support the existing concept that oral contraceptives alter sympathetic baroreflex sensitivity. Furthermore, the present study is the first to determine sympathetic baroreflex sensitivity during an orthostatic challenge in women taking oral contraceptives, and, although sensitivity appears to increase during orthostatic stress, these increases were not different between LH and HH phases.
Although discrepancies exist regarding sympathetic baroreflex sensitivity, our results support the resting MSNA data reported by Minson et al. (11). Specifically, our results demonstrate that oral contraceptives did not alter resting MSNA. This is important because the influence of endogenous fluctuations of estrogen and progesterone on resting MSNA remains controversial in eumenorrheic women (1–4, 9, 10, 14). In particular, some studies demonstrate an augmentation of resting MSNA during the midluteal phase of the menstrual cycle compared with the early follicular phase (10, 14), whereas others report no difference in resting MSNA across menstrual phases (1–4, 9). The reasons underlying these differences remain unclear.
We recognize two important limitations. First, the type of oral contraceptive was not uniform. Nine of the 12 subjects took similar oral contraceptives (35 μg of ethinyl estradiol and 0.25 mg of norethindrone during the HH phase), whereas 3 of the 12 took slightly varied doses of ethinyl estradiol and type of progestin. Because the goal of this study was to prevent the endogenous fluctuations of estrogen and progesterone, it was determined that a slight variation in the type of oral contraceptive, although not ideal, was acceptable. Second, central venous pressure was not measured in the present study. Exogenous and endogenous hormones can influence intravascular volume (13, 17). Thus, despite applying similar levels of progressive LBNP during both the LH and HH phases, we cannot exclude the possibility that these similar levels of LBNP had different influences on intravascular volume shifts.
In conclusion, the present study demonstrates that oral contraceptives do not modulate sympathetic neural and cardiovascular responses at rest or during an orthostatic challenge. Similarly, sympathetic baroreflex sensitivities were similar during the LH and HH phases of oral contraception. Taken together with recent reports that the menstrual cycle alters MSNA responses to orthostatic stress in eumenorrheic women (2, 4), our results suggest normal fluctuations of endogenous estradiol and progesterone influence sympathoexcitatory patterns during an orthostatic challenge in young, healthy women. The physiological significance of these relations remains uncertain, but they could be important to understanding why women are more prone than men to orthostatic intolerance.
Perspectives and Significance
The present and previous studies (2, 4) examining the influence of the menstrual cycle on MSNA responses during an orthostatic stress have focused on young women (i.e., ∼20 years). Future studies that examine elderly women may be warranted. Specifically, menopause leads to dramatic decreases in plasma levels of estrogen and progesterone, yet the influence of menopause on sympathetic neural responses to orthostatic challenge have not been determined. Furthermore, the influence of long-term oral contraceptive use (i.e., prolonged suppression of endogenous fluctuations of estrogen and progesterone) is unclear. Such research could be helpful in explaining why the elderly, and particularly elderly women, are more prone to autonomic dysfunction and subsequent othostatic hypotension (15).
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grant HL-088689.
ACKNOWLEDGMENTS
The authors thank Jessica Cross and Johnathan Lawrence for technical assistance.
REFERENCES
- 1.Carter JR, Lawrence JE. Effects of the menstrual cycle on sympathetic neural responses to mental stress in humans. J Physiol 585: 635– 641, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 297: E85– E91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol 85: 2075– 2081, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019– 2031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichinose M, Saito M, Fujii N, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during severe orthostatic stress. J Physiol 576: 947– 958, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichinose M, Saito M, Kitano A, Hayashi K, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during mild orthostatic stress in humans. J Physiol 557: 321– 330, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445– 451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861– 869, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence JE, Ray CA, Carter JR. Vestibulosympathetic reflex during the early follicular and midluteal phases of the menstrual cycle. Am J Physiol Endocrinol Metab 294: E1046– E1050, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862– 868, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation 102: 1473– 1476, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H2202– H2209, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Oian P, Tollan A, Fadnes HO, Noddeland H, Maltau JM. Transcapillary fluid dynamics during the menstrual cycle. Am J Obstet Gynecol 156: 952– 955, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Park J, Middlekauff HR. Altered pattern of sympathetic activity with the ovarian cycle in female smokers. Am J Physiol Heart Circ Physiol 297: H564– H568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study CHS Collaborative Research Group. Hypertension 19: 508– 519, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol 53: 221– 231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stachenfeld NS, Keefe DL, Palter SF. Estrogen and progesterone effects on transcapillary fluid dynamics. Am J Physiol Regul Integr Comp Physiol 281: R1319– R1329, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Sundlof G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol 278: 525– 532, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]